- 1Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Radiology, Peking Union Medical College Hospital, Chinese Academy Medical Sciences and Peking Union Medical College, Beijing, China
- 3Department of Gastroenterology, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 4Department of Gastroenterology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 5State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, China
- 6Department of Gastroenterology, Sir Run Run Shaw Hospital, College of Medicine Zhejiang University, Hangzhou, China
- 7Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology and Hepatology, Ministry of Health; Shanghai Inflammatory Bowel Disease Research Center; Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 8Department of Gastroenterology, The Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China
- 9Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 10Department of Gastroenterology, Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 11Department of Epidemiology and Biostatistics, Institute of Basic Medical Sciences, Peking Union Medical College, Chinese Academy of Medical Sciences, School of Basic Medicine, Beijing, China
Background: Differential diagnosis of Crohn’s disease (CD) and ulcerative primary intestinal lymphoma (UPIL) is a tough problem in clinical practice.
Aims: Our study identified key differences between CD and UPIL patients and aimed to further establish a scoring model for differential diagnosis.
Methods: A total of 91 CD and 50 UPIL patients from 9 tertiary inflammatory bowel disease centers were included. Univariate and multivariate analyses were used to determine significant markers for differentiating CD and UPIL. A differential scoring model was established by logistic regression analysis.
Results: The differential model was based on clinical symptoms, endoscopic and imaging features that were assigned different scores: intestinal bleeding (−2 points), extraintestinal manifestation (2 points), segmental lesions (1 point), cobblestone sign (2 points), homogeneous enhancement (−1 point), mild enhancement (−1 point), engorged vasa recta (1 point). A total score of ≥1 point indicates CD, otherwise UPIL was indicated. This model produced an accuracy of 83.66% and an area under the ROC curve of 0.947. The area under the ROC curve for validation using the 10-fold validation method was 0.901.
Conclusion: This study provided a convenient and useful model to differentiate CD from UPIL.
Highlights
What is known:
● CD and UPIL have different therapy and prognosis
● Differential diagnosis of CD and UPIL is difficult
What is new here
● Endoscopic and imaging indicators significantly improved the ability to differentiate between CD and UPIL
● This model in our study is useful to differentiate CD from UPIL.
Introduction
Ileocolonic ulcers appear in many clinical conditions such as Crohn’s disease (CD), intestinal tuberculosis (ITB), and primary intestinal lymphoma (PIL) (1). CD is a chronic inflammatory disease that affects the whole gastrointestinal tract, especially the terminal ileum and ileocecal region. The incidence rate of CD has recently increased in China. PIL is a heterogeneous disease that can be classified as fungating, ulcerative, or other types according to endoscopic morphology (2). A fungating lesion is a warning sign for physicians to keep vigilant watch for potential malignancy, whereas ulcerative lesions are easily ignored. There are many similarities between CD and ulcerative primary intestinal lymphoma (UPIL), and some reports have suggested that UPIL can be easily misdiagnosed as CD (3). Since UPIL often requires intense sequential chemotherapy treatment and carries an unfavorable prognosis (4), and physicians should effectively differentiate CD from UPIL.
The gold diagnostic criteria for CD and UPIL rely on pathology, but it is difficult to obtain typical pathological manifestations through biopsy specimens for both diseases. A recent article reported a potential model for the differential diagnosis between CD and PIL (5). However, since all types of PIL were enrolled in this study, it is not conducive to summarize the characteristics of UPIL. Differential diagnosis for CD and UPIL is still a tough problem in clinical practice.
To enhance the differential diagnostic efficiency and reduce the misdiagnosis rate, we analyzed the key differences in the clinical, endoscopic, and imaging characteristics between CD and UPIL patients. Furthermore, we evaluated the diagnostic value of different markers and established a scoring model for differential diagnosis.
Methods
Patients
A total of 91 CD patients and 50 UPIL patients were enrolled in this study between 1 January 2004, and 30 December 2018. These patients were diagnosed and treated at nine centers, namely, the Peking Union Medical College Hospital, the Sixth Affiliated Hospital of Sun Yat-sen University, the First Affiliated Hospital of Sun Yat-sen University, the Xijing Hospital, the Sir Run Shaw Hospital, the Shanghai Renji Hospital, the Shanghai Tenth People’s Hospital, the Affiliated Hospital of Anhui Medical University, and the Wuhan Union Hospital. Clinical, endoscopic, and imaging data were collected from all patients. The Institutional Review Board of Peking Union Medical College Hospital (S-K1100) approved the study.
Inclusion and Exclusion Criteria
The inclusion criteria are as follows: (1) clinically or pathologically confirmed CD or pathologically confirmed PIL; and (2) clinical, endoscopic, and radiographic data were available for the majority of the patients. Endoscopic and imaging data include the original images and reports. For each item, the proportion of missing cases was lower than 20%.
The exclusion criteria are as follows: (1) PIL with fungating or other non-ulcerative lesions; and (2) PIL with post-surgery or treatment data (patients with data before the surgery or treatment could be included).
Diagnostic Criteria
All patients were diagnosed with CD according to the European Crohn’s and Colitis Organization (ECCO) guidelines and Chinese consensus based on clinical manifestations, endoscopic features, and imaging or pathological features (6, 7).
All patients were diagnosed with PIL by histological results according to Dawson’s criteria (8).
Data Collection
Demographic and Clinical Data
Demographic and clinical data included the sex, age at the onset of gastrointestinal symptoms, clinical manifestations, intestinal complications, extraintestinal manifestations (EIMs) (oral and vulvar ulcers, skin lesions, joint lesions, ocular lesions, fatty liver, cholelithiasis, thromboembolic disease, and myelodysplastic syndromes), past, and personal history of the patient (shown in Tables 1, 2). Skin lesions are mainly referred to as nodular erythema, pyoderma gangrenosum, pseudofolliculitis, papules, and acne-like nodules, and are diagnosed by dermatologists. Ocular lesions mainly include uveitis, iritis, scleritis, and retinal vasculitis, and are diagnosed by ophthalmologists.
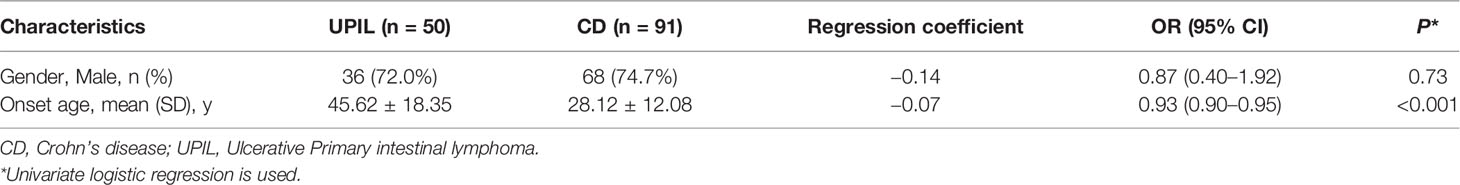
Table 1 Demographic Characteristics of participants with Crohn’s disease or Ulcerative Primary Intestinal Lymphoma.

Table 2 Clinical Characteristics of Participants with Crohn’s disease or Ulcerative Primary Intestinal Lymphoma.
Endoscopic Data
Endoscopic indicators included lesion locations, segmental lesions, ulcer morphology (shallow, deep, longitudinal, irregular, annular, oval, and aphthous ulcers), number of ulcers (1, 2–5, and >5), ulcer diameter (<5 mm, 5–20 mm, and >20 mm), inflammatory polyps, mucosal bridges, and cobblestone signs (Figure 1). The definitions of the above variables were taken from the articles published by Li and He (9, 10).
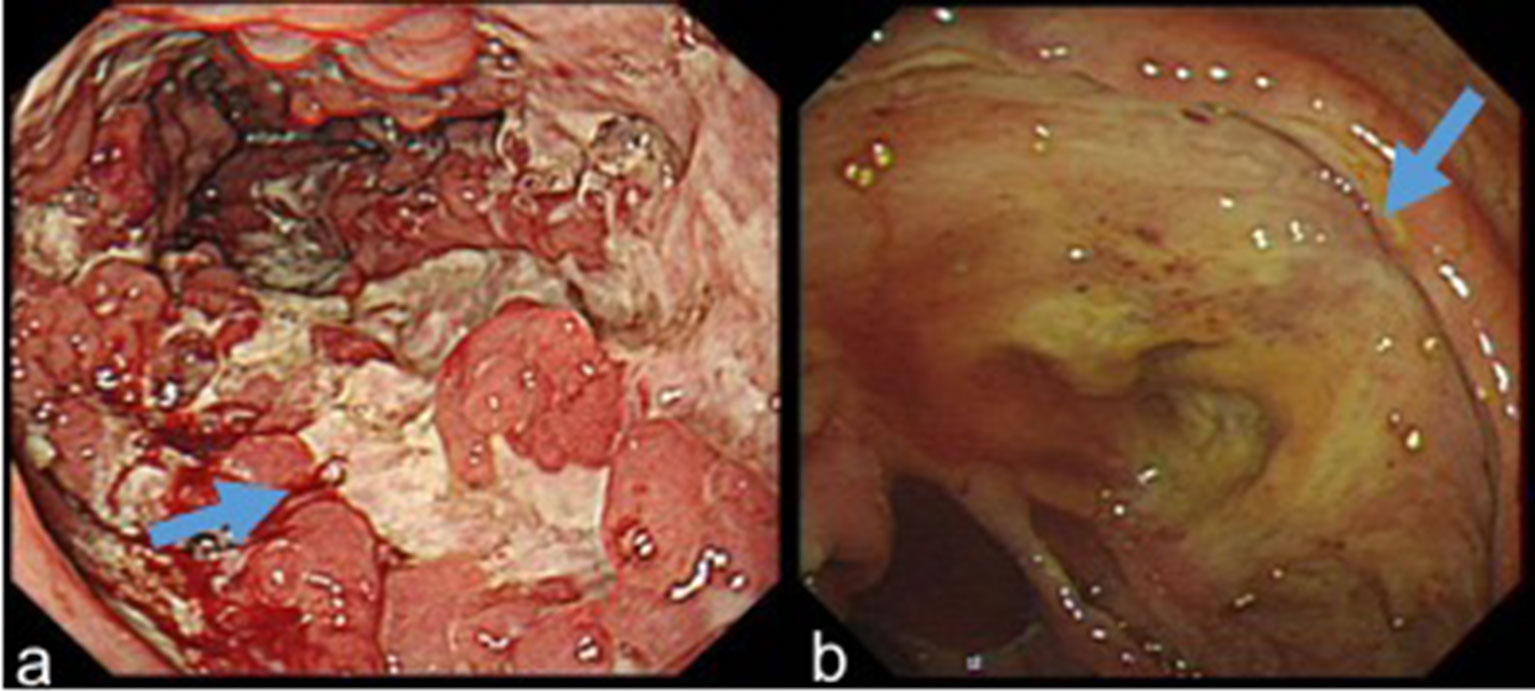
Figure 1 (A) Longitudinal ulcers, inflammatory polyps and cobblestone sign in a patient with CD. (B) A large and deep ulcer in a patient with UPIL.
Two experienced endoscopic physicians independently read all the endoscopic data. If their opinions were inconsistent, the final diagnosis was made after discussion.
Imaging Data
Imaging items included the length of the longest lesion segment, the thickness of the lesion, enhancement degree, homogeneous hyperenhancement, asymmetric mural hyperenhancement, polypoid lesion of the mucosal surface, fibrofatty proliferation, and engorged vasa recta (Figure 2). The definition of the above variables refers to the article published by Guglielmo (11).
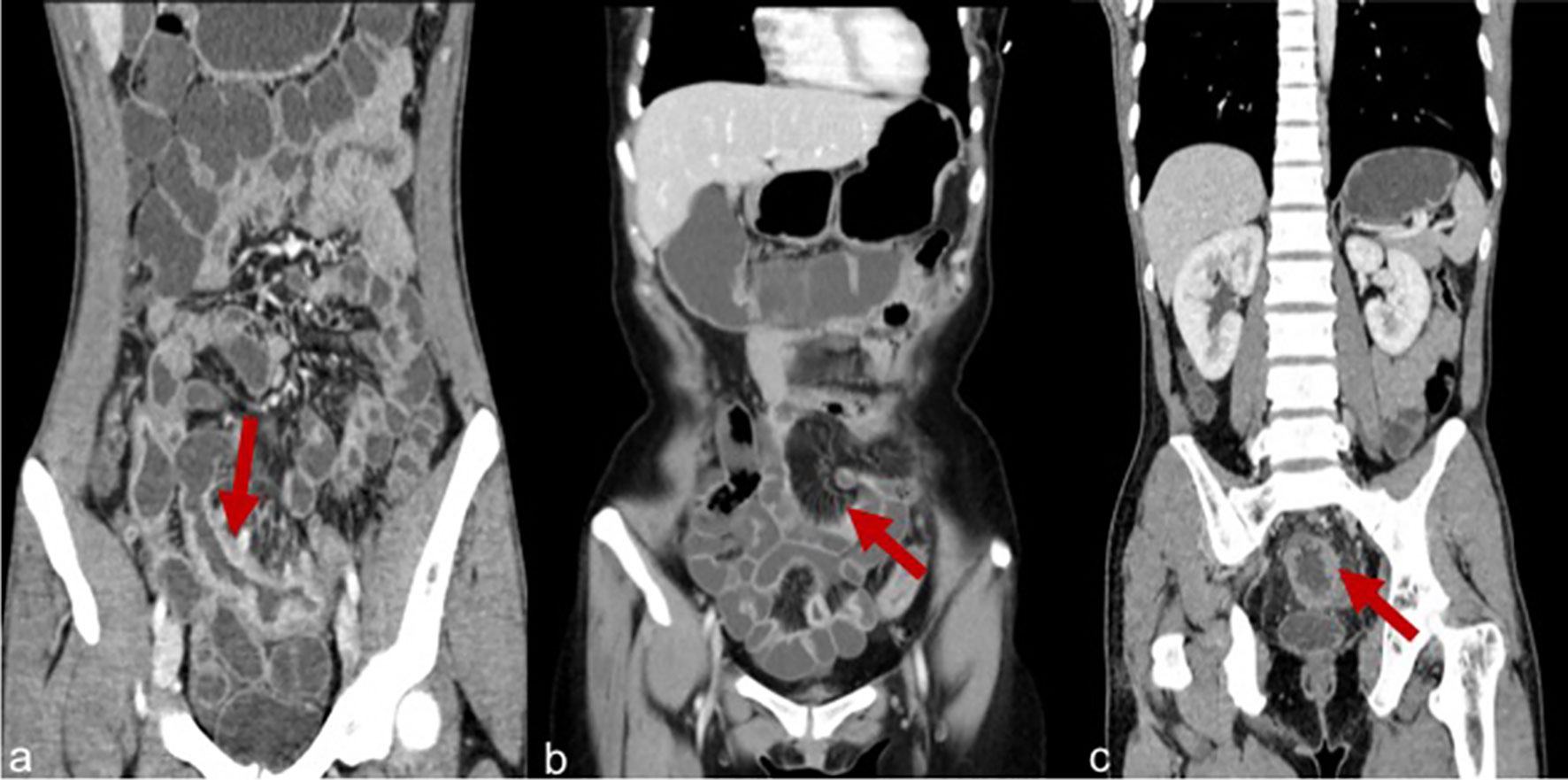
Figure 2 (A) Severe enhancement in a patient with CD. (B) Engorged vasa recta in a patient with CD. (C) Homogeneous enhancement in a patient with CD.
Two radiologists independently reviewed all the imaging data. When their opinions were inconsistent, the final diagnosis was made after discussion.
Statistical Analysis
Continuous variables following an apparently normal distribution were summarized by the mean [standard deviation (SD)]; otherwise, they were summarized by the median and interquartile range. Categorical variables were presented as proportions. Univariate analysis was conducted using logistic regression with one independent variable. Receiver operating characteristic (ROC) curve analysis was used to determine the threshold value for continuous variables presenting a linear assumption, otherwise Lowess smoothing function was used. A multivariate logistic regression model was built after univariate analysis, and a further variable selection procedure was conducted, and a final multivariate model was developed by incorporating variables with statistical significance (P-value <0.05) variables. Variables with marginal statistical significance (P-value slightly >0.05) were also included in the final multivariate model due to their clinical significance.
A scoring system was built based on the final multivariate model. Taking the variable with the minimum regression coefficient as 1 point, the scores of other variables were obtained by dividing their regression coefficients with the minimum regression coefficient and rounding to the nearest integer. The Youden Index was used to determine the cutoff value of the scoring model. The total risk score of each patient was the sum of all the scores of predictors assigned to him or her.
Evaluating the predictive performance (AUC) of the fitted model using all cases from the original analysis sample tends to result in an overly optimistic estimate of the performance. Therefore, a 10-fold cross-validation procedure was employed to calculate a more realistic estimate of predictive performance (12). To calculate the 10-fold cross-validation AUC, the original data set was randomly divided into ten parts, and the first tenth of the data was held out as a validation set, with a logistic model being fitted using the remaining observations. Then, the predicted probability for each observation in the validation set was calculated using the training model. This procedure was repeated 10 times, and an AUC score was calculated for each of the 10 runs, and then the average AUC was calculated.
All the data were analyzed by SAS9.2 (SAS Institute, Inc, Cary, NC).
Results
Demographic Characteristics
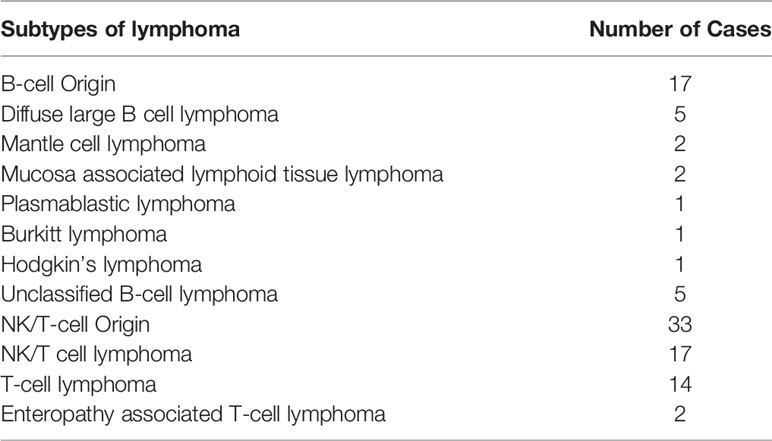
Among the 50 UPIL patients, 17 lymphomas (34%) were of B-cell origin, and the rest of the cases (33 cases, 66%) were of NK-cell and T-cell origins. The details are shown in Table 3.
As shown in Table 1, there was no significant difference in gender between the CD and UPIL patients. The age of onset of gastrointestinal symptoms in UPIL was significantly higher than that in CD patients (P <0.001).
Univariate Analysis to Compare the Clinical Characteristics Between CD and UPIL
Comparative Analysis of Clinical Symptoms
The clinical data (Table 2) showed that the incidence rates of intestinal stenosis and EIMs were significantly higher in patients with CD than in those with UPIL (all P <0.05). In contrast, the incidence rates of intestinal perforation and bleeding were significantly lower in CD patients compared to UPIL patients (both P <0.05).
Comparative Analysis of Endoscopic Characteristics
The endoscopic characteristics (Table 4) showed that for lesion location(s), the proportion of the ileocecal and ascending colon involvement in patients with CD was significantly higher than in patients with UPIL (both P <0.05). Regarding the distribution of lesions, patients with CD were also more likely to have segmental lesions (P <0.001). In terms of ulcer morphology, the proportions of patients with CD who had shallow and longitudinal ulcers were higher than those of patients with UPIL (both P <0.05). Furthermore, patients with UPIL are more likely to have a single and large ulcer with a diameter exceeding 20 mm compared to CD (P <0.05). Additionally, the proportions of inflammatory polyps, mucosal bridges, and cobblestone signs in patients with CD were higher (P <0.05).
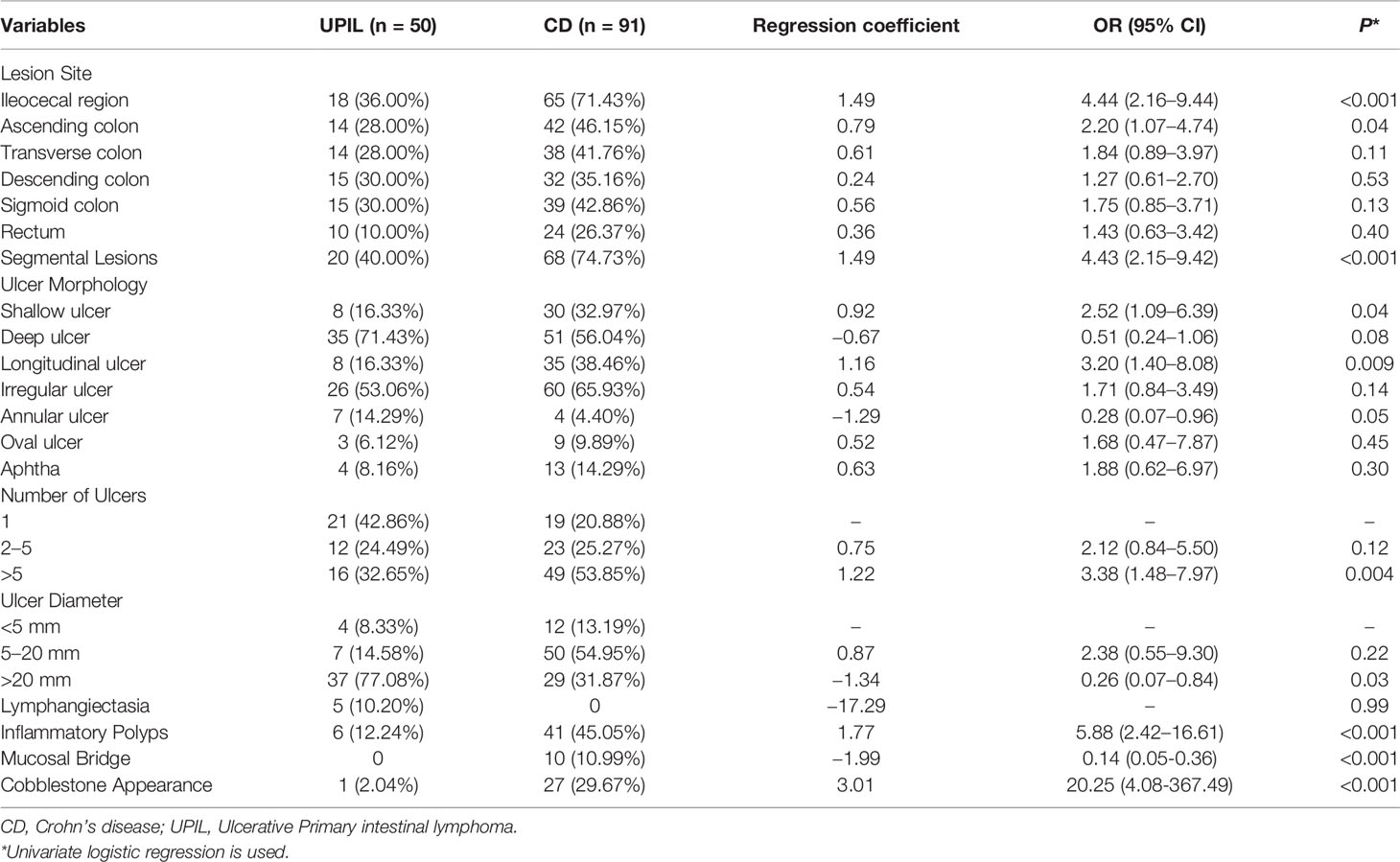
Table 4 Endoscopic Characteristics of Participants with Crohn’s disease or Ulcerative Primary Intestinal Lymphoma.
Comparative Analysis of Imaging Characteristics
The imaging data (Table 5) showed that patients with CD had significantly longer but thinner lesions than UPIL patients (P <0.05). In terms of lesion enhancement characteristics, most patients with UPIL displayed mild enhancement, while most CD patients displayed moderate to severe enhancement. CD patients are more prone to asymmetric mural hyperenhancement, while UPIL patients are more likely to have homogeneous enhancement. Additionally, compared to patients with UPIL, patients with CD were more prone to mucosal polypoid bulges, fibrofatty proliferation, and engorged vasa recta (all P <0.05).
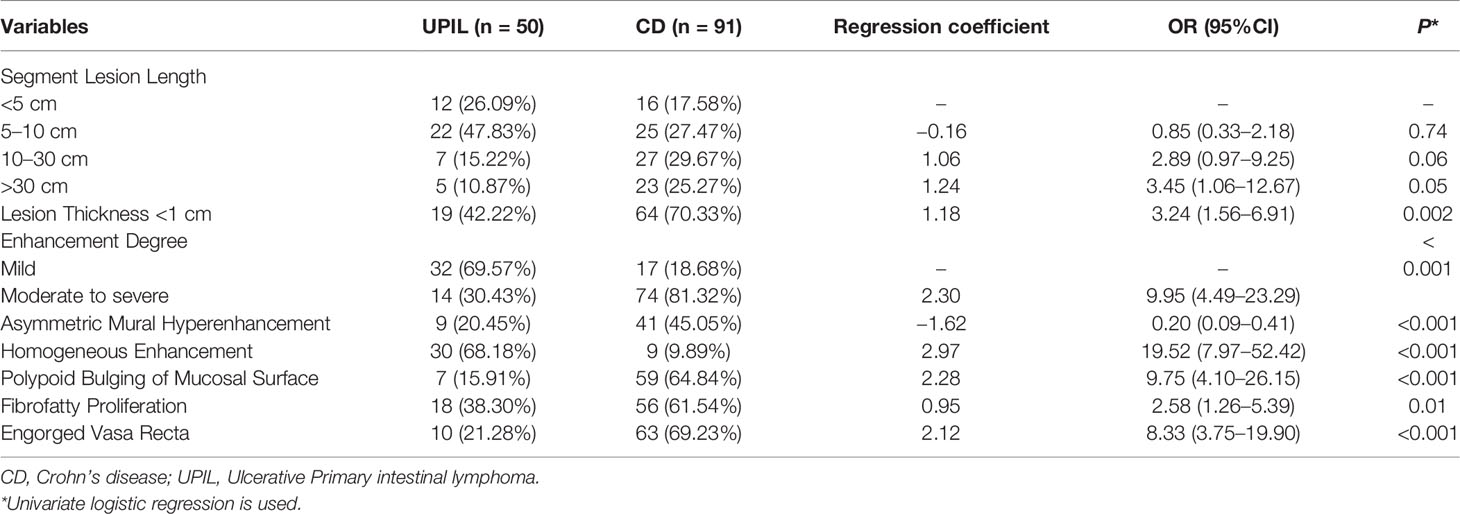
Table 5 Imaging Characteristics of Participants with Crohn’s disease or Ulcerative Primary Intestinal Lymphoma.
The Diagnostic Value of Different Indicators in CD and UPIL
To comparatively analyze the diagnostic value of clinical symptoms and various endoscopic and imaging indicators in CD and UPIL, we performed multivariate and ROC curve analyses of the clinical symptoms individually, clinical symptoms combined with endoscopic indicators, and clinical symptoms combined with endoscopic and imaging indicators to select the model with the best differential diagnostic power.
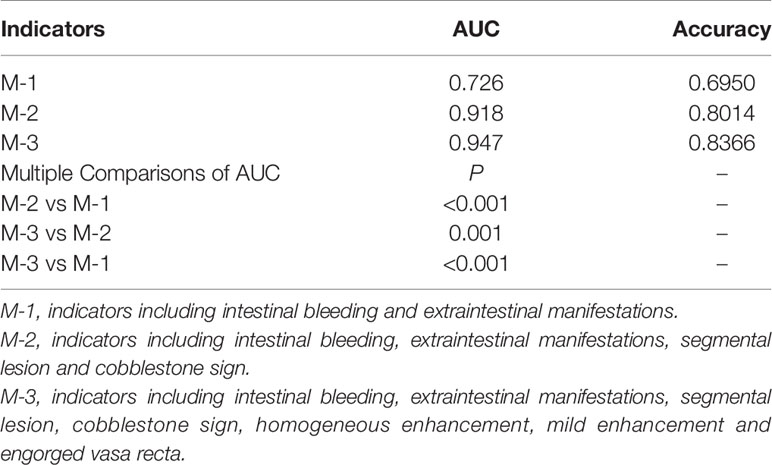
First, we included clinical symptom indicators that produced P <0.05 with the univariate analysis in the multivariate logistic regression analysis. We found that the indicators with statistical significance were intestinal bleeding and EIMs. The AUC for differentiating CD and UPIL based on these two indicators was 0.726. We then performed a multivariate logistic regression analysis of clinical and endoscopic indicators that produced a P <0.05 in the univariate analysis. These indicators included intestinal bleeding, EIMs, segmental lesions, and cobblestone signs. Based on these four indicators, the AUC for differentiating CD and UPIL based on these four indicators was 0.918. Finally, we performed a multivariate logistic regression analysis of clinical, endoscopic, and imaging indicators with a P <0.05 in the univariate analysis, which included intestinal bleeding, EIMs, segmental lesions, cobblestone signs, homogeneous enhancement, mild enhancement, and engorged vasa recta (Table 6). Based on these seven indicators, the AUC for differentiating CD and UPIL was 0.947 (Figure 3). Statistical analysis showed that the last AUC was significantly higher than the other two AUCs (P <0.05) (Table 7).
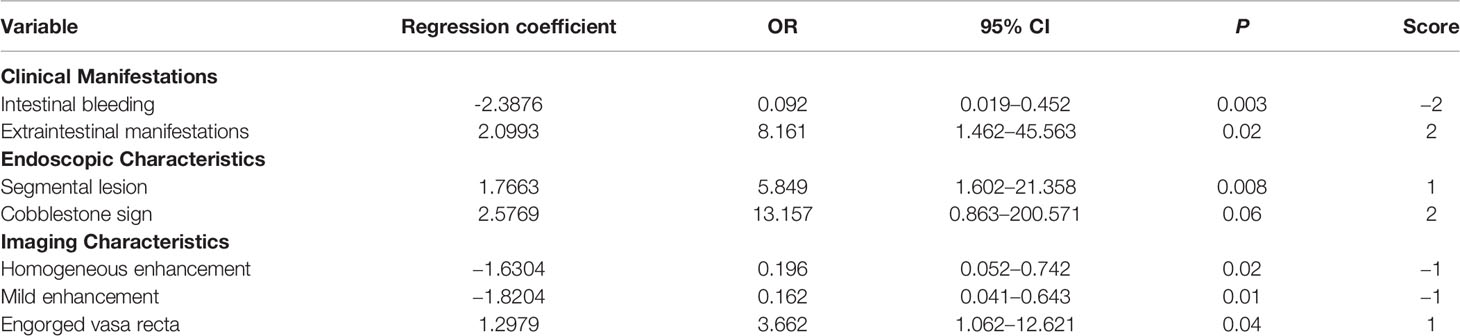
Table 6 Multivariate Analysis and Scores Based on Clinical Manifestations, Endoscopic, and Imaging Characteristics.
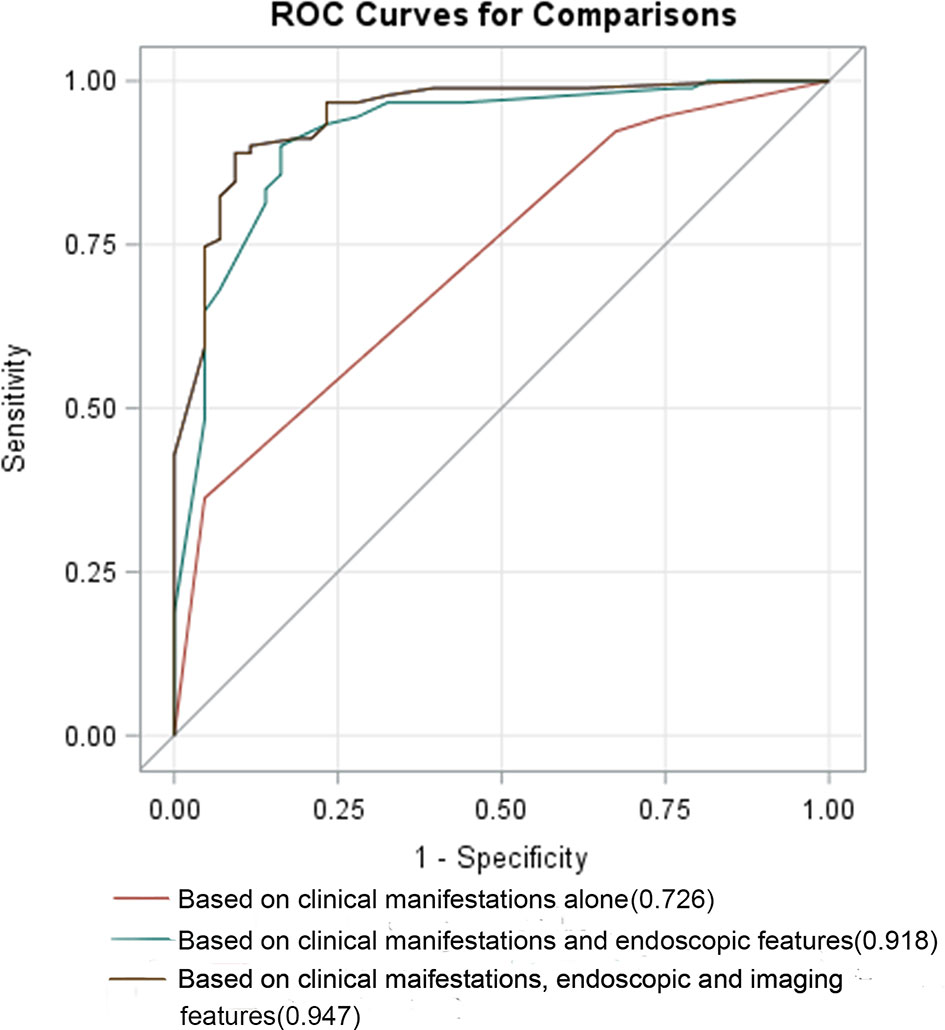
Figure 3 ROC curves based on different variables. The AUC for differentiating CD and UPIL based on clinical manifestations alone, combined clinical manifestations and endoscopic features, combined clinical manifestations, endoscopic, and imaging features was 0.726, 0.918, and 0.947, respectively.
Therefore, including endoscopic and imaging indicators significantly improved the ability to differentiate between CD and UPIL.
Establishment of a Differential Diagnosis Scoring Model for CD and UPIL
The differential diagnostic scoring model for CD and UPIL was ultimately established based on the logistic regression model of clinical symptoms combined with endoscopic and imaging features. According to the previous multivariate analysis, we got the scores of each variable using the method shown in the statistical analysis subsection (Table 6). A patient was diagnosed with CD if the total score was ≥1; otherwise, he or she was diagnosed with UPIL. The accuracy of the differential diagnosis using this model was as high as 83.66%. The calibration plot also demonstrated the good performance of this score model (Figure 4).
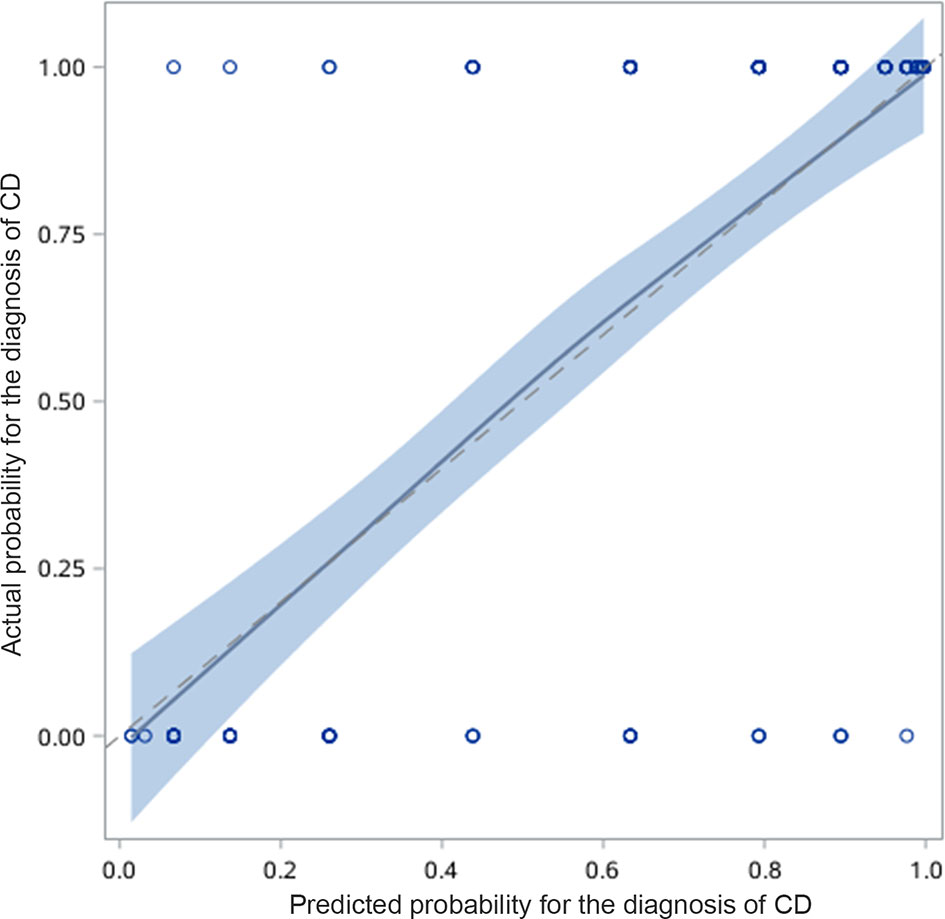
Figure 4 Calibration curve for predicting the possibility of CD. The calibration plot also demonstrated good performance of this score model.
Validation of the Differential Diagnosis Scoring Model
The above results were validated using a 10-fold validation method. The AUC for 10-fold validation was 0.901 (Figure 5), suggesting that this model can robustly differentiate between CD and UPIL.
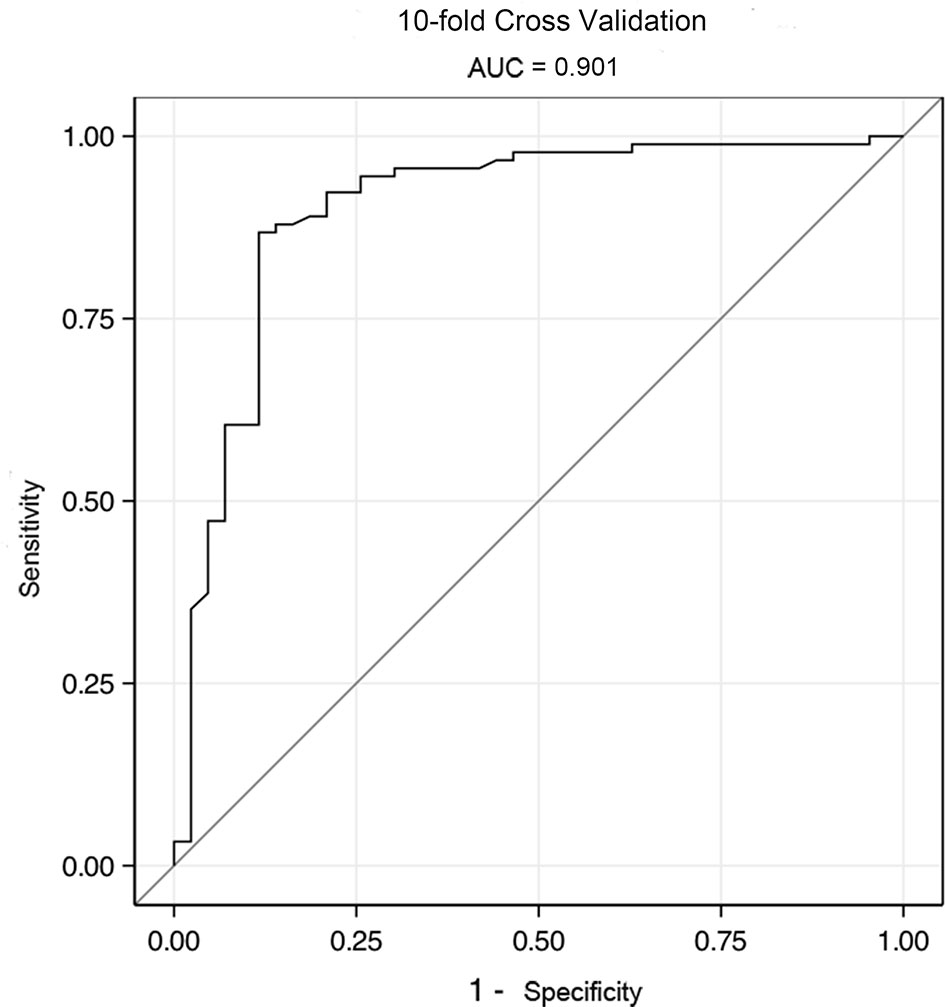
Figure 5 The ROC of validation model by 10-fold cross validation. The AUC for 10-fold validation was 0.901.
Discussion
Approximately 30 to 50% of extranodal lymphomas primarily involve the gastrointestinal tract, two-thirds of which are classified as ulcerative lesions (13). Pathology is the gold standard for the diagnosis of CD and UPIL. However, it is very difficult to obtain typical pathological manifestations and make a diagnosis through biopsy specimens. Granulomas are found only in 15–65% of the mucosal biopsy from patients with CD, while only 21% of intestinal non-Hodgkin’s lymphomas can be diagnosed by endoscopic biopsy (14, 15). Due to the lack of standardized diagnostic criteria for endoscopy features for CD and UPIL and the inherent limitations of endoscopic biopsies, 76.47% PIL patients were misdiagnosed as other diseases (16). Therefore, it is important for clinicians to be able to actively differentiate CD from UPIL.
In this study, we established a differential model using multivariate logistic regression that could robustly differentiate between CD and UPIL. This scoring model identified the most meaningful variables based on clinical symptoms and imaging and endoscopic characteristics. A total score ≥1 point indicates a diagnosis of CD, and less than 1 point indicates UPIL. Our predictive model produced an accuracy of 83.66% and an area under the ROC curve of 0.947, which will be conductive and helpful in clinical practice for differentiating between CD and UPIL.
In this study, demographic and clinical characteristics were compared between CD and UPIL patients. From univariate analyses, the results indicated that onset age, perianal lesions, intestinal perforation, intestinal bleeding, and EIMs appeared significantly different between CD and UPIL. It was significant that UPIL patients had an older age at onset, which was consistent with the results of the large-sample epidemiological studies of lymphoma (17). EIMs and perianal lesions were regarded as critical indicators of CD patients (18). Additionally, UPIL and CD can cause additional intestinal complications. However, more patients with CD than UPIL display intestinal stenosis, and more patients with UPIL than CD display intestinal perforation and hemorrhage. Sun et al. reported that 61.76% suffered from intestinal perforation and 2.94% from massive hematochezia in patients with intestinal T-cell lymphoma (16). The incidence of perforation is higher in UPIL. This may be due to the adherence of lymphoma cells to the vascular wall, which causes vascular occlusion, ischemic necrosis, and finally perforation.
Although endoscopic evaluation plays a key role in the diagnosis of CD and UPIL, there was no consensus on typical endoscopic features for UPIL; some cases depicted a diffuse or irregular ulcer that indicated UPIL, and others did not exhibit any specific characteristic (2, 13, 19). In univariate analyses, we found some different endoscopic features between these two groups, such as ulcer type, size quantity, pseudo-polyps, cobblestone appearance, and so on. Endoscopic features of these ulcers represent the different biological behaviors and histopathology of the two diseases. In multivariate analysis, the incidence rates of segmental distribution and cobblestone appearance were significantly different between CD and UPIL.
In terms of imaging, there are few comparative studies on CD and UPIL. Many studies show that the enhancement of gastrointestinal lymphoma is homogeneous, which mean it is equal to or lower in attenuation than the normal tissues (20). Necrosis of neoplasms may account for the low attenuation (21). For CD patients, Bodily et al. showed that mucosal surface hyperenhancement is highly correlated with histopathological activity (22). Moreover, mural stratification and engorged vasa recta are common in CD patients (23). An engorged vasa recta is also a specific manifestation of CTE in patients with active CD. However, this mechanism was not found in UPIL. The preservation of wall stratification is also a helpful CT criterion for differentiating benign from malignant diseases (24). Our multivariate analysis also showed that CD patients were more likely to have engorged vasa recta, while homogeneous and low attenuation were more common in UPIL patients. This result is similar to other studies (5, 25). As far as we are aware, this is the first study to compare imaging features of CD and UPIL, and we think this result is particularly interesting and useful as it provides a new method for differentiating CD and UPIL using imaging features.
To comparatively analyze the diagnostic value of clinical symptoms, endoscopic and imaging features in CD and UPIL, ROC curve analyses based on different multivariate analyses were performed. The AUCs for differentiating CD and UPIL were 0.726 based on clinical manifestations, 0.918 based on combined clinical manifestations combined with endoscopic features, and 0.947 based on the clinical manifestations combined with endoscopic and imaging features. Statistical analysis showed that the last AUC was significantly higher than the other two AUCs (P <0.05) (26). The results showed that endoscopic and imaging characteristics can improve the ability of clinical symptoms to differentiate between CD and UPIL.
Lastly, we scored the results of the multivariate analysis based on clinical symptoms, and endoscopic and imaging (CTE) data according to the coefficients of logistic regression analysis and established a diagnostic scoring model for CD and UPIL. The results suggest that the combination of clinical symptoms, CTE, and endoscopic features can robustly differentiate CD and UPIL. The AUC obtained from 10-fold cross-validation of this model was 0.901, indicating that it has strong predictive power for distinguishing CD and UPIL. It is worth noting that the indicator “cobblestone appearance” did not attain statistical significance in the scoring system. But the cobblestone sign is deemed the “colonoscopic marker” of CD, and it is visualized by multiple deep ulcers with elevated edematous mucosa interconnecting the ulcers. According to the published references, the cobblestone sign may not be very specific for CD, and it may also occur in other diseases like infection, but it is rare in UPIL. We therefore think the cobblestone sign is of great value in excluding UPIL. Furthermore, a label of statistical significance (P <0.05) does not mean or imply that an association or effect is highly probable, and many statisticians think that we should re-examine the meaning of P-value and move forward to a world beyond “P <0.05” (27). So we included this variable in the scoring system.
This is the first study to provide a combined clinical, endoscopic, and imaging-based model for differentiating UPIL from CD. However, the first notable limitation of this study is that there were various pathological types of lymphoma included, which could have affected the diagnostic model efficiency and the ultimate prediction accuracy. Secondly, 10-fold cross validation belongs to internal validation, and the objectivity is indeed inferior to external validation, which needs a larger sample size. We have tried our best to collect enough patients for external validation in this multicenter study. Unfortunately, UPIL is uncommon in clinical practice, and we did not collect enough patients to perform external validation. Because of the limited sample size, a ten-fold validation was performed, which verified the robustness of the model at present and had a lower variance than a single hold-out set estimator. However, it is still essential to further collect data for external verification, which is our future work plan; Thirdly, some markers like lactate dehydrogenase that are sensitive to the diagnosis of UPIL were excluded from this study. Fourthly, patients in the CD group suffered from a heavy level of activity restriction (moderate to severe), which may not represent the full spectrum of CD patients. Fifthly, this study was a retrospective with a small sample size, and future studies with larger populations must validate these results in full.
In conclusion, our results suggest that the combination of endoscopy, imaging features, and clinical symptoms is of great value for differentiating of CD and UPIL. The inclusion of more different subtypes of UPIL in the future will help establish a more accurate and meaningful differential model.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HY and JQ: Study design, data collection and analysis support, and critical revision of manuscript. HZ: Data collection and analysis and drafting the manuscript. WL, BT, TG, XG, RF, KW, QC, ZR, ZL, NH, LZ, YL, and CW: Data collection. WH Data analysis. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the National Nature Science Foundation of China [81570505 and 81970495], the Beijing Municipal Natural Science Foundation [7202161], the Health Research & Special Projects Grant of China [201502005], and the CAMS Innovation Fund for Medical Sciences (CIFMS) [2016-I2M-3-001 and 2019-I2M-2-007].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the following for their great help and support to this work: all participating units and participating doctors, Mingli Su from The Six Affiliated Hospital of Sun Yat-sen University, Yao He from The First Affiliated Hospital of Sun Yat-sen University, Jie Liang from Xijing Hospital Affiliated to the Fourth Military Medical University, Juntao Lu from Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Leilei Fang from The Tenth People’s Hospital Affiliated to Tongji University, and Qiuyuan Liu from the First Affiliated Hospital of Anhui Medical University.
Abbreviations
CD, Crohn’s disease; UPIL, ulcerative primary intestinal lymphoma; ECCO, European Crohn’s and Colitis Organization; EIM, extraintestinal manifestations; SD, standard deviation; ROC, Receiver operating characteristic.
References
1. Lee JM, Lee KM. Endoscopic Diagnosis and Differentiation of Inflammatory Bowel Disease. Clin Endosc (2016) 49(4):370–5. doi: 10.5946/ce.2016.090
2. Myung SJ, Joo KR, Yang SK, Jung HY, Chang HS, Lee HJ, et al. Clinicopathologic Features of Ileocolonic Malignant Lymphoma: Analysis According to Colonoscopic Classification. Gastrointest Endosc (2003) 57(3):343–7. doi: 10.1067/mge.2003.135
3. Wu PH, Chu KE, Lin YM, Huang SH, Wu CC. T-Cell Lymphomas Presenting as Colon Ulcers and Eosinophilia. Case Rep Gastroenterol (2015) 9(2):246–52. doi: 10.1159/000437294
4. Lightner AL, Shannon E, Gibbons MM, Russell MM. Primary Gastrointestinal Non-Hodgkin’s Lymphoma of the Small and Large Intestines: A Systematic Review. J Gastrointest Surg (2016) 20(4):827–39. doi: 10.1007/s11605-015-3052-4
5. Zhang TY, Lin Y, Fan R, Hu SR, Cheng MM, Zhang MC, et al. Potential Model for Differential Diagnosis Between Crohn’s Disease and Primary Intestinal Lymphoma. World J Gastroenterol (2016) 22(42):9411–18. doi: 10.3748/wjg.v22.i42.9411
6. Gomollon F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al. 3rd European Evidence-Based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis (2017) 11(1):3–25. doi: 10.1093/ecco-jcc/jjw168
7. Inflammatory Bowel Disease Group, Chinese Society Of Gastroenterology. Consensus on Diagnosis and Treatment of Inflammatory Bowel Disease (2018, Beijing). Chin J Dig (2018) 5:292–311.
8. Dawson IM, Cornes JS, Morson BC. Primary Malignant Lymphoid Tumours of the Intestinal Tract. Report of 37 Cases With a Study of Factors Influencing Prognosis. Br J Surg (1961) 49:80–9. doi: 10.1002/bjs.18004921319
9. Li J, Li P, Bai J, Lyu H, Li Y, Yang H, et al. Discriminating Potential of Extraintestinal Systemic Manifestations and Colonoscopic Features in Chinese Patients With Intestinal Behcet’s Disease and Crohn’s Disease. Chin Med J (Engl) (2015) 128(2):233–8. doi: 10.4103/0366-6999.149213
10. He Y, Zhu Z, Chen Y, Chen F, Wang Y, Ouyang C, et al. Development and Validation of a Novel Diagnostic Nomogram to Differentiate Between Intestinal Tuberculosis and Crohn’s Disease: A 6-Year Prospective Multicenter Study. Am J Gastroenterol (2019) 114(3):490–9. doi: 10.14309/ajg.0000000000000064
11. Guglielmo FF, Anupindi SA, Fletcher JG, Al-Hawary MM, Dillman JR, Grand DJ, et al. Small Bowel Crohn Disease at CT and MR Enterography: Imaging Atlas and Glossary of Terms. Radiographics (2020) 40(2):354–75. doi: 10.1148/rg.2020190091
12. Cherlin S, Plant D, Taylor JC, Colombo M, Cordell HJ. Prediction of Treatment Response in Rheumatoid Arthritis Patients Using Genome-Wide SNP Data. Genet Epidemiol (2018) 42(8):754–71. doi: 10.1002/gepi.22159
13. Ding D, Pei W, Chen W, Zuo Y, Ren S. Analysis of Clinical Characteristics, Diagnosis, Treatment and Prognosis of 46 Patients With Primary Gastrointestinal Non-Hodgkin Lymphoma. Mol Clin Oncol (2014) 2(2):259–64. doi: 10.3892/mco.2013.224
14. Makharia GK, Srivastava S, Das P, Goswami P, Singh U, Tripathi M, et al. Clinical, Endoscopic, and Histological Differentiations Between Crohn’s Disease and Intestinal Tuberculosis. Am J Gastroenterol (2010) 105(3):642−51. doi: 10.1038/ajg.2009.585
15. Daum S, Ullrich R, Heise W, Dederke B, Foss HD, Stein H, et al. Intestinal Non-Hodgkin’s Lymphoma: A Multicenter Prospective Clinical Study From the German Study Group on Intestinal Non-Hodgkin’s Lymphoma. J Clin Oncol (2003) 21(14):2740–46. doi: 10.1200/JCO.2003.06.026
16. Sun ZH, Zhou HM, Song GX, Zhou ZX, Bai L. Intestinal T-Cell Lymphomas: A Retrospective Analysis of 68 Cases in China. World J Gastroenterol (2014) 20(1):296–302. doi: 10.3748/wjg.v20.i1.296
17. Ding W, Zhao S, Wang J, Yang Q, Sun H, Yan J, et al. Gastrointestinal Lymphoma in Southwest China: Subtype Distribution of 1,010 Cases Using the WHO (2008) Classification in a Single Institution. Acta Haematol (2016) 135(1):21–8. doi: 10.1159/000437130
18. Feuerstein JD, Cheifetz AS. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin Proc (2017) 92(7):1088–103. doi: 10.1016/j.mayocp.2017.04.010
19. Liu YY, Chen MK, Cao Z, Liu SZ, Ding BJ. Differential Diagnosis of Intestinal Tuberculosis From Crohn’s Disease and Primary Intestinal Lymphoma in China. Saudi J Gastroenterol (2014) 20(4):241–7. doi: 10.4103/1319-3767.136979
20. Choi D, Lim HK, Lee SJ, Lim JH, Kim SH, Lee WJ, et al. Gastric Mucosa-Associated Lymphoid Tissue Lymphoma: Helical CT Findings and Pathologic Correlation. AJR Am J Roentgenol (2002) 178(5):1117–22. doi: 10.2214/ajr.178.5.1781117
21. Lewis RB, Mehrotra AK, Rodriguez P, Manning MA, Levine MS. From the Radiologic Pathology Archives: Gastrointestinal Lymphoma: Radiologic and Pathologic Findings. Radiographics (2014) 34(7):1934–53. doi: 10.1148/rg.347140148
22. Bodily KD, Fletcher JG, Solem CA, Johnson CD, Fidler JL, Barlow JM, et al. Crohn Disease: Mural Attenuation and Thickness at Contrast-Enhanced CT Enterography–Correlation With Endoscopic and Histologic Findings of Inflammation. Radiology (2006) 238(2):505–16. doi: 10.1148/radiol.2382041159
23. Meyers MA, McGuire PV. Spiral CT Demonstration of Hypervascularity in Crohn Disease: "Vascular Jejunization of the Ileum" or the "Comb Sign". Abdom Imaging (1995) 20(4):327–32. doi: 10.1007/bf00203365
24. Chen CY, Jaw TS, Wu DC, Kuo YT, Lee CH, Huang WT. Am J Roentgenol (2010) 195(5):1124–30. doi: 10.2214/ajr.09.3129
25. Kim HJ, Ha HK, Kim HJ, Byeon JS, Kim MJ, Lee SS, et al. Gastrointestinal Dissemination of Mucosa-Associated Lymphoid Tissue Lymphoma: Computed Tomographic Findings. J Comput Assist Tomogr (2010) 34(2):187–92. doi: 10.1097/RCT.0b013e3181bbd21e
26. Hanley JA, McNeil BJ. A Method of Comparing the Areas Under Receiver Operating Characteristic Curves Derived From the Same Cases. Radiology (1983) 148(3):839–43. doi: 10.1148/radiology.148.3.6878708
Keywords: Crohn’s disease, ulcerative primary intestinal lymphoma, diagnosis, imaging, scoring model
Citation: Yang H, Zhang H, Liu W, Tan B, Guo T, Gao X, Feng R, Wu K, Cao Q, Ran Z, Liu Z, Hu N, Zhu L, Lai Y, Wang C, Han W and Qian J (2022) Differential Diagnosis of Crohn’s Disease and Ulcerative Primary Intestinal Lymphoma: A Scoring Model Based on a Multicenter Study. Front. Oncol. 12:856345. doi: 10.3389/fonc.2022.856345
Received: 17 January 2022; Accepted: 28 March 2022;
Published: 02 May 2022.
Edited by:
Alessandro Isidori, AORMN Hospital, ItalyReviewed by:
Zimu Gong, Houston Methodist Hospital, United StatesNguyen Minh Duc, Pham Ngoc Thach University of Medicine, Vietnam
Copyright © 2022 Yang, Zhang, Liu, Tan, Guo, Gao, Feng, Wu, Cao, Ran, Liu, Hu, Zhu, Lai, Wang, Han and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaming Qian, qianjiaming1957@126.com; Wei Han, weihancool@aliyun.com
†These authors have contributed equally to this work
 Hong Yang
Hong Yang Huimin Zhang1†
Huimin Zhang1† Bei Tan
Bei Tan Zhanju Liu
Zhanju Liu Wei Han
Wei Han Jiaming Qian
Jiaming Qian