- 1Department of Surgery, Austin Health, University of Melbourne, Melbourne, VIC, Australia
- 2Department of Preventive Medicine, Monash University, Melbourne, VIC, Australia
- 3Department of Radiation Oncology, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia
- 4Urology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Oligometastatic prostate cancer (OMPC) has been proposed as an intermediary state between localised disease and widespread metastases, with varying definitions including 1, 3, or ≤5 visceral or bone metastasis. Traditional definitions of OMPC are based on staging with conventional imaging, such as computerised tomography (CT) and whole-body bone scan (WBBS). Novel imaging modalities such as prostate-specific membrane antigen positron emission tomography (PSMA PET) have improved diagnostic utility in detecting early metastatic prostate cancer (PC) metastases compared with conventional imaging. Specifically, meta-analytical data suggest that PSMA PET is sensitive in detecting oligometastatic disease in patients with biochemical recurrence (BCR) post-radical treatment of PC. Recent trials have evaluated PSMA PET-guided metastases-directed therapy (MDT) in oligometastatic recurrent disease, typically with salvage surgery or radiotherapy (RT). To date, these preliminary studies demonstrate promising results, potentially delaying the need for systemic therapy. We aim to report a comprehensive, multidisciplinary review of PSMA-guided MDT in OMPC. In this review, we highlight the utility of PMSA PET in biochemically recurrent disease and impact of PSMA PET on the definition of oligometastatic disease and outline data pertaining to PSMA-guided MDT.
Introduction
Prostate cancer (PC) is the second most frequent cancer (37.5 per 100,000) and the fifth most common cause of cancer death in men (1). With significant burden of disease, PC management has steadily advanced throughout the years with improved treatment pathways for primary localised disease, locally advanced disease, oligometastatic cancer, and high-volume metastatic disease. Despite a shift towards early diagnosis with the introduction of screening with prostate-specific antigen (PSA) (2), a proportion of cases harbour an aggressive disease course. Optimal treatment pathways for patients with localised high-risk disease are ill-defined, but broadly, these patients may receive prostatectomy or radiotherapy (RT)—which may provide oncological control and improvements of local symptoms (3). However, the risk of recurrent disease exists in these patients, even when treated during the localised disease course. Indeed, previous high-volume series suggests a 50% risk of biochemical recurrence and 37% risk of salvage therapy 10 years after prostatectomy in high-risk patients (4)
In the setting of disease recurrence, oligometastatic PC (OMPC) can be considered an intermediary state between localised disease and widespread metastases with heterogeneous definitions including 1, 3, or ≤5 visceral or bone metastasis (5–10). Given the limited deposits of metastatic disease, several groups proposed consideration of metastasis-directed therapy (MDT) with the aim of optimising oncological outcomes (11). However, most MDT has been based on conventional imaging with computed tomography (CT), 99mTc-methylene diphosphonate (MDP) bone scintigraphy, and choline-positron emission tomography (PET) (12–15).
More recently, novel imaging techniques have been developed including prostate-specific membrane antigen (PSMA) PET (16). Of these, the most widely studied radioligands include 68Ga-PSMA-11 (17) and 18F-PSMA-DCFPyL (18). These techniques have provided improved diagnostic accuracy in the management of advanced PC, especially in biochemical recurrence (BCR) (17, 19, 20). Specifically, PSMA PET techniques allow visualisation of metastatic disease prior to metastatic deposits, reaching morphological criteria required for diagnosis on conventional imaging (21). Hence, recent trials have evaluated PSMA PET-guided MDT in oligometastatic recurrent disease, typically with salvage surgery or radiotherapy, which potentially delays the need for systemic therapy (22–24). Therefore, we aim to comprehensively review PSMA PET-guided MDT in OMPC. Our report will highlight PSMA PET in BCR and paradigm shifting definitions of oligometastatic disease and discuss current trials in PSMA PET-guided MDT.
The role of PSMA PET in detection of biochemically recurrent disease
PSMA is a cellular surface protein with high expression in prostate tissue and limited extraprostatic expression. It is a 750-amino-acid, 100-kDa, type II transmembrane glycoprotein consisting of intracellular, transmembrane, and extracellular components (25). It may also be expressed in other organs including the kidney, small bowel, neuroendocrine tissue, and neural tissue (26). However, PSMA has been found to have 12 times greater expression on prostatic tissue compared to the next highest organ (27). Furthermore, studies have demonstrated PSMA expression in dysplastic prostatic changes and subsequent marked expression in prostatic adenocarcinoma and lymph node metastases (LNMs), whilst it is lowest in benign prostatic tissue (28, 29). Increased PSMA expression also occurs in the setting of increasing grade and stage of PC (26, 30). Additionally, in oligometastatic disease, only 2% of LNMs have been found to be negative for PSMA expression (29). Hence, PSMA represents an attractive target for imaging and therapeutic intervention in PC.
Following radical therapy, such as prostatectomy or radiotherapy, PSMA PET in the setting of BCR has been extensively investigated; however, definitions of BCR have been varied. A prostate-specific antigen (PSA) > 0.4 ng/ml and rising has been noted to best predict further metastases after radical prostatectomy (RP) (31, 32), although a PSA ≥ 0.2 ng/ml and confirmed on subsequent check post-RP has also been proposed (33). Hence, the European Association of Urology (EAU) Prostate Cancer Guidelines Panel recommends evaluating a patient’s life expectancy when considering further treatment and should not be based on meeting a PSA threshold. Rather, the EAU suggests utilising an externally validated, patient-specific risk stratification system dividing into EAU Low-Risk BCR [PSA-doubling time > 1 year and Primary Gleason Score <8 (ISUP grade < 4) for RP] and EAU High-Risk BCR [PSA-doubling time ≤ 1 year or Primary Gleason Score 8-10 (ISUP grade 4–5)] (34). Furthermore, the EAU guidelines recommend early restaging and early immediate post-operative RT in high-risk BCR.
With the definition of biochemical recurrence in mind, a systematic review and meta-analysis of 37 articles involving 4,790 patients by Perera et al. (17, 19) noted 76% overall percentage positivity for 68Ga-PSMA PET in BCR. Increasing risk of positivity was associated with increasing post-treatment PSA. Specifically, for PSA between 0 and 0.19, 0.2 and 0.49, 0.5 and 0.99, and 1 and 1.99 or ≥2 ng/ml, the proportion of positive PSMA PET was 33%, 45%, 75% and 95%, respectively. These articles demonstrate the usefulness of PSMA PET in the setting of BCR PC, particularly at low levels of pre-PET PSA >0.2 ng/ml.
However, head-to-head comparison of novel imaging with conventional staging (CT and 99mTc-MDP bone scintigraphy) is limited, particularly in the biochemically recurrent setting. In the primary staging setting, Hofman et al. highlighted superiority of PSMA PET versus conventional imaging in a randomised open-label cross-over trial (21). In the setting of BCR, a recent prospective single-centre clinical trial by Joshi et al. (35) compared PSMA PET and MRI with conventional imaging in 30 patients with BCR following radical curative therapy for PC. Histological correlation was performed to assess clinical efficacy of PSMA PET. Median PSA was 0.69 ng/ml, and PSMA avid lesions were present in 21 patients (70%) compared to 5 patients in conventional imaging (17%) (35). Detection of local recurrence was significantly more likely in PSMA PET/MRI when compared to conventional imaging (p=0.005) and eight of nine biopsied lesions were positive (88.9%) for metastatic PC with a positive predictive value of 95.2% (35). Eissa et al. (20) further corroborated these findings, noting superiority of PSMA PET to conventional imaging techniques including CT and magnetic resonance imaging (MRI). Furthermore, Fendler et al. (36) retrospectively investigated 200 patients with non-metastatic castrate-resistant PC for which 55% had M1 disease on PSMA PET despite negative conventional staging, emphasising superiority of PSMA PET.
Defining oligometastatic prostate cancer and impact of PSMA imaging
Oligometastatic cancer was first hypothesised by Hellman and Weichselbaum (37) determining the oligometastatic state as a subgroup of patients with potentially curable and limited number of metastases, hence defined as intermediary between localised and widespread metastases. Traditional definitions of OMPC are based on conventional imaging, such as CT and 99mTc-MDP bone scintigraphy. OMPC can be biologically divided into de novo (metastatic synchronous) tumour at the time of diagnosis as compared to oligorecurrent disease (post-treatment of the primary cancer) and oligoprogressive disease and development of a second primary tumour (metachronous) (38). Aggressive management of OMPC as a distinct disease state is at the forefront of improving patient survival of an otherwise poorly prognosticated disease, hence the need for a clear definition. However, a universal definition for oligometastatic (recurrent) PC is lacking, with maximum number and location being deliberated.
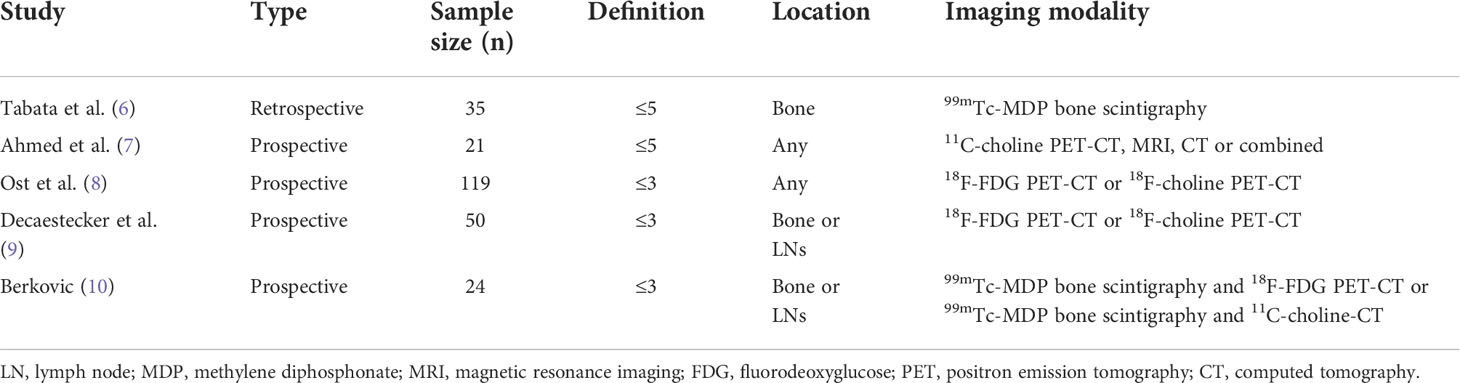
Varying definitions including ≤3 or ≤5 visceral or bone metastasis have been proposed (Table 1) (5). Tabata et al. (6) and Ahmed et al. (7) utilised ≤5 to define OMPC; however, it differed on the location (bone only vs. not specified) and imaging modality (99mTc-MDP bone scintigraphy vs. 11C-choline PET, MRI, CT, or combined), respectively. Ost et al. (8), Decaestecker et al. (9), and Berkovic et al. (10) defined OMPC as ≤3, but differed based on location (any vs. bone or LNs vs. bone or LNs) and imaging modality (18F-fluorodeoxyglucose [FDG] PET-CT or 18F-choline PET-CT vs. 18F-FDG PET-CT or 18F-choline PET-CT vs. 99mTc-MDP bone scintigraphy and 18F-FDG PET-CT or 99mTc-MDP bone scintigraphy and 11C-choline-CT), respectively.
Due to the heterogeneity of OMPC definitions, more recent clinical trials take into account the disease burden, stratifying into low and high risk/volume as defined in the LATITUDE (39) and CHAARTED (40) criteria. The LATITUDE trial defined high risk as having two or more of the following criteria: ≥3 bone metastases, visceral metastases, and ≥ ISUP grade 4. The CHAARTED trial defined high-volume disease as ≥4 bone metastases (including ≥1 in vertebral column or spine) or visceral metastases. Therefore, OMPC can be defined as low risk and low volume based on the noted criteria in hormone-sensitive PC, which aids in the determination of treatment, such as RT to the primary for low-volume disease (41). However, these two trials also utilised conventional imaging in identifying metastatic disease. Novel imaging, such as PSMA PET, has subsequently changed the definition of OMPC due to its significant detection of disease at low pre-PET PSA. Interestingly, Barbato et al. (42) attempted to combine the CHAARTED low-/high-volume disease criteria on PSMA PET compared to CT, using 40 ml as an arbitrary cutoff. PSMA PET was concluded to have improved tumour volume assessment due to detection of additional lesions in 62% of patients. However, the study was retrospective and had low numbers (n=105) and omitted bone scintigraphy, highlighting the need for larger studies.
As noted, definitions of OMPC can be classified based on biology (de novo vs. oligorecurrent vs. oligoprogressive), location (visceral vs. bone vs. both), and volume/risk (low vs. high). Diagnosis of nodal metastatic disease, historically, is based on achieving morphological criteria such as size criteria >10 mm based on conventional imaging. PSMA PET has enabled earlier detection of oligometastatic disease when compared to conventional imaging and may detect lymph node metastases <10 mm. As such, PSMA PET detection rates of OMPC compared to conventional imaging was significantly higher (p=0.005) with a positive predictive value of 95.2% (35) and best at pre-PET PSA levels of >0.2 ng/ml (17). Furthermore, the earlier diagnosis of OMPC must be considered with caution. For example, patients with subcentimetre PSMA avid nodes diagnosed as OMPC may have previously deemed localised disease on conventional imaging. Accordingly, it is probable that the OMPC population diagnosed on PSMA PET is impacted by the Will Rogers’ stage-migration phenomenon. Moreover, with earlier detection of metastatic disease with PSMA PET, we may be artificially observing prolonged overall survival through lead-time bias (43). Despite earlier detection, the natural history of disease course may not be altered. Although more and more studies are investigating the use of metastasis-directed therapy (MDT), correction of lead-time bias is important to gain accurate measurement of improvement in overall survival.
Historic data on MDT in the pre-PSMA era
Given that OMPC is considered an intermediate state of tumour spread with limited metastatic capacity (44), the importance of oligometastatic disease is increasingly acknowledged, as evidence grows for the treatment of limited metastatic lesions. The rationale for MDT in oligometastatic cancer can be addressed twofold—biologically and clinically. From a biological standpoint, overall reduction in tumour burden (cytoreductive therapy) may explain improved outcomes with treatment of primary and metastatic sites (45), whereas clinically, MDTs may potentially delay further metastatic progression and postpone the use of systemic treatment, reducing the burden of adverse drug effects, as this is indeed true for other tumour types such as colorectal cancer, sarcomas, and renal cell carcinoma (13). Focal ablative therapies, such as stereotactic body radiation therapy (SBRT), surgery, or focal thermal ablation, are examples of MDTs (11).
The success of MDTs relies on imaging modalities with high diagnostic accuracy to sensitively guide targeted therapy. However, conventional imaging with CT and 99mTc-MDP bone scintigraphy demonstrates poor sensitivity to detect oligometastatic disease (12). The advent of modern imaging techniques including whole-body MRI and PET/CT scans using tracers, such as 18F-NaF or 18F-choline, have been frequently incorporated into guidelines and trials in recent years (12). Indeed, prior to the widespread use of PSMA PET/CT, MDTs for OMPC were predominantly diagnosed with choline PET/CT, and the literature consists of small heterogeneous studies. In a systematic review by Ost et al. (13), a total of 450 patients were pooled from 15 single-arm case series, whereby PET/CT was used for diagnosis in 98%, using either choline (91%) or FDG (7%) as tracer. Treated metastases were predominantly nodal (78%), bone metastases (21%), and less frequently visceral metastases (1%). MDT modality was either high-dose radiotherapy (66%) or surgical metastasectomy (34%). Although there was great heterogeneity among patient populations, the authors found that 51% of men were progression free 1–3 years after MDT. Results should be interpreted with caution, however, as 61% had adjuvant androgen deprivation therapy (ADT) and 49% had adjuvant nodal irradiation. Due to the overall low number and heterogeneity of patients, and lack of comparative or randomised trials, the review concluded that MDT should not be considered the standard of care. In an attempt to overcome the limitations of retrospective studies, a multi-institutional analysis used fixed inclusion and exclusion criteria, demonstrating an ADT-free survival of 28 months after SBRT for oligorecurrent PC (8). However, 50% of these patients also received a temporary course of adjuvant ADT at the time of SBRT.
There are several small case series that demonstrate benefit of MDT without ADT, showing a median progression-free survival of 24 and 19 months following SBRT (9, 46), and 4 years following salvage LND (47). Recently, the Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence (STOMP) trial aimed to validate the observations of previous retrospective studies showing benefits of MDT (14). STOMP was the first prospective, randomised, phase II trial that demonstrated that MDT using SBRT delayed the initiation of ADT in men with hormone-sensitive metastatic PC with ≤3 detectable metastases on choline PET/CT. The primary outcome of the STOMP trial was ADT-free survival in men assigned to MDT versus surveillance alone. The results showed that those undergoing MDT experienced a longer median ADT-free survival of 21 months compared to 13 months in the surveillance arm [HR, 0.60 (0.40–0.90, 80% CI), log-rank p=0.11]. At the last update, the 5 year ADT-free survival was 34% for the MDT group and 8% for the surveillance group (15). However, 30% of patients treated with MDT progressed to polymetastatic disease (>3 metastases) within the first year. Authors suggest that this may be due to microscopic metastatic disease, which is not seen by choline PET/CT, but may be overcome in future studies utilising PSMA PET/CT, which has much greater sensitivity and specificity (14).
Stereotactic ablative body radiotherapy (SABR) was utilised by Siva et al. (48), who performed the second prospective trial demonstrating delayed initiation of ADT on 33 patients with one to three metastases utilising NaF PET/CT. A single fraction of 20-Gy SABR was prescribed to 50 lesions and reviewed with imaging at 12 and 24 months. Local progression-free survival was 97% (91%–100%, 95% CI) at 12 months and 93% (84%–100%, 95% CI) at 24 months, and distant progression-free survival was 58% (43%–77%, 95% CI) at 12 months and 39% (25–60%, 95% CI) at 24 months. As opposed to the STOMP trial, ADT was initiated on clinician discretion instead of pre-defined protocols and was delayed by 24 months in 48% (31%–75%, 95% CI). The authors also note the low sensitivity and specificity of NaF PET for the detection of nodal metastases, which may exclude truly oligometastatic disease patients.
Another treatment modality for patients with recurrent oligometastatic disease is salvage lymph node dissection (SLND). A retrospective study by Rischke et al. (49) found that adjuvant RT delayed BCR in 93 patients who underwent SLND in comparison to SLND alone with the 5-year relapse-free rate of 70.7% vs. 26.3% (p<0.0001), respectively. However, given the small numbers, retrospective analysis, and superseded imaging modality for the identification of oligometastases (11C or 18F PET/CT), the authors suggest that prospective randomised trials are required for confirmation of adjuvant RT.
Current data on MDT guided by PSMA
Given the sensitivity of PSMA PET for the localization of sites of recurrence, this new emerging imaging modality has the potential to directly impact MDT in several ways. Not only will it redefine the treatment paradigms for oligometastatic disease, but it will also allow MDT to target involved areas that would not normally be included on historical consensus guidelines (50). In the post-operative setting, a recent retrospective multicentre study evidenced the high detection rate (from 40.9% for a PSA value range of 0.2–0.4 ng/ml to 64.2% for a value in the range from 0.8 to 1 ng/ml) of 68Ga-PSMA PET/CT in a population of early biochemical recurrence, with PSA ≤1 ng/ml after radical prostatectomy. These results suggest that PSMA imaging pre-salvage radiotherapy might significantly influence disease management of early biochemical recurrence, with PSMA PET guiding optimal clinical approach (51). Additionally, in a recent multicentre analysis of 270 patients who presented for salvage radiotherapy, 19% of patients had at least one lesion identified on PSMA PET/CT that was outside a consensus prostate fossa + pelvic lymph node radiation field, with 12% overall having extra-pelvic disease (52).
In a recent systematic review of next-generation imaging modalities of recurrent oligometastatic disease, PSMA PET-directed salvage therapy was used in 50% of studies (22). Of note, in the recent phase II Observation vs. Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer (ORIOLE) trial, randomised 80 patients received SBRT vs. observation alone (23). SBRT planning was based on conventional imaging alone; although a 18F-DCFPyL-PSMA PET was performed, it was not used for treatment planning. PSMA PET-positive lesions that were not prescribed in the treatment fields were found in 44% of patients. A post-hoc analysis based on the extent of untreated disease seen on PSMA PET found progression-free survival advantages for men who had received treatment to all PSMA-avid disease (HR, 0.26; 95% CI, 0.09–0.76; p=0.006) (23). In another recent retrospective multicentre study comparing choline-PET with PSMA PET-directed MDT, disease-free survival rates were 34% (n=15) and 64% (n=28) (p=0.06), respectively (24). The ADT administration rate was also higher after choline PET-guided SBRT due to the higher incidence of polymetastatic disease after first-course SBRT compared with 68Ga-PSMA-based SBRT (20 vs. 5 patients, p=0.001). Furthermore, a large multicentre retrospective study evaluated 394 patients with oligorecurrent disease comparing 68Ga-PSMA PET/CT-directed RT to combined elective RT (RT to prostate bed and pelvic and para-aortic nodes) plus focal therapy (53). Biochemical recurrence-free survival was significantly more in the combined PSMA PET directed and elective RT group compared with PSMA PET-directed therapy alone at 36 months (53% vs. 37%, p=0.001). These studies suggest that PSMA PET may delay the initiation of systemic ADT and prolong progression-free survival.
SLND represents another treatment option for patients with recurrent oligometastatic disease. However, only retrospective studies are available for the evaluation of PSMA-directed SLND. A recent systematic review highlighted that most are single-centre series with small and highly heterogeneous cohorts in terms of endpoints, adjuvant treatments, and definitions of progression (54). Of 27 studies included in the review, the majority (15/27) used choline as a tracer, whilst PSMA-labelled radionuclides were used in 11/27 (54). In the first evaluation of PLND in OMPC detected by 68Ga-PSMA PET, diagnostic accuracies per nodal lesion showed a sensitivity and specificity of 94% and 99%, respectively, in a total of 213 nodes from 35 patients (55). A retrospective series by Linxweiler et al. (56) compared SLND directed by 68Ga-labelled PSMA versus choline PET/CT, demonstrating an improvement in biochemical complete response rate (44% vs. 18%), a greater PSA decrease (mean −57% vs. mean +10%, p = 0.015), and a longer ADT-free period (4.7 vs. 12 months, p=0.001). Further advancements in minimally invasive approaches such as laparoscopic robotic-assisted SLND may also improve perioperative morbidity compared with a standard open surgical approach; however, only small retrospective case series have been published where 68Ga-PSMA PET (56–59) and 99mTechnetium-PSMA PET were used (60). Interestingly, Bravi et al. (61) found on long-term follow-up (10 years) of patients with lymph node recurrence on 11C-chole or 68Ga-labelled PSMA PET/CT that one in three men treated with SLND died due to PC in the setting of PET-detected nodal PC recurrence. The authors concluded that a multimodal approach including use of ADT to maximise patient outcomes and MDT may be curative in a select population of patients.
A recent prospective phase II study by Glicksman et al. (62) used 18F-DCFPyL-PSMA PET to identify patients with oligorecurrent PC in the setting of rising PSA (0.4–3.0 ng/ml) post-definitive therapy (RP and post-operative RT). Out of 72 patients, 38 (53%) were found to have PSMA-detected oligorecurrent disease amenable to MDT. Ten patients underwent surgery, 27 had SABR, and 1 patient was not based on discussions with a urologist and radiation oncologist. For those treated with MDT, 60% (n=22) of patients had a biochemical response (7 surgery and 15 SABR) with 22% (n=8) meeting undetectable PSA levels (complete biochemical response) and a median follow-up duration of 7.7 months. Although this study presents promising results, data cannot be extrapolated to patients with BCR post-RP alone.
A novel surgical approach to patients with recurrent disease on 68Ga-PSMA PET/CT post-RP, Li et al. (63) enrolled 19 patients into integrated indocyanine green (ICG)-guided fluorescent laparoscopic SLND. The authors aimed to use ICG-guided SLND to effectively remove affected LNs and to minimise complications. The specificity of 68Ga-PSMA PET/CT was 96.6% (and a sensitivity of 42.9%), whilst ICG had a sensitivity of 92.8% (and a specificity of 39.1%). The authors concluded that in patients with BCR with recurrent lymph node disease, a combined approach with 68Ga-PSMA PET/CT and ICG fluorescence-guided SLND is an effective and safe treatment; however, further validation and long-term results are warranted.
In contrast to the high level of evidence supporting PSMA-targeted PET for post-RP BCR, fewer studies have investigated the post-primary RT population. Meta-analyses have pooled post-RT and post-RP patients together, with majority being post-RP (17, 64). In a prospective study comparing 18F-DCFPyL PSMA-targeted PET restaging to conventional imaging exclusively in post-RT patients, PSMA PET was able to detect more recurrence at any site [87% (78%–94%, 95% CI) vs. 67% (56%–77%, 95% CI)] and extra-prostatic sites [39% (28%–51%, 95% CI) vs. 19% (11%–29%, 95% CI), p<0.001] (65). The distribution of disease detected on PSMA PET was 48% prostatic, 27% regional nodes, and 30% distant. Interestingly, this differs from post-RP BCR, where failures tend to be regional nodal or extra-pelvic, with a smaller proportion exhibiting isolated prostate bed recurrence (66). The high rates of extra-prostatic disease detected in patients who meet Phoenix criteria (PSA rise ≥2 ng/ml above nadir) (67) suggest that there may be a role for PSMA-targeted PET at earlier time points post-RT to maximize detection of local failure.
Future directions and active clinical trials
As demonstrated in an international meta-analysis on OMPC recurrence (≤3 lesions), the majority of patients treated with SBRT for nodal recurrence had a relapse within 2 years in nearby lymph node regions, with an estimated median time of 12–18 months (8). Similar results were seen in a large multi-institutional study exploring the role of SLND after nodal recurrence (59). PEACE V-Salvage Treatment of OligoRecurrent nodal prostate cancer Metastases (STORM) is a randomised phase II study aiming to assess the potential of combined whole pelvic radiotherapy and MDT against MDT alone (68). Another single-centre study (NCT04271579) (ProsTone) aims to investigate whether a unilateral pelvic lymph node dissection on the side of conspicuous PSMA PET is sufficient, without the need to perform a dissection of the contralateral side. SLND may also be carried out with the aid of experimental preoperative labelling with PSMA ligands for easier intraoperative localisation (PSMA radio-guided surgery); a comparison of conventional salvage surgery and the PSMA-radioguided surgery is also planned (NCT04271579). There are currently no randomised studies on oncological outcomes for patients who received MDT for BCR based on PSMA-targeted PET compared to other imaging. However, randomized phase III trials are ongoing (NCT03582774 and NCT03762759), with estimated completion dates in 2023 and 2025. Another emerging trend is the use of ADT and/or pelvic nodal RT combined with salvage prostate bed RT. For example in SPPORT, freedom from progression was superior in men who received RT plus 6 months of ADT (69).
In addition to diagnostic uses, PSMA-targeting agents are also being used therapeutically in a field called theranostics by utilising radiopharmaceuticals (70). Currently, the most used is Lutetium-177 (177Lu) labelling, which emits beta particles with approximately 1-mm path length to deliver radiation to sites of disease. A phase II trial investigating its use in metastatic castrate-resistant PC showed a PSA response rate of 96.7%, low toxic effects, and improvements in pain palliation (71). The most common toxic effects included xerostomia (87%), nausea (50%), and fatigue (50%), whilst a minority of patients (13%) experienced thrombocytopenia. Current active studies include the Australian LuPARP (NCT03874884) and American NCT03805594, which assess the efficacy of 177Lu in combination with targeted therapy or immunotherapy, whilst promising preliminary results are seen in trials such as TheraP (NCT03392428), which investigates its efficacy compared with conventional chemotherapy (72). Additionally, for castrate-sensitive PC, the international PSMAddition (NCT04720157) and Australian UpFrontPSMA (NCT04343885) (73) are large randomised trials evaluating the efficacy of 177Lu-PSMA-617 compared with the standard of care. The Australian POPSTAR II (NCT PENDING) phase II trial also aims to investigate the castrate-sensitive PC group. It aims to investigate patients with <5 metastases and compare SABR with or without Lu-PSMA.
Furthermore, trials (Table 2) for PSMA PET in OMPC patients include the use of radiopharmacy, SBRT, and SLND. A phase II trial from the Netherlands (NCT04443062) aims to compare 177Lu to delayed ADT in patients with BCR [18F-PSMA PET-CT-positive metastases in bones and/or lymph nodes (≤5 metastases)] and inability to perform local treatment for oligometastases (74). An American phase I trial (NCT05079698) combining SBRT and 177Lu infusion in PSMA PET-detected lesions (≤3 metastases) aims to identify the dose-limiting toxicity in their pilot study. The DETECT trial (NCT04300673) provides an exciting take on SLND in patients with ≥1 18F/68Ga-PSMA-PET/CT suspected positive metastasis pelvic lymph nodes. All patients receive 111Indium (111In) PSMA tracer 24 h prior to surgery with the aim to evaluate the feasibility of 111In guided detection of lymph node metastases with intra-operative gamma-probe. Finally, a phase II trial (NCT03525288) comparing PSMA PET-guided definitive RT to standard care RT without PSMA PET aims to identify failure-free survival.
The advent of PSMA PET has been paradigm shifting in the world of PC, which has propagated an exciting field of discovery. From its use as a diagnostic tool and identification of early BCR to PSMA-targeted therapies, the current landscape of MDT in OMPC is promising. However, PSMA PET in the setting of MDT is still in its infancy and is not ready for prime time. We therefore eagerly await the results of upcoming clinical trials.
Author contributions
MA: draft preparation. AY: draft preparation. NP: draft revision. SS: draft revision. JI: draft revision, supervision. KT: draft revision, supervision. JE: draft revision, supervision. DB: draft revision, supervision, funding. MP: draft revision, supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers at MSK and the NIH/NCI Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center (P30 CA008748). Marlon Perera is sponsored by the Australian–America Fulbright Commission administered through a 2021–2022 Fulbright Future Scholarship funded by The Kinghorn Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. New Engl J Med (2009) 360(13):1320–8. doi: 10.1056/NEJMoa0810084
3. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol (2017) 71(4):618–29. doi: 10.1016/j.eururo.2016.08.003
4. Abdollah F, Sood A, Sammon JD, Hsu L, Beyer B, Moschini M, et al. Long-term cancer control outcomes in patients with clinically high-risk prostate cancer treated with robot-assisted radical prostatectomy: Results from a multi-institutional study of 1100 patients. Eur Urol. (2015) 68(3):497–505. doi: 10.1016/j.eururo.2015.06.020
5. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: A European society for radiotherapy and oncology and European organisation for research and treatment of cancer consensus recommendation. Lancet Oncol (2020) 21(1):e18–28. doi: 10.1016/S1470-2045(19)30718-1
6. Tabata K, Niibe Y, Satoh T, Tsumura H, Ikeda M, Minamida S, et al. Radiotherapy for oligometastases and oligo-recurrence of bone in prostate cancer. Pulm Med (2012) 2012:541656. doi: 10.1155/2012/541656
7. Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol (2012) 2:215. doi: 10.3389/fonc.2012.00215
8. Ost P, Jereczek-Fossa BA, As NV, Zilli T, Muacevic A, Olivier K, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: A multi-institutional analysis. Eur Urol. (2016) 69(1):9–12. doi: 10.1016/j.eururo.2015.07.004
9. Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol (2014) 9:135. doi: 10.1186/1748-717X-9-135
10. Berkovic P, De Meerleer G, Delrue L, Lambert B, Fonteyne V, Lumen N, et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: Deferring androgen deprivation therapy. Clin Genitourin Cancer. (2013) 11(1):27–32. doi: 10.1016/j.clgc.2012.08.003
11. Lecouvet FE, Oprea-Lager DE, Liu Y, Ost P, Bidaut L, Collette L, et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: A consensus recommendation from the EORTC imaging group. Lancet Oncol (2018) 19(10):e534–e45. doi: 10.1016/S1470-2045(18)30571-0
12. Crawford ED, Stone NN, Yu EY, Koo PJ, Freedland SJ, Slovin SF, et al. Challenges and recommendations for early identification of metastatic disease in prostate cancer. Urology. (2014) 83(3):664–9. doi: 10.1016/j.urology.2013.10.026
13. Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: A systematic review of the literature. Eur Urology. (2015) 67(5):852–63. doi: 10.1016/j.eururo.2014.09.004
14. Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol (2017) 36(5):446–53. doi: 10.1200/JCO.2017.75.4853
15. Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J Clin Oncol (2020) 38 10(6_suppl):446–53. doi: 10.1200/JCO.2020.38.6_suppl.10
16. Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. (2017) 44(8):1258–68. doi: 10.1007/s00259-017-3711-7
17. Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: A systematic review and meta-analysis. Eur Urol. (2020) 77(4):403–17. doi: 10.1016/j.eururo.2019.01.049
18. Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, et al. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: Results from the CONDOR phase III, multicenter study. Clin Cancer Res (2021) 27(13):3674–82. doi: 10.1158/1078-0432.CCR-20-4573
19. Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur Urol. (2016) 70(6):926–37. doi: 10.1016/j.eururo.2016.06.021
20. Eissa A, Elsherbiny A, Coelho RF, Rassweiler J, Davis JW, Porpiglia F, et al. The role of 68Ga-PSMA PET/CT scan in biochemical recurrence after primary treatment for prostate cancer: A systematic review of the literature. Minerva Urol Nefrol. (2018) 70(5):462–78. doi: 10.23736/S0393-2249.18.03081-3
21. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet. (2020) 395(10231):1208–16. doi: 10.1016/S0140-6736(20)30314-7
22. Farolfi A, Hadaschik B, Hamdy FC, Herrmann K, Hofman MS, Murphy DG, et al. Positron emission tomography and whole-body magnetic resonance imaging for metastasis-directed therapy in hormone-sensitive oligometastatic prostate cancer after primary radical treatment: A systematic review. Eur Urol Oncol (2021) 4(5):714–30. doi: 10.1016/j.euo.2021.02.003
23. Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: The ORIOLE phase 2 randomized clinical trial. JAMA Oncol (2020) 6(5):650–9. doi: 10.1001/jamaoncol.2020.0147
24. Mazzola R, Francolini G, Triggiani L, Napoli G, Cuccia F, Nicosia L, et al. Metastasis-directed therapy (SBRT) guided by PET-CT 18F-CHOLINE versus PET-CT 68Ga-PSMA in castration-sensitive oligorecurrent prostate cancer: A comparative analysis of effectiveness. Clin Genitourinary Cancer (2021) 19(3):230–6. doi: 10.1016/j.clgc.2020.08.002
25. Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. (2013) 40(4):486–95. doi: 10.1007/s00259-012-2298-2
26. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res (1997) 3(1):81–5.
27. Cunha AC, Weigle B, Kiessling A, Bachmann M, Rieber EP. Tissue-specificity of prostate specific antigens: Comparative analysis of transcript levels in prostate and non-prostatic tissues. Cancer Lett (2006) 236(2):229–38. doi: 10.1016/j.canlet.2005.05.021
28. Wright GL Jr., Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol (1995) 1(1):18–28. doi: 10.1016/1078-1439(95)00002-Y
29. Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. (1998) 52(4):637–40. doi: 10.1016/S0090-4295(98)00278-7
30. Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. (68)Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. (2017) 44(6):941–9. doi: 10.1007/s00259-017-3631-6
31. Stephenson AJ, Kattan MW, Eastham JA, Dotan ZA, Bianco FJ Jr., Lilja H, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: A proposal for a standardized definition. J Clin Oncol (2006) 24(24):3973–8. doi: 10.1200/JCO.2005.04.0756
32. Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. Defining prostate specific antigen progression after radical prostatectomy: What is the most appropriate cut point? J Urol (2001) 165(4):1146–51. doi: 10.1016/S0022-5347(05)66452-X
33. Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D'Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American urological association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. (2007) 177(2):540–5. doi: 10.1016/j.juro.2006.10.097
34. Van den Broeck T, van den Bergh RCN, Briers E, Cornford P, Cumberbatch M, Tilki D, et al. Biochemical recurrence in prostate cancer: The European association of urology prostate cancer guidelines panel recommendations. Eur Urol Focus. (2020) 6(2):231–4. doi: 10.1016/j.euf.2019.06.004
35. Joshi A, Roberts MJ, Perera M, Williams E, Rhee H, Pryor D, et al. The clinical efficacy of PSMA PET/MRI in biochemically recurrent prostate cancer compared with standard of care imaging modalities and confirmatory histopathology: results of a single-centre, prospective clinical trial. Clin Exp Metastasis. (2020) 37(4):551–60. doi: 10.1007/s10585-020-10043-1
36. Fendler WP, Weber M, Iravani A, Hofman MS, Calais J, Czernin J, et al. Prostate-specific membrane antigen ligand positron emission tomography in men with nonmetastatic castration-resistant prostate cancer. Clin Cancer Res (2019) 25(24):7448–54. doi: 10.1158/1078-0432.CCR-19-1050
37. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol (1995) 13(1):8–10. doi: 10.1200/JCO.1995.13.1.8
38. Lancia A, Zilli T, Achard V, Dirix P, Everaerts W, Gomez-Iturriaga A, et al. Oligometastatic prostate cancer: The game is afoot. Cancer Treat Rev (2019) 73:84–90. doi: 10.1016/j.ctrv.2019.01.005
39. Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol (2019) 20(5):686–700. doi: 10.1016/S1470-2045(19)30082-8
40. Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol (2018) 36(11):1080–7. doi: 10.1200/JCO.2017.75.3657
41. Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet (2018) 392(10162):2353–66. doi: 10.1016/S0140-6736(18)32486-3
42. Barbato F, Fendler WP, Rauscher I, Herrmann K, Wetter A, Ferdinandus J, et al. PSMA-PET for the assessment of metastatic hormone-sensitive prostate cancer volume of disease. J Nucl Med (2021) jnumed.121.2621:62. doi: 10.2967/jnumed.121.262120
43. Feinstein AR, Sosin DM, Wells CK. The will Rogers phenomenon. stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med (1985) 312(25):1604–8. doi: 10.1056/NEJM198506203122504
44. Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol (2011) 8(6):378–82. doi: 10.1038/nrclinonc.2011.44
45. Rao A, Vapiwala N, Schaeffer EM, Ryan CJ. Oligometastatic prostate cancer: A shrinking subset or an opportunity for cure? Am Soc Clin Oncol Educ Book (2019) 39:309–20. doi: 10.1200/EDBK_239041
46. Casamassima F, Masi L, Menichelli C, Bonucci I, Casamassima E, Lazzeri M, et al. Efficacy of eradicative radiotherapy for limited nodal metastases detected with choline PET scan in prostate cancer patients. Tumori (2011) 97:49–55. doi: 10.1177/030089161109700110
47. Jilg CA, Rischke HC, Reske SN, Henne K, Grosu AL, Weber W, et al. Salvage lymph node dissection with adjuvant radiotherapy for nodal recurrence of prostate cancer. J Urology. (2012) 188(6):2190–7. doi: 10.1016/j.juro.2012.08.041
48. Siva S, Bressel M, Murphy DG, Shaw M, Chander S, Violet J, et al. Stereotactic abative body radiotherapy (SABR) for oligometastatic prostate cancer: A prospective clinical trial. Eur Urol. (2018) 74(4):455–62. doi: 10.1016/j.eururo.2018.06.004
49. Rischke HC, Schultze-Seemann W, Wieser G, Kronig M, Drendel V, Stegmaier P, et al. Adjuvant radiotherapy after salvage lymph node dissection because of nodal relapse of prostate cancer versus salvage lymph node dissection only. Strahlenther Onkol. (2015) 191(4):310–20. doi: 10.1007/s00066-014-0763-5
50. Kishan AU, Nickols NG, Spratt DE. Prostate-specific membrane antigen positron emission tomography–guided radiotherapy. Eur Urol Focus. (2021) 7(2):250–3. doi: 10.1016/j.euf.2020.09.020
51. Francolini G, Detti B, Bottero M, Zilli T, Lancia A, Bruni A, et al. Detection rate, pattern of relapse and influence on therapeutic decision of PSMA PET/CT in patients affected by biochemical recurrence after radical prostatectomy, a retrospective case series. Clin Transl Oncol (2021) 23(2):364–71. doi: 10.1007/s12094-020-02427-2
52. Calais J, Czernin J, Cao M, Kishan AU, Hegde JV, Shaverdian N, et al. Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: Impact on salvage radiotherapy planning. J Nucl Med (2018) 59(2):230. doi: 10.2967/jnumed.117.201749
53. Kirste S, Kroeze SGC, Henkenberens C, Schmidt-Hegemann NS, Vogel MME, Becker J, et al. Combining (68)Ga-PSMA-PET/CT-Directed and elective radiation therapy improves outcome in oligorecurrent prostate cancer: A retrospective multicenter study. Front Oncol (2021) 11:640467. doi: 10.3389/fonc.2021.640467
54. Ploussard G, Gandaglia G, Borgmann H, de Visschere P, Heidegger I, Kretschmer A, et al. Salvage lymph node dissection for nodal recurrent prostate cancer: A systematic review. Eur Urology. (2019) 76(4):493–504. doi: 10.1016/j.eururo.2018.10.041
55. Hijazi S, Meller B, Leitsmann C, Strauss A, Meller J, Ritter CO, et al. Pelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga-PSMA-positron emission tomography/computerized tomography. Prostate. (2015) 75(16):1934–40. doi: 10.1002/pros.23091
56. Linxweiler J, Saar M, Al-Kailani Z, Janssen M, Ezziddin S, Stöckle M, et al. Robotic salvage lymph node dissection for nodal-only recurrences after radical prostatectomy: Perioperative and early oncological outcomes. Surg Oncol (2018) 27(2):138–45. doi: 10.1016/j.suronc.2018.02.010
57. Siriwardana A, Thompson J, van Leeuwen PJ, Doig S, Kalsbeek A, Emmett L, et al. Initial multicentre experience of 68gallium-PSMA PET/CT guided robot-assisted salvage lymphadenectomy: Acceptable safety profile but oncological benefit appears limited. BJU Int (2017) 120(5):673–81. doi: 10.1111/bju.13919
58. Montorsi F, Gandaglia G, Fossati N, Suardi N, Pultrone C, De Groote R, et al. Robot-assisted salvage lymph node dissection for clinically recurrent prostate cancer. Eur Urology. (2017) 72(3):432–8. doi: 10.1016/j.eururo.2016.08.051
59. Fossati N, Suardi N, Gandaglia G, Bravi CA, Soligo M, Karnes RJ, et al. Identifying the optimal candidate for salvage lymph node dissection for nodal recurrence of prostate cancer: Results from a Large, multi-institutional analysis. Eur Urol. (2019) 75(1):176–83. doi: 10.1016/j.eururo.2018.09.009
60. Maurer T, Robu S, Schottelius M, Schwamborn K, Rauscher I, van den Berg NS, et al. (99m)Technetium-based prostate-specific membrane antigen-radioguided surgery in recurrent prostate cancer. Eur Urol. (2019) 75(4):659–66. doi: 10.1016/j.eururo.2018.03.013
61. Bravi CA, Fossati N, Gandaglia G, Suardi N, Mazzone E, Robesti D, et al. Long-term outcomes of salvage lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy: Not as good as previously thought. Eur Urol. (2020) 78(5):661–9. doi: 10.1016/j.eururo.2020.06.043
62. Glicksman RM, Metser U, Vines D, Valliant J, Liu Z, Chung PW, et al. Curative-intent metastasis-directed therapies for molecularly-defined oligorecurrent prostate cancer: A prospective phase II trial testing the oligometastasis hypothesis. Eur Urol. (2021) 80(3):374–82. doi: 10.1016/j.eururo.2021.02.031
63. Li TC, Wang Y, Xiao CT, Li MZ, Liu XP, Huang WT, et al. (68)Ga-PSMA ligand PET/CT integrating indocyanine green-guided salvage lymph node dissection for lymph node metastasis after radical prostatectomy. Asian J Androl. (2022) 24(1):97–101. doi: 10.4103/aja.aja_44_21
64. Treglia G, Annunziata S, Pizzuto DA, Giovanella L, Prior JO, Ceriani L. Detection rate of 18F-labeled PSMA PET/CT in biochemical recurrent prostate cancer: A systematic review and a meta-analysis. Cancers. (2019) 11(5):710. doi: 10.3390/cancers11050710
65. Liu W, Zukotynski K, Emmett L, Chung HT, Chung P, Wolfson R, et al. A prospective study of 18F-DCFPyL PSMA PET/CT restaging in recurrent prostate cancer following primary external beam radiotherapy or brachytherapy. Int J Radiat Oncol Biol Phys (2020) 106(3):546–55. doi: 10.1016/j.ijrobp.2019.11.001
66. Gupta SK, Watson T, Denham J, Shakespeare TP, Rutherford N, McLeod N, et al. Prostate-specific membrane antigen positron emission tomography-computed tomography for prostate cancer: Distribution of disease and implications for radiation therapy planning. Int J Radiat Oncol Biol Phys (2017) 99(3):701–9. doi: 10.1016/j.ijrobp.2017.06.2448
67. Roach M 3rd, Hanks G, Thames H Jr., Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO phoenix consensus conference. Int J Radiat Oncol Biol Phys (2006) 65(4):965–74. doi: 10.1016/j.ijrobp.2006.04.029
68. De Bruycker A, Spiessens A, Dirix P, Koutsouvelis N, Semac I, Liefhooghe N, et al. PEACE V–salvage treatment of oligorecurrent nodal prostate cancer metastases (STORM): A study protocol for a randomized controlled phase II trial. BMC cancer. (2020) 20(1):1–12. doi: 10.1186/s12885-020-06911-4
69. Pollack A, Karrison TG, Balogh AG Jr., Low D, Bruner DW, Wefel JS, et al. Short term androgen deprivation therapy without or with pelvic lymph node treatment added to prostate bed only salvage radiotherapy: The NRG Oncology/RTOG 0534 SPPORT trial. Int J Radiat Oncology Biology Physics. (2018) 102(5):1605. doi: 10.1016/j.ijrobp.2018.08.052
70. Plichta KA, Graves SA, Buatti JM. Prostate-specific membrane antigen (PSMA) theranostics for treatment of oligometastatic prostate cancer. Int J Mol Sci (2021) 22(22):12095. doi: 10.3390/ijms222212095
71. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol (2018) 19(6):825–33. doi: 10.1016/S1470-2045(18)30198-0
72. Hofman MS, Emmett L, Sandhu SK, Iravani A, Joshua AM, Goh JC, et al. TheraP: A randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: Initial results (ANZUP protocol 1603). J Clin Oncol (2020) 38(15_suppl):5500. doi: 10.1200/JCO.2020.38.15_suppl.5500
73. Dhiantravan N, Emmett L, Joshua AM, Pattison DA, Francis RJ, Williams S, et al. UpFrontPSMA: a randomized phase 2 study of sequential 177 Lu-PSMA-617 and docetaxel vs docetaxel in metastatic hormone-naïve prostate cancer (clinical trial protocol). BJU Int (2021) 128(3):331–42. doi: 10.1111/bju.15384
Keywords: prostate cancer, PSMA PET, metastatic disease, oligometastatic, metastastases directed therapy
Citation: Alberto M, Yim A, Papa N, Siva S, Ischia J, Touijer K, Eastham JA, Bolton D and Perera M (2022) Role of PSMA PET-guided metastases-directed therapy in oligometastatic recurrent prostate cancer. Front. Oncol. 12:929444. doi: 10.3389/fonc.2022.929444
Received: 26 April 2022; Accepted: 28 July 2022;
Published: 18 August 2022.
Edited by:
Shafak Aluwini, University Medical Center Groningen, NetherlandsReviewed by:
Gianluca Ingrosso, University of Perugia, ItalyRoman Sosnowski, Maria Sklodowska-Curie National Research Institute of Oncology, Poland
Copyright © 2022 Alberto, Yim, Papa, Siva, Ischia, Touijer, Eastham, Bolton and Perera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marlon Perera, marlonlperera@gmail.com
 Matthew Alberto
Matthew Alberto Arthur Yim
Arthur Yim Nathan Papa2
Nathan Papa2 Shankar Siva
Shankar Siva James A. Eastham
James A. Eastham Damien Bolton
Damien Bolton Marlon Perera
Marlon Perera