- 1Department of Pharmacy, The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei, China
- 2Anhui Province Key Laboratory of Chinese Medicinal Formula, Synergetic Innovation Center of Anhui Authentic Chinese Medicine Quality Improvement, Anhui University of Chinese Medicine, Hefei, China
Taohong Siwu decoction (THSWD) is a classic traditional Chinese medicine (TCM) prescription that is widely used in the clinical treatment of gynecological and cerebrovascular diseases. Here we used a method that coupled ultra-performance liquid chromatography to quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS) in which both positive and negative ion modes were established to investigate the major constituents in THSWD. A Waters ACQUITY UPLC BEH C18 column (2.1 mm×100 mm, 1.7 μm) was used to separate the aqueous extract of THSWD. The mobile phase consisted of 0.1% aqueous formic acid (A) and acetonitrile (B). Ninety-five components were identified in two different ion modes, including aromatic acids, flavones, polysaccharides, volatile oils monoterpene glycosides, aromatic cyanogenic glycosides, and others. Pathological changes in tumors and serum expression of interleukin-4 in a mouse model of breast cancer were detected after THSWD treatment. The results showed that THSWD had obvious therapeutic effects. This study establishes a material basis for the use of THSWD in the treatment of breast cancer.
Introduction
Traditional Chinese medicine (TCM) is an important part of Chinese culture (Zhen, 2013). As the most representative and integral part of traditional Chinese culture, TCM has a long, profound history. At present, the Party and State attach great importance to TCM, which also has a far-reaching influence all over the world (Weiwei, 2017). Formulae are the main form of TCM clinical medicine (Chen and Luo, 2018). They are characterized by multiple components, which affect multiple pathways and targets, so their mechanisms of action are largely unknown (Li et al., 2012). The “Traditional Chinese Medicine Law of the People’s Republic of China” is its official implementation, marking the first time that China identified the important status, development guidelines, and supporting measures of TCM from a legal perspective and provided a legal guarantee for TCM development.
Taohong Siwu decoction (THSWD) consists of six species of medicinal herbs: Prunus persica (L.) Batsch (Taoren, TR), Carthamustinctorius L. (Honghua, HH), Angelica sinensis (Oliv.) Diels (Danggui, DG), Ligusticum chuanxiong hort (Chuanxiong, CX), Paeoniae Radix Alba (Baishao, BS), and Rehmanniaglutinosa (Gaertn.) DC. (Shudi, SD) (Ding and Zhang, 2010). THSWD was documented in “Yi Zong Jin Jian” compiled by Wu Qian in the Qing Dynasty, on the basis that the Siwu decoction enriches the blood, adds TR and HH into the blood, eliminates blood stasis, and promotes blood circulation and nourishment (Liu et al., 2015). It has been widely used in the treatment of gynecological and cerebrovascular diseases (Kim et al., 2016). Ischemic stroke, also known as cerebral infarction due to thrombosis, belongs to the category of “stroke” in TCM (Yu et al., 2007). It is an acute cerebrovascular disease characterized by sudden stupor, unconsciousness, hemiplegia, slurred speech, and speech disorder (Cheng et al., 2018).
Considering the characteristics of a TCM compound, the composition is complex. Moreover, factors like origin, cultivation, harvesting, processing, preparation, and more all influence the clinical treatment effect (Zhang, 2010). Therefore, verifying the chemical composition of TCM compounds through analysis and quality testing is particularly important. It is quite time-consuming to chemically profile TCM preparations with conventional high-performance liquid chromatography coupled with ultraviolet detection (HPLC-UV) or HPLC coupled with mass spectrometry (HPLC-MS) (Han et al., 2007; Li et al., 2014). Compared with traditional HPLC technology, which remains constant over a wider linear range, ultra-performance liquid chromatography (UPLC) has higher efficiency. Its advantages include increasing the mobile phase velocity, shortening the analysis time, and increasing throughput. The columns show superior performance, and the ultra-high pressure liquid chromatography pump offers precise gradient control, low diffusion, a low cross-contamination auto-sampler system, a high-speed detector, and comprehensive hardware and software. The peak capacity, analysis efficiency, and sensitivity are greatly improved compared with conventional HPLC, providing a good platform for the separation analysis of complex systems (Gumustas et al., 2014; Hedaya et al., 2017; Wu et al., 2017). Time-of-flight MS (TOF-MS) can provide accurate molecular mass (Zhang et al., 2018). MassHunter software can calculate the molecular formula of the test substance, so the chemical composition represented by each chromatographic peak can be directly identified with very small errors, generally at 5 ppm. This approach is widely used in the identification of compounds. In this study, UPLC-Q-TOF-MS technology was used to separate and identify the main components of THSWD.
THSWD is used to treat cerebrovascular and gynecological diseases. DG and CX have good therapeutic effects on gynecological conditions, especially breast diseases. However, there have been no reports of the study of THSWD in the treatment of breast cancer. We analyzed the components of THSWD and assessed its therapeutic effect in a mouse model of breast cancer to provide a basis for its clinical use.
Materials and Methods
Materials
Prunus persica (L.) Batsch (Taoren, TR, batch number: 17033101), Carthamus tinctorius L. (Honghua, HH, batch number: 17041401), Angelica sinensis (Oliv.) Diels (Danggui, DG, batch number: 16070501), Conioselinum anthriscoides ‘Chuanxiong’ (syn. Ligusticum chuanxiong Hort) (Chuanxiong, CX, batch number: 17061601), Paeoniae lactiflora Pall. (Baishao, BS, batch number: 17050301), and Rehmannia glutinosa (Gaertn.) DC (Shudi, SD, batch number: 17042501) were purchased from Anqing Huashi Chinese Herbal Medicine Co. Ltd. (Anqing, China). All TCM materials were qualified by Professor Huasheng Peng (hspeng@126.com). All voucher specimens were deposited at the Herbarium of Anhui University of Chinese Medicine, Hefei, China (Herbarium code: ACM, voucher numbers: 17021, 17025, 17034, 17051, 17068, 17080). A Waters ACQUITY™ UPLC I-Class and a SYNAPTG2-Si MS were obtained from Waters Corporation (Milford, MA, USA). The 4T1 breast cancer cell line and fetal bovine serum (batch number: 20160720) were from Zhejiang Tianhang Biotechnology Co., Ltd. RPMI 1640 medium (batch number: ZI110516) was purchased from Hangzhou Northrend Biotechnology Co., Ltd.
Preparation and Extraction
In this system, the herbs TR, HH, DG, SD, CX, and BS (3:2:3:4:2:3) were soaked then decocted twice with 10 vol. of boiling water for 2 h and 8 vol. of boiling water for 1.5 h. The extraction solutions were then filtered and combined. Next, 95% ethanol was added for alcohol precipitation to obtain Taohong-Siwu decoction alcohol precipitation. A small amount of alcohol solution was filtered through a 0.22-μm microporous membrane filter, and 2 μL of filtrate was used for injection analysis.
UPLC–MS and UPLC–MS2 Analysis
A Waters ACQUITY UPLC BEH C18 column (2.1 mm×100 mm, 1.7 μm) was used to separate the aqueous extract of THSWD. The mobile phase was 0.1% aqueous formic acid (A) and acetonitrile (B). Chromatographic separation was performed at 35°C in this system. Gradient elution with a flow rate of 0.3 mL/min was performed as follows: 3% B at 0-2 min; 3%-8% B at 2-8 min; 8%-25%B at 8-12 min; 25%-25% B at 12-15 min; 25%-45% B at 15-16 min; 45%-90% B at 16-22 min; 90%-100% B at 22-26 min; 100% B at 26-28 min. The MS analysis was carried out with the electrospray ionization (ESI) source in both positive and negative ion modes, and leucine enkephalin was used as the accurate mass calibration solution. The desolvation gas temperature was 350°C. The flow rates of cone and desolvation gases were set at 50 L/h and 600 L/h, respectively. The capillary, cone, and extraction cone voltages were set to 3.0 and 2.5 V in positive and negative ion modes, respectively. MSE was applied for MS/MS analysis with a low collision energy of 6 V and a high collision energy of 20-80V. The scan area was set at m/z 50-1200.
Animal Treatment and Sample Collection
BALB/c mice (specific pathogen-free grade, 22 ± 2g), were purchased from the Laboratory Animal Center, Medical University of Anhui Province (permit number: 2016AH-031-11). They were randomly divided into six groups (10 mice each): normal; model; THSWD low-dose, middle-dose, and high-dose (L, M, and H); and cisplatin. After 4T1 cells were resuscitated, they were routinely cultured in RPMI 1640 medium containing 10% fetal bovine serum at 5% CO2 and 37°C. The culture medium was changed every 2 to 3 days and passaged. Cells cultured to logarithmic growth phase were collected and diluted 10 times with sterile saline, and 0.2 mL was injected into each mouse. After 48 hours, there was a small pink protrusion of about 2 mm × 2 mm, indicating successful modeling. Mice in the THSWD H, THSWD M, and THSWD L groups were treated with THSWD (25.2, 12.6, and 6.3 g/kg, respectively, three times a day) by gavage. The cisplatin group received cisplatin (0.1 mg/mL) by gavage, the normal and model groups were administered with an equivalent amount of regular saline. The dosing period was 18 days. After the last administration, blood and tumor samples were collected and animals were sacrificed by cervical vertebrae dislocation. All experiments were subject to approval by the Committee on the Ethics of Animal Experiments of Anhui University of Chinese medicine (Permit Number: LLSC20160336).
Hematoxylin and Eosin (H&E) Staining
Tumor tissues were fixed, paraffin-embedded, and sectioned. Changes in tumor cell arrangement and pathological alterations of the tumor were observed with conventional H&E staining and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Interleukin (IL)-4, IL-10, IL-13, and Transforming Growth Factor (TGF)-β1 Expression
Serum was collected from mice and centrifuged at 350 g for 5 min at 4°C. Supernatants were stored at -80°C for cytokine analysis. Enzyme-linked immunosorbent assays (ELISAs) were performed to quantify the concentrations of IL-4, IL-10, IL-13, and TGF-β1, according to the manufacturer’s instructions. Optical density (OD) values were read at 450 nm, and the levels of IL-4, IL-10, IL-13, and TGF-β1 were determined based on standard curves.
CD44, CD117, and CD133 Expression
At 24 hours after transfection, the cells were removed and washed with phosphate-buffered saline (PBS; 5 min, 3 times), fixed in methanol (-20°C, 5 min), fixed in 70% ethanol for 5 min, and washed with PBS (5 min, 3 times). They were then blocked in 1% skimmed milk powder for 30 min, and the mixture was incubated for 2 h at room temperature with primary antibody. The cells were washed with PBS, incubated with secondary antibody for 1 h, stained with 4′,6-diamidino-2-phenylindole for 2 min, washed with PBS (5 min, 3 times), mounted in DAKO medium (DAKO, Glostrup, Denmark), observed under a fluorescent microscope, and photographed.
Data Processing
Establishment of a Component Analysis Database
We used the UNIFI software library management system to collect data from offline and online MS databases such as PubMed (http://www.ncbi.nlm.nih.gov/pubmed), MassBank (http://www.massbank.jp/), Chemspider (http://www.chemspider.com/), and METLIN (https://en.wikipedia.org/) and identify relevant literature on the chemical composition of THSWD. We collected the names and molecular and structural formulas to build a database of the chemical constituents of THSWD.
Data Acquisition and Analysis
The MSE Continuum model was used to collect data and determine the chemical composition of THSWD, which was automatically identified by the UNIFI data processing system. MassLynx 4.1 software (Waters Corporation) was used to deal with the base-peak ion current patterns of positive and negative ion modes. The main chemical components were identified and confirmed by the accurate mass of fragment ions theory, relative retention time, offline and online mass spectral database, and related literature.
Results and Discussion
Optimization of Chromatographic Conditions and Q-TOF-MS/MS Method Development
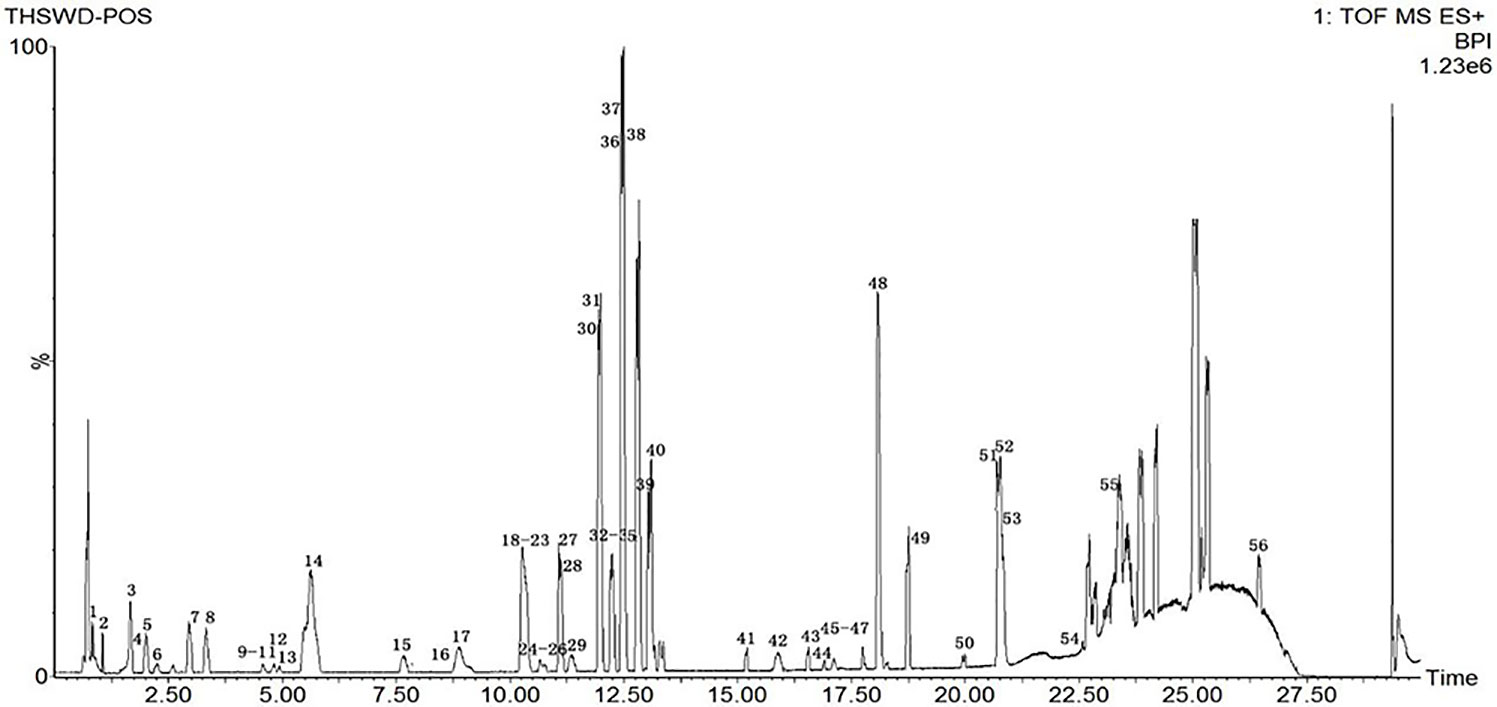
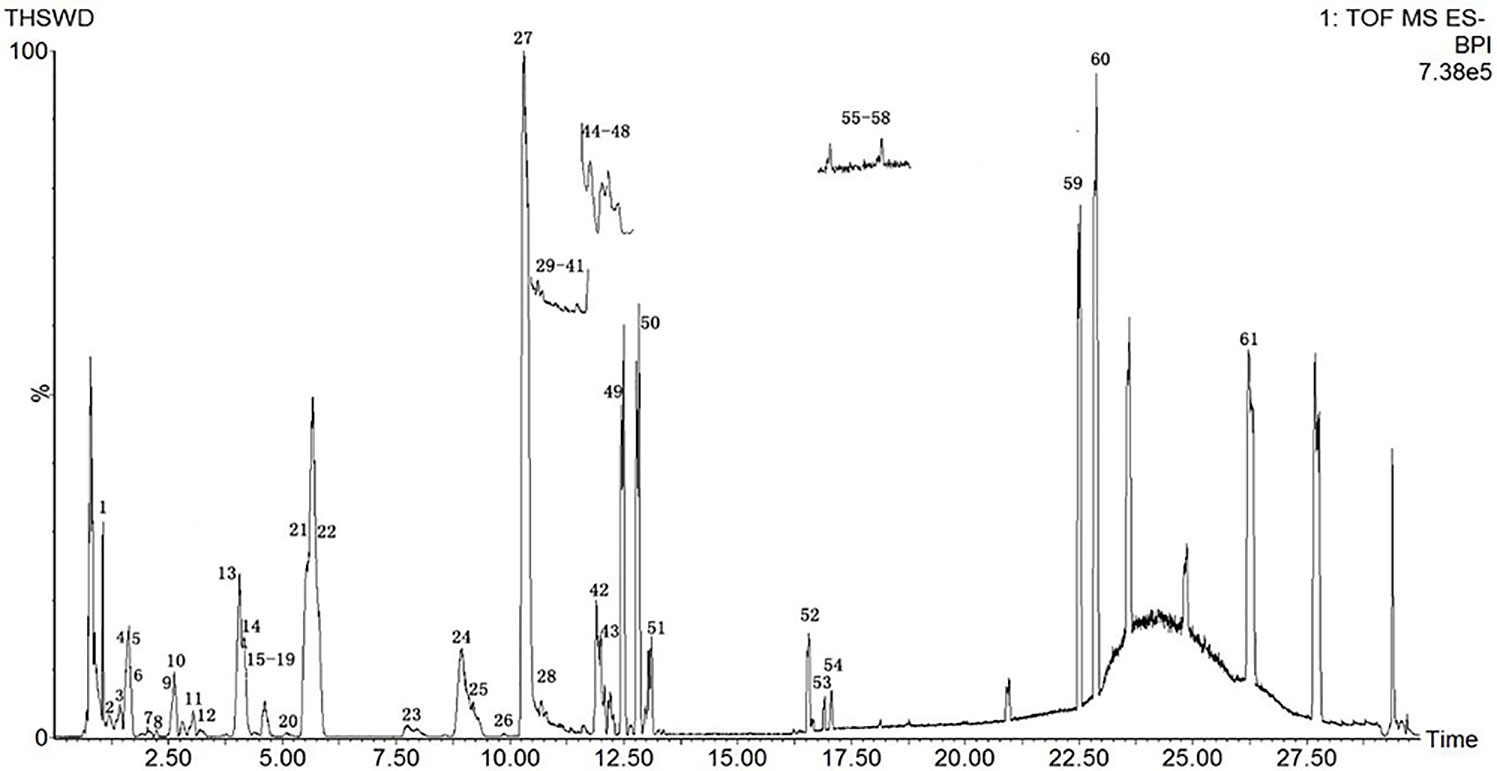
Due to the diversity of TCM prescriptions, the complexity of each single herb, and the large variety of structures, it is necessary to optimize the chromatographic conditions to obtain and separate the compounds quickly and improve the resolution. The items to be improved are the mobile phase, flow rate, column temperature, and MS parameters. For the mobile phase, we chose aqueous formic acid (A) and acetonitrile (B). We set the column temperature at 35°C. The detection wavelength was based on the literature, and full-band scans were performed at 320 nm with a flow rate of 0.3 mL/min to reduce the column pressure and shorten the analytical time. The elution procedures and MS conditions were set according to section 2.3. The standard sample solution and medicinal sample solution of each ingredient in THSWD were precisely absorbed. The total ion chromatogram of the THSWD solution was obtained according to the liquid-phase and MS conditions and the injection analysis. The base peak chromatograms of THSWD in positive and negative ion modes are shown in Figures 1 and 2, respectively.
Analysis of THSWD by UPLC-Q-TOF-MS and Identification of Its Main Constituents
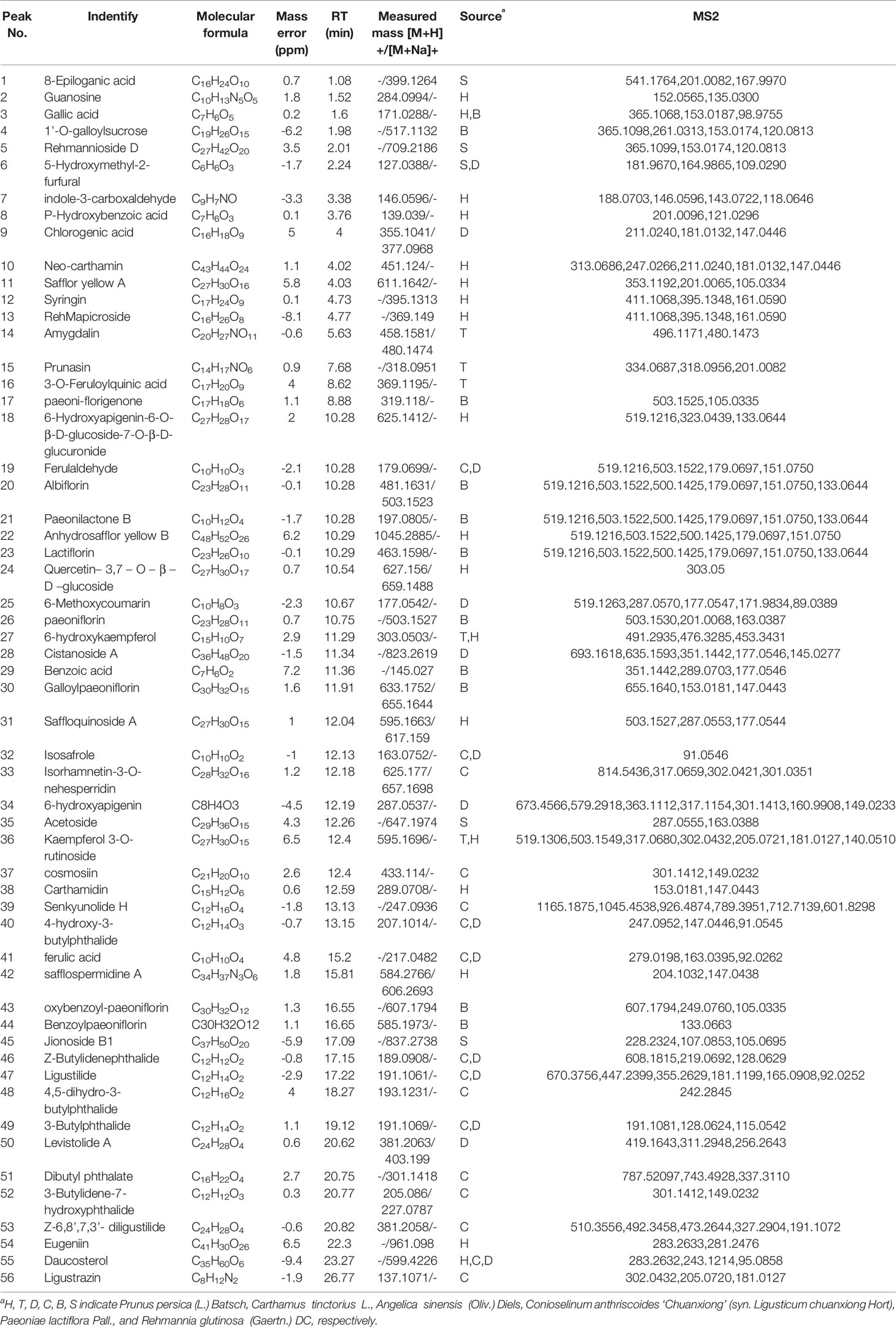
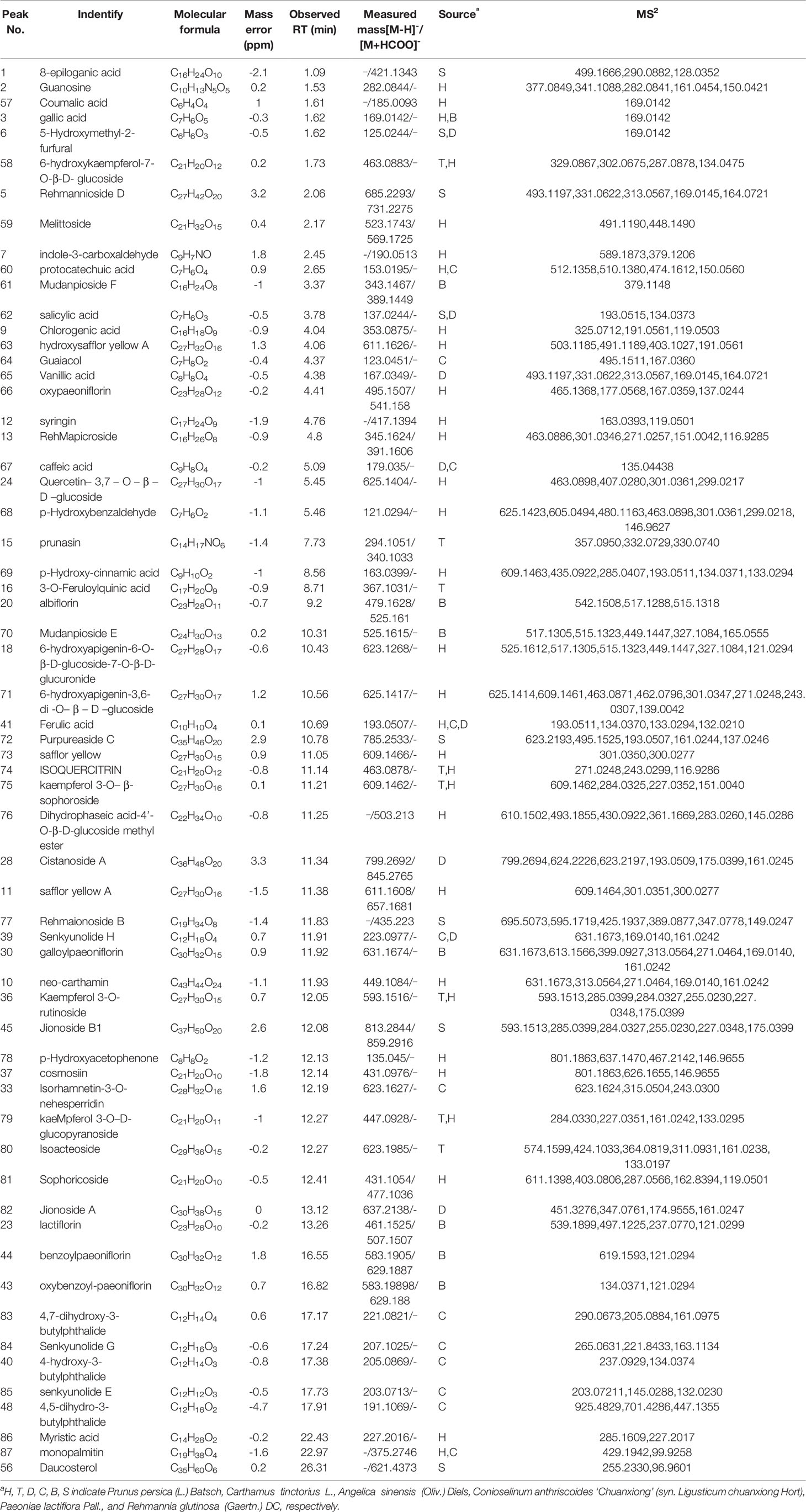
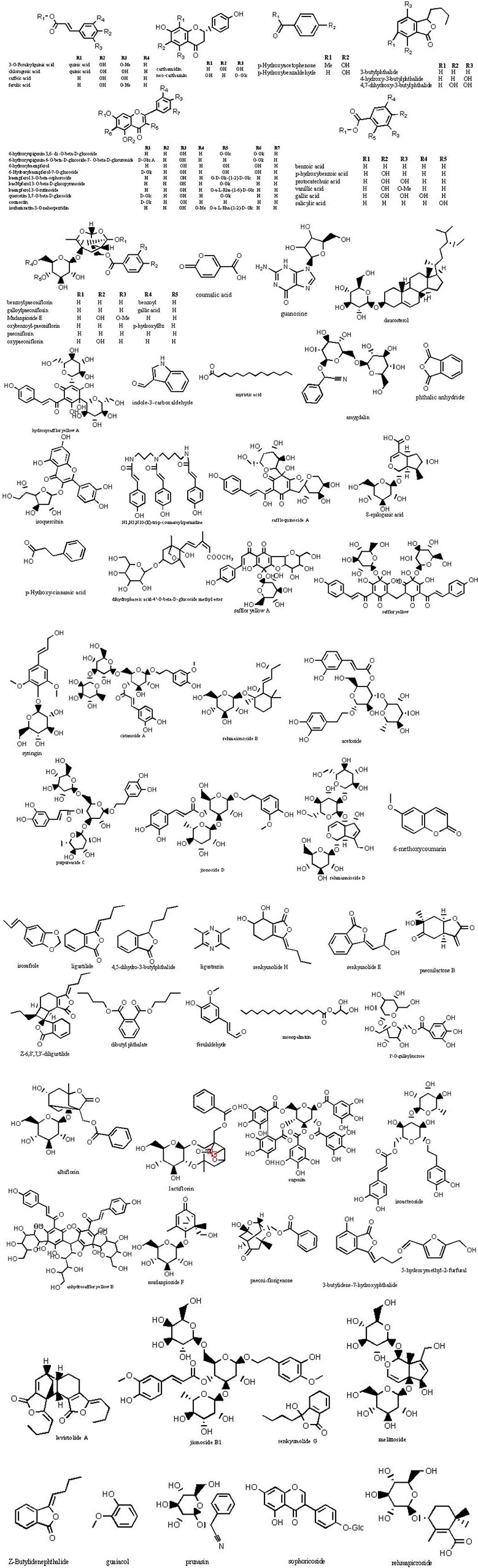
The molecular weights of the 87 peaks extracted from the total ion chromatogram of the extract of THSWD were consistent with the data of the previously built pool, including aromatic acids, flavones, polysaccharides, volatile oils, monoterpene glycosides, aromatic cyanogenic glycosides, and others (Table 1 and Table 2). Their sources were confirmed by comparing the base peak chromatograms of THSWD with its six separate herbal extracts. Standard compounds available were identified by comparing retention time with the exact mass. For compounds that were not standard, the structure was primarily based on accurate mass and tandem MS. In this study, the formulas were based on the high-precision excimer [M+H]+, [M+Na]+, [M-H]-, and [M+HCOO]- within a mass error of 10 ppm and a partial isotopic abundance. We then searched chemical databases such as Chemspider (www.chemspider.com) and Massbank (http://www.massbank.jp) for the most rational formulas. When all isomers were matched, the structures previously reported from the six herbs of THSWD had a higher probability than the other isomers. Finally, fragment ions were used to confirm the chemical structure further. The structures underlying the main activity in THSWD are shown in Figure 3.
Phenolic Acids
Seven phenolic acids of THSWD were identified in positive ion mode. Most from Conioselinum anthriscoides ‘Chuanxiong’ and Angelica sinensis (Oliv.) Diels were ferulic acid, chlorogenic acid, caffeic acid, and vanillic acid, and gallic acid was from Paeonia lactiflora Pall. The retention time and MS information of each chemical component in THSWD were determined by UPLC-MS, and the chemical composition was confirmed by combining the extracted ion current map with data in the relevant literature. Compound 41 showed the tR at the 15.2-min peak obtained in ESI-mode at the m/z 194.059 ion peak. The literature (Lin et al., 1998; Huang and Song, 2001; Lu et al., 2004) reports that the relative molecular mass of ferulic acid in Conioselinum anthriscoides ‘Chuanxiong’ (syn. Ligusticum chuanxiong Hort) was 194. It was speculated that m/z 194.059 was its quasi-molecular ion peak, and MS2 analysis was performed on m/z 193, which were m/z 279.0198, 163.0395, 92.0262, and other fragment peaks. According to the elemental composition analysis, the molecular formula is C10H10O4. It showed a theoretical value of the relative molecular mass of 193.0506 and a measured value of 194.059. This peak adds Na in ESI+ mode to obtain the ion peak with m/z 217. MS2 analysis was performed on m/z 217 to obtain fragment peaks such as m/z 123 and 151. According to the elemental composition analysis, the molecular formula was C10H10O4, and the theoretical value of the relative molecular mass was 217.0471. The actual value was 217.0475. Based on this, the compound was assumed to be ferulic acid.
Compound 9 showed a [M+H] +ion at m/z 355.1041, and more abundant ions in the MS2 spectrum were m/z 211.0240, 181.0132, and 147.0446. It was identified as chlorogenic acid from Angelica sinensis (Oliv.) Diels. The MS2 spectrum and possible fragmentation pathways of chlorogenic acid are depicted in Figure 4. Compound 67 had a retention time of 5.09 min, and the characteristic ion fragment is 179[M-H]-. It can be deduced that the molecular formula may be C9H8O4. In the further MS cleavage process, its quasi-molecular ion removes 1 molecule of CO2 to form a characteristic fragment ion m/z 135.04438 [M-H-CO2]-. By comparison with the standard spectrum, it was confirmed to be caffeic acid.
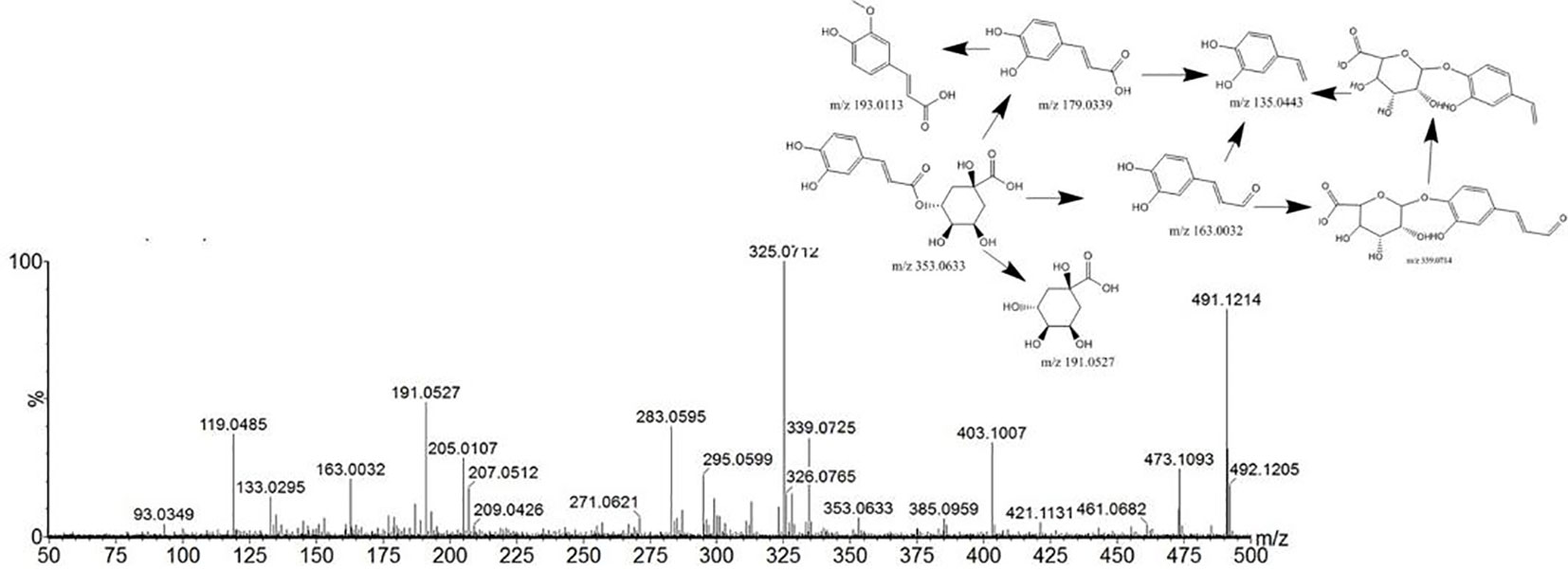
Figure 4 Tandem mass spectra and possible fragment pathways of chlorogenic acid in positive ion mode.
In negative ion mode, the retention time of compound 3 was 1.62 min, and the peak of its excimer ion was m/z 169.0142 [M -H]-. It can be deduced that the molecular formula may be C7H6O5; the error is 0.3. In positive ion mode, the excimer ion peak was m/z 171.0227 [M + H]+ with an error of 0.2. In the subsequent MS cleavage process, the quasi-molecular ion removed one molecule of CO2 to form the characteristic fragment ion m/z 125.0215 [M-H-CO2]-, indicating that the molecular structure may contain carboxylic acid groups. The related literature (Sun et al., 2009; Jiang et al., 2011; Liu et al., 2017) is consistent with the main characteristics of gallic acid, so that was inferred to be the identity of compound 3.
Flavones
Most of the six drugs of THSWD contain flavonoids that have a high response in positive and negative ion modes, but some compounds only respond in negative ion mode with [M-H]- and [M+HCOO]-. To facilitate the discussion of MS cleavage of different flavonoids in THSWD, the nomenclature of fragment ions for flavonoids was used (Ma et al., 1997; Feng et al., 2017). The main active ingredient in Carthamus tinctorius L. is safflor yellow. Viewing the information on the mass spectrum of compound 63, it showed a molecular weight at 612.1698. The fragment m/z 611[M-H]- was the excimer ion peak. By reading the literature (Ning et al., 2012; Zhang et al., 2014), it was inferred to be hydroxy safflor yellow A with a molecular formula of C27H32O16. At the same time, compound 11 was identified as safflor yellow A. Compound 10 was identified as neocarthamin.
Some flavonoids are mostly present in the form of flavonoid glycosides. By comparison with the reference substance, it was determined that compounds 24, 35, 19, and 12 were quercetin, apigenin, kaempferol, and isorhamnetin, respectively. The retention time and lysis of the reference substance were in accordance with these inferences.
Polysaccharides
Polysaccharides are one of the main active ingredients of THSWD and are mainly from Angelica sinensis (Oliv.) Diels and Rehmannia glutinosa (Gaertn.) DC. Compound 4 had a retention time of 1.98 min, the excimer ion peak was m/z 493 [M-H]-, the fragment m/z 331 was formed as an excimer ion peak, and a glucose residue (162 u) was formed. The characteristic fragment was m/z 169. The ions were derived from galloyl anions, and fragment ions of m/z 125 were generated after the gallic acyl anion underwent hydroxyl alpha cleavage to lose one molecule of CO2 (44 u). According to the literature, the three isomers 1’-O-galloyl sucrose, 6’-O-galloyl sucrose, and 6-O-galloyl sucrose exist in the white peony, but from the total ion chromatogram A m/z 493 was extracted, and only one peak appeared at 4.549 min. Therefore, it is presumed that compound 4 is galloyl sucrose, but the linking position of its sugar needs to be confirmed.
Volatile Compounds
The major volatile oils were from Angelica sinensis (Oliv.) Diels and Conioselinum anthriscoides ‘Chuanxiong’ (syn. Ligusticum chuanxiong Hort). Compound 47 showed an [M+H]+ ion at m/z 191.1061. The literature (Wang and Han, 2011) has reported that the relative molecular mass of ligustilide in C. anthriscoides ‘Chuanxiong’ was 190. It was speculated that m/z 191 was its quasi-molecular ion peak. MS2 analysis was performed on m/z 191, which yielded fragment peaks such as m/z 670, 447, and 355. It showed a retention time of 17.22 in positive ion mode. According to the elemental composition analysis, the molecular formula was C12H14O2, the theoretical value of the relative molecular mass was 191.1061, and the measured value was 191.1061. Based on this, it was assumed that the compound was ligustilide.
Compound 40 showed a retention time of 13.15 min in positive ion mode, with an [M+H]+ ion at m/z 207.1014. The peak with tR of 17.38 min yielded an m/z 205.0869 ion peak in the ESI-. The literature (Yang et al., 2007) reports that the relative molecular mass of 4-hydroxy-3-butyl- benzoquinone in C. anthriscoides ‘Chuanxiong’ is 206. According to the MS2 results, it was assumed that the compound was 4-hydroxy-3-butyl-benzoquinone. The MS2 spectrum and possible fragmentation pathways are depicted in Figure 5.
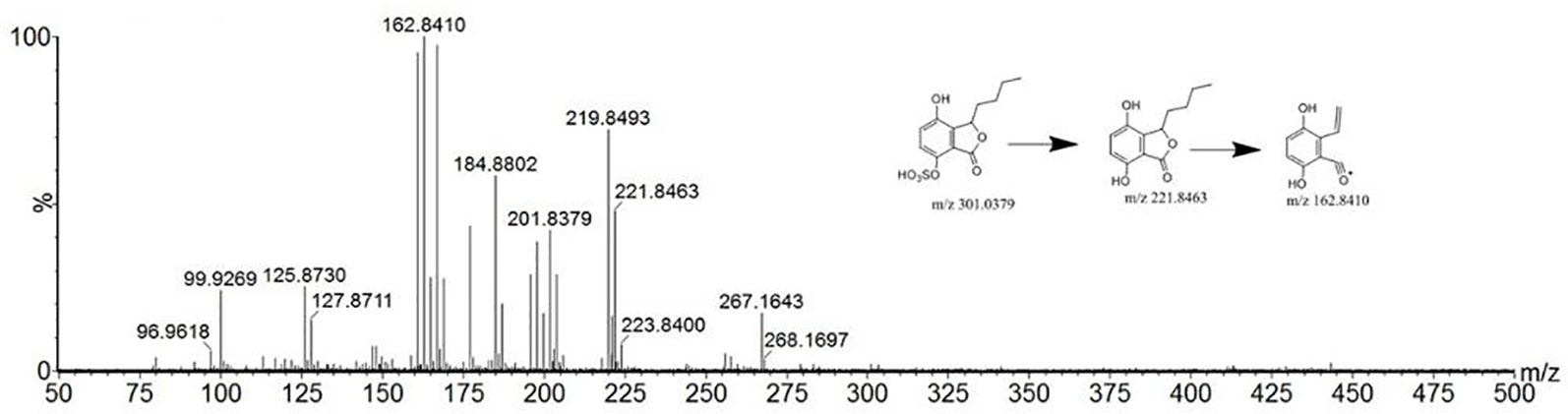
Figure 5 Tandem mass spectra and possible fragment pathways of 4-hydroxy-3-butyl-benzoquinone in positive ion mode.
Monoterpene Glycosides
Compound 21 showed a retention time of 10.28 min. The fragment ions in the mass spectrum were m/z 519.1216, 503.1522, 500.1425, 179.0697, 151.0750, and 133.0644, and the excimer ion peak was m/z 479 [M-H]-. The MS2 spectrum and possible fragmentation pathways are depicted in Figure 6. Fragment ion m/z 525 formed for the molecular ion peak plus one molecule of formic acid (46 u). Due to the presence of a lactone ring in the structure of paeoniflorin, its acyloxy groups can be broken on both sides, losing one molecule of CO2 (44 u) at m/z 435. The fragment m/z 357 was formed as the excimer peak and lost one molecule of benzoic acid (122 u), and m/z 121 was the missing one of the benzoic acid fragment ions. A combination of fragment m/z 481 [M+H]+ and fragment m/z 503.1522 [M+Na]+ appeared in positive ion mode, indicating that compound 21 is paeonolide B.
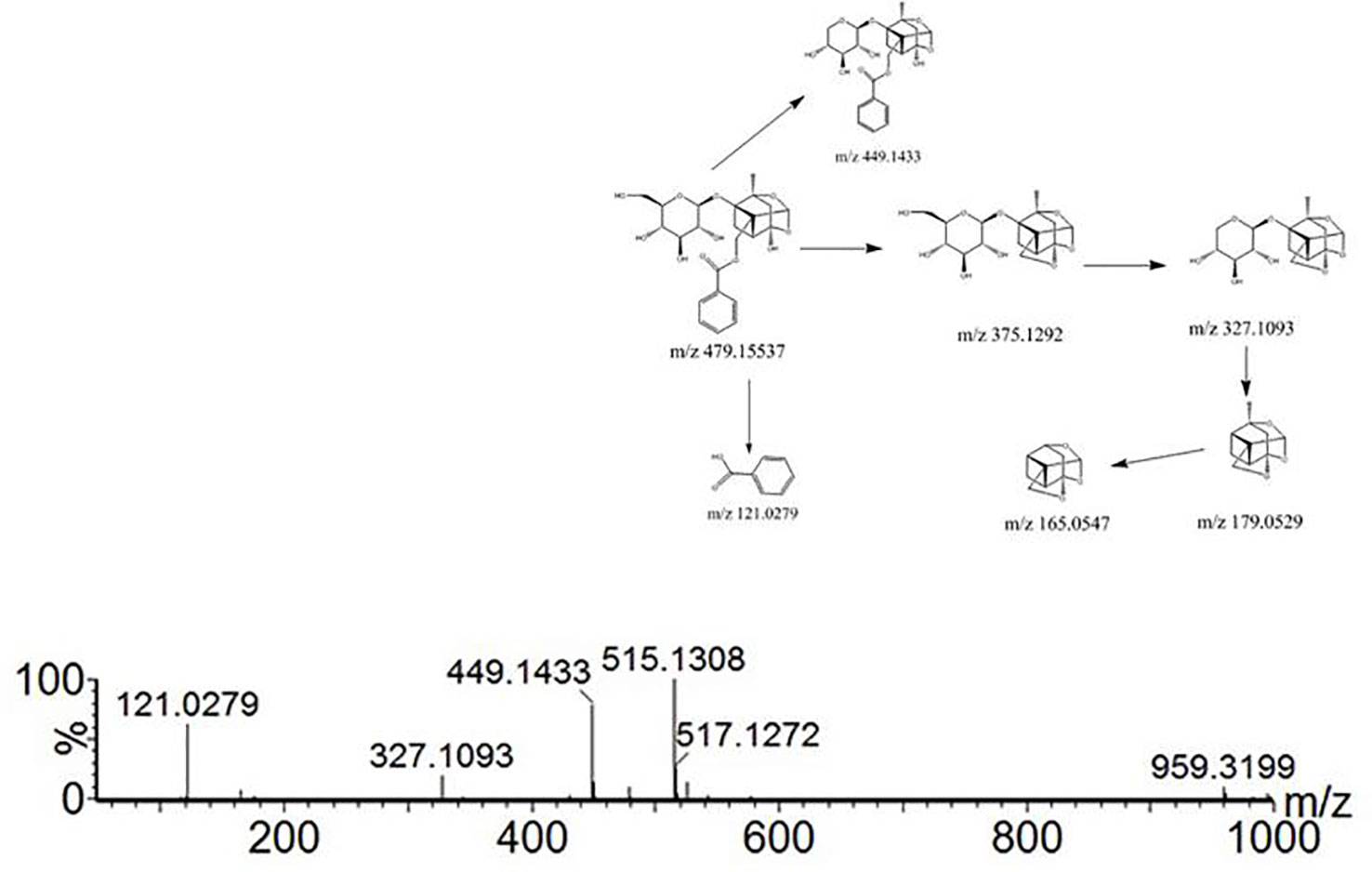
For compound 26, the retention time was 12.393 min. The fragment ions in the mass spectrum had m/z 525, 449, 327, and 165. The molecular ion peak was m/z 479 [M+H]-, and the fragment ion m/z 525 was the molecular ion peak plus a molecule of formic acid (46 u). m/z 449 is the molecular ion peak (m/z 479 [MH]-) that loses a molecule of formaldehyde (30 u) on the 6-carbon residue of the glucosyl group, while the m/z 449 fragment ion further loses a molecule of benzoic acid (122 u) to form the m/z 327 fragment ion. m/z 165 is a fragment of the decyl alkyl skeleton structure, and since the decane skeleton is connected with a benzoyl substituent, m/z 121 fragments were generated here. Combining the fragments m/z 503 [M + Na]+ appearing in positive ion mode, the literature suggests that compound 26 is peoniflorin.
Compounds 43 and 44 showed the same molecular weight at 584 Da and almost consistent tandem MS behavior with paeonolide. The fragment ions in the mass spectrum were m/z 629, 553, and 431. The fragment ion m/z 629 was the molecular ion peak (m/z 583 [M-H]-) plus one molecule of formic acid (46 u), and m/z 553 was the molecule. The ion peak (m/z 583 [M-H]-) loses the formation of a molecule of formaldehyde (30 u) on the 6-carbon residue of the glucosyl group, and the fragment ion m/z 553 further loses a molecule of benzoic acid (122 u), resulting in m/z 431 fragment ions. Positive ion mode yielded m/z 585[M + H]+ and m/z 607 [M + Na]+, which identified compound 44 as benzoyl glycoside according to the literature.
Aromatic Cyanogenic Glycosides
Most of the common cyanogenic glycosides have the same skeleton structure. According to the substituents on the cyanohydrin derivatives, they can be divided into two categories: aliphatic and aromatic. A given species of plant generally contains only one or two cyanogenic glycosides, such as Rosaceae. Compounds 14 and 15 were all derived from dry mature seeds of the Rosaceae plant Prunus persica (L.) Batsch or Prunus davidiana (Carr.) Franch.
Others
THSWD also contains human essential amino acids, vitamins, and other ingredients.
H&E
H&E stained samples for each group are shown in Figure 7. Tumor cells in the model group were arranged closely, with less necrosis and a large nuclear-to-plasma ratio. The cell morphology was abnormal, and there was more vascular filling. Compared with the model group, tumor cells in the THSWD groups and cisplatin group showed large areas of necrosis with obvious cell debris. The necrotic cells were loosely arranged with large gaps. The results indicate that THSWD exerts a destructive effect on mouse breast cancer cells. This is the first evidence that THSWD has a significant therapeutic effect on breast cancer tumor cells.
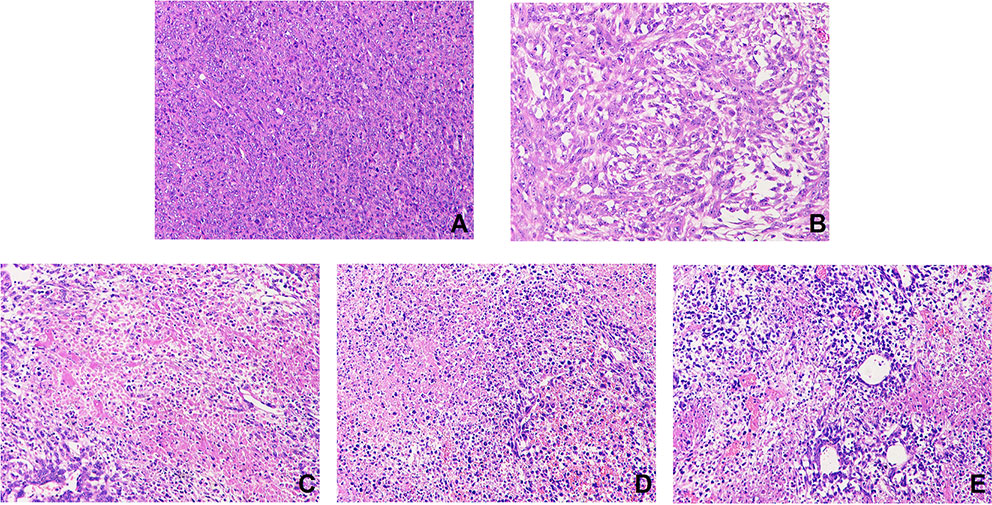
Figure 7 Effect of THSWD on tumor cells in mice. Sections were stained with H&E. Magnification, ×200; scale bars, 50 μm. Model group (A), cisplatin group (B), THSWD H group (C), THSWD M group (D), THSWD L group (E).
IL-4, IL-10, IL-13, and TGF-β1 Expression
To explore the mechanism of THSWD on breast cancer, levels of IL-6, IL-8, and IL-1β were measured by ELISA (Table 3). IL-5 was significantly increased in the model group. After treatment with THSWD or cisplatin, IL-5 was significantly reduced. The results indicate that THSWD may regulate immune function in breast cancer mice.
CD44, CD117, and CD133 Expression
Tumor cell CD44 expression was highest in the model group and lowest in the cisplatin group (Figure 8). There was no obvious difference between the three THSWD dose groups. The results showed that cisplatin and THSWD significantly inhibited CD44 expression in tumor cells of breast cancer mice.
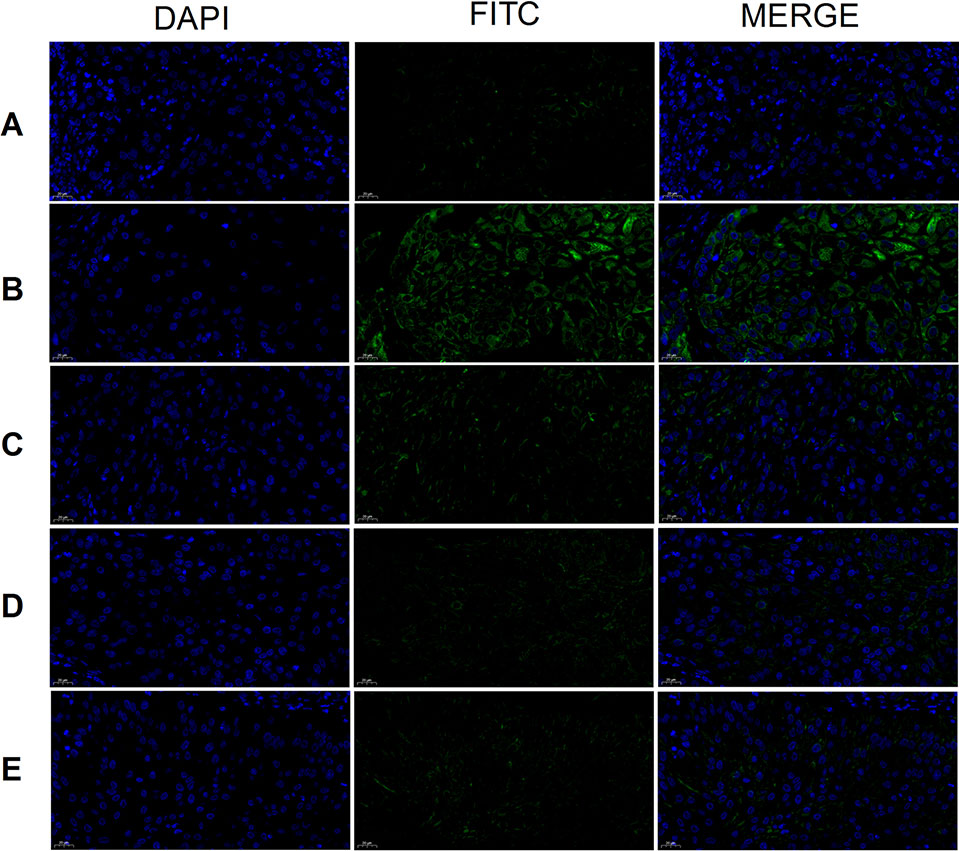
Figure 8 CD44 expression (×400). Model group (A), cisplatin group (B), THSWD H group (C), THSWD M group (D), THSWD L group (E).
CD117 expression was highest in the tumor cells of the model group and lowest in the cisplatin and THSWD middle-dose groups (Figure 9). The results showed that cisplatin and THSWD significantly inhibited CD117 expression in tumor cells of breast cancer mice.
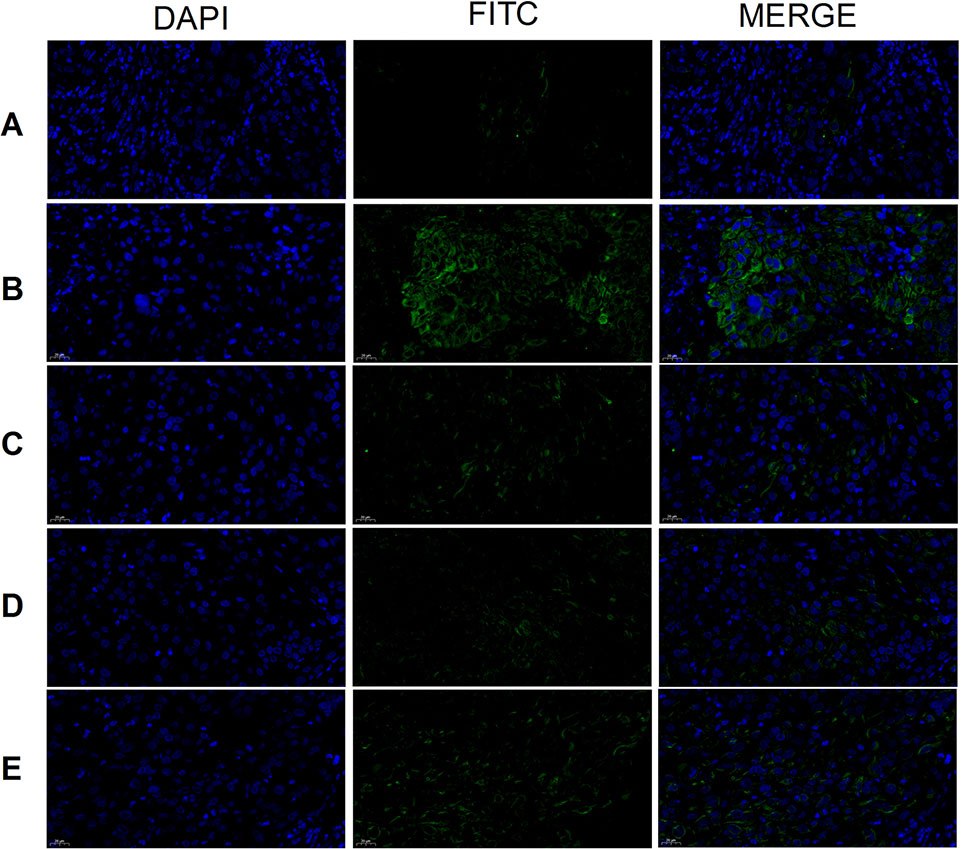
Figure 9 CD117 expression (×400). Model group (A), cisplatin group (B), THSWD H group (C), THSWD M group (D), THSWD L group (E).
The expression of CD133 was highest in tumor cells of the model group and lowest in the cisplatin group (Figure 10). Results were similar for the THSWD high-, middle-, and low-dose groups. Both cisplatin and THSWD significantly inhibited CD133 expression in tumor cells of breast cancer mice.
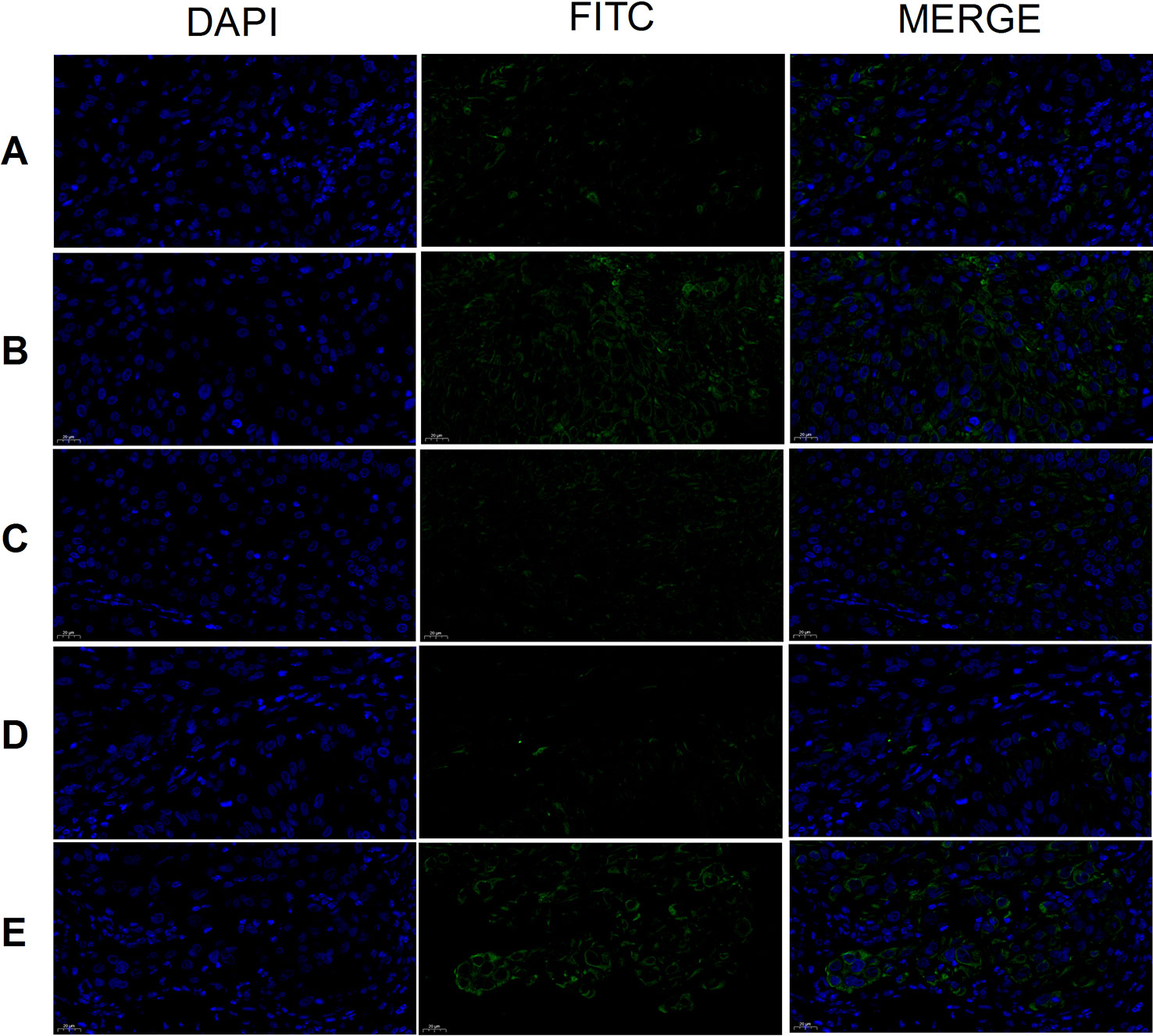
Figure 10 CD133 expression (×400). Model group (A), cisplatin group (B), THSWD H group (C), THSWD M group (D), THSWD L group (E).
Conclusion
In this study, UPLC-Q-TOF-MS was used for the full chemical characterization of THSWD in both positive and negative ion modes. A total of 87 compounds were identified in 32 minutes, including aromatic acids, flavones, polysaccharides, volatile oils, monoterpene glycosides, aromatic cyanogenic glycosides, and others. This method and the chemical material findings may provide useful information for quality control when producing THSWD and related individual herbs, despite the ingredient complexity and specificity for TCM. The results can be used to develop a quality standard of the TCM compound THSWD and provide a theoretical basis for further study of THSWD metabolism in vivo to clarify the material basis and mechanism of its clinical application.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
All experiments were subject to approval by the Committee on the Ethics of Animal Experiments of Anhui University of Chinese Medicine.
Author Contributions
XD is responsible for designing the experiment private. LP is responsible for article writing. QB is responsible for the experimental operation. DP is responsible for data analysis.
Funding
This research was supported by the National Natural Science Fund Regional Innovation and Development Joint Fund Project (No. U19A2009), Anhui University Collaborative Innovation Project (GXXT-2019-043), the Anhui Provincial College Natural Science Research Key Project (No. KJ2019A0466), Excellent and Top Talents Program in Colleges and Universities (No. gxyq2019034), Anhui Provincial Key Laboratory of Traditional Chinese Medicine Compounds (2019AKLCMF03), Open Project of National Key Clinical Specialty (Traditional Chinese Medicine Surgery)(2019zdzk04) and the Natural Science Research Project of Colleges and Universities in Anhui Province (2019fyyb038).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Chen, Q., Luo, B. (2018). Study on the naming characteristics of typhoid prescription in Qianjin Fang. J. Shandong Univ. Tradit. Chin. Med. 42 (1), 66–69.
Cheng, L., Wan, H., Fang, Y., Wang, H., Yang, J., Wan, H. (2018). Mechanism of treating ischemic stroke with Yangyin Yiqi Huoxue therapy. Chin. J. Emerg. Med. 27 (1), 94–96.
Ding, Y., Zhang, Q. (2010). Progress in clinical and experimental research of Taohong Siwu Decoction. Jiangsu J. Tradit. Chin. Med. 42 (1), 77–79.
Feng, Y., Chen, Y., Xin, H. (2017). Analysis of flavonoids in rosae laevigatae fructus by UPLC-Q-TOF-MS. Chin. J. Exp. Formulaics. 23 (12), 71–76.
Gumustas, M., Uslu, B., Ozkan, S. A., Aboul-Enien, H. Y. (2014). Validated stability-indicating HPLC and UPLC assay methods for the determination of entacapone in pharmaceutical dosage forms. Chromatographia 77, 1721–1726. doi: 10.1007/s10337-014-2758-x
Han, J., Ye, M., Guo, H., Yang, M., Wang, B. R., Guo, D. A. (2007). Analysis of multiple constituents in a Chinese herbal preparation Shuang-Huang-Lian oral liquid by HPLC–DAD-ESI-MSn. J. Pharm. Biomed. Anal. 44, 430–438. doi: 10.1016/j.jpba.2007.02.023
Hedaya, M. A., Thomas, V., Abdel-Hamid, M. E., Kehinde, E. O., Phillips, O. A. (2017). A validated UPLC–MS/MS method for the analysis of linezolid and a novel oxazolidinone derivative (PH027) in plasma and its application to tissue distribution study in rabbits. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 1040, 89–96. doi: 10.1016/j.jchromb.2016.11.034
Huang, W. H., Song, C. Q. (2001). Chemical and pharmacological advances of the study on Angelica sinensis. China J. Chin. Mater. Med. 26, 147–151.
Jiang, H., Rong, R., Lu, Q. (2011). Liquid chromatography - mass spectrometry identification of Rhubarb chemical composition. Lishizhen Med. Mater. Med. Res. 22 (7), 1705.
Kim, J., Fann, D. Y., Seet, R. C., Jo, D. G., Mattson, M. P., Arumugam, T. V. (2016). Phytochemicals in ischemic stroke. Neuromol. Med. 18 (3), 283–305.
Li, J., Lu, C., Jiang, M., Niu, X., Guo, H., Li, L., et al. (2012). Traditional Chinese medicine-based network pharmacology could lead to new multicompound drug discovery. Evid. Based Complement. Alternat. Med. 2012, 149762. doi: 10.1155/2012/149762
Li, P. L., Liu, M. H., Hu, J. H., Su, W. W. (2014). Systematic chemical profiling of Citrus grandis ‘Tomentosa’ by ultra-fast liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 90, 167–179. doi: 10.1016/j.jpba.2013.11.030
Lin, L. Z., He, X. G., Lian, L. Z., King, W., Elliott, J. (1998). Liquid chromatographic electrospray mass spectrometric study of the phthalides of Angelica sinensis and chemical changes of Z-ligustilide. J. Chromatogr A. 810, 71–79. doi: 10.1016/S0021-9673(98)00201-5
Liu, L., Duan, J. A., Su, S. L., Liu, P., Tang, Y. P., Qian, D. W. (2015). Research progress of Taowu Siwu Decoction for Siwu Decoction for gynecological blood stasis syndrome dysmenorrhea. Zhongguo Zhong Yao Za Zhi. 40 (5), 814–821.
Liu, M. J., Wang, Y., Li, L., Li, X., Dai, Y., Zhang, C., et al. (2017). UPLC-Q-TOF-MS/MS rapid identification analysis of chemical composition of Sanhuang Tablet. China J. Chin. Mater. Medica. 42 (9), 1685–1692. doi: 10.19540/j.cnki.cjcmm.20170223.010
Lu, X. H., Zhang, J. J., Liang, H., Zhao, Y. Y. (2004). Chemical constituents of Angelica sinensis. J. Chin. Pharm. Sci. 13, 1–3. doi: 10.1097/TP.0b013e3181821c25
Ma, Y. L., Li, Q. M., Van den Heuvel, H., Claeys, M. (1997). Characterization of flavone and flavonolaglycones by collision-induced dissociation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 11 (12), 1357–1364. doi: 10.1002/(SICI)1097-0231(199708)11:12<1357::AID-RCM983>3.0.CO;2-9
Ning, Y., Zeng, S., Peng, G., Wang, D. (2012). Content determination of hydroxysafflor yellow A in Tibetan Medicine Shisanwei Honghua Pills by LC-MS/MS. Chin. Pharm. 23 (27), 2538–2541.
Sun, H., Zhu, C., Zhang, H., Wang, Y., Luo, G., Hu, P., et al. (2009). Rhubarb and its processed products of liquid chromatography and mass analysis and material basis comparison. Chin. Patent Med. 31 (3), 421.
Wang, Y., Han, Y. (2011). Study on Fingerprint of ligusticun chuanxiong Hort by HPLC. Chin. Pharm. Aff. 25 (10), 1017–1021.
Weiwei, H. (2017). Thoroughly and comprehensively study and understand the “Chinese Medicine Law” to support the development of traditional chinese medicine in accordance with the law. J. Nanjing Univ. Chin. Med. (Soc. Sci. Edition). 18 (1), 6–13.
Wu, J., Fang, X., Yuan, Y., Dong, Y., Liang, Y., Xie, Q., et al. (2017). UPLC/Q-TOF-MS profiling of phenolics from Canarium pimela leaves and its vasorelaxant and antioxidant activities. Rev. Bras. Farmacogn. 27 (6), 716–723. doi: 10.1016/j.bjp.2017.10.005
Yang, L., Xie, X., Wan, L., Wang, S. B., Zhan, K.. (2007). Chemical constituents from ligusticun chuanxiong Hort. Lishizhen Med. Mater. Med. Res. 18 (7), 1576–1577.
Yu, L., Chen, Y., Xue, M. (2007). The pathogenesis of ischemic stroke is discussed. Mod. J. Integ. Tradit. Chin. West. Med. 16 (11), 1478–1479.
Zhang, J., Chen, X., Sun, Y. (2014). Simultaneous determination of hydroxysafflor yellow A, ferulic acid and ammonium glycyrrhizinate in Xinshenghua Granules by RP-HPLC. Chin. Patent Med. 36 (2), 310–313.
Zhang, X., Liang, C., Li, C., Bu, M., Bu, L., Xiao, Y., et al. (2018). Simultaneous qualitative and quantitative study of main compounds in Commelina communis Linn by UHPLC-Q-TOF-MS-MS and HPLC-ESI-MS-MS. J. Chromatogr. Sci. 56 (7), 582–594. doi: 10.1093/chromsci/bmy030
Zhang, X. (2010). Discussion on the factors affecting the quality of chinese medicinal materials. Chin. Mod. Doctor. 48 (16), 54–55.
Keywords: Taohong Siwu decoction, breast cancer, UPLC-Q-TOF-MS, components, rats
Citation: Duan X, Pan L, Bao Q and Peng D (2020) UPLC-Q-TOF-MS Study of the Mechanism of THSWD for Breast Cancer Treatment. Front. Pharmacol. 10:1625. doi: 10.3389/fphar.2019.01625
Received: 06 November 2019; Accepted: 13 December 2019;
Published: 24 January 2020.
Edited by:
Jiarui Wu, Beijing University of Chinese Medicine, ChinaReviewed by:
Jianye Dai, Lanzhou University, ChinaHaiyang Yu, Tianjin University of Traditional Chinese Medicine, China
Xinkui Liu, Beijing University of Chinese Medicine, China
Copyright © 2020 Duan, Pan, Bao and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daiyin Peng, pengdaiyin@163.com
 Xianchun Duan
Xianchun Duan Lingyu Pan
Lingyu Pan Qiuyu Bao2
Qiuyu Bao2 Daiyin Peng
Daiyin Peng