- 1Surgical Oncology Department, National Institute of Oncology, University Mohammed V in Rabat, Rabat, Morocco
- 2Medical Oncology Department, National Institute of Oncology, University Mohammed V in Rabat, Rabat, Morocco
- 3Département d’oncologie médicale Centre Hospitalier Lyon-Sud, Hospices Civils de Lyon, Lyon, France
- 4Département de Chirurgie Digestive et Endocrinienne, Centre Hospitalier Lyon-Sud, Hospices Civils de Lyon, Lyon, France
Background: The utility of heated intraperitoneal chemotherapy (HIPEC) in the management of epithelial ovarian cancer (EOC) has been assessed in several randomised clinical trials and meta-analyses, and it is still a subject of controversy. Therefore, we performed an umbrella review of existing meta-analyses to summarise the outcomes of HIPEC and cytoreductive surgery (CRS) association in ovarian cancer.
Methods: We examined the MEDLINE, Cochrane Library, Scopus, Prospero, Web of Science and Science Direct from inception to May 30, 2020, for meta-analyses of randomised controlled trials and observational studies. Analyses of overall survival, disease free survival and progression survival were performed separately for primary and recurrent ovarian cancers.
Results: We identified 6 meta-analyses investigating the association of HIPEC with CRS in the management of ovarian cancer. Three year overall survival was significantly improved by the association of CRS and HIPEC for primary (HR: 0.66, 95%CI:0.56-0.78) and recurrent ovarian cancers (HR:0.50, 95%CI:0.38-0.64). This benefit was also demonstrated on disease-free survival for primary (HR: 0.54, 95%CI:0.48-0.61) and recurrent ovarian cancer (HR: 0.60, 95%CI:0.46-0.78). The pooled hazard ratios confirmed the advantage of HIPEC and CRS association with respect to CRS alone on progression free survival for primary and recurrent ovarian cancer respectively with HR: 0.50, 95%CI: 0.43-0.58 and HR: 0.59, 95%CI: 0.41-0.85.
Conclusion: While waiting for the results of the current prospective studies, the present umbrella study suggests that HIPEC performed at the end of CRS may be a complementary effective asset for ovarian cancer patient management.
1 Introduction
Epithelial ovarian cancer (EOC) is the most common cause of gynaecological cancer death worldwide, with a late onset diagnosis, high death-to-incidence rate and poor overall prognosis (1). While 70% of cases are detected in advanced stages, late stage presentations of the disease are associated with a 5-year relative overall survival rate of 29%, as opposed to 92% for early-stage disease (2, 3). Unlike other malignancies, the dissemination of ovarian cancer cells has a particular pattern which selectively invades the mesothelium of the peritoneal surface, spreading within the peritoneal cavity in a highly aggressive and rapidly growing manner, up to the encasement of reproductive organs and viscera (4). Consequently, the standard treatment consists of cytoreductive surgery (CRS), associated with systemic platinum-based chemotherapy (5–7).
CRS is an aggressive locoregional treatment involving the resection of the disseminated intra-abdominal disease (7, 8). This includes, hysterectomy, bilateral salpingo-oophorectomy, omentectomy as well as additional procedures such as peritonectomies (9), bowel resections, diaphragm peritonectomy with or without segmental full-thickness diaphragm resection, splenectomy with or without distal pancreatectomy, segmental liver resection, cholecystectomy, partial stomach resection, and partial bladder/ureteral resection (10). Postoperative residual disease was shown to be a major prognostic factor of overall survival (11–13), hence the necessity of surgery to be complete. In fact, surgery used to be qualified as “optimal” in reference to a residual tumour of less than 1 cm, but this is not the goal anymore. Currently, surgery is performed when expected to be complete with the aim of removing all macroscopic tumours, which can be challenging in cases of invasive carcinomatosis. In this context, the peritoneal cancer index (PCI) assessment at the time of surgical exploration is a valuable indicator which enables the estimation of the extent of carcinomatosis, the probability of complete cytoreduction and overall the oncological outcome (14, 15).
Besides its indications in the management of rare peritoneal cancers, the use of heated intraperitoneal chemotherapy (HIPEC) in association to CRS has been the subject of controversy. In fact, the OVHIPEC study was the first extensive phase III Randomised Clinical Trial to assess HIPEC benefit in the first line management of ovarian cancer (16). These results were subject to criticism (17), and although they led to guidelines change in some countries such as France, they did not have a similar impact on a more international level (18–20). At present, 17 ongoing clinical trials are examining the impact of HIPEC and CRS association in the management of primary or recurrent ovarian cancer (21). Whilst the results from these studies are pending, the synthesis of all available and robust data up to date is crucial. To assimilate the vast amount of research available on the effect of HIPEC and CRS in ovarian cancer, we performed an umbrella review of existing meta-analyses on this association to look at the impact on overall, disease free and progression free survival as well as morbidity and quality of life in primary and recurrent ovarian cancer.
2 Materials and Methods
Umbrella reviews can be referred to as overviews of reviews, reviews of reviews, a summary of systematic reviews and consist of the overall examination of the body of information on a specific subject/intervention in order to highlight similarities or contradictions in the results (22, 23). In view of the lack of widely accepted guidelines for umbrella reviews’ carry out, we followed the Cochrane Collaboration guidelines in conducting and reporting the results of this review (24). A protocol was priorly designed in accordance with the reporting guidance provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) statement, then registered within the international Prospective Register of Systematic Reviews (PROSPERO) database for systematic reviews and meta-analyses (CRD42020171008) (25).
2.1 Search Strategy
Two researchers (AS and HE) independently searched the MEDLINE, Cochrane Library, Scopus, Prospero, Web of Science and Science Direct from inception until May 30, 2020, to identify peer-reviewed meta-analyses of observational studies and randomised controlled trials. We also searched the references listed in eligible articles. No language restrictions were applied. Detailed search strategy is provided in Appendix 1 and key words and MESH were defined as follows:
[Hyperthermic intraperitoneal chemotherapy’ OR ‘HIPEC’ OR ‘intraperitoneal’ OR ‘Intraperitoneal Chemotherapy, Hyperthermic’]
AND
[‘ovarian’ OR ‘ovary’ OR ‘Ovarian Neoplasm’ OR ‘Ovary Neoplasms’ OR ‘Neoplasm, Ovary’ OR ‘Ovarian Cancer OR Cancer’ OR ‘Cancer of the Ovary’]
AND
[“Cytoreduction Surgical Procedures”]
AND
[‘Meta-Analysis as Topic’ OR ‘Meta-Analysis ‘ [Publication Type].
2.2 Eligibility Criteria and Data Extraction
Review articles fulfilling the following criteria included (1): Meta-analyses of randomised controlled trials and observational prospective cohort studies (2) addressing ovarian cancer (3) examining the outcome of HIPEC in association with CRS (4) assessing overall survival and progression free or disease free survival.
Studies were excluded if they were primary studies, included the evaluation of HIPEC in other malignancies without separate analysis for ovarian cancer, and those for which it is not possible to retrieve the full article.
2.3 Data Collection and Analysis
The reviewed articles meeting the inclusion criteria were imported by each reviewer separately using a Zotero © software (version 5.0.80 for macOS), which is an open-source and free research tool for reference management (26). The duplicates were removed, and then the titles and abstracts of all articles were screened independently prior to full article review and final selection. A manual search of references cited in the selected articles was also performed to identify additional studies. Disagreements were resolved through consensus. When a consensus could not be reached a third investigator made the final decision. A list of excluded studies is provided.
2.4 Data Extraction and Management
Data extraction was performed independently by two investigators (AS and HE) and in case of discrepancies a third investigator was involved. A pre-established data extraction form was used to collect the following Information: (1) general information: title, author, journal name, year of publication (2) study characteristics: country, period of publication, number of original studies, type of intervention, comparator and type of outcomes. (3) outcome assessment: summary information on overall survival, disease free or progression free survival with estimate of effect and 95% confidence interval.
2.5 Methodological Quality and Risk of Bias Assessment
Two independent investigators (AS and HE) evaluated the quality of the included meta-analysis using the Assessing the Methodological Quality of Systematic Reviews version 2.0 (AMSTAR 2.0) checklist. The AMSTAR 2.0 includes 16 items categorised into critical and non-critical domains allowing to rate studies based on weaknesses in critical domains. Confidence in the results of the studies is classified as high, moderate, low, or critically low confidence instead of using an overall score (27).
Risk of bias was also examined by means of the ‘Risk of Bias in Systematic Reviews’ (ROBIS) tool which is completed in three phases: (1) assess relevance (optional), (2) identify concerns with the review process, and (3) judge risk of bias. Our analysis comprised phase 2 and 3 which cover four domains through which bias may be introduced into the review, namely study eligibility criteria; identification and selection of studies; data collection and study appraisal; synthesis and findings as well as the overall risk of bias in the interpretation of review findings and whether priorly identified limitations were taken into consideration (28).
2.6 Statistical Analysis
We synthesised the data from the meta-analyses in terms of: search period, type of intervention, comparison, primary and secondary outcome, number of included randomised controlled trials and relative risk estimates including odds ratio (OR), and/or hazard ratio (HR).
For each meta-analysis, we synthesised the summary effect as well as the 95% confidence interval (CI). We quantified the degree of heterogeneity using the I2 statistic, which represents the percentage of the total variability across studies which is due to heterogeneity. I2 values of 25%, 50% and 75% corresponded to low, moderate and high degrees of heterogeneity respectively. Subgroup analysis was conducted on the basis of primary and recurrent ovarian cancer. Publication bias was assessed through examining asymmetry in the funnel plot. All statistical analyses were performed using Revman 5.3. Ethical approval was not necessary as this study did not involve patient consent.
3 Results
3.1Summary of Meta-Analyses
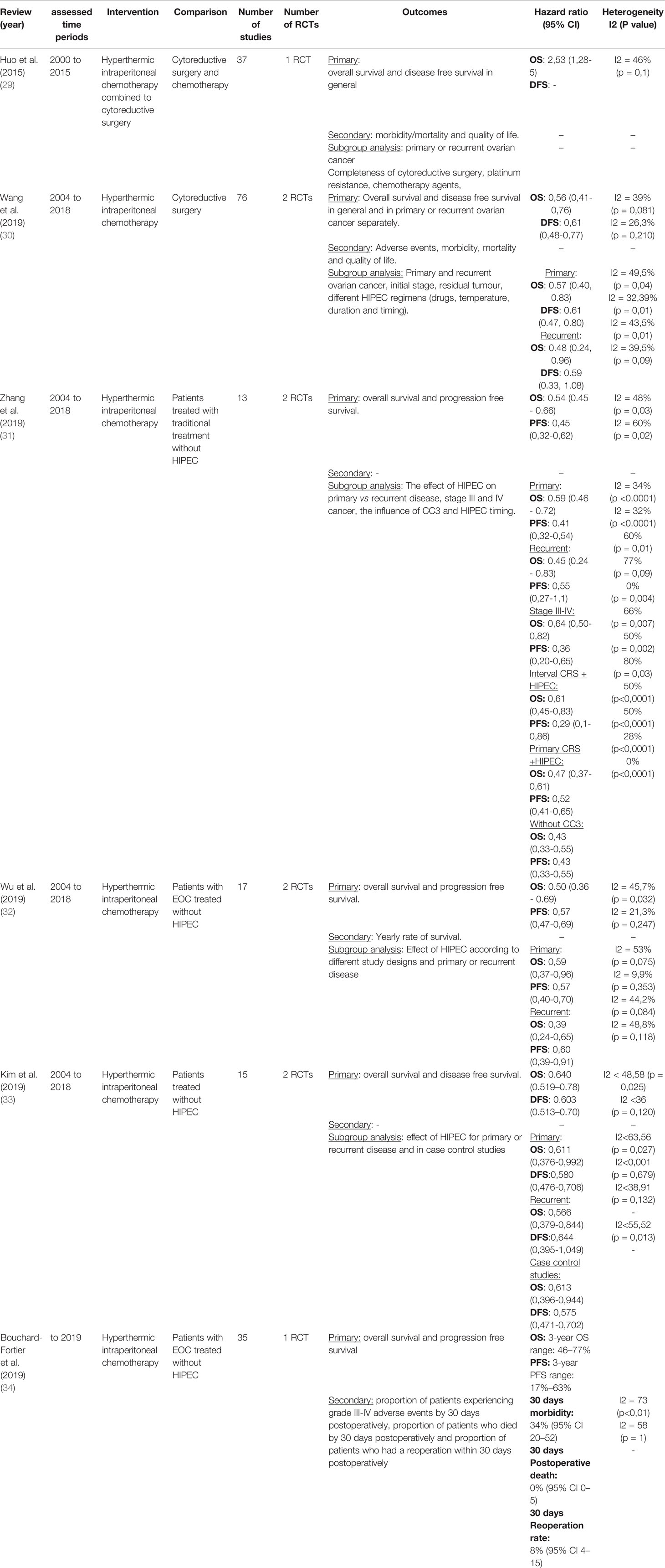
We initially identified 1311 articles, of which 1261 were excluded as considered irrelevant to our search for not addressing CRS and HIPEC according to title and abstract screening. Among 50 articles examined in full text, 44 were not included in the umbrella review as they did not meet the inclusion criteria decided in the protocol (List of excluded articles Appendix). We ultimately identified 6 meta-analyses investigating the association of HIPEC with CRS in the management of ovarian cancers. Five meta-analyses reported overall survival outcomes. Moreover, 2 meta-analyses assessed disease free survival; 3 investigated progression free survival; and one studied both. Figure 1 shows the process of study selection. The publication dates of eligible meta-analyses ranged from 2015 to 2020. As the methodological quality of the meta-analyses is relatively equal, we disregarded the overlap in some primary studies to avoid excluding eligible meta-analyses. The characteristics of the extracted data is presented in Table 1.
Figure 1 Search flowchart. *A list of excluded studies is provided in Appendix 1 (List of excluded articles Appendix).
3.2 Methodological Quality of Included Reviews
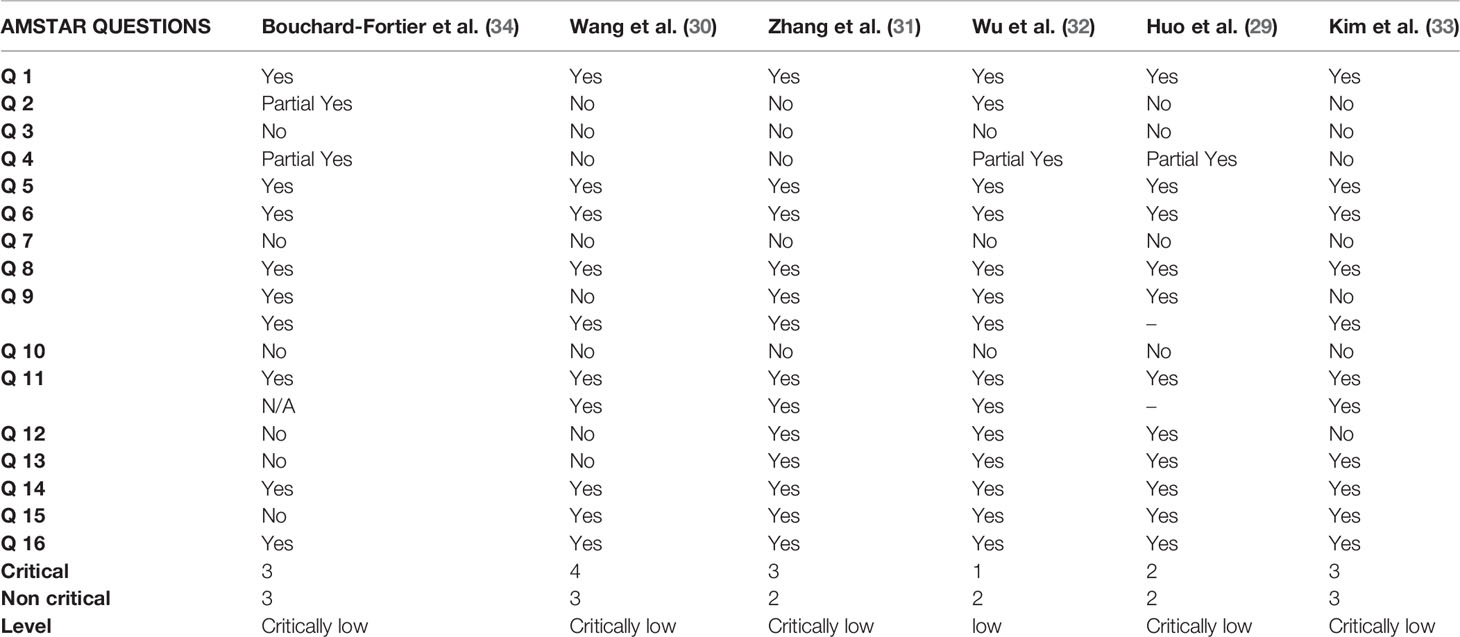
Using the AMSTAR 2 quality checklist, only 2 meta-analyses had a priorly registered protocol, and 3 studies had a comprehensive literature search strategy conducted in at least two bibliographic databases and supplemented by searching grey literature. None of the meta-analyses provided a list of excluded studies. Overall, 1 meta-analysis had low quality rating, while the 5 other meta-analyses had a critically low quality. Detailed findings from the AMSTAR 2 analysis are summarised in Table 2.
3.3 Risk of Bias Assessment
We assessed the risk of bias in all included meta-analyses and all 6 studies had low risk of bias. Details are shown in Table 3.
3.4 Significant Findings and Heterogeneity
3.4.1 Overall Survival
Five of the included studies investigated the overall survival of patients undergoing HIPEC + CRS in comparison to CRS alone, with separated outcomes for primary and recurrent ovarian cancer. The meta-analyses showed that HIPEC + CRS was associated with improved 3 year survival for primary and recurrent cancers with calculated pooled hazard ratios and 95% confidence intervals of HR: 0.66, 95%CI: 0.56-0.78 and HR: 0.50, 95%CI: 0.38-0.64 respectively (Figure 2).
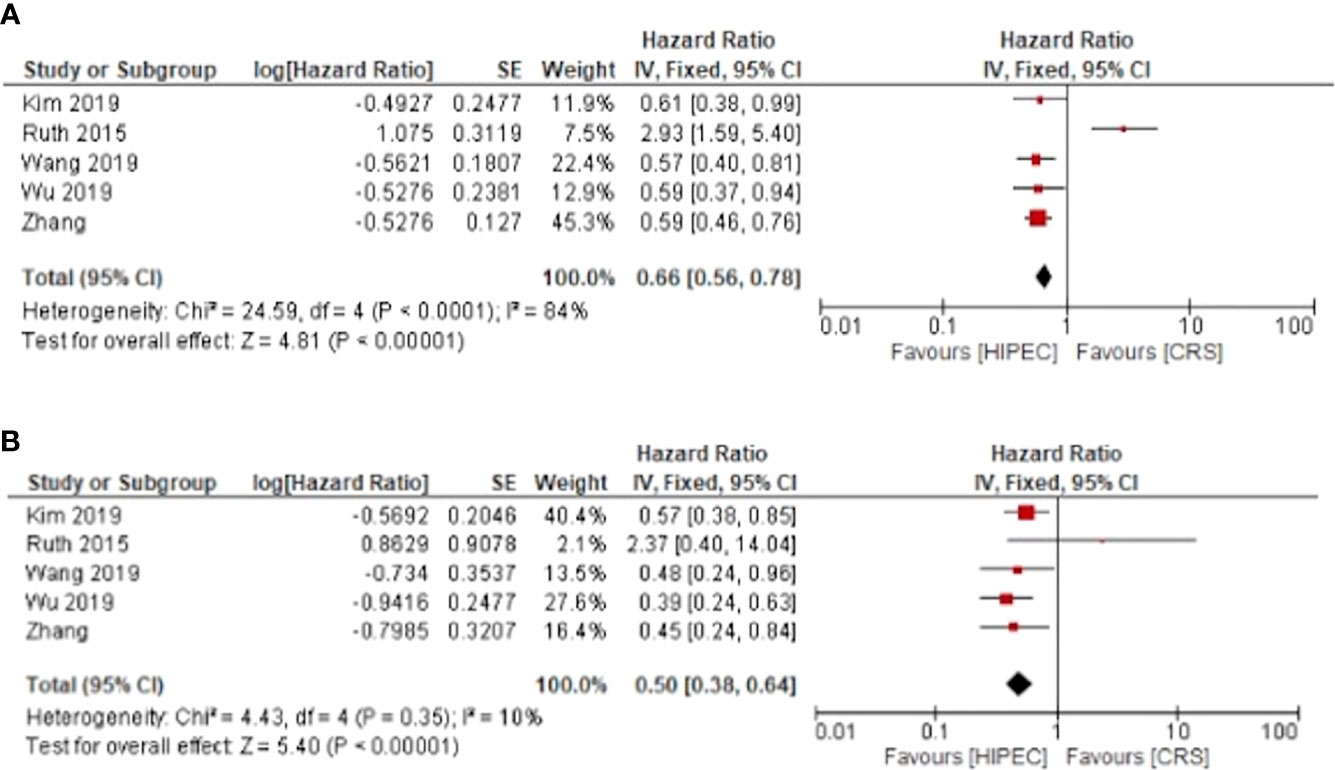
Figure 2 HIPEC + CRS versus CRS overall survival forest plots (A) primary ovarian cancer (B) recurrent ovarian cancer.
3.4.2 Disease Free Survival
Only four meta-analyses investigated disease free survival. HIPEC + CRS was associated with a disease-free survival benefit for primary and recurrent ovarian cancer with pooled hazard ratios of HR: 0.54, 95%CI: 0.48-0.61 and HR: 0.60, 95%CI: 0.46-0.78 respectively (Figure 3).
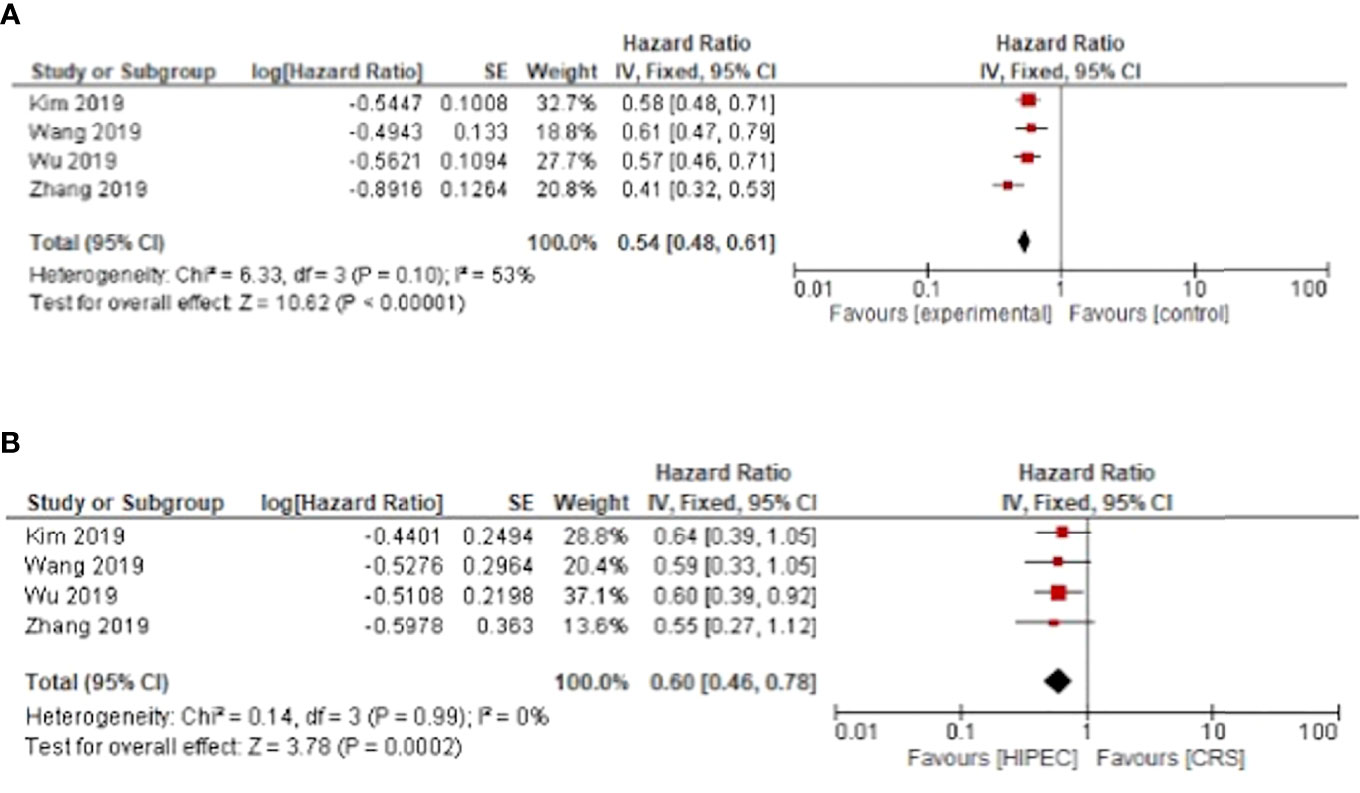
Figure 3 HIPEC + CRS versus CRS disease free survival forest plots (A) primary ovarian cancer (B) recurrent ovarian cancer.
3.4.3 Progression Free Survival
The progression free survival was improved for primary and recurrent ovarian cancer respectively with HR: 0.50, 95%CI: 0.43-0.58 and HR: 0.59, 95%CI: 0.41-0.85 (Figure 4).
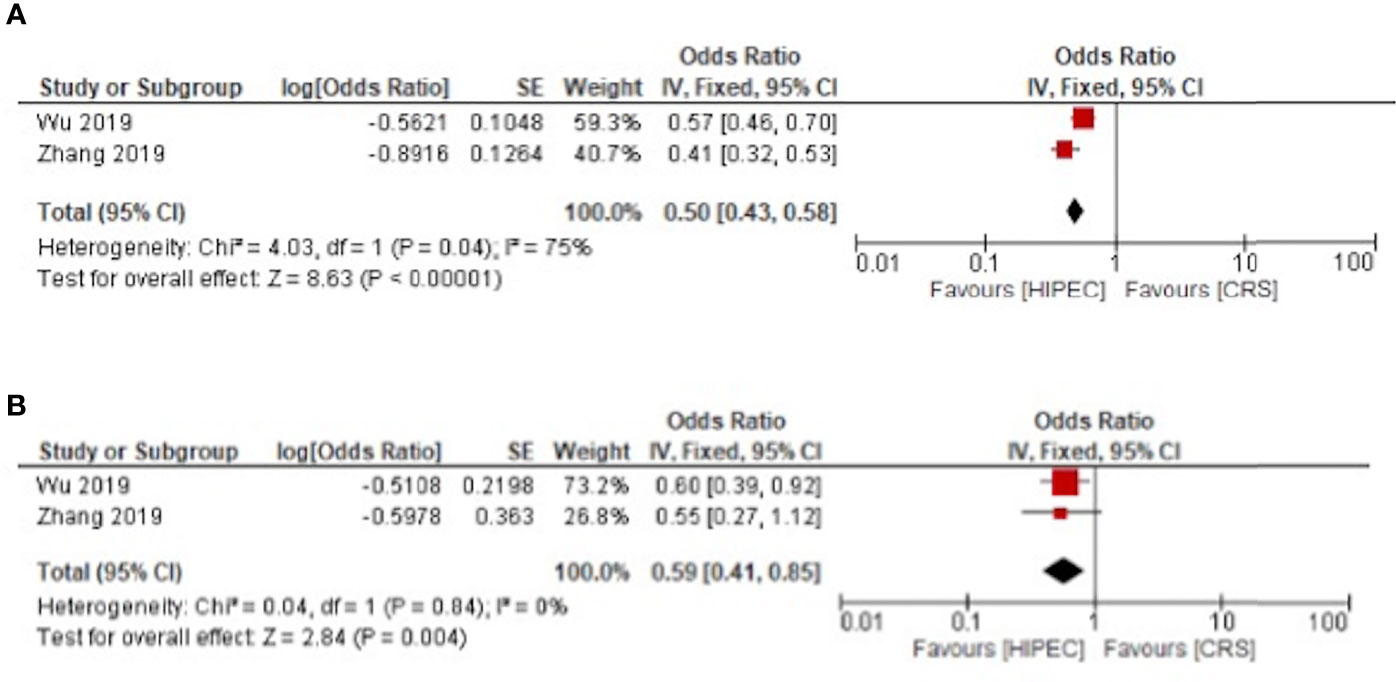
Figure 4 HIPEC + CRS versus CRS progression free survival forest plots (A) primary ovarian cancer (B) recurrent ovarian cancer.
3.4.4 Heterogeneity Assessment
For primary ovarian cancer, heterogeneity was present for both overall and disease-free survival at three years with I²= 84%, p < 0.01 and I²= 53%, p = 0.1 respectively. Regarding recurrent cancers, the heterogeneity was low for overall survival with I²= 10%, p = 0.35. Heterogeneity was not present for disease free and progression free survival in recurrent cancer (Figures 3 and 4).
3.4.5 Morbidity
Y. Wang et al. qualitatively reviewed available data on adverse events and morbidity in patients undergoing HIPEC and CRS. On the other hand, the meta-analyses by G.Bouchard Fortier et al. and Y.R. Huo et al. calculated in primary EOC settings, the pooled proportions of 30-day grade III-IV morbidity, estimated at 34% (95% CI 20-52) and 31.3% (range: 1.8-55.6%) respectively. In addition, Y.R. Huo et al. reported pooled Grade III-IV morbidity rate of 26.2% (1.8-55.6%) in recurrent settings.
3.4.6 Quality of Life
Both the meta-analyses by Y.Ruth and Y. Wang reported studies assessing quality of life in patients undergoing HIPEC + CRS with the following quality of life assessment tools developed by the European Organization for Research and Treatments of Cancer (EORTC): Quality of Life Questionnaire-Core 30 (QLQ-C30), Quality of Life Questionnaire-OVarian Cancer Module (QLQ-OV28), Quality of Life Questionnaire-ColoRectal Cancer Module (QLQ- CR38) and the V FACT-QOL questionnaire. Overall, although data on quality of life remains insufficient, patients undergoing HIPEC + CRS either showed no significant difference, or a worsening followed by an improvement within 90% of the baseline by 6 months but while remaining below the baseline (p<0.05).
4 Discussion
The addition of HIPEC to CRS has been discussed as a potential therapeutic option for peritoneal malignancy management (12, 16, 35–37). The efficacy of HIPEC would result from the specific ovarian cancer’s pattern of spread, the heat effect on anticancer drugs permeability, and the increased chemosensitivity of cancer cells to heat therapeutics (38, 39). This is the first umbrella review of meta-analyses giving an overview and synthesis of the available evidence on the association of HIPEC and CRS in the management of primary and recurrent ovarian cancer.
Our umbrella review confirms the benefit of using CRS and HIPEC for the management of primary ovarian cancer on overall survival, disease free and progression free survival. These results are in line with the OVHIPEC 1 study outcomes by Van Driel et al. (16). The use of HIPEC was associated with a 25% reduction in relapse (HR: umbrella 0.54 vs Van Driel 0.68) and death risk (HR: umbrella 0.66 vs Van Driel 0.67). Moreover, although the relative effect of HIPEC is more marked on recurrence-free survival compared to overall survival, the absolute benefit is higher on overall survival. Despite the existence of high-quality evidence supporting the use of HIPEC, numerous questions are still pending, such the optimal surgery timing between immediate or interval surgery when HIPEC is to be combined to CRS, along with the best time-point for CRS and HIPEC, or the optimised doses and temperatures to be used.
Regarding recurrent ovarian cancers, the HIPEC + CRS association was also associated with overall survival, disease free and progression free survival improvements compared to CRS alone. In fact, the utility of HIPEC for survival has previously been reported by Spiliotis and al (40) in patients with platinum-sensitive, or platinum-resistant recurrent ovarian cancers. Nonetheless, these results were criticised because the statistical hypothesis and primary endpoints had not been clearly defined (41), thereby urging the need for higher scientific evidence, which should be provided by the results from the Italian trial HORSE (42) and the French randomised trial CHIPOR (43)
Historically, HIPEC was developed as an alternative to the increasing use of intraperitoneal chemotherapy (IP) (44) in the 2010’s, which explains the frequent comparison of their mechanisms. That being said, the efficacy of the IP chemotherapy has been reconsidered following the GOG 252 study (45). The randomised trial comparing Intravenous Versus IP Chemotherapy in addition to Bevacizumab showed no disease free survival (DFS) improvement with IP therapy, irrespective of the residual tumour size following CRS. The DFS of patients receiving intravenous chemotherapy, IP Carboplatin and IP Cisplatin were 31.3 months, 31,8 and 33,8 months respectively, therefore showing no significant benefit. A major difference between HIPEC and IP chemotherapy relates to the repetitive administration of chemotherapy in the peritoneum through an intraperitoneal catheter, in addition to the IC administration. Platinum salts, especially cisplatin, are characterised by a strong absorption from peritoneum to blood which limits the peritoneal lesion drug exposure while inducing strong systemic side effects, eventually interfering with the dose intensity of the systemic chemotherapy. Moreover, the peritoneum catheter is associated with local complications such as numerous adhesions formation, infection and pain that hampers extensive locoregional chemotherapy. In contrast, HIPEC is a targeted locoregional therapy meant to complement radical surgery and allow an exhaustive treatment for peritoneal surface malignancies at the end of surgery. Indeed, the surgical procedure is most likely associated with cancer cells’ release within the peritoneal cavity, which could be effectively eliminated by the local application of chemotherapy after the procedure. This may explain why the survival improvement observed with HIPEC may not be the result of an additional Cisplatin dose (46). The systemic passage handoff HIPEC is limited by the decreased duration of exposure, as the abdomen is washed out at the end of the procedure, in addition to the use of Thiosulfate to mitigate Cisplatin-induced toxicity, the reason why this type of treatment is now recommended.
Wang et al’s review on morbidity in patients undergoing HIPEC and CRS, along with two studies, namely Cascales-Campos et al. (47) and Munoz-Casares (48), agreed on the similarity of the overall postoperative morbidity rate between patients undergoing HIPEC and CRS. On the other hand, a study by Ryu et al. reported a non-statistically significant increased rate of major complications for patients with HIPEC (49), while another study reported more grade III-IV complications in the HIPEC group (P=0.02) (50). These complications included minor leaks, ileus, transient hepatitis, leucopenia, abdominal pain, infection and fistulas (30, 32).
Regarding the complications of HIPEC procedures, only 2 meta-analyses reported the 30 days rate of complications for grade III and IV with Y.R Huo et al. (29) describing separate morbidity pooled rates for primary and recurrent EOC of 31.3% (range: 1.8-55.6%) and 26.2% (1.8-55.6%), while G. Bouchard Fortier et al. (34) rounded up the 30 day morbidity pooled rate for primary and recurrent epithelial cancer to 34% (95% CI 20–52). These complication rates are thought to be more related to the effects of extensive CRS rather than the HIPEC therapy (34), especially as Van Driel was the only study comparing the adverse events rates for patients undergoing surgery alone and those with combined surgery and HIPEC and where no statistically significant difference was noted (25% vs. 27%, p = 0.76).
Both the meta-analyses by Y. Ruth (29) and Y. Wang (30) described the quality of life in patients undergoing HIPEC + CRS. Using the V FACT-O QoL questionnaire, Huo et al. showed that if the immediate post-operative quality of life following surgery was worse than the presurgery period (FACT-O score 126 vs 108, p<0.05), it improved later with a 90% baseline improvement at 6 months post-surgery (p<0.05) (29, 51). The European Organization for Research and Treatments of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30), Quality of Life Questionnaire-OVarian Cancer Module (QLQ-OV28) and Quality of Life Questionnaire-ColoRectal Cancer Module (QLQ-CR38) were also used to assess quality of life following HIPEC. No visible quality of life difference between HIPEC + CRS and CRS only patients were observed (16). In another study, Chia et al. used the EORTC QLQ-C3o to assess the quality of life of HIPEC + CRS patients and demonstrated a decrease in quality of life, particularly of the physical and role functioning scales (52). However, this decline was short in time, and improvement or return to baseline 6-12 months after surgery was observed in most cases (52–54). Risk factors associated with worse Qol were higher age, prolonged operation time, extensive disease, residual disease, adjuvant chemotherapy, complications, stoma placement, and recurrent disease (53).
The present study has some limitations. The main weakness linked to HIPEC studies resides in the heterogeneity of the used intraperitoneal regimens, in terms of chemotherapy, dose, temperature and timing with relation to neoadjuvant treatment. For example, while a phase 1 dose-escalation found that cisplatin optimal dose was 70 mg/m2 (55), a subsequent phase II reported 40% morbidity rate using cisplatin at 75 mg/m2 (56). These discrepancies make the data difficult to interpret when we have to choose the best regimen, especially as the difference in applied HIPEC regimens by each institute may be one of the reasons for different treatment outcomes. That being said, several networks are carrying out the work of harmonising these protocols into more standardised recommendations. Some additional analyses would have been interesting in the present project, such as the assessment of BRCA status distribution and the comparison of HIPEC regimens, CRS-HIPEC intervals and whether CRS was primary debulking or interval debulking. However, they were not possible since these data were not examined in the included studies. It would also be useful to develop criteria for the selection of patients, surgeon’s level of experience, as well as the choice of cytostatic (such as dose, temperature, duration of cytostatic application) in order to standardise regimens. Another important limitation relates to the current change of practice with the increasing prescription of PARP inhibitors both in first line and recurrent settings. None of the studies we investigated assessed the role of PARP inhibitors in patients treated with CRS and HIPEC. Well designed RCT are warranted to confirm that CRS + HIPEC and PARP inhibitors can be safely combined, and that HIPEC still contributes to improving PFS and OS. Another limitation is the reduced number of meta-analyses and RCTs which were published in a close interval of time, nevertheless, the umbrella review was conducted with the aim of contrasting the results from these studies as determined in the forest plots. The umbrella review methodology that we used also integrates limitations. The meta‐analyses in this umbrella review contained a small proportion of randomised controlled trials compared to the number of observational studies, which could have decreased the quality of evidence. The methodological quality of the included meta-analyses is overall considered to be low, thereby urging research teams to improve the methodological quality of future meta-analyses by following methodology guidelines, especially when addressing controversial subjects.
Despite these limitations, the implementation of HIPEC in routine is facing an opposition exerted by many clinicians beyond scientific rationality and objectivity. A part of the problem may be related to the natural reluctance of teams to change their organisations, along with conflicts between gynaecologic oncologists and general surgeons. The latter ones Indeed initiated the HIPEC technology for GI cancers with peritoneal involvement before subsequently developing this technique for gynaecologic cancers with their experience of peritoneal carcinomatosis management. This evolution might have led to some conflicts with gynaecologists. In that context, further evidence confirming or not the benefit related to HIPEC will be needed to help the ovarian cancer specialists agree on the utility of this approach.
At present, the utility of heated intraperitoneal chemotherapy in the management of epithelial ovarian cancer (EOC) is still a controversial topic in the scientific community, which pending prospective studies are expected to settle. However, for the time being, the present umbrella study suggests that HIPEC performed at the end of CRS seems to benefit patients treated for primary disease after neoadjuvant chemotherapy as demonstrated in OVHIPEC-1. Further studies are awaited to assess HIPEC in recurrent settings and confirm its utility in primary ovarian cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conceptualization: AS, HE, and NB. Data curation: AS and HE. Formal analysis, AS, HE, and MM. Investigation: AS and HE. Methodology: AS, HE, and MM. Project administration: AS. Supervision: HE and NB. Validation: AB, SB, BY, OG, and RM. Writing—original draft: AS, HE, and NB. Writing—review and editing: MM, AB, SB, BY, OG, and RM. All authors contributed to the article and approved the submitted version.
Funding
Publication fees were supported by the ARCO (association pour la recherche et l'enseignement en chirurgie oncologique) and ThermaSolutions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Miss Hanane Benkhouya for her support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.809773/full#supplementary-material
References
1. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial Ovarian Cancer. Lancet (2019) 393:1240–53. doi: 10.1016/S0140-6736(18)32552-2
2. Reid BM, Permuth JB, Sellers TA. Epidemiology of Ovarian Cancer: A Review. Cancer Biol Med (2017) 14:9–32. doi: 10.20892/j.issn.2095-3941.2016.0084
3. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian Cancer in the World: Epidemiology and Risk Factors. Int J Womens Health (2019) 11:287–99. doi: 10.2147/IJWH.S197604
4. Lengyel E. Ovarian Cancer Development and Metastasis. Am J Pathol (2010) 177:1053–64. doi: 10.2353/ajpath.2010.100105
5. Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2016) 34:3460–73. doi: 10.1200/JCO.2016.68.6907
6. Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N Engl J Med (2010) 363:943–53. doi: 10.1056/NEJMoa0908806
7. Souadka A, Benkabbou A, Majbar MA, Essangri H, Amrani L, Ghannam A, et al. CRS and HIPEC: The Need for an Adaptable Learning Curve Model. J Surg Oncol (2020) 122(6):1187–8. doi: 10.1002/jso.26123
8. Smith ME, Nathan H. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Safety Is Only Half of the Story. JAMA Netw Open (2019) 2:e186839. doi: 10.1001/jamanetworkopen.2018.6839
9. Martín-Cameán M, Delgado-Sánchez E, Piñera A, Diestro MD, De Santiago J, Zapardiel I. The Role of Surgery in Advanced Epithelial Ovarian Cancer. Ecancermedicalscience (2016) 10:666. doi: 10.3332/ecancer.2016.666
10. Wang EW, Wei CH, Liu S, Lee SJ, Shehayeb S, Glaser S, et al. Frontline Management of Epithelial Ovarian Cancer-Combining Clinical Expertise With Community Practice Collaboration and Cutting-Edge Research. J Clin Med Res (2020) 9(9):2830. doi: 10.3390/jcm9092830
11. Chang S-J, Hodeib M, Chang J, Bristow RE. Survival Impact of Complete Cytoreduction to No Gross Residual Disease for Advanced-Stage Ovarian Cancer: A Meta-Analysis. Gynecol Oncol (2013) 130:493–8. doi: 10.1016/j.ygyno.2013.05.040
12. Bakrin N, Bereder JM, Decullier E, Classe JM, Msika S, Lorimier G, et al. Peritoneal Carcinomatosis Treated With Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Advanced Ovarian Carcinoma: A French Multicentre Retrospective Cohort Study of 566 Patients. Eur J Surg Oncol (2013) 39:1435–43. doi: 10.1016/j.ejso.2013.09.030
13. Souadka A, Essangri H, Majbar MA, Benkabbou A, Boutayeb S, Amrani L, et al. Mid-Term Audit of a National Peritoneal Surface Malignancy Program Implementation in a Low Middle Income Country: The Moroccan Experience. Cancers (2021) 13:1088. doi: 10.3390/cancers13051088
14. Harmon RL, Sugarbaker PH. Prognostic Indicators in Peritoneal Carcinomatosis From Gastrointestinal Cancer. Int Semin Surg Oncol (2005) 2:3. doi: 10.1186/1477-7800-2-3
15. Jónsdóttir B, Lomnytska M, Poromaa IS, Silins I, Stålberg K. The Peritoneal Cancer Index is a Strong Predictor of Incomplete Cytoreductive Surgery in Ovarian Cancer. Ann Surg Oncol (2020) 28(1):244–51. doi: 10.1245/s10434-020-08649-6
16. van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med (2018) 378:230–40. doi: 10.1056/NEJMoa1708618
17. Vergote I, Chiva L, du Bois A. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med (2018) 378:1362–3. doi: 10.1056/NEJMc1802033
18. Harter P, du Bois A, Mahner S, Pfisterer J, Ortmann O, Marth C, et al. Statement of the AGO Kommission Ovar, AGO Study Group, NOGGO, AGO Austria and AGO Switzerland Regarding the Use of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Ovarian Cancer. Geburtshilfe und Frauenheilkunde (2016) 76:147–9. doi: 10.1055/s-0035-1568169
19. Lavoue V, Huchon C, Akladios C, Alfonsi P, Bakrin N, Ballester M, et al. Management of Epithelial Cancer of the Ovary, Fallopian Tube, Primary Peritoneum. Long Text of the Joint French Clinical Practice Guidelines Issued by FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY, Endorsed by INCa. (Part 2: Systemic, Intraperitoneal Treatment, Elderly Patients, Fertility Preservation, Follow-Up). J Gynecol Obstet Hum Reprod (2019) 48:379–86. doi: 10.1016/j.jogoh.2019.03.018
20. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2021) 19:191–226. doi: 10.6004/jnccn.2021.0007
21. Search of: Hipec. Available at: https://www.clinicaltrials.gov/ct2/results?term=hipec&cond=Ovarian+Cancer&recrs=a&age_v=&gndr=&type=Intr&rslt=&Search=Apply (Accessed October 19, 2020).
22. Hartling L, Chisholm A, Thomson D, Dryden DM. A Descriptive Analysis of Overviews of Reviews Published Between 2000 and 2011. PloS One (2012) 7:e49667. doi: 10.1371/journal.pone.0049667
23. Aromataris E, Fernandez RS, Godfrey C, Holly C, Khalil H, Tungpunkom P. Methodology for JBI Umbrella Reviews. (2014) 1.
24. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons (2011).
25. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ (2016) 354:i4086. doi: 10.1136/bmj.i4086
27. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or non-Randomised Studies of Healthcare Interventions, or Both. BMJ (2017) 358:j4008. doi: 10.1136/bmj.j4008
28. Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. A New Tool to Assess Risk of Bias in Systematic Reviews was Developed. J Clin Epidemiol (2016) 69:225–34. doi: 10.1016/j.jclinepi.2015.06.005
29. Huo YR, Richards A, Liauw W, Morris DL. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) and Cytoreductive Surgery (CRS) in Ovarian Cancer: A Systematic Review and Meta-Analysis. Eur J Surg Oncol (2015) 41:1578–89. doi: 10.1016/j.ejso.2015.08.172
30. Wang Y, Ren F, Chen P, Liu S, Song Z, Xiaoxin Ma X. Effects of CytoReductive Surgery Plus Hyperthermic IntraPEritoneal Chemotherapy (HIPEC) Versus CytoReductive Surgery for Ovarian Cancer Patients: A Systematic Review and Meta-Analysis. Eur J Surg Oncol (2019) 45:301–9. doi: 10.1016/j.ejso.2018.10.528
31. Zhang G, Zhu Y, Liu C, Chao G, Cui R, Zhang Z. The Prognosis Impact of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Plus Cytoreductive Surgery (CRS) in Advanced Ovarian Cancer: The Meta-Analysis. J Ovarian Res (2019) 12:1–9. doi: 10.1186/s13048-019-0509-1
32. Wu Q, Wu Q, Xu J, Cheng X, Wang X, Lu W, et al. Efficacy of Hyperthermic Intraperitoneal Chemotherapy in Patients With Epithelial Ovarian Cancer: A Meta-Analysis. Int J Hyperthermia (2019) 36:562–72. doi: 10.1080/02656736.2019.1612101
33. Kim SI, Cho J, Lee EJ, Park S, Park SJ, Seol A, et al. Selection of Patients With Ovarian Cancer Who may Show Survival Benefit From Hyperthermic Intraperitoneal Chemotherapy: A Systematic Review and Meta-Analysis. Medicine (2019) 98:e18355. doi: 10.1097/MD.0000000000018355
34. Bouchard-Fortier G, Cusimano MC, Fazelzad R, Sajewycz K, Lu L, Espin-Garcia O, et al. Oncologic Outcomes and Morbidity Following Heated Intraperitoneal Chemotherapy at Cytoreductive Surgery for Primary Epithelial Ovarian Cancer: A Systematic Review and Meta-Analysis. Gynecol Oncol (2020) 158:218–28. doi: 10.1016/j.ygyno.2020.03.034
35. Cascales-Campos PA, Gil J, Gil E, Feliciangeli E, González-Gil A, Parrilla JJ, et al. Treatment of Microscopic Disease With Hyperthermic Intraoperative Intraperitoneal Chemotherapy After Complete Cytoreduction Improves Disease-Free Survival in Patients With Stage IIIC/IV Ovarian Cancer. Ann Surg Oncol (2014) 21:2383–9. doi: 10.1245/s10434-014-3599-4
36. Di Giorgio A, De Iaco P, De Simone M, Garofalo A, Scambia G, Pinna AD, et al. Cytoreduction (Peritonectomy Procedures) Combined With Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Advanced Ovarian Cancer: Retrospective Italian Multicenter Observational Study of 511 Cases. Ann Surg Oncol (2017) 24:914–22. doi: 10.1245/s10434-016-5686-1
37. Honoré C, Goéré D, Messager M, Souadka A, Dumont F, Piessen G, et al. Risk Factors of Peritoneal Recurrence in Eso-Gastric Signet Ring Cell Adenocarcinoma: Results of a Multicentre Retrospective Study. Eur J Surg Oncol (2013) 39:235–41. doi: 10.1016/j.ejso.2012.12.013
38. Alter R, Turaga K, Lengyel E. Are We Ready for Hyperthermic Intraperitoneal Chemotherapy in the Upfront Treatment of Ovarian Cancer? JAMA Netw Open (2020) 3:e2014184. doi: 10.1001/jamanetworkopen.2020.14184
39. Markman M. Intraperitoneal Antineoplastic Drug Delivery: Rationale and Results. Lancet Oncol (2003) 4:277–83. doi: 10.1016/S1470-2045(03)01074-X
40. Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, et al. Cytoreductive Surgery and HIPEC in Recurrent Epithelial Ovarian Cancer: A Prospective Randomized Phase III Study. Ann Surg Oncol (2015) 22:1570–5. doi: 10.1245/s10434-014-4157-9
41. Harter P, Reuss A, Sehouli J, Chiva L, du Bois A. Brief Report About the Role of Hyperthermic Intraperitoneal Chemotherapy in a Prospective Randomized Phase 3 Study in Recurrent Ovarian Cancer From Spiliotis Et al. Int J Gynecol Cancer (2017) 27:246–7. doi: 10.1097/igc.0000000000000864
42. Hyperthermic Intra-Peritoneal Chemotherapy (HIPEC) in Ovarian Cancer Recurrence - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT01539785 (Accessed October 20, 2020).
43. Hyperthermic Intra-Peritoneal Chemotherapy (HIPEC) in Relapse Ovarian Cancer Treatment - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT01376752 (Accessed March 4, 2021).
44. Neuwirth MG, Alexander HR, Karakousis GC. Then and Now: Cytoreductive Surgery With Hyperthermic Intraperitoneal Chemotherapy (HIPEC), a Historical Perspective. J Gastrointest Oncol (2016) 7:18–28. doi: 10.3978/j.issn.2078-6891.2015.106
45. Walker JL, Brady MF, Wenzel L, Fleming GF, Huang HQ, DiSilvestro PA, et al. Randomized Trial of Intravenous Versus Intraperitoneal Chemotherapy Plus Bevacizumab in Advanced Ovarian Carcinoma: An NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol (2019) 37:1380–90. doi: 10.1200/JCO.18.01568
46. Bolis G, Favalli G, Danese S, Zanaboni F, Mangili G, Scarabelli C, et al. Weekly Cisplatin Given for 2 Months Versus Cisplatin Plus Cyclophosphamide Given for 5 Months After Cytoreductive Surgery for Advanced Ovarian Cancer. J Clin Oncol (1997) 15:1938–44. doi: 10.1200/JCO.1997.15.5.1938
47. Cascales-Campos PA, Gil J, Feliciangeli E, Gil E, González-Gil A, López V, et al. The Role of Hyperthermic Intraperitoneal Chemotherapy Using Paclitaxel in Platinum-Sensitive Recurrent Epithelial Ovarian Cancer Patients With Microscopic Residual Disease After Cytoreduction. Ann Surg Oncol (2015) 22:987–93. doi: 10.1245/s10434-014-4049-z
48. Muñoz-Casares FC, Rufián S, Rubio MJ, Díaz CJ, Díaz R, Casado Á, et al. The Role of Hyperthermic Intraoperative Intraperitoneal Chemotherapy (HIPEC) in the Treatment of Peritoneal Carcinomatosis in Recurrent Ovarian Cancer. Clin Trans Oncol (2009) 11:753–9. doi: 10.1007/s12094-009-0438-3
49. Ryu KS, Kim JH, Ko HS, Kim JW, Ahn WS, Park YG, et al. Effects of Intraperitoneal Hyperthermic Chemotherapy in Ovarian Cancer. Gynecol Oncol (2004) 94:325–32. doi: 10.1016/j.ygyno.2004.05.044
50. Baiocchi G, Ferreira FO, Mantoan H, da Costa AABA, Faloppa CC, Kumagai LY, et al. Hyperthermic Intraperitoneal Chemotherapy After Secondary Cytoreduction in Epithelial Ovarian Cancer: A Single-Center Comparative Analysis. Ann Surg Oncol (2016) 23:1294–301. doi: 10.1245/s10434-015-4991-4
51. Argenta PA, Sueblinvong T, Geller MA, Jonson AL, Downs LS Jr, Carson LF, et al. Hyperthermic Intraperitoneal Chemotherapy With Carboplatin for Optimally-Cytoreduced, Recurrent, Platinum-Sensitive Ovarian Carcinoma: A Pilot Study. Gynecol Oncol (2013) 129:81–5. doi: 10.1016/j.ygyno.2013.01.010
52. Chia CS, Tan GHC, Lim C, Soo KC, Teo MCC. Prospective Quality of Life Study for Colorectal Cancer Patients With Peritoneal Carcinomatosis Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol (2016) 23:2905–13. doi: 10.1245/s10434-016-5203-6
53. Leimkühler M, Hentzen JEKR, Hemmer PHJ, Been LB, van Ginkel RJ, Kruijff S, et al. Systematic Review of Factors Affecting Quality of Life After Cytoreductive Surgery With Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol (2020) 27:3973–83. doi: 10.1245/s10434-020-08379-9
54. Steffens D, Koh C, Ansari N, Solomon MJ, Brown K, McBride K, et al. Quality of Life After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Early Results From a Prospective Cohort Study of 115 Patients. Ann Surg Oncol (2020) 27:3986–94. doi: 10.1245/s10434-020-08443-4
55. Gouy S, Ferron G, Glehen O, Bayar A, Marchal F, Pomel C, et al. Results of a Multicenter Phase I Dose-Finding Trial of Hyperthermic Intraperitoneal Cisplatin After Neoadjuvant Chemotherapy and Complete Cytoreductive Surgery and Followed by Maintenance Bevacizumab in Initially Unresectable Ovarian Cancer. Gynecol Oncol (2016) 142:237–42. doi: 10.1016/j.ygyno.2016.05.032
Keywords: umbrella review, epithelial ovarian cancer, HIPEC, meta-analysis, peritoneal carcinomatosis (PC), cytoreductive surgery and HIPEC
Citation: Souadka A, Essangri H, Majbar MA, Benkabbou A, Boutayeb S, You B, Glehen O, Mohsine R and Bakrin N (2022) Hyperthermic Intraperitoneal Chemotherapy and Cytoreductive Surgery in Ovarian Cancer: An Umbrella Review of Meta-Analyses. Front. Oncol. 12:809773. doi: 10.3389/fonc.2022.809773
Received: 05 November 2021; Accepted: 28 February 2022;
Published: 09 May 2022.
Edited by:
Alberto Di Leo, Ospedale San Camillo, ItalyReviewed by:
Jan Baptist Vermorken, University of Antwerp, BelgiumMaja Pavlov, University Clinical Center of Serbia, Serbia
Galina Kireeva, N.I. Pirogov National Medical and Surgical Center (NMSC), Russia
Arianna Corvasce, Santa Maria Hospital, Italy
Copyright © 2022 Souadka, Essangri, Majbar, Benkabbou, Boutayeb, You, Glehen, Mohsine and Bakrin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amine Souadka, a.souadka@um5s.net.ma
 Amine Souadka
Amine Souadka Hajar Essangri
Hajar Essangri Mohammed Anass Majbar
Mohammed Anass Majbar Amine Benkabbou1
Amine Benkabbou1 Naoual Bakrin
Naoual Bakrin