- 1Department of Radiotherapy, Tangdu Hospital, Air Force Military Medical University, Xi’an, China
- 2Cancer Center, Faculty of Health Sciences, University of Macau, Macau, Macau SAR, China
Background: Intraoperative radiotherapy (IORT) and whole-breast irradiation (WBI) are both effective radiotherapeutic interventions for early breast cancer patients undergoing breast-conserving surgery; however, an issue on whether which one can entail the better prognosis is still controversial. Our study aimed to investigate the 5-year oncological efficacy of the IORT cohort and the WBI cohort, respectively, and compare the oncological efficacy between the cohorts.
Materials and Methods: We conducted a computerized retrieval to identify English published articles between 2000 and 2021 in the PubMed, the Web of Science, the Cochrane Library, and APA PsycInfo databases. Screening, data extraction, and quality assessment were performed in duplicate.
Results: A total of 38 studies were eligible, with 30,225 analyzed participants. A non-comparative binary meta-analysis was performed to calculate the weighted average 5-year local recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), and overall survival (OS) in the two cohorts, respectively. The LRFS, DMFS, and OS (without restriction on the 5-year outcomes) between the two cohorts were further investigated by a comparative binary meta-analysis. The weighted average 5-year LRFS, DMFS, and OS in the IORT cohort were 96.3, 96.6, and 94.1%, respectively, and in the WBI cohort were 98.0, 94.9, and 94.9%, respectively. Our pooled results indicated that the LRFS in the IORT cohort was significantly lower than that in the WBI cohort (pooled odds ratio [OR] = 2.36; 95% confidential interval [CI], 1.66–3.36). Nevertheless, the comparisons of DMFS (pooled OR = 1.00; 95% CI, 0.76–1.31), and OS (pooled OR = 0.95; 95% CI, 0.79–1.14) between the IORT cohort with the WBI cohort were both not statistically significant.
Conclusions: Despite the drastically high 5-year oncological efficacy in both cohorts, the LRFS in the IORT cohort is significantly poorer than that in the WBI cohort, and DMFS and OS do not differ between cohorts.
Research in Context
Evidence Before This Study
We searched PubMed, without language and date restrictions, on July 1, 2021, by using a retrieval strategy: (early breast cancer) AND (breast-conserving surgery) AND ((intraoperative radiotherapy) OR (whole-breast irradiation)) AND survival. The use of IORT and WBI was supported by a growing body of high-quality clinical studies. Since the advent of WBI was earlier than that of IORT, WBI was utilized more frequently than IORT for early breast cancer patients undergoing breast-conserving surgery. All studies showed similar disease-free survival (DFS) and overall survival (OS) outcomes between the IORT cohort and the WBI cohort but the results for local control, i.e., local recurrence-free survival (LRFS), were divergent. The current degree of uncertainty might be attributable to the heterogeneity of irradiation techniques used, the eligibility criteria of participants, and the overall reporting time of outcomes. To diminish this heterogeneity, a non-comparative binary meta-analysis was conducted to assess the 5-year oncological efficacy of a single dose of 21 Gy IORT delivered during surgery (i.e., excluded as a tumor bed boost before WBI), and a 45–50 Gy WBI with a 10–16 Gy boost, respectively. However, for including the largest number of eligible studies in a comparative binary meta-analysis that investigated the comparison of the oncological efficacy between the cohorts, we did not restrict the radiotherapeutic strategy and the overall reporting time in the inclusion criteria.
Added Value of This Study
This meta-analysis supported the use of IORT and WBI in clinical settings. The substantial to considerable heterogeneity of all included studies for the non-comparative meta-analysis gave the opportunity to better understand the importance of patient characteristics (e.g., mean age, tumor biology, histology, and molecular subtype) on prognostic outcomes. Our present study implicated the optimal 5-year oncological efficacy in the IORT cohort and the WBI cohort, and showed a significant decrease in the LRFS in the IORT cohort compared with the WBI cohort, despite no significant difference of DMFS and OS between the cohorts. Additionally, we emphasized the significance of choosing the most appropriate participants for the IORT cohort in the discussion section, because the local control failure was a devastating situation for the patients.
Implications of all the Available Evidence
Although early breast cancer patients undergoing breast-conserving surgery combined with IORT or WBI can achieve great oncological efficacy and similar DMFS and OS between the cohorts, criteria are awaited to establish the delivery strategies of techniques and the conditions of the patient for IORT. The established criteria need to involve some easily attained parameters before surgery (e.g., age, menopausal status, tumor size, hormone receptor status, molecular subtypes, and proliferation index), and the optimal dose and the delivery timeframe of IORT. The preoperative parameters should be stricter than the American Society of Radiation Oncology criteria for accelerated partial breast irradiation. About the irradiation strategy of IORT, two uncertainties require the answer. Is a single dose of 21 Gy the best dose of IORT? When the postoperative histopathology of the patient is at any of the following high risks, grade 3 tumor, lymph node-positive, and lymphatic vessel infiltration, whether IORT is considered as a tumor bed boost followed by WBI?
Introduction
Female breast cancer has surpassed lung cancer to become the most common diagnostic cancer in 2020; among women, breast cancer ranks first for the incidence of new diagnostic cancer and cancer-related mortality worldwide, with an estimated 2.254 million new cases (24.5%) and 0.682 million new deaths (15.5%) (1). Breast cancer in different stages is tailored to use corresponding treatment strategies. Several randomized controlled trials (RCTs) (2–6) and a meta-analysis of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (7) revealed the equivalent overall survival (OS) of early breast cancer patients treated with breast-conserving surgery followed by whole-breast irradiation (WBI) and those undergoing mastectomy. Another meta-analysis of the EBCTCG found that WBI significantly reduced the long-term risk of any first recurrence (i.e., local–regional relapse or distant metastasis) and long-term mortality of early breast cancer patients (8). The above findings demonstrate that breast-conserving surgery followed by WBI, conventionally consisting of 45–50 Gy over 4.5–5 weeks with or without a tumor bed boost, has been widely accepted as the standard care for early breast cancer.
In the past two decades, with the deep understanding of tumor biology and the introduction of many modern high-precision technologies into radiotherapy, we have witnessed the rapid development of radiotherapy and realized several limitations of the current WBI (e.g., lengthy treatment schedule, radiation effect, and long-term toxicity), which severely affect the acceptability, accessibility, and practical management of WBI (9). Serious medical challenges occur every day in the transitioning countries with limited resources or in the areas with a substantial distance from a radiotherapy center within the transitioned countries. In this context, an accelerated partial breast irradiation (APBI) with an attenuated planning target volume and shortened treatment duration is introduced to solve these problems.
Intraoperative radiotherapy (IORT) is an innovative form of APBI and has been applied by several radiotherapeutic/oncological institutes to treat early breast cancer during the past decade. The European Institute of Oncology (Milan, Italy) uses IORT to surrogate the postoperative WBI; after completion of lumpectomy, an intraoperative single shot of radiation with at least of the same biologically equivalent dose is delivered to the breast volume where the tumor is previously located (10). By contrast, the TARGIT-IORT trial group utilizes IORT as a treatment alternative for WBI or the tumor bed boost; when postoperative histopathology finds any of the unsuspected high risks, i.e., grade 3 tumor, lymph node-positive, and lymphatic vessel infiltration, IORT is supplemented by WBI (11). IORT provides the following advantages: an excellent delineation of tumor bed under visual control, a good dose homogeneity, an excellent normal tissue sparing (12), improved quality of life of patients, avoidance of tumor growth between the duration from the completion of surgery to the beginning of adjuvant radiotherapy, and the averted radiation to heart and lungs when a shield between breast and the pectoralis muscle is positioned (13).
The standardized dose of IORT introduced by the European Institute of Oncology is 21 Gy (13). In fact, the intraoperative single dose of 21 Gy is biologically equivalent to 1.5–2.5 folds of the total dose of WBI with or without boost (14). Since the advent of WBI and IORT, increasing bodies of radiation/oncology centers have evaluated the oncological efficacy and the possible local side-effects of both techniques (9, 15–17). In the recent decade, many RCTs have centered on the comparison of local–regional control, distant metastasis, and OS between the early breast cancer patients undergoing WBI (defined as the WBI cohort) and those undergoing IORT (defined as the IORT cohort) (18–20). For example, end-of-study results of an RCT after a median follow-up of 12.4 years indicated that the IORT cohort had a 9% higher of ipsilateral breast tumor recurrence rate than the WBI cohort (hazard ratio [HR] = 4.62; 95% CI, 2.68–7.95; P <0.0001) (18). However, because of the diversity in demography, histopathology, and systematic treatment modality across different clinical studies, the reported results in the oncological efficacy of the cohorts, and the efficacy comparison between them are discordant. In this context, our present study aimed to settle this issue by performing a non-comparative binary meta-analysis to investigate the individually weighted average proportion of 5-year oncological efficacy in the WBI cohort and the IORT cohort, respectively, and a comparative binary meta-analysis to compare the oncological efficacy between the cohorts.
Materials and Methods
This meta-analysis complied with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (21, 22). There was no need for Ethical or Institutional Review Board Approval for the study design due to the nature of our work.
Literature Search
A computerized retrieval was conducted on July 9, 2021 in the PubMed, the Web of Science, the Cochrane Library, and the APA PsycInfo databases to identify English published articles. The following terms were used: ((“breast cancer[MesH]” AND early) OR (early breast tumor) OR (early breast tumour) OR (early breast carcinoma) OR (early breast cancer) OR (early-stage breast tumor) OR (early-stage breast tumour) OR (early-stage breast carcinoma) OR (early-stage breast cancer)) AND (((intra-operative OR intraoperative) AND (irradiation OR radiation OR radiotherapy)) OR (whole-breast irradiation) OR (whole breast radiotherapy)) AND (recurrence OR relapse OR reappearance OR metastasis OR survival).
Inclusion and Exclusion Criteria
Clinical articles reporting the oncological efficacy of the WBI cohort or the IORT cohort, or comparing that between the cohorts was considered to be eligible. Additionally, potential studies with publication year from 2000 to 2021 were required to meet the following inclusion criteria: (1) populations—patients with early breast cancer; (2) treatment strategy—breast-conserving surgery plus WBI or IORT; (3) endpoints—local recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), and/or OS. As a significantly decreased local recurrence (LR) rate in WBI with a boost compared with WBI without a boost (23), only the clinical studies that patients underwent 45–50 Gy WBI with a 10–16 Gy boost or a single dose of 21 Gy IORT were included into computing the weighted average proportion of 5-year oncological efficacy in the WBI cohort or the IORT cohort, respectively. Of note, comparison of the oncological efficacy between the cohorts did not merely focus on the 5-year outcomes and did not confine to the same delivery paradigm (i.e., the hypofractionated radiotherapy or WBI without a boost was also eligible to the WBI cohort, and IORT as a boost followed by WBI when postoperative histopathology experienced any of the previously mentioned high risks was also involved in the IORT cohort). Besides, retrieved citations that emerged any of the following criteria were removed: (1) article type—reviews, case reports, case series that involved less than 10 patients, editorials, letters, comments, and conference papers; (2) populations—female patients who underwent neoadjuvant treatment prior to breast-conserving surgery, and male patients; (3) the studies for calculating the weighted average proportion did not apply the above-mentioned delivery paradigm and/or did not report the 5-year outcomes; and (4) overlapping study populations.
Data Extraction and Quality Assessment
We extracted the following data from the included studies by using a standardized form: (1) study characteristics—family name of the first author, publication year, recruitment duration, original nation, study type, number of patients, and median follow-up; (2) demographic characteristics—mean age, molecular subtype, tumor size, lymph node status, and tumor grade; and (3) outcome characteristics—the event number of LRFS, DMFS, and OS in WBI cohort and/or IORT cohort. The LRFS was defined as the time from diagnosis to the time of tumor reappearing at the site of the surgical resection. We defined the DMFS as the time from diagnosis to the time of any recurrence of carcinoma to distant organs and/or tissues. The OS was defined as the time from diagnosis to last follow-up or time of death. Quality assessment of the analyzed studies for the comparative meta-analysis was judged by drawing figures of the risk of bias summary and the risk of bias graph with Review Manager 5.4 (https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman). The included studies only for calculating the weighted average proportion did not require quality assessment due to the non-comparative data that were impossible or fair to assign equal weight to different quality aspects. Two co-authors (JZ and QW) independently assessed the literature search, study selection, and data extraction. If there were any inconsistencies, they were addressed by a third co-author (YQ).
Data Synthesis and Statistical Analysis
The primary outcome for calculating the weighted average proportion of the 5-year oncological efficacy in the cohorts included the rates of LRFS, DMFS, and OS with corresponding 95% confidence intervals (CIs), and for comparing the oncological efficacy between the cohorts was presented as odds ratio (OR) with 95% CIs. The crude proportions or ORs were independently calculated and then pooled together. The number of events, if it was not provided in the article, was calculated in terms of the endpoint percentage or other relevant information. The heterogenicity that implicated the degree of variability in results across the included studies was assessed by Cochran’s Q test and Higgins I2 statistic test (24); P <0.10 suggested significant heterogeneity, and different cutoff intervals of I2 values at 0–25%, 25–50%, 50–75%, and 75–100% corresponded to nonsignificant, moderate, substantial, and considerable heterogeneity, respectively. When the heterogeneity test was not statistically significant (P ≥0.10), a binary fixed-effect model, the Mantel–Haenszel method was used to pool data, and if not so, a binary random-effect model, the Dersimonian–Laird method was employed (25). The publication bias in the comparative meta-analysis was evaluated by an Egger’s test with a significant level of P <0.05. All statistical analyses were performed by the software Open Meta-Analyst (http://www.cebm.brown.edu/openmeta/download.html).
Results
Literature Search
A PRISMA flow diagram of the literature screening selection is outlined in Figure 1. A total of 1,996 citations were obtained from the PubMed, the Web of Science, the Cochrane Library, and the APA PsycInfo databases, and 449 reduplications, 248 conference papers, 45 reviews, and 6 case reports were excluded. The remaining 1,248 citations were evaluated by title and abstract screening, and 1,422 of them were removed; fundamental characteristics of the abstracts were judged with respect to the inclusion and exclusion criteria, and 73 full-length articles were chosen. After full-text scrutinization, 35 of them were further omitted for the following reasons: (1) no provision of the 5-year outcomes in 15 potential articles for calculating the weighted average proportion; (2) no application of a 10–16 Gy boost to the patient in 13 potential studies for calculating the weighted average proportion in the WBI cohort; (4) no usage of a single dose of 21 Gy in five potential studies for calculating the weighted average proportion in the IORT cohort; and (5) 2 articles with other reasons. Ultimately, 38 articles with 30,225 early breast cancer patients were involved (9–12, 15–20, 26–53), in which 18 exclusively analyzed the 5-year oncological efficacy in the WBI cohort (15, 16, 26–41), 9 exclusively analyzed that in the IORT cohort (9, 12, 17, 42–47), and 11 compared the oncological efficacy between the cohorts (10, 11, 18–20, 48–53). Three of the 11 studies for the comparative meta-analysis had the available data to calculate the weighted average 5-year oncological efficacy in the non-comparative meta-analysis (10, 18, 20).
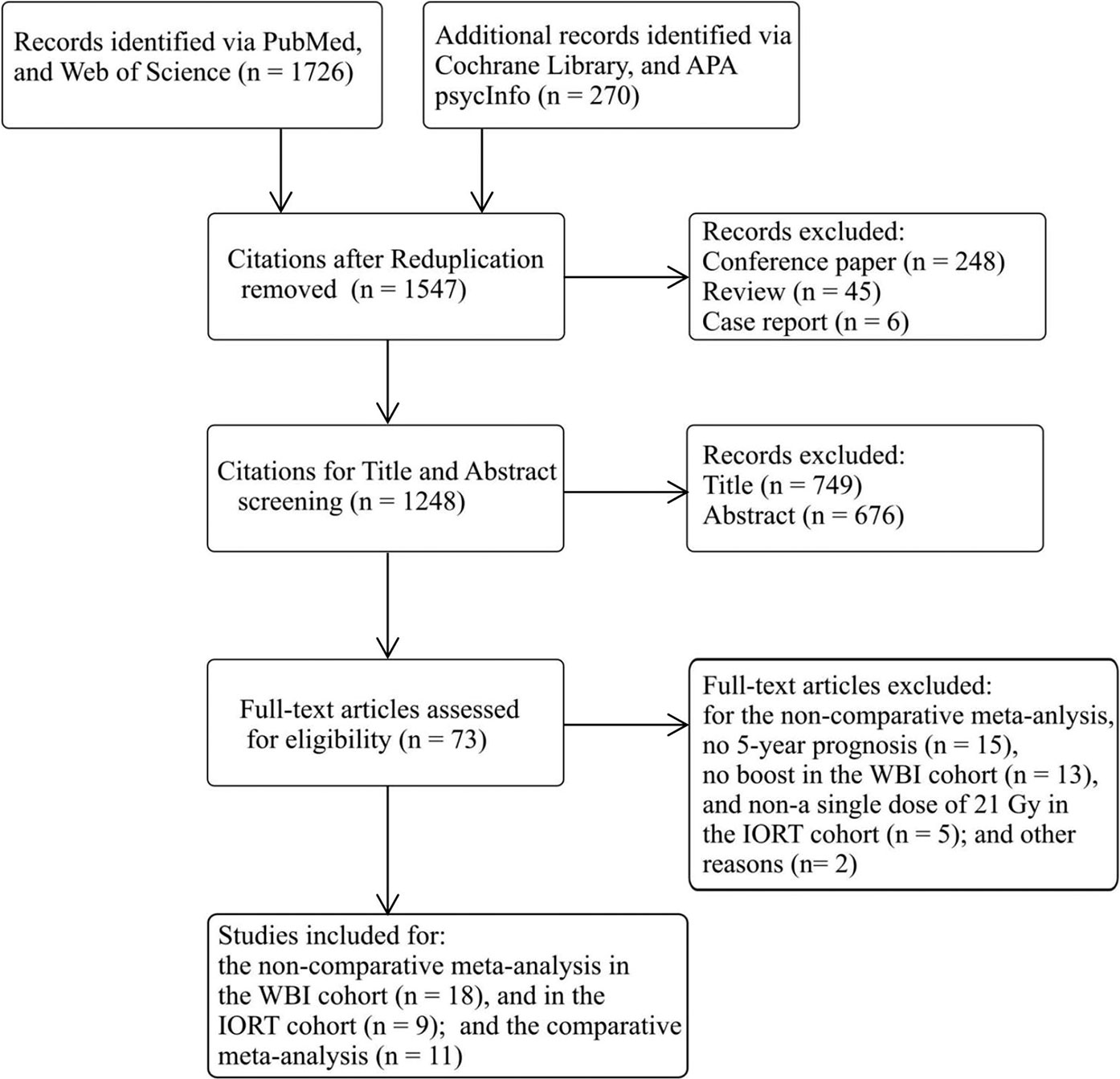
Figure 1 PRISMA flow diagram of study selection. IORT, intraoperative radiotherapy; WBI, whole-breast irradiation.
Characteristics of the Studies Included for Meta-Analysis
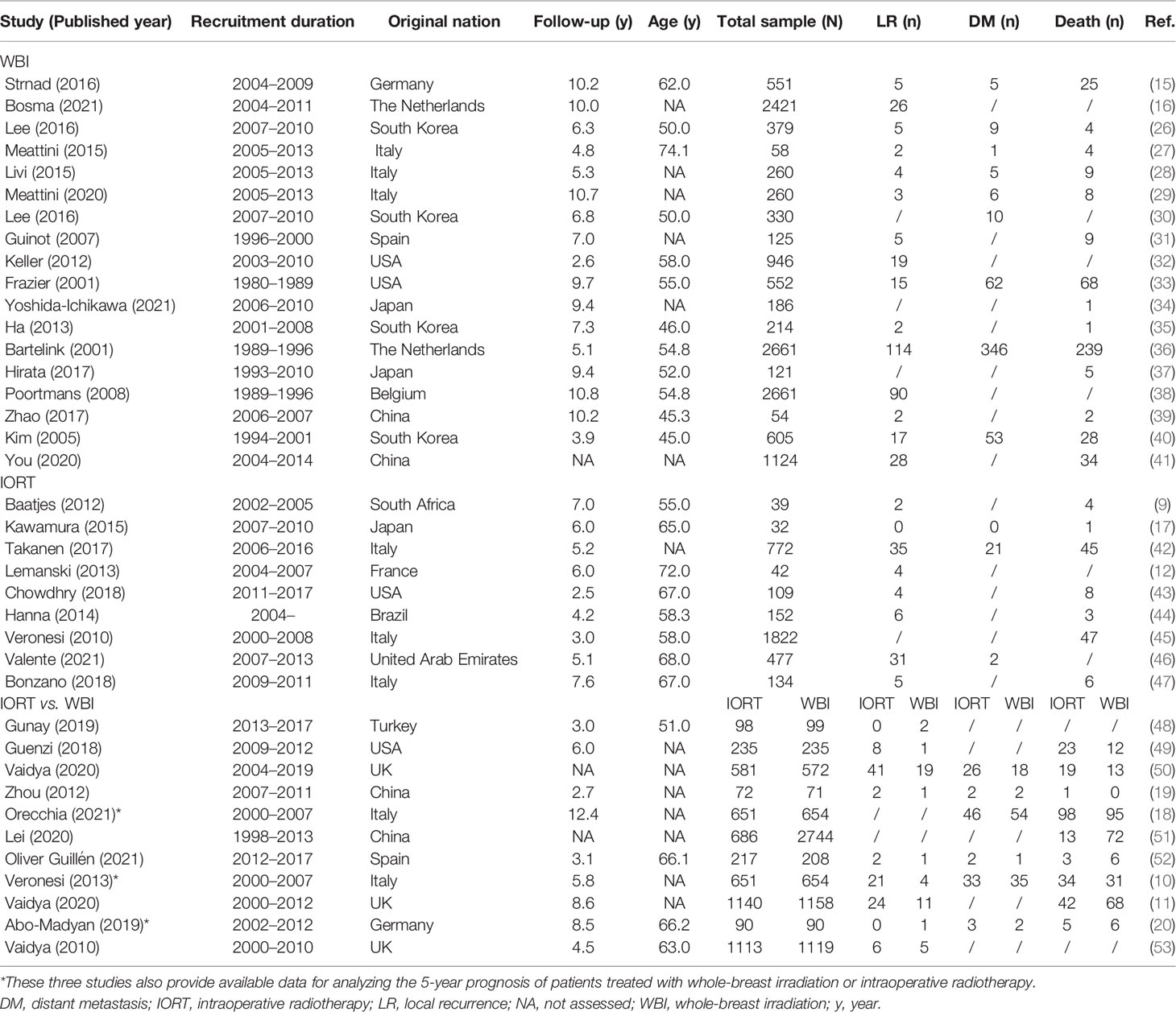
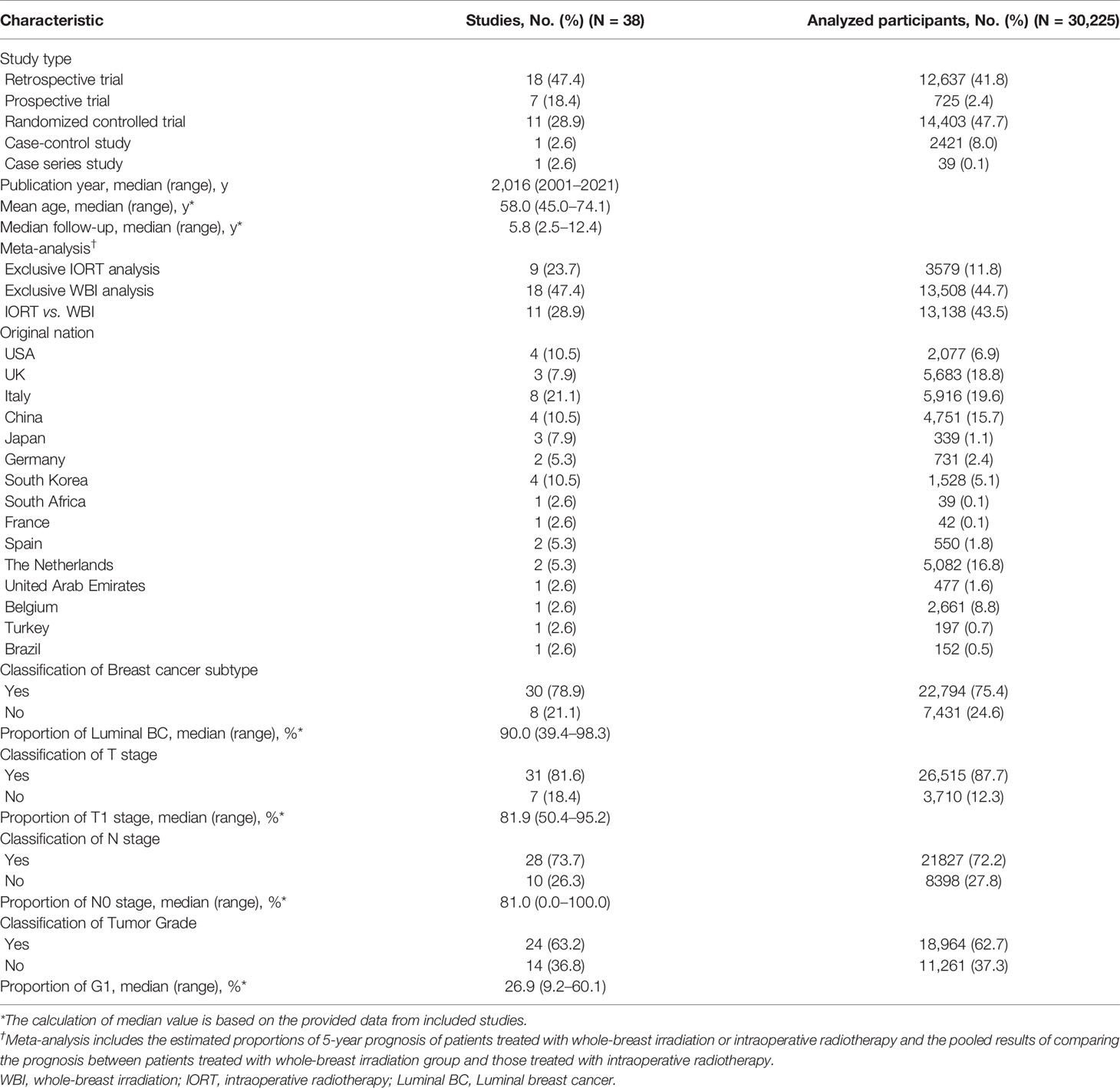
The characteristics of the 38 included studies in the “study-level” analysis are presented in Table 1, and those in the “patient-level” analysis are summarized in Table 2. Nearly half of the studies (n = 18) were categorized as retrospective trials; the publication year ranged from 2001 to 2021 (median: 2016); the median value of the mean age calculated from all the available studies was 58.0 years (45.0–74.1), and that of the median follow-up was 5.8 years (2.5–12.4); Italy ranked first for the original nation of all the involved studies (n = 8). Thirty studies provided the information of breast cancer subtype, with the median proportion of Luminal breast cancer of 90.0% (39.4–98.3); 31 studies provided the information of tumor size, with the median proportion of T1 stage of 81.9% (50.4–95.2); 28 studies provided the information of lymph node status, with the median proportion of N0 stage of 81.0% (0.0–100.0); and 24 studies provided the information of tumor grade, and the median proportion of tumor of grade 1 was 26.9% (9.2–60.1). Additionally, Table 1 provides the event number of LR, DM, and death from all the analyzed studies.
LRFS
We collected 10 (9, 10, 12, 17, 20, 42–44, 46, 47) and 17 (10, 15, 16, 20, 26–29, 31–33, 35, 36, 38–41) articles for the calculation of the weighted average 5-year LRFS rates in the IORT cohort and the WBI cohort, respectively; the pooled result indicated them were 96.3% (95% CI, 94.9–97.7%) and 98.0% (95% CI, 97.3–98.6%), respectively (Table 3). There were nine studies included for the comparison of LRFS rate between the cohorts (10, 11, 19, 20, 48–50, 52, 53). The pooled result showed a significantly lower LRFS rate in the IORT cohort than the WBI cohort (OR = 2.36; 95% CI, 1.66–3.36) (Figure 2A).
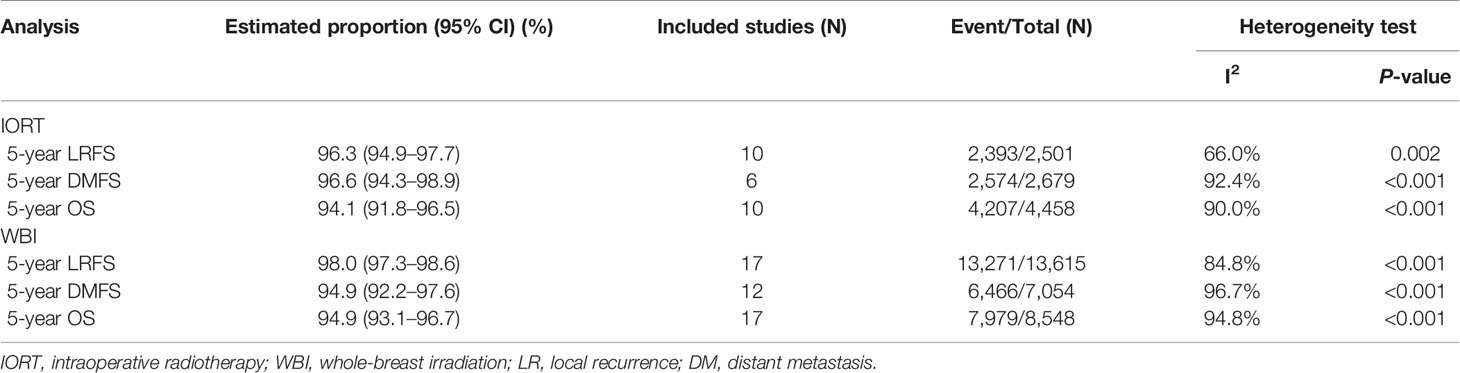
Table 3 The weighted average proportion of 5-year oncological efficacy via meta-analysis with random-effect model.
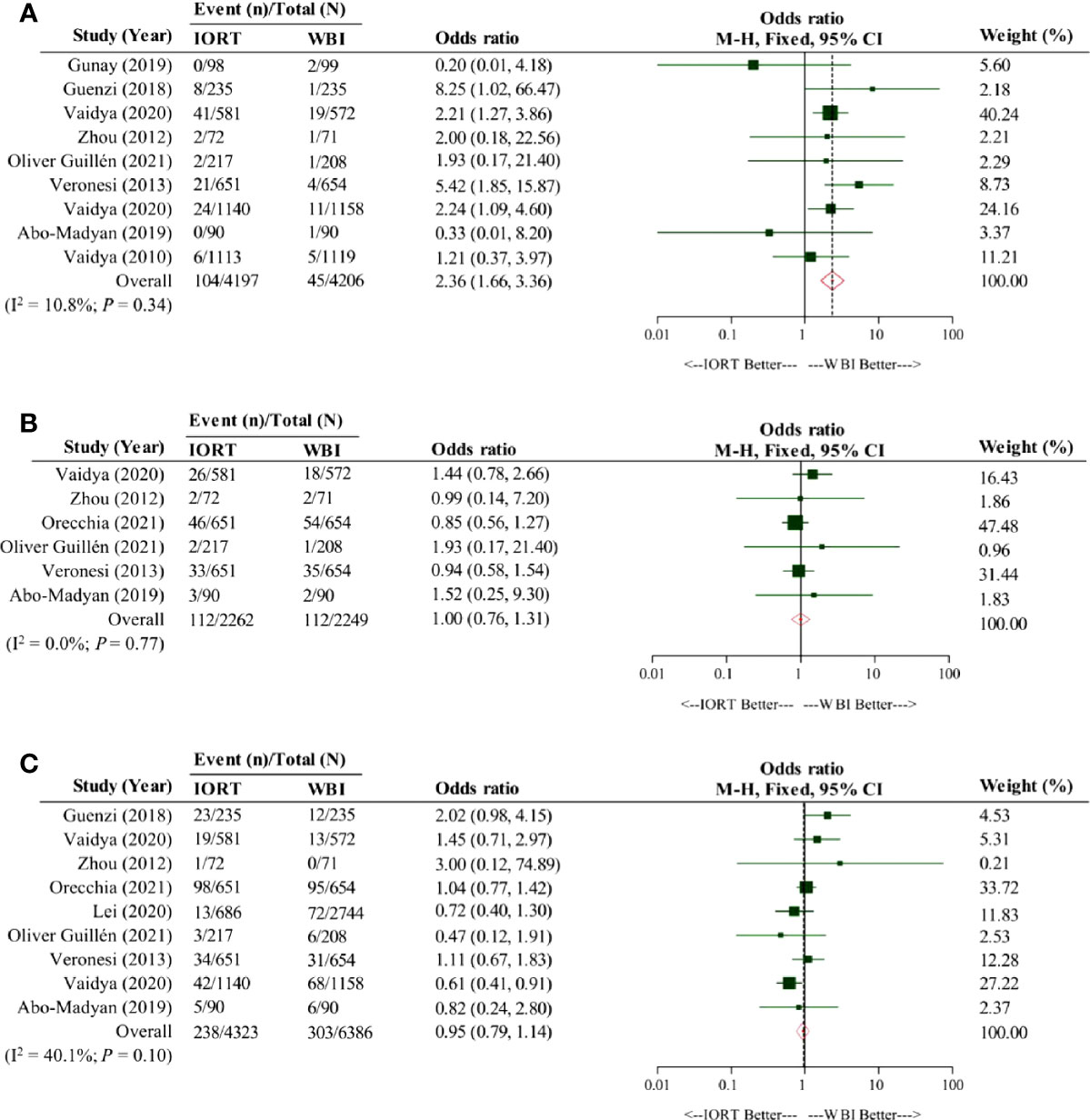
Figure 2 Pooled forest plot for comparison of the oncological efficacy between intraoperative radiotherapy cohort and whole-breast irradiation cohort. Panel (A) shows the pooled forest plot for comparison of local relapse-free survival, Panel (B) shows the pooled forest plot for comparison of distant metastasis-free survival, and Panel (C) shows the pooled forest plot for comparison of overall survival. IORT, intraoperative radiotherapy cohort; WBI, whole-breast irradiation cohort.
DMFS
Six (10, 17, 18, 20, 42, 46) and 12 (10, 15, 18, 20, 26–30, 33, 36, 40) studies were respectively involved for the analysis of the weighted average 5-year DMFS rates in the IORT cohort and the WBI cohort. Our pooled result showed that the weighted average 5-year DMFS rate in the IORT cohort (96.6% [95% CI, 94.3–98.9%]) outnumbered that in the WBI cohort (94.9% [95% CI, 92.2–97.6%]) (Table 3). Based on the original data from six analyzed articles (10, 18–20, 50, 52), the pooled result suggested that the DMFS rate in the IORT cohort was not significantly different from that in the WBI cohort (OR = 1.00; 95% CI, 0.76–1.31) (Figure 2B).
OS
The OS of early breast cancer patients undergoing IORT or WBI was the greatest concerning issue for which ten (9, 10, 17, 18, 20, 42–45, 47) and 17 (10, 15, 18, 20, 26–29, 31, 33–37, 39–41) studies were respectively obtained to calculate the weighted average 5-year OS rates in the IORT cohort and the WBI cohort; the pooled result revealed that the weighted average 5-year OS rate in the IORT cohort (94.1% [95% CI, 91.8–96.5%]) was nearly equivalent to that in the WBI cohort (94.9% [95% CI, 93.1–96.7%]) (Table 3). By analyzing the crude data from nine included studies (10, 11, 18, 19, 49–53), our pooled result consistently implicated a similar OS between the cohorts (OR = 0.95; 95% CI, 0.79–1.14) (Figure 2C).
Risk of Bias
The 11 studies for the comparative meta-analysis were combined to judge each risk of bias domain. The risk of bias summary and the risk of bias graph are shown in Figures 3A, B. We moreover assessed the detailed risk of bias in the 11 articles (Supplementary Materials: eTable 1, page 1–2). Overall, all clinical trials were at high risk of bias concerning the allocation concealment, the blinding of participants and personnel, and also the blinding of outcome assessment.
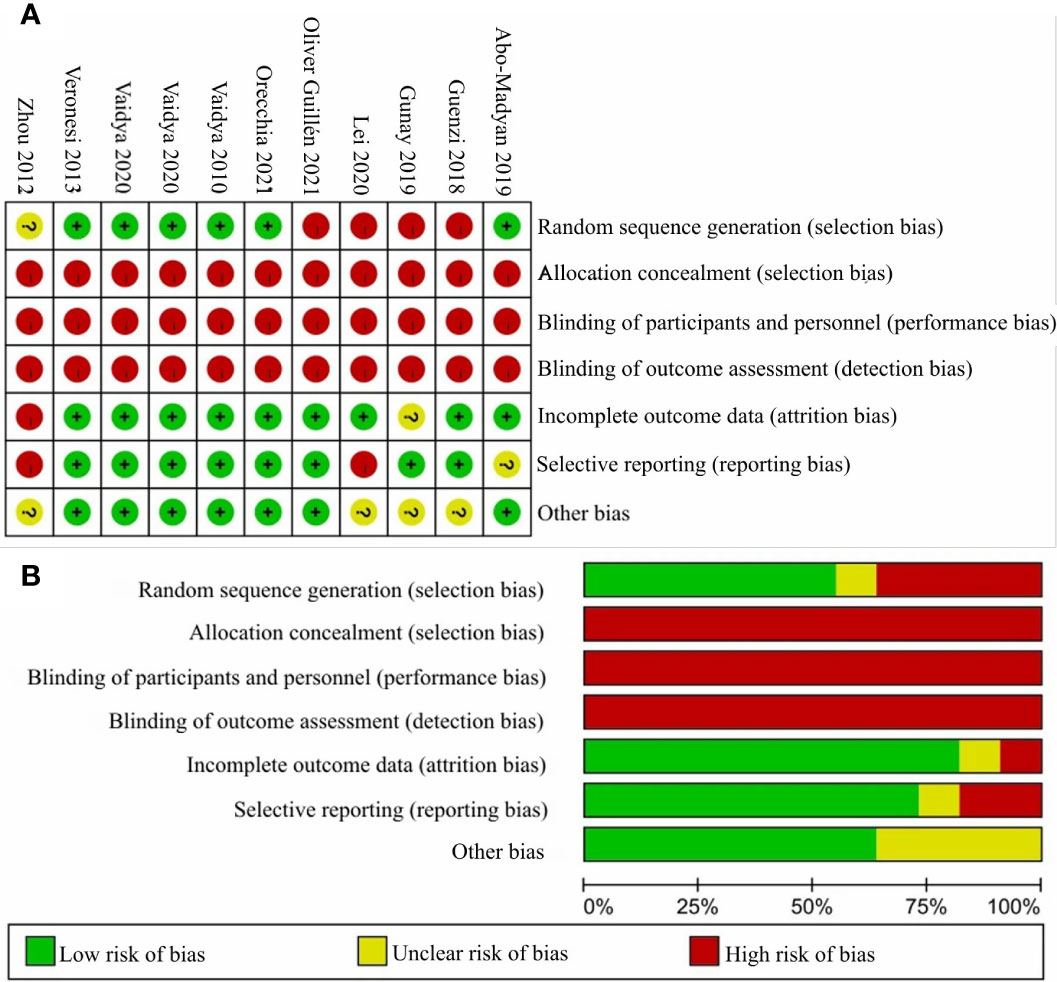
Figure 3 The judgments of risk of bias summary and risk of bias graph. Panel (A) shows the judgement of risk of bias summary and Panel (B) shows the judgement of risk of bias graph.
Publication Bias
The publication bias results in the comparison of LRFS, DMFS, and OS between the cohorts in terms of the Egger’s test all were statistically nonsignificant (P = 0.474, 0.269, and 0.680, respectively) (Supplementary Materials: eTable 2, page 2). The results indicated that no publication bias existed in all comparative meta-analysis.
Discussion
The non-comparative meta-analysis demonstrates the outstanding 5-year oncological efficacy in early breast cancer patients who undergo breast-conserving surgery combined with a single 21 Gy IORT or a 45–50 Gy WBI plus a 10–16 Gy boost. The 5-year weighted average LRFS, DMFS, and OS in the IORT cohort were 96.3, 96.6, and 94.1%, respectively, and in the WBI cohort were 98.0, 94.9, and 94.9%, respectively. As suggested by Vaidya and colleagues, the rates of overall complications (17.6% in the IORT cohort vs 15.5% in the WBI cohort; P = 0.19) and major toxicity (3.3% in the IORT cohort vs 3.9% in the WBI cohort; P = 0.44) were similar in two cohorts (53). Nonetheless, some women after WBI always suffer from breast atrophy, malformation of the irradiated breast, pigmentation, and rough skin (19). By contrast, the quality of life (e.g., cosmesis, and breast pain) of patients and the economization of healthcare system resources are optimized by the introduction of IORT (54, 55). An estimated savings of around £15 million is ascertained annually with the use of IORT in countries like the UK (56). The popularity of IORT for the treatment of early breast cancer is witnessed in the USA, with a tenfold increment from 2010 to 2013 in light of an analysis in the Surveillance, Epidemiology, and End Results Program database (51). Thanks to its unique advantages, IORT is more desirable than WBI in early breast cancer patients, even in the face of a hypothetically poorer local control.
LRFS has received great attention when planning the treatment strategy for early breast cancer patients. Our findings confirm the inferiority of LRFS in the IORT cohort compared with the WBI cohort, which is consistent with the conclusion of a retrospective observational study with a median follow-up of six years (49) but is marginally discordant to that of the phase 3 TARGIT-A RCT (50) that only shows a statistically significant trend. Demographically, the proportional distributions of Luminal breast cancer, T1 grade, N0 grade, and HER2-negative status in the cohorts of the two studies were well-balanced. In contrast, tumors of grade 2 predominantly accounted for 3/4 cases of the cohorts in the observational study (74.9% in the IORT cohort and 75.7% in the WBI cohort), but tumors of grade 1 were the main part of both cohorts in the TARGIT-A RCT (56.5% in the IORT cohort and 63.8% in the WBI cohort); WBI used in the observational study, in fact, was a hypofractionated schedule, i.e., hypofractionated radiotherapy, and the TARGIT-A RCT applied IORT as an intraoperative boost to the patients when they had any of high risks. As such, the difference in tumor grade and irradiation delivery may influence the incidence of LRFS. Given no heterogeneity among all studies (I2 = 10.8%; P = 0.34) (Figure 2A), the present study concludes that in the significantly poorer LRFS of early breast cancer patients undergoing IORT than WBI.
Clear evidence for no impact of the inferior LRFS in the IORT cohort on the DMFS, cancer-specific survival, and OS exists (10, 18, 51, 57). Our present results mirror these data, that is, an insignificant difference of DMFS and OS between the cohorts. The weighted average 5-year OS in both cohorts was nearly equivalent (94.1% in the IORT cohort and 94.9% in the WBI cohort, respectively); however, the weighted average 5-year DMFS in the IORT cohort (96.6%) was numerically greater than that in the WBI cohort (94.3%), corresponding to an absolute excess of 2.3% weighted average 5-year DMFS in the IORT cohort. The inconsistent DMFS outcomes between the non-comparative meta-analysis and the comparative meta-analysis may have the following explanations: (1) imbalanced tumor histological distribution, and (2) diverse radiotherapy doses and irradiation delivery techniques across all included studies for the two meta-analyses. To obviate the interference of different irradiation strategies, we conducted a subgroup analysis that compared the difference of DMFS between the IORT cohort and the WBI cohort, with the delivery strategy mapping to the non-comparative meta-analysis, and still observed that the difference was not statistically significant (OR = 0.90; 95%CI, 0.66–1.22) (Supplementary Materials: eFigure 1, page 2).
Albeit a decreased LRFS in the IORT cohort cannot compromise the DMFS and OS of patients, the improper treatment selection-caused local control failure in itself is an undesirable situation for patients and an additional economic burden to the healthcare system resources. It is therefore needed to determine which patients undergoing IORT are at high risk or at very low risk to develop LR. For the patients at high risk of LR, WBI with a boost or IORT supplemented by WBI is the more suitable alternative. Veronesi et al. (10) assessed the association between the characteristics of the patient in the IORT cohort and LR and identified that tumor size >2 cm, tumor of grade 3, positive lymph nodes ≥4, and triple-negative breast cancer were the high-risk factors for LR. An unplanned analysis in the long-term ELIOT phase 3 equivalence RCT (18) proposed a criterion for the very low-risk group: well-differentiated Luminal A tumor with a proliferation index (Ki-67) <14%, and tumor size <1 cm; the 5-year, 10-year, and 15-year rates of ipsilateral breast tumor recurrence in the IORT cohort and the WBI cohort were all very low and not significantly different (HR = 1.97; 95% CI, 0.36–10.8; P = 0.45). However, the definitive criteria for the high-risk group and the very low-risk group need to be verified in several independent datasets before they can be considered as any reliable statement in the clinical guidelines and consensus.
There were some limitations in the present study. First, the majority of analyzed studies for the non-comparative meta-analysis were performed retrospectively, which might indicate other biases due to the data collection and subject selection. Second, because the characteristics of the patient across the included studies for the non-comparative meta-analysis were divergent, the heterogeneity test indicated substantial to considerable heterogeneity, and thus the random-effect model was used to pool the data. Furthermore, we did not perform the subgroup analysis in terms of the high-risk group or the very low-risk group due to the limited data. Lastly, our study was absent from the analysis of long-term toxicity between the cohorts.
Conclusions
This study demonstrates the optimal 5-year LRFS, DMFS, and OS in early breast cancer patients who undergo IORT or WBI. Additionally, the LRFS in the IORT cohort is significantly lower than that in the WBI cohort, while the DMFS and OS between the cohorts are devoid of significant difference.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
LH: Writing manuscript, Statistical analysis. JZ: Writing manuscript, data collection. YQ: Writing manuscript, supervision. DH: Table drawing. CY: Figure drawing. HC: Validity. QW: Supervision. GL: Data collection. QS: Conception/Design, final approval of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.759903/full#supplementary-material
Abbreviations
RCTs, randomized controlled trials; EBCTCG, Early Breast Cancer Trialists’ Collaborative Group; OS, overall survival; WBI, whole-breast irradiation; APBI, accelerated partial breast irradiation; IORT, intraoperative radiotherapy; PRISMAl, Preferred Reporting Items for Systematic Reviews and Meta-analyses; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival; LR, local recurrence; CIs, confidence intervals; OR, odds ratio.
References
1. Sung H, Ferlay J, Siegel RL. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Sarrazin D, Lê MG, Arriagada R, Contesso G, Fontaine F, Spielmann M, et al. Ten-Year Results of a Randomized Trial Comparing a Conservative Treatment to Mastectomy in Early Breast Cancer. Radiother Oncol (1989) 14:177–84. doi: 10.1016/0167-8140(89)90165-5
3. Blichert-Toft M, Rose C, Andersen JA, Overgaard M, Axelsson CK, Andersen KW, et al. Danish Randomized Trial Comparing Breast Conservation Therapy With Mastectomy: Six Years of Life-Table Analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr (1992) 19–25.
4. Veronesi U, Salvadori B, Luini A, Greco M, Saccozzi R, del Vecchio M, et al. Breast Conservation Is a Safe Method in Patients With Small Cancer of the Breast. Long-Term Results of Three Randomised Trials on 1,973 Patients. Eur J Cancer (1995) 31a:1574–9. doi: 10.1016/0959-8049(95)00271-j
5. Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and Results After 12 Years of Follow-Up in a Randomized Clinical Trial Comparing Total Mastectomy With Lumpectomy With or Without Irradiation in the Treatment of Breast Cancer. N Engl J Med (1995) 333:1456–61. doi: 10.1056/NEJM199511303332203
6. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-Term Results of a Randomized Trial Comparing Breast-Conserving Therapy With Mastectomy: European Organization for Research and Treatment of Cancer 10801 Trial. J Natl Cancer Inst (2000) 92:1143–50. doi: 10.1093/jnci/92.14.1143
7. (EBCTCG) EBCTCG. Effects of Radiotherapy and Surgery in Early Breast Cancer. An Overview of the Randomized Trials. N Engl J Med (1995) 333:1444–55. doi: 10.1056/NEJM199511303332202
8. (EBCTCG) EBCTCG. Effect of Radiotherapy After Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10,801 Women in 17 Randomised Trials. Lancet (2011) 378:1707–16. doi: 10.1016/S0140-6736(11)61629-2
9. Baatjes KJ, Apffelstaedt JP. 7-Year Follow Up of Intra-Operative Radiotherapy for Early Breast Cancer in a Developing Country. Breast (2012) 21:326–9. doi: 10.1016/j.breast.2012.01.021
10. Veronesi U, Orecchia R, Maisonneuve P, Viale G, Rotmensz N, Sangalli C, et al. Intraoperative Radiotherapy Versus External Radiotherapy for Early Breast Cancer (ELIOT): A Randomised Controlled Equivalence Trial. Lancet Oncol (2013) 14:1269–77. doi: 10.1016/S1470-2045(13)70497-2
11. Vaidya JS, Bulsara M, Baum M, Wenz F, Massarut S, Pigorsch S, et al. Long Term Survival and Local Control Outcomes From Single Dose Targeted Intraoperative Radiotherapy During Lumpectomy (TARGIT-IORT) for Early Breast Cancer: TARGIT-A Randomised Clinical Trial. Bmj (2020) 370:m2836. doi: 10.1136/bmj.m2836
12. Lemanski C, Azria D, Gourgou-Bourgade S, Ailleres N, Pastant A, Rouanet P, et al. Electrons for Intraoperative Radiotherapy in Selected Breast-Cancer Patients: Late Results of the Montpellier Phase II Trial. Radiat Oncol (2013) 8:191. doi: 10.1186/1748-717X-8-191
13. Orecchia R, Ciocca M, Lazzari R, Garibaldi C, Leonardi MC, Luini A, et al. Intraoperative Radiation Therapy With Electrons (ELIOT) in Early-Stage Breast Cancer. Breast (2003) 12:483–90. doi: 10.1016/S0960-9776(03)00156-5
14. Hanna GG, Kirby AM. Intraoperative Radiotherapy in Early Stage Breast Cancer: Potential Indications and Evidence to Date. Br J Radiol (2015) 88:20140686. doi: 10.1259/bjr.20140686
15. Strnad V, Ott OJ, Hildebrandt G, Kauer-Dorner D, Knauerhase H, Major T, et al. 5-Year Results of Accelerated Partial Breast Irradiation Using Sole Interstitial Multicatheter Brachytherapy Versus Whole-Breast Irradiation With Boost After Breast-Conserving Surgery for Low-Risk Invasive and in-Situ Carcinoma of the Female Breast: A Randomised, Phase 3, Non-Inferiority Trial. Lancet (2016) 387:229–38. doi: 10.1016/S0140-6736(15)00471-7
16. Bosma SCJ, Hoogstraat M, van Werkhoven E, de Maaker M, van der Leij F, Elkhuizen PHM, et al. A Case-Control Study to Identify Molecular Risk Factors for Local Recurrence in Young Breast Cancer Patients. Radiother Oncol (2021) 156:127–35. doi: 10.1016/j.radonc.2020.11.025
17. Kawamura M, Itoh Y, Sawaki M, Kikumori T, Tsunoda N, Kamomae T, et al. A Phase I/II Trial of Intraoperative Breast Radiotherapy in an Asian Population: 5-Year Results of Local Control and Cosmetic Outcome. Radiat Oncol (2015) 10:150. doi: 10.1186/s13014-015-0469-6
18. Orecchia R, Veronesi U, Maisonneuve P, Galimberti VE, Lazzari R, Veronesi P, et al. Intraoperative Irradiation for Early Breast Cancer (ELIOT): Long-Term Recurrence and Survival Outcomes From a Single-Centre, Randomised, Phase 3 Equivalence Trial. Lancet Oncol (2021) 22:597–608. doi: 10.1016/S1470-2045(21)00080-2
19. Zhou SF, Shi WF, Meng D, Sun CL, Jin JR, Zhao YT. Interoperative Radiotherapy of Seventy-Two Cases of Early Breast Cancer Patients During Breast-Conserving Surgery. Asian Pac J Cancer Prev (2012) 13:1131–5. doi: 10.7314/APJCP.2012.13.4.1131
20. Abo-Madyan Y, Welzel G, Sperk E, Neumaier C, Keller A, Clausen S, et al. Single-Center Long-Term Results From the Randomized Phase-3 TARGIT-A Trial Comparing Intraoperative and Whole-Breast Radiation Therapy for Early Breast Cancer. Strahlenther Onkol (2019) 195:640–7. doi: 10.1007/s00066-019-01438-5
21. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PloS Med (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
22. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. Jama (2018) 319:388–96. doi: 10.1001/jama.2017.19163
23. Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, et al. Impact of a Higher Radiation Dose on Local Control and Survival in Breast-Conserving Therapy of Early Breast Cancer: 10-Year Results of the Randomized Boost Versus No Boost EORTC 22881-10882 Trial. J Clin Oncol (2007) 25:3259–65. doi: 10.1200/JCO.2007.11.4991
24. Liang Z, Zhang Q, Wang C, Shi F, Cao H, Yu Y, et al. Hyaluronic Acid/Hyaluronidase as Biomarkers for Bladder Cancer: A Diagnostic Meta-Analysis. Neoplasma (2017) 64:901–8. doi: 10.4149/neo_2017_612
25. Sutton AJ AK, Jones DR. Methods for Meta Analysis in Medical Research. Wiley Series in Probability and Statistics-Applied Probability and Statistics Section. Wiley Hoboken (2000).
26. Lee SW, Kim YJ, Shin KH, Kim K, Chie EK, Han W, et al. A Comparative Study of Daily 3-Gy Hypofractionated and 1.8-Gy Conventional Breast Irradiation in Early-Stage Breast Cancer. Med (Baltimore) (2016) 95:e3320.
27. Meattini I, Saieva C, Marrazzo L, Di Brina L, Pallotta S, Mangoni M, et al. Accelerated Partial Breast Irradiation Using Intensity-Modulated Radiotherapy Technique Compared to Whole Breast Irradiation for Patients Aged 70 Years or Older: Subgroup Analysis From a Randomized Phase 3 Trial. Breast Cancer Res Treat (2015) 153:539–47. doi: 10.1007/s10549-015-3565-2
28. Livi L, Meattini I, Marrazzo L, Simontacchi G, Pallotta S, Saieva C, et al. Accelerated Partial Breast Irradiation Using Intensity-Modulated Radiotherapy Versus Whole Breast Irradiation: 5-Year Survival Analysis of a Phase 3 Randomised Controlled Trial. Eur J Cancer (2015) 51:451–63. doi: 10.1016/j.ejca.2014.12.013
29. Meattini I, Marrazzo L, Saieva C, Desideri I, Scotti V, Simontacchi G, et al. Accelerated Partial-Breast Irradiation Compared With Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial. J Clin Oncol (2020) 38:4175–83. doi: 10.1200/JCO.20.00650
30. Lee SW, Shin KH, Chie EK, Kim JH, Im SA, Han W, et al. Accelerated Whole Breast Irradiation in Early Breast Cancer Patients With Adverse Prognostic Features. Oncotarget (2016) 7:81888–98. doi: 10.18632/oncotarget.11702
31. Guinot JL, Roldan S, Maroñas M, Tortajada I, Carrascosa M, Chust ML, et al. Breast-Conservative Surgery With Close or Positive Margins: Can the Breast be Preserved With High-Dose-Rate Brachytherapy Boost? Int J Radiat Oncol Biol Phys (2007) 68:1381–7. doi: 10.1016/j.ijrobp.2007.01.055
32. Keller LM, Sopka DM, Li T, Klayton T, Li J, Anderson PR, et al. Five-Year Results of Whole Breast Intensity Modulated Radiation Therapy for the Treatment of Early Stage Breast Cancer: The Fox Chase Cancer Center Experience. Int J Radiat Oncol Biol Phys (2012) 84:881–7. doi: 10.1016/j.ijrobp.2012.01.069
33. Frazier RC, Kestin LL, Kini V, Martinez AA, Chen PY, Baglan KL, et al. Impact of Boost Technique on Outcome in Early-Stage Breast Cancer Patients Treated With Breast-Conserving Therapy. Am J Clin Oncol (2001) 24:26–32. doi: 10.1097/00000421-200102000-00004
34. Yoshida-Ichikawa Y, Horimoto Y. Ipsilateral Breast Tumor Control Following Hypofractionated and Conventional Fractionated Whole-Breast Irradiation for Early Breast Cancer: A Long-Term Follow-Up. Breast Cancer (2021) 28:92–8. doi: 10.1007/s12282-020-01134-8
35. Ha B, Suh HS, Lee J, Lee KJ, Lee R, Moon BI. Long-Term Results of Forward Intensity-Modulated Radiation Therapy for Patients With Early-Stage Breast Cancer. Radiat Oncol J (2013) 31:191–8. doi: 10.3857/roj.2013.31.4.191
36. Bartelink H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I, et al. Recurrence Rates After Treatment of Breast Cancer With Standard Radiotherapy With or Without Additional Radiation. N Engl J Med (2001) 345:1378–87. doi: 10.1056/NEJMoa010874
37. Hirata K, Yoshimura M, Inoue M, Yamauchi C, Ogura M, Toi M, et al. Regional Recurrence in Breast Cancer Patients With One to Three Positive Axillary Lymph Nodes Treated With Breast-Conserving Surgery and Whole Breast Irradiation. J Radiat Res (2017) 58:79–85. doi: 10.1093/jrr/rrw071
38. Poortmans PM, Collette L, Bartelink H, Struikmans H, Van den Bogaert WF, Fourquet A, et al. The Addition of a Boost Dose on the Primary Tumour Bed After Lumpectomy in Breast Conserving Treatment for Breast Cancer. A Summary of the Results of EORTC 22881-10882 “Boost Versus No Boost” Trial. Cancer Radiother (2008) 12:565–70. doi: 10.1016/j.canrad.2008.07.014
39. Zhao S, Liu Y, Huang F, Chen X, Cao X, Yu J. The Long-Term Outcome of Adjuvant Hypofractionated Radiotherapy and Conventional Fractionated Radiotherapy After Breast-Conserving Surgery for Early Breast Cancer: A Prospective Analysis of 107 Cases. J Thorac Dis (2017) 9:3840–50. doi: 10.21037/jtd.2017.09.125
40. Kim KJ, Huh SJ, Yang JH, Park W, Nam SJ, Kim JH, et al. Treatment Results and Prognostic Factors of Early Breast Cancer Treated With a Breast Conserving Operation and Radiotherapy. Jpn J Clin Oncol (2005) 35:126–33. doi: 10.1093/jjco/hyi039
41. You KY, Bi ZF. Identifying Risk Factors for Regional Recurrence in Early-Stage Breast Cancer With Pt1-2 and Negative Sentinel Lymph Node Biopsy. Cancer Manag Res (2020) 12:9211–9. doi: 10.2147/CMAR.S264267
42. Takanen S, Gambirasio A, Gritti G, Källi M, Andreoli S, Fortunato M, et al. Breast Cancer Electron Intraoperative Radiotherapy: Assessment of Preoperative Selection Factors From a Retrospective Analysis of 758 Patients and Review of Literature. Breast Cancer Res Treat (2017) 165:261–71. doi: 10.1007/s10549-017-4321-6
43. Chowdhry VK, Bushey JA, Kwait RM, Goldberg S, Ritchie J, Ji YL, et al. Intraoperative Radiation Therapy as Part of Planned Monotherapy for Early-Stage Breast Cancer. J Radiat Oncol (2018) 7:167–73. doi: 10.1007/s13566-017-0338-z
44. Hanna SA, de Barros A, de Andrade FEM, Bevilacqua JLB, Piato JRM, Pelosi EL, et al. Intraoperative Radiation Therapy in Early Breast Cancer Using a Linear Accelerator Outside of the Operative Suite: An “Image-Guided” Approach. Int J Radiat Oncol Biol Phys (2014) 89:1015–23. doi: 10.1016/j.ijrobp.2014.04.038
45. Veronesi U, Orecchia R, Luini A, Galimberti V, Zurrida S, Intra M, et al. Intraoperative Radiotherapy During Breast Conserving Surgery: A Study on 1,822 Cases Treated With Electrons. Breast Cancer Res Treat (2010) 124:141–51. doi: 10.1007/s10549-010-1115-5
46. Valente SA, Tendulkar RD, Cherian S, Shah C, Ross DL, Lottich SC, et al. TARGIT-R (Retrospective): 5-Year Follow-Up Evaluation of Intraoperative Radiation Therapy (IORT) for Breast Cancer Performed in North America. Ann Surg Oncol (2021) 28:2512–21. doi: 10.1245/s10434-020-09432-3
47. Bonzano E, Belgioia L, Fregatti P, Friedman D, Agostinelli S, Cavagnetto F, et al. Tumor Size-Driven Dose of Intraoperative Radiotherapy for Breast Cancer: 18 Gy Versus 21 Gy. Anticancer Res (2018) 38:5475–9. doi: 10.21873/anticanres.12880
48. Gunay S, Kandemir O, Dönmez Yilmaz B, Akan A, Yalcin O. Comparison of Intraoperative and Postoperative Boost Radiotherapy in Terms of Local Recurrence and Cosmetic Outcomes in Patients With Early-Stage Breast Cancer. Indian J Surg (2018) 81:253–8. doi: 10.1007/s12262-018-1794-4
49. Guenzi M, Bonzano E, Corvò R, Merolla F, Pastorino A, Cavagnetto F, et al. Comparison of Local Recurrence Among Early Breast Cancer Patients Treated With Electron Intraoperative Radiotherapy vs Hypofractionated Photon Radiotherapy an Observational Study. Front Oncol (2018) 8:207. doi: 10.3389/fonc.2018.00207
50. Vaidya JS, Bulsara M, Saunders C, Flyger H, Tobias JS, Corica T, et al. Effect of Delayed Targeted Intraoperative Radiotherapy vs Whole-Breast Radiotherapy on Local Recurrence and Survival: Long-Term Results From the TARGIT-A Randomized Clinical Trial in Early Breast Cancer. JAMA Oncol (2020) 6:e200249. doi: 10.1001/jamaoncol.2020.0249
51. Lei J, Wang Y, Bi Z, Xue S, Ou B, Liu K. Intraoperative Radiotherapy (IORT) Versus Whole-Breast External Beam Radiotherapy (EBRT) in Early Stage Breast Cancer: Results From SEER Database. Jpn J Radiol (2020) 38:85–92. doi: 10.1007/s11604-019-00891-7
52. Oliver Guillén JR, Hernando Almudi E, Molina Osorio G, Ibañez Carreras R, Font Gómez JA, Vicente Gómez I, et al. Intraoperative Radiotherapy in Early Breast Cancer: Observational Comparison With Whole Breast Radiotherapy. Cir Esp (Engl Ed) (2021) 99:132–9. doi: 10.1016/j.ciresp.2020.04.024
53. Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, et al. Targeted Intraoperative Radiotherapy Versus Whole Breast Radiotherapy for Breast Cancer (TARGIT-A Trial): An International, Prospective, Randomised, Non-Inferiority Phase 3 Trial. Lancet (2010) 376:91–102. doi: 10.1016/S0140-6736(10)60837-9
54. Keshtgar MR, Williams NR, Bulsara M, Saunders C, Flyger H, Cardoso JS, et al. Objective Assessment of Cosmetic Outcome After Targeted Intraoperative Radiotherapy in Breast Cancer: Results From a Randomised Controlled Trial. Breast Cancer Res Treat (2013) 140:519–25. doi: 10.1007/s10549-013-2641-8
55. Corica T, Nowak AK, Saunders CM, Bulsara M, Taylor M, Vaidya JS, et al. Cosmesis and Breast-Related Quality of Life Outcomes After Intraoperative Radiation Therapy for Early Breast Cancer: A Substudy of the TARGIT-A Trial. Int J Radiat Oncol Biol Phys (2016) 96:55–64. doi: 10.1016/j.ijrobp.2016.04.024
56. Vaidya JS, Tobias JS, Baum M, Keshtgar M, Joseph D, Wenz F, et al. Intraoperative Radiotherapy for Breast Cancer. Lancet Oncol (2004) 5:165–73. doi: 10.1016/S1470-2045(04)01412-3
57. Kolberg HC, Loevey G, Akpolat-Basci L, Stephanou M, Fasching PA, Untch M, et al. Targeted Intraoperative Radiotherapy Tumour Bed Boost During Breast Conserving Surgery After Neoadjuvant Chemotherapy in HER2 Positive and Triple Negative Breast Cancer. Rev Recent Clin Trials (2017) 12:93–100. doi: 10.2174/1574887112666170201142458
Keywords: intraoperative radiotherapy, whole-breast irradiation, early breast cancer, oncological efficacy, meta-analysis
Citation: He L, Zhou J, Qi Y, He D, Yuan C, Chang H, Wang Q, Li G and Shao Q (2021) Comparison of the Oncological Efficacy Between Intraoperative Radiotherapy With Whole-Breast Irradiation for Early Breast Cancer: A Meta-Analysis. Front. Oncol. 11:759903. doi: 10.3389/fonc.2021.759903
Received: 17 August 2021; Accepted: 29 November 2021;
Published: 17 December 2021.
Edited by:
Alexandra Resch, Franziskus Spital, AustriaReviewed by:
Govind Babu Kanakasetty, HCG Cancer Hospital, IndiaStefan Konrad, Medical University of Vienna, Austria
Copyright © 2021 He, Zhou, Qi, He, Yuan, Chang, Wang, Li and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuju Shao, shaoqjfmmu@163.com
†These authors have contributed equally to this work
 Lin He
Lin He Jiejing Zhou
Jiejing Zhou Yuhong Qi
Yuhong Qi Dongjie He
Dongjie He Canliang Yuan
Canliang Yuan Hao Chang
Hao Chang Qiming Wang
Qiming Wang Gaiyan Li
Gaiyan Li Qiuju Shao
Qiuju Shao