- 1Institute for Liver and Digestive Health, University College London, London, United Kingdom
- 2St. Bartholomew’s hospital, Barts Health NHS Trust, London, United Kingdom
- 3Division of Surgery and Interventional Science – University College London, London, United Kingdom
Cholangiocarcinoma is an uncommon and highly aggressive biliary tract malignancy with few manifestations until late disease stages. Diagnosis is currently achieved through a combination of clinical, biochemical, radiological and histological techniques. A number of reported cancer biomarkers have the potential to be incorporated into diagnostic pathways, but all lack sufficient sensitivity and specificity limiting their possible use in screening and early diagnosis. The limitations of standard serum markers such as CA19-9, CA125 and CEA have driven researchers to identify multiple novel biomarkers, yet their clinical translation has been slow with a general requirement for further validation in larger patient cohorts. We review recent advances in the diagnostic pathway for suspected CCA as well as emerging diagnostic biomarkers for early detection, with a particular focus on non-invasive approaches.
1. Introduction
Cholangiocarcinomas (CCAs) are a heterogenous and highly aggressive group of tumours which arise from the biliary epithelium (1, 2). Depending on their anatomical location within the biliary tree, CCAs are classified as intrahepatic (iCCA), perihilar (pCCA) or distal (dCCA). Striking differences in their biology and clinical management, challenge the historical classification of pCCA and dCCA under the umbrella term ‘extrahepatic CCA’ (eCCA). Aside from their anatomical localisation, a common feature of CCAs is their poor prognoses, with overall five-year survival rates below 20% (3–5).
CCA is the second most common primary hepatic malignancy, accounting for 15% of all primary liver tumours (6). Epidemiological data suggest a rise in incidence in western countries (0.3–6 per 100,000 people per year) (7), while in some regions of East Asia higher rates (>6 per 100,000) are observed which are associated with liver fluke infections. Albeit a rare disease, CCA is relatively common in those with primary sclerosing cholangitis (PSC); a chronic, fibro-inflammatory and cholestatic liver disease, characterised by progressive fibrosis and biliary stricturing (8, 9). Up to 15% of patients with PSC will develop CCA, with the highest incidence 2-5 years into diagnosis (10). Other common risks include genetic, environmental (liver fluke), lifestyle (alcohol consumption and smoking), chronic infections (HBV and HCV), metabolic syndromes (diabetes mellitus, NAFLD) and obesity, as well as certain chronic inflammatory states (inflammatory bowel disease, chronic pancreatitis) (11). The lack of aetiological factors linked to patients at risk for CCA, however, makes early detection more challenging (12).
Surgical resection or liver transplantation remain the only curative option, with added benefit when followed by adjuvant chemotherapy such as capecitabine (median OS 36.4 vs 51.1 months respectively) (13). Most cases (>70%) are unfortunately non-resectable at time of diagnosis (14, 15), where therapeutic options are limited to systemic therapy and palliation (12, 13, 16). For these cases, first-line gemcitabine plus cisplatin and second-line FOLFOX (folinic acid, 5-fluorouracil and oxaliplatin) is recommended (13). Reports of improved R0 resection rates (>83%) following neoadjuvant chemo-radiotherpay, are limited to small cohort studies and case series, and determination of the exact role of neoadjuvant treatment in the setting of CCA, requires further validation using large sampled - randomized controlled trials (15). CCA tumours often consist of small nests of epithelial cancer cells surrounded by an abundant desmoplastic stroma and a complex tumour microenvironment, formed by cancer-associated fibroblasts, immune cells, endothelial cells and extracellular matrix, making pathological diagnosis challenging (7).
Current diagnostic modalities include clinical, biochemical, radiological and histological techniques – all of which are beset by relatively low sensitivity or specificity, often making the accurate diagnosis of CCA, particularly in patients with PSC, difficult (8, 17). Additionally, none of the currently available tissue or liquid biomarkers are sufficiently sensitive or specific to reliably aid in the early diagnosis of CCA (18). The relatively low incidence of CCA coupled with the high frequency of concomitant cholestasis and cholangitis, as well as difficulties obtaining adequate tissue samples, have hampered the identification of more accurate biomarkers (19).
2. Diagnosis and Management of Benign and Malignant Biliary Strictures
Differentiating benign from malignant biliary strictures (BBS and MBS respectively) remains a challenge despite advances in imaging and tissue cytogenetic profiling techniques (20–22). BBS are most commonly iatrogenic in aetiology, and are frequently observed following liver and pancreato-biliary surgery, including cholecystectomy (23–25). The formation of biliary anastomosis is often complicated by strictures which can be a single, localised (anastomotic strictures) or multiple and rather more proximal to the anastomosis site in their non-anastomotic counterparts (23). Other aetiologies of BBS include Inflammatory (i.e. chronic pancreatitis), autoimmune (i.e. PSC and IgG4 sclerosing cholangiopathy), infectious (tuberculosis, parasitic), vascular pathologies as well as radiotherapy induced biliary duct sclerosis (26, 27). MBS arise as consequence of a malignant process within the biliary tree (i.e. CCA), primary or metastatic liver, pancreatic (ductal adenocarcinoma) or ampullary, as well as gallbladder primary neoplasms (25, 28).
Indeterminate strictures in which laboratory parameters, imaging and histology are inconclusive, would prompt for urgent surgical intervention. The differentiation of malignant from benign peri-hilar strictures in particular, is more challenging (29). Despite the use of a multi-modal approach, and considering the low diagnostic accuracy of existing modalities - the presence of malignancy cannot be excluded on the basis of a negative biopsy with confidence (29). Surgical resection of pCCA is guided by the Bismuth-Corlette classification (30) with types II-IV requiring extensive surgery which involves major hepatic resection in the form of extended hepatectomy and caudate lobectomy (Figure 1) (29, 31). Reports of up to 25% of patients who underwent surgical resection for suspected CCA and subsequently found to have benign disease, highlight an urgent need for more accurate diagnostics (32–34). A diagnostic modality with improved sensitivity over standard cytology and histology, may therefore prevent unnecessary extensive surgery which has been linked with morbidity and mortality rates as high as 60% and 18%, respectively (29, 35).
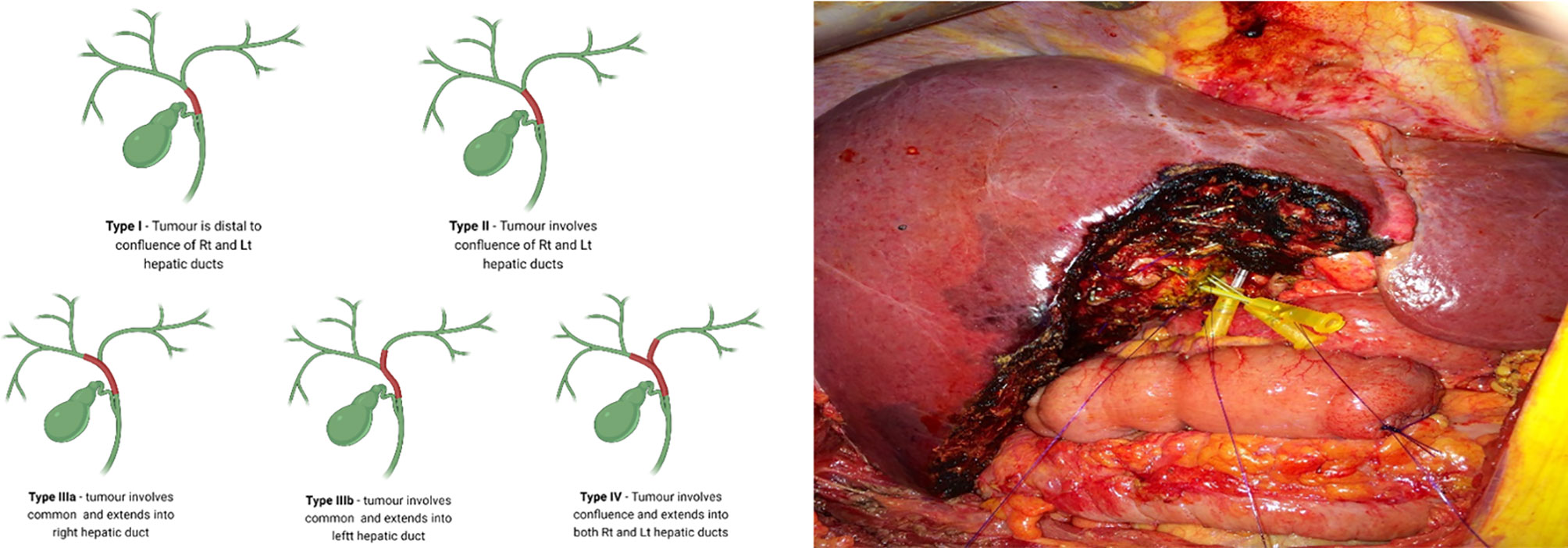
Figure 1 Surgical resection of perihilar CCA is guided by the Bismuth-Corlette classification with types II-IV (Left) requiring extensive surgery, which involves major hepatic resection in the form of extended hepatectomy and caudate lobectomy (Right). (Right) - central hepatic and biliary confluence resection for a Bismuth-Corlette type II hilar CCA. Right anterior, right posterior and Left hepatic ducts are cannulated.
2.1 Endoscopic Retrograde Cholangiopancreatography
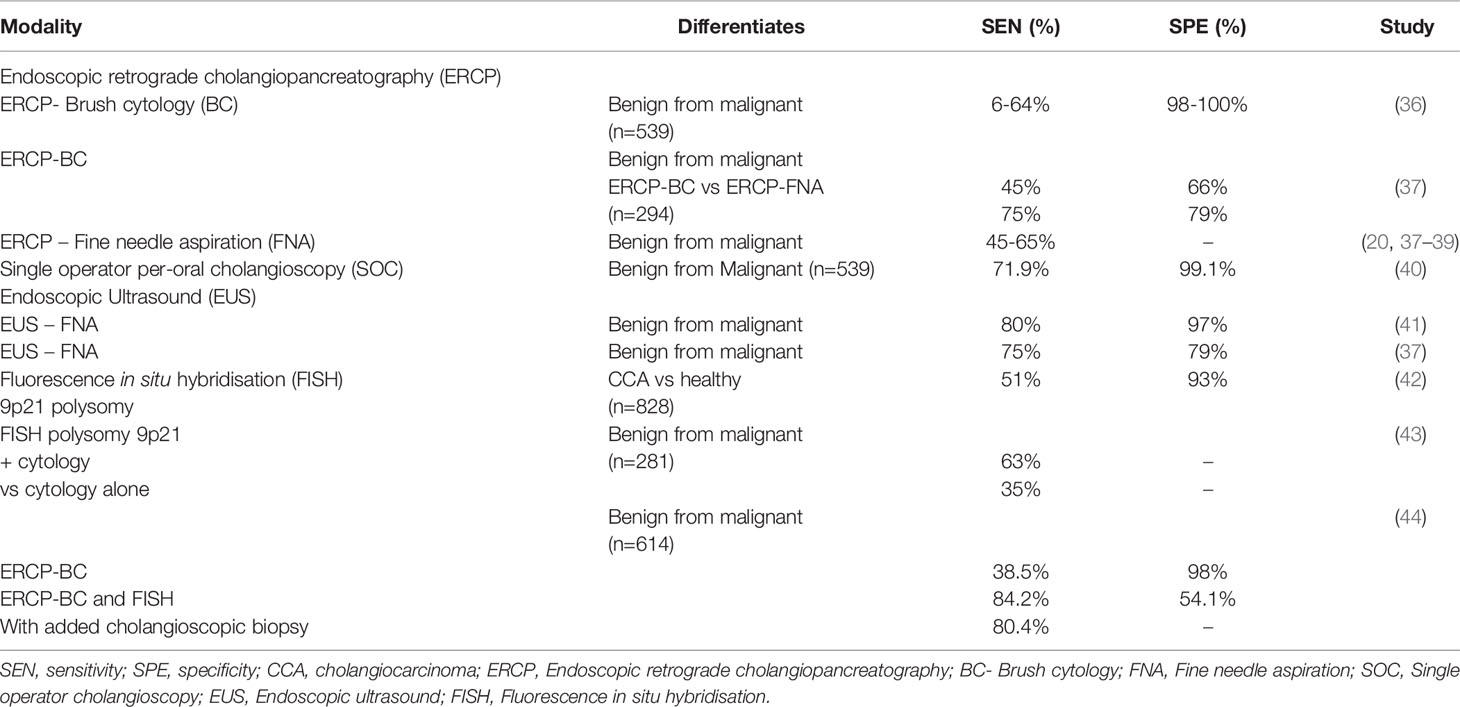
A number of diagnostic approaches can be applied in the workup of CCA (Table 1). Endoscopic retrograde cholangiopancreatography (ERCP) fluoroscopy with brush cytology (ERCP-BC) (and/or forceps biopsy) is the primary sampling technique. However, the predictive value (sensitivity ranging 6-64%, specificity 98-100%) of ERCP-BC is limited by the often inadequate amount of sampled tissue, cellular atypia (due to underlying inflammation or long term biliary stenting), or the well differentiated appearance of certain carcinomas – factors that challenge conclusive differentiation of benign from malignant epithelium (36). The diagnostic utility of ERCP and fine needle aspiration (FNA) is similarly limited by poor sensitivity for the detection of CCA (pooled sensitivity of 45%-65%) and is challenged by location, size and type of lesion, interpretation of cytology and operator skills (20, 37–39). Intraductal biopsy under fluoroscopic guidance may also increase the positive predictive value for CCA during ERCP (45), although this has not been proven in prospective studies for patients with PSC.
2.2 Single Operator Cholangioscopy
Cholangioscopy enables the direct visualisation of the biliary epithelium, characterisation of filling defects as well as targeted biopsy of suspicious strictures (Figures 2, 3). Morphological differences between BBS and MBS seen using cholangioscopy (i.e. surface irregularity, nodularity, neovascularisation) are often indicative, with dilated and tortuous vessel presence allowing for positive predictive values as high as 100% (46, 47). Moreover, it allows for minimally invasive ablative techniques (endoscopic, photodynamic and radiofrequency ablation) to be applied, and offers a non-surgical alternative in management of patients with type 2 Mirizzi syndrome (46). Single operator peroral cholangioscopy (SOC) with cholangioscopy-guided biopsy was reported to have a pooled sensitivity and specificity of 66% and 98% in a 2015 systematic review (20). The use of novel optical techniques which augment the visualised mucosa during cholangioscopy is increasing, and these include chromoendoscopy, biliary narrow band imaging and probe-based confocal laser endomicroscopy (pCLE) (48–50). In one prospective study of 136 patients with indeterminate biliary strictures, the addition of pCLE to standard ERCP with brush cytology increased sensitivity from 56% to 89%, with an overall diagnostic accuracy of 88% (51). A more recent meta-analysis of 15 studies which included a total of 539 subjects, reported a pooled sensitivity of 71.9% (95% CI 0.66-0.77) and specificity of 99.1% (95% CI 0.97-0.99) of peroral-cholangioscopy (POC) directed biopsies in the differentiation of malignant from benign strictures (40). This meta-analysis however, did not include any randomised-controlled studies but only reports of small, single centre studies. With respect to PSC related biliary strictures, a report from a single-centre retrospective study showed a limited impact of SOC guided biopsies on clinical management of study subjects, with lower accuracy observed compared to brush cytology (sensitivity and specificities of 15%, 65% and 47%, 95% respectively) (52). In this cohort of 80 patients, however, a higher prevalence of PSC (40%) with previous plastic stenting was described. In these patients, the clinical impact of SOC was limited, and using SOC guided tissue sampling changed management in only 17% of patients (52).
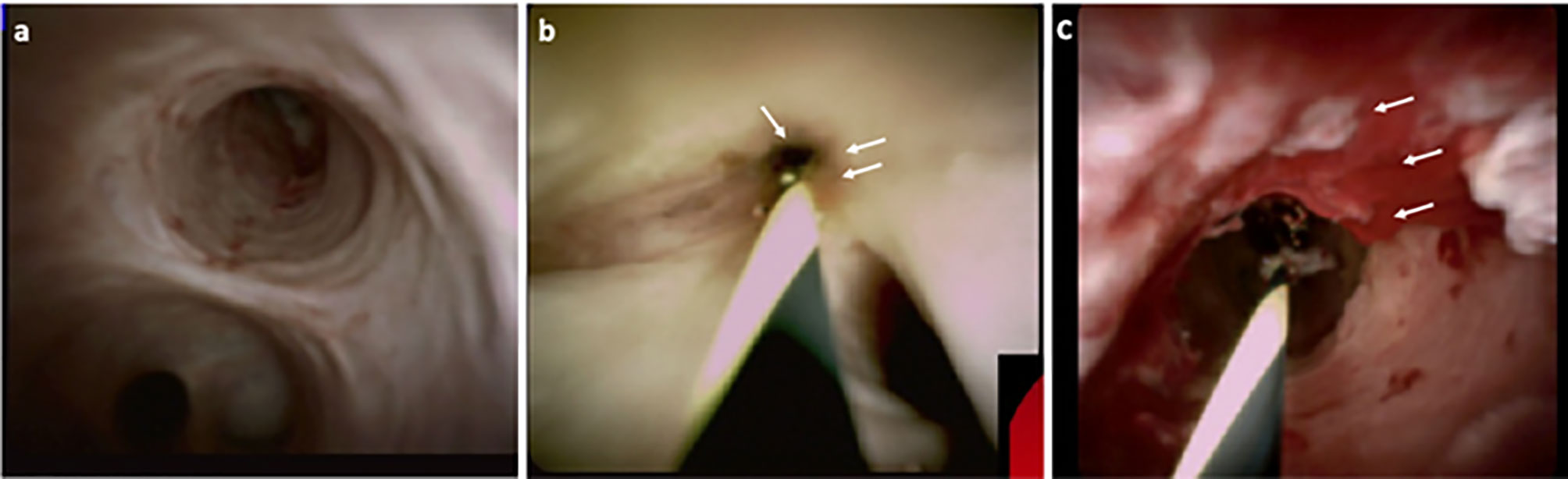
Figure 2 Direct visualisation of the biliary epithelium using cholangioscopy, in healthy (A), PSC stricture (B) and CCA (C). Characteristics such as surface irregularity, filling defects, nodularity and neovascularisation (C – white arrows) are often indicative of malignancy, with high positive predictive value.
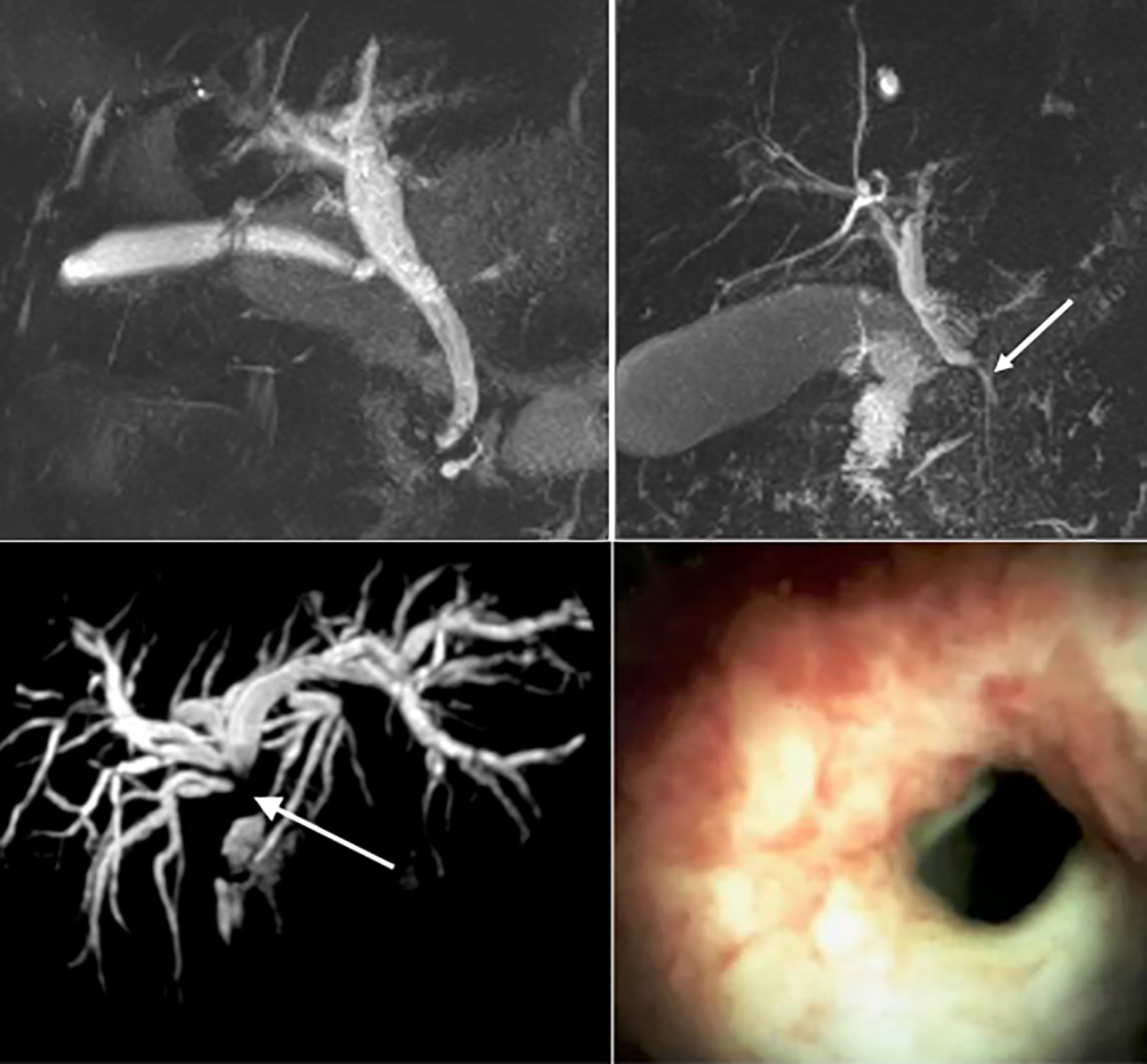
Figure 3 Top left: Magnetic resonance imaging (MRI) of a normal biliary tree. Top right: MRI imaging of a benign biliary stricture secondary to acute pancreatitis (white arrow). Bottom Left: MRI of a dilated intrahepatic biliary tree secondary to a cholangiocarcinoma at the liver hilum (white arrow). Bottom right: Cholangioscopy demonstrating mucosal nodularity and neovascularisation in the 7 -o’clock position, secondary to cholangiocarcinoma.
2.3 Endoscopic Ultrasound
Considering the low diagnostic yield of ERCP- FNA and forceps biopsy, the use of endoscopic ultrasound (EUS) together with fine needle aspiration as well as intraductal ultrasound (IDUS) (sensitivity and a specificity of 98% in one retrospective study) (53), has gained popularity. The role of the latter in diagnosis of biliary strictures has not yet been established due to it being an emerging modality. EUS-FNA can differentiate benign from malignant strictures with high accuracy (pooled sensitivity and specificity of 80% and 97% respectively) (41), and compared to ERCP-BC, demonstrated significantly improved diagnostic yield. In a prospective study evaluating EUS-FNA alongside ERCP-BC under same sedation in 50 patients with intermediate biliary strictures, Moura et al. reported accuracy of 100% vs. 54.8% (p=0.019) for EUS-FNA vs ERCP-BC in the diagnosis of extra-ductal lesions, respectively (54). A comparable diagnostic accuracy was observed with both modalities, in the assessment of intra-ductal or smaller lesion (less than 1.5cm in size) (54). In a meta-analysis which included 294 patients subjected to either EUS-FNA or ERCP-BC for tissue diagnosis of MBS, improved sensitivity and specificity were similarly observed when using EUS for guiding FNA (75% vs 45% and 79% vs 66%, respectively) with accuracies of 79% vs 61% respectively (37). In a meta-analysis of 10 studies evaluating the incremental benefit of EUS-FNA in patients with previously non-diagnostic ERCP-BC, Chiang et al. concluded that EUS has value in the diagnosis of extrahepatic biliary strictures in patients with previously indeterminate strictures following ERCP. Taken together, the data showed that a definitive diagnosis of malignancy was confirmed in an additional 14% of cases when a non-diagnostic ERCP-BC was followed by EUS-FNA (55). The tandem use of these two modalities has shown to improve the diagnostic accuracy of ERCP (around 60-70%) by as much as 30% when EUS-FNA followed. With respect to the nature of the strictures, the highest diagnostic accuracy (97% for both sensitivity and specificity) was observed when a non-diagnostic ERCP was followed by a second ERCP-BC in intrinsic lesions, and by EUS-FNA in case of extrinsic lesions (38, 56). An important consideration emerging from similar studies concerns needle tract seeding, where peritoneal metastases following EUS guided biopsies in CCA and pancreatic cancer patients was observed (57, 58). The risk of needle tract seeding following EUS-FNA however remains unclear (ranging ~2-4%) (57, 59, 60), with several studies describing lack of actual impact on disease progression and overall survival, in both CCA and pancreatic cancer (60–62). Nevertheless, inclusion of the puncture site in those proceeding to surgical resection, reduces the risk of dissemination (62). Real time microscopic diagnosis using CLE allows interrogation of the epithelium with high resolution, using intra-ductal fluorescein dye injection. Due to reduced positive predictive value and specificity when used in combination with ERCP, CLE is reserved for strictures still indeterminate following assessment using POC and IDUS – despite a sensitivity of 98% compared to ERCP alone (45%) (63).
2.4 Fluorescence In Situ Hybridisation
The addition of fluorescence in situ hybridisation (FISH) was shown to improve detection rates. By using fluorescent probes that target specific chromosomal aberrations, the diagnostic accuracy of brush cytology is enhanced (64). Aneuploidy of chromosomes 3, 7, and 17 as well as 9p21 polysomy are most commonly assessed, and have reported to improve the overall sensitivity for detecting malignancy by as much as 50% compared to cytology (43, 44, 65). A systematic review and meta-analysis (SRMA) published in 2014 of eight studies involving 828 patients, demonstrated that the pooled sensitivity and specificity of FISH polysomy to detect CCA was 51% and 93%, respectively (42). Similarly, more recent reports in larger cohorts support the diagnostic benefit of FISH for detection of malignant strictures. Brooks et al., reported their findings in a cohort of 281 patients (49% with underlying malignancy) who were evaluated using either EUS-FNA, or cholnagioscopic biopsy with or without FISH assays. In this study, FISH polysomy 9p21 and cytology had a significantly higher sensitivity compared to cytology alone [63 versus 35% (p < 0.05)] (43). EUS-FNA (in distal stricture evaluation) and cholangioscopic biopsy (proximal strictures) increased test sensitivity by 60% (from 33 to 93%; p < 0.001) and 48–76% (p = 0.05) in previously indeterminate strictures on cytology (43). In a single centre retrospective study of 614 patients, Han et al., reported that the addition of FISH to brush cytology resulted in increased sensitivity for detection of malignancy (84.2% (95% CI 68.8–94%) vs. 38.5% (95% CI 31.6–45.8%) observed with BC and fluoroscopic biopsy) (44). The added use of cholangioscopy however, reduced the sensitivity to 80.4% (95% CI 67.6–89.8%). Of note, highest specificity (nearing 98%) was reported with BC alone (95% CI 95.8–98.9%), while the use of FISH reduced test specificity to 54.1% (95% CI 42.9–65%) (44). The low sensitivities in these studies highlight the need for better performing markers for the detection of CCA.
3. Diagnostic and Prognostic Biomarkers in CCA
3.1 Disease Markers in Clinical Use: CA19-9, CEA and CA125
Biomarkers can be applied in the diagnosis, prognostication and management of a given disease. An ideal biomarker allows early disease detection with high sensitivity and specificity, is technically simple to obtain and quantify - at an acceptable cost. No such biomarker for the detection of cholangiocarcinoma, has been identified so far. Moreover, considering the low incidence as well as the heterogenous clinical picture at presentation of biliary tract cancers, screening the general population for CCA is neither feasible nor cost effective. Biomarkers aimed at differentiating high risk biliary tract lesions associated with CCA in clinical use include carbohydrate-antigens 19-9, 125 (CA19-9 and CA125 respectively) and carcino-embryonic antigen (CEA). CA19-9 is the primary tumour marker used in the diagnosis of CCA, although its lack of specificity and limited sensitivity hampers its clinical utility. A meta-analysis included 1,264 patients with CCA reported pooled sensitivity and specificity of 72% and 84%, respectively, for the differentiation of CCA from healthy controls and those with PSC (AUC 0.83) (66). Furthermore, in the 5-10% of the general population who are Lewis (a) antigen negative, CA 19-9 will remain undetectable (67). CA19-9 is elevated in a variety of hepatic and extra-hepatic conditions including cholangitis and cholestasis, further limiting its use in PSC, where these conditions commonly co-exist (7, 68). One retrospective study of 79 patients with PSC found that more than one-third of patients with a dominant biliary stricture and CA 19-9 >129 IU/ml did not have CCA after a median follow-up of 30 months (69). Due to the limited sensitivity of CA19-9 in the detection of CCA, any result must be interpreted in the context of the clinical picture and findings from cross-sectional imaging (70). Current screening strategies for patients with PSC may include annual magnetic resonance cholangiopancreatography (MRCP) (Figure 3) coupled with serum CA19-9, although this surveillance strategy is not currently recommended in the European Society of Gastroenterological Endoscopy (ESGE) (70) or British Society of Gastroenterology (71) guidelines, due to a lack of supportive outcome data.
CEA is a cell-surface anchored glycoprotein that is involved in cell adhesion. Its elevation is most often associated with colorectal malignancies, however, it is raised in up to 30% of patients with CCA showing a sensitivity and specificity of 72% and 84%, respectively (12). CA125 is a membrane associated glycoprotein encoded by the MUC16 gene which is often elevated in ovarian cancer, although it is also raised in up to 50% of CCA cases (72, 73).
3.2 Bile and Tissue Markers of CCA
3.2.1 Markers in Bile
Nucleic acids, proteins, circulating tumour cells as well as extracellular vesicles can be obtained from various bodily fluids (e.g. serum, bile, urine, saliva) or tissue, with variable diagnostic values in the context of CCA. The presence of tumour specific proteins (which are either shed or directly secreted by the malignant biliary epithelium), as well as tissue specific oncogenic proteins (e.g. enzymes) and their metabolites (onco-metabolites) make bile a particularly valuable reservoir of disease markers. The presence and levels of such diagnostic targets can be assessed using omics-based analyses, in bile obtained at time of endoscopic assessment (Table 2).
3.2.1.1 Metabolites and Proteins
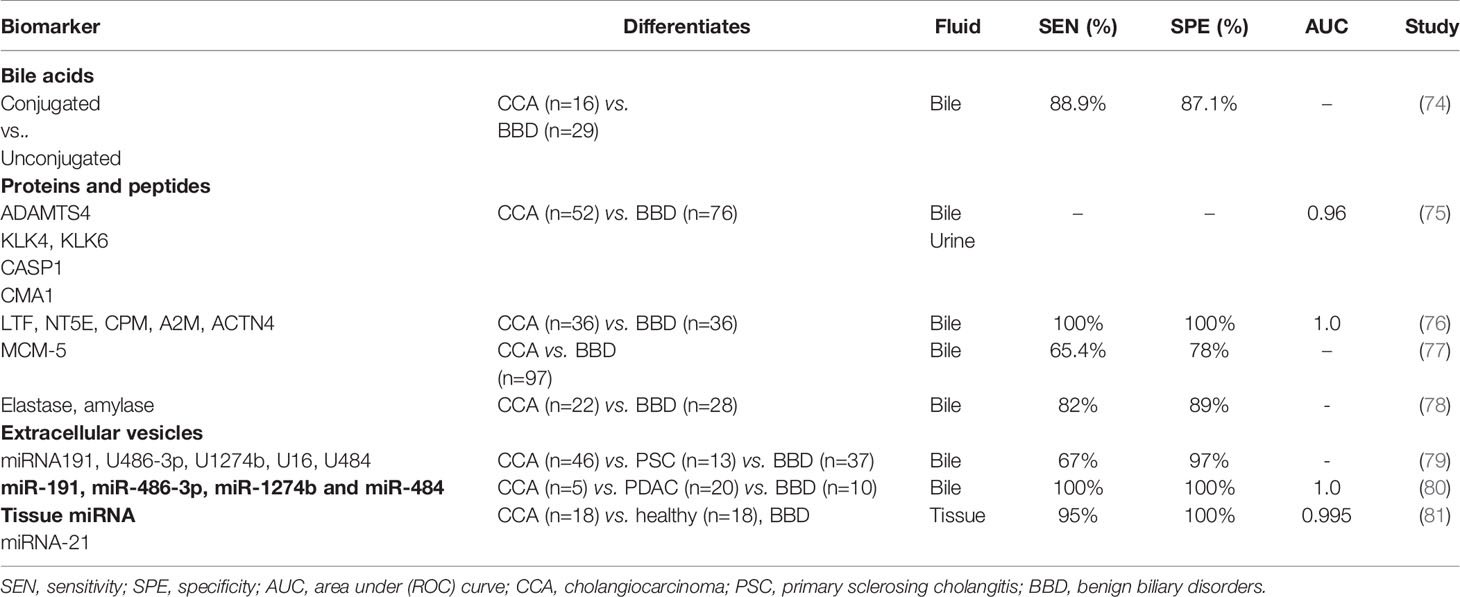
Specific changes in the composition of bile can both induce and be suggestive of an underlying malignant process in the biliary tree (11, 82, 83). A reduction in total biliary bile acid content was noted by several groups as a unique feature in CCA, compared to other malignant (PDAC) and benign causes of biliary obstruction (76, 83–85). Differences between conjugated to unconjugated-bile acid ratios, can be used in the differentiation of these pathologies with a relatively high accuracy (88.9% sensitivity and 87.1% specificity) (74). Specifically, Song et al., suggested a role for glycocholic acid (GCA) and taurochenodeoxycholic acid (TDCA) as specific to CCA with a higher average composition ratio of GCA (35.6%), compared to patients with pancreatic cancer or other benign biliary conditions (85). The higher concentrations of GCA and TDCA in states of chronic cholestasis, regardless of their aetiology, stimulate malignant cell proliferation, invasion and downregulation of apoptosis, through receptor mediated activation of the (MAPK) ERK 1/2 and PI3k/AKT signalling cascades, among others (11, 86). Secondly, the activation of receptors mediating these signalling pathways [i.e. G-protein coupled bile acid receptor 1 (GPBAR1) and sphingosine 1 phosphate receptor 2 (S1PR2), or nuclear factor kappa B (NF-κB)] in CCA tissue samples is enhanced (82, 86–89). Furthermore, differences between serum/stool ratios of GCA and tauroursodeoxycholic acid (TUDCA), have been linked with a specific composition of intestinal microbiota (Lactobacillus, Actinomyces, Peptostreptococcaceae, and Alloscardovia) as a feature in iCCA vs. patients with liver cirrhosis, HCC, as well as compared to healthy subjects (90).
Proteomic profiling of bile can distinguish CCA from PSC and non-malignant conditions such as biliary tract lithiasis with high accuracy (91–93). Peptide fingerprints of three proteases identified in bile - ‘a disintegrin and metalloproteinase with thrombospondin motifs 4′ (ADAMTS4), kallikrein-4 (KLK4) and chymase (CMA1) differentiate CCA from benign strictures (p < 0.05). When combined with a urinary panel (ADAMTS4, proteases caspase-1 (CASP1) and KLK6), these proteins differentiated CCA patients (n=36) from PSC (33) and others with benign conditions (n=18), as observed in a case-control phase II study on 87 patients (AUC 0.96 at 95% CI 0.89 to 0.99) (75). Using an artificial intelligence- machine learning based approach to biomarker discovery, Urman et al., reported diagnostic utility of two 5-protein based biomarker panels identified in bile (and urine) taken from patients with benign (n = 36) and malignant conditions, CCA (n=36) or PDAC (n=57). The biliary panel (LTF, NT5E, CPM, A2M, and ACTN4) differentiated patients with benign cholangiopathy and CCA with an AUC of 1 (at 100% sensitivity and 100% specificity). The urinary panel included MUC5B, FAT4, ALB, AMY2A and ENPP7 which allowed differentiation of PDAC patients from controls with similarly high accuracy (AUC 1, at 100% sensitivity and 100% specificity) (76).
3.2.1.2 Mini-chromosome Maintenance Proteins
Mini-chromosome maintenance (MCM) proteins have key roles in DNA replication, and are known markers of oncogenesis and cancer progression (94–97). Ayaru et al., reported the value of biliary MCM-5 as a marker of pancreato-biliary malignancy with a sensitivity of 66% compared to 20% for brush cytology (97). Similar sensitivity (65.4%) of biliary MCM-5 in differentiating malignant (n=50) from benign strictures (n=47), compared with 25% for brush cytology, was reported by Keane et al. (77). Levels of certain pancreatic enzymes (elastase, amylase) have also been reported as altered in patients with CCA (compared to benign obstructive aetiologies) and showed a high sensitivity (82%) at 89% specificity in their differentiation (78).
3.2.1.3 Extracellular Vesicles and Micro RNAs
Extracellular vesicles (EVs) are cell secreted exosomes, microvesicles, or apoptotic bodies which contain biomolecules such as nucleic acids, proteins, as well as lipids. EVs encase their cargo within a lipid membrane which keeps them stable in various bodily fluids, from which they can be isolated for diagnostic purposes (98, 99). EVs act as means of inter-cellular communication and in cancers, mediate tumour formation and shaping of metastatic niche landscapes (100). EVs have been found to retain key membrane markers from their cell of origin, making them excellent candidates for cancer detection (101). One of the first studies reporting the value of bile derived nucleic acids as a diagnostic tool in biliary pathologies included a panel of five miRNAs (191 U 486‐3p U 1274b U 16 U 484). In this study, which was published in 2014, a panel sensitivity of 67% at 96% specificity, highlighted the value of miRNAs in differentiating malignancy (n=46) and PSC (n=16) from benign cholestatic conditions (n=34) such as BBSs, papillary stenoses, choledocholithiasis, pancreatic cysts and cholangitis (79).
Severino et al., showed that the total amounts of EVs isolated from bile samples obtained from CCA patients exceed those identified in BBSs - a finding which can be explained by the high metabolic turnover in malignancy (80). Furthermore, the authors described five outstanding miRNAs (miR-191, miR-486-3p, miR-1274b and miR-484) which were identified in bile samples taken from patients with CCA, pancreatic cancer (5 and 20 patients respectively), and from those with chronic pancreatitis (n=15) and biliary stones (n=10), at time of ERCP. An exceptional accuracy in differentiating CCA from other benign strictures (100% sensitivity and 100% specificity) was reported (80).
3.2.2 Tissue Markers
3.2.2.1 Genomic Alterations in CCA
The mutational landscape of CCA is heterogeneous although alterations in some genes have a higher prevalence. The differential abundance of metabolites or proteins can guide toward a diagnosis of CCA. Striking changes in the metabolic profile of glucose, lipids and nucleotides can already be observed in early disease stages (84). These can be explained by the high metabolic demand involved in constitutive activation of proliferative (e.g., RAS, MAPK, PI3/AKT/mTOR) and inflammatory (e.g. STAT3) pathways, as well as loss of tumour suppression mechanisms (e.g. TP53, CDKN2A, BAP1) as part of tumorigenesis (5, 84, 102). Fibroblast growth factor receptor (FGFR) fusions have been described in 10-15% of iCCA (103), but not in other subtypes, and lead to a constitutive activation of the receptor and its signalling pathway, deregulating key processes such as cell proliferation, survival and migration (103, 104). Targeted inhibition (using pemigatinib) in previously treated iCCA patients with FGFR2 gene alteration (fusions/rearrangements), showed objective response in 35.5% of patients compared to patients with other FGFR alterations or FGFR negative controls. The promising results of this phase 2 study (FIGHT-202), are suggestive of a role for FGFR inhibition in selected cases where iCCA is driven by FGFR2 aberrations (104). The benefit of combining pemigatinib with gemcitabine and cisplatin as first-line therapy in unresectable or metastatic CCA is under assessment in a multi-centre phase 3, randomized controlled trial (FIGHT-302; NCT03656536). Enhanced activation of inflammatory signalling pathways (cytokine, chemokine and dendritic pathways) as well as an interplay with certain cytokines (e.g. IL-4, IL-10, IL-17) and their transducer STAT3 – is characteristic of an inflammatory biological subclass of CCA. The enrichment of EGF (BRAF mutations), RAS (KRAS mutations), AKT, VGF and MET proliferative pathways (p<0.05) and specific DNA aberrations (11q 13.2 and 14q22.1) on the other hand, are features in the genetic signature of a proliferative subtype (p<0.001) (102). A survival and disease recurrence analysis in tissue samples from 149 patients with CCA (38% inflammatory and 62% proliferative subtypes), revealed unfavourable outcomes in those patients with a proliferative molecular class (24.3 vs 47.2 months in the inflammatory class; P = .048) (102).
3.2.2.2 Metabolites and Proteins
Tissue specific markers (metabolites, enzymes) of glucose, lipid or nucleotide metabolism (e.g. GLUT2, HK2, GFAT, PKM2 and LDHA) enable differentiation between hepatobiliary neoplasms and healthy tissue. Mutations in certain genes that code for metabolic enzymes (i.e. IDH1; isocitrate dehydrogenase 1 - α-ketoglutarate metabolism) or differences in metabolite levels (e.g. glucose, glucose-6-phosphate) allow more accurate differentiation between primary biliary tract and hepatic cancers (84). Mutations in IDH1 and IDH2 are present in 10-20% of CCA patients (105), and result in the accumulation of 2-hydroxyglutarate (2-HG)-increasing oxidative stress (106). Specific genetic signatures can also differentiate between intra and extra- hepatic CCA. Higher frequency of alterations in FGFR, IDH1, IDH2, BAP1, PBRM1, MCL1, CDKN2A, BRAF and BRCA1/2 are observed in iCCA, while KRAS, TP53, CDKN2B, SMAD4, ErbB2 (HER2), CTNNB1 and MLH1 mutations are more suggestive of eCCA (107, 108). Similarly, the presence of specific mutation can be used for the purpose of staging and disease prognostication. Transcriptomic studies in large patient cohorts (137 in one and 292 in a second) assessing the prognostic utilities of KRAS, TP53 and IDH mutations, demonstrated worse prognosis and higher iCCA recurrence in the presence of the former two compared to IDH mutations (109, 110).
3.2.2.3 Mucin Glycoproteins
The abundance of cancer associated proteins in biopsies are other predictors of disease outcomes. The differential expression of several mucin glycoproteins (MUC1, MUC2, MUC4, MUC5) can be used for prognostication in CCA (111–113). A meta-analysis which included 4,126 CCA patients highlighted MUC1 and MUC4 as reliable predictors of survival (HR 2.52; 95 % CI 1.49–4.26, and HR 2.45; 95 % CI 1.56–3.86, respectively), especially when combined with EGFR (HR 1.79; 95 % CI 1.14–2.8), fascin (HR 2.58; 95 % CI 1.19–5.58) and the cell cycle marker p27 (HR 0.29; 95 % CI 0.14–0.6) (114).
3.2.2.4 The Role of Cadherins in CCA
A downregulation of cadherins is observed as part of the epithelial to mesenchymal transition (EMT) process, promoting CCA progression through the TGF-β axis (115, 116). Cadherin-17 (CDH17) plays a key role in the development of the gastrointestinal and pancreato-biliary systems (117). Aberrant expression of CDH17 is a feature in various gastrointestinal malignancies including stomach, colon, pancreatic and liver, and is a marker of advanced disease and poor outcomes (117–119). A recently reported multi-variate analysis in a cohort of 180 CCA patients, identified CDH17 elevated expression as a predictor of malignancy as well as a reliable predictor of postoperative survival. Interestingly, a positive correlation with nodal (p=0.04) and distant disease spread was noted, which outperformed widely used disease staging systems (p=0.04) (TNM, LCSGJ and Okabayashi) (117).
3.2.2.5 mIRNA Tissue Profiles Predict Malignancy and Metastatic Disease
Tissue microarrays-based RNA profiling revealed diagnostic as well as prognostic utilities for miRNAs in CCA. miRNAs are non-coding nucleotide sequences (RNA) which are key regulators in carcinogenesis (120). miRNA -191, miRNA-29a and miRNA 21/221 have been implicated in haematological as well as gastrointestinal (pancreatic, gastric, CCA) malignancies, and can be detected in both serum and tissue of patients (120–123). Similarly, their expression levels have been described as independent risk factors in CCA with implications on survival and disease progression (120). miRNA-21 outstands across various reports as a differentiator between biliary tract cancers and healthy tissue (Table 2) (81, 121, 123–126). The diagnostic utility of miRNA-21 demonstrated a 95% sensitivity and 100% specificity in distinguishing CCA patients from healthy and benign controls (AUC 0.995) (81). The expression of miRNA-21 can be indicative of metastatic spread (P = 0.037) and shorter survival (P < 0.05) in liver fluke induced CCA (125).
3.3 Novel Blood Biomarkers of CCA
3.3.1 Proteins
Serum pre-fractionation prior to mass-spectrometry enables the detection of low-abundance proteins, which are often masked by highly abundant serum proteins such as globulin and albumin. Analyses of the serum proteome in blood samples from healthy, PSC and CCA have shown changes in serum protein composition which were diagnostic, albeit only in small cohorts of patients (2) (Table 3).
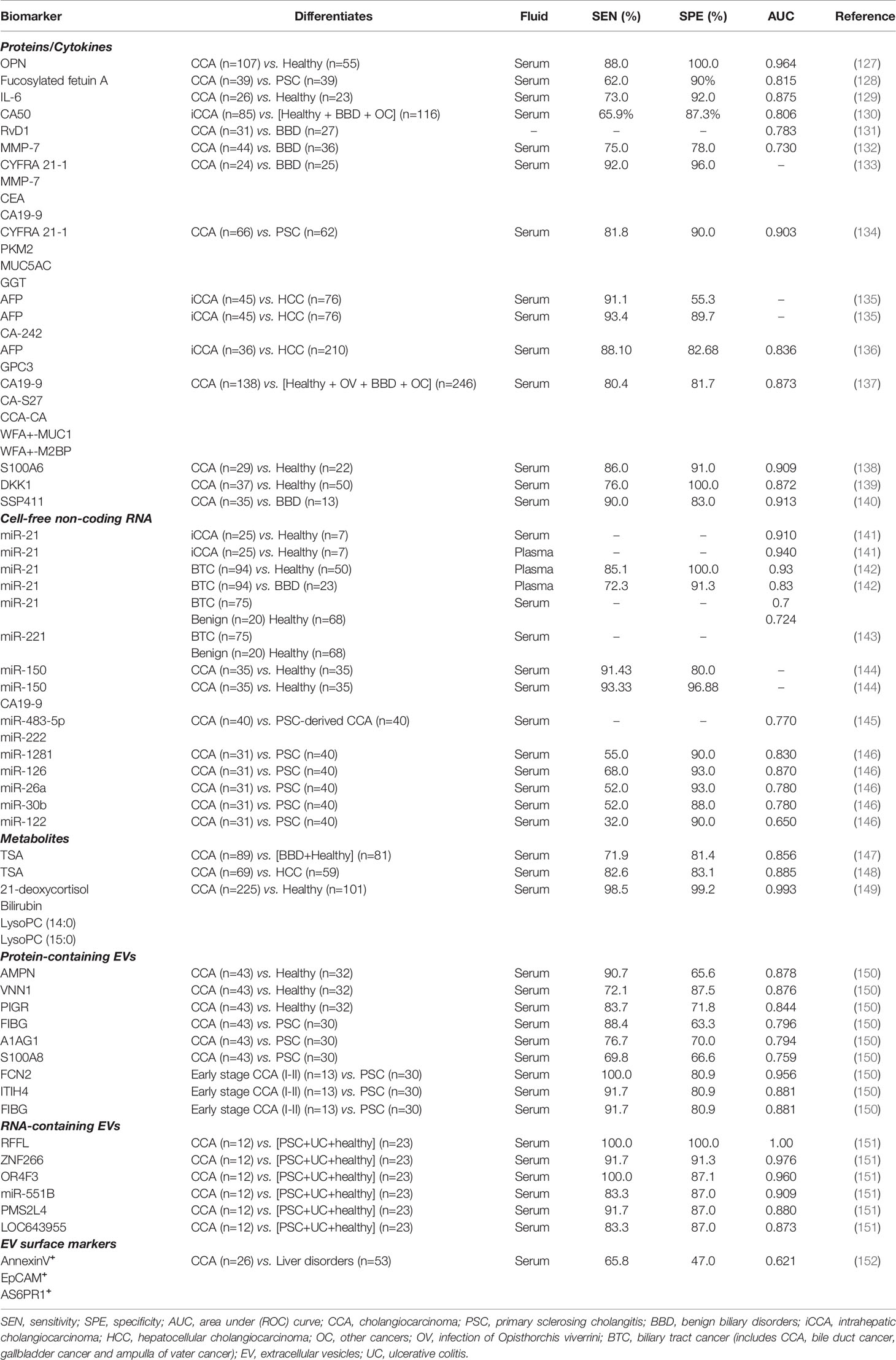
Osteopontin is a matricellular protein previously linked with multiple types of cancer and implicated in several pathological processes such as inflammation and tumorigeneses (153). Loosen et al., reported elevated levels of osteopontin in sera of patients with CCA (n=27) compared to samples taken from patients with PSC (n=10) (H test p = 0.001) (127). Remarkably, elevated levels of circulating osteopontin were associated with poor survival following tumour resection.
Investigators from the Mayo Clinic performed serum glycomic and proteomic analysis on 117 patient samples, 39 of which had CCA and 39 - PSC. In this study Betesh et al., identified differences in expression of certain glycans in blood taken from patients with CCA. One protein, fucosylated fetuin A, was able to differentiate CCA patients from those with PSC with reasonable performance (AUC 0.815 versus AUC 0.63 for CA19-9), suggesting a role for this protein in the surveillance of patients with PSC (128).
Anti-glycoprotein (GP)-2 immunoglobulin A autoantibodies are associated with CCA development in patients with PSC (154). In a European cohort of 250 patients, anti-GP2 positivity in PSC patients was associated with a significantly higher risk of developing CCA, independently of disease duration, bilirubin level and age (154).
Different cytokines identified in sera of CCA patients have shown diagnostic and/or prognostic values. The pro-inflammatory cytokine interleukin 6 (IL-6) was found elevated in serum samples of patients with CCA compared to healthy individuals, with test sensitivity and specificity of 73% and 92%, respectively (129). IL-6 however, can also be elevated in other hepatobiliary cancers like HCC, accentuating the need for more CCA specific cytokines (12).
Carbohydrate antigen 50 (CA50) is a cancer-associated cell surface antigen known to be expressed in malignancies of the digestive tract, including pancreatic and colorectal cancers, but has also been reported to be elevated in cirrhosis, pancreatitis or type 2 diabetes mellitus (155). In a recent study, serum levels of CA50 taken from 85 patients with iCCA were compared to healthy individuals (n=110), patients with benign biliary disorders (n = 23) and other cancers (n = 33). CA50 levels differentiated iCCA from non-CCA cases with 65.9% sensitivity and 87.3% specificity (AUC = 0.806) (130).
Another study linked lower levels of the circulating anti-inflammatory agent resolving D1 (RvD1) with CCA. Patients with CCA had lower levels (<380 ng/mL) of RvD1 when compared to serum levels in patients with benign biliary disorders (AUC = 0.783) – with suggested correlation with disease stage (131). In this study however, RvD1 did not perform any better than CA19-9 in the same cohort of patients (AUC = 0.940).
Elevated levels of matrix metalloproteinase 7 (MMP-7), an enzyme with key roles in extracellular matrix remodelling during tissue repair and tumour progression, have been found in the serum of patients with CCA. A study by Leelawat and co-workers compared the diagnostic potential of MMP-7, with CEA and CA19-9 in differentiating CCA patients from those with benign biliary tract diseases. MMP-7 showed better diagnostic value (AUC 0.730) compared to CEA and CA19-9 (AUC of 0.63) in this report (132).
Combinations of proteins into multi-marker panels have been reported to increase their individual diagnostic performance by several authors. A study by Lumachi et al. compared the individual performance of serum cytokeratin-19 fragment (CYFR21.1), MMP-7, CEA and CA19-9 and in combination, in the detection of CCA. Serum levels of these proteins in twenty-four patients with histologically confirmed CCA and 25 matched patients with benign liver were measured. The mean value of each marker was significantly higher (p<0.01) in CCA compared to controls and the combination of all serum markers was reported to have test sensitivity of 92% at 96% specificity for detecting CCA, with an overall diagnostic accuracy of 94% (133).
More recently, our group evaluated a number of biomarkers with diagnostic utility in the differentiation of CCA from benign biliary disease. In a cohort of 66 patients with CCA and 62 with PSC, a panel combining serum levels of PKM2, CYFR21.1, MUC5AC and GGT was able to differentiate CCA from PSC with test sensitivity of 82% and specificity of 90% (AUC 0.90) (134).
Another proposed CCA biomarker is serum alpha fetoprotein (AFP). In one study, AFP levels differentiated HCC from iCCA with 91.1% sensitivity but only at 55.3% specificity. Combination of AFP with carbohydrate antigen 242 (CA-242) increased test specificity to 93.4%, albeit with lowered sensitivity (135). More recently, another study tested the combination of AFP with serum glypican-3 (GPC3) to differentiate HCC from iCCA in a larger cohort of patients (n=210 and n=36 respectively). The results showed that even though test sensitivity was increased compared to AFP alone (67.62% to 88.10%), the overall accuracy did not improve (AUC 0.836 to 0.853) (136).
The performance of a novel biomarker panel combining five cancer-associated glycans and glycoproteins, (known as glycobiomarkers or “GlycoBiomarker (GB)-score”), has been recently evaluated in patients with CCA. More specifically – levels of CA19-9, carbohydrate antigen-S27 (CA-S27), CCA-associated carbohydrate antigen (CCA-CA), WFA-positive MUC1 (WFA+-MUC1), and WFA-positive M2BP (WFA+-M2BP) were measured in serum taken from 138 CCA patients and 246 non-CCA controls, showing a 80.4% sensitivity and 81.7% specificity (AUC = 0.873) (137).
Other serum proteins have been reported as stage specific and prognostic in CCA. A recent study analysed the serum proteome of 148 HCC and 60 CCA patients by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and found 25 differently expressed proteins in CCA patients, with AUC ranging from 0.701 to 0.823 (156). Interestingly, the authors reported that levels of plasma serine protease inhibitor inversely correlated with tumour development (stage I to IV). In addition, levels of afamain - a human plasma vitamin E-binding glycoprotein, and previously described ovarian cancer tumour marker (157), were associated with poor prognosis in CCA (156). Despite their potential, these similarly require further validation in larger and randomized cohorts.
3.3.2 Serum Metabolites
Specific changes in the concentration of serum metabolites can also point towards a diagnosis of CCA. Quantitative metabolomic analyses of serum taken from patients with CCA identified a role for serum metabolites as biomarkers of this cancer. Total sialic acid (TSA), a nine-carbon sugar present in oligosaccharide chains of many glycoproteins and glycolipids, has been associated with CCA development. In a study comparing serum TSA of CCA patients and a control group formed by healthy individuals and patients with benign biliary conditions, the sensitivity and specificity were 71.9% and 81.4% respectively (AUC 0.856) (147). Similarly, Kongtawelert et al. compared TSA levels in CCA and HCC patients and reported an overall AUC of 0.885 (148), highlighting the relevance of this metabolite in CCA detection.
Lastly, serum metabolomes of a larger group of CCA patients were compared with those of healthy (n = 176 and 85, respectively), and identified 75 differently expressed metabolites between these cohorts. As reported by Liang et al., following further validation (n=225), 21-deoxycortisol, lysophosphatidylcholine 14:0 (lysoPC 14:0), lysophosphatidylcholine (lysoPC 15:0) and bilirubin (over-expressed) (the latter 2 over-expressed in CCA), were selected based on their superior diagnostic performance (AUC 0.918, 0.954, 0.927 and 0.922). The combination of these four markers into a panel showed an even higher diagnostic accuracy (AUC of 0.993) (149).
3.3.3 Liquid Biopsies
The presence of specific genetic signatures which are identified using targeted genomic analysis of tumour tissue, are increasingly used for diagnosis, prognostication and evaluation of treatment response (158, 159). Image guided tissue acquisition for diagnosis confirmation is required pre-operatively, yet is frequently limited by insufficient tissue yield which is required for accurate molecular profiling. Specifically, in the presence of a highly fibrotic tumour stroma, the retrieval of adequate tissue material which sufficiently captures the heterogeneity of biliary cancers, is imperative. Technological advancements in genomic analysis have enabled the identification of genetic (and epigenetic) aberrations in blood, through isolation of tumour-derived circulating nucleic acids (circulating free DNA (cfDNA), cell free RNA) or tumour cells (CTCs) (159). Known as ‘liquid biopsy’, such tests are increasing in popularity, as they provide a minimally invasive access to a rich source of cancer derived genetic material which can be used for diagnosis and monitoring of tumour evolution and progression (159).
3.3.3.1 Cell-Free Non-Coding RNA
Micro-RNAs (miRNAs) are small, highly conserved RNA molecules involved in post-transcriptional regulation of genes (160). An important characteristic of miRNAs is their presence and stability in biofluids, which can often be sampled less invasively. Researchers have reported key roles for miRNAs in the pathogenesis (proliferation, invasion and metastasis) of various cancers, including CCA (161). In one small study, miR-21 and miR-221 were found to be significantly overexpressed in plasma (AUC 0.94) and serum (AUC 0.91) of patients with iCCA (n=25), compared to healthy controls (n=7) (141). Similarly, miR-21 differential expression in tissue, showed outstanding diagnostic performance (AUC=0.995) (81) in differentiating CCA from benign disease and healthy controls (141). A similar study confirmed the value of serum miR-21 in differentiating CCA from healthy controls (AUC 0.93), as well as CCA from benign biliary disease including PSC (AUC 0.83) (142). However, miR-21 was also found upregulated in other cancers including HCC, questioning its specificity (162). Outstanding from a panel of five miRs (miR-10a, miR-21, miR-135b, miR-221, and miR-214), exosome derived miR-221 levels alone differentiated malignant (n=75) from benign (n=20) and 68 healthy samples (p<0.01) in a recent study published by Han et al. (143). The authors suggested a role for miR-221, in the early detection of BTCs (143).
Serum level differences of miR-150 demonstrated test sensitivity and specificities of 91.43% and 80%, respectively, when measured in CCA and compared to healthy controls, with improved performance when assayed together with CA19-9 (93.33% sensitivity and 96.88% specificity) (144). A similar observation was reported by Wang et al. (163). A small study which included 30 patients with PSC and 30 patients with CCA, found that serum miR-483-5p and miR-222 show different expression patterns in PSC compared to CCA cohorts (145). Another study by Voigtländer and collaborators, reported five other miRs (miR-1281, -126, -26a, -30b, -122) to be differentially expressed in serum of patients with PSC and CCA (ROC AUCs ranging from 0.70 to 0.91), although their combination did not significantly improve diagnostic accuracy (146).
Whilst most reported diagnostic miRs appear to be up-regulated in CCA, others such as miR-150-5p or miR-106a may be down-regulated (164, 165).
3.3.3.2 Circulating Tumour Cells
Circulating tumour cells (CTCs) are released into the blood stream by primary tumours, and their serum levels showed both diagnostic and prognostic value in several malignancies including HCC, gastric, pancreatic, breast as well as colorectal cancers (166–170). However, their low abundance limit their use; even in metastatic settings, CTCs represent as little as one out of 109 of total circulating cells (171). Despite these, investigators further evaluated the diagnostic potential of CTCs in the context of CCA. A number of technologies have been developed in an attempt to isolate CTCs from peripheral blood, including the CellSearch® System, which is licenced by the US Food and Drug Administration. This semi-automated platform identifies, isolates and enumerates CTCs using cell specific EpCAM antibodies and immunofluorescent markers. The often lack of sufficient expression of EpCAM by tumours is however a disadvantage. In one study, up to 20% of CCAs did not overexpress this protein (172). CellSearch® further differentiates cell types based on their positivity to DAPI, cytokeratins (such as 8, 18 and 19) or negativity to CD45 (173). One early study demonstrated the ability of this system to detect CTCs in patients with metastatic cancer versus healthy controls and those with benign disease. Of 344 patients that were either healthy or had an underlying benign disease, only 0.3% had >2 CTCs per 7.5ml of blood, compared to 36% of specimens collected from patients with metastatic disease, although it is not stated whether any had metastatic CCA (174). In another small study of 13 patients with CCA reported by Al-Ustwani et al., only 3 patients had significantly elevated number of CTCs (>2 per 7.5ml of blood) in this small cohort (173). Other studies have suggested that levels of CTCs may be associated with a poor prognosis in patients with advanced CCA (175, 176), however, their diagnostic role in CCA is yet to be fully determined.
3.3.6 Extracellular Vesicles
In a study performed by Arbelaiz et al., serum EVs from healthy controls and patients with CCA (n=43), PSC (n=30) and hepatocellular carcinoma (HCC), were isolated and characterised. These were supplemented by EVs previously derived from human CCA cell lines and normal cholangiocytes in vitro (150). Proteomic analysis revealed that the proteins with best performance in differentiation of CCA from healthy controls were AMPN, VNN1 and PIGR, showing AUCs of 0.878, 0.876 and 0.844 respectively. Additionally, several differentially expressed proteins were identified in serum EVs of CCA versus PSC patients, including FIBG, A1AG1 and S100A8 (maximum AUC 0.80). Some candidates (including FCN2, ITIH4 and FIBG) also showed higher diagnostic values for early stage CCA (stages I-II) versus PSC than CA19-9 (AUC 0.956, 0.881 and 0.881, respectively), showing the potential usefulness of these serum EV proteomic signatures, as early diagnostic tools in CCA. Further validation in larger cohorts are however pending.
Lapitz and colleagues performed transcriptomic analysis of the content of serum EVs isolated from CCA patients, and a control group which included PSC, ulcerative colitis and healthy individuals (151). EV contained miRNAs RFFL (E3 ubiquitin-protein ligase rififylin), ZNF266 (zinc finger protein 266) and OR4F3 (olfactory receptor family 4 subfamily F member 3) showed the best diagnostic performance (AUC 1.00, 0.976 and 0.960, respectively). With respect to non-coding RNAs, miR-551B, PMS2L4 and LOC643955 distinguished these patients with highest accuracies (AUC 0.909, 0.880 and 0.873, respectively).
Lastly, Julich-Haertel et al. used a different approach which was based on the identification of membrane bound markers of large EVs or tumour-associated microparticles (taMPs). The group compared the level of serum taMPs containing specific surface markers in patients with liver malignancies (including CCA and HCC) and non-liver cancers and cirrhosis. taMPs carrying Annexin V, EpCAM and ASGPR1 showed a sensitivity of 65.8% but low specificity of 47.0% (AUC 0.621) (152).
3.4 Urinary Biomarkers of CCA
3.4.1 Volatile Organic Compounds
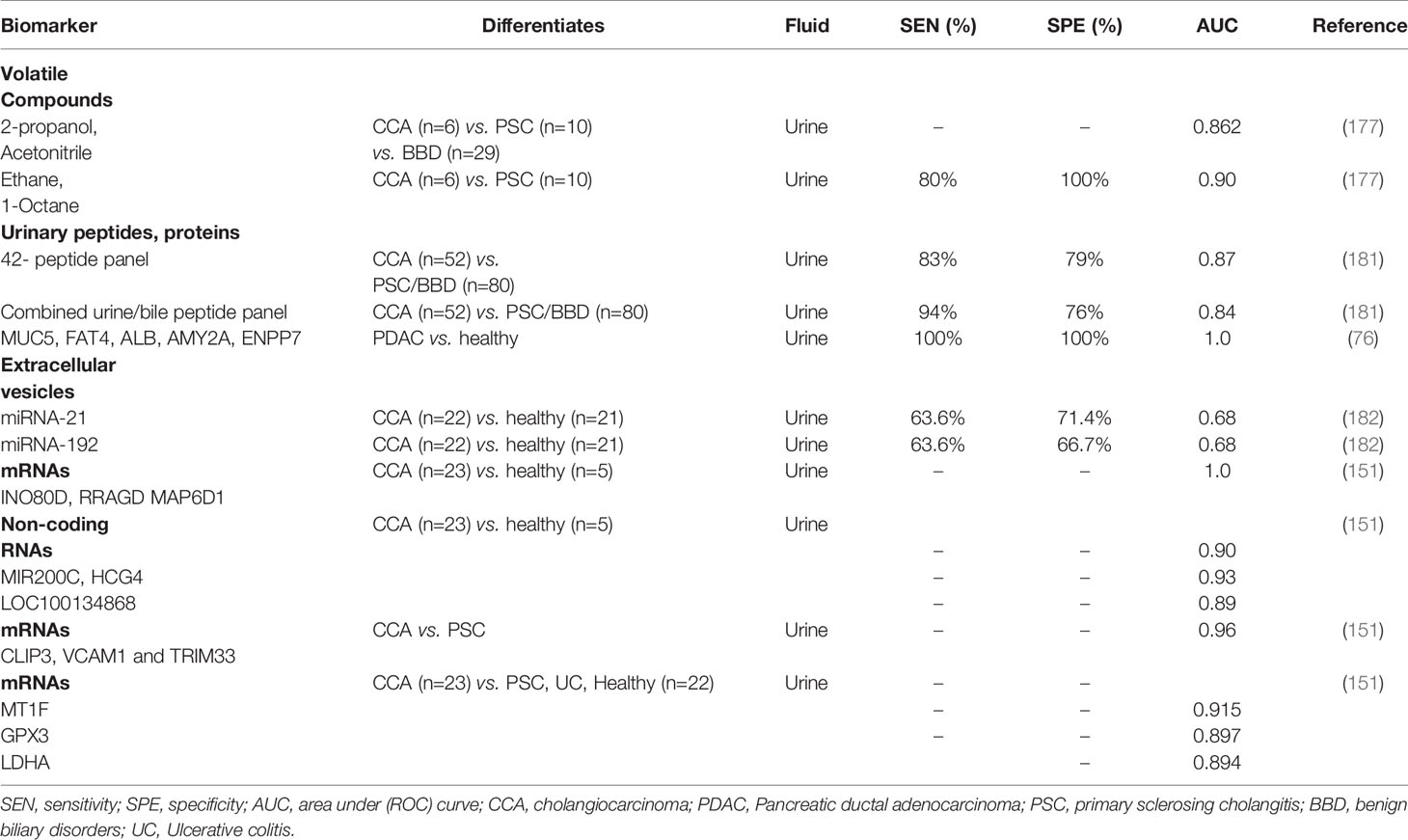
Urine is an excellent sample matrix as it is non-invasively accessible, can be obtained in large quantities and is stable in its composition if handled correctly. Urine is source abundant in volatile organic compounds (VOCs) which can differentiate CCA from benign biliary conditions. Selected-ion flow-tube mass spectrometry (SIFT-MS) allows the measurement of their concentration in urine. Navaneethan et al., showed that ethane and 1-octene distinguished CCA from PSC patients with an AUC of 0.90 (80% sensitivity at 100% specificity) (177). Other VOCs such as 2-propanol and acetonitrile have also been reported as having value in differentiation CCA from patients with PSC or other benign lesions (AUC 0.862) (177).
3.4.2 Proteins and peptides
Furthermore, since urine is an ultrafiltrate of plasma, the urinary proteome is highly sensitive to changes in renal function and a wide range of non-renal diseases, including certain cancers (178). Urinary proteomic biomarkers have been described in many tumours including pancreatic, renal, prostate, bladder, lung, breast, ovarian cancer and CCA (179). Metzger and colleagues used capillary electrophoresis-mass spectrometry to evaluate the urinary proteome in early CCA (180). A 42-biomarker panel was initially identified based on the differentially excreted urinary peptides of 41 patients including 14 with CCA, 13 with PSC and 14 with other benign biliary disease. In a subsequent cross-sectional validation of 123 patients, the urinary peptide panel accurately diagnosed 35 of 42 CCA patients and 64 of 81 patients with benign biliary disease (including those with PSC), with an AUC of 0.87, 83% sensitivity and 79% specificity. Evaluation of 101 healthy controls gave 86% specificity. More recently, combined bile and urine proteome analysis was performed in a case-control phase II study of 87 patients (36 CCA, including 13 with CCA on a background of PSC, 33 PSC and 18 other benign disorders). A logistic regression model was developed and subsequently validated in a prospective cohort of 45 patients undergoing ERCP for the evaluation of biliary strictures (181). The combination of both urine and bile markers gave an accuracy of 92% in the detection of CCA (sensitivity 94%, specificity 76%, AUC 0.84). Other groups have suggested that urinary miRNAs may be useful in the diagnosis of CCA. In a recent study of 192 patients with either Opisthorchis viverrini infection, periductal fibrosis or CCA, miR-21 and miR-192 were found to be elevated in the urine of patients with CCA versus healthy controls (AUC 0.849). Of these two biomarkers, miR-21 discriminated CCA with most accuracy (AUC 0.682) (182). Table 4 summarises the performance of urinary2 biomarker panels in the diagnosis of CCA.
Recent interest in EV isolation from body fluids (such as serum and bile) has also included urine as a diagnostic target. Urine isolated EVs can be screened using transcriptomics showing distinct signatures of CCA as compared to patients with PSC and healthy controls. Lapitz et al., identified specific messenger RNAs [INO80D, RRAGD and MAP6D1 (AUC 1.00)] as well as certain non-coding RNAs [MIR200C, HCG4 and LOC100134868; AUC of 0.904, 0.930 and 0.896, respectively)] in EVs isolated from urine of patients with CCA (n=23), differentiating them from healthy controls (n=5). The authors have also reported higher expression of CLIP3, VCAM1 and TRIM33 messenger RNAs in CCA versus PSC (AUC 0.965), while others such as MT1F, GPX3 and LDHA were able to distinguish CCA from a mixed cohort of patients with PSC, ulcerative colitis and healthy patients with high accuracy (AUC 0.915, 0.897, 0.894, respectively) (151).
4 Concluding Remarks
CCAs are a group of heterogenous malignancies which are a devastating form of cancer that is most often diagnosed at a late stage. Due to their aggressiveness, the only curative option for all subtypes is radical surgical resection, which is often supplemented with adjuvant chemotherapy (13). CCA is however most often diagnosed at a non-resectable stage, where associated 5-year survival is less than 5%. The exact role of neoadjuvant chemotherapy in improving oncological resection rates has not yet been fully established, and larger scale trials are required for validation of the few studies that have showed benefit (15). The early detection of CCA remains a major challenge, particularly in patients with PSC and currently available diagnostic modalities are not sufficiently sensitive to differentiate malignant from benign strictures with ideal accuracy (20, 37–39, 183). Tissue, cytological and bile-based biomarkers may provide additional diagnostic information over standard methods, with variable sensitivities and specificities - ranging from 58%-87% and up to 98%-100%, respectively (183). Owing to technological developments in the field of genomics, molecular profiling of lesions and identification of tumour specific genetic and epigenetic alterations, can guide toward diagnosis (and appropriate differentials), prognosis, as well as direct treatment (159). Similarly, the evolvement of proteomic and metabolomic techniques and their application in biliary tract cancer research, identified striking changes in protein or metabolite compositions in tissue and bile, which can be used to differentiate malignant from benign biliary lesions. However, these ‘invasive’ markers can often only be obtained by subjecting patients to invasive procedures, such as ERCP with biliary brush cytology or EUS with biopsy, therefore their use in screening is limited. In attempts to minimise the invasiveness of such diagnostic tests, molecular signatures and metabolic changes in other bodily fluids (such as blood an urine) have also been investigated. Although showing only modest diagnostic accuracies at best, these findings which have been reported in small cohorts, justify further interrogation in the form of larger scale validation studies.
Liquid biopsies in which tumour derived genetic material can be isolated from blood (circulating DNA, RNA or circulating tumour cells), offer an appealing and minimally invasive approach for both diagnostic (and surveillance) purposes, as well as means for monitoring treatment response. Various nucleic acids (e.g.miRNAs), freely circulating or exosome bound, and CTCs with potential theranostic utilities have been described, although many still require validation in collaborative studies (5). Among these, mIR-21 and miR-221 levels have been commonly reported to have diagnostic value across several studies (141–143, 182). The correlation of such novel biomarkers with CA19-9 levels is likely to further improve their diagnostic accuracies (81, 143). Advances in molecular profiling and sampling techniques have improved researchers’ understanding of CCA evolution. Moreover, recent evidence points to improved clinical outcomes, when therapeutic targeting of specific genetic aberrations (e.g. FGFR fusions) in selected cohorts of patients, is guided by tumour genomic analyses (104, 108). Despite increasing reports of biomarker studies in CCA, these have mostly been observed in limited numbers of subjects and clinical samples. Their translation into clinic however, requires larger cohort validations - which are challenged by the low incidence of this devastating subtype of cancer.
Author Contributions
AN, concept, literature search, writing and edits, revision of manuscript. AG-S, literature search, writing of manuscript. GG, literature search, writing of manuscript. PA, critical revision and edits, supervision. GF, critical revision and supervision. SP, concept, supervision and critical revision of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This work was supported in part by Pancreatic Cancer UK and the UCLH/UCL Comprehensive Biomedical Centre which receives a proportion of funding from the Department of Health’s National Institute for Health Research (NIHR) Biomedical Research Centres funding scheme.
References
1. Chen Z, Guo P, Xie X, Yu H, Wang Y, Chen G. The Role of Tumour Microenvironment: A New Vision for Cholangiocarcinoma. J Cell Mol Med (2019) 23(1):59–69. doi: 10.1111/jcmm.13953
2. Macias RIR, Banales JM, Sangro B, Muntané J, Avila MA, Lozano E, et al. The Search for Novel Diagnostic and Prognostic Biomarkers in Cholangiocarcinoma. Biochim Biophys Acta - Mol Basis Dis (2018) 1864(4):1468–77. doi: 10.1016/j.bbadis.2017.08.002
3. Beal EW, Tumin D, Moris D, Zhang X-F, Chakedis J, Dilhoff M, et al. Cohort Contributions to Trends in the Incidence and Mortality of Intrahepatic Cholangiocarcinoma. HepatoBiliary Surg Nutr (2018) 7(4):270–6. doi: 10.21037/hbsn.2018.03.16
4. Strijker M, Belkouz A, van der Geest LG, van Gulik TM, van Hooft JE, de Meijer VE, et al. Treatment and Survival of Resected and Unresected Distal Cholangiocarcinoma: A Nationwide Study. Acta Oncol (Madr) (2019) 58(7):1048–55. doi: 10.1080/0284186X.2019.1590634
5. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: The Next Horizon in Mechanisms and Management. Nat Rev Gastroenterol Hepatol (2020) 17(9):557–88. doi: 10.1038/s41575-020-0310-z
6. Shaib Y, El-Serag H. The Epidemiology of Cholangiocarcinoma. Semin Liver Dis (2004) 24(02):115–25. doi: 10.1055/s-2004-828889
7. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Cholangiocarcinoma: Current Knowledge and Future Perspectives Consensus Statement From the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol (2016) 13(5):261–80. doi: 10.1038/nrgastro.2016.51
8. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary Sclerosing Cholangitis–A Comprehensive Review. J Hepatol (2017) 67(6):1298–323. doi: 10.1016/j.jhep.2017.07.022
9. Cardinale V. Intra-Hepatic and Extra-Hepatic Cholangiocarcinoma: New Insight Into Epidemiology and Risk Factors. World J Gastrointest Oncol (2010) 2(11):407. doi: 10.4251/wjgo.v2.i11.407
10. Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and Risk Factors for Cholangiocarcinoma in Primary Sclerosing Cholangitis. Am J Gastroenterol (2004) 99(3):523–6. doi: 10.1111/j.1572-0241.2004.04067.x
11. Labib PL, Goodchild G, Pereira SP. Molecular Pathogenesis of Cholangiocarcinoma. BMC Cancer (2019) 19(1):185. doi: 10.1186/s12885-019-5391-0
12. Macias RIR, Kornek M, Rodrigues PM, Paiva NA, Castro RE, Urban S, et al. Diagnostic and Prognostic Biomarkers in Cholangiocarcinoma. Liver Int (2019) 39(S1):108–22. doi: 10.1111/liv.14090
13. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine Compared With Observation in Resected Biliary Tract Cancer (BILCAP): A Randomised, Controlled, Multicentre, Phase 3 Study. Lancet Oncol (2019) 20(5):663–73. doi: 10.1016/S1470-2045(18)30915-X
14. Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical Presentation, Diagnosis and Staging of Cholangiocarcinoma. Liver Int (2019) 39:98–107. doi: 10.1111/liv.14086
15. Rizzo A, Brandi G. Neoadjuvant Therapy for Cholangiocarcinoma: A Comprehensive Literature Review. Cancer Treat Res Commun (2021) 27:100354. doi: 10.1016/j.ctarc.2021.100354
16. Marin JJG, Prete MG, Lamarca A, Tavolari S, Landa-Magdalena A, Brandi G, et al. Current and Novel Therapeutic Opportunities for Systemic Therapy in Biliary Cancer. Br J Cancer (2020) 123(7):1047–59. doi: 10.1038/s41416-020-0987-3
17. Blechacz B, Gores GJ. Cholangiocarcinoma: Advances in Pathogenesis, Diagnosis, and Treatment. Hepatology (2008) 48(1):308–21. doi: 10.1002/hep.22310
18. Loosen SH, Vucur M, Trautwein C, Roderburg C, Luedde T. Circulating Biomarkers for Cholangiocarcinoma. Dig Dis (2018) 36(4):281–8. doi: 10.1159/000488342
19. Viterbo D, Gausman V, Gonda T. Diagnostic and Therapeutic Biomarkers in Pancreaticobiliary Malignancy. World J Gastrointest Endosc (2016) 8(3):128. doi: 10.4253/wjge.v8.i3.128
20. Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative Effectiveness of Biliary Brush Cytology and Intraductal Biopsy for Detection of Malignant Biliary Strictures: A Systematic Review and Meta-Analysis. Gastrointest Endosc (2015) 81(1):168–76. doi: 10.1016/j.gie.2014.09.017
21. Singhi AD, Klimstra DS. Well-Differentiated Pancreatic Neuroendocrine Tumours (PanNETs) and Poorly Differentiated Pancreatic Neuroendocrine Carcinomas (PanNECs): Concepts, Issues and a Practical Diagnostic Approach to High-Grade (G3) Cases. Histopathology (2018) 72(1):168–77. doi: 10.1111/his.13408
22. Singhi AD, Nikiforova MN, Chennat J, Papachristou GI, Khalid A, Rabinovitz M, et al. Integrating Next-Generation Sequencing to Endoscopic Retrograde Cholangiopancreatography (ERCP)-Obtained Biliary Specimens Improves the Detection and Management of Patients With Malignant Bile Duct Strictures. Gut (2020) 69(1):52–61. doi: 10.1136/gutjnl-2018-317817
23. Keane MG, Devlin J, Harrison P, Masadeh M, Arain MA, Joshi D. Diagnosis and Management of Benign Biliary Strictures Post Liver Transplantation in Adults. Transplant Rev (2021) 35(1):100593. doi: 10.1016/j.trre.2020.100593
24. Elwir S, Thompson J, Amateau SK, Trikudanathan G, Attam R, Hassan M, et al. Endoscopic Management of Biliary Leaks and Strictures After Living Donor Liver Transplantation: Optimizing Techniques for Successful Management. Dig Dis Sci (2017) 62(1):244–52. doi: 10.1007/s10620-016-4367-z
25. Bowlus CL, Olson KA, Gershwin ME. Evaluation of Indeterminate Biliary Strictures. Nat Rev Gastroenterol Hepatol (2016) 13(1):28–37. doi: 10.1038/nrgastro.2015.182
26. Ma MX, Jayasekeran V, Chong AK. Benign Biliary Strictures: Prevalence, Impact, and Management Strategies. Clin Exp Gastroenterol (2019) 12:83–92. doi: 10.2147/CEG.S165016
27. Kapoor BS, Mauri G, Lorenz JM. Management of Biliary Strictures: State-Of-the-Art Review. Radiology (2018) 289(3):590–603. doi: 10.1148/radiol.2018172424
28. Leng J, Zhang N, Dong J. Percutaneous Transhepatic and Endoscopic Biliary Drainage for Malignant Biliary Tract Obstruction: A Meta-Analysis. World J Surg Oncol (2014) 12(1):272. doi: 10.1186/1477-7819-12-272
29. Otsuka S, Ebata T, Yokoyama Y, Igami T, Mizuno T, Yamaguchi J, et al. Benign Hilar Bile Duct Strictures Resected as Perihilar Cholangiocarcinoma. Br J Surg (2019) 106(11):1504–11. doi: 10.1002/bjs.11257
30. Zeng N, Tao H, Fang C, Fan Y, Xiang N, Yang J, et al. Individualized Preoperative Planning Using Three-Dimensional Modeling for Bismuth and Corlette Type III Hilar Cholangiocarcinoma. World J Surg Oncol (2016) 14(1):44. doi: 10.1186/s12957-016-0794-8
31. Izbicki JR, Tsui TY, Bohn BA, Bockhorn M. Surgical Strategies in Patients With Advanced Hilar Cholangiocarcinoma (Klatskin Tumor). J Gastrointest Surg (2013) 17(3):581–5. doi: 10.1007/s11605-012-2109-x
32. Lee HJ, Cho KB. Diagnosis of Malignant Biliary Stricture: More Is Better. Clin Endosc (2018) 51(2):115–7. doi: 10.5946/ce.2018.035
33. Clayton RAE, Clarke DL, Currie EJ, Madhavan KK, Parks RW, Garden OJ. Incidence of Benign Pathology in Patients Undergoing Hepatic Resection for Suspected Malignancy. Surgery (2003) 1(1):32–8. doi: 10.1016/S1479-666X(03)80006-9
34. Scheuermann U, Widyaningsih R, Hoppe-Lotichius M, Heise M, Otto G. Detection of Benign Hilar Bile Duct Stenoses – A Retrospective Analysis in 250 Patients With Suspicion of Klatskin Tumour. Ann Med Surg (2016) 8:43–9. doi: 10.1016/j.amsu.2016.05.001
35. Franken LC, Schreuder AM, Roos E, van Dieren S, Busch OR, Besselink MG, et al. Morbidity and Mortality After Major Liver Resection in Patients With Perihilar Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Surgery (2019) 165(5):918–28. doi: 10.1016/j.surg.2019.01.010
36. Avadhani V, Hacihasanoglu E, Memis B, Pehlivanoglu B, Hanley KZ, Krishnamurti U, et al. Cytologic Predictors of Malignancy in Bile Duct Brushings: A Multi-Reviewer Analysis of 60 Cases. Mod Pathol (2017) 30(9):1273–86. doi: 10.1038/modpathol.2017.51
37. De Moura DH, Moura EH De, Bernardo W, De Moura EH, Baraca F, Kondo A, et al. Endoscopic Retrograde Cholangiopancreatography Versus Endoscopic Ultrasound for Tissue Diagnosis of Malignant Biliary Stricture: Systematic Review and Meta-Analysis. Endosc Ultrasound (2018) 7(1):10. doi: 10.4103/2303-9027.193597
38. Lee YN, Moon JH, Choi HJ, Kim HK, Choi S-Y, Choi MH, et al. Diagnostic Approach Using ERCP-Guided Transpapillary Forceps Biopsy or EUS-Guided Fine-Needle Aspiration Biopsy According to the Nature of Stricture Segment for Patients With Suspected Malignant Biliary Stricture. Cancer Med (2017) 6(3):582–90. doi: 10.1002/cam4.1034
39. Ahmed W, Karim S, Wadsworth C. P3 Improving ERCP Tissue Acquisition in Malignant Strictures With Transpapillary Endobiliary Biopsies. In: Posters. BMJ Publishing Group Ltd Br Soc Gastroenterology (2021) 70:A43.1–A43. doi: 10.1136/gutjnl-2020-bsgcampus.78
40. Badshah MB, Vanar V, Kandula M, Kalva N, Badshah MB, Revenur V, et al. Peroral Cholangioscopy With Cholangioscopy-Directed Biopsies in the Diagnosis of Biliary Malignancies: A Systemic Review and Meta-Analysis. Eur J Gastroenterol Hepatol (2019) 31(8):935–40. doi: 10.1097/MEG.0000000000001402
41. Sadeghi A, Mohamadnejad M, Islami F, Keshtkar A, Biglari M, Malekzadeh R, et al. Diagnostic Yield of EUS-Guided FNA for Malignant Biliary Stricture: A Systematic Review and Meta-Analysis. Gastrointest Endosc (2016) 83(2):290–8. doi: 10.1016/j.gie.2015.09.024
42. Navaneethan U, Njei B, Venkatesh PGK, Vargo JJ, Parsi MA. Fluorescence In Situ Hybridization for Diagnosis of Cholangiocarcinoma in Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis. Gastrointest Endosc (2014) 79(6):943–50. doi: 10.1016/j.gie.2013.11.001
43. Brooks C, Gausman V, Kokoy-Mondragon C, Munot K, Amin SP, Desai A, et al. Role of Fluorescent In Situ Hybridization, Cholangioscopic Biopsies, and EUS-FNA in the Evaluation of Biliary Strictures. Dig Dis Sci (2018) 63(3):636–44. doi: 10.1007/s10620-018-4906-x
44. Han S, Tatman P, Mehrotra S, Wani S, Attwell AR, Edmundowicz SA, et al. Combination of ERCP-Based Modalities Increases Diagnostic Yield for Biliary Strictures. Dig Dis Sci (2020) 66(4):1276–1284. doi: 10.1007/s10620-020-06335-x
45. Ehlken H, Zenouzi R, Schramm C. Risk of Cholangiocarcinoma in Patients With Primary Sclerosing Cholangitis. Curr Opin Gastroenterol (2017) 33(2):78–84. doi: 10.1097/MOG.0000000000000335
46. Parsa N, Khashab MA. The Role of Peroral Cholangioscopy in Evaluating Indeterminate Biliary Strictures. Clin Endosc (2019) 52(6):556–64. doi: 10.5946/ce.2019.011
47. Kim H-J, Kim M-H, Lee S-K, Yoo K-S, Seo D-W, Min Y-I. Tumor Vessel: A Valuable Cholangioscopic Clue of Malignant Biliary Stricture. Gastrointest Endosc (2000) 52(5):635–8. doi: 10.1067/mge.2000.108969
48. Shieh FK, Drumm H, Nathanson MH, Jamidar PA. High-Definition Confocal Endomicroscopy of the Common Bile Duct. J Clin Gastroenterol (2012) 46(5):401–6. doi: 10.1097/MCG.0b013e31822f3fcd
49. Choi HJ, Moon JH, Lee YN. Advanced Imaging Technology in Biliary Tract Diseases:Narrow-Band Imaging of the Bile Duct. Clin Endosc (2015) 48(6):498–502. doi: 10.5946/ce.2015.48.6.498
50. Fugazza A, Gaiani F, Carra MC, Brunetti F, Lévy M, Sobhani I, et al. Confocal Laser Endomicroscopy in Gastrointestinal and Pancreatobiliary Diseases: A Systematic Review and Meta-Analysis. BioMed Res Int (2016) 2016:1–31. doi: 10.1155/2016/4638683
51. Slivka A, Gan I, Jamidar P, Costamagna G, Cesaro P, Giovannini M, et al. Validation of the Diagnostic Accuracy of Probe-Based Confocal Laser Endomicroscopy for the Characterization of Indeterminate Biliary Strictures: Results of a Prospective Multicenter International Study. Gastrointest Endosc (2015) 81(2):282–90. doi: 10.1016/j.gie.2014.10.009
52. de Vries AB, van der Heide F, ter Steege RWF, Koornstra JJ, Buddingh KT, Gouw ASH, et al. Limited Diagnostic Accuracy and Clinical Impact of Single-Operator Peroral Cholangioscopy for Indeterminate Biliary Strictures. Endoscopy (2020) 52(02):107–14. doi: 10.1055/a-1061-7067
53. Meister T. Intraductal Ultrasound Substantiates Diagnostics of Bile Duct Strictures of Uncertain Etiology. World J Gastroenterol (2013) 19(6):874. doi: 10.3748/wjg.v19.i6.874
54. Moura D, de Moura E, Matuguma S, dos Santos M, Moura E, Baracat F, et al. EUS-FNA Versus ERCP for Tissue Diagnosis of Suspect Malignant Biliary Strictures: A Prospective Comparative Study. Endosc Int Open (2018) 06(06):E769–77. doi: 10.1055/s-0043-123186
55. Chiang A, Theriault M, Salim M, James P. The Incremental Benefit of EUS for the Identification of Malignancy in Indeterminate Extrahepatic Biliary Strictures: A Systematic Review and Meta-Analysis. Endosc Ultrasound (2019) 8(5):310. doi: 10.4103/eus.eus_24_19
56. Lee Y, Moon J, Choi H, Kim H, Lee H, Lee T, et al. Tissue Acquisition for Diagnosis of Biliary Strictures Using Peroral Cholangioscopy or Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Endoscopy (2019) 51(01):50–9. doi: 10.1002/cam4.1034
57. Minaga K, Takenaka M, Katanuma A, Kitano M, Yamashita Y, Kamata K, et al. Needle Tract Seeding: An Overlooked Rare Complication of Endoscopic Ultrasound-Guided Fine-Needle Aspiration. Oncology (2017) 93(1):107–12. doi: 10.1159/000481235
58. Levy MJ, Gleeson FC, Campion MB, Caudill JL, Clain JE, Halling K, et al. Prospective Cytological Assessment of Gastrointestinal Luminal Fluid Acquired During EUS: A Potential Source of False-Positive FNA and Needle Tract Seeding. Am J Gastroenterol (2010) 105(6):1311–8. doi: 10.1038/ajg.2010.80
59. Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, et al. Lower Frequency of Peritoneal Carcinomatosis in Patients With Pancreatic Cancer Diagnosed by EUS-Guided FNA vs. Percutaneous FNA. Gastrointest Endosc (2003) 58(5):690–5. doi: 10.1016/S0016-5107(03)02009-1
60. Yane K, Kuwatani M, Yoshida M, Goto T, Matsumoto R, Ihara H, et al. Non-Negligible Rate of Needle Tract Seeding After Endoscopic Ultrasound-Guided Fine-Needle Aspiration for Patients Undergoing Distal Pancreatectomy for Pancreatic Cancer. Dig Endosc (2020) 32(5):801–11. doi: 10.1111/den.13615
61. Ngamruengphong S, Swanson KM, Shah ND, Wallace MB. Preoperative Endoscopic Ultrasound-Guided Fine Needle Aspiration Does Not Impair Survival of Patients With Resected Pancreatic Cancer. Gut (2015) 64(7):1105–10. doi: 10.1136/gutjnl-2014-307475
62. Chafic A, Dewitt J, LeBlanc J, El Hajj I, Cote G, House M, et al. Impact of Preoperative Endoscopic Ultrasound-Guided Fine Needle Aspiration on Postoperative Recurrence and Survival in Cholangiocarcinoma Patients. Endoscopy (2013) 45(11):883–9. doi: 10.1055/s-0033-1344760
63. Dorrell R, Pawa S, Zhou Y, Lalwani N, Pawa R. The Diagnostic Dilemma of Malignant Biliary Strictures. Diagnostics (2020) 10(5):337. doi: 10.3390/diagnostics10050337
64. Kipp BR, Stadheim LM, Halling SA, Pochron NL, Harmsen S, Nagorney DM, et al. A Comparison of Routine Cytology and Fluorescence In Situ Hybridization for the Detection of Malignant Bile Duct Strictures. Am J Gastroenterol (2004) 99(9):1675–81. doi: 10.1111/j.1572-0241.2004.30281.x
65. Barr Fritcher EG, Voss JS, Brankley SM, Campion MB, Jenkins SM, Keeney ME, et al. An Optimized Set of Fluorescence In Situ Hybridization Probes for Detection of Pancreatobiliary Tract Cancer in Cytology Brush Samples. Gastroenterology (2015) 149(7):1813–24.e1. doi: 10.1053/j.gastro.2015.08.046
66. Liang B, Zhong L, He Q, Wang S, Pan Z, Wang T, et al. Diagnostic Accuracy of Serum CA19-9 in Patients With Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Med Sci Monit (2015) 21:3555–63. doi: 10.12659/MSM.895040
67. Keane MG, Shah A, Pereira SP, Joshi D. Novel Biomarkers and Endoscopic Techniques for Diagnosing Pancreaticobiliary Malignancy. F1000Research (2017) 6:1643. doi: 10.12688/f1000research.11371.1
68. Berretta M, Cavaliere C, Alessandrini L, Stanzione B, Facchini G, Balestreri L, et al. Serum and Tissue Markers in Hepatocellular Carcinoma and Cholangiocarcinoma: Clinical and Prognostic Implications. Oncotarget (2017) 8(8):14192–220. doi: 10.18632/oncotarget.13929
69. Sinakos E, Saenger AK, Keach J, Kim WR, Lindor KD. Many Patients With Primary Sclerosing Cholangitis and Increased Serum Levels of Carbohydrate Antigen 19-9 Do Not Have Cholangiocarcinoma. Clin Gastroenterol Hepatol (2011) 9(5):434–9. doi: 10.1016/j.cgh.2011.02.007
70. Aabakken L, Karlsen T, Albert J, Arvanitakis M, Chazouilleres O, Dumonceau J-M, et al. Role of Endoscopy in Primary Sclerosing Cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy (2017) 49(06):588–608. doi: 10.1055/s-0043-107029
71. Chapman MH, Thorburn D, Hirschfield GM, Webster GGJ, Rushbrook SM, Alexander G, et al. British Society of Gastroenterology and UK-PSC Guidelines for the Diagnosis and Management of Primary Sclerosing Cholangitis. Gut (2019) 68(8):1356–78. doi: 10.1136/gutjnl-2018-317993
72. Qiu Y, He J, Chen X, Huang P, Hu K, Yan H. The Diagnostic Value of Five Serum Tumor Markers for Patients With Cholangiocarcinoma. Clin Chim Acta (2018) 480:186–92. doi: 10.1016/j.cca.2018.02.008
73. Higashi M, Yamada N, Yokoyama S, Kitamoto S, Tabata K, Koriyama C, et al. Pathobiological Implications of MUC16/CA125 Expression in Intrahepatic Cholangiocarcinoma-Mass Forming Type. Pathobiology (2012) 79(2):101–6. doi: 10.1159/000335164
74. Albiin N, Smith ICP, Arnelo U, Lindberg B, Bergquist A, Dolenko B, et al. Detection of Cholangiocarcinoma With Magnetic Resonance Spectroscopy of Bile in Patients With and Without Primary Sclerosing Cholangitis. Acta Radiol (2008) 49(8):855–62. doi: 10.1080/02841850802220092
75. Voigtländer T, Metzger J, Husi H, Kirstein MM, Pejchinovski M, Latosinska A, et al. Bile and Urine Peptide Marker Profiles: Access Keys to Molecular Pathways and Biological Processes in Cholangiocarcinoma. J BioMed Sci (2020) 27(1):13. doi: 10.1186/s12929-019-0599-5
76. Urman JM, Herranz JM, Uriarte I, Rullán M, Oyón D, González B, et al. Pilot Multi-Omic Analysis of Human Bile From Benign and Malignant Biliary Strictures: A Machine-Learning Approach. Cancers (Basel) (2020) 12(6):1644. doi: 10.3390/cancers12061644
77. Keane MG, Huggett MT, Chapman MH, Johnson GJ, Webster GJ, Thorburn D, et al. Diagnosis of Pancreaticobiliary Malignancy by Detection of Minichromosome Maintenance Protein 5 in Biliary Brush Cytology. Br J Cancer (2017) 116(3):349–55. doi: 10.1038/bjc.2016.447
78. Chen C-Y, Tsai W-L, Wu H-C, Syu M-J, Wu C-C, Shiesh S-C. Diagnostic Role of Biliary Pancreatic Elastase for Cholangiocarcinoma in Patients With Cholestasis. Clin Chim Acta (2008) 390(1–2):82–9. doi: 10.1016/j.cca.2008.01.011
79. Li L, Masica D, Ishida M, Tomuleasa C, Umegaki S, Kalloo AN, et al. Human Bile Contains MicroRNA-Laden Extracellular Vesicles That Can Be Used for Cholangiocarcinoma Diagnosis. Hepatology (2014) 60(3):896–907. doi: 10.1002/hep.27050
80. Severino V, Dumonceau J-M, Delhaye M, Moll S, Annessi-Ramseyer I, Robin X, et al. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology (2017) 153(2):495–504.e8. doi: 10.1053/j.gastro.2017.04.043
81. He Q, Cai L, Shuai L, Li D, Wang C, Liu Y, et al. Ars2 Is Overexpressed in Human Cholangiocarcinomas and Its Depletion Increases PTEN and PDCD4 by Decreasing microRNA-21. Mol Carcinog (2013) 52(4):286–96. doi: 10.1002/mc.21859
82. Zhang X, Yang Z, Shi Z, Zhu Z, Li C, Du Z, et al. Analysis of Bile Acid Profile in Plasma to Differentiate Cholangiocarcinoma From Benign Biliary Diseases and Healthy Controls. J Steroid Biochem Mol Biol (2021) 205:105775. doi: 10.1016/j.jsbmb.2020.105775
83. Park JY, Park BK, Ko JS, Bang S, Song SY, Chung JB. Bile Acid Analysis in Biliary Tract Cancer. Yonsei Med J (2006) 47(6):817. doi: 10.3349/ymj.2006.47.6.817
84. Satriano L, Lewinska M, Rodrigues PM, Banales JM, Andersen JB. Metabolic Rearrangements in Primary Liver Cancers: Cause and Consequences. Nat Rev Gastroenterol Hepatol (2019) 16(12):748–66. doi: 10.1038/s41575-019-0217-8
85. Song W-S, Park H-M, Ha JM, Shin SG, Park H-G, Kim J, et al. Discovery of Glycocholic Acid and Taurochenodeoxycholic Acid as Phenotypic Biomarkers in Cholangiocarcinoma. Sci Rep (2018) 8(1):11088. doi: 10.1038/s41598-018-29445-z
86. Liu R, Zhao R, Zhou X, Liang X, Campbell DJW, Zhang X, et al. Conjugated Bile Acids Promote Cholangiocarcinoma Cell Invasive Growth Through Activation of Sphingosine 1-Phosphate Receptor 2. Hepatology (2014) 60(3):908–18. doi: 10.1002/hep.27085
87. Yoon J, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile Acids Induce Cyclooxygenase-2 Expression via the Epidermal Growth Factor Receptor in a Human Cholangiocarcinoma Cell Line. Gastroenterology (2002) 122(4):985–93. doi: 10.1053/gast.2002.32410
88. Keitel V, Stindt J, Häussinger D. Bile Acid-Activated Receptors: GPBAR1 (TGR5) and Other G Protein-Coupled Receptors. Handb Exp Pharmacol (2019) 256:19–49. doi: 10.1007/164_2019_230
89. Erice O, Labiano I, Arbelaiz A, Santos-Laso A, Munoz-Garrido P, Jimenez-Agüero R, et al. Differential Effects of FXR or TGR5 Activation in Cholangiocarcinoma Progression. Biochim Biophys Acta - Mol Basis Dis (2018) 1864(4):1335–44. doi: 10.1016/j.bbadis.2017.08.016
90. Jia X, Lu S, Zeng Z, Liu Q, Dong Z, Chen Y, et al. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology (2020) 71(3):893–906. doi: 10.1002/hep.30852
91. Lankisch TO, Metzger J, Negm AA, Voβkuhl K, Schiffer E, Siwy J, et al. Bile Proteomic Profiles Differentiate Cholangiocarcinoma From Primary Sclerosing Cholangitis and Choledocholithiasis. Hepatology (2011) 53(3):875–84. doi: 10.1002/hep.24103
92. Voigtländer T, Lankisch TO. Endoscopic Diagnosis of Cholangiocarcinoma: From Endoscopic Retrograde Cholangiography to Bile Proteomics. Best Pract Res Clin Gastroenterol (2015) 29(2):267–75. doi: 10.1016/j.bpg.2015.02.005
93. Scarlett CJ, Saxby AJ, Nielsen A, Bell C, Samra JS, Hugh T, et al. Proteomic Profiling of Cholangiocarcinoma: Diagnostic Potential of SELDI-TOF MS in Malignant Bile Duct Stricture. Hepatology (2006) 44(3):658–66. doi: 10.1002/hep.21294
94. Tye BK. MCM Proteins in DNA Replication. Annu Rev Biochem (1999) 68(1):649–86. doi: 10.1146/annurev.biochem.68.1.649
95. Kim D-W, Kim J-Y, Moon JH, Kim K-B, Kim T-S, Hong S-J, et al. Transcriptional Induction of Minichromosome Maintenance Protein 7 (Mcm7) in Human Cholangiocarcinoma Cells Treated With Clonorchis Sinensis Excretory–Secretory Products. Mol Biochem Parasitol (2010) 173(1):10–6. doi: 10.1016/j.molbiopara.2010.03.005
96. Ren B, Yu G, Tseng GC, Cieply K, Gavel T, Nelson J, et al. MCM7 Amplification and Overexpression Are Associated With Prostate Cancer Progression. Oncogene (2006) 25(7):1090–8. doi: 10.1038/sj.onc.1209134
97. Ayaru L, Stoeber K, Webster GJ, Hatfield ARW, Wollenschlaeger A, Okoturo O, et al. Diagnosis of Pancreaticobiliary Malignancy by Detection of Minichromosome Maintenance Protein 5 in Bile Aspirates. Br J Cancer (2008) 98(9):1548–54. doi: 10.1038/sj.bjc.6604342
98. Ikeda C, Haga H, Makino N, Inuzuka T, Kurimoto A, Ueda T, et al. Utility of Claudin-3 in Extracellular Vesicles From Human Bile as Biomarkers of Cholangiocarcinoma. Sci Rep (2021) 11(1):1195. doi: 10.1038/s41598-021-81023-y
99. Yanez-Mo M, Siljander PR-M, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J Extracell vesicles (2015) 4:27066. doi: 10.3402/jev.v4.27066
100. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-To-Cell Mediators of Metastasis. Cancer Cell (2016) 30(6):836–48. doi: 10.1016/j.ccell.2016.10.009
101. Lapitz A, Arbelaiz A, Olaizola P, Aranburu A, Bujanda L, Perugorria MJ, et al. Extracellular Vesicles in Hepatobiliary Malignancies. Front Immunol (2018) 9:2270. doi: 10.3389/fimmu.2018.02270
102. Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, et al. Integrative Molecular Analysis of Intrahepatic Cholangiocarcinoma Reveals 2 Classes That Have Different Outcomes. Gastroenterology (2013) 144(4):829–40. doi: 10.1053/j.gastro.2013.01.001
103. Saborowski A, Lehmann U, Vogel A. FGFR Inhibitors in Cholangiocarcinoma: What’s Now and What’s Next? Ther Adv Med Oncol (2020) 12:1758835920953293. doi: 10.1177/1758835920953293
104. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol (2020) 21(5):671–84. doi: 10.1016/S1470-2045(20)30109-1
105. Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, et al. Frequent Mutation of Isocitrate Dehydrogenase (IDH) 1 and IDH2 in Cholangiocarcinoma Identified Through Broad-Based Tumor Genotyping. Oncologist (2012) 17(1):72. doi: 10.1634/theoncologist.2011-0386
106. de la Fouchardiere C. Towards Greater Clarity in the Treatment of Cholangiocarcinoma. Lancet Oncol (2020) 21(6):738–739. doi: 10.1016/S1470-2045(20)30214-X
107. Lendvai G, Szekerczés T, Illyés I, Dóra R, Kontsek E, Gógl A, et al. Cholangiocarcinoma: Classification, Histopathology and Molecular Carcinogenesis. Pathol Oncol Res (2020) 26(1):3–15. doi: 10.1007/s12253-018-0491-8
108. Lee H, Ross JS. The Potential Role of Comprehensive Genomic Profiling to Guide Targeted Therapy for Patients With Biliary Cancer. Therap Adv Gastroenterol (2017) 10(6):507–20. doi: 10.1177/1756283X17698090
109. Nepal C, O’Rourke CJ, Oliveira DVNP, Taranta A, Shema S, Gautam P, et al. Genomic Perturbations Reveal Distinct Regulatory Networks in Intrahepatic Cholangiocarcinoma. Hepatology (2018) 68(3):949–63. doi: 10.1002/hep.29764
110. Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. Genomic Spectra of Biliary Tract Cancer. Nat Genet (2015) 47(9):1003–10. doi: 10.1038/ng.3375
111. Shibahara H, Tamada S, Higashi M, Goto M, Batra SK, Hollingsworth MA, et al. MUC4 Is a Novel Prognostic Factor of Intrahepatic Cholangiocarcinoma-Mass Forming Type. Hepatology (2004) 39(1):220–9. doi: 10.1002/hep.20031
112. Higashi M, Yonezawa S, Ho JJL, Tanaka S, Irimura T, Kim YS, et al. Expression of MUC1 and MUC2 Mucin Antigens in Intrahepatic Bile Duct Tumors: Its Relationship With a New Morphological Classification of Cholangiocarcinoma. Hepatology (1999) 30(6):1347–55. doi: 10.1002/hep.510300609
113. Mall AS, Tyler MG, Ho SB, Krige JEJ, Kahn D, Spearman W, et al. The Expression of MUC Mucin in Cholangiocarcinoma. Pathol - Res Pract (2010) 206(12):805–9. doi: 10.1016/j.prp.2010.08.004
114. Ruys AT, Groot Koerkamp B, Wiggers JK, Klümpen H-J, ten Kate FJ, van Gulik TM. Prognostic Biomarkers in Patients With Resected Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Ann Surg Oncol (2014) 21(2):487–500. doi: 10.1245/s10434-013-3286-x
115. Araki K, Shimura T, Suzuki H, Tsutsumi S, Wada W, Yajima T, et al. E/N-Cadherin Switch Mediates Cancer Progression via TGF-β-Induced Epithelial-to-Mesenchymal Transition in Extrahepatic Cholangiocarcinoma. Br J Cancer (2011) 105(12):1885–93. doi: 10.1038/bjc.2011.452
116. Techasen A, Loilome W, Namwat N, Khuntikeo N, Puapairoj A, Jearanaikoon P, et al. Loss of E-Cadherin Promotes Migration and Invasion of Cholangiocarcinoma Cells and Serves as a Potential Marker of Metastasis. Tumor Biol (2014) 35(9):8645–52. doi: 10.1007/s13277-014-2087-6
117. Zheng B, Shen S, Wong K, Gong Z, Sun W, Ni X, et al. Clinical Correlation of Cadherin-17 Marker With Advanced Tumor Stages and Poor Prognosis of Cholangiocarcinoma. J Surg Oncol (2021) 123(5):1253–62. doi: 10.1002/jso.26399
118. Wang Y, Shek FH, Wong KF, Liu LX, Zhang XQ, Yuan Y, et al. Anti-Cadherin-17 Antibody Modulates Beta-Catenin Signaling and Tumorigenicity of Hepatocellular Carcinoma. Lee JW, Editor. PloS One (2013) 8(9):e72386. doi: 10.1371/journal.pone.0072386
119. Altree-Tacha D, Tyrrell J, Haas T. CDH17 Is a More Sensitive Marker for Gastric Adenocarcinoma Than CK20 and CDX2. Arch Pathol Lab Med (2017) 141(1):144–50. doi: 10.5858/arpa.2015-0404-OA
120. Zheng B, Jeong S, Zhu Y, Chen L, Xia Q. miRNA and lncRNA as Biomarkers in Cholangiocarcinoma(CCA). Oncotarget (2017) 8(59):100819–30. doi: 10.18632/oncotarget.19044
121. Huang W-K, Yeh C-N. The Emerging Role of MicroRNAs in Regulating the Drug Response of Cholangiocarcinoma. Biomolecules (2020) 10(10):1396. doi: 10.3390/biom10101396
122. Amato F, Rae C, Prete MG, Braconi C. Cholangiocarcinoma Disease Modelling Through Patients Derived Organoids. Cells (2020) 9(4):832. doi: 10.3390/cells9040832
123. Selaru FM, Olaru AV, Kan T, David S, Cheng Y, Mori Y, et al. MicroRNA-21 Is Overexpressed in Human Cholangiocarcinoma and Regulates Programmed Cell Death 4 and Tissue Inhibitor of Metalloproteinase 3. Hepatology (2009) 49(5):1595–601. doi: 10.1002/hep.22838
124. Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in Patients With Hepatocellular Carcinoma or Intrahepatic Cholangiocarcinoma and Its Prognostic Significance. Mol Carcinog (2013) 52(4):297–303. doi: 10.1002/mc.21864
125. Chusorn P, Namwat N, Loilome W, Techasen A, Pairojkul C, Khuntikeo N, et al. Overexpression of microRNA-21 Regulating PDCD4 During Tumorigenesis of Liver Fluke-Associated Cholangiocarcinoma Contributes to Tumor Growth and Metastasis. Tumor Biol (2013) 34(3):1579–88. doi: 10.1007/s13277-013-0688-0
126. Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science (2009) 324(5933):1457–61. doi: 10.1126/science.1171362
127. Loosen SH, Roderburg C, Kauertz KL, Pombeiro I, Leyh C, Benz F, et al. Elevated Levels of Circulating Osteopontin Are Associated With a Poor Survival After Resection of Cholangiocarcinoma. J Hepatol (2017) 67(4):749–57. doi: 10.1016/j.jhep.2017.06.020
128. Betesh L, Comunale MA, Wang M, Liang H, Hafner J, Karabudak A, et al. Identification of Fucosylated Fetuin-A as a Potential Biomarker for Cholangiocarcinoma. PROTEOMICS–Clinical Appl (2017) 11(9–10):1600141. doi: 10.1002/prca.201600141
129. Cheon YK, Cho YD, Moon JH, Jang JY, Kim YS, Kim YS, et al. Diagnostic Utility of Interleukin-6 (IL-6) for Primary Bile Duct Cancer and Changes in Serum IL-6 Levels Following Photodynamic Therapy. Am J Gastroenterol (2007) 102(10):2164–70. doi: 10.1111/j.1572-0241.2007.01403.x
130. Luang S, Teeravirote K, Saentaweesuk W, Ma-In P, Silsirivanit A. Carbohydrate Antigen 50: Values for Diagnosis and Prognostic Prediction of Intrahepatic Cholangiocarcinoma. Medicina (B Aires) (2020) 56(11):616. doi: 10.3390/medicina56110616
131. Gül-Utku Ö, Karatay E, Ergül B, Kisa Ü, Erdin Z, Oğuz D. The Role of Resolvin D1 in the Differential Diagnosis of the Cholangiocarcinoma and Benign Biliary Diseases. Clin Lab (2020) 66(5). doi: 10.7754/Clin.Lab.2020.200212
132. Leelawat K, Sakchinabut S, Narong S, Wannaprasert J. Detection of Serum MMP-7 and MMP-9 in Cholangiocarcinoma Patients: Evaluation of Diagnostic Accuracy. BMC Gastroenterol (2009) 9:30. doi: 10.1186/1471-230X-9-30
133. Lumachi F, Re G, Tozzoli R, D’Aurizio F, Facomer F, Chiara GB, et al. Measurement of Serum Carcinoembryonic Antigen, Carbohydrate Antigen 19-9, Cytokeratin-19 Fragment and Matrix Metalloproteinase-7 for Detecting Cholangiocarcinoma: A Preliminary Case-Control Study. Anticancer Res (2014) 34(11):6663–7.
134. Cuenco J, Wehnert N, Blyuss O, Kazarian A, Whitwell HJ, Menon U, et al. Identification of a Serum Biomarker Panel for the Differential Diagnosis of Cholangiocarcinoma and Primary Sclerosing Cholangitis. Oncotarget (2018) 9(25):17430–42. doi: 10.18632/oncotarget.24732
135. Tao L-Y, Cai L, He X-D, Liu W, Qu Q. Comparison of Serum Tumor Markers for Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma. Am Surg (2010) 76(11):1210–3. doi: 10.1177/000313481007601119
136. Liu S, Wang M, Zheng C, Zhong Q, Shi Y, Han X. Diagnostic Value of Serum Glypican-3 Alone and in Combination With AFP as an Aid in the Diagnosis of Liver Cancer. Clin Biochem (2020) 79:54–60. doi: 10.1016/j.clinbiochem.2020.02.009
137. Silsirivanit A, Matsuda A, Kuno A, Tsuruno C, Uenoyama Y, Seubwai W, et al. Multi-Serum Glycobiomarkers Improves the Diagnosis and Prognostic Prediction of Cholangiocarcinoma. Clin Chim Acta (2020) 510:142–9. doi: 10.1016/j.cca.2020.07.017
138. Onsurathum S, Haonon O, Pinlaor P, Pairojkul C, Khuntikeo N, Thanan R, et al. Proteomics Detection of S100A6 in Tumor Tissue Interstitial Fluid and Evaluation Of Its Potential as a Biomarker of Cholangiocarcinoma. Tumour Biol (2018) 40(4):1010428318767195. doi: 10.1177/1010428318767195
139. Shi R-Y, Yang X-R, Shen Q-J, Yang L-X, Xu Y, Qiu S-J, et al. High Expression of Dickkopf-Related Protein 1 Is Related to Lymphatic Metastasis and Indicates Poor Prognosis in Intrahepatic Cholangiocarcinoma Patients After Surgery. Cancer (2013) 119(5):993–1003. doi: 10.1002/cncr.27788
140. Shen J, Wang W, Wu J, Feng B, Chen W, Wang M, et al. Comparative Proteomic Profiling of Human Bile Reveals SSP411 as a Novel Biomarker of Cholangiocarcinoma. PloS One (2012) 7(10):e47476–6. doi: 10.1371/journal.pone.0047476
141. Correa-Gallego C, Maddalo D, Doussot A, Kemeny N, Kingham TP, Allen PJ, et al. Circulating Plasma Levels of MicroRNA-21 and MicroRNA-221 Are Potential Diagnostic Markers for Primary Intrahepatic Cholangiocarcinoma. PloS One (2016) 11(9):e0163699. doi: 10.1371/journal.pone.0163699
142. Kishimoto T, Eguchi H, Nagano H, Kobayashi S, Akita H, Hama N, et al. Plasma Mi R-21 Is a Novel Diagnostic Biomarker for Biliary Tract Cancer. Cancer Sci (2013) 104(12):1626–31. doi: 10.1111/cas.12300
143. Han Y, Zhang H, Zhou Z, Liu R, Liu D, Bai M, et al. Serum microRNAs as Biomarkers for the Noninvasive Early Diagnosis of Biliary Tract Cancer. Int J Gen Med (2021) 14:1185–95. doi: 10.2147/IJGM.S297371
144. Salem PES, Ghazala RA, El Gendi AM, Emara DM, Ahmed NM. The Association Between Circulating MicroRNA-150 Level and Cholangiocarcinoma. J Clin Lab Anal (2020) 34(11):e23397. doi: 10.1002/jcla.23397
145. Bernuzzi F, Marabita F, Lleo A, Carbone M, Mirolo M, Marzioni M, et al. Serum microRNAs as Novel Biomarkers for Primary Sclerosing Cholangitis and Cholangiocarcinoma. Clin Exp Immunol (2016) 185(1):61–71. doi: 10.1111/cei.12776
146. Voigtlander T, Gupta SK, Thum S, Fendrich J, Manns MP, Lankisch TO, et al. MicroRNAs in Serum and Bile of Patients With Primary Sclerosing Cholangitis and/or Cholangiocarcinoma. PloS One (2015) 10(10):e0139305. doi: 10.1371/journal.pone.0139305
147. Wongkham S, Boonla C, Kongkham S, Wongkham C, Bhudhisawasdi V, Sripa B. Serum Total Sialic Acid in Cholangiocarcinoma Patients: An ROC Curve Analysis. Clin Biochem (2001) 34(7):537–41. doi: 10.1016/S0009-9120(01)00265-X
148. Kongtawelert P, Tangkijvanich P, Ong-Chai S, Poovorawan Y. Role of Serum Total Sialic Acid in Differentiating Cholangiocarcinoma From Hepatocellular Carcinoma. World J Gastroenterol (2003) 9(10):2178–81. doi: 10.3748/wjg.v9.i10.2178
149. Liang Q, Liu H, Zhang T, Jiang Y, Xing H, Zhang H. Serum Metabolomics Uncovering Specific Metabolite Signatures of Intra- and Extrahepatic Cholangiocarcinoma. Mol Biosyst (2016) 12(2):334–40. doi: 10.1039/C5MB00572H
150. Arbelaiz A, Azkargorta M, Krawczyk M, Santos-Laso A, Lapitz A, Perugorria MJ, et al. Serum Extracellular Vesicles Contain Protein Biomarkers for Primary Sclerosing Cholangitis and Cholangiocarcinoma. Hepatology (2017) 66(4):1125–43. doi: 10.1002/hep.29291
151. Lapitz A, Arbelaiz A, O’Rourke CJ, Lavin JL, Casta A, Ibarra C, et al. Patients With Cholangiocarcinoma Present Specific RNA Profiles in Serum and Urine Extracellular Vesicles Mirroring the Tumor Expression: Novel Liquid Biopsy Biomarkers for Disease Diagnosis. Cells (2020) 9(3):721. doi: 10.3390/cells9030721
152. Julich-Haertel H, Urban SK, Krawczyk M, Willms A, Jankowski K, Patkowski W, et al. Cancer-Associated Circulating Large Extracellular Vesicles in Cholangiocarcinoma and Hepatocellular Carcinoma. J Hepatol (2017) 67(2):282–92. doi: 10.1016/j.jhep.2017.02.024
153. Icer MA, Gezmen-Karadag M. The Multiple Functions and Mechanisms of Osteopontin. Clin Biochem (2018) 59:17–24. doi: 10.1016/j.clinbiochem.2018.07.003
154. Jendrek ST, Gotthardt D, Nitzsche T, Widmann L, Korf T, Michaels MA, et al. Anti-GP2 IgA Autoantibodies Are Associated With Poor Survival and Cholangiocarcinoma in Primary Sclerosing Cholangitis. Gut (2017) 66(1):137–44. doi: 10.1136/gutjnl-2016-311739
155. Shan M, Tian Q, Zhang L. Serum CA50 Levels in Patients With Cancers and Other Diseases. Prog Mol Biol Transl Sci (2019) 162:187–98. doi: 10.1016/bs.pmbts.2018.12.006
156. Chang T-T, Ho C-H. Plasma Proteome Atlas for Differentiating Tumor Stage and Post-Surgical Prognosis of Hepatocellular Carcinoma and Cholangiocarcinoma. PloS One (2020) 15(8):e0238251. doi: 10.1371/journal.pone.0238251
157. Dieplinger H, Dieplinger B. Afamin—a Pleiotropic Glycoprotein Involved in Various Disease States. Clin Chim Acta (2015) 446:105–10. doi: 10.1016/j.cca.2015.04.010
158. Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid Biopsy: Monitoring Cancer-Genetics in the Blood. Nat Rev Clin Oncol (2013) 10(8):472–84. doi: 10.1038/nrclinonc.2013.110
159. Rizzo A, Ricci AD, Tavolari S, Brandi G. Circulating Tumor DNA in Biliary Tract Cancer: Current Evidence and Future Perspectives. Cancer Genomics - Proteomics (2020) 17(5):441–52. doi: 10.21873/cgp.20203
160. Kori M, Yalcin Arga K. Potential Biomarkers and Therapeutic Targets in Cervical Cancer: Insights From the Meta-Analysis of Transcriptomics Data Within Network Biomedicine Perspective. PloS One (2018) 13(7):e0200717. doi: 10.1371/journal.pone.0200717
161. Haga H, Yan I, Takahashi K, Wood J, Patel T. Emerging Insights Into the Role of microRNAs in the Pathogenesis of Cholangiocarcinoma. Gene Expr J Liver Res (2014) 16(2):93–9. doi: 10.3727/105221614X13919976902174
162. Huang C-S, Yu W, Cui H, Wang Y-J, Zhang L, Han F, et al. Increased Expression of miR-21 Predicts Poor Prognosis in Patients With Hepatocellular Carcinoma. Int J Clin Exp Pathol (2015) 8(6):7234–8.
163. Wang S, Yin J, Li T, Yuan L, Wang D, He J, et al. Upregulated Circulating miR-150 Is Associated With the Risk of Intrahepatic Cholangiocarcinoma. Oncol Rep (2015) 33(2):819–25. doi: 10.3892/or.2014.3641
164. Wu X, Xia M, Chen D, Wu F, Lv Z, Zhan Q, et al. Profiling of Downregulated Blood-Circulating miR-150-5p as a Novel Tumor Marker for Cholangiocarcinoma. Tumour Biol (2016) 37(11):15019–29. doi: 10.1007/s13277-016-5313-6
165. Cheng Q, Feng F, Zhu L, Zheng Y, Luo X, Liu C, et al. Circulating miR-106a Is a Novel Prognostic and Lymph Node Metastasis Indicator for Cholangiocarcinoma. Sci Rep (2015) 5(1):16103. doi: 10.1038/srep16103
166. Qi L-N, Xiang B-D, Wu F-X, Ye J-Z, Zhong J-H, Wang Y-Y, et al. Circulating Tumor Cells Undergoing EMT Provide a Metric for Diagnosis and Prognosis of Patients With Hepatocellular Carcinoma. Cancer Res (2018) 78(16):4731–44. doi: 10.1158/0008-5472.CAN-17-2459
167. Liu J, Wang J, Song Y, Ma B, Luo J, Ni Z, et al. A Panel Consisting of Three Novel Circulating lncRNAs, Is It a Predictive Tool for Gastric Cancer? J Cell Mol Med (2018) 22(7):3605–13. doi: 10.1111/jcmm.13640
168. Arnoletti JP, Fanaian N, Reza J, Sause R, Almodovar AJ, Srivastava M, et al. Pancreatic and Bile Duct Cancer Circulating Tumor Cells (CTC) Form Immune-Resistant Multi-Cell Type Clusters in the Portal Venous Circulation. Cancer Biol Ther (2018) 19(10):887–97. doi: 10.1080/15384047.2018.1480292
169. Bidard F-C, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, et al. Clinical Validity of Circulating Tumour Cells in Patients With Metastatic Breast Cancer: A Pooled Analysis of Individual Patient Data. Lancet Oncol (2014) 15(4):406–14. doi: 10.1016/S1470-2045(14)70069-5
170. Tan CRC, Zhou L, El-Deiry WS. Circulating Tumor Cells Versus Circulating Tumor DNA in Colorectal Cancer: Pros and Cons. Curr Colorectal Cancer Rep (2016) 12(3):151–61. doi: 10.1007/s11888-016-0320-y
171. Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of Rare Circulating Tumour Cells in Cancer Patients by Microchip Technology. Nature (2007) 450(7173):1235–9. doi: 10.1038/nature06385
172. Kawashima R, Abei M, Fukuda K, Nakamura K, Murata T, Wakayama M, et al. EpCAM- and EGFR-Targeted Selective Gene Therapy for Biliary Cancers Using Z33-Fiber-Modified Adenovirus. Int J Cancer (2011) 129(5):1244–53. doi: 10.1002/ijc.25758
173. Al Ustwani O, Iancu D, Yacoub R, Iyer R. Detection of Circulating Tumor Cells in Cancers of Biliary Origin. J Gastrointest Oncol (2012) 3(2):97–104. doi: 10.3978/j.issn.2078-6891.2011.047
174. Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas But Not in Healthy Subjects or Patients With Nonmalignant Diseases. Clin Cancer Res (2004) 10(20):6897–904. doi: 10.1158/1078-0432.CCR-04-0378
175. Yang JD, Campion MB, Liu MC, Chaiteerakij R, Giama NH, Ahmed Mohammed H, et al. Circulating Tumor Cells Are Associated With Poor Overall Survival in Patients With Cholangiocarcinoma. Hepatology (2016) 63(1):148–58. doi: 10.1002/hep.27944
176. Backen AC, Lopes A, Wasan H, Palmer DH, Duggan M, Cunningham D, et al. Circulating Biomarkers During Treatment in Patients With Advanced Biliary Tract Cancer Receiving Cediranib in the UK ABC-03 Trial. Br J Cancer (2018) 119(1):27–35. doi: 10.1038/s41416-018-0132-8
177. Navaneethan U, Parsi MA, Lourdusamy D, Grove D, Sanaka MR, Hammel JP, et al. Volatile Organic Compounds in Urine for Noninvasive Diagnosis of Malignant Biliary Strictures: A Pilot Study. Dig Dis Sci (2015) 60(7):2150–7. doi: 10.1007/s10620-015-3596-x
178. Thongboonkerd V. Recent Progress in Urinary Proteomics. Proteomics – Clin Appl (2007) 1(8):780–91. doi: 10.1002/prca.200700035
179. Frantzi M, Latosinska A, Belczacka I, Mischak H. Urinary Proteomic Biomarkers in Oncology: Ready for Implementation? Expert Rev Proteomics (2019) 16(1):49–63. doi: 10.1080/14789450.2018.1547193
180. Metzger J, Negm AA, Plentz RR, Weismüller TJ, Wedemeyer J, Karlsen TH, et al. Urine Proteomic Analysis Differentiates Cholangiocarcinoma From Primary Sclerosing Cholangitis and Other Benign Biliary Disorders. Gut (2013) 62(1):122–30. doi: 10.1136/gutjnl-2012-302047
181. Voigtländer T, Metzger J, Schönemeier B, Jäger M, Mischak H, Manns MP, et al. A Combined Bile and Urine Proteomic Test for Cholangiocarcinoma Diagnosis in Patients With Biliary Strictures of Unknown Origin. United Eur Gastroenterol J (2017) 5(5):668–76. doi: 10.1177/2050640616687836
182. Silakit R, Loilome W, Yongvanit P, Thongchot S, Sithithaworn P, Boonmars T, et al. Urinary microRNA-192 and microRNA-21 as Potential Indicators for Liver Fluke-Associated Cholangiocarcinoma Risk Group. Parasitol Int (2017) 66(4):479–85. doi: 10.1016/j.parint.2015.10.001
Keywords: cholangiocarcinoma, primary sclerosing cholangitis (PSC), biliary strictures, ERCP (Endoscopic Retrograde Cholangiopancreatography), early detection, biomarkers
Citation: Ney A, Garcia-Sampedro A, Goodchild G, Acedo P, Fusai G and Pereira SP (2021) Biliary Strictures and Cholangiocarcinoma – Untangling a Diagnostic Conundrum. Front. Oncol. 11:699401. doi: 10.3389/fonc.2021.699401
Received: 23 April 2021; Accepted: 15 September 2021;
Published: 30 September 2021.
Edited by:
Francesco Giuseppe Carbone, Azienda Provinciale per i Servizi Sanitari (APSS), ItalyReviewed by:
Alessandro Rizzo, Sant’Orsola-Malpighi Polyclinic, ItalyGuido Carpino, Foro Italico University of Rome, Italy
Copyright © 2021 Ney, Garcia-Sampedro, Goodchild, Acedo, Fusai and Pereira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Ney, alexander.ney.15@ucl.ac.uk; Stephen P. Pereira, stephen.pereira@ucl.ac.uk
 Alexander Ney
Alexander Ney Andres Garcia-Sampedro
Andres Garcia-Sampedro George Goodchild2
George Goodchild2 Pilar Acedo
Pilar Acedo Stephen P. Pereira
Stephen P. Pereira