- 1Department of Gastroenterology, West China Medical School, Sichuan University, Chengdu, China
- 2Department of West China School of Public Health, Sichuan University, Chengdu, China
- 3Department of Abdominal Oncology, West China Medical School, Sichuan University, Chengdu, China
- 4Department of Stomatology, Hospital of Stomatology, Sichuan University, Chengdu, China
- 5West China Medical School, Sichuan University, Chengdu, China
Background and Objectives: This study aimed to compare the efficacy of transarterial chemoembolization (TACE) plus sorafenib (TACE-S) to TACE plus lenvatinib (TACE-L) for the treatment of HCC with portal vein tumor thrombus (PVTT).
Methods: This cohort study recruited patients from September 2017 to September 2020. A total of 59 and 57 consecutive patients were treated with TACE-L and TACE-S, respectively.
Results: Before propensity score matching (PSM), comparing TACE-L to TACE-S, the median overall survival (OS) time was 16.4 months and 12.7 months, respectively [hazard ratio (HR) 1.34; 95% confidence interval (CI): 0.81–2.20; p = 0.25]. The median progression-free survival (PFS) time was 8.4 months and 7.43 months, respectively (HR 1.54; 95% CI: 0.98–2.41; p = 0.081). After PSM, the median OS time was 18.97 months and 10.77 months, respectively (HR 2.21; 95% CI: 1.12–4.38; p = 0.022); the median PFS time was 10.6 months (95% CI: 6.6–18.0 months) and 5.4 months (95% CI: 4.2–8.1 months), respectively (HR 2.62; 95% CI: 1.43–4.80; p = 0.002). After PSM, the overall response rate (ORR) was 66.8% vs. 33.3% [odds ratio (OR) 0.85; 1.05–6.90; p = 0.037].
Conclusion: Both TACE-L and TACE-S are safe, well-tolerated treatments for HCC with PVTT. In HCC with PVTT, TACE-L was significantly superior to TACE-S with respect to OS, PFS, and ORR. A larger-scale randomized clinical trial is needed.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most frequent cancer worldwide (1). Unfortunately, 30%–62% of patients with HCC tend to develop portal vein tumor thrombus (PVTT), which is a poor risk factor for overall survival (OS) (2).
For patients with HCC and PVTT, sorafenib is recommended as the first-line treatment by the Barcelona Clinic Liver Cancer (BCLC) staging system and European Association for the Study of the Liver (3, 4). Unfortunately, HCC with PVTT treated with sorafenib only achieved a 5.6 months of OS time (2). Peng et al. (5) demonstrated that combining TACE with sorafenib (TACE-S) significantly prolonged median OS compared to mono-sorafenib (16.0 vs. 10.0 months). Similarly, the corresponding time to progression (TTP) was longer in the TACE-S group than the mono-sorafenib group (10.0 vs. 5.0 months, p < 0.001). According to the PVTT classification validated by Yamada et al. (6) in patients with right or left PVTT (Vp3), the median OS in the TACE-S group and mono-sorafenib group was 11.0 months and 7.0 months, respectively (p = 0.001). The corresponding TTP was 6.0 months and 3.5 months in the TACE-S group and mono-sorafenib group, respectively (p < 0.001) (5). This finding suggests that HCC patients with PVTT benefit from TACE-S treatment. According to the REFLECT study (7), compared to sorafenib, lenvatinib, exhibits a better overall response rate (ORR) (29.6% vs. 6.9%; p < 0.001), progression-free survival (PFS) (7.2 vs. 4.6 months, p < 0.001), and non-inferior OS (13.6 vs. 12.3 months). TACE combined with lenvatinib (TACE-L) also presented a significantly longer OS than lenvatinib alone (8). Hence, for HCC with PVTT, TACE-L may have better clinical efficacy than TACE-S. However, there is currently a lack of evidence on TACE-L versus TACE-S. Therefore, we performed this study to compare the efficacy of TACE-L vs. TACE-S in the treatment of HCC with PVTT.
Methods
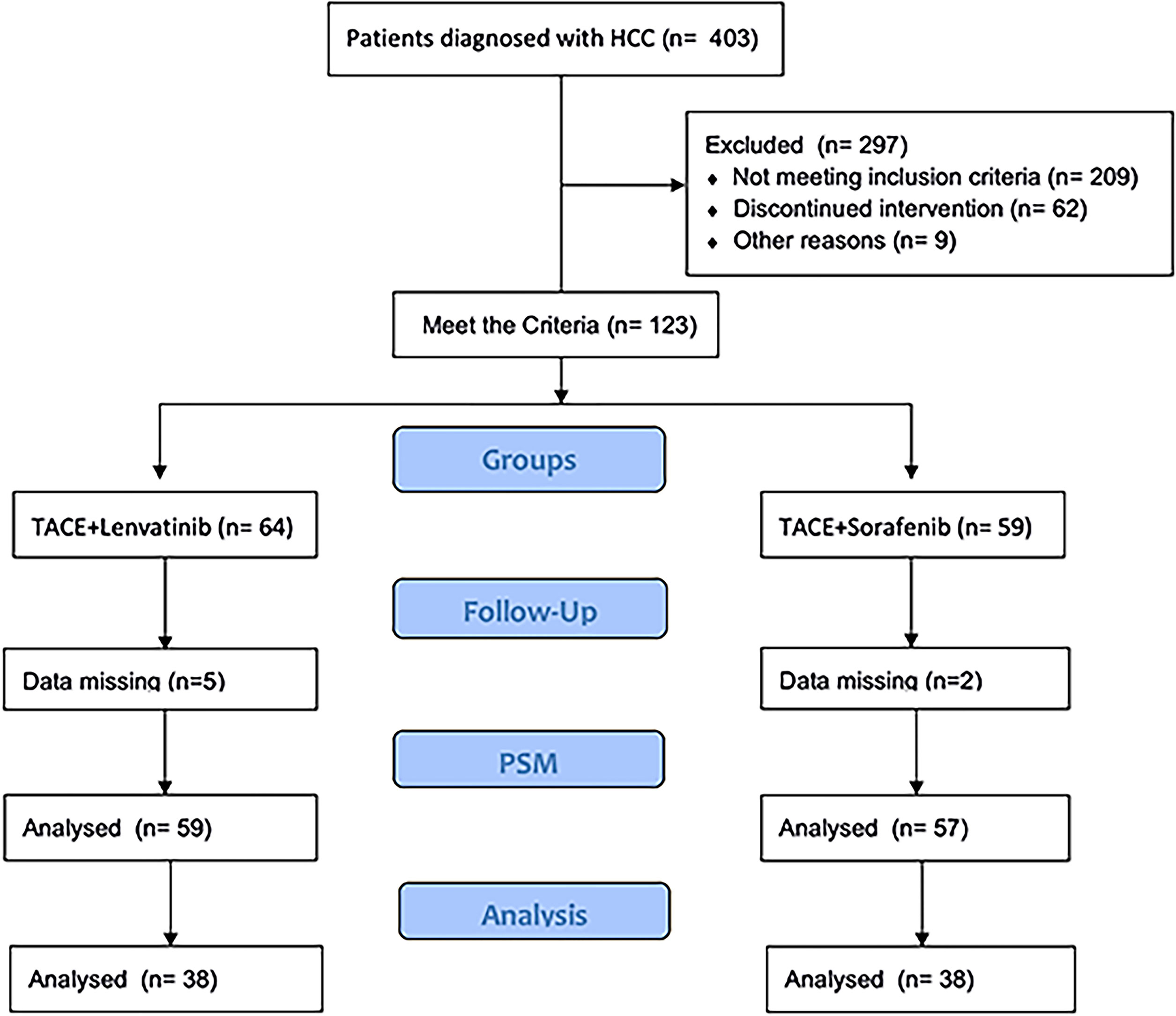
This cohort study was approved by the ethics committee of our institution. The trial registration ID was ChiCTR2100046490 (chictr.org. cn). This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from every patient. The HCC was diagnosed by histopathological examination. PVTT was diagnosed by magnetic resonance imaging (MRI), abdominal dynamic computer tomography (CT) or ultrasonic examination. All procedures were strictly according to the CONSORT guidelines. Patients were recruited from September 2017 to December 2019. Patients were recruited into two groups according to their willingness. All patients were followed up to February 2021. Among these patients, 59 and 57 were included in the TACE-L and TACE-S groups, respectively (Figure 1).
Eligibility Criteria
All patients were preoperatively evaluated by MRI, abdominal dynamic CT, and/or abdominal ultrasonography. Inclusion criteria were as follows: (1) unresectable HCC with PVTT; (2) international normalized ratio was lower than 1.5; (3) no history of any anti-tumor treatment in the previous 6 months; (4) liver function with Child-Pugh A or B; (5) no serious concurrent medical illness; (6) Eastern Cooperative Oncology Group (ECOG) score ≤ 2; and (7) historically or cytologically proven HCC.
Exclusion criteria: (1) <18 years old or ≥75 years old; (2) esophageal variceal bleeding within the previous 3 months; (3) patients who previously used targeted therapy, systemic chemotherapy, programmed death ligand-1 (PD-L1) inhibitors, PD-L2 inhibitors, cytotoxic T lymphocyte associate protein (CTLA-4) antibody inhibitors, or antitumor Chinese medicines; (4) pregnant females; (5) hepatic artery thrombosis; (6) acute tumor rupture with hemoperitoneum; (7) extrahepatic organs not including regional lymph node metastasis; (8) a history of hepatic encephalopathy; (9) heart failure or concurrent ischemic heart disease; and (10) patients with malignant tumors in other organs.
Procedures
The treatment protocol was described in a previous study (9). Each patient received the TACE treatment. Briefly, after local anesthesia with 3–5 ml of 1% lidocaine administered in the groin, hepatic arteriography was performed. Then, 2–20 ml of an injectable suspension of iodized oil (10-20 ml, Guerbet) and epirubicin (40-45 mg, Pfizer) was injected into the target artery using a 2-F microcatheter (Terumo), followed by gelatin sponge particle (350–560 μm, Alicon) embolization until arterial flow stasis was achieved. Treatment was continued until progression to the TACE refractory criteria, unacceptable toxicity, or withdrawal of consent.
Sorafenib (Pfizer) or lenvatinib (Merck) was administered within 7 days before or after TACE. Drug dosage was based on the recommended doses in the SHARP (10) and REFLECT (7) studies, respectively: sorafenib: 400 mg orally, twice per day; lenvatinib: 8 mg orally, once per day (body weight < 60 kg) or 12 mg orally, once per day (body weight ≥ 60 kg). Doses were adjusted according to the grade of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE version 4.0). Lenvatinib or sorafenib was continually administered until disease progression developed or unacceptable adverse events (AEs) appeared.
Follow-Up and Assessment
After 4 weeks of TACE, tumors were assessed by dynamic CT or MRI every 3 months, with tumor marker tests performed at the same time (11). All patients were followed up by the same multidisciplinary team after treatment. Complete response (CR), partial response (PR), progressive disease (PD), and stable disease (SD) assessment of liver tumors was performed according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (12) within 1 month after the first TACE session (13). The disease control rate (DCR) and overall response rate (ORR) were defined as CR/PR/SD and CR/PR, respectively. PFS was defined as the time from patients’ TACE treatment to disease progression. AEs were evaluated using CTCAE version 4.0. OS was calculated as the time from TACE treatment to death or the last follow-up.
Statistical Analysis
SPSS 20.0 (IBM, USA) and R-studio (version 4.1.2) were used for statistical analysis. Normally distributed data were expressed as the median and 95% confidence interval (CI), and enumeration data were expressed as n (%). Independent-samples t-test or χ2 test was used to assess two groups of baseline characteristics. OS and PFS were evaluated using the Kaplan–Meier method. The Cox model was used to identify risk factors. A p-value < 0.05 was defined as statistically significant.
Results
Patient Demographics
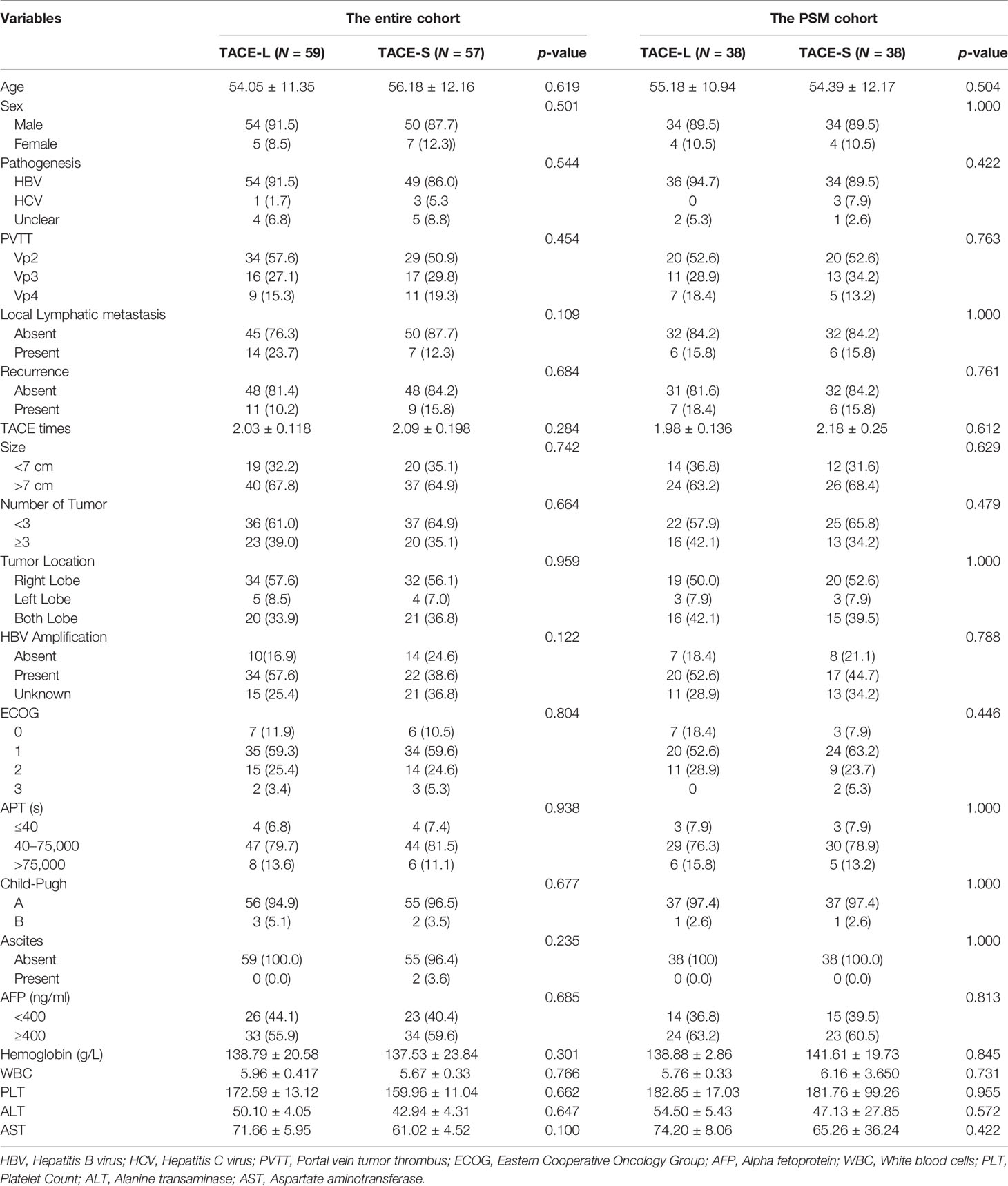
A total number of 116 patients were recruited in this study (TACE-L, 59; TACE-S, 57). The mean age was 54.05 ± 11.35 years in the TACE-L group and 55.18 ± 10.94 in the TACE-S group. The baseline characteristics of sex, ECOG score, PVTT classification, AFP level, and pre- and post-propensity score matching (PSM) are presented in Table 1.
Overall Survival
In the TACE-L and TACE-S groups, the median OS was 16.4 months (95% CI: 10.93–21.81 months) and 12.7 months (95% CI: 10.8–17.9 months), respectively (HR 1.34; 95% CI: 0.81–2.20; p = 0.25) (Figure 2). At 6, 12, and 24 months, the estimated OS rates were 81.4% vs. 78.9%, 40.7% vs. 40.4%, and 8.5% vs. 8.8% in the TACE-L and TACE-S groups, respectively (Supplementary Table 1). In the subgroup analysis, based on the number of TACE procedures (<3 times), the median OS was 14.97 months (95% CI: 9.49–20.45 months) vs. 11.5 months (95% CI: 10.09–12.92 months) (HR 2.93; 95% CI: 1.27–2.00; p = 0.017). For patients with α-fetal protein (AFP) ≥ 400 ng/ml, the median OS was 17.6 months (95% CI: 9.64–25.56 months) vs. 11.5 months (95% CI: 9.35–13.65 months) (HR 1.03; 95% CI: 0.61-1.75; p = 0.009). The median OS was 20.37 months (95% CI: 13.78–26.96 months) vs. 14.8 months (95% CI: 11.76–17.84 months) (p = 0.433) in the subgroup of ECOG < 2 (HR 2.71; 95% CI: 1.11–6.62; p = 0.027) (Figure 3).
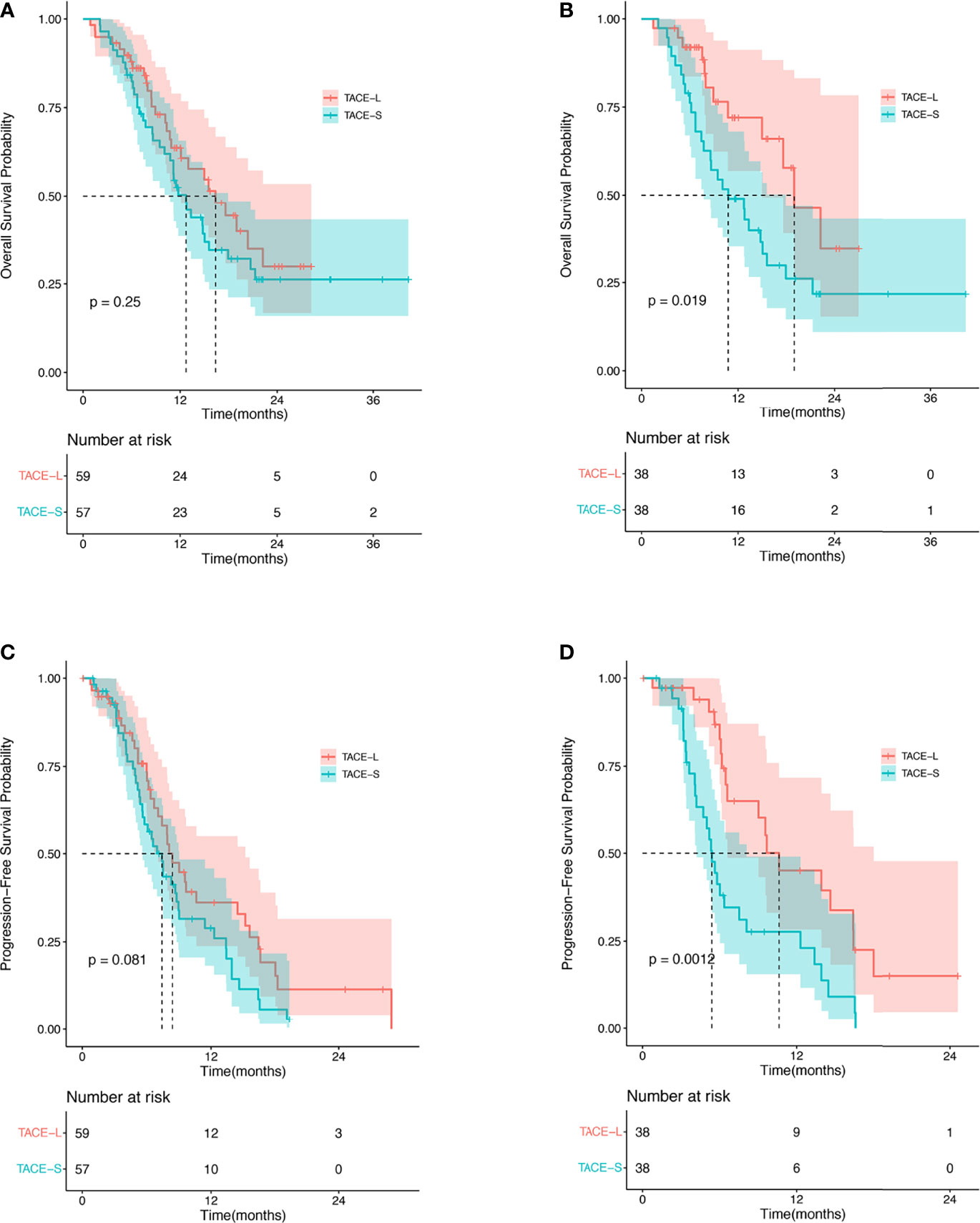
Figure 2 Kaplan–Meier analysis for OS before PSM (A), OS after PSM (B), PFS before PSM (C), and PFS after PSM (D).
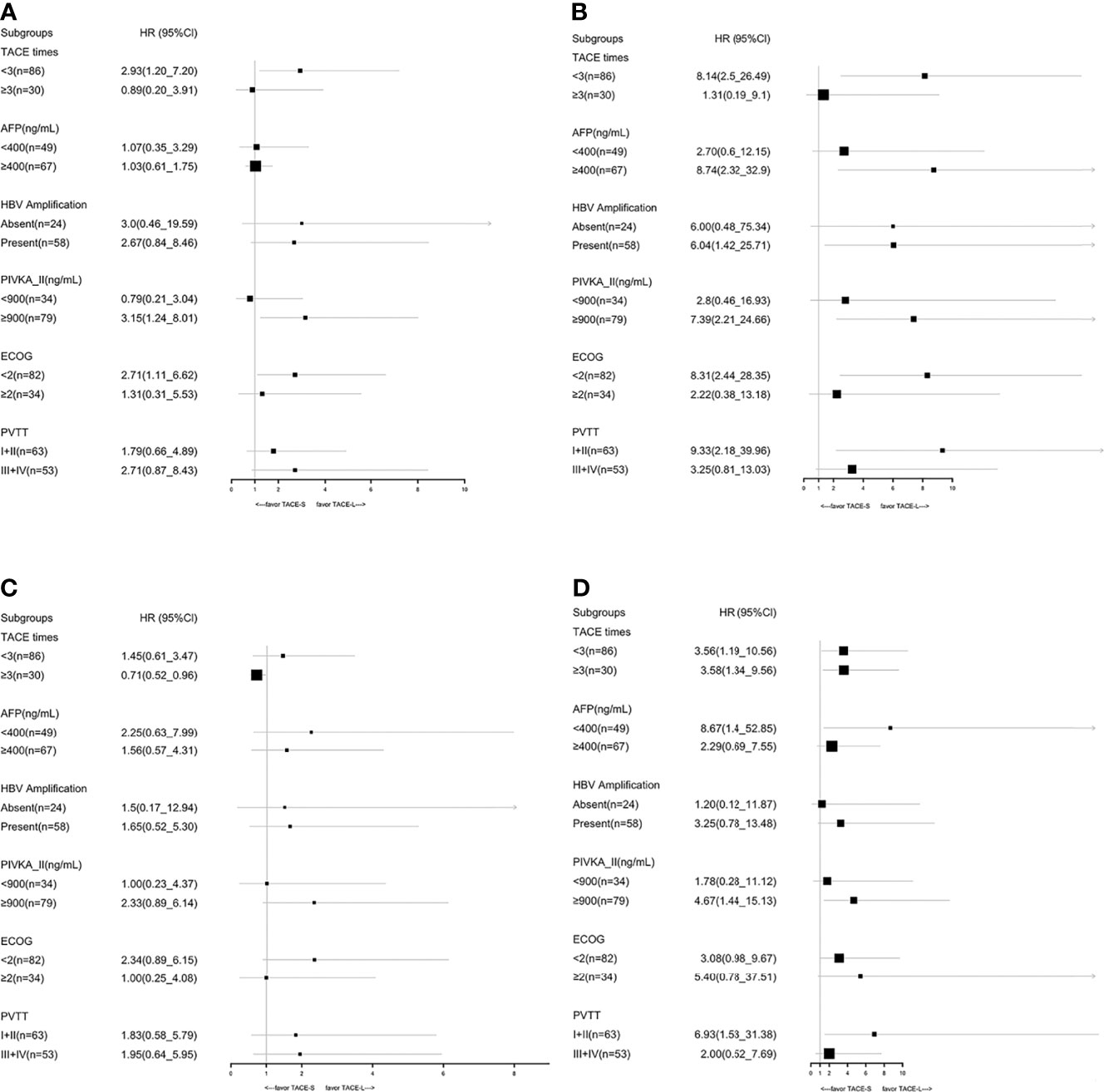
Figure 3 Subgroup analyses of OS before PSM (A), OS after PSM (B), PFS before PSM (C), and PFS after PSM (D) for known prognostic factors.
After PSM, the median OS was 18.97 months (95% CI: 13.53–24.41 months) and 10.77 months (95% CI: 6.07–15.47 months) in the TACE-L and TACE-S groups, respectively (HR 2.21; 95% CI: 1.12–4.38; p = 0.022) (Figure 2). The estimated OS rates at 6, 12, 24, and 36 months were 81.6% vs. 73.7%, 34.2% vs. 42.1%, and 8.9% vs. 5.3% in the TACE-L and TACE-S groups, respectively. In the subgroup analysis, the median OS of Vp2 PVTT was 22.23 months (95% CI: 12.78–31.61 months) vs. 12.8 months (95% CI: 8.01–17.59 months) (HR 9.33; 95% CI: 2.18–28.35; p < 0.001) in TACE-L compared to TACE-S, respectively. Based on the number of TACE procedures (<3 times), the median OS was 14.97 months vs. 10.77 months (HR 8.14; 95% CI: 2.53-26.49; p < 0.001). For patients with AFP ≥ 400 ng/ml, the median OS was 22.23 months vs. 10.77 months (HR 8.74; 95% CI: 2.32–32.90; p = 0.001). The median OS was 22.23 months vs. 12.8 months in subgroup of ECOG < 2 (HR 8.31; 95% CI: 2.44–28.35; p < 0.001) (Figure 3).
Progression-Free Survival
The median PFS time was 8.4 months (95% CI: 7.07–15.2 months) and 7.43 months (95% CI: 5.63–9.03 months) in the TACE-L and TACE-S groups, respectively (HR 1.54; 95% CI: 0.98–2.41; p = 0.081) (Figure 2). The estimated PFS rates at 6, 12, and 24 months were 59.3% vs. 42.1%, 20.3% vs. 17.5%, and 3.4% vs. 1.8% in the TACE-L and TACE-S groups, respectively (Supplementary Table 1). Before PSM, the subgroup analysis showed no difference in PVTT, the number of TACE procedures or ECOG.
After PSM, the median PFS time was 10.6 months (95% CI: 6.6–18.0 months) and 5.4 months (95% CI: 4.2–8.1 months) in the TACE-L and TACE-S groups, respectively (HR 2.62; 95% CI: 1.43–4.80; p = 0.002) (Figure 2). The estimated PFS rates at 6, 12, and 24 months were 52.6% vs. 34.2%, 23.7% vs. 15.8%, and 2.6% vs. 0% in the TACE-L and TACE-S groups, respectively. In the subgroup analysis based on PVTT classification, the median PFS of Vp2 PVTT was 9.67 months (95% CI: 8.76–10.58 months) vs. 5.33 months (95% CI: 4.38–6.28 months) (HR 6.93; 95% CI: 1.53-31.34; p < 0.001) in TACE-L vs. TACE-S, respectively. Based on the number of TACE procedures < 3 times, the median PFS was 9.03 months (95% CI: 4.68–13.38 months) vs. 5.2 months (95% CI: 3.77–6.63 months) (HR 3.56; 95% CI: 1.19–10.59; p = 0.02). For the number of TACE procedures ≥ 3 times, the median PFS was 16.4 months (95% CI: 7.80–25.00 months) vs. 6.37 months (95% CI: 5.50–7.24 months) (HR 1.99; 95% CI: 0.66–5.98; p = 0.222). The median PFS was 10.63 months (95% CI: 6.06–15.20 months) vs. 9.69 months (95% CI: 3.11–5.29 months) in the subgroup of patients with AFP < 400 ng/ml (HR 8.67; 95% CI: 1.40–53.85; p = 0.021) (Figure 3).
Tumor Response
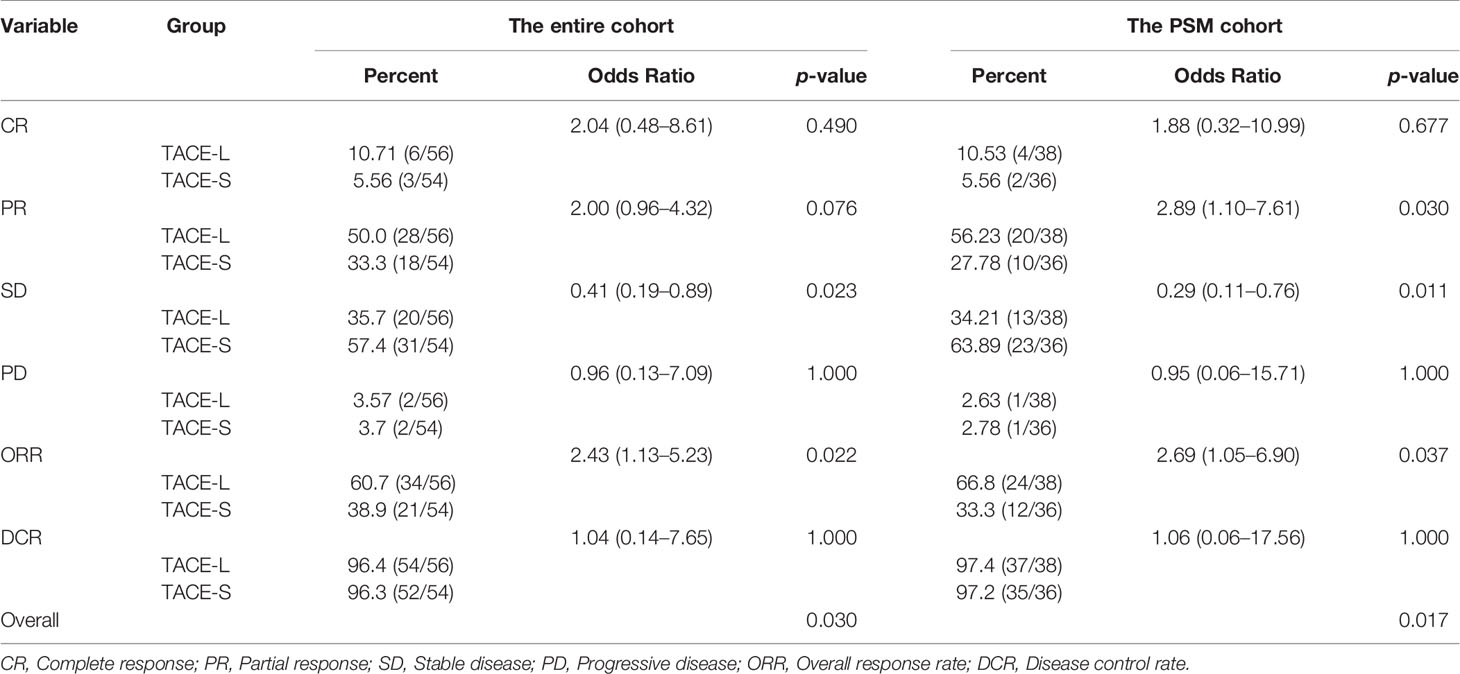
A total of 56 and 54 patients were evaluated for tumor response in the TACE-L and TACE-S groups. Comparing TACE-L and TACE-S, the CR was 10.71% vs. 5.56% (OR 2.04; 95% CI: 0.48–8.61; p = 0.49); the PR was 50.5% vs. 33.3% (OR 2.00; 95% CI: 0.96–4.32; p = 0.076); ORR was 60.7% vs. 38.9% (OR 2.43; 95% CI: 1.13–5.23; p = 0.022); the DCR was 96.4% vs. 96.3% (OR 1.04; 95% CI: 0.14–7.65; p = 1.00) (Table 2).
After PSM, the CR was 10.53% vs. 5.56% (OR 0.85; 0.32–10.99; p = 0.67), and the PR was 56.23% vs. 27.78% (OR 2.89; 1.10–7.61; p = 0.03) (Table 2). The ORR was 66.8% vs. 33.3% (OR 0.85; 1.05–6.90; p = 0.037) (Table 2), and the DCR was 97.4% vs. 97.2% (OR 1.06; 95% CI: 0.06–17.56; p = 1.00).
Univariable and Multivariable Analyses of OS in the PSM Cohort
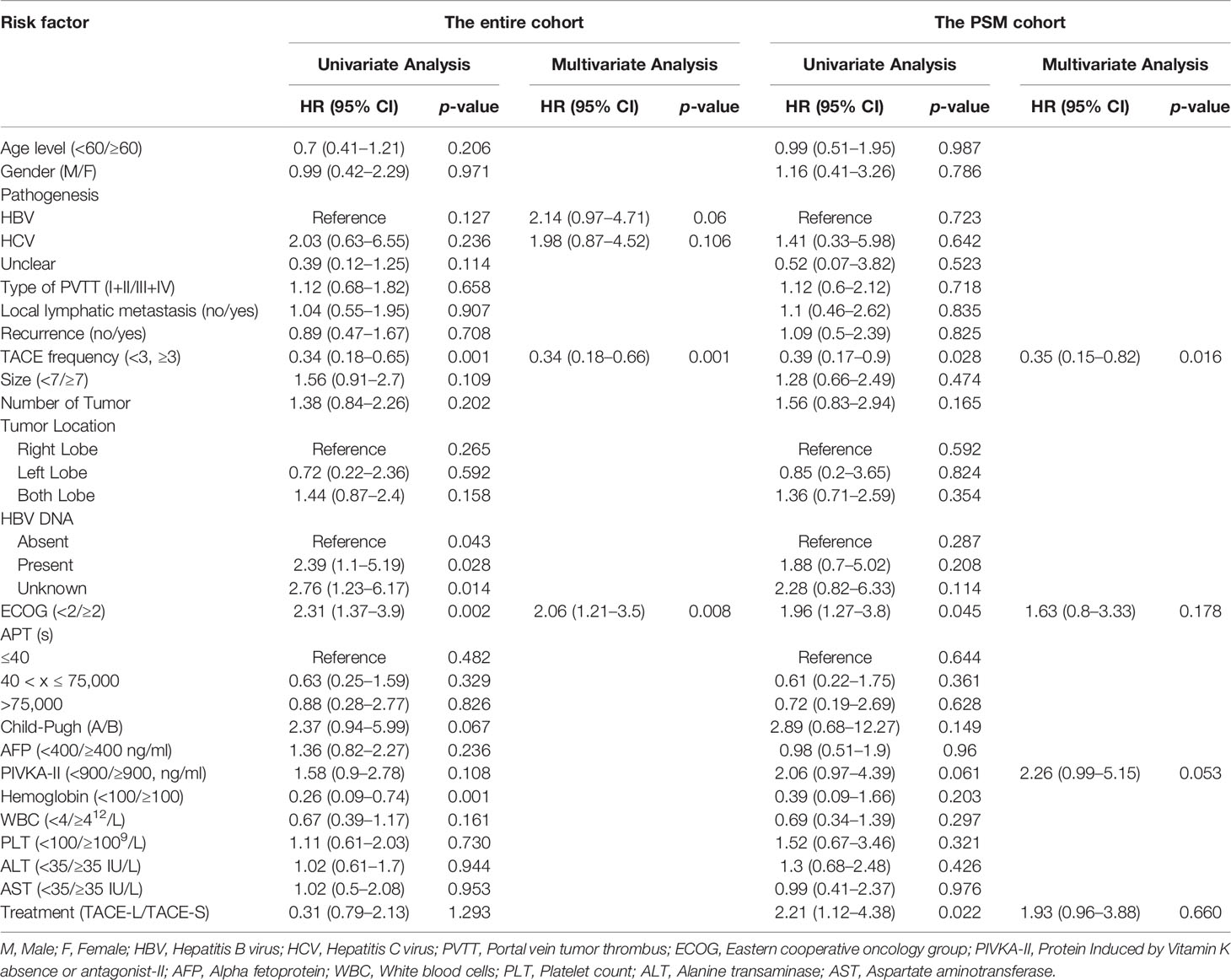
Additional univariable and multivariable Cox regression analyses with robust estimators were performed in the PSM cohort (Table 3). TACE frequency < 3 (HR, 0.39; 95% CI: 0.17–0.9, p = 0.028), ECOG < 2 (HR, 1.96; 95% CI: 1.02–1.75, p = 0.045), and treatment method (HR, 2.21; 95% CI: 1.12–4.38, p = 0.02) were critically important factors for longer overall survival.
Adverse Events
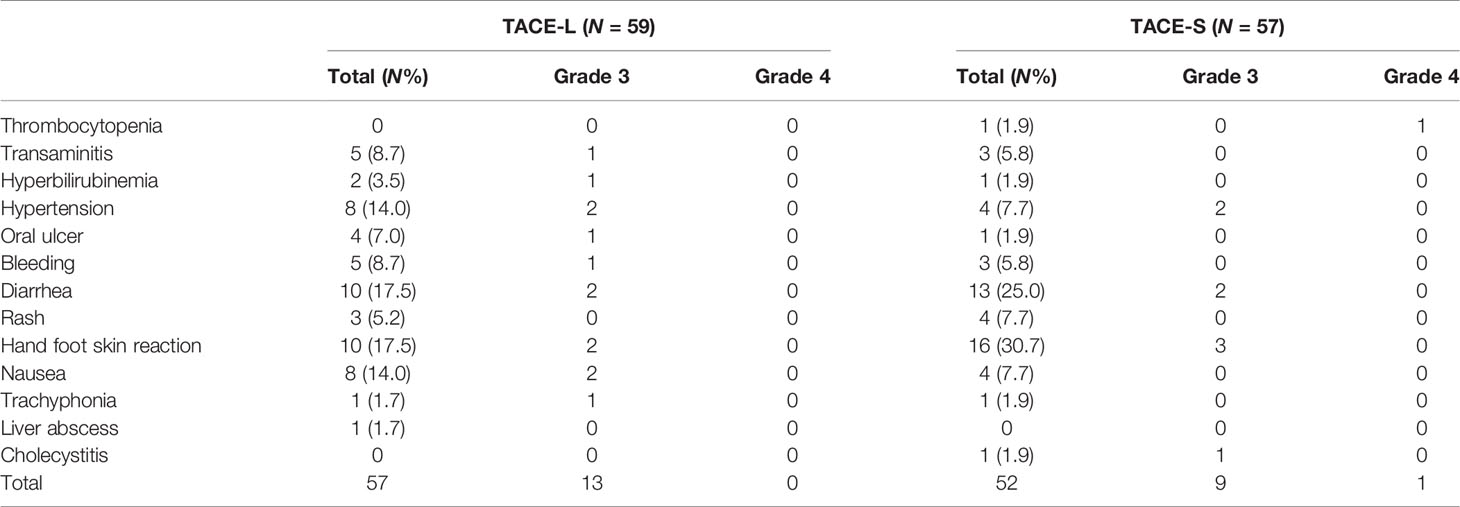
No treatment-related death was observed in either group (Table 4). One patient treated with TACE-S experienced cholecystitis and recovered through surgical resection. One patient treated with TACE-L had a liver abscess after the first chemoembolization, whose condition was improved after puncture drainage and antibiotics treatment. In the TACE-L group, a total of 57 complications were observed in 38 patients. The most frequent adverse events were hand–foot syndrome and diarrhea (17.5%). Grade 3–4 toxicities were observed in 13 (24.0%) patients. Three patients (7.9%) stopped using lenvatinib due to intolerance. Fifty-two complications were observed in 40 patients in the TACE-S group. The highest incidences were hand–foot syndrome (30.7%) and diarrhea (25.0%). Grade 3–4 toxicities were observed in 10 patients (17.50%). Two patients (5.0%) stopped using sorafenib due to intolerance.
Discussion
In the treatment of HCC with PVTT, accumulating evidence suggests that TACE-S has a higher OS rate than mono-sorafenib. Recently, the REFLECT study (7) showed that lenvatinib was superior to sorafenib with respect to PFS. Moreover, lenvatinib has a 64.87% cost-saving advantage than sorafenib (14). Ding et al. have reported TACE-L vs. TACE-S in HCC patients (15). Our study offers a comparison of the efficacy between TACE-L and TACE-S in HCC patients with PVTT.
Previously, several studies have demonstrated that lenvatinib is superior to sorafenib in PFS (p = 0.009) and TTP (p = 0.005) with a similar result in OS (HR 0.92, 95% CI: 0.79–1.06) (7). Our results revealed that TACE-L achieved significantly better benefits with respect to PFS, OS, and ORR than TACE-S in HCC patients with PVTT. These results are similar to those of a previously published study (15), in which TACE-L was better than TACE-S in HCC with PVTT in terms of median TTP (4.7 vs. 3.1 months, p = 0.037) and numerically better median OS (15.6 vs. 10.8 months, p = 0.15) and ORR (50.0 vs. 25.0%, p = 0.07). In our study, we did not observe any significant differences in either OS or PFS in the entire cohort but only after PSM. Subgroup analysis showed that TACE-L achieved a better PFS and OS than TACE-S in Vp2 PVTT and procedure times < 3.
For sorafenib treatment, TACE-S exhibited a better PFS benefit than TACE alone (p < 0.01) (16). Furthermore, Peng et al. (5) demonstrated that patients with PVTT had a better median OS (p < 0.001) and TTP (p < 0.001) in the TACE-S group than in the monosorafenib group. This is similar to a previous study reporting that TACE-S is superior to sorafenib with respect to TTP and OS (17). Furthermore, another study reported a significantly improved TTP (p = 0.003), PFS (p = 0.01), and ORR (p = 0.005) (18). Wu et al. (19) reported no difference between TACE-S and sorafenib in OS (p = 0.223). A similar study reported that TACE-S cannot improve OS compared to sorafenib (HR 0.91; 90% CI 0.69–1.21; p = 0.29) in patients with advanced HCC (20).
Only a few studies have tested the combination of TACE with lenvatinib. Previously, Zhang et al. (21) presented a DCR (92.3%) in TACE-L treatment of HCC with PVTT. This is similar to the DCR (97.4%) observed in our study. Chen et al. (22) demonstrated that the median disease-free survival was 12.0 months in the TACE-L group, which is similar to another study (8). He et al. (23) compared lenvatinib alone to transcatheter arterial infusion plus lenvatinib or toripalimab. The combination group achieved better PFS (11.1 versus 5.1 months, p < 0.001), OS (p < 0.001), and ORR (59.2% versus 9.3%, p < 0.001). The combination group reported a similar PFS and ORR to the TACE-L group in our study.
Compared to TACE-S, TACE-L resulted in a higher incidence of grade ≥3 adverse events in our study. This is entirely contradictory to a previous study (7), which reported fewer grade ≥3 adverse events with lenvatinib treatment. Similar to a previous study (24), no differences in serious adverse events or treatment-related deaths were observed between the TACE-L and TACE-S groups in our study. In another study, the most frequent adverse events were hypertension (17%) and proteinuria (12.2%) (8). However, the most frequent adverse events in our study were hand–foot syndrome and diarrhea. These differences might arise from different procedures. The previous study used both TACE with epirubicin and/or cisplatin and performed hepatic arterial infusion chemotherapy with cisplatin and 5-Fu (8).
Limitations
There are several limitations in this study that must be addressed. Although we performed PSM, this method does not entirely eliminate selection bias. This study had a relatively small sample size and did not allow us to do some analyses of subgroups. Especially after PSM, the sample size was further decreased. Hence, a large-scale and randomized study is needed.
Conclusions
Both TACE-L and TACE-S are safe, well-tolerated treatments for HCC patients with PVTT. For HCC patients with PVTT, TACE-L may be significantly superior to TACE-S with respect to OS, PFS, and ORR. A larger-scale randomized clinical trial is needed.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: www.medresman.org.
Ethics Statement
The studies involving human participants were reviewed and approved by West China Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study has received funding from the Department of Science and Technology of Sichuan Province of China (21YYJC2782), Health Commission of Sichuan Province (20PJ043), and Post-Doctor Research Project, West China Hospital, Sichuan University (2021HXBH023).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.821599/full#supplementary-material
References
1. Zhu K, Cheng J, Lai J, Meng X, Zhou B, Huang W. Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: Treatment With Transarterial Chemoembolization Combined With Sorafenib–a Retrospective Controlled Study. Radiol (2014) 272(1):284–93. doi: 10.1148/radiol.14131946
2. Yang B, Li C, Guo W, Qin T, Jiao H, Fei Z, et al. Intra-Arterial Ethanol Embolization Augments Response to TACE for Treatment of HCC With Portal Venous Tumor Thrombus. BMC Cancer (2018) 18(1):101. doi: 10.1186/s12885-018-3989-2
3. Bruix J, Sherman M, Llovet J, Beaugrand M, Lencioni R, Burroughs A, et al. Clinical Management of Hepatocellular Carcinoma. Conclusions of the Barcelona-2000 EASL Conference. European Association for the Study of the Liver. J Hepatol (2001) 35(3):421–30. doi: 10.1002/hep.24199
4. Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology (2011) 53(3):1020–2. doi: 10.1002/hep.24199
5. Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J. Advanced Recurrent Hepatocellular Carcinoma: Treatment With Sorafenib Alone or in Combination With Transarterial Chemoembolization and Radiofrequency Ablation. Radiology (2018) 287(2):705–14. doi: 10.1148/radiol.2018171541
6. Yamada K, Izaki K, Sugimoto K, Mayahara H, Yoshitaka M, Yoden E, et al. Prospective Trial of Combined Transcatheter Arterial Chemoembolization and Three-Dimensional Conformal Radiotherapy for Portal Vein Tumor Thrombus in Patients With Unresectable Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys (2003) 57(1):113–9. doi: 10.1016/S0360-3016(03)00434-6
7. Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, et al. REFLECT-A Phase 3 Trial Comparing Efficacy and Safety of Lenvatinib to Sorafenib for the Treatment of Unresectable Hepatocellular Carcinoma: An Analysis of Japanese Subset. J Gastroenterol (2020) 55(1):113–22. doi: 10.1007/s00535-019-01642-1
8. Shimose S, Iwamoto H, Tanaka M, Niizeki T, Shirono T, Noda Y, et al. Alternating Lenvatinib and Trans-Arterial Therapy Prolongs Overall Survival in Patients With Inter-Mediate Stage HepatoCellular Carcinoma: A Propensity Score Matching Study. Cancers (Basel) (2021) 13(1):160. doi: 10.3390/cancers13010160
9. Zhang Y, Miao H, Xie W, Jiang S, Song Z, Huang G, et al. The PPRD Score Stratifies Patients With Hepatocellular Carcinoma and Portal Vein Tumor Thrombus Treated With Sorafenib Plus Transarterial Chemoembolization. Eur Radiol (2021) 31(1):232–43. doi: 10.1007/s00330-020-07078-z
10. Rimassa L, Santoro A. Sorafenib Therapy in Advanced Hepatocellular Carcinoma: The SHARP Trial. Expert Rev Anticancer Ther (2009) 6(1744-8328(1744-8328 (Electronic). doi: 10.1586/era.09.41
11. Yang B, You X, Yuan M, Qin T, Duan L, He J, et al. Transarterial Ethanol Ablation Combined With Transarterial Chemoembolization for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus. Hepat Mon (2016) 16(8):e37584. doi: 10.5812/hepatmon.37584
12. Lencioni R, Llovet JM. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin Liver Dis (2010) 1098-8971(Electronic):052–60. doi: 10.1055/s-0030-1247132
13. Li X, Guo W, Guo L, Lau W, Ge N, Wang K, et al. Should Transarterial Chemoembolization be Given Before or After Intensity-Modulated Radiotherapy to Treat Patients With Hepatocellular Carcinoma With Portal Vein Tumor Thrombus? A Propensity Score Matching Study. Oncotarget (2018) 9(36):24537–47. doi: 10.18632/oncotarget.25224
14. Kim JJ, McFarlane T, Tully S, Wong W. Lenvatinib Versus Sorafenib as First-Line Treatment of Unresectable Hepatocellular Carcinoma: A Cost-Utility Analysis. oncologist (2020) 25(3):e512–9. doi: 10.1634/theoncologist.2019-0501
15. Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, et al. Transarterial Chemoembolization Plus Lenvatinib Versus Transarterial Chemoembolization Plus Sorafenib as First-Line Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Prospective Randomized Study. Cancer (2021) 127(20):3782–93. doi: 10.1002/cncr.33677
16. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, Multicentre Prospective Trial of Transarterial Chemoembolisation (TACE) Plus Sorafenib as Compared With TACE Alone in Patients With Hepatocellular Carcinoma: TACTICS Trial. Gut (2020) 69(8):1492–501. doi: 10.1136/gutjnl-2019-318934
17. Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, et al. Sorafenib Alone Versus Sorafenib Combined With Transarterial Chemoembolization for Advanced-Stage Hepatocellular Carcinoma: Results of Propensity Score Analyses. Radiology (2013) 269(2):603–11. doi: 10.1148/radiol.13130150
18. Park JW, Kim YJ, Kim DY, Bae SH, Park SW, Lee YJ, et al. Sorafenib With or Without Concurrent Transarterial Chemoembolization in Patients With Advanced Hepatocellular Carcinoma: The Phase III STAH Trial. J Hepatol (2019) 70(4):684–91. doi: 10.1016/j.jhep.2018.11.029
19. Wu FX, Chen J, Bai T, Zhu SL, Yang TB, Qi LN, et al. The Safety and Efficacy of Transarterial Chemoembolization Combined With Sorafenib and Sorafenib Mono-Therapy in Patients With BCLC Stage B/C Hepatocellular Carcinoma. BMC Cancer (2017) 17(1):645. doi: 10.1186/s12885-017-3545-5
20. Harper KAntibiotics Protect Against Liver Tumors in Mice. Cancer Discov (2018) 8(8):905–6. doi: 10.1158/2159-8290.CD-NB2018-078
21. Zhang L, Wang Y, Ge N, Gan Y, Ren Z, Chenn Y, et al. When Will it be Better? Lenvatinib Combined With TACE for Unresectable Hepatocellular Carcinoma: A Retrospective Analysis of Real-World Evidence in China. J Clin Oncol (2021) 39(3_suppl):281–1. doi: 10.1200/JCO.2021.39.3_suppl.281
22. Chen J-H, Lu L, Wen T, Huang Z, Zhang T, Zeng YY, et al. Adjuvant Lenvatinib in Combination With TACE for Hepatocellular Carcinoma Patients With High Risk of Postoperative Relapse (LANCE): Interim Results From a Muticenter Prospective Cohort Study. J Clin Oncol (2020) 38(15_suppl):4580–0. doi: 10.1200/JCO.2020.38.15_suppl.4580
23. He MK, Liang RB, Zhao Y, Xu YJ, Chen HW, Zhou YM, et al. Lenvatinib, Toripalimab, Plus Hepatic Arterial Infusion Chemotherapy Versus Lenvatinib Alone for Advanced Hepatocellular Carcinoma. Ther Adv Med Oncol (2021) 13:17588359211002720. doi: 10.1177/17588359211002720
24. Ding X, Sun W, Chen J, Li W, Shen Y, Guo X, et al. 171p Transarterial Chemoembolization (TACE) Plus Lenvatinib Versus TACE Plus Sorafenib for Hepatocellular Carcinoma (HCC) With Portal Vein Tumour Thrombus (PVTT): A Prospective Randomized Study. Ann Oncol (2020) 31:S1306. doi: 10.1016/j.annonc.2020.10.192
Keywords: hepatocellular carcinoma, lenvatinib, sorafenib, portal vein thrombosis, transcatheter arterial chemoembolization
Citation: Yang B, Jie L, Yang T, Chen M, Gao Y, Zhang T, Zhang Y, Wu H and Liao Z (2021) TACE Plus Lenvatinib Versus TACE Plus Sorafenib for Unresectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Prospective Cohort Study. Front. Oncol. 11:821599. doi: 10.3389/fonc.2021.821599
Received: 24 November 2021; Accepted: 01 December 2021;
Published: 23 December 2021.
Edited by:
Zongbing You, Tulane University, United StatesReviewed by:
Quan Wang, Peking University, ChinaXie Fujia, The First Affiliated Hospital of Kunming Medical University, China
Copyright © 2021 Yang, Jie, Yang, Chen, Gao, Zhang, Zhang, Wu and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biao Yang, landayb@163.com; Hao Wu, haowuwch@126.com; Zhengyin Liao, liaozhengyin@163.com
 Biao Yang
Biao Yang Luo Jie2
Luo Jie2 Mingyang Chen
Mingyang Chen Hao Wu
Hao Wu