- 1National Clinical Research Center for Metabolic Diseases, Key Laboratory of Diabetes Immunology, Ministry of Education, and Department of Metabolism and Endocrinology, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Phase I Clinical Trial Center and Department of Pharmacy, The Second Xiangya Hospital of Central South University, Changsha, China
- 3Department of Clinical Research and Development, Jiangsu Hengrui Pharmaceuticals Co., Ltd., Shanghai, China
- 4National Clinical Research Center for Metabolic Diseases, Hunan Provincial Key Laboratory of Metabolic Bone Diseases, Department of Metabolism and Endocrinology, and Health Management Center, The Second Xiangya Hospital of Central South University, Changsha, China
SHR-1222 is a humanized monoclonal antibody targeting sclerostin and has the potential to promote bone formation and reduce bone resorption. This study was aimed to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and immunogenicity of SHR-1222 in healthy men and postmenopausal women with low bone mass (BMD). It was a randomized, double-blind, placebo-controlled, dose-escalation, phase I study. Subjects received SHR-1222 at 50, 100, 200, 300, and 400 mg sequentially or matching placebo subcutaneously. Totally, 50 subjects with low BMD were enrolled and randomly assigned; 10 received placebo and 40 received SHR-1222 (50 mg, n = 4; 100, 200, 300, or 400 mg, n = 9). The most common adverse events that occurred at least 10% higher in subjects with SHR-1222 treatment than those with placebo were decreased blood calcium, blood urine present, increased blood cholesterol, electrocardiogram T wave abnormal, urinary tract infection, increased blood pressure diastolic, and positive bacterial test. All the above adverse events were mild in severity and well resolved except one of increased blood cholesterol in a subject lost to follow-up. The serum SHR-1222 concentration increased in a dose-dependent manner. Administration of SHR-1222 upregulated the bone-formation markers N-terminal propeptide of type 1 procollagen, osteocalcin, and bone-specific alkaline phosphatase, while downregulated the bone-resorption marker β-C-telopeptide. The BMD at the lumbar spine notably rose after a single dose of SHR-1222. The largest increase occurred in the 400 mg cohort (3.8, 6.7, and 6.1% on day 29, 57, and 85, respectively; compared with 1.4, 0.8, and 1.0% in the placebo group). Although 10.0% of subjects receiving SHR-1222 tested positive for anti–SHR-1222 antibodies, no obvious effects of antibody formation were found on pharmacokinetics. Overall, SHR-1222 was well tolerated at doses from 50 to 400 mg and is a promising new remedy for osteoporosis.
Clinical Trial Registration: http://www.clinicaltrials.gov, NCT03870100.
Introduction
Osteoporosis is a skeletal metabolic disorder characterized by low bone density, reduced bone quality, and increased skeletal fragility and fractures (Armas and Recker, 2012). Many risk factors are associated with osteoporosis, including ageing, female gender, estrogen deficiency, alcohol, smoking, low body mass index, low dietary calcium intake, vitamin D deficiency, and insufficient exercise. Due to loss of protective effect of estrogen on bone, postmenopausal women are at a high risk of osteoporosis. It is estimated that the prevalence of osteoporosis at the spine or hip in China was 6.46 and 29.13% for men and women aged 50 years and older, respectively (Zeng et al., 2019). Approximately 50% of women and 20% of men with osteoporosis aged over 50 years will develop a fracture (Coughlan and Dockery, 2014).
Pharmacological treatments for osteoporosis are either anti-resorptive or anabolic drugs. Bisphosphonates, the most widely used anti-osteoporosis medication, inhibit bone resorption by inducing the apoptosis of osteoclasts actively engaged in the degradation of mineral on the bone surface and reduce the risk of fracture (Drake et al., 2008). However, patients should be noticed of gastrointestinal toxicities of oral administration, osteonecrosis of the mandible, and atypical femoral fractures caused by long-term use. Besides, the activity of osteoblasts is also blocked by bisphosphonates, which may subsequently lead to suppressed bone remodeling (Burr et al., 2003). Otherwise, anabolic drugs such as parathyroid hormone analogs and sclerostin inhibitors can promote bone formation and bone remodeling (Solling et al., 2019). Teriparatide, a typical parathyroid hormone analog, improves bone quality and bone mass by stimulating osteoblastic bone formation and reduces the risk of fracture in osteoporosis patients (Hodsman et al., 2005). However, the approved lifetime duration of treatment with teriparatide is limited to only 24 months because of the rat toxicology finding of osteosarcoma (Lindsay et al., 2016).
Sclerostin is not only a negative regulator of bone formation by upregulating the Wnt pathway, but also an enhancer of osteoclastogenesis by increasing the synthesis of receptor activators of NF-κB ligand (Poole et al., 2005; Paszty et al., 2010; Wijenayaka et al., 2011). Sclerostin inhibitors such as romosozumab have the prominent potential to enhance bone mass by promoting bone formation and reducing bone resorption (Padhi et al., 2011; Padhi et al., 2014). It has been demonstrated that romosozumab prevented new vertebral fracture more significantly than placebo or alendronate did in postmenopausal osteoporosis patients (Cosman et al., 2016; Saag et al., 2017). Currently, romosozumab is the only monoclonal antibody of sclerostin that has been approved by the US FDA and EU EMA since 2019 (EMA, 2019; FDA, 2019). Regrettably, romosozumab was found of possible cardiovascular disease risks, which may restrict its application (Saag et al., 2017). Moreover, romosozumab is not available in countries or regions outside the United States and the European Union. Developing alternatives to romosozumab is then an urgent need.
SHR-1222 is a newly generated humanized monoclonal antibody targeting sclerostin. Our preclinical studies showed that SHR-1222 could bind to human sclerostin with a high affinity (data on file, Jiangsu Hengrui Pharmaceuticals Co., Ltd.). This study was conducted to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and immunogenicity of SHR-1222 in healthy men and postmenopausal women with low bone mass.
Methods
Study Design
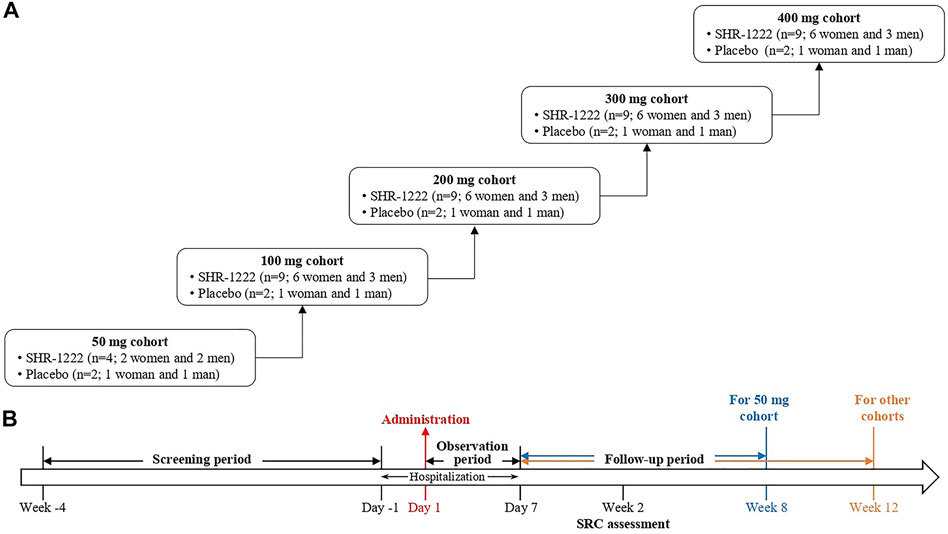
This was a randomized, double-blind, placebo-controlled, dose-escalation, phase I study of SHR-1222 in China (ClinicalTrials.gov Identifier: NCT03870100). Subjects received SHR-1222 at doses of 50, 100, 200, 300, and 400 mg sequentially or matching placebo subcutaneously (Figure 1). In the 50 mg cohort, 6 subjects were randomized, 4 to receive SHR-1222 (2 women and 2 men) and 2 to placebo (1 woman and 1 man). In each of the 100, 200, 300, and 400 mg cohorts, 11 subjects were randomized, 9 to receive SHR-1222 (6 women and 3 men) and 2 to placebo (1 woman and 1 man). The decision to proceed to the next dose cohort was made by the safety review committee composed of investigator and sponsor representatives after all subjects in the previous cohort had been monitored for at least 2 weeks since dosing.
The primary objectives of this study were to evaluate the safety and tolerability of a single subcutaneous injection of SHR-1222 and explore the safe dose range. The secondary objectives were to assess the pharmacokinetics, pharmacodynamics, and immunogenicity of SHR-1222.
The study protocol and all amendments were approved by the Ethics Committee of the Second Xiangya Hospital of Central South University and conducted in accordance with the Good Clinical Practice and Declaration of Helsinki. All subjects provided written informed consent before enrollment. The sponsor participated in study design and statistical analysis. All authors had access to the data, made contributions to the article writing or reviewing, and vouch for its accuracy and completeness.
Study Population
Eligible subjects were healthy men or postmenopausal women between 45 and 59 years of age with low bone mineral density (BMD). Postmenopausal was defined as having bilateral oophorectomy 6 weeks before or having at least 12 months of spontaneous amenorrhea confirmed by a serum follicle-stimulating hormone concentration greater than 40 U/L. Low BMD was defined as a T-score between −2.5 and −1.0 (not including −2.5, −1.0) at the lumbar spine (L1–L4, one or more vertebra) or femoral neck, with modified WHO criteria (Kanis, 2002). Key exclusion criteria included any condition that would affect bone metabolism; prior estrogen/progesterone replacement therapy, calcitonin, parathyroid hormone and its analogs, doses of vitamin D greater than 1000 IU/day, glucocorticosteroids, anabolic steroids, or calcitriol and its analogs within the previous 6 months; prior bisphosphonates or fluoride for osteoporosis within the previous 12 months; a bone fracture within the previous 6 months; or a T-score of −2.5 or less at the lumbar spine (L1–L4) or femoral neck.
Study Procedures
Laboratory examinations, vital signs, physical examinations, 12-lead electrocardiogram and echocardiography were performed for safety assessment. Adverse events were evaluated and coded according to MedDRA (Medical Dictionary for Regulatory Activities, version 22.0). Dual energy X-ray absorptiometry scans were performed for BMD values by the same Hologic Discovery Wi Bone Densitometer (Hologic, Bedford, MA, United States) at baseline, week 4 and 8 for the 50 mg cohort and at baseline, week 4, 8, and 12 for other cohorts. Instrument quality control was produced to ensure the coefficient of variation (CV) under 1% throughout the study. The least significant change of BMD was 2.8% according to the calculation based on the CV value (Hangartner, 2007).
Blood samples were collected on day 1 (pre-dose, 1 and 8 h post dose), 2, 3, 4, 5, 6 and 7 during the observation period, and from day 8 to week 12 (except for the 50 mg cohort, to week 8) during the follow-up period. Pharmacokinetic analyses were performed at the Shanghai InnoStar Bio-tech Co., Ltd., including time to maximum serum concentration (Tmax), maximum serum concentration (Cmax), area under the plasma concentration-time profile (AUC), half-life (t½), apparent clearance (CL/F) and apparent volume of distribution (Vd/F), and mean residence time (MRT). PD biomarkers of N-terminal propeptide of type 1 procollagen (P1NP), osteocalcin (OST), bone-specific alkaline phosphatase (BSAP), and β-C-telopeptide (β-CTx) were analyzed at the Guangzhou KingMed Center using chemiluminescence methods (Roche Diagnostics Test Kits for P1NP, OST and β-CTx; Beckman Coulter Diagnostics Test Kits for BASP). Anti–SHR-1222 antibodies were determined at the Shanghai InnoStar Bio-tech Co., Ltd. using a validated Meso Scale Discovery electrochemiluminescence immunoassay.
Statistical Analysis
All subjects who received placebo in different dose cohorts were combined into one placebo group. Subjects who had ever received SHR-1222 or placebo were included for safety analysis. Serum SHR-1222 concentration-time data were analyzed by noncompartmental methods. The PK analyses included all treated subjects for whom the PK parameters could be estimated. Percentage changes in bone metabolic markers and BMD from baseline were calculated. Data of safety, the serum SHR-1222 concentration, pharmacokinetic parameters, and percentage changes in PD biomarkers and BMD were grouped by the treatment and dose and summarized with descriptive statistics.
Results
Study Population
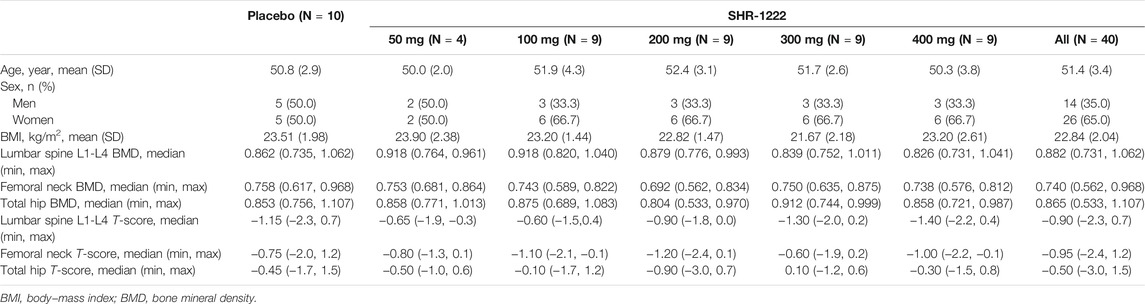
A total of 50 subjects with low BMD were enrolled and randomly assigned; 10 received placebo and 40 received SHR-1222 (50 mg, n = 4; 100, 200, 300, or 400 mg, n = 9). Of these subjects, 49 completed the study except one in the 100 mg SHR-1222 cohort due to loss to follow-up. Table 1 shows the demographics and baseline characteristics of the study population. There were 31 (62.0%) women and 19 (38.0%) men. The mean age of each cohort ranged from 50.0 to 52.4 years. The median T-score of each cohort ranged from −1.40 to −0.60 at the lumbar spine, −1.20–−0.60 at the femoral neck, and −0.90–0.10 at the total hip.
Safety
SHR-1222 was well tolerated at all doses from 50 to 400 mg. All subjects experienced at least one adverse event during the study, but the majority were mild in severity. Only 7.5% (3/40) of subjects who received SHR-1222 had moderate or worse adverse events. No serious adverse events or adverse events leading to death occurred. No subjects withdrew from the study due to adverse events.
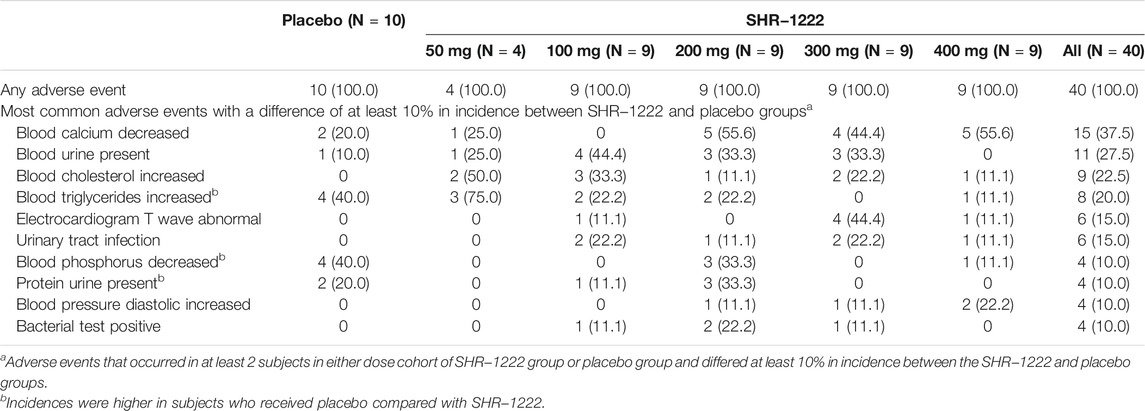
The most common adverse events that occurred at least 10% higher in subjects with SHR-1222 treatment than those with placebo were decreased blood calcium (37.5 versus 20.0%), blood urine present (27.5 versus 10.0%), increased blood cholesterol (22.5% versus 0), electrocardiogram T wave abnormal (15.0% versus 0), urinary tract infection (15.0% versus 0), increased blood pressure diastolic (10.0% versus 0), and positive bacterial test (10.0% versus 0) (Table 2). No dose-dependent trend was observed in the above adverse events except decreased blood calcium (25.0%, 0, 55.6, 44.4, and 55.6% in the 50, 100, 200, 300, and 400 mg cohort respectively). All the above adverse events were mild in severity and well resolved except one of increased blood cholesterol in the SHR-1222 100 mg cohort because the subject was lost to follow-up.
Pharmacokinetics
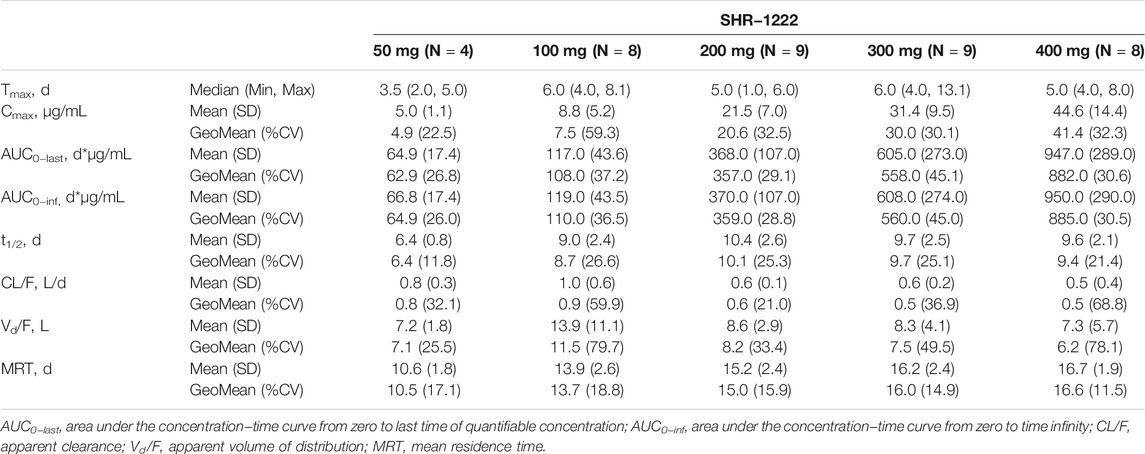
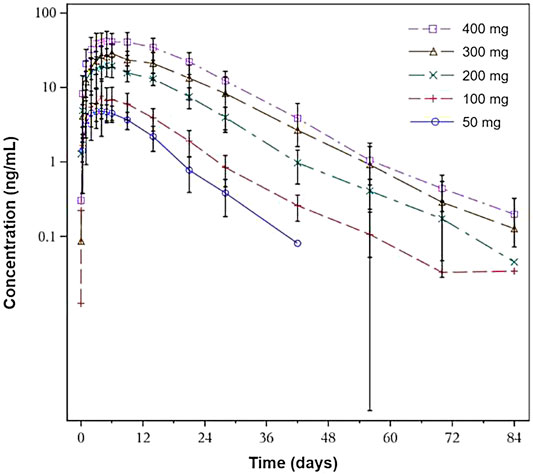
The pharmacokinetic parameters are summarized in Table 3. After a single dose of SHR-1222 ranging from 50 to 400 mg, serum concentrations in terms of Cmax, AUC0-last, and AUC0-inf increased with dose. The serum SHR-1222 concentration increased in a dose-dependent manner (Figure 2; Table 3). Peak SHR-1222 serum concentrations occurred 3.5 days after administration in the 50 mg cohort and ranged from 5.0 to 6.0 days in the 100, 200, 300, and 400 mg cohorts. The mean t1/2 was 6.4 days in the 50 mg cohort and ranged from 9.0 to 10.4 days in other cohorts. The Geometric mean CL/F, Vd/F and MRT ranged from 5.0 to 9.0 L/d, 6.2–11.5 L, and 10.5–16.6 days respectively.
Bone Turnover Markers
Figure 3 shows the percentage changes in levels of the bone-formation markers P1NP, BSAP, and OST and bone-resorption marker β-CTx.
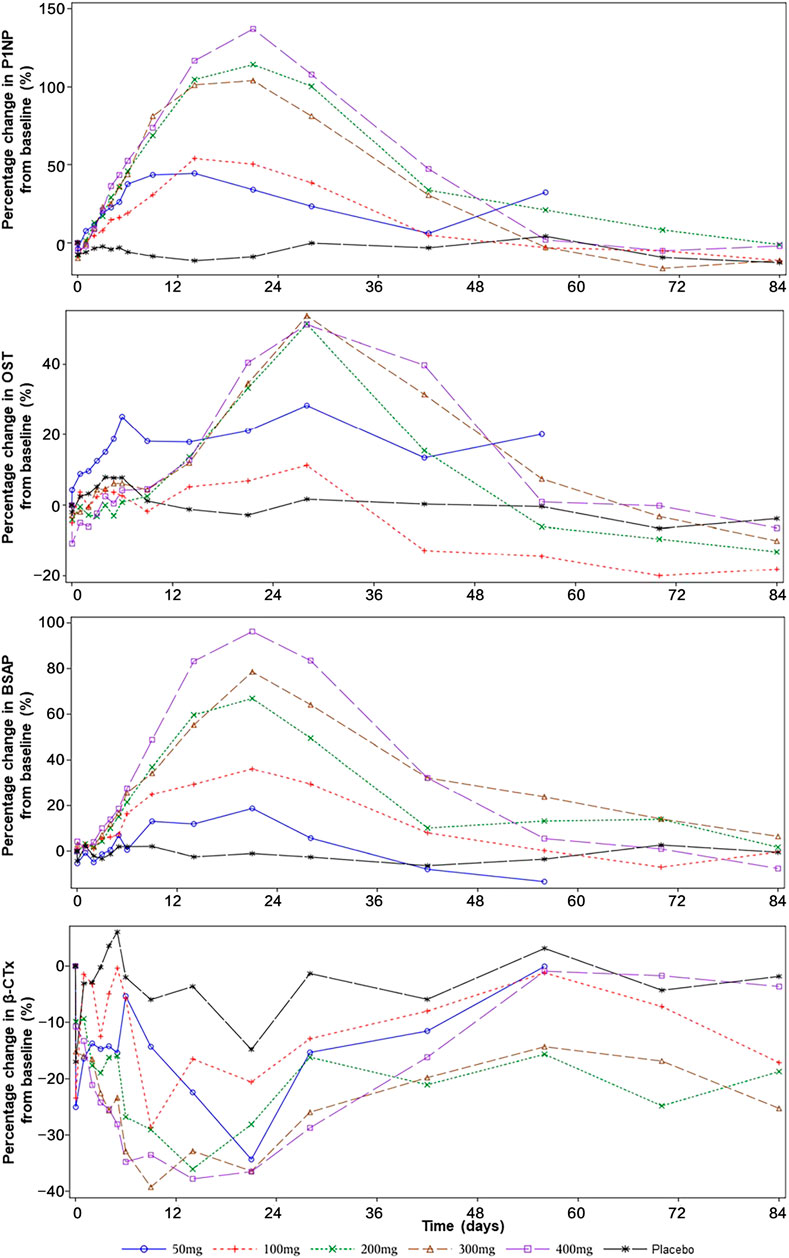
FIGURE 3. The effect of SHR-1222 on bone turnover markers. P1NP, N-terminal propeptide of type 1 procollagen; OST, osteocalcin; BSAP, bone-specific alkaline phosphatase; CTx, C-telopeptide.
After a single dose of SHR-1222 from 50 to 400 mg, the serum levels of P1NP, OST, and BSAP increased from the baseline with the peak changes reached on day 15–22, day 29, and day 22 respectively. Also, dose-dependent increases were noted. The maximum changes of P1NP were 44.5, 54.0, 114.1, 103.9, and 137.0% in the 50, 100, 200, 300, and 400 mg SHR-1222 cohort respectively compared with 4.2% in the placebo group. Similarly, the maximum changes of BSAP were 18.7, 36.2, 66.9, 78.6, and 96.2% in the 50, 100, 200, 300, and 400 mg SHR-1222 cohort respectively compared with 2.7% in the placebo group. A little difference happened in the maximum changes of OST, which increased with the dose from 50 to 200 mg and reached a platform at doses of 200, 300 and 400 mg (28.2, 11.2, 51.2, 53.5, and 51.1% for 50, 100, 200, 300, and 400 mg, respectively; compared with 7.9% for placebo).
As for the bone-resorption marker β-CTx, its level decreased in all SHR-1222 cohorts after a single dose. The bottom levels were reached during day 10–22 (−34.3%, −28.6%, −36.1%, −39.3%, and −37.8% for 50, 100, 200, 300, and 400 mg, respectively; compared with −17.0% for placebo).
BMD at the Lumbar Spine
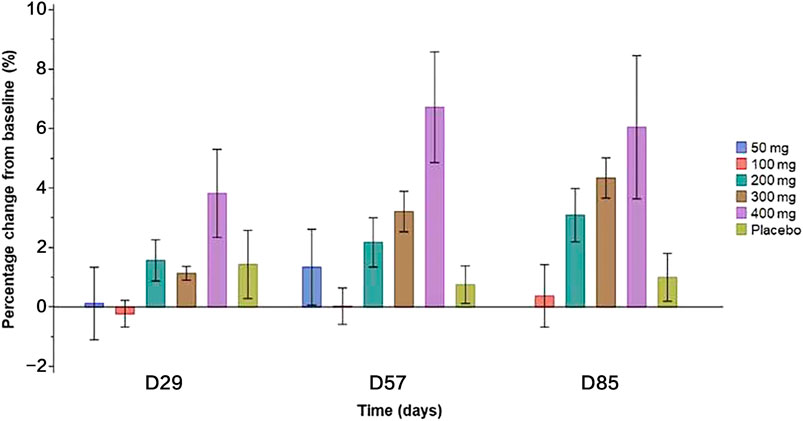
Compared with placebo, a single dose of SHR-1222 at 200, 300, and 400 mg caused increased BMD at the lumbar spine since day 29 (Figure 4), which appeared to be dose-dependent (Figure 4). The largest increase was observed in the 400 mg SHR-1222 cohort with the percentage change of 3.8, 6.7 and 6.1% on day 29, 57, and 85 respectively (compared with 1.4, 0.8 and 1.0% in the placebo group).
Immunogenicity
Of the 40 subjects who received SHR-1222, 4 subjects (10.0%) tested positive for binding anti–SHR-1222 antibodies. However, no obvious effects of antibody formation were found on main pharmacokinetic parameters standardized by dose (AUC0-last/Dose, AUC0-inf/Dose, and Cmax/Dose).
Discussion
This was a study assessing the safety and efficacy of SHR-1222 in healthy men and postmenopausal women with low BMD. Generally, SHR-1222 was safe and well-tolerated. Seven adverse events showed 10–22.5% higher incidences in subjects with SHR-1222 treatment than those with placebo. Among them, decreased blood calcium showed a dose-dependent trend. It was an expected adverse event for sclerostin inhibitors, which had been reported in the preclinical studies of SHR-1222 as well as the phase I study of romosozumab (Padhi et al., 2011). By direct blocking the interaction between sclerostin and low-density lipoprotein receptor-related proteins 5/6, sclerostin inhibitors could promote bone formation, inhibit bone resorption, and make blood calcium to be deposited in the bones, which eventually leads to a decrease in the serum blood calcium level (Winkler et al., 2003; Li et al., 2005; Semenov et al., 2005). Decreased blood calcium is a common adverse event in the treatment of osteoporosis, and vitamin D and calcium supplements are strongly recommended (Weaver et al., 2016; Paschalis et al., 2017; Cano et al., 2018). In phase III clinical trials of romosozumab for osteoporosis, daily calcium and vitamin D were given to all patients (Cosman et al., 2016; Saag et al., 2017). The incidence of hypocalcemia did not exceed 0.2% during the 2-years study period (Cosman et al., 2016; Saag et al., 2017). In our study, calcium and vitamin D were not supplied, and the incidence was 17.5% higher in subjects treated with SHR-1222 compared with placebo. This may indicate a powerful effect of SHR-1222 on calcium deposition in bone. Meanwhile, the adverse events of decreased calcium in this study were all mild in severity and transient in most of the subjects. No subject discontinued the study due to this event. In the following studies of SHR-1222, prophylaxis for decreased blood calcium should be considered by calcium and vitamin D supplementation.
Urinary disorders (blood urine present, bacterial test positive, and urinary tract infection) and cardiovascular disorders (electrocardiogram T wave abnormal and increased blood pressure diastolic) were unexpected adverse events for sclerostin inhibitors. However, in subjects aged between 45 and 59 indexes of urine system, blood pressure and electrocardiogram tend to be easily influenced by many factors. Slightly increased risks of cardiovascular events, heart attack, and stroke were observed in the efficacy trial of romosozumab (n = 4,093, Saag et al., 2017), which brought about a black box warning in romosozumab’s label (FDA, 2019). This kind of cardiovascular safety issue did not occur in this SHR-1222 study. Due to the small sample size, evidence is insufficient to say that SHR-1222 has disadvantages in the urinary tract or cardiovascular system. Further studies and more data are needed.
Compared with subjects receiving placebo, those with SHR-1222 showed a higher incidence of increased blood cholesterol but a lower incidence of increased blood triglycerides. The lower incidence of increased triglycerides could be explained as inhibition of sclerostin could increase fatty acid oxidation, reduce de novo fatty acid synthesis in adipocytes (Kim et al., 2017) and promote beige adipogenesis (Fulzele et al., 2017). The mechanism of the effect of SHR-1222 on cholesterol metabolism is unclear. Increased blood cholesterol found in this study was manageable: mild in severity; transient without obvious clinical meaningful impact; spontaneously resolved in all subjects except in one with the outcome unknown because of the loss to follow-up; and causing no withdrawal from the study. It is suggested that blood lipid levels should be monitored closely in the following studies.
After a single subcutaneous injection of SHR-1222, the serum level of SHR-1222 increased with the doses ranging from 50 to 400 mg in terms of Cmax, AUC0-last, and AUC0-inf. The increase ratio was greater than that of the dose. The Tmax was 5.0–6.0 days and the half-life was 9.0–10.4 days at doses of 100–400 mg, similar to those parameters of romosozumab (5 and 12.8 days, respectively) (FDA, 2019).
SHR-1222 showed optimistic effects to promote bone formation, inhibit bone resorption, and increase bone density. The increase in BMD at the lumbar spine caused by SHR-1222 was similar to that by romosozumab at 3 mg/kg 3 months after a single dose (Padhi et al., 2011). Also, the maximum changes in P1NP, BSAP, and β-CTx following a single dose of SHR-1222 at 200 mg were comparable with those following a single dose of romosozumab at 3 mg/kg which is the approved recommended dosage for a 70 kg woman (Padhi et al., 2011).
SHR-1222 showed similar immunogenicity results compared with romosozumab. The drug-related anti-drug antibodies were detected in 10.0% of SHR-1222 and 11.0% of romosozumab treated subjects (Padhi et al., 2011). We did not find any obvious effect of antibody formation on pharmacokinetic parameters of SHR-1222. Due to the lack of data regarding SHR-1222 neutralizing antibodies and the small sample size in this study, further investigations are required.
In conclusion, a single subcutaneous injection of SHR-1222 was safe and well-tolerated within the doses ranging from 50 to 400 mg. Decreased blood calcium was a common adverse event of SHR-1222 in a dose-dependent manner, which may be prevented by supplementation of vitamin D and calcium. The pharmacodynamics analysis demonstrated the efficacy of SHR-1222 in BMD promotion, which supports the further clinical development of SHR-1222 as an attractive alternative treatment for osteoporosis. By the time of submission, another phase I study is in progress to assess the safety and efficacy of multiple doses of SHR-1222 in postmenopausal osteoporosis patients (ClinicalTrials.gov Identifier: NCT04435158).
Data Availability Statement
The datasets presented in this article are not readily available because of potential intellectual property. Requests to access the datasets should be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Conception and design of the study: ZD, PF, XY, RZ, ZS, LZ, YL, SZ, ZY and ZZ. Clinical investigators (recruited and treated patients): ZD, QF, XF, and YX. Project implementation: PF, QY, RZ, LY, and XY. Data collection and analysis: ZD, PF, ZS, LZ, YL, SF, SZ, ZY, and ZZ. Writing and/or revision of the manuscript: ZD, PF, ZS, and ZZ. Principal investigator: ZZ. All authors approved the final version for submission.
Funding
This study received funding from Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Conflict of Interest
Authors LZ, SF, YL, SZ and ZY were employed by Jiangsu Hengrui Pharmaceuticals Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Jiangsu Hengrui Pharmaceuticals Co., Ltd.. The funder had the following involvement with the study: design, data collection and analysis.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the subjects and their families, as well as all the investigators and research staff for participating in this study.
References
Armas, L. A., and Recker, R. R. (2012). Pathophysiology of Osteoporosis: New Mechanistic Insights. Endocrinol. Metab. Clin. North. Am. 41 (3), 475–486. doi:10.1016/j.ecl.2012.04.006
Burr, D. B., Miller, L., Grynpas, M., Li, J., Boyde, A., Mashiba, T., et al. (2003). Tissue Mineralization Is Increased Following 1-year Treatment with High Doses of Bisphosphonates in Dogs. Bone 33 (6), 960–969. doi:10.1016/j.bone.2003.08.004
Cano, A., Chedraui, P., Goulis, D. G., Lopes, P., Mishra, G., Mueck, A., et al. (2018). Calcium in the Prevention of Postmenopausal Osteoporosis: EMAS Clinical Guide. Maturitas 107, 7–12. doi:10.1016/j.maturitas.2017.10.004
Cosman, F., Crittenden, D. B., Adachi, J. D., Binkley, N., Czerwinski, E., Ferrari, S., et al. (2016). Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 375 (16), 1532–1543. doi:10.1056/NEJMoa1607948
Coughlan, T., and Dockery, F. (2014). Osteoporosis and Fracture Risk in Older People. Clin. Med. (Lond) 14 (2), 187–191. doi:10.7861/clinmedicine.14-2-187
Drake, M. T., Clarke, B. L., and Khosla, S. (2008). Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 83 (9), 1032–1045. doi:10.4065/83.9.1032
EMA (2019). Evenity (Romosozumab) an Overview of Evenity and Why it Is Authorised in the EU. Available at: https://www.ema.europa.eu/en/documents/overview/evenity-epar-medicine-overview_en.pdf (Accessed September 25, 2021).
FDA (2019). Prescribing Information for Romosozumab. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s002lbl.pdf (Accessed May 25, 2021).
Fulzele, K., Lai, F., Dedic, C., Saini, V., Uda, Y., Shi, C., et al. (2017). Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots. J. Bone Miner Res. 32 (2), 373–384. doi:10.1002/jbmr.3001
Hangartner, T. N. (2007). A Study of the Long-Term Precision of Dual-Energy X-ray Absorptiometry Bone Densitometers and Implications for the Validity of the Least-Significant-Change Calculation. Osteoporos. Int. 18 (4), 513–523. doi:10.1007/s00198-006-0280-1
Hodsman, A. B., Bauer, D. C., Dempster, D. W., Dian, L., Hanley, D. A., Harris, S. T., et al. (2005). Parathyroid Hormone and Teriparatide for the Treatment of Osteoporosis: a Review of the Evidence and Suggested Guidelines for its Use. Endocr. Rev. 26 (5), 688–703. doi:10.1210/er.2004-0006
Kanis, J. A. (2002). Diagnosis of Osteoporosis and Assessment of Fracture Risk. Lancet 359 (9321), 1929–1936. doi:10.1016/S0140-6736(02)08761-5
Kim, S. P., Frey, J. L., Li, Z., Kushwaha, P., Zoch, M. L., Tomlinson, R. E., et al. (2017). Sclerostin Influences Body Composition by Regulating Catabolic and Anabolic Metabolism in Adipocytes. Proc. Natl. Acad. Sci. U S A. 114, E11238. doi:10.1073/pnas.1707876115
Li, X., Zhang, Y., Kang, H., Liu, W., Liu, P., Zhang, J., et al. (2005). Sclerostin Binds to LRP5/6 and Antagonizes Canonical Wnt Signaling. J. Biol. Chem. 280 (20), 19883–19887. doi:10.1074/jbc.M413274200
Lindsay, R., Krege, J. H., Marin, F., Jin, L., and Stepan, J. J. (2016). Teriparatide for Osteoporosis: Importance of the Full Course. Osteoporos. Int. 27 (8), 2395–2410. doi:10.1007/s00198-016-3534-6
Padhi, D., Allison, M., Kivitz, A. J., Gutierrez, M. J., Stouch, B., Wang, C., et al. (2014). Multiple Doses of Sclerostin Antibody Romosozumab in Healthy Men and Postmenopausal Women with Low Bone Mass: a Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Pharmacol. 54 (2), 168–178. doi:10.1002/jcph.239
Padhi, D., Jang, G., Stouch, B., Fang, L., and Posvar, E. (2011). Single-dose, Placebo-Controlled, Randomized Study of AMG 785, a Sclerostin Monoclonal Antibody. J. Bone Miner Res. 26 (1), 19–26. doi:10.1002/jbmr.173
Paschalis, E. P., Gamsjaeger, S., Hassler, N., Fahrleitner-Pammer, A., Dobnig, H., Stepan, J. J., et al. (2017). Vitamin D and Calcium Supplementation for Three Years in Postmenopausal Osteoporosis Significantly Alters Bone mineral and Organic Matrix Quality. Bone 95, 41–46. doi:10.1016/j.bone.2016.11.002
Paszty, C., Turner, C. H., and Robinson, M. K. (2010). Sclerostin: a Gem from the Genome Leads to Bone-Building Antibodies. J. Bone Miner Res. 25 (9), 1897–1904. doi:10.1002/jbmr.161
Poole, K. E., van Bezooijen, R. L., Loveridge, N., Hamersma, H., Papapoulos, S. E., Löwik, C. W., et al. (2005). Sclerostin Is a Delayed Secreted Product of Osteocytes that Inhibits Bone Formation. FASEB J. 19 (13), 1842–1844. doi:10.1096/fj.05-4221fje
Saag, K. G., Petersen, J., Brandi, M. L., Karaplis, A. C., Lorentzon, M., Thomas, T., et al. (2017). Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 377 (15), 1417–1427. doi:10.1056/NEJMoa1708322
Semënov, M., Tamai, K., and He, X. (2005). SOST Is a Ligand for LRP5/LRP6 and a Wnt Signaling Inhibitor. J. Biol. Chem. 280 (29), 26770–26775. doi:10.1074/jbc.M504308200
Sølling, A. S. K., Harsløf, T., and Langdahl, B. (2019). Current Status of Bone-Forming Therapies for the Management of Osteoporosis. Drugs Aging 36 (7), 625–638. doi:10.1007/s40266-019-00675-8
Weaver, C. M., Alexander, D. D., Boushey, C. J., Dawson-Hughes, B., Lappe, J. M., LeBoff, M. S., et al. (2016). Calcium Plus Vitamin D Supplementation and Risk of Fractures: an Updated Meta-Analysis from the National Osteoporosis Foundation. Osteoporos. Int. 27 (1), 367–376. doi:10.1007/s00198-015-3386-5
Wijenayaka, A. R., Kogawa, M., Lim, H. P., Bonewald, L. F., Findlay, D. M., and Atkins, G. J. (2011). Sclerostin Stimulates Osteocyte Support of Osteoclast Activity by a RANKL-dependent Pathway. PLoS One 6 (10), e25900. doi:10.1371/journal.pone.0025900
Winkler, D. G., Sutherland, M. K., Geoghegan, J. C., Yu, C., Hayes, T., Skonier, J. E., et al. (2003). Osteocyte Control of Bone Formation via Sclerostin, a Novel BMP Antagonist. EMBO J. 22 (23), 6267–6276. doi:10.1093/emboj/cdg599
Keywords: SHR-1222, low bone mass, sclerostin, bone resorption, bone formation
Citation: Dai Z, Fang P, Yan X, Zhu R, Feng Q, Yan Q, Yang L, Fan X, Xie Y, Zhuang L, Feng S, Liu Y, Zhong S, Yang Z, Sheng Z and Zhou Z (2021) Single Dose of SHR-1222, a Sclerostin Monoclonal Antibody, in Healthy Men and Postmenopausal Women With Low Bone Mass: A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation, Phase I Study. Front. Pharmacol. 12:770073. doi: 10.3389/fphar.2021.770073
Received: 03 September 2021; Accepted: 07 October 2021;
Published: 20 October 2021.
Edited by:
Mariusz Skwarczynski, The University of Queensland, AustraliaReviewed by:
Dongqing Yang, University of Adelaide, AustraliaDaisuke Inoue, Teikyo University Chiba Medical Center, Japan
Copyright © 2021 Dai, Fang, Yan, Zhu, Feng, Yan, Yang, Fan, Xie, Zhuang, Feng, Liu, Zhong, Yang, Sheng and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhifeng Sheng, shengzhifeng@csu.edu.cn; Zhiguang Zhou, zhouzhiguang@csu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Zhijie Dai
Zhijie Dai Pingfei Fang
Pingfei Fang Xiang Yan
Xiang Yan Ronghua Zhu2
Ronghua Zhu2 Zhifeng Sheng
Zhifeng Sheng Zhiguang Zhou
Zhiguang Zhou