- 1Cancer Center, Department of Affiliated People’ Radiation Oncology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, China
- 2Graduate Department, Jinzhou Medical University, Jinzhou, China
- 3Graduate Department, Bengbu Medical College, Bengbu, China
- 4Cancer Center, Department of Medical Oncology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, China
Long non-coding RNA LINC00152 (cytoskeleton regulator, or LINC00152) is an 828-bp lncRNA located on chromosome 2p11.2. LINC00152 was originally discovered during research on hepatocarcinogenesis and has since been regarded as a crucial oncogene that regulates gene expression in many cancer types. LINC00152 is aberrantly expressed in various cancers, including gastric, breast, ovarian, colorectal, hepatocellular, and lung cancer, and glioma. Several studies have indicated that LINC00152 is correlated with cell proliferation, apoptosis, migration, invasion, cell cycle, epithelial-mesenchymal transition (EMT), chemotherapy and radiotherapy resistance, and tumor growth and metastasis. High LINC00152 expression in most tumors is significantly associated with poor patient prognosis. Mechanistic analysis has demonstrated that LINC00152 can serve as a competing endogenous RNA (ceRNA) by sponging miRNA, regulating the abundance of the protein encoded by a particular gene, or modulating gene expression at the epigenetic level. LINC00152 can serve as a diagnostic or prognostic biomarker, as well as a therapeutic target for most cancer types. In the present review, we discuss the roles and mechanisms of LINC00152 in human cancer, focusing on its functions in chemotherapy and radiotherapy resistance.
Introduction
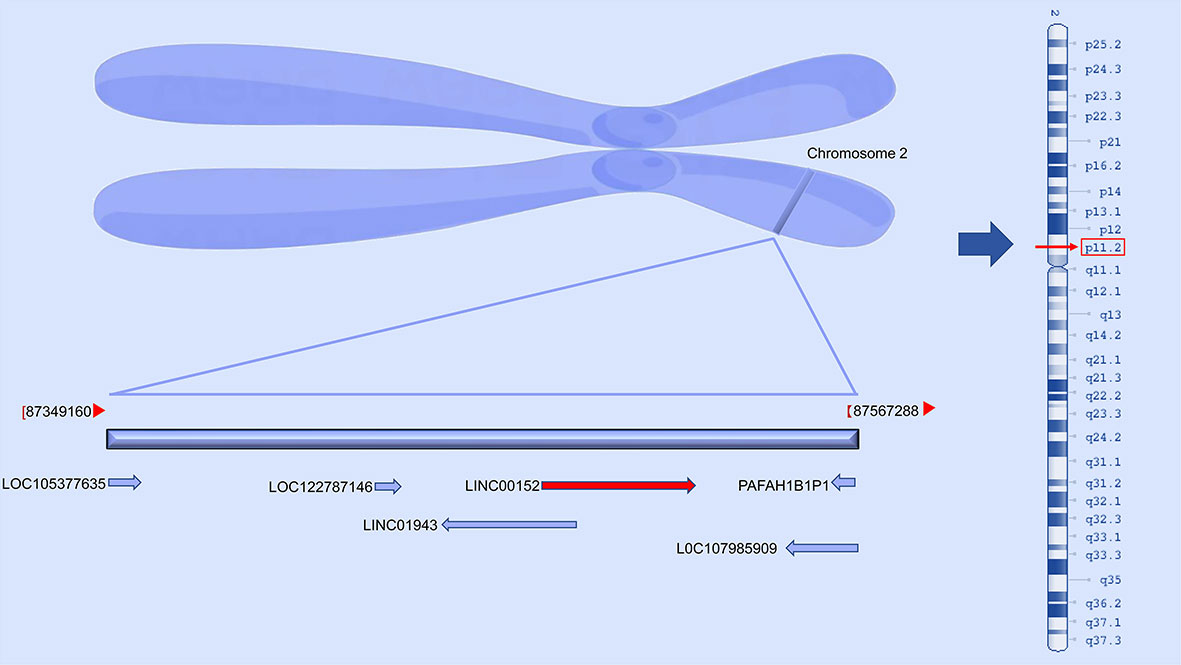
Long non-coding RNAs (lncRNAs) are transcripts of more than 200 nucleotides that generally do not encode proteins and include cyclic RNAs (circRNAs) and pseudogenes (1). These lncRNAs play a vital role in regulating cell homeostasis and disease progression by serving as competitive endogenous RNAs (ceRNAs) or binding directly to regulate tumor occurrence and growth. The lncRNA cytoskeletal regulatory RNA (CYTOR), also known as LINC00152, is located in the chromosomal region 2p11.2, and is overexpressed in many cancers (Figure 1). LINC00152 was initially detected with variable hypomethylation levels during the development of hepatocellular cancer (2).
The function of lncRNAs is highly correlated with their subcellular distribution. LncRNAs act as endogenous miRNA sponges to modulate miRNA targets in the cytoplasm. Cytoskeletal regulators, such as long intergenic non-coding RNA 00152 (LINC00152), can regulate gene expression through various mechanisms. LINC00152 acts as a ceRNA in the cytoplasm and binds to multi-comb inhibition complex 2 (PRC2) in the nucleus to regulate epigenetic gene regulation. LINC00152 is primarily found in the cytoplasm, where cytoplasmic lncRNAs operate as microRNA sponges, thus inhibiting the action of target microRNA (3). Mechanistic investigations have revealed that LINC00152 can act as a ceRNA by sponging miRNA, thus influencing the amount of protein encoded by a gene and altering gene expression at the epigenetic level.
LINC00152, which was later called STAiR18, was identified in 2013 by analyzing the expression profile of signal transducer and activator of transcription 3 (STAT3)-dependent genes in gastric cancer (4). The capacity of LINC00152 to sponge various miRNAs influences cell cycle arrest, apoptosis, EMT, migration, and invasion. Sponging miRNAs eliminates their inhibitory effect on target genes, thereby altering their expression level (4). Subsequent studies have demonstrated that LINC00152 is overexpressed in many human malignancies, including lung, liver, pancreatic, and breast cancers. In addition, LINC00152 has been implicated in regulating cancer cell proliferation, the cell cycle, epithelial-mesenchymal transition (EMT), and chemotherapy and radiotherapy resistance. Ongoing investigations into the role of LINC00152 are therefore required. LINC00152 is a pivotal oncogenic long non‐coding RNA in human cancers (5). The expression of LINC00152 could contribute to tumor diagnosis, targeted therapy and curative effect evaluation (6).
The present review highlights the current research on the function, regulatory mechanisms, and chemotherapy and radiotherapy resistance of LINC00152 in human cancers.
The role of LINC00152 in various cancers
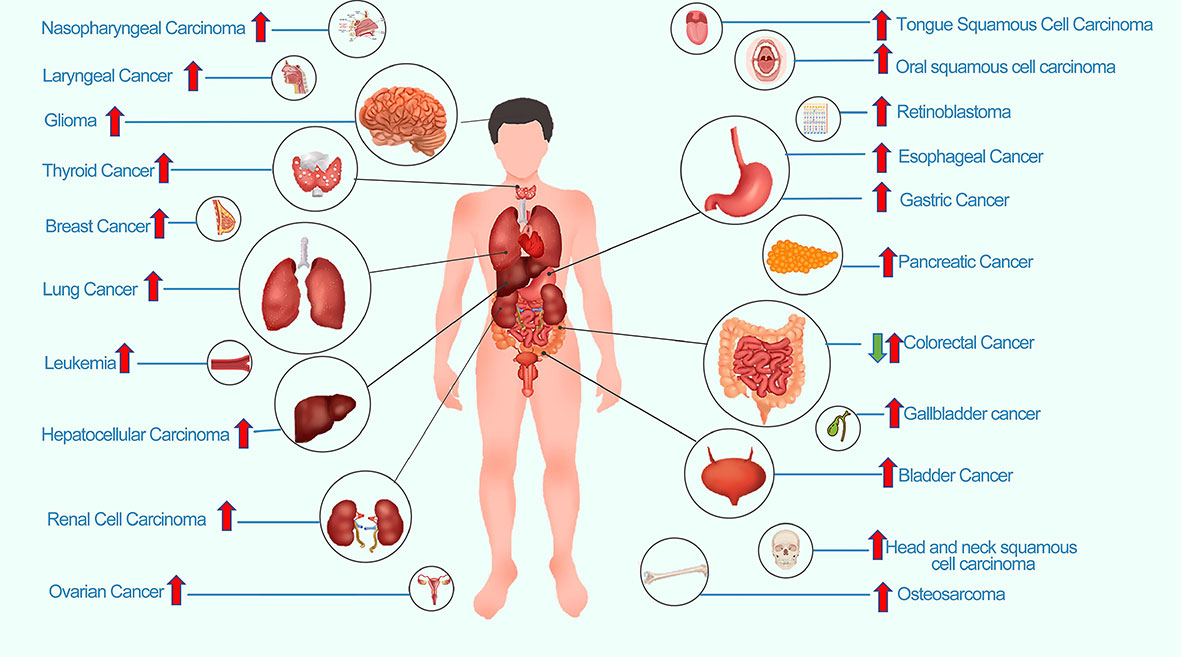
The role of LINC00152 in human cancer has been explored in numerous clinical, translational, and basic studies (5). Accumulating evidence has demonstrated that the expression of LINC00152 is abnormally dysregulated in most tumor types. High expression of LINC00152 has been observed in multiple types of tumors, including breast cancer, ovarian cancer, hepatocellular cancer, lung cancer, leukemia, bladder cancer, nasopharyngeal cancer, gallbladder cancer, osteosarcoma, laryngeal cancer, thyroid cancer, retinoblastoma, head and neck squamous cell cancer, and pancreatic cancer (Figure 2). In contrast, LINC00152 is expressed at low levels in colon cancer tissue and cells (7). A specific explanation for the downregulation of LINC00152 expression in colon cancer remains unknown.
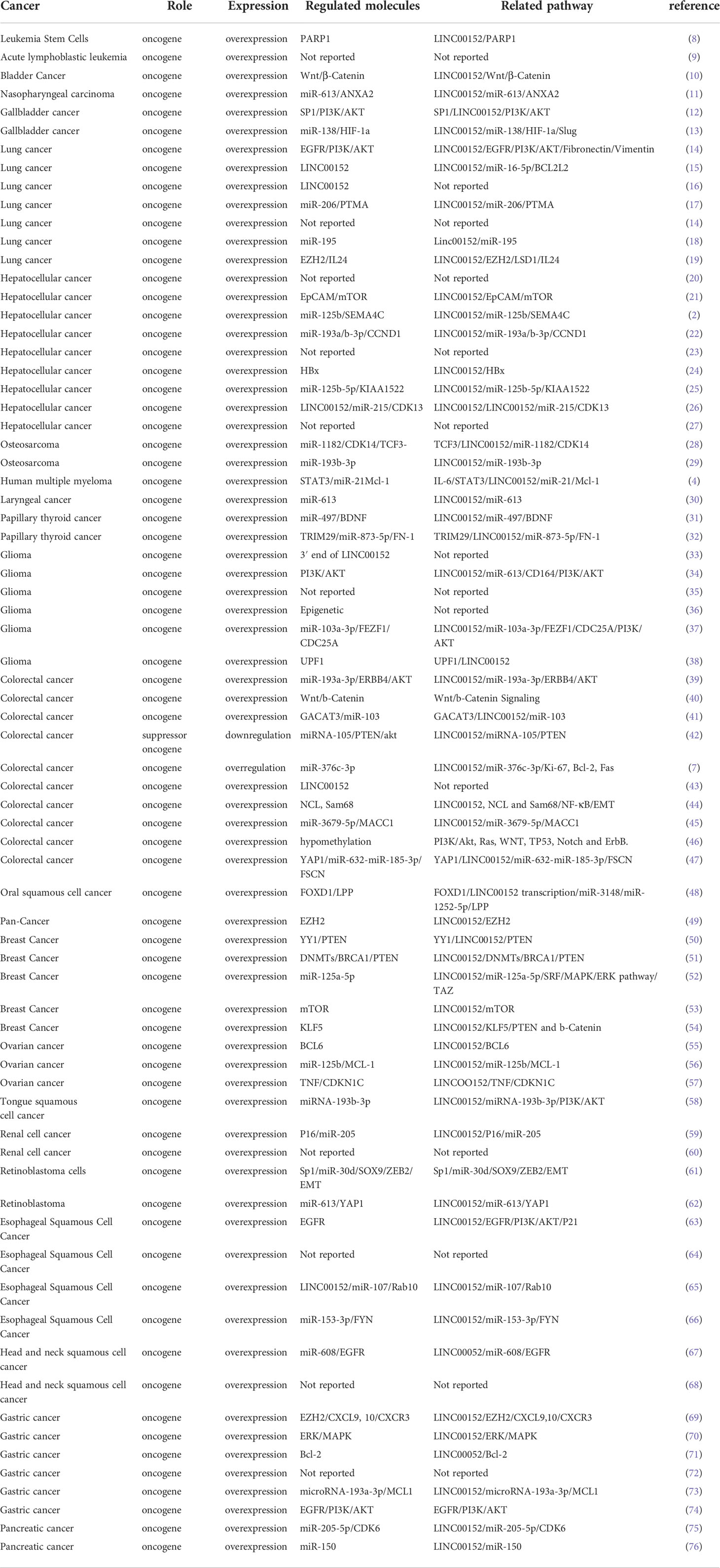
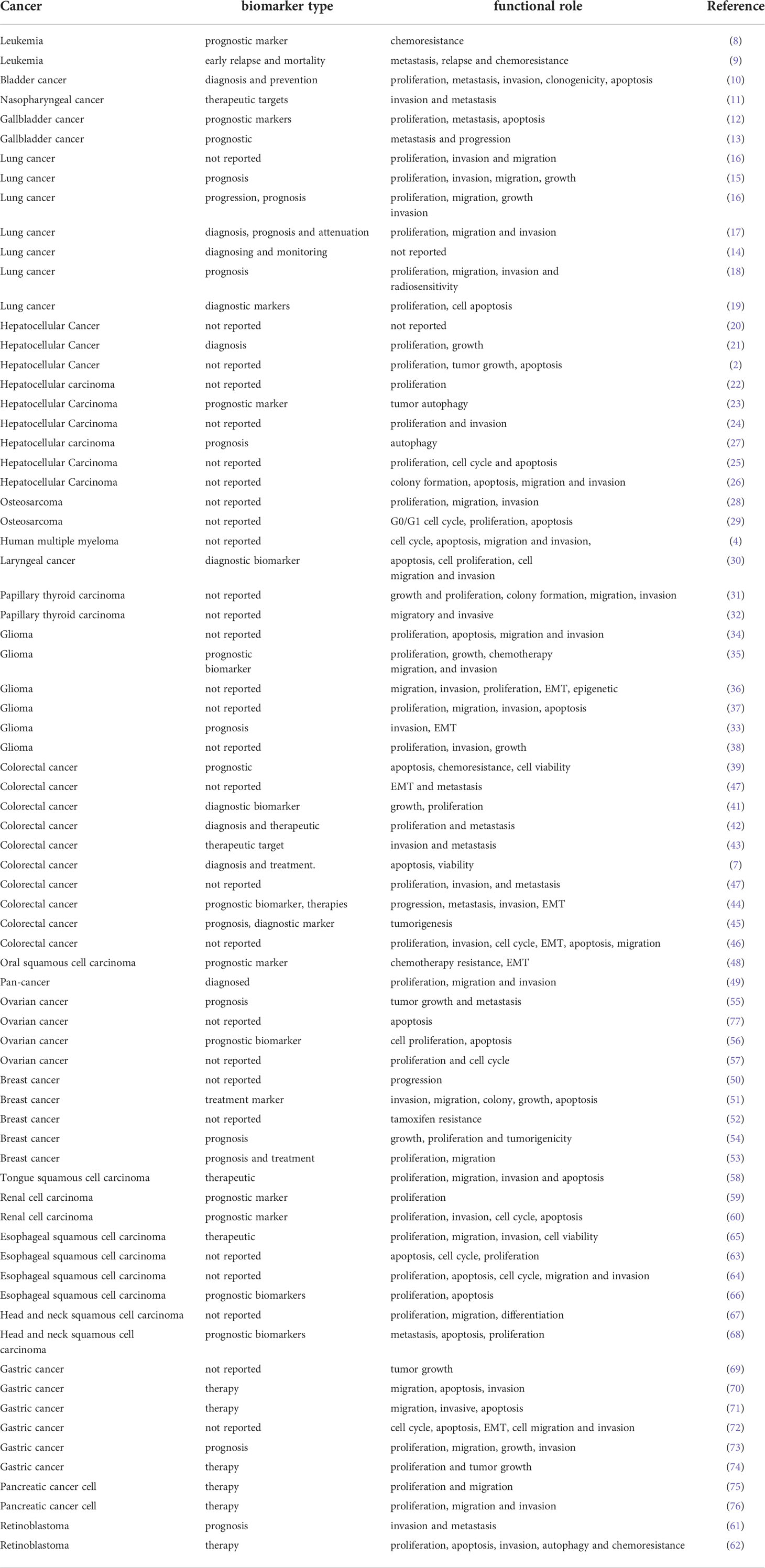
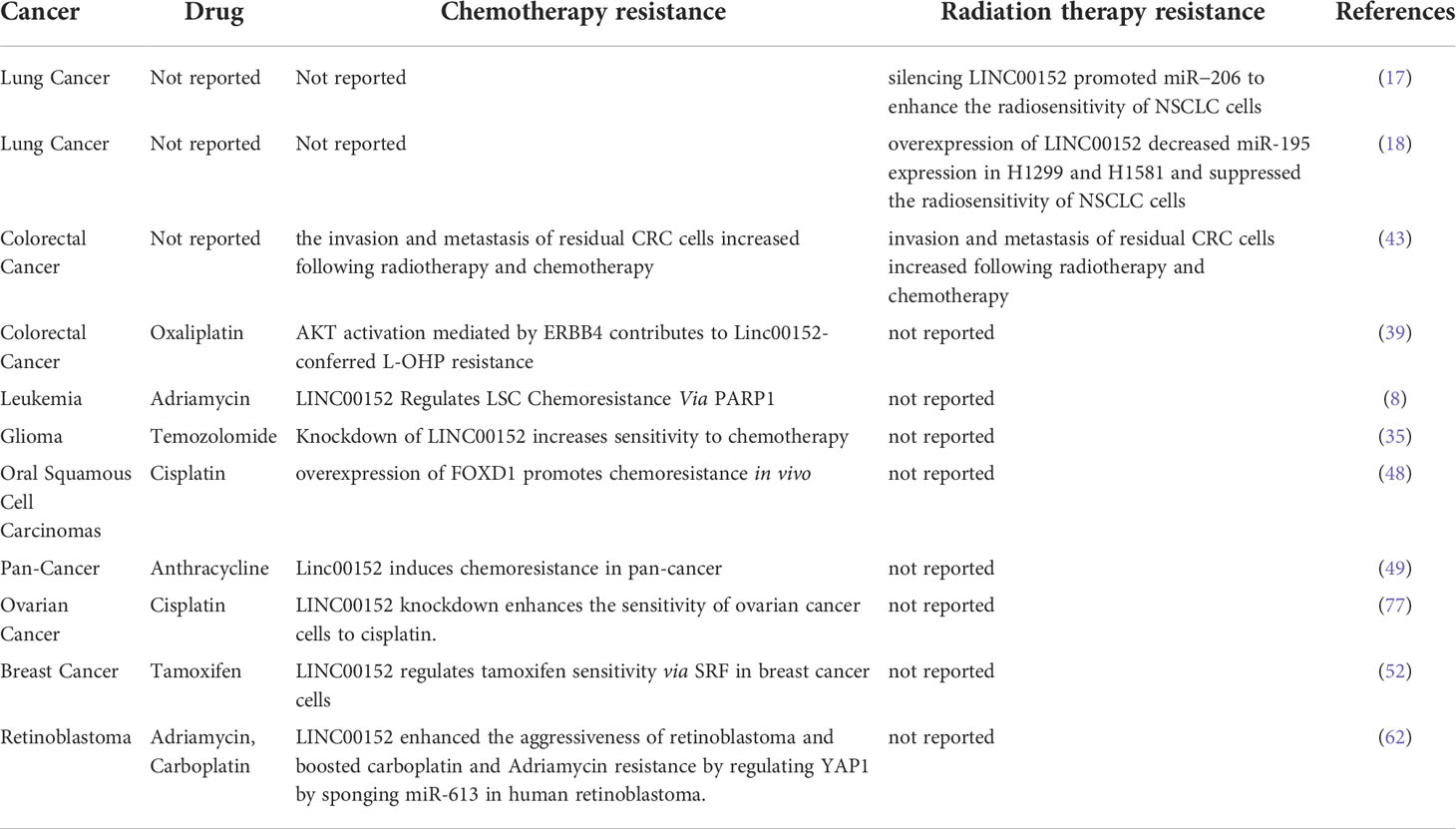
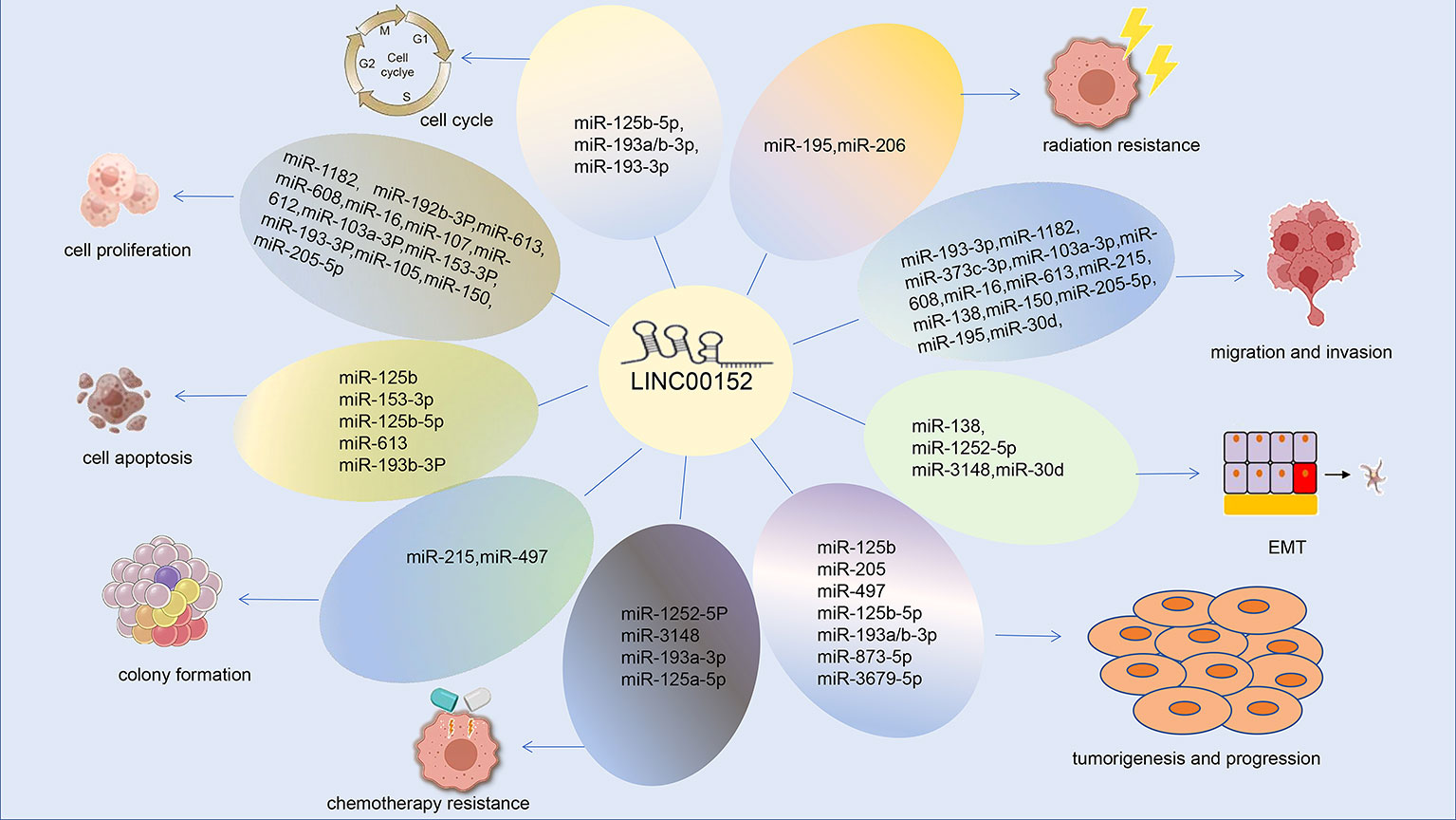
LINC00152 has essential roles in almost all aspects of tumor occurrence and progression, including tumorigenesis, cancer cell proliferation, apoptosis, invasion, metastasis, autophagy, and the response to anti-tumor treatment. The functions and underlying molecular mechanisms of LINC00152 in various cancers are summarized in Table 1. Potential biomarkers for the diagnosis and prognosis of LINC00152 in cancer are presented in Table 2. Table 3 summarizes the role of LINC00152 in chemotherapy and radiation resistance and will be explained in detail in later sections.
Function and mechanisms of LINC00152 in human cancer
Oral squamous cell cancer
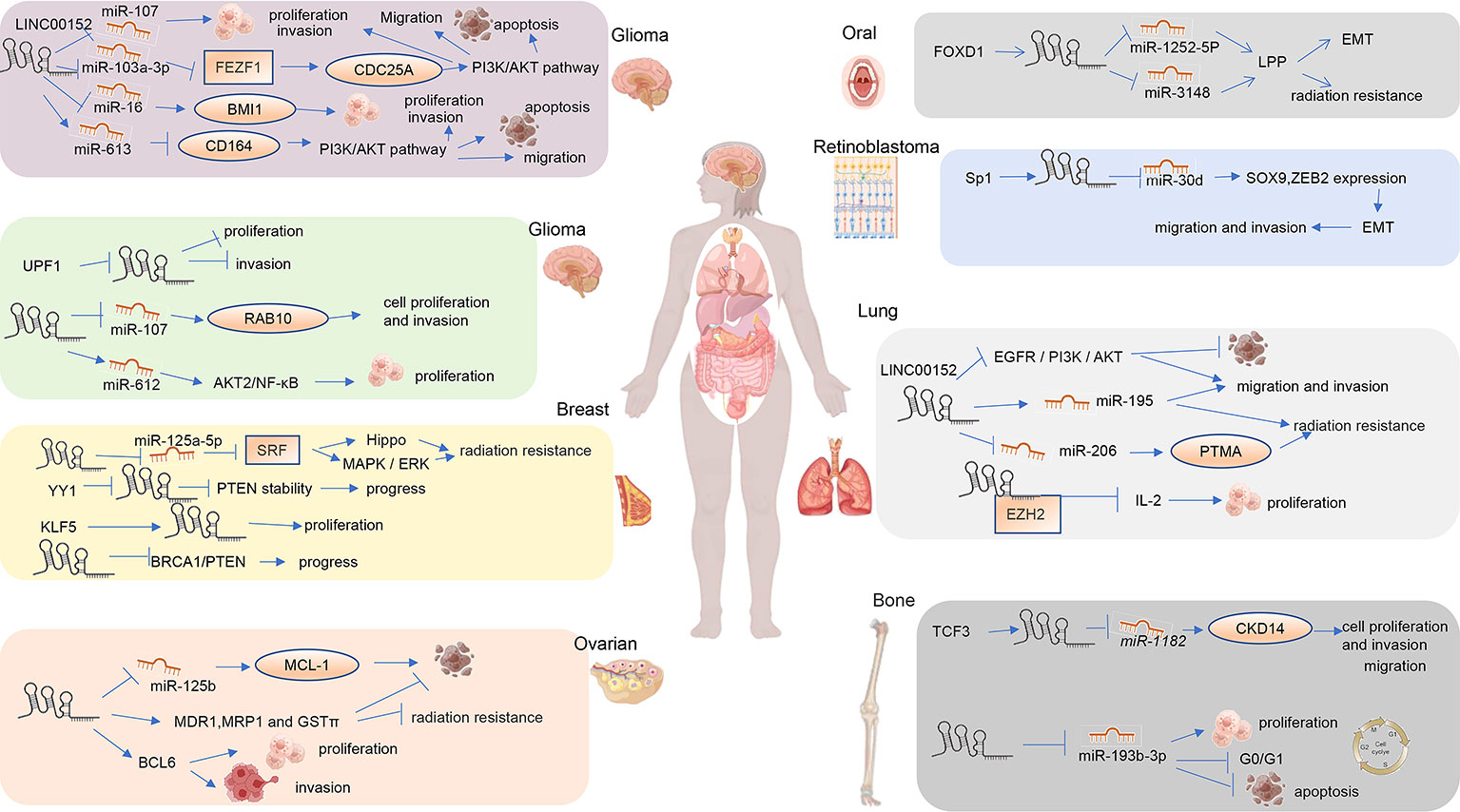
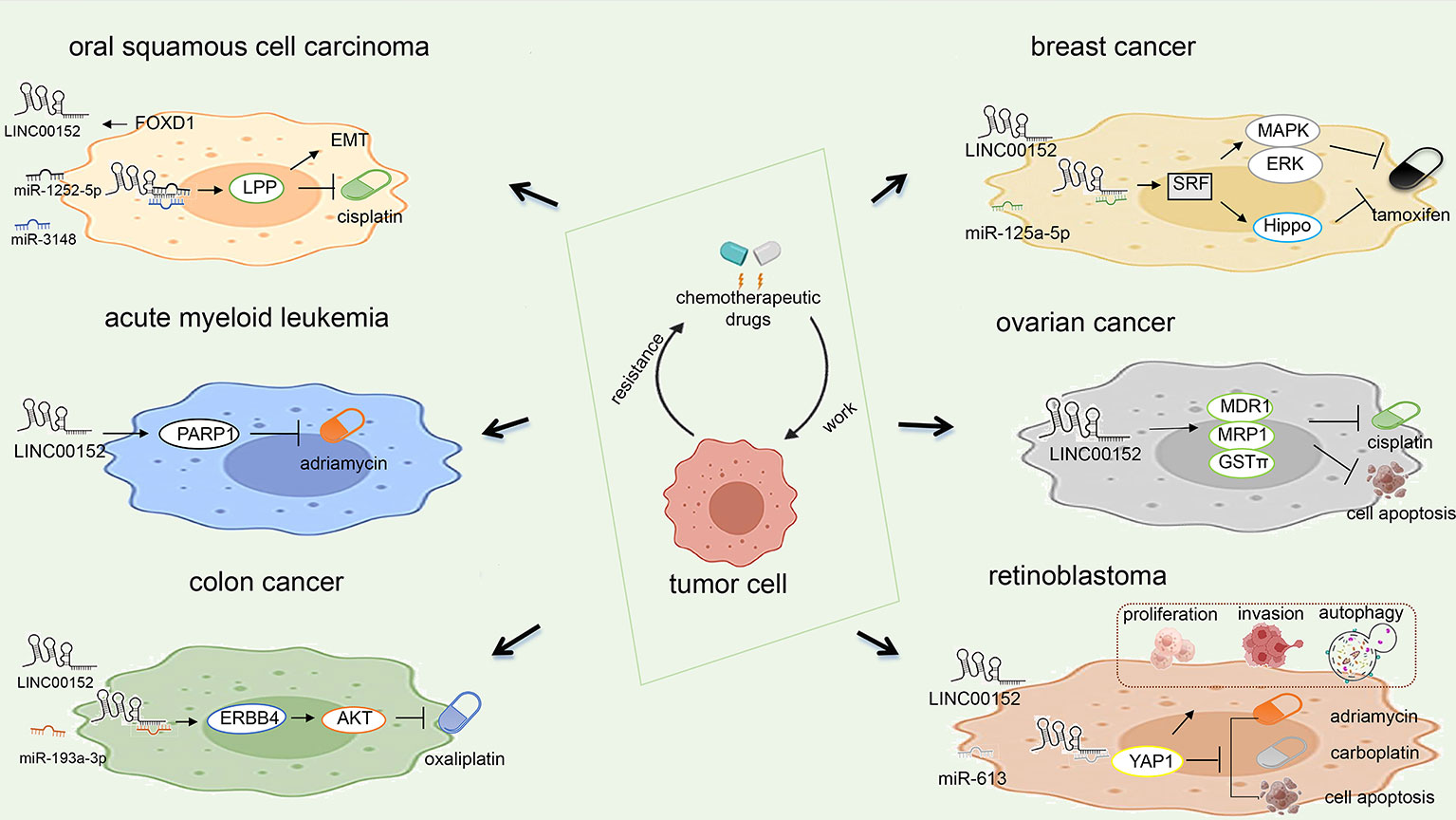
Oral squamous cell cancer (OSCC) is an aggressive form of head and neck squamous cell cancer (HNSCC) (78). OSCC accounts for 4% of all newly diagnosed cancers and ranks eighth among all estimated new cases among men worldwide (79).The five-year survival rate of patients with OSCC can reach 68%. Chen et al. found that the LINC00152/lipoma preferred partner (LPP) axis is the key to Forkhead boxD1 (FOXD1)-induced EMT and chemotherapy resistance in OSCC. FOXD1 may bind directly to the LINC00152 promoter and activate LINC00152 transcription. LINC00152 then specifically inhibits miR-1252-5p and miR-3148, thus upregulating the expression of LPP and promoting EMT and chemoresistance in OSCC (48).
Tongue squamous cell cancer
Squamous cell carcinoma of the tongue (TSCC) is the most common oral malignancy and has a poor prognosis. The five-year survival rate of patients with TSCC can reach 68.8%. Li et al. demonstrated that LINC00152 expression is significantly upregulated in TSCC tissue compared to that in normal tissue. Li et al. also revealed that increased LINC00152 expression could promote TSCC cell growth and cell cycle progression, migration and invasion, as well as inhibit apoptosis. Mechanistic analyses have indicated that LINC00152 acts as a sponge for miR-193b-3p to promote the phosphorylation and activation of the phosphoinositide 3-kinase (PI3K) signaling pathway and downstream protein kinase B (AKT), which contributes to the development of TSCC (58). LINC00152, therefore, promotes the oncogenic potential of TSCC and may be a potential therapeutic target.
Esophageal cancer
Esophageal cancer (EC) is one of the most common cancers of the digestive system (Figure 3), ranking seventh among the causes of cancer-related death (79). EC has a unique geographical distribution and is widespread in Eastern Asia and Southern Africa but rare in Central America (80). The five-year survival rate of patients with EC only reaches 20.6% (https://seer.cancer.gov/). EC is frequently identified at advanced cancer stages owing to the lack of early clinical signs and symptoms (81).
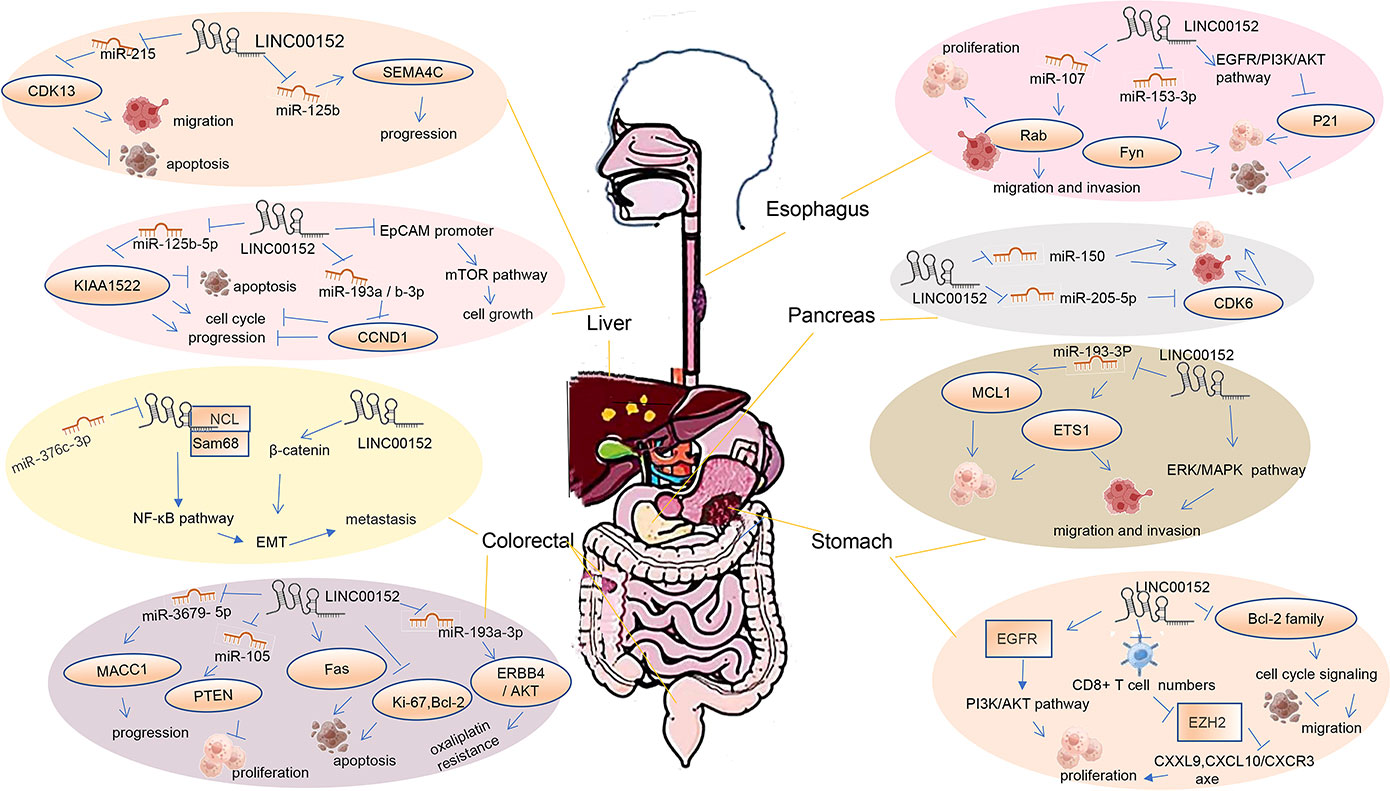
Yang et al. (64). studied LINC00152 overexpression in esophageal squamous cell carcinoma (ESCC) tissue. LINC00152 is closely related to TNM staging and lymphatic metastasis in ESCC. High expression of LINC00152 is related to poor prognosis in ESCC patients. Functionally, the overexpression of LINC00152 promotes the proliferation, invasion, and migration of ESCC cells in vitro and also regulates the interaction between mitotic arrest-deficient 2-like 1 (MAD2L1) and cyclin-dependent kinase 6 (CDK6) in vesicle transport pathway proteins, and syntaxin 3 (STX3) and STX12 soluble N-ethylmaleimide-sensitive factor-attachment protein (SNAP) receptor (SNARE) family members (64). Ding et al. (63) found that LINC00152 knockdown might inhibit proliferation and induce apoptosis of Eca-109 and KYSE-150 cells by inhibiting the anti-tumor epidermal growth factor receptor EGFR/PI3K/AKT pathway and enhancing P21 expression in EC (63). In addition, Zhou et al. (38) found that LINC00152 regulates Rab10 by sponging miR-107 to promote cell proliferation, migration, and invasion in ESCC (65). Liu et al. (66) found that LINC00152 promotes ESCC proliferation and inhibits apoptosis by downregulating miR-153-3p and promoting FYN expression (66). Therefore, LINC00152 is an optimal candidate as a therapeutic target for the treatment of EC.
Gastric cancer
Gastric cancer (GC) is the fifth most common cancer and the third most common cause of cancer-related deaths worldwide (82). Stomach cancer has a unique geographic distribution and is common in Eastern Asian countries such as Japan and Mongolia but uncommon in Southern Africa (80). Men are twice more likely than women to have GC. As a result, novel molecular targets for GC treatment are urgently required.
LINC00152 is highly expressed in GC tissue and cells. Huang et al. (73) showed that LINC 00152 overexpression promotes GC cell proliferation through the LINC00152/miR-193a-3p/myeloid leukemia 1 (MCL1) pathway (73). In vivo experiments have confirmed that knockdown of LINC00152 inhibits the growth of GC xenografts by upregulating mir-193b-3p and downregulating ETS1 (72). Further research revealed that LINC00152 might directly bind to Bcl-2 to activate cell cycle signaling, promote migration and invasion, and suppress apoptosis (71). LINC00152 activates PI3K/AKT signaling by directly binding to EGFR to increase GC cell proliferation (74). An enhanced extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signaling pathway significantly reverses the biological effects of GC caused by LINC00152 (70). LINC00152 can also promote the growth of tumor cells, both in vivo and in vitro, by binding enhancer of zeste homolog 2 (EZH2) and regulating the CXC motif chemokine ligand 9 (CXCL9) and CXCL10/CXCR3 axes in CD8T cells (69). LINC00152 may, therefore, be a potential prognostic biomarker and therapeutic target for GC in the future.
Colorectal cancer
Colorectal cancer (CRC) is the fourth most common cancer globally. Li et al. (45) monitored the overexpression of LINC00152 in colon cancer and found that it was significantly associated with poor prognosis. LINC00152 is positively linked to invasion depth, TNM stage, lymph node metastasis, and carbohydrate antigen 19-9 (CA19-9) levels according to clinicopathological examinations (41). LINC00152 was reported to regulate the biological characteristics of residual CRC cells after radiotherapy and chemotherapy, and promote the migration and increased invasion of residual cells (43). The heterotrimeric complex of LINC00152, NCL, and SAM68 activates the nuclear factor-kappa B (NF-κB) pathway and EMT and thus promotes CRC progression (44). High SAM68 expression was inversely related to the overall survival rate. Our current research suggests that SAM68 can specifically recognize the binding site in exon1 of LINC00152, and the formation of the NCL-LINC00152-SAM68 complex can activate the NF-κB signaling pathway, thus promoting the EMT and metastasis of CRC (44). In addition, LINC00152 can promote tumor progression and proliferation through the LINC00152/miR-3679-5p/MACC1 axi (45). LINC00152 was used as a competitive endogenous RNA to make oxaliplatin-resistant colon cancer-sponging miR-193a-3p via the LINC00152/miR-193a-3p/erbb4/Akt signaling axis (39). LINC00152 is negatively regulated by miR-376c-3p and may suppress the viability of colon cancer cells and contribute to apoptosis by regulating the expression of Ki-67, Bcl-2, and Fas (7). Hypomethylation of the LINC00152 promoter is closely related to its increased expression (46). In addition, Yue et al. (40) found a positive feedforward loop between LINC00152 and Wnt/β-catenin signaling that promotes colon cancer metastasis and EMT (40). Ou et al. (2019) identified that YAP1 target LINC00152, which promoted the biological characteristics of CRC cells by sponging miR‐185‐3p and miR‐632 for upregulating its target FSCN1, as an “YAP1/LINC00152/FSCN1” axis to promote the malignant proliferation, migration and metastasis in CRC (47).
However, Zhang et al. (42) reported that LINC00152 inhibits proliferation and metastasis of colon cancer cells through regulating miRNA-105/PTEN axis (42). This is oppositive with other studies in colon cancer. More experiments need to identify the function of LINC00152 in colon cancers.
Hepatocellular cancer
Hepatocellular carcinoma (HCC) is the most prevalent primary liver cancer and is the sixth most common neoplasm (80). LINC00152 expression was elevated in HCC tissue compared to that in normal and precancerous tissue. Hu et al. (25) reported that interfering with LINC00152 can inhibit proliferation, arrest the cell cycle, and promote apoptosis of hepatocellular cancer cells by regulating the miR-125b-5p/KIAA1522 axis (25). Wang et al. (26) demonstrated that silencing LINC00152 inhibited HCC development by modulating miR-215 to upregulate CDK13 (26). Deng et al. (24) found that HBx enhances the expression of LINC00152 and promotes the proliferation and invasion of HCC cells (24). LINC00152 acts as a ceRNA by sponging miR-193a/b-3p to regulate CCND1 expression to inhibit cell cycle progression (22). Deng et al. (23) found that autophagy-associated genes (ARG) are associated with the prognosis of HCC patients (23). LINC00152 promotes the proliferation and tumor growth of HCC cells by sponging miR-125b and upregulating the expression of semaphorin-4C (SEMA4C) (2). Similarly, Ji et al. (21) showed that LINC00152 could activate the mammalian target of rapamycin (mTOR) signaling pathway through a combination of EpCAM promoters in a cis-regulated manner, which promotes HCC cell proliferation in vitro and tumor growth in vivo (21). In hepatocellular cancer, a signature of immunoautophagy-related lncRNA (IAR-lncRNA) predicts survival (27). LINC00152 can be used as a biomarker for the differential diagnosis of liver cancer (20). Our growing understanding of LINC00152 suggests that targeting it may be a unique therapeutic strategy for hepatocellular carcinoma.
Gallbladder cancer
Gallbladder cancer (GBC) is the most common and aggressive malignancy of the biliary system (83). Some gallbladder cancers can be cured by radical cholecystectomy, whereas metastases to other organs require chemotherapy, and some patients also require postoperative adjuvant chemotherapy (84). LINC00152 is significantly upregulated in gallbladder cancer, and the upregulation of LINC00152 by SP1 promotes gallbladder cancer cell growth and tumor metastasis by targeting the PI3K/AKT signaling pathway (12). LINC00152 can inhibit the expression of HIF-1a by functioning as a miRNA sponge to abrogate the endogenous effect of miR-138, which promotes GBC metastasis and EMT (13). This suggests that LINC00152 could be used as a therapeutic target for GBC treatment.
Pancreatic cancer
Pancreatic cancer (PC) remains a life-threatening disease, with a five-year survival rate of only 10% and an overall poor prognosis. PC lacks tools for early diagnosis, and treatment choices are limited. LINC00152 is remarkably upregulated in PC tissue and cell lines. Yuan et al. (76) found that LINC00152 promotes the proliferation, migration, and invasion of pancreatic cancer cells by inhibiting the expression of miR-150 (76). Inhibition of CDK6 expression by LINC00152 sponges miR-205-5p and promotes the proliferation and migration of tumor cells (75). These results indicate that LINC00152 may be an effective diagnostic biomarker and therapeutic target for PC.
Nasopharyngeal cancer
The expression of LINC00152 in nasopharyngeal cancer tissue and cells is increased compared to in normal tissue and cells. LINC00152 competitively binds to miR-613 to induce ANXA2 upregulation, thus promoting the invasion and metastasis of nasopharyngeal cancer cells (11)
Laryngeal cancer
LINC00152 is significantly upregulated in laryngeal squamous cell carcinoma (LSCC) tissue and is correlated with poor prognosis (85). LINC00152 sponges miR-613, thus promoting the proliferation, migration, and invasion of laryngeal cancer cells, and inducing apoptosis (30). Our results highlight the role of LINC00152 as a therapeutic target for laryngeal cancer.
Lung cancer
Lung cancer is the second most common type of cancer worldwide. LINC00152 promotes the growth, invasion, and migration of lung adenocarcinoma cells and is associated with a poor prognosis (16). Chen et al. (19) found that the interaction of LINCOO152 with EZH2 inhibits interleukin-24 (IL24) transcription to promote lung adenocarcinoma proliferation, and ectopic expression of IL24 partially reversed the LAD cell growth promotion induced by LINC00152 overexpression (19)(Figure 4). LINC00152 enhances non-small cell lung cancer (NSCLC) cell proliferation, migration, and invasion, and decreases radiosensitivity in NSCLC cells in vitro by sponging miR-195 (18). LINC00152 knockdown inhibits the proliferation, invasion, and migration of lung cancer cells through the EGFR/PI3K/AKT pathway, and improves apoptosis and the G1 phase ratio (14).Silencing LINC00152 enhanced the radiosensitivity of NSCLC cells by upregulating miR-206 and inhibiting prothymosin alpha (PTMA). Gaining an understanding of the role of LINC00152 in the radiosensitivity of NSCLC identified new potential targets for the clinical treatment of NSCLC (17). Hu et al. (15) found that LINC00152 silencing restrained tumorigenesis in NSCLC by regulating the miR-16-5p/BCL2L2 axis (15). LINC00152 may be a valuable biomarker for diagnosing and monitoring NSCLC (15).
Ovarian cancer
The expression of LINC00152 in epithelial ovarian cancer tissue was significantly upregulated compared to in normal tissue (57). An in vitro study found that LINC00152 regulates cell proliferation and cell cycle in SKOV3 cells (57). LINC00152 may competitively inhibit miR125b upregulation of MCL1 expression, which modulates the mitochondrial apoptosis pathway during ovarian cancer progression (56). LINC00152 knockdown boosted epithelial ovarian cancer cell chemosensitivity to cisplatin by enhancing apoptosis and reducing the expression levels of MDR1, MRP1, and GST (77). Wang et al. (55) found that LINC00152 binds to Ser333/Ser343 of B-cell lymphoma 6 (BCL6) and stabilizes it against ubiquitination to promote ovarian tumor proliferation and invasion (55).
Breast cancer
Breast cancer (BC) is the most common malignancy worldwide, accounting for 15% of deaths among women (79). The expression of LINC00152 was significantly increased in triple-negative breast cancer tissue and cells. YY1 binds to the LINC00152 promoter to inhibit the transcription of LINC00152, which weakens the stability of PTEN and promotes the progression of triple-negative breast cancer (50). LINC00152 regulates genes involved in the rapamycin pathway of EGFR/mTOR and is required for cell proliferation, migration, and cytoskeleton organization (53). Positive feedback loops of LINC00152 and KLF5 promote breast cancer growth and proliferation (54). LINC00152 binding to miR-125a-5p promotes tamoxifen resistance by inhibiting serum response factor (SRF), thereby activating the MAPK/ERK and Hippo pathways. LINC00152 also promotes tamoxifen resistance in breast cancer cells by sponging miR-125a-5p (52). Wu et al. (51) found that LINC00152 knockdown inhibits breast cancer cell invasion, migration, tumor growth, and colony growth, and triggers apoptosis through a mechanism that activates breast cancer type 1 (BRCA1)/phosphatase and tensin homolog (PTEN) via DNA methyltransferase (DNMT) inactivation (51).
Bladder cancer
Tang et al. (2019) found that LINC00152 promotes bladder cancer cell viability, migration, invasion, and EMT by activating the Wnt/ß-Catenin signaling pathway (10). This is rare research about the role of LINC00152 in bladder cancer. This is an area for urgent attention, and more intensive research is warranted going forward.
Renal cell cancer
LINC00152 is involved in the progression of clear cell renal cell cancer (ccRCC) and is a potential prognostic biomarker and therapeutic target for ccRCC (60). By epigenetically suppressing P16 expression and interacting with miR-205, LINC00152 may contribute to renal cell cancer progression (59). Despite the potential role of LINC00152 in ccRCC, there has not been sufficient focus on this field in recent years. Additional research is required to determine if LINC00152 is a suitable diagnostic or prognostic biomarker for renal cell carcinoma.
Leukemia
Leukemia is the most common childhood cancer, accounting for 28% of cases. High LINC00152 expression is associated with poor survival in acute myeloid leukemia (AML) patients. LINC00152 promotes poly [ADP-ribose] polymerase 1 (PARP1) expression to induce chemoresistance and regulate the self-renewal of leukemic stem cell (LSC) self-renewal. The inhibition of LINC00152 increased the sensitivity of leukemic cells to doxorubicin. These results suggest that LINC00152 may serve as a potential prognostic marker in AML patients (8). Transcriptome analysis has identified LINC00152 as a biomarker for early relapse and mortality in acute lymphoblastic leukemia (9)
Thyroid cancer
Thyroid cancer (PTC) is the most common endocrine cancer. TRIM29 reduces miR-873-5p expression by upregulating LINC00152 to upregulate FN1, thereby promoting PTC migration and invasion (32). LINC00152 acts as a ceRNA miR-497 sponge, downregulating its downstream target brain-derived neurotrophic factor (BDNF) to promote cell proliferation, colony formation, migration, and invasion (31).
Glioma
LINC00152 is upregulated in glioma tissue and cells and negatively correlates with UPF1 levels. LINC00152 promotes the proliferation and invasion of glioma cells by inducing BMI1 expression by sponging miR-16 (86). Peng et al. (87) found that LINC00152 promotes tumor proliferation and invasion through the LINC00152/miR-107/RAB10 axis (87). LINC00152 functions as an oncogene in glioblastoma cells, promoting cell proliferation and invasion, in part by targeting miR-107 expression (88). Zou et al. (38) found that UPF1 downregulates LINC00152 to suppress the growth and invasion of glioma cells (38). Functionally, LINC00152 promotes the proliferation, migration, invasion, and induction of apoptosis of glioma cells, and reduces their sensitivity to in vitro chemotherapy (35). Mechanistically, LINC00152 binds to miR-103a-3p to suppress FEZ family zinc finger 1 (FEZF1), thereby promoting cell division cycle 25 A (CDC25A) expression to promote the PI3K/AKT pathway to exert these functions in malignant glioma (37). Through the PI3K/AKT pathway, the LINC00152/miR-613/CD164 axis affects cell proliferation, apoptosis, migration, and invasion in glioma (34). LINC00152 promotes invasion through a 3′-hairpin structure and is related to glioblastoma prognosis (33). Blocking LINC00152 reduces glioblastoma malignancy by affecting the mesenchymal phenotype via the miR-612/AKT2/NF-B pathway (89). Consequently, blocking LINC00152 decreases glioblastoma malignancy (33). However, LINC00152 has opposing effects in different types of glioblastoma cells (36). LINC00152 knockdown stimulates migration and invasion of A172 GBM cells, whereas knockdown of LINC00152 in other glioblastoma cell lines (U87-MG and LN299) leads to tumor suppression, as it serves as an oncogene (36). In summary, LINC00152 may serve as a prognostic marker and new therapeutic target for glioma.
Head and neck squamous cell cancer
LINC00152 is involved in multi-step pathological processes in head and neck squamous cell cancer (HNSCC), such as ribosomal biogenesis and maintenance of genomic stability (68). LINC000152 is positively correlated with lymph node metastasis and negatively correlated with overall survival (OS) and disease-free survival (DFS) in HNSCC patients (68). Upregulated LINC00052 expression in head and neck cancers is associated with poor prognosis. LINC00052 acts as a ceRNA for miR-608 to regulate the expression of epidermal growth factor receptor (EGFR), thus promoting the proliferation, migration, and invasion of HNSCC (67).
Osteosarcoma
LINC00152 acts as a ceRNA binding miR-193b-3p, leading to increased cell proliferation, G0/G1 cell cycle arrest, and reduced apoptosis, thus promoting osteosarcoma development (29). Zheng et al. (28) found that transcription factor 3 (TCF3) activates LINC00152 to act as a ceRNA to sponge miR-1182 and upregulate the expression of CDK14, thus promoting the proliferation, migration, and invasion of osteosarcoma cells (28).
Multiple myeloma
IL-6 mediates STAT3 activation, and positive feedback induces LINC00152 expression, which is a critical factor for the survival of INA-6 multiple myeloma cells (4). LINC00152 is overexpressed in osteosarcoma cells (4). At present, there are rare studies on the role and mechanism of LINC00152 in multiple myeloma, and further studies are needed.
Retinoblastoma
LINC00152 is upregulated in retinoblastoma tumor tissue. 61 found that LINC00152, which is activated by Sp1, can sponge miR-30d, thus significantly increasing the expression of SOX9 and zinc finger E-box-binding homeobox 2 (ZEB2), inducing EMT, and promoting the invasion and metastasis of retinoblastoma cells (61). LINC00152 regulates the expression of YAP1 in retinoblastoma cells by sponging miR-613, thus promoting proliferation, invasion, apoptosis, autophagy, and chemical resistance of retinoblastoma cells (62).
We summarized the role and mechanisms of LINC00152 in various cancer types. It indicated the potential cancer diagnosis and prognosis value of LINC00152. More importantly, LINC00152 also play an important role in radiotherapy and chemotherapy resistance.
The role and mechanism of LINC00152 in radiotherapy and chemotherapy resistance
LINC00152 plays a vital role in the resistance to radiotherapy and chemotherapy. We summarized the mechanisms by which LINC00152 confers resistance to chemotherapy in Figure 5. LINC00152 is highly expressed in NSCLC, and silencing of LINC00152 enhances the radiosensitivity of NSCLC cells by upregulating miR-206 and inhibiting prothymosin α(PTMA). LINC00152 knockdown and control cells were administered subcutaneously into mice as part (17). The tumor weight and size in the knockdown group were significantly reduced after radiation, demonstrating that LINC00152 knockdown improved the radiosensitivity of xenograft tumors in mice in an animal study (17). Silencing LINC00152 may therefore represent a strategy for the treatment of NSCLC. However, this study was conducted in the context of radiation therapy in NSCLC and did not explore the role of LINC00152 in other cancer cells, which should be explored further in the future.
LINC00152 enhances NSCLC proliferation, migration, and invasion, and alleviates radiosensitivity in vitro by sponging miR-195 (18). Further research showed LINC00152 inhibited radiosensitivity of NSCLC cells in vitro and in vivo. The increased radiosensitivity achieved by knocking down LINC00152 sponging of miR-195 can improve the prognosis of patients with NSCLC. LINC00152 may serve as a prognostic marker and promising therapeutic target for patients with NSCLC. However, the role of LINC00152 in chemotherapy or molecular-targeted therapy has not been reported for lung cancer. Therefore, the regulatory role of LINC00152 in drug resistance in lung cancer remains unknown.
Chen et al. (43) found that LINC00152 is involved in regulating the invasion and metastasis of residual CRC cells after chemoradiotherapy. Author established residual CRC cells models, which was intended to mimic the clinical treatment model as far as possible. Transwell experiments prove that the migration and invasion of the residual CRC cells were significant increased compared with the original cells. LINC00152 is a potential biomarker of altered biological characteristics caused by chemoradiotherapy in CRC cells (43). There is a solid theoretical basis for further research to improve the CRC therapy and improve the prognosis of patients with CRC.
Cui et al. (8) demonstrated that LINC00152 promotes PARP1 expression, which induces chemoresistance in acute myeloid leukemia and regulates the self-renewal of LSCs (8). In addition, knockdown of LINC00152 can inhibit PARP1 expression to improve the sensitivity of leukemia cells to chemotherapy, thus improving the prognosis of leukemia patients. These findings indicate that the LINC00152/PARP1 pathway could be used as a new therapeutic target for AML.
LINC00152 may serve as a potential prognostic biomarker for high-grade glioma (HGG) patients and is, therefore, a potential therapeutic target for gliomas. Further studies are needed to identify the mechanisms by which LINC00152 regulates glioma and verify its clinical application in patients with glioma. In addition, further research suggests that patients with low expression of LINC00152 had longer OS than that of the other groups. Moreover, assay showed knockdown of LINC00152 increased the sensitivity of chemotherapy in TMZ‐resistant LN229 and SNB19 cells. Wang et al. (44) reported that knockdown of LINC00152 suppresses the proliferation, invasion, and migration of glioma cells in vitro and increases their sensitivity to chemotherapy (35).
Yue et al. found that colon cancer cells display different response to oxaliplatin treatment and LINC00152 antagonize oxaliplatin-induced apoptosis LINC00152 regulates oxaliplatin resistance by sponging miR-193a-3p and then regulates ERBB4 in vitro. Besides, LINC00152 mediates oxaliplatin resistance through sponging miR-193a-3p in xenograft model. Further research found that AKT activation mediated by ERBB4 contributes to LINC00152-conferred oxaliplatin resistance. Collectively, LINC00152 promotes oxaliplatin resistance by sponging miR-193A-3P to participate in the LINC00152/miR-193A-3P/ERBB4/AKT signal axis as a competitive endogenous RNA (39).
Chen et al. (11) found that FOXD1 upregulates LINC00152 as a ceRNA to inhibit miR-1252-5p and miR-3148, thereby upregulating LPP expression to promote EMT and chemotherapy resistance in OSCC (90). In this previous study, Further studies reduced the role of EMT in OSCC by silencing FOXD1, thus increasing chemosensitivity and promoting apoptosis. This finding indicates that overexpression of FOXD1 promotes cisplatin resistance in vitro and in vivo by regulating the EMT of OSCC cells. Whereas silencing FOXD1 inhibits cisplatin resistance, suggesting that FOXD1 may be a potential prognostic marker and anti-drug resistance therapeutic target. New evidence is expected for the role of FOXD1 and the chemical resistance of OSCC involved by FOXD1. However, the detailed mechanisms of FOXD1 upregulation in OSCC remain unexplored and will be the focus of our future research.
Xu et al. found that LINC00152 induces chemoresistance in pan-cancer, resulting in a poor prognosis for pan-cancer patients (49). The mechanisms underlying LINC00152’s upregulation in pancreatic cancer is unknown. This research broadened the carcinogenic role of lncRNA in pancreatic cancer and revealed that LINC00152 might be a potential therapeutic target and contribute to the comprehensive management of pancreatic cancer.
The expression level of LINC00152 in epithelial ovarian cancer cells is upregulated. The knock-down of LINC00152 increases the chemosensitivity of epithelial ovarian cancer cells to cisplatin by increasing apoptosis and decreasing the expression levels of MDR1, MRP1, and GSTπ (77). This study only investigated the effect of LINC00152 silencing on cisplatin resistance in COC1 and COC1/DDP cells but did not explore the effect of LINC00152 overexpression on drug resistance. This needs to be further verified on other ovarian cancer cell lines and animal models.
Liu et al. (66) found that LINC00152 improves serum response factor (SRF) expression by sponging miR-125a-5p to activate the MAPK/ERK and Hippo pathways to promote tamoxifen resistance in breast cancer cells. In addition, the prognosis of patients with breast cancer can be improved by promoting tamoxifen sensitivity in breast cancer cells by knocking down LINC00152 to inhibit SRF (52).
LINC00152 regulates the expression of YAP1 in retinoblastoma cells by sponging miR-613, and knockdown of YAP1 eliminates the miR-613-mediated effects on retinoblastoma cell proliferation, invasion, apoptosis, autophagy, and chemical resistance (62). In addition, Wang et al. (55) also found that knockdown of LINC00152 increased the chemosensitivity of retinoblastoma to carboplatin and doxorubicin by regulating miR-613.
In total, LINC00152 plays an important role in chemotherapy and radiotherapy resistance through regulating microRNA, protein, or classical signaling pathway. LINC00152 may be a potential sensitizer for radiotherapy and chemotherapy in the future.
The role and mechanism of LINC00152 in cancer recurrence
LINC00152 as a tumor marker to predict tumor recurrence has been reported in various cancers. A meta-analysis showed that LINC00152 overexpression is significantly related to poor overall survival and poor disease-free survival (91). Meanwhile, LINC00152 is a biomarker of early relapse and mortality in acute lymphoblastic leukemia according to transcriptome analysis (9). In retinoblastoma, LINC00152 is activated by SP1 to inhibit miR-30d and thus regulate the expression of SOX9 and ZEB2 to promote tumor recurrence (61). The Kaplan-Meier analysis suggested that high LINC00152 expression leads to significantly lower DFS rates in lung adenocarcinoma. CCK8 assay and the colony-forming assay showed LINC00152 stimulated tumor cell proliferation in lung adenocarcinoma (92). Immunochemistry staining found that LINC00152 was related to nuclear accumulation of β-catenin in colon cancer tissues and have a prognostic value (40). Li et al. (14) found that LINC00152 binds to KLF5 to induce breast cancer cell proliferation and predicts poor prognosis. Yu et al. (5) found that LINC00152 expression was significantly upregulated in tongue squamous cell carcinoma and high LINC00152 expression was closely associated with progression and poor prognosis (93).More studies about the role and mechanism of linc00152 in cancer recurrence are needed.
The role and mechanism of LINC00152 in immunotherapy response
LINC00152 is also reported to involved in immunotherapy response. Ou et al. (69) found that LINC00152 mediates CD8+ T cell infiltration in gastric cancer by binding to EZH2 and regulating CXCL9,10/CXCL9 axis. The inhibition of LINC00152 may inhibit the progression of gastric cancer in vivo by promoting CD8+ T cell infiltration immune response (69). TCGA database indicated that LINC00152 and HMGA1 regulate each other. Chen et al. (92) found that LINC00152 acts as a competitive endogenous RNA to regulate the expression of HMGA1. LINC00152 and HMGA1 play an important role in the cell cycle and proliferation of GC cells, through reducing the infiltration of immune cells and the 28 types of tumor‐infiltrating lymphocytes (TILs) found in human cancers (94). More studies about the role and mechanism of LINC00152 in immunotherapy response are needed.
Discussion
Dysregulation of lncRNAs is associated with various malignant behaviors of cancer cells, such as cancer progression and metastasis. LINC00152 is significantly upregulated in most cancer tissue and cell lines, and is associated with poor prognosis. Clinicopathological analysis has shown that LINC00152 is positively associated with tumor infiltration depth, TNM stage, lymph node metastasis, and CA19-9 levels (41). LINC00152 research has recently flourished, confirming their role in regulating diverse functions such as proliferation, apoptosis, EMT, migration, invasion, cell cycle, and chemotherapy and radiotherapy resistance in various human cancers.
LINC00152 is overexpressed and plays an oncogenic role in many types of tumors, including lung, hepatocellular, ovarian, and esophageal cancer. LINC00152 can contribute to tumor progression in certain cancer types. Chen et al. (19) found that the interaction between LINCOO152 and EZH2 inhibits IL24 transcription to promote lung adenocarcinoma proliferation. However, downregulation of LINC00152 in serum-derived exosomes has been observed in CRC patients (95).
The mechanisms by which LINC00152 promotes tumor development are highly complex, including serving as a ceRNA sponge for miRNA, interacting with proteins, activating signaling pathways, and regulating epigenetic regulation. LINC00152 is involved in various signaling pathways leading to cancer progression, including the ERK/MAPK, β-catenin, mTOR, and PI3K signaling pathways. Several experiments have confirmed the role of lncRNAs in epigenetics, transcription, and gene expression, and lncRNAs, circRNAs, and miRNAs can act as ceRNAs to interact with mRNA and regulate cell function (96) (Figure 6). LINC00152 can act as a ceRNA to regulate HMGA1 expression in GC cells (94). The molecular mechanism by which LINC00152 participates in multiple cancers has been preliminarily explored. However, further in-depth analysis is warranted, particularly in cancers that are poorly understood or have limited treatment options.
LINC00152 play an important role in radiotherapy and chemotherapy. LINC00152 was reported to induce chemoresistance in pan-cancer, resulting in poor patient prognosis (49). Wang et al. (44) reported that knockdown of LINC00152 increases the sensitivity to chemotherapy in glioma (35). In addition, knockdown of LINC00152 increased the chemosensitivity of carboplatin and doxorubicin in retinoblastoma (62). We summarized the role and mechanism of LINC00152 in chemotherapy in Figure 5. There are rare studies about the role and mechanism of LINC00152 in radiotherapy. Only 2 papers reported that LINC00152 reduced the radiosensitivity by sponging miR-195 or miR-206 in NSCLC (17, 18). It remains unknow about the role of LINC00152 in radiotherapy in other cancers. LINC00152 could be used as a potential chemotherapy and radiotherapy sensitization targets and may contribute to the prognosis of cancer patients.
This review provides a comprehensive description of the role and mechanisms of LINC00152 in various cancer types, with an emphasis on chemotherapy and radiotherapy resistance. More studies are needed on LINC00152 to elucidate the mechanisms of chemoradiotherapy resistance and improve the prognosis of patients with cancer.
LINC00152 could be a potential biomarker for cancer diagnosis and prognosis, and may be a promising therapeutic target due to its important role in cancer. The source of LINC00152, the mechanism of LINC00152, and its clinical application require further investigation. Only once these mechanisms are fully understood can LINC00152 be used in the clinical setting for treating cancer.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This study was supported in part by grants from the National Natural Science Foundation of China (82003236, to HZ); Zhejiang Provincial Nature Science Foundation of China (Grant number: LGF20H160030 to LG); Zhejiang Health Science and Technology Project (Grant number: 2019KY280, to LG; 2022KY596, to HZ).
Acknowledgments
The authors thank Figdraw because some image elements are utilized from Figdraw (www.figdraw.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xing C, Sun S-G, Yue Z-Q, Bai F. Role of lncrna Lucat1 in cancer. BioMed Pharmacother (2021) 134:111158. doi: 10.1016/j.biopha.2020.111158
2. Tian Q, Yan X, Yang L, Liu Z, Yuan Z, Zhang Y. Lncrna cytor promotes cell proliferation and tumor growth via mir-125b/Sema4c axis in hepatocellular carcinoma. Oncol Lett (2021) 22(5):796. doi: 10.3892/ol.2021.13057
3. Feng C, Shen J-M, Lv P-P, Jin M, Wang L-Q, Rao J-P, et al. Construction of implantation failure related lncrna-mrna network and identification of lncrna biomarkers for predicting endometrial receptivity. Int J Biol Sci (2018) 14(10):1361–77. doi: 10.7150/ijbs.25081
4. Binder S, Zipfel I, Friedrich M, Riedel D, Ende S, Kämpf C, et al. Master and servant: Linc00152 - a Stat3-induced long noncoding rna regulates Stat3 in a positive feedback in human multiple myeloma. BMC Med Genomics (2020) 13(1):22. doi: 10.1186/s12920-020-0692-3
5. Yu Y, Yang J, Li Q, Xu B, Lian Y, Miao L. Linc00152: A pivotal oncogenic long non-coding rna in human cancers. Cell Prolif (2017) 50(4):e12349. doi: 10.1111/cpr.12349
6. Xu J, Guo J, Jiang Y, Liu Y, Liao K, Fu Z, et al. Improved characterization of the relationship between long intergenic non-coding rna Linc00152 and the occurrence and development of malignancies. Cancer Med (2019) 8(10):4722–31. doi: 10.1002/cam4.2245
7. Zhang YH, Fu J, Zhang ZJ, Ge CC, Yi Y. Lncrna-Linc00152 down-regulated by mir-376c-3p restricts viability and promotes apoptosis of colorectal cancer cells. Am J Transl Res (2016) 8(12):5286–97.
8. Cui C, Wang Y, Gong W, He H, Zhang H, Shi W, et al. Long non-coding rna Linc00152 regulates self-renewal of leukemia stem cells and induces chemo-resistance in acute myeloid leukemia. Front Oncol (2021) 11:694021. doi: 10.3389/fonc.2021.694021
9. Bárcenas-López DA, Núñez-Enríquez JC, Hidalgo-Miranda A, Beltrán-Anaya FO, May-Hau DI, Jiménez-Hernández E, et al. Transcriptome analysis identifies Linc00152 as a biomarker of early relapse and mortality in acute lymphoblastic leukemia. Genes (Basel) (2020) 11(3):302. doi: 10.3390/genes11030302
10. Xian-Li T, Hong L, Hong Z, Yuan L, Jun-Yong D, Peng X, et al. Higher expression of Linc00152 promotes bladder cancer proliferation and metastasis by activating the Wnt/β-catenin signaling pathway. Med Sci Monit (2019) 25:3221–30. doi: 10.12659/MSM.913944
11. Chen W, Du M, Hu X, Ma H, Zhang E, Wang T, et al. Long noncoding rna cytoskeleton regulator rna promotes cell invasion and metastasis by titrating mir-613 to regulate Anxa2 in nasopharyngeal carcinoma. Cancer Med (2020) 9(3):1209–19. doi: 10.1002/cam4.2778
12. Cai Q, Wang ZQ, Wang SH, Li C, Zhu ZG, Quan ZW, et al. Upregulation of long non-coding rna Linc00152 by Sp1 contributes to gallbladder cancer cell growth and tumor metastasis via Pi3k/Akt pathway. Am J Transl Res (2016) 8(10):4068–81.
13. Cai Q, Wang Z, Wang S, Weng M, Zhou D, Li C, et al. Long non-coding rna Linc00152 promotes gallbladder cancer metastasis and epithelial-mesenchymal transition by regulating hif-1α via mir-138. Open Biol (2017) 7(1):160274. doi: 10.1098/rsob.160247
14. Li N, Feng XB, Tan Q, Luo P, Jing W, Zhu M, et al. Identification of circulating long noncoding rna Linc00152 as a novel biomarker for diagnosis and monitoring of non-Small-Cell lung cancer. Dis Markers (2017) 2017:7439698. doi: 10.1155/2017/7439698
15. Hu H, Chen C, Chen F, Sun N. Linc00152 knockdown suppresses tumorigenesis in non-small cell lung cancer via sponging mir-16-5p. J Thorac Dis (2022) 14(3):614–24. doi: 10.21037/jtd-22-59
16. Zhang PP, Wang YQ, Weng WW, Nie W, Wu Y, Deng Y, et al. Linc00152 promotes cancer cell proliferation and invasion and predicts poor prognosis in lung adenocarcinoma. J Cancer (2017) 8(11):2042–50. doi: 10.7150/jca.18852
17. Jiang G, Yu H, Li Z, Zhang F. Lncrna cytoskeleton regulator reduces Non−Small cell lung cancer radiosensitivity by downregulating Mirna−206 and activating prothymosin α. Int J Oncol (2021) 59(5):88. doi: 10.3892/ijo.2021.5268
18. Zhang J, Li W. Long noncoding rna cytor sponges mir-195 to modulate proliferation, migration, invasion and radiosensitivity in nonsmall cell lung cancer cells. Biosci Rep (2018) 38(6):BSR20181599. doi: 10.1042/BSR20181599
19. Chen QN, Chen X, Chen ZY, Nie FQ, Wei CC, Ma HW, et al. Long intergenic non-coding rna 00152 promotes lung adenocarcinoma proliferation via interacting with Ezh2 and repressing Il24 expression. Mol Cancer (2017) 16(1)17. doi: 10.1186/s12943-017-0581-3
20. Burenina OY, Lazarevich NL, Kustova IF, Shavochkina DA, Moroz EA, Kudashkin NE, et al. Panel of potential lncrna biomarkers can distinguish various types of liver malignant and benign tumors. J Cancer Res Clin Oncol (2021) 147(1):49–59. doi: 10.1007/s00432-020-03378-5
21. Ji J, Tang J, Deng L, Xie Y, Jiang R, Li G, et al. Linc00152 promotes proliferation in hepatocellular carcinoma by targeting epcam via the mtor signaling pathway. Oncotarget (2015) 6(40):42813–24. doi: 10.18632/oncotarget.5970
22. Ma P, Wang H, Sun J, Liu H, Zheng C, Zhou X, et al. Linc00152 promotes cell cycle progression in hepatocellular carcinoma via mir-193a/B-3p/Ccnd1 axis. Cell Cycle (2018) 17(8):974–84. doi: 10.1080/15384101.2018.1464834
23. Deng X, Bi Q, Chen S, Chen X, Li S, Zhong Z, et al. Identification of a five-Autophagy-Related-Lncrna signature as a novel prognostic biomarker for hepatocellular carcinoma. Front Mol Biosci (2020) 7:611626. doi: 10.3389/fmolb.2020.611626
24. Deng X, Zhao XF, Liang XQ, Chen R, Pan YF, Liang J. Linc00152 promotes cancer progression in hepatitis b virus-associated hepatocellular carcinoma. BioMed Pharmacother (2017) 90:100–8. doi: 10.1016/j.biopha.2017.03.031
25. Hu B, Yang X-B, Yang X, Sang X-T. Lncrna cytor affects the proliferation, cell cycle and apoptosis of hepatocellular carcinoma cells by regulating the mir-125b-5p/Kiaa1522 axis. Aging (2020) 13(2):2626–39. doi: 10.18632/aging.202306
26. Wang J, Zhang Y, Lu L, Lu Y, Tang Q, Pu J. Insight into the molecular mechanism of Linc00152/Mir-215/Cdk13 axis in hepatocellular carcinoma progression. J Cell Biochem (2019) 120(11):18816–25. doi: 10.1002/jcb.29197
27. Wang Y, Ge F, Sharma A, Rudan O, Setiawan MF, Gonzalez-Carmona MA, et al. Immunoautophagy-related long noncoding rna (Iar-lncrna) signature predicts survival in hepatocellular carcinoma. Biology (2021) 10(12):1301. doi: 10.3390/biology10121301
28. Zheng L, Hu N, Zhou X. Tcf3-activated Linc00152 exerts oncogenic role in osteosarcoma through regulating mir-1182/Cdk14 axis. Pathol Res Pract (2019) 215(2):373–80. doi: 10.1016/j.prp.2018.12.031
29. Liu P, He W, Lu Y, Wang Y. Long non-coding rna Linc00152 promotes tumorigenesis via sponging mir-193b-3p in osteosarcoma. Oncol Lett (2019) 18(4):3630–6. doi: 10.3892/ol.2019.10700
30. Zheng X, Dong S, Sun L, Xu J, Liu J, Hao R. Lncrna Linc00152 promotes laryngeal cancer progression by sponging mir-613. Open Med (Wars) (2020) 15:240–8. doi: 10.1515/med-2020-0035
31. Sun Z, Guo X, Zang M, Wang P, Xue S, Chen G. Long non-coding rna Linc00152 promotes cell growth and invasion of papillary thyroid carcinoma by regulating the mir-497/Bdnf axis. J Cell Physiol (2019) 234(2):1336–45. doi: 10.1002/jcp.26928
32. Wu T, Zhang D-L, Wang J-M, Jiang J-Y, Du X, Zeng X-Y, et al. Trim29 inhibits mir-873-5p biogenesis via cytor to upregulate fibronectin 1 and promotes invasion of papillary thyroid cancer cells. Cell Death Dis (2020) 11(9):813. doi: 10.1038/s41419-020-03018-3
33. Reon BJ, Takao Real Karia B, Kiran M, Dutta A. Promotes invasion through a 3'-hairpin structure and associates with prognosis in glioblastoma. Mol Cancer Res (2018) 16(10):1470–82. doi: 10.1158/1541-7786.MCR-18-0322
34. Zhang L, Wang Y, Su H. Long non-coding rna Linc00152/Mir-613/Cd164 axis regulates cell proliferation, apoptosis, migration and invasion in glioma via Pi3k/Akt pathway. Neoplasma (2020) 67(4):762–72. doi: 10.4149/neo_2020_190706N598
35. Wang W, Wu F, Zhao Z, Wang K-Y, Huang R-Y, Wang H-Y, et al. Long noncoding rna Linc00152 is a potential prognostic biomarker in patients with high-grade glioma. CNS Neurosci Ther (2018) 24(10):957–66. doi: 10.1111/cns.12850
36. Binder S, Zipfel I, Müller C, Wiedemann K, Schimmelpfennig C, Pfeifer G, et al. The noncoding rna Linc00152 conveys contradicting effects in different glioblastoma cells. Sci Rep (2021) 11(1):18499. doi: 10.1038/s41598-021-97533-8
37. Yu M, Xue Y, Zheng J, Liu X, Yu H, Liu L, et al. Linc00152 promotes malignant progression of glioma stem cells by regulating mir-103a-3p/Fezf1/Cdc25a pathway. Mol Cancer (2017) 16(1):110. doi: 10.1186/s12943-017-0677-9
38. Zou SF, Yang XY, Li JB, Ding H, Bao YY, Xu J. Upf1 alleviates the progression of glioma via targeting lncrna cytor. Eur Rev Med Pharmacol Sci (2019) 23(22):10005–12. doi: 10.26355/eurrev_201911_19567
39. Yue B, Cai D, Liu C, Fang C, Yan D. Linc00152 functions as a competing endogenous rna to confer oxaliplatin resistance and holds prognostic values in colon cancer. Mol Ther (2016) 24(12):2064–77. doi: 10.1038/mt.2016.180
40. Yue B, Liu C, Sun H, Liu M, Song C, Cui R, et al. A positive feed-forward loop between lncrna-cytor and Wnt/β-catenin signaling promotes metastasis of colon cancer. Mol Ther (2018) 26(5):1287–98. doi: 10.1016/j.ymthe.2018.02.024
41. Ye S, Lu Y, Ru Y, Wu X, Zhao M, Chen J, et al. Lncrnas Gacat3 and Linc00152 regulate each other through mir-103 and are associated with clinicopathological characteristics in colorectal cancer. J Clin Lab Anal (2020) 34(9):e23378. doi: 10.1002/jcla.23378
42. Zhang Y, Jin W, Ma D, Cao J, Fu T, Zhang Z, et al. Long non-coding rna cytor regulates proliferation and metastasis of colon cancer cells through regulating mirna-105/Pten axis. Int J Clin Exp Pathol (2021) 14(4):434–43.
43. Chen Z, Cai X, Chang L, Xia Y, Wang L, Hou Y, et al. Linc00152 is a potential biomarker involved in the modulation of biological characteristics of residual colorectal cancer cells following chemoradiotherapy. Oncol Lett (2018) 15(4):4177–84. doi: 10.3892/ol.2018.7833
44. Wang X, Yu H, Sun W, Kong J, Zhang L, Tang J, et al. The long non-coding rna cytor drives colorectal cancer progression by interacting with ncl and Sam68. Mol Cancer (2018) 17(1):110. doi: 10.1186/s12943-018-0860-7
45. Li M, Wang Q, Xue F, Wu Ya. Lncrna- works as an oncogene through the /Mir-3679-5p/ axis in colorectal cancer. DNA Cell Biol (2019) 38(6):572–82. doi: 10.1089/dna.2018.4548
46. Galamb O, Kalmár A, Sebestyén A, Dankó T, Kriston C, Fűri I, et al. Promoter hypomethylation and increased expression of the long non-coding rna Linc00152 support colorectal carcinogenesis. Pathol Oncol Res POR (2020) 26(4):2209–23. doi: 10.1007/s12253-020-00800-8
47. Ou C, Sun Z, He X, Li X, Fan S, Zheng X, et al. Targeting Yap1/Linc00152/Fscn1 signaling axis prevents the progression of colorectal cancer. Adv Sci (Weinh) (2020) 7(3):1901380. doi: 10.1002/advs.201901380
48. Chen S, Yang M, Wang C, Ouyang Y, Chen X, Bai J, et al. Forkhead box D1 promotes emt and chemoresistance by upregulating lncrna cytor in oral squamous cell carcinoma. Cancer Lett (2021) 503:43–53. doi: 10.1016/j.canlet.2020.11.046
49. Xu S, Wan L, Yin H, Xu H, Zheng W, Shen M, et al. Long noncoding rna Linc00152 functions as a tumor propellant in pan-cancer. Cell Physiol Biochem (2017) 44(6):2476–90. doi: 10.1159/000486170
50. Shen X, Zhong J, Yu P, Zhao Q, Huang T. Yy1-regulated Linc00152 promotes triple negative breast cancer progression by affecting on stability of pten protein. Biochem Biophys Res Commun (2019) 509(2):448–54. doi: 10.1016/j.bbrc.2018.12.074
51. Wu J, Shuang Z, Zhao J, Tang H, Liu P, Zhang L, et al. Linc00152 promotes tumorigenesis by regulating dnmts in triple-negative breast cancer. BioMed Pharmacother (2018) 97:1275–81. doi: 10.1016/j.biopha.2017.11.055
52. Liu Y, Li M, Yu H, Piao H. Lncrna cytor promotes tamoxifen resistance in breast cancer cells via sponging Mir−125a−5p. Int J Mol Med (2020) 45(2):497–509. doi: 10.3892/ijmm.2019.4428
53. Van Grembergen O, Bizet M, De Bony EJ, Calonne E, Putmans P, Brohée S, et al. Portraying breast cancers with long noncoding rnas. Sci Adv (2016) 2(9):e1600220. doi: 10.1126/sciadv.1600220
54. Li Q, Wang X, Zhou L, Jiang M, Zhong G, Xu S, et al. A positive feedback loop of long noncoding rna Linc00152 and Klf5 facilitates breast cancer growth. Front Oncol (2021) 11:619915. doi: 10.3389/fonc.2021.619915
55. Wang S, Weng W, Chen T, Xu M, Wei P, Li J, et al. Linc00152 promotes tumor progression and predicts poor prognosis by stabilizing Bcl6 from degradation in the epithelial ovarian cancer. Front Oncol (2020) 10:555132. doi: 10.3389/fonc.2020.555132
56. Chen P, Fang X, Xia B, Zhao Y, Li Q, Wu X. Long noncoding rna Linc00152 promotes cell proliferation through competitively binding endogenous mir-125b with mcl-1 by regulating mitochondrial apoptosis pathways in ovarian cancer. Cancer Med (2018) 7(9):4530–41. doi: 10.1002/cam4.1547
57. Ni H, Niu LL, Tian SC, Jing LK, Zhang LT, Lin QQ, et al. Long non-coding rna Linc00152 is up-regulated in ovarian cancer tissues and regulates proliferation and cell cycle of Skov3 cells. Eur Rev Med Pharmacol Sci (2019) 23(22):9803–13. doi: 10.26355/eurrev_201911_19543
58. Li X, Rui B, Cao Y, Gong X, Li H. Long non-coding rna Linc00152 acts as a sponge of mirna-193b-3p to promote tongue squamous cell carcinoma progression. Oncol Lett (2020) 19(3):2035–42. doi: 10.3892/ol.2020.11293
59. Wang Y, Liu J, Bai H, Dang Y, Lv P, Wu S. Long intergenic non-coding rna 00152 promotes renal cell carcinoma progression by epigenetically suppressing P16 and negatively regulates mir-205. Am J Cancer Res (2017) 7(2):312–22.
60. Wu Y, Tan C, Weng WW, Deng Y, Zhang QY, Yang XQ, et al. Long non-coding rna Linc00152 is a positive prognostic factor for and demonstrates malignant biological behavior in clear cell renal cell carcinoma. Am J Cancer Res (2016) 6(2):285–99.
61. Gao Y, Luo X, Zhang J. Sp1-mediated up-regulation of Lnc00152 promotes invasion and metastasis of retinoblastoma cells via the mir-30d/Sox9/Zeb2 pathway. Cell Oncol (Dordrecht) (2021) 44(1):61–76. doi: 10.1007/s13402-020-00522-8
62. Wang Y, Xin D, Zhou L. Lncrna Linc00152 increases the aggressiveness of human retinoblastoma and enhances carboplatin and adriamycin resistance by regulating mir-613/Yes-Associated protein 1 (Yap1) axis. Med Sci Monit (2020) 26:e920886. doi: 10.12659/msm.920886
63. Ding Y, Guo H, Zhu L, Xu L, Pei Q, Cao Y. Linc00152 knock-down suppresses esophageal cancer by egfr signaling pathway. Open Med (Warsaw Poland) (2020) 15:126–33. doi: 10.1515/med-2020-0019
64. Yang Y, Sun X, Chi C, Liu Y, Lin C, Xie D, et al. Upregulation of long noncoding rna Linc00152 promotes proliferation and metastasis of esophageal squamous cell carcinoma. Cancer Manag Res (2019) 11:4643–54. doi: 10.2147/CMAR.S198905
65. Zhou Z, Huang F. Long non-coding rna Linc00152 regulates cell proliferation, migration and invasion in esophageal squamous cell carcinoma via mir-107/Rab10 axis. Onco Targets Ther (2019) 12:8553–67. doi: 10.2147/OTT.S221515
66. Liu D, Gao M, Wu K, Zhu D, Yang Y, Zhao S. Linc00152 facilitates tumorigenesis in esophageal squamous cell carcinoma via mir-153-3p/Fyn axis. BioMed Pharmacother (2019) 112:108654. doi: 10.1016/j.biopha.2019.108654
67. Ouyang T, Zhang Y, Tang S, Wang Y. Long non-coding rna Linc00052 regulates mir-608/Egfr axis to promote progression of head and neck squamous cell carcinoma. Exp Mol Pathol (2019) 111:104321. doi: 10.1016/j.yexmp.2019.104321
68. Guo Y-Z, Sun H-H, Wang X-T, Wang M-T. Transcriptomic analysis reveals key lncrnas associated with ribosomal biogenesis and epidermis differentiation in head and neck squamous cell carcinoma. J Zhejiang Univ Sci B (2018) 19(9):674–88. doi: 10.1631/jzus.B1700319
69. Ou J, Lei P, Yang Z, Yang M, Luo L, Mo H, et al. Linc00152 mediates Cd8 T-cell infiltration in gastric cancer through binding to Ezh2 and regulating the Cxcl9, 10/Cxcr3 axis. J Mol Histol (2021) 52(3):611–20. doi: 10.1007/s10735-021-09967-z
70. Shi Y, Sun H. Down-regulation of lncrna Linc00152 suppresses gastric cancer cell migration and invasion through inhibition of the Erk/Mapk signaling pathway. Onco Targets Ther (2020) 13:2115–24. doi: 10.2147/ott.S217452
71. Mao Y, Tie Y, Du J, He J. Linc00152 promotes the proliferation of gastric cancer cells by regulating b-cell lymphoma-2. J Cell Biochem (2019) 120(3):3747–56. doi: 10.1002/jcb.27655
72. Zhao J, Liu Y, Zhang W, Zhou Z, Wu J, Cui P, et al. Long non-coding rna Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle (2015) 14(19):3112–23. doi: 10.1080/15384101.2015.1078034
73. Huang Y, Luo H, Li F, Yang Ye, Ou G, Ye X, et al. Linc00152 down-regulated to enhance Mcl1 expression and promote gastric cancer cells proliferation. Biosci Rep (2018) 38(3):BSR20171607. doi: 10.1042/BSR20171607
74. Zhou J, Zhi X, Wang L, Wang W, Li Z, Tang J, et al. Linc00152 promotes proliferation in gastric cancer through the egfr-dependent pathway. J Exp Clin Cancer Res CR (2015) 34:135. doi: 10.1186/s13046-015-0250-6
75. Zhu H, Shan Y, Ge K, Lu J, Kong W, Jia C. Lncrna cytor promotes pancreatic cancer cell proliferation and migration by sponging mir-205-5p. Pancreatology (2020) 20(6):1139–48. doi: 10.1016/j.pan.2020.05.004
76. Yuan ZJ, Yu C, Hu XF, He Y, Chen P, Ouyang SX. Linc00152 promotes pancreatic cancer cell proliferation, migration and invasion via targeting mir-150. Am J Transl Res (2020) 12(5):2241–56.
77. Zou H, Li H. Knockdown of long non-coding rna Linc00152 increases cisplatin sensitivity in ovarian cancer cells. Exp Ther Med (2019) 18(6):4510–6. doi: 10.3892/etm.2019.8066
78. Siegel R, Naishadham D, Jemal A. Cancer statistic. CA Cancer J Clin (2013) 63(1):11–30. doi: 10.3322/caac.21166
79. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistic. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
80. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
81. Encinas de la Iglesia J, Corral de la Calle MA, Fernández Pérez GC, Ruano Pérez R, Álvarez Delgado A. Esophageal cancer: Anatomic particularities, staging, and imaging techniques. Radiologia (2016) 58(5):352–65. doi: 10.1016/j.rx.2016.06.004
82. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
83. Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist (2010) 15(2):168–81. doi: 10.1634/theoncologist.2009-0302
85. Zhao L, Chi WW, Cao H, Meng WX, Cui WN, Wang BS. [Expression of long-chain non-coding rna Linc00152 in laryngeal squamous cell carcinoma and its clinical significance]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi = J Clin Otorhinolaryngology Head Neck Surg (2019) 33(8):721–5. doi: 10.13201/j.issn.1001-1781.2019.08.010
86. Chen X, Li D, Gao Y, Tang W, Iw L, Cao Y, et al. Long intergenic noncoding rna 00152 promotes glioma cell proliferation and invasion by interacting with mir-16. Cell Physiol Biochem (2018) 46(3):1055–64. doi: 10.1159/000488836
87. Peng G, Su J, Xiao S, Liu Q. Linc00152 acts as a potential marker in gliomas and promotes tumor proliferation and invasion through the Linc00152/Mir-107/Rab10 axis. J Neurooncol (2021) 154(3):285–99. doi: 10.1007/s11060-021-03836-1
88. Liu X, Yidayitula Y, Zhao H, Luo Y, Ma X, Xu M. Lncrna Linc00152 promoted glioblastoma progression through targeting the mir-107 expression. Environ Sci pollut Res (2018) 25(18):17674–81. doi: 10.1007/s11356-018-1784-x
89. Cai J, Zhang J, Wu P, Yang W, Ye Q, Chen Q, et al. Blocking Linc00152 suppresses glioblastoma malignancy by impairing mesenchymal phenotype through the mir-612/Akt2/Nf-κb pathway. J Neurooncol (2018) 140(2):225–36. doi: 10.1007/s11060-018-2951-0
90. Chen S, Yang M, Wang C, Ouyang Y, Chen X, Bai J, et al. Forkhead box D1 promotes emt and chemoresistance by upregulating lncrna cytor in oral squamous cell carcinoma. Cancer Lett (2021) 503:43–53. doi: 10.1016/j.canlet.2020.11.046
91. Wang H, Liu Y, Tang A. Prognostic values of long noncoding rna Linc00152 in various carcinomas: An updated systematic review and meta-analysis. Oncologist (2020) 25(1):e31–e8. doi: 10.1634/theoncologist.2018-0358
92. Zhang P-P, Wang Y-Q, Weng W-W, Nie W, Wu Y, Deng Y, et al. Linc00152 promotes cancer cell proliferation and invasion and predicts poor prognosis in lung adenocarcinoma. J Cancer (2017) 8(11):2042–50. doi: 10.7150/jca.18852
93. Yu J, Liu Y, Guo C, Zhang S, Gong Z, Tang Y, et al. Upregulated long non-coding rna Linc00152 expression is associated with progression and poor prognosis of tongue squamous cell carcinoma. J Cancer (2017) 8(4):523–30. doi: 10.7150/jca.17510
94. Chen J, Zheng Q, Liu F, Jin H, Wu X, Xi Y. Linc00152 acts as a competing endogenous rna of Hmga1 to promote the growth of gastric cancer cells. J Clin Lab Anal (2022) 36(2):e24192. doi: 10.1002/jcla.24192
95. Ng CT, Azwar S, Yip WK, Zahari Sham SY, Faisal Jabar M, Sahak NH, et al. Isolation and identification of long non-coding rnas in exosomes derived from the serum of colorectal carcinoma patients. Biol (Basel) (2021) 10(9):918. doi: 10.3390/biology10090918
Keywords: long non-coding RNA, LINC00152, cancer, chemotherapy resistance, radiotherapy resistance
Citation: Li S, Yao W, Liu R, Gao L, Lu Y, Zhang H and Liang X (2022) Long non-coding RNA LINC00152 in cancer: Roles, mechanisms, and chemotherapy and radiotherapy resistance. Front. Oncol. 12:960193. doi: 10.3389/fonc.2022.960193
Received: 02 June 2022; Accepted: 20 July 2022;
Published: 10 August 2022.
Edited by:
Aamir Ahmad, University of Alabama at Birmingham, United StatesReviewed by:
Hailin Tang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaGabriel Tao, Merck, United States
Copyright © 2022 Li, Yao, Liu, Gao, Lu, Zhang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Liang, lxdctopone@sina.com; Haibo Zhang, zhbdoctor@163.com
†These authors have contributed equally to this work
 Shuang Li
Shuang Li Weiping Yao
Weiping Yao Ruiqi Liu
Ruiqi Liu Liang Gao4
Liang Gao4 Yanwei Lu
Yanwei Lu Haibo Zhang
Haibo Zhang Xiaodong Liang
Xiaodong Liang