- 1Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Operating Room of Anesthesia Surgery Center, West China Hospital/West China School of Nursing, Sichuan University, Chengdu, China
- 3Laboratory of Mitochondria and Metabolism, Department of Anesthesiology, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
- 4Department of day surgery center, West China Hospital, Sichuan University, Chengdu, China
Background: Current diagnostic criteria for cancer cachexia are inconsistent, and arguments still exist about the impact of cachexia on the survival of patients with colorectal cancer. In this study, we aim to investigate the prognostic value of a novel cachexia indicator, the cachexia index (CXI), in patients with colorectal cancer.
Methods: The CXI was calculated as skeletal muscle index (SMI) × serum albumin/neutrophil-lymphocyte ratio. The cut-off value of CXI was determined by the receiver operating characteristic (ROC) curves and Youden’s index. The major outcomes were major complications, overall survival (OS), and recurrence-free survival (RFS).
Results: A total of 379 patients (234 men and 145 women) were included. The ROC curves indicated that CXI had a significantly diagnostic capacity for the detection of major complications. Based on Youden’s index, there were 231 and 148 patients in the low and high CXI groups, respectively. Patients in the low CXI group had significantly older age, lower BMI, and a higher percentage of cachexia and TNM stage II+III. Besides, Patients in low CXI group were associated with a significantly higher rate of major complications, blood transfusion, and longer length of stay. Logistic regression analysis indicated that low CXI, cachexia, and coronary heart disease were independent risk factors for the major complications. Kaplan Meier survival curves indicated that patients with high CXI had a significantly more favorable OS than those with low CXI, while no significant difference was found in RFS between the two groups. Besides, there were no significant differences in OS or RFS between patients with and without cachexia. The univariate and multivariate Cox regression analysis indicated that older age, low CXI, and coronary heart disease instead of cachexia were associated with a decreased OS.
Conclusion: CXI was better than cachexia in predicting OS and could be a useful prognostic indicator in patients with colorectal cancer, and greater attention should be paid to patients with low CXI.
Introduction
Cancer cachexia is a multifactorial syndrome, and its key characteristic is the loss of skeletal muscle mass (1). It is driven by various metabolic changes such as increased energy expenditure, excess catabolism, and elevated inflammation (2). Colorectal cancer is the third most commonly diagnosed cancer worldwide, and it ranks second in the cause of cancer death because of its high fatality rate (3). Cachexia is highly prevalent in colorectal cancer. Based on the diagnostic criteria that weight loss over 5% in the previous 6 months, the prevalence of cachexia in colorectal cancer is nearly 50% (2).
Current diagnostic criteria for cancer cachexia are inconsistent across studies (4). One of the most accepted international consensus in 2011, the Fearon criteria, recommended a combination of weight loss, body mass index (BMI), and skeletal muscle index (SMI) for the diagnosis of cancer cachexia, in which an accurate estimate of weight loss is indispensable (1). However, weight loss is not a completely objective indicator because not all patients can provide an accurate estimate of weight loss over the past 6 months, which could increase the risk of recalling bias. There are few studies investigating the impact of cancer cachexia on the survival of patients with colorectal cancer, and the results are inconsistent. For example, Gannavarapu et al. and Thoresen et al. indicate that cachexia is a poor prognostic factor for patients with colorectal cancer (5, 6). While Shibata et al. suggest that cachexia couldn`t completely predict the survival in patients with colorectal cancer (7). Furthermore, a cohort study by Brown et al. finds that a stable body weight does not mean that there is no loss of skeletal muscle in colorectal cancer (8). As a consequence, arguments still exist for the diagnostic criteria of cachexia between clinical assessment and the Fearon criteria in patients with colorectal cancer (9, 10).
Cachexia index (CXI), a new measure of cachexia, is consisted of three objective indicators including SMI, serum albumin, and neutrophil-lymphocyte ratio (NLR) (11). Recent studies have indicated that CXI is significantly associated with treatment response and prognosis in patients with malignancies such as lung cancer, liver cancer, and aggressive lymphomas (11–15). In this study, we aim to evaluate the prognostic value of CXI in patients with colorectal cancer, which might be helpful to distinguish the potentially cachexic patients.
Methods
Patients
Patients with colorectal cancer undergoing radical surgery at the Department of Gastrointestinal Surgery of West China Hospital from October 2020 to September 2021 were retrospectively collected in this study. The inclusion criteria for the patients were (1): pathology confirmed colorectal cancer (2); between 18 and 80 years old; (3) the preoperative CT scan was performed in our hospital. Besides, the exclusion criteria were: (1) patients undergoing emergency or non-radical surgery; (2) having a history of other malignancies. All the clinicopathological data were collected from the medical records, examination reports, and pathological reports through the Hospital Information System (HIS) of West China Hospital. All patients were routinely followed up after surgery, and the last follow-up data were collected in May 2022. All the included patients were anonymized before analysis. This study was conducted according to the Declaration of Helsinki and approved by the ethics committee of West China Hospital.
Assessment of preoperative CXI, cachexia, and postoperative major complications
The preoperative CXI was calculated as the following formula: SMI (cm2/m2) × serum albumin (g/L)/NLR (11). The SMI was measured based on the preoperative abdominal CT images of included patients. The skeletal muscle area of the third lumbar vertebra (L3) level was analyzed using the software of syngo MultiModality Workplace (Siemens Medical Solutions, Forchheim, Germany) and BMI_CT (Seoul, South Korea), and the Hounsfield unit (HU) threshold for the skeletal muscle was set as -29 to 150 (16). The SMI was reported as total skeletal muscle area (cm2) of L3/height squared (m2) (17, 18). NLR was reported as the number of peripheral neutrophils/the number of peripheral lymphocytes (19). The postoperative major complications were recognized as surgical complications ≥ Grade III (20). Cachexia was diagnosed according to the Fearon criteria: weight loss > 5% over the past 6 months; or BMI < 20 and any degree of weight loss > 2%; or sarcopenia and any degree of weight loss > 2% (1). The L3 SMI cut-offs for sarcopenia were defined as <52.4 (men) and <38.5 cm2/m2 (women), respectively (21–24).
Statistical analysis
A two-sided P-value of < 0.05 meant statistical significance in this study. For continuous data, the t-test or Mann–Whitney U test was used for comparison according to the normality, and the Chi-squared test or Fisher’s exact test was used for categorical data. To investigate the diagnostic capacity of CXI in the detection of major complications, receiver operating characteristic (ROC) curves were conducted. The cut-off value of CXI for defining the low and high CXI was determined according to Youden’s index. The univariate logistic regression analysis was applied for investigating the associations between multiple clinicopathological variables and the risk of major complications, and variables with a P-value of < 0.2 in the univariate analysis were further analyzed in multivariate analysis. Kaplan–Meier survival curves were used for analyzing the survival data, and the differences in survival curves were analyzed by log-rank tests. The univariate Cox proportional hazards model was also used for analyzing the overall survival (OS). Then, variables with a P-value of < 0.2 in the univariate analysis were further analyzed in multivariate analysis. The SPSS version 25.0 and GraphPad Prism version 8.0 were used for statistical analyses in this study.
Results
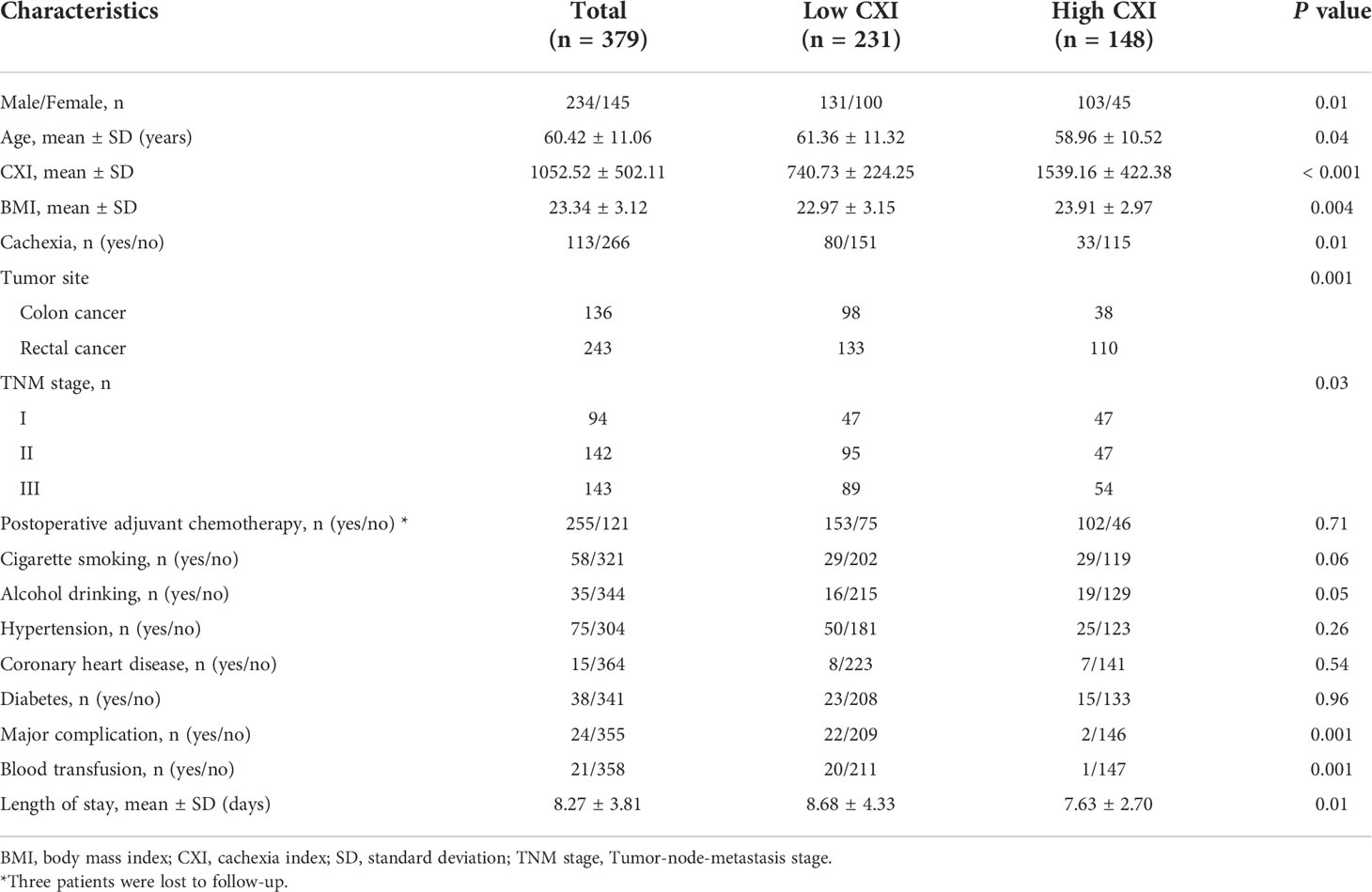
A total of 379 patients (234 men and 145 women) with a mean age of 60.42 (± 11.06) years old were included in this study, and three patients were lost to follow-up. There were 136 patients with colon cancer and 243 with rectal cancer, respectively. 113 patients (29.82%) were diagnosed with cachexia based on the Fearon criteria. Postoperative pathology indicated that there were 94, 142, and 143 patients classified as Tumor-node-metastasis (TNM) stage I, II, and III, respectively. Besides, 24 patients (6.33%) had major surgical complications and 21 patients (5.54%) had a blood transfusion. Detailed clinical characteristics of included patients were shown in Table 1.
The representative CT image for assessing the skeletal muscle area of the L3 level was shown in Figure 1, and the mean CXI for all included patients was 1052.52 (± 502.11). Through ROC curve, we observed that CXI had a significantly diagnostic capacity for the detection of major complications (AUC: 0.671; 95%CI: 0.566 to 0.775; P= 0.005) (Figure 2).
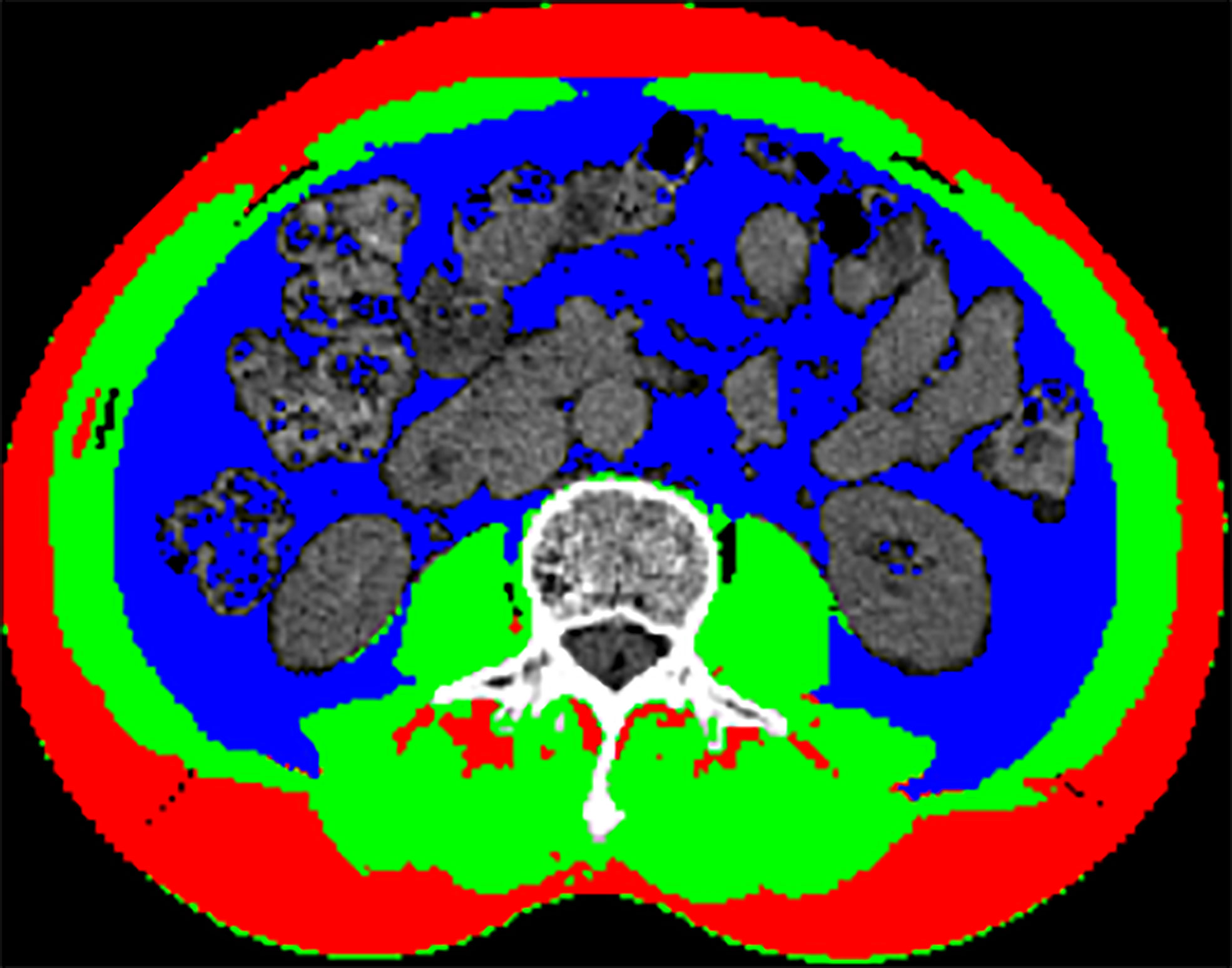
Figure 1 Representative CT image of the skeletal muscle area at L3 level: (Green) skeletal muscle; (Blue) visceral adipose tissue; (Red) subcutaneous adipose tissue.
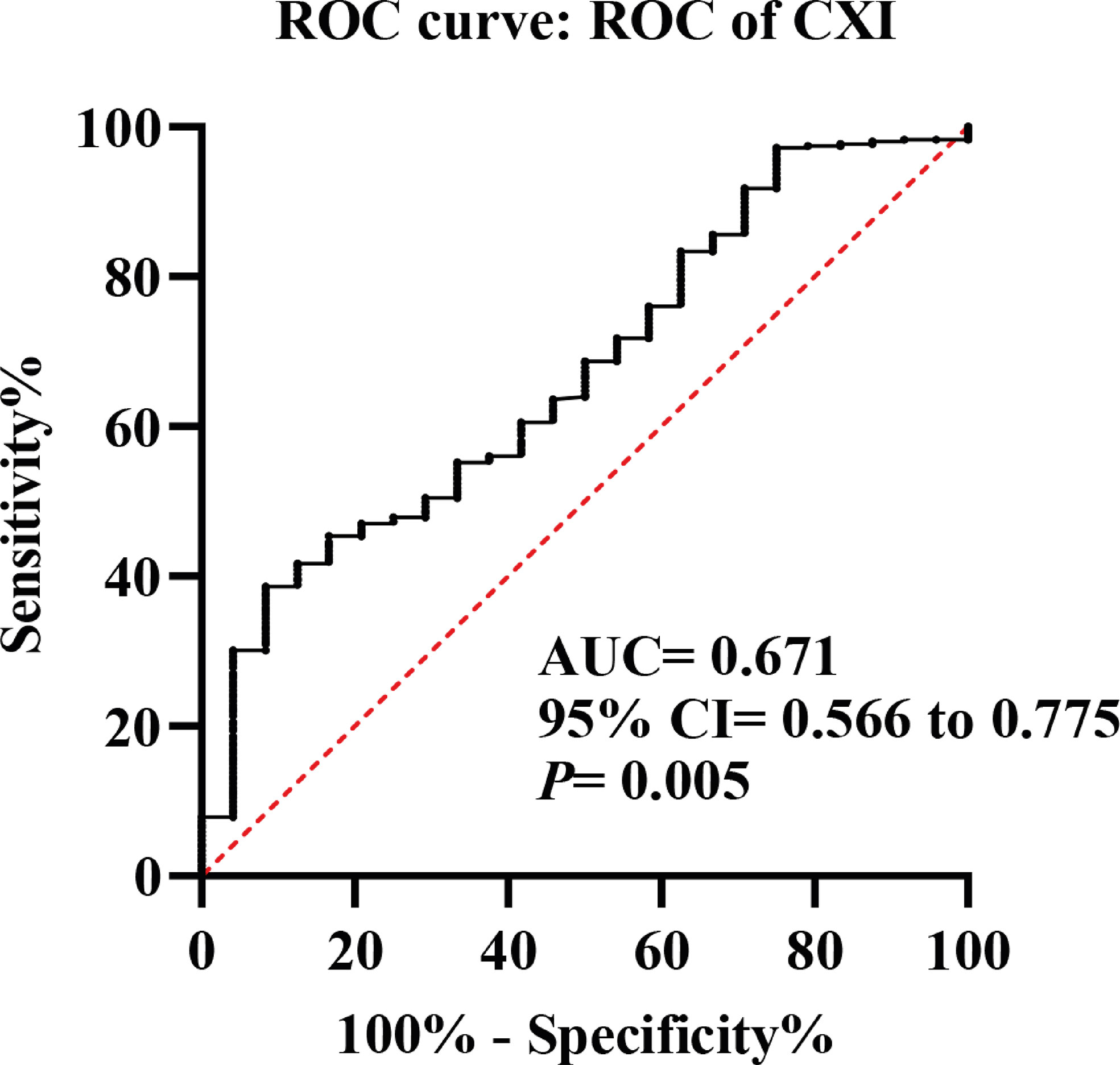
Figure 2 Receiver operating characteristic (ROC) curves of cachexia index (CXI) for the detection of major complications.
Based on the ROC curves of major complications and Youden’s index, patients with a CXI of < 1087 (male) or < 1164 (female) were classified as low CXI group, and patients with a CXI of ≥ 1087 (male) or ≥ 1164 (female) were classified as high CXI group (Figure S1). Eventually, there were 231 patients with low CXI and 148 patients with high CXI, respectively. Patients in the low CXI group had a significantly older age (61.36 ± 11.32 vs. 58.96 ± 10.52, P= 0.04), lower BMI (22.97 ± 3.15 vs. 23.91 ± 2.97, P= 0.004), and a higher percentage of cachexia (P= 0.01) and TNM stage II+III (P= 0.03). Besides, Patients in low CXI group were associated with a significantly higher rate of major complications (9.52% vs. 1.35%, P= 0.001), blood transfusion (8.66% vs. 0.68%, P= 0.001), and longer length of stay (8.68 ± 4.33 vs. 7.63 ± 2.70, P= 0.01) (Table 1). No significant differences were found in postoperative adjuvant chemotherapy, cigarette smoking, alcohol drinking, hypertension, coronary heart disease, and diabetes between the two groups (Table 1).
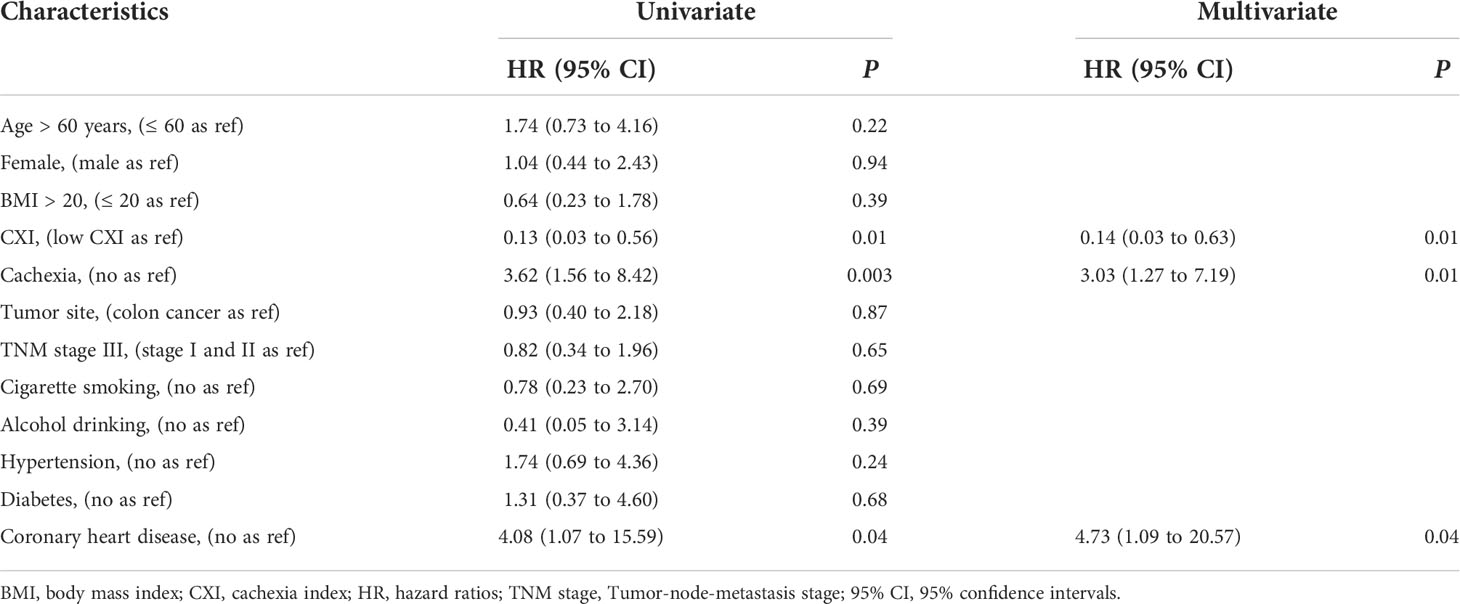
In logistic regression analysis for the associations between multiple clinical variables and risk of major complications, both univariate and multivariate analysis indicated that low CXI (multivariate analysis: HR 0.14, 95% CI 0.03 to 0.63; P= 0.01), cachexia (multivariate analysis: HR 3.03, 95% CI 1.27 to 7.19; P= 0.01), and coronary heart disease (multivariate analysis: HR 4.73, 95% CI 1.09 to 20.57; P= 0.04) were independent risk factors for the major complications (Table 2).
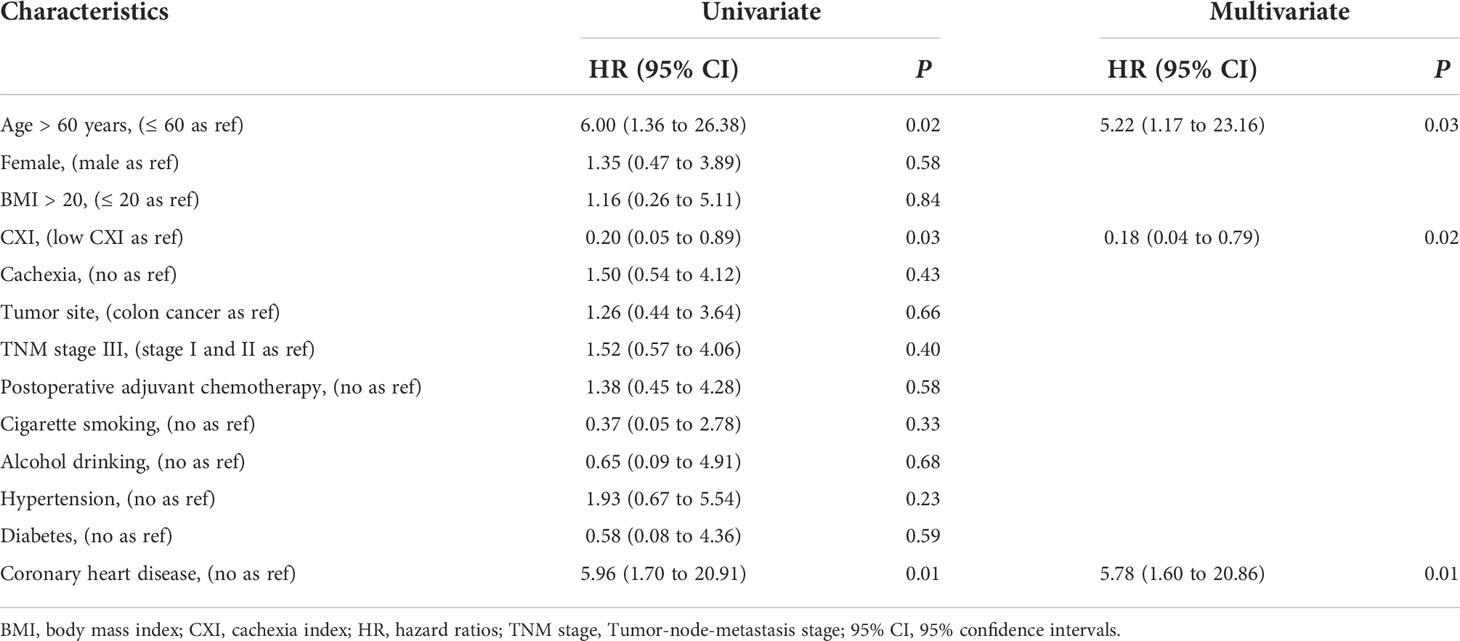
To investigate the prognostic value of CXI and cachexia in the survival of colorectal patients, the Kaplan–Meier survival analyses for OS and recurrence-free survival (RFS) were conducted. The results indicated that patients with high CXI had a significantly more favorable OS than those with low CXI (P= 0.02, Figure 3A), while no significant difference was found in RFS between the two groups (P= 0.91, Figure 3B). There were no significant differences in OS (P= 0.43, Figure 4A) or RFS (P= 0.07, Figure 4B) between patients with and without cachexia. Univariate and multivariate regression analysis indicated that older age (multivariate analysis: HR 5.22, 95% CI 1.17 to 23.16; P= 0.03), low CXI (multivariate analysis: HR 0.18, 95% CI 0.04 to 0.79; P= 0.02), and coronary heart disease (multivariate analysis: HR 5.78, 95% CI 1.60 to 20.86; P= 0.01) instead of cachexia were significantly associated with a decreased OS (Table 3).
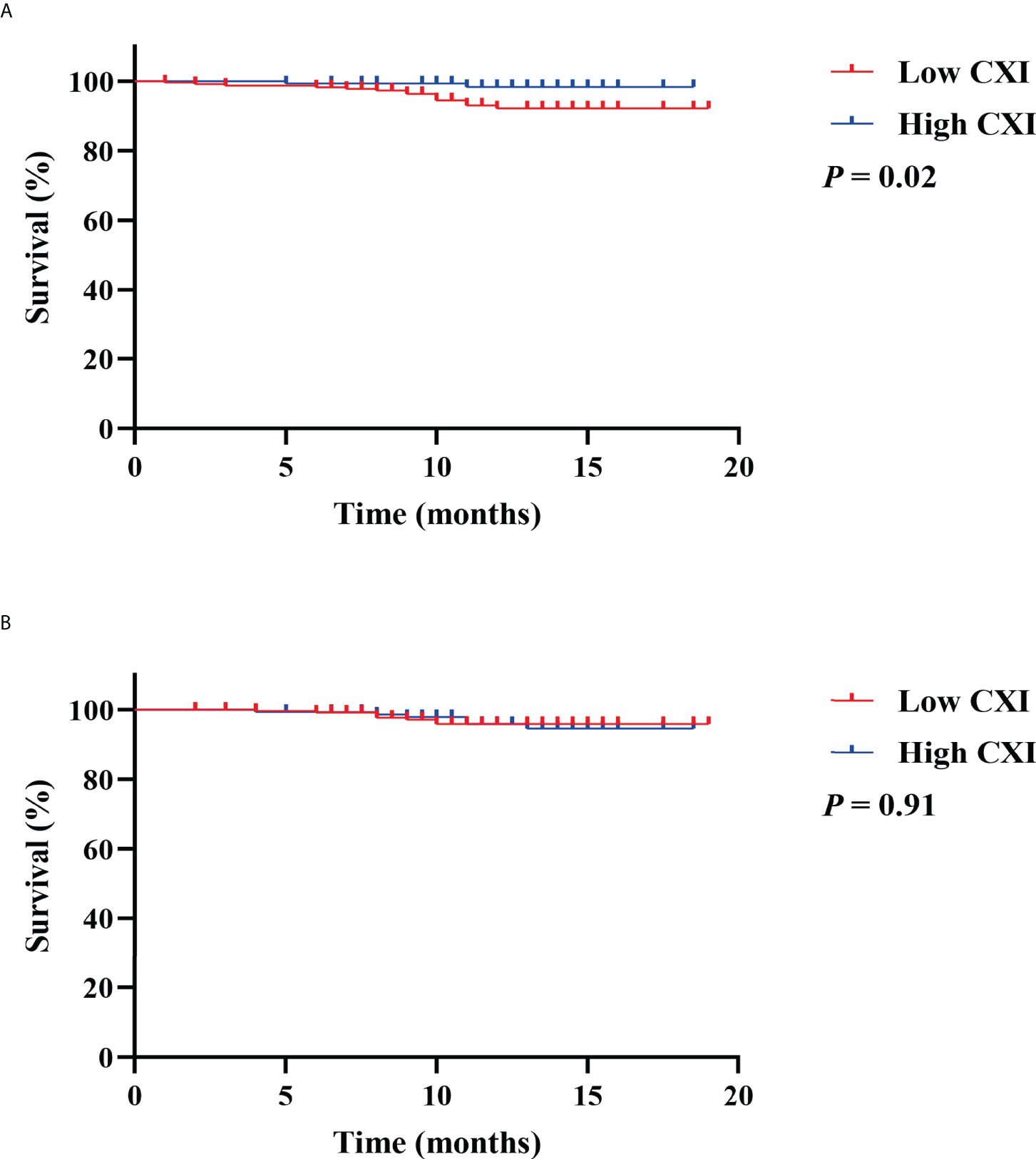
Figure 3 Kaplan Meier survival curves for the associations between cachexia index (CXI) and (A) overall survival; (B) recurrence free survival.
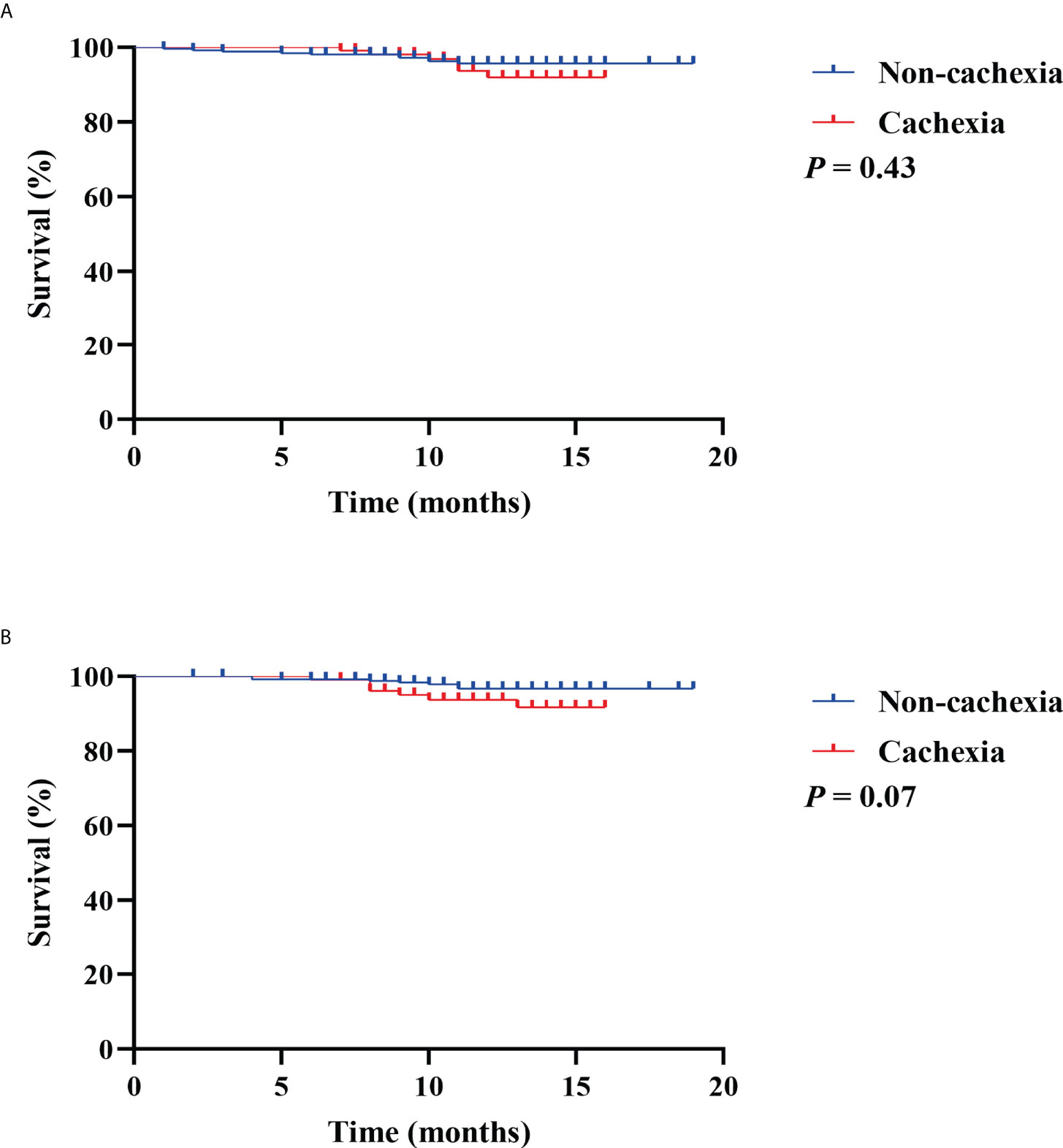
Figure 4 Kaplan Meier survival curves for the associations between cachexia and (A) overall survival; (B) recurrence free survival.
Discussion
In this study of 379 patients, we firstly investigated the prognostic value of CXI in patients with colorectal cancer. We found that a lower CXI was significantly associated with older age, lower BMI, and a more advanced TNM stage, which might reflect a more serious disease state. Besides, patients with low CXI were more likely to be diagnosed with cachexia, indicating that CXI could be a measurement of cachexia. Be similar to the previous studies that low CXI was an independent negative prognostic factor for OS in patients with lung cancer, liver cancer, and aggressive lymphomas (11–15), the Kaplan Meier survival curves and Cox regression analysis in this study also indicated that patients with high CXI had a significantly more favorable OS than those with low CXI. Besides, we found that CXI had no associations with the RFS, indicating that CXI is a prognostic indicator for OS instead of RFS. Furthermore, we first found that low CXI was significantly associated with poor perioperative outcomes, including a higher rate of major complications, blood transfusion, and longer length of stay. We also investigated the prognostic of cachexia diagnosed according to the Fearon criteria. However, Kaplan Meier survival curves and Cox regression analysis indicated that cachexia had no significant associations with the postoperative survival. These findings demonstrated that CXI was better than cachexia in predicting OS and could be a useful prognostic indicator in patients with colorectal cancer.
Cancer cachexia is widely recognized as a disorder characterized by an ongoing loss of skeletal muscle mass (regardless of the loss of fat mass) and cannot be fully reversed by conventional nutritional support (1, 2). Skeletal muscle loss, malnutrition, and increased inflammatory response are three key features of cancer cachexia (2, 4, 25). Current diagnostic criteria for cachexia mainly include the cancer-specific and general criteria (4). Although these diagnostic criteria of cancer cachexia have differences from each other, an estimate of weight loss is indispensable. However, weight loss as the main diagnostic criterion of cancer cachexia might increase the risk of recalling bias. Besides, a loss of total body weight cannot reflect a specific loss of skeletal muscle and fat. Furthermore, in some patients with advanced cancer, the fluid gains such as edema and malignant ascites could mask the actual weight loss (26, 27), which might increase the risk of bias when investigating cancer cachexia.
CXI is a new measure of cachexia that is calculated as SMI (cm2/m2) × serum albumin (g/L)/NLR, and these three parameters are objective and easily accessed from abdominal CT scans, routine peripheral blood, and biochemical tests. CT-determined SMI is a useful indicator to reflect the skeletal muscle mass and has been widely applied for investigating sarcopenia in many clinical studies (28–30). Our previous umbrella review also summarized that SMI-determined sarcopenia was significantly associated with multiple health-related outcomes in older populations and patients with or without tumors (31). An abdominal CT scan is a routine examination for patients with colorectal cancer before surgery, and CT-determined SMI of the L3 level is recognized as reflecting the whole-body muscle mass (32, 33). Therefore, CT-determined SMI is available for almost every patient with colorectal cancer and could be a key indicator for assessing the cancer cachexia. For malnutrition, albumin is an important nutritional marker in patients with gastrointestinal cancer (34, 35). It is reported that hypoalbuminemia can reflect cancer-induced malnutrition and have a negative impact on prognosis in patients with cancer (36, 37). NLR is an inflammation-related marker in the calculation of CXI, and it is also recognized as an indicator of cancer-related systemic inflammation in gastrointestinal cancer (36, 38–40). Therefore, the three objective indicators in the calculation formula of CXI could reflect the skeletal muscle status, malnutrition, and systematic inflammatory response of cancer cachexia, respectively.
Notably, the reality of CXI might be influenced by some drugs and diseases. In patients with diabetes, for example, the use of insulin could affect the level of serum albumin (41, 42). Besides, patients with liver cirrhosis usually have significantly decreased serum albumin (43). Furthermore, some studies suggest that the use of steroids could lead to an increase in NLR (44, 45). Therefore, the use of specific drugs should be noted when calculating CXI. Be limited by lacking relevant data, we did not investigate the impact of the use of specific drugs on the prognostic value of CXI in this study, and further studies about this issue are required in the future.
The strengths of this study are that we first investigate the prognostic value of CXI in patients with colorectal cancer, and our sample is the largest among the relevant studies about CXI and prognosis of malignancies. Our results indicate that low CXI is an independent negative prognostic factor for OS, and we also first suggest that low CXI was significantly associated with poor perioperative outcomes including a higher rate of major complications, blood transfusion, and longer length of stay. There are also several limitations of this study. Because this is a single-center and retrospective study, the risk of selection bias might be increased. Besides, it is the first study investigating CXI in colorectal cancer, thus, no external validation is available. The cut-off value for determining the low and high CXI needs further prospective studies to verify in the future. Furthermore, we only included Chinese patients in this study, and whether CXI could be a prognostic indicator for other races remains to be verified.
In conclusion, our study identified that CXI was better than cachexia in predicting OS and could be a useful prognostic indicator in patients with colorectal cancer, and greater attention should be paid to patients with low CXI. Considering the limitations of this study, our results need more prospective studies to verify in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of West China Hospital, Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
QW, QY, and RZ contributed equally to this study. All of the authors contributed to the research and development process that resulted in this article. QW, QY, and RZ wrote the manuscript. All of the authors read the manuscript and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81970715); Key Research and Development Program of Sichuan Province (22ZDYF2138).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.984459/full#supplementary-material
Supplementary Figure 1 | Receiver operating characteristic (ROC) curve of cachexia index (CXI) and major complications for determining the cut-off values of low and high CXI groups (A) male patients; (B) female patients.
References
1. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol (2011) 12(5):489–95. doi: 10.1016/S1470-2045(10)70218-7
2. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers (2018) 4:17105. doi: 10.1038/nrdp.2017.105
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. van der Meij BS, Teleni L, McCarthy AL, Isenring EA. Cancer cachexia: an overview of diagnostic criteria and therapeutic approaches for the accredited practicing dietitian. J Hum Nutr Diet (2021) 34(1):243–54. doi: 10.1111/jhn.12811
5. Gannavarapu BS, Lau SKM, Carter K, Cannon NA, Gao A, Ahn C, et al. Prevalence and survival impact of pretreatment cancer-associated weight loss: A tool for guiding early palliative care. J Oncol Pract (2018) 14(4):e238–e50. doi: 10.1200/JOP.2017.025221
6. Thoresen L, Frykholm G, Lydersen S, Ulveland H, Baracos V, Prado CM, et al. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. different assessment criteria for nutritional status provide unequal results. Clin Nutr (2013) 32(1):65–72. doi: 10.1016/j.clnu.2012.05.009
7. Shibata M, Fukahori M, Kasamatsu E, Machii K, Hamauchi S. A retrospective cohort study to investigate the incidence of cachexia during chemotherapy in patients with colorectal cancer. Adv Ther (2020) 37(12):5010–22. doi: 10.1007/s12325-020-01516-6
8. Brown JC, Caan BJ, Cespedes Feliciano EM, Xiao J, Weltzien E, Prado CM, et al. Weight stability masks changes in body composition in colorectal cancer: A retrospective cohort study. Am J Clin Nutr (2021) 113(6):1482–9. doi: 10.1093/ajcn/nqaa440
9. van der Werf A, van Bokhorst QNE, de van der Schueren MAE, Verheul HMW, Langius JAE. Cancer cachexia: Identification by clinical assessment versus international consensus criteria in patients with metastatic colorectal cancer. Nutr Cancer (2018) 70(8):1322–9. doi: 10.1080/01635581.2018.1504092
10. Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer cachexia: Beyond weight loss. J Oncol Pract (2016) 12(11):1163–71. doi: 10.1200/JOP.2016.016832
11. Go SI, Park MJ, Park S, Kang MH, Kim HG, Kang JH, et al. Cachexia index as a potential biomarker for cancer cachexia and a prognostic indicator in diffuse large b-cell lymphoma. J Cachexia Sarcopenia Muscle (2021) 12(6):2211–9. doi: 10.1002/jcsm.12837
12. Go SI, Park MJ, Lee GW. Clinical significance of the cachexia index in patients with small cell lung cancer. BMC Cancer (2021) 21(1):563. doi: 10.1186/s12885-021-08300-x
13. Jafri SH, Previgliano C, Khandelwal K, Shi R. Cachexia index in advanced non-Small-Cell lung cancer patients. Clin Med Insights Oncol (2015) 9:87–93. doi: 10.4137/CMO.S30891
14. Karmali R, Alrifai T, Fughhi IAM, Ng R, Chukkapalli V, Shah P, et al. Impact of cachexia on outcomes in aggressive lymphomas. Ann Hematol (2017) 96(6):951–6. doi: 10.1007/s00277-017-2958-1
15. Goh MJ, Kang W, Jeong WK, Sinn DH, Gwak GY, Paik YH, et al. Prognostic significance of cachexia index in patients with advanced hepatocellular carcinoma treated with systemic chemotherapy. Sci Rep (2022) 12(1):7647. doi: 10.1038/s41598-022-11736-1
16. Kurk S, Peeters P, Stellato R, Dorresteijn B, de Jong P, Jourdan M, et al. Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle (2019) 10(4):803–13. doi: 10.1002/jcsm.12436
17. Kim YY, Lee J, Jeong WK, Kim ST, Kim JH, Hong JY, et al. Prognostic significance of sarcopenia in microsatellite-stable gastric cancer patients treated with programmed death-1 inhibitors. Gastric Cancer (2021) 24(2):457–66. doi: 10.1007/s10120-020-01124-x
18. Oke SM, Rye B, Malietzis G, Baldwin-Cleland R, Bottle A, Gabe SM, et al. Survival and CT defined sarcopenia in patients with intestinal failure on home parenteral support. Clin Nutr (2020) 39(3):829–36. doi: 10.1016/j.clnu.2019.03.015
19. Huang CM, Huang MY, Tsai HL, Huang CW, Su WC, Chang TK, et al. Pretreatment neutrophil-to-Lymphocyte ratio associated with tumor recurrence and survival in patients achieving a pathological complete response following neoadjuvant chemoradiotherapy for rectal cancer. Cancers (Basel) (2021) 13(18). doi: 10.3390/cancers13184589
20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
21. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol (2008) 9(7):629–35. doi: 10.1016/S1470-2045(08)70153-0
22. Mishra A, Bigam KD, Extermann M, Faramand R, Thomas K, Pidala JA, et al. Sarcopenia and low muscle radiodensity associate with impaired FEV1 in allogeneic haematopoietic stem cell transplant recipients. J Cachexia Sarcopenia Muscle (2020) 11(6):1570–9. doi: 10.1002/jcsm.12604
23. Fehrenbach U, Wuensch T, Gabriel P, Segger L, Yamaguchi T, Auer TA, et al. CT body composition of sarcopenia and sarcopenic obesity: Predictors of postoperative complications and survival in patients with locally advanced esophageal adenocarcinoma. Cancers (Basel) (2021) 13(12). doi: 10.3390/cancers13122921
24. Wan Q, Wang Z, Zhao R, Tu T, Shen X, Shen Y, et al. CT-determined low skeletal muscle mass predicts worse overall survival of gastric cancer in patients with cachexia. Cancer Med (2022). doi: 10.1002/cam4.5040
25. Moreira-Pais A, Ferreira R, Oliveira PA, Duarte JA. Sarcopenia versus cancer cachexia: The muscle wasting continuum in healthy and diseased aging. Biogerontology (2021) 22(5):459–77. doi: 10.1007/s10522-021-09932-z
26. Roeland EJ, Ma JD, Nelson SH, Seibert T, Heavey S, Revta C, et al. Weight loss versus muscle loss: Re-evaluating inclusion criteria for future cancer cachexia interventional trials. Support Care Cancer (2017) 25(2):365–9. doi: 10.1007/s00520-016-3402-0
27. Mahmoud AM, Ismail YM, Hussien A, Debaky Y, Ahmed IS, Mikhael HSW, et al. Peritoneal carcinomatosis in colorectal cancer: Defining predictive factors for successful cytoreductive surgery and hyperthermic intraperitoneal chemotherapy - a pilot study. J Egyptian Natl Cancer Inst (2018) 30(4):143–50. doi: 10.1016/j.jnci.2018.10.004
28. Xie H, Gong Y, Kuang J, Yan L, Ruan G, Tang S, et al. Computed tomography-determined sarcopenia is a useful imaging biomarker for predicting postoperative outcomes in elderly colorectal cancer patients. Cancer Res Treat (2020) 52(3):957–72. doi: 10.4143/crt.2019.695
29. Rinninella E, Cintoni M, Raoul P, Pozzo C, Strippoli A, Bria E, et al. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: A systematic review and meta-analysis. Clin Nutr (2020) 39(7):2045–54. doi: 10.1016/j.clnu.2019.10.021
30. Pamoukdjian F, Bouillet T, Levy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin Nutr (2018) 37(4):1101–13. doi: 10.1016/j.clnu.2017.07.010
31. Xia L, Zhao R, Wan Q, Wu Y, Zhou Y, Wang Y, et al. Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies. Cancer Med (2020) 9(21):7964–78. doi: 10.1002/cam4.3428
32. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing (2019) 48(1):16–31. doi: 10.1093/ageing/afy169
33. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutrition Metab = Physiol Appliquee Nutr Metabol (2008) 33(5):997–1006. doi: 10.1139/H08-075
34. Gupta A, Gupta E, Hilsden R, Hawel JD, Elnahas AI, Schlachta CM, et al. Preoperative malnutrition in patients with colorectal cancer. Can J Surg (2021) 64(6):E621–E9. doi: 10.1503/cjs.016820
35. Wan GY, Zheng LY, Li HQ, Yuan H, Xue H, Zhang XY. Effects of enteral nutritional rich in n-3 polyunsaturated fatty acids on the nutritional status of gastrointestinal cancer patients: A systematic review and meta-analysis. Eur J Clin Nutr (2020) 74(2):220–30. doi: 10.1038/s41430-019-0527-5
36. Yamamoto T, Kawada K, Obama K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci (2021) 22(15). doi: 10.3390/ijms22158002
37. Stares M, Swan A, Cumming K, Ding TE, Leach J, Stratton C, et al. Hypoalbuminaemia as a prognostic biomarker of first-line treatment resistance in metastatic non-small cell lung cancer. Front Nutr (2021) 8:734735. doi: 10.3389/fnut.2021.734735
38. Li Z, Zhao R, Cui Y, Zhou Y, Wu X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I-III colon cancer. Sci Rep (2018) 8(1):9453. doi: 10.1038/s41598-018-27896-y
39. Ming-Sheng F, Mei-Ling D, Xun-Quan C, Yuan-Xin H, Wei-Jie Z, Qin-Cong P. Preoperative neutrophil-to-Lymphocyte ratio, platelet-to-Lymphocyte ratio, and CEA as the potential prognostic biomarkers for colorectal cancer. Can J Gastroenterol Hepatol (2022) 2022:3109165. doi: 10.1155/2022/3109165
40. Schiefer S, Wirsik NM, Kalkum E, Seide SE, Nienhuser H, Muller B, et al. Systematic review of prognostic role of blood cell ratios in patients with gastric cancer undergoing surgery. Diagnostics (2022) 12(3). doi: 10.3390/diagnostics12030593
41. Chen Q, Lu M, Monks BR, Birnbaum MJ. Insulin is required to maintain albumin expression by inhibiting forkhead box O1 protein. J Biol Chem (2016) 291(5):2371–8. doi: 10.1074/jbc.M115.677351
42. Wasko J, Wolszczak M, Kaminski ZJ, Steblecka M, Kolesinska B. Human serum albumin binds native insulin and aggregable insulin fragments and inhibits their aggregation. Biomolecules (2020) 10(10). doi: 10.3390/biom10101366
43. Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med (2012) 7 Suppl 3:S193–9. doi: 10.1007/s11739-012-0802-0
44. Fuca G, Galli G, Poggi M, Lo Russo G, Proto C, Imbimbo M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open (2019) 4(1):e000457. doi: 10.1136/esmoopen-2018-000457
Keywords: colorectal cancer, cachexia index, cancer cachexia, major complications, overall survival
Citation: Wan Q, Yuan Q, Zhao R, Shen X, Chen Y, Li T and Song Y (2022) Prognostic value of cachexia index in patients with colorectal cancer: A retrospective study. Front. Oncol. 12:984459. doi: 10.3389/fonc.2022.984459
Received: 02 July 2022; Accepted: 02 September 2022;
Published: 23 September 2022.
Edited by:
Aneesha Dasgupta, Purdue University Indianapolis, United StatesReviewed by:
Shrey Kanvinde, BioMarin, United StatesTanmay Kulkarni, Bristol Myers Squibb, United States
Copyright © 2022 Wan, Yuan, Zhao, Shen, Chen, Li and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinghan Song, yinghan2222@163.com; Tao Li, scutaoli1981@scu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Qianyi Wan1†
Qianyi Wan1† Tao Li
Tao Li Yinghan Song
Yinghan Song