Development of sarcopenia-based nomograms predicting postoperative complications of benign liver diseases undergoing hepatectomy: A multicenter cohort study
- 1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Key Laboratory of Diagnosis and Treatment of Severe Hepato-Pancreatic Diseases of Zhejiang Province, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 3Department of Oncology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 4Department of Epidemiology and Biostatistics, School of Public Health and Management, Wenzhou Medical University, Wenzhou, China
Background: Sarcopenia has a remarkable negative impact on patients with liver diseases. We aimed to evaluate the impact of preoperative sarcopenia on the short-term outcomes after hepatectomy in patients with benign liver diseases.
Methods: A total of 558 patients with benign liver diseases undergoing hepatectomy were prospectively reviewed. Both the muscle mass and strength were measured to define sarcopenia. Postoperative outcomes including complications, major complications and comprehensive complication index (CCI) were compared among four subgroups classified by muscle mass and strength. Predictors of complications, major complications and high CCI were identified by univariate and multivariate logistic regression analysis. Nomograms based on predictors were constructed and calibration cures were performed to verify the performance.
Results: 120 patients were involved for analysis after exclusion. 33 patients were men (27.5%) and the median age was 54.0 years. The median grip strength was 26.5 kg and the median skeletal muscle index (SMI) was 44.4 cm2/m2. Forty-six patients (38.3%) had complications, 19 patients (15.8%) had major complications and 27 patients (22.5%) had a CCI ≥ 26.2. Age (p = 0.005), SMI (p = 0.005), grip strength (p = 0.018), surgical approach (p = 0.036), and operation time (p = 0.049) were predictors of overall complications. Child-Pugh score (p = 0.037), grip strength (p = 0.004) and surgical approach (p = 0.006) were predictors of major complications. SMI (p = 0.047), grip strength (p < 0.001) and surgical approach (p = 0.014) were predictors of high CCI. Among the four subgroups, patients with reduced muscle mass and strength showed the worst short-term outcomes. The nomograms for complications and major complications were validated by calibration curves and showed satisfactory performance.
Conclusion: Sarcopenia has an adverse impact on the short-term outcomes after hepatectomy in patients with benign liver diseases and valuable sarcopenia-based nomograms were constructed to predict postoperative complications and major complications.
1. Introduction
Along with the change of diet habit and life style, many people are diagnosed with benign liver diseases, such as focal nodular hyperplasia, hepatolithiasis and hemangioma (1). Liver resection remains the main curative treatment and many factors are related to the postoperative outcomes after hepatectomy (2, 3). Identifying predictive factors is important to minimize the risk of adverse outcomes and improve the quality of life of patients.
Sarcopenia, defined as a degenerative loss of muscle mass, strength and function, has gained increasing interest and is associated with adverse outcomes in patients with maligancies (4–6). Many patients with liver disease would experience sarcopenia, which is closely associated with poor clinical outcomes including survival, quality of life and complications (7, 8). Sarcopenia had an important impact on postoperative morbidity and overall survival (OS) after hepatectomy or liver transplantation (9–12). However, most studies defined sarcopenia only based on radiological images without assessing muscle strength, which was a better predictor affecting postoperative outcomes than muscle mass (13). Our previously published studies have confirmed the adverse impact of sarcopenia on the outcomes in hepatocellular carcinoma and intrahepatic cholangiocarcinoma following surgery, and identified the importance of muscle strength in defining sarcopenia (14, 15).
However, limited works have been reported on the impact of sarcopenia on benign liver diseases. Therefore, we performed this prospective study to assess the impact of sarcopenia on short-term outcomes in patients with benign liver diseases undergoing hepatectomy.
2. Materials and methods
2.1. Patients
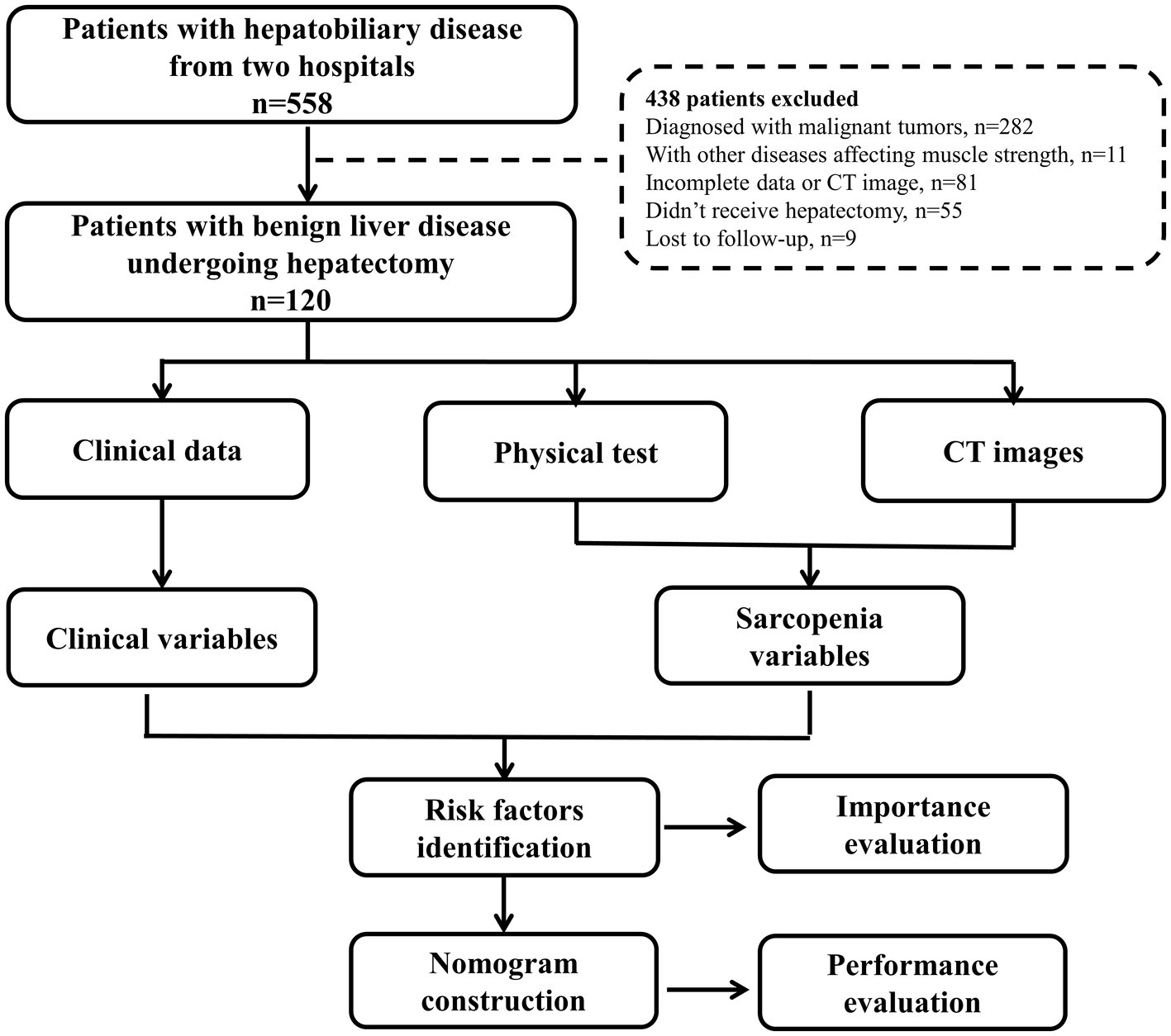
Between May 2021 and April 2022, 558 patients with hepatobiliary diseases who admitted to the first affiliated hospital of Wenzhou Medical University and the first affiliated hospital of Zhejiang Chinese Medical University were prospectively enrolled. All patients received muscle strength test (grip strength and chair stand test), physical performance (gait speed), and imaging evaluation before treatment following the European Working Group on Sarcopenia in Older People (EWGSOP) standard (13). The study protocol was detailed in Supplementary data 1. Clinical data and follow-up information within 90 days after surgery were collected. The inclusion criteria were: (1) pathologically diagnosed benign liver disease, (2) receive liver resection, (3) without other diseases affecting muscle weakness, (4) Eastern Cooperative Oncology Group performance status (ECOG-PS) 0–2, (5) Child-Pugh grade A-B, (6) computed tomography (CT) performed within 1 month before surgery, (7) complete clinical and follow-up information. The flowchart of the study was shown in Figure 1.
Multidisciplinary meeting and essential supportive therapies were performed before surgery to optimize the treatment strategy. The relevant clinical data were collected, including epidemiological characteristics, laboratory tests, operation-related factors, image data, physical tests and postoperative outcomes (complications, major complications and mortality).
The study was approved by the Ethics Committee of local institutional review boards (Number 2021–066) and adhered to the Declaration of Helsinki. Written informed consents were obtained from each patient before research.
2.2. Definition of short-term outcomes
Patients were followed up once every 1 month after surgery through out-patient service. The primary outcomes of the study were postoperative complications and major complications. The postoperative complications were classified according to the Clavien-Dindo classification system and major complications were defined as grade III or higher (16). In addition, we used comprehensive complication index (CCI) to evaluate the burden of complications, which is calculated based on the Clavien–Dindo classification grade (17). We used an online tool provided at https://www.assessurgery.com to calculate CCI score and a CCI ≥26.2 was used as a threshold to define the severity of complications according to the previous studies (18, 19). Specifically, the complications included cardiovascular complications (e.g., heart insufficient and atrial fibrillation), infectious complications (e.g., wound infections, abdominal abscess, peritonitis and sepsis), pulmonary complications (e.g., pleural effusion, pneumonia and respiratory insufficiency), gastrointestinal complications (e.g., intestinal obstruction, vomit, diarrhea and biliary leakage), and others (e.g., fever, ascites, abdominal hemorrhage, organ failure and death). Postoperative biliary leak, bleeding, and organ failure were defined according to the international study group of liver surgery and other studies (18, 20, 21).
The secondary outcomes were hospital stay, hospital cost and unplanned 90-day readmission rate. Hospital cost was extracted from the electronic medical records database, which contains the cost associated with the treatment (e.g., surgery, anesthesia, and medication) and basic care of patients during hospitalization.
2.3. Definition of sarcopenia and clinical factors
Preoperative abdominal CT images at the third lumbar (L3) vertebra level were acquired. Image J software was used to segment the region of interest including the area of skeletal muscle, area of visceral adipose tissue (VAT) and area of subcutaneous adipose tissue (SAT) according to the tissue Hounsfield unit (HU) thresholds (Figure 2). The threshold of attenuation value was-29 to 150 HU for skeletal muscle tissue, −150 to −50 HU for VAT, and −190 to −30 HU for SAT. The muscle density was evaluated by the mean CT attenuation value (HU) of the muscle tissue at the L3 level. Skeletal muscle index (SMI) was used to define reduced muscle mass, which was calculated as the total cross-sectional area of skeletal muscle in the L3 plane (cm2) /height (m2) based on the CT image. Two researchers who were blinded to the clinical information segmented the CT images independently and discordance was resolved by consultation. According to the receiver operating characteristic (ROC) curve based on complications, the optimal cut-off values of SMI were defined as 51.1 cm2/m2 in males and 37.1 cm2/m2 in females. Handgrip strength, gait speed, and chair stand test data were recorded with a standardized protocol prior to surgery (22). According to the Asian consensus of sarcopenia, a cut-off value of less than 28 kg in men and less than 18 kg in women was used to define reduced muscle strength (23).
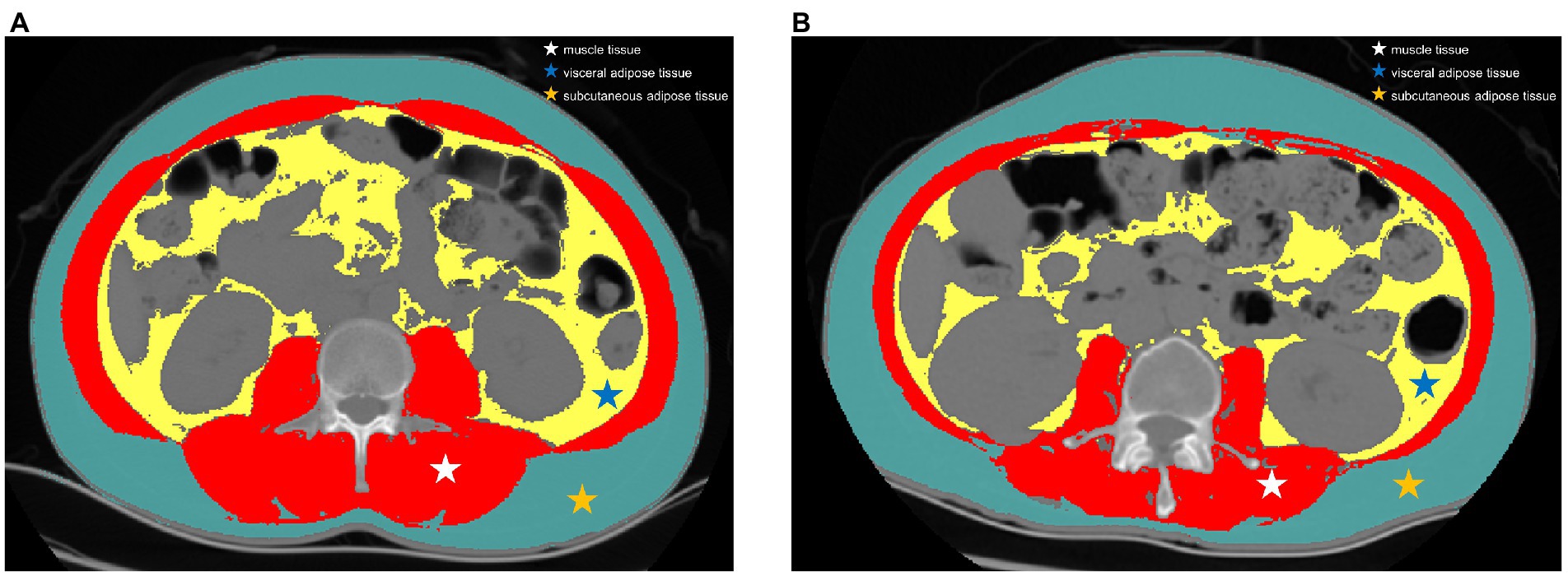
Figure 2. Representative computed tomography scans at the third lumbar vertebra level in patients with and without sarcopenia. (A) a patient with high skeletal muscle index and (B) a patient with low skeletal muscle index. Red region: skeletal muscle mass, assessed using thresholds of-29 to 150 Hounsfield units; yellow region: visceral adipose tissue, assessed using thresholds of-150 to-50 Hounsfield units; green region: subcutaneous adipose tissue, assessed using thresholds of −190 to −30 Hounsfield units.
Body mass index (BMI) was calculated as weight (kg) /height2 (m2). Controlling nutritional status (CONUT) score was calculated and classified based on serum albumin concentration, total lymphocyte count, and total cholesterol concentration (24) (Supplementary Table S1). Prognostic nutritional index (PNI) score was calculated according to the formula: 10 × serum albumin (g/dl) + 0.005 × total lymphocyte count (/mm3) (25). Albumin-bilirubin (ALBI) score was calculated according to the formula: (log10 bilirubin × 0.66) + (albumin × −0.085) (26). Major resection was defined as the resection of three or more segments, and minor resection was defined as the removal of less than three segments (18).
2.4. Statistical analysis
SPSS software (version 25.0) and R software (version 4.2.1) were used to perform statistic analysis and draw nomograms. Python (version 3.10.5) software was used to perform random forest algorithm and Shapley additive explanation (SHAP) analysis. PASS 15 software was used to calculate the sample size. Continuous data were presented as mean ± standard deviation (SD) or medians (interquartile range, IQR). Categorical data were presented as count (percentage). ROC curves using complication as a marker of endpoint were used to determine the optimal cut-off values of factors. T test or Mann–Whitney U test was used to analyze continuous data and Chi-square test or Fisher exact test was used for categorical data. Univariate and multivariate logistic regression analysis were performed to identify risk factors. Random forest algorithm was used to evaluate the importance of each feature and SHAP values were used to provide a local explanation for the direction of each feature’s effect. Nomogram was established according to the results of multivariate logistic analysis. C-index and calibration curve were performed to evaluate the predictive performance. p < 0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
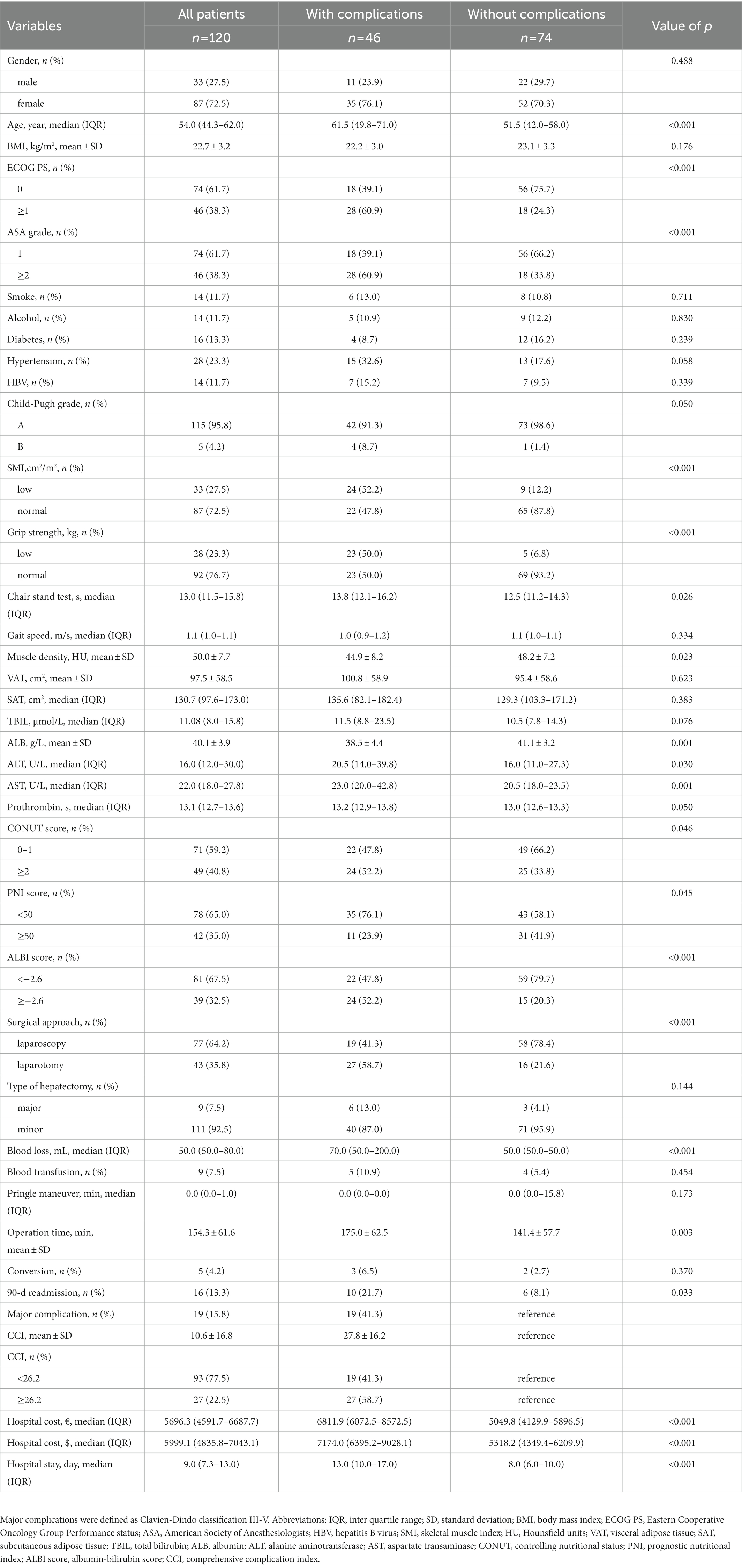
A total 120 patients with benign liver diseases undergoing hepatectomy were enrolled for analysis after exclusion. Sixteen patients had focal nodular hyperplasia, four patients had hepatic cyst, 53 patients had hepatolithiasis and 47 patients had hepatic hemangioma. Forty-six patients (38.3%) had postoperative complications and 19 patients (15.8%) developed major complications. The baseline characteristics of patients with and without complications are shown in Table 1. There were 33 men (27.5%) and most patients (95.8%) had a Child-Pugh class A. The median age was 54.0 years (IQR, 44.3–62.0 years) and mean BMI was 22.7 ± 3.2 kg/m2. The median grip strength was 26.5 kg (IQR, 21.4–33.8 kg) and the median SMI was 44.4 cm2/m2 (IQR, 39.1–52.1 cm2/m2). Thirty-three patients (27.5%) experienced low SMI and 28 patients (23.3%) experienced low grip strength. Seventy-seven patients (64.2%) underwent laparoscopic hepatectomy and 43 patients (35.8%) underwent open surgery. The mean operation time was 154.3 ± 61.6 min for all patients, 175.0 ± 62.5 min for patients with complications, and 141.4 ± 57.7 for patients without complications (p = 0.003). Five patients (4.2%) converted from laparoscopic operation to open surgery according to the following reasons: one case had severe abdominal adhesion, two cases had an unsuitable lesion location and two cases had serious intraoperative bleeding. One patient (0.8%) died because of postoperative multiple organ failure (MOF). All the other 119 patients went home for rehabilitation after discharge. Twenty-seven patients (22.5%) had a high CCI ≥26.2 and 16 patients (13.3%) had unplanned 90-day readmission. Patients with complications had a higher readmission rate (p = 0.033), a higher hospital cost (p < 0.001) and a longer hospital stay (p < 0.001) than patients without complications.
3.2. Predictors of overall complications
According to the univariate logistic regression analysis, age, PS score, SMI, grip strength, chair stand test, muscle density, albumin (ALB), aspartate transaminase (AST), prothrombin time, CONUT score, ALBI score, surgical approach and operation time were associated with overall complications (Table 2). Then variables with p value <0.05 were brought into the subsequent multivariate logistic regression analysis, which showed that age (p = 0.005), SMI (p = 0.005), grip strength (p = 0.018), surgical approach (p = 0.036), and operation time (p = 0.049) were independent risk factors of overall complications. The nomogram based on the results of multivariate logistic analysis were developed and the calibration plot showed favorable performance (Figures 3A,B). The C-index of the nomogram was 0.889 [95% confidence interval (CI), 0.827–0.951].
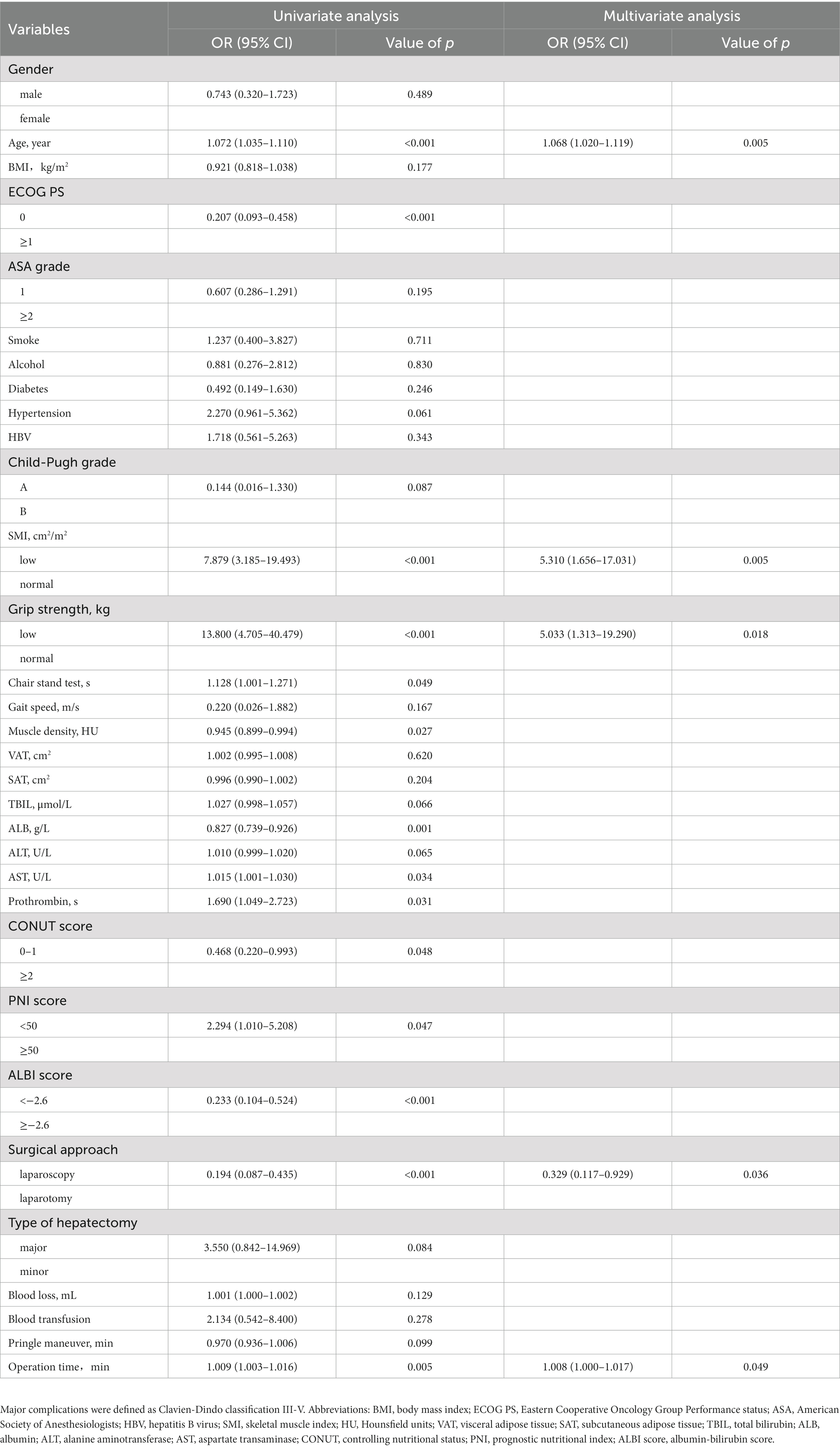
Table 2. Univariate and multivariate logistic regression analysis of predictors of overall complications.
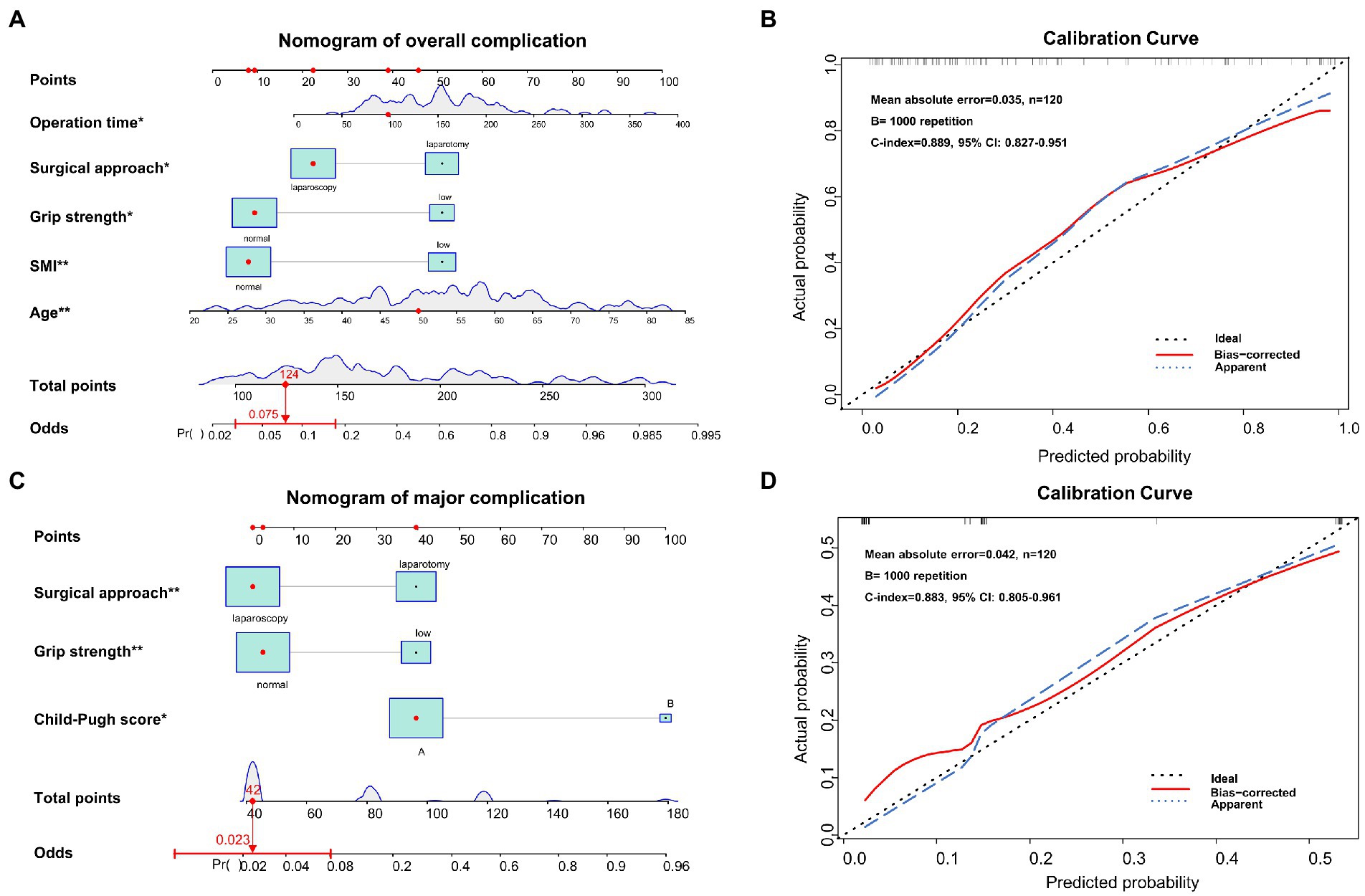
Figure 3. Nomograms and calibration curves for predicting overall complication and major complication after hepatectomy. (A) the nomogram predicting overall complications, (B) the calibration curve of the complication prediction model, (C) the nomogram predicting major complications, and (D) the calibration curve of the major complication prediction model.
3.3. Predictors of major complications
Through univariate logistic regression analysis, Child-Pugh grade, SMI, grip strength, total bilirubin (TBIL), ALB, alanine aminotransferase (ALT), AST and surgical approach were associated with major complications (Table 3). Through multivariate logistic analysis, Child-Pugh grade (p = 0.037), grip strength (p = 0.004) and surgical approach (p = 0.006) were independent risk factors of major complications. Then the nomogram based on Child-Pugh grade, grip strength and surgical approach was developed to predict major complications (Figure 3C). The C-indexes was 0.883 (95% CI, 0.805–0.961) and the calibration plots showed good agreement between observed outcomes and predicted outcomes (Figure 3D).
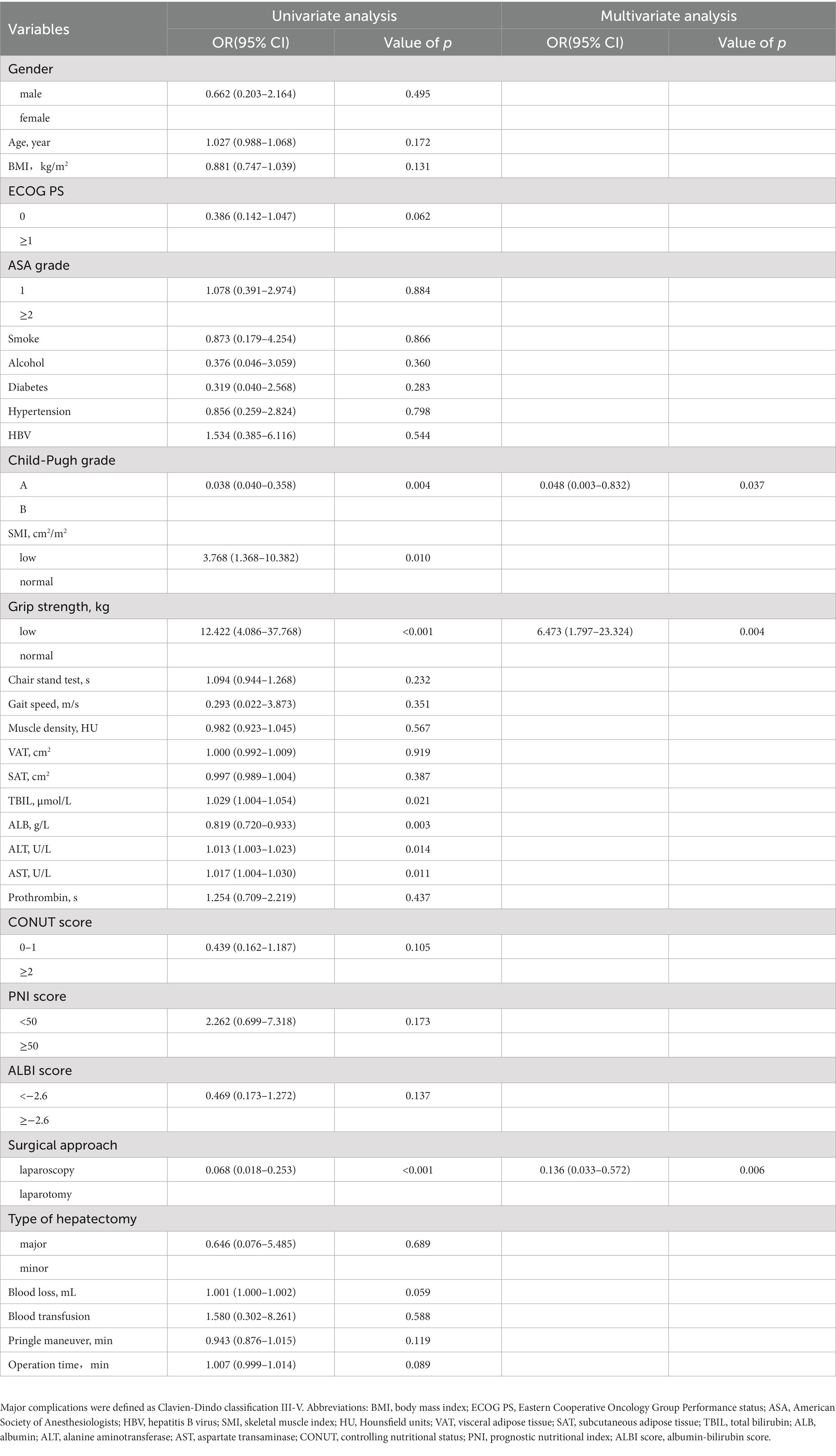
Table 3. Univariate and multivariate logistic regression analysis of predictors of major complications (Clavien-Dindo classification III-V).
3.4. Predictors of high comprehensive complication index ≥26.2
According to the previous studies, we also identified risk factors of high CCI ≥26.2. As shown in Supplementary Table S2, PS score, Child-Pugh grade, SMI, grip strength, chair stand test, TBIL, ALB, ALT, AST, surgical approach and operation time were associated with high CCI score. Multivariate logistic regression analysis showed that SMI (p = 0.047), grip strength (p < 0.001) and surgical approach (p = 0.014) were independent risk factors of high CCI score.
3.5. Subgroup analysis according to muscle mass and muscle strength
Based on the thresholds of SMI and grip strength to define sarcopenia, patients were further divided into four subgroups: patients with normal muscle mass and strength (77/120), patients with reduced muscle mass (15/120), patients with reduced muscle strength (10/120), and patients with reduced muscle mass and strength (18/120). As shown in Table 4, there are significant differences in overall complication, major complication, high CCI score, hospital cost, hospital stay and 90-day readmission rate among these four groups. Patients with reduced muscle mass and strength experienced the worst postoperative outcomes. No difference was seen in conversion rate among the four groups (p = 0.662).
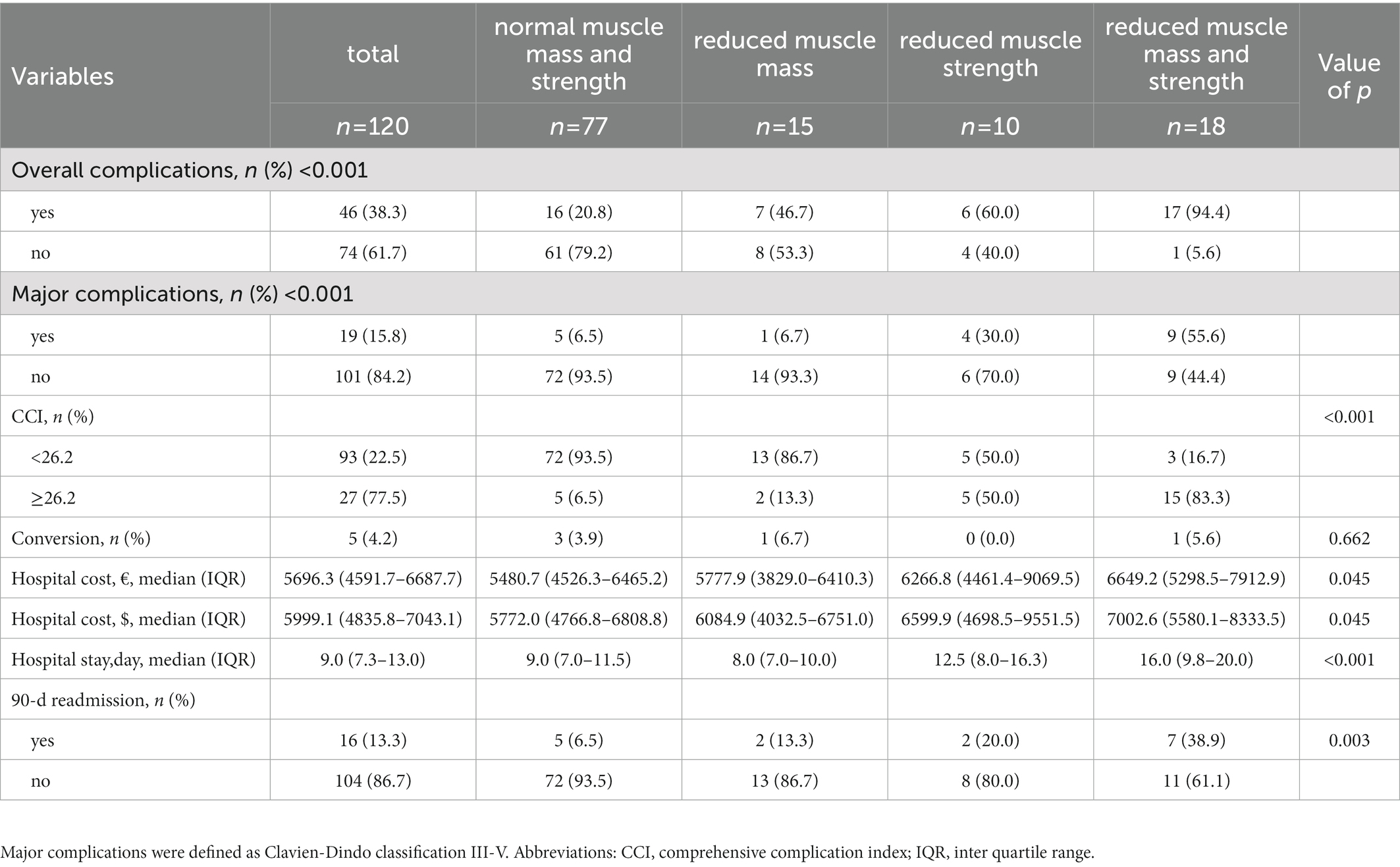
Table 4. Postoperative outcomes after hepatectomy in patients with benign liver diseases classified by muscle mass and muscle strength.
3.6. Evaluation of feature importance associated with major complication
In order to evaluate the importance of factors obtained from the results of univariate logistic analysis which are recognized clinically important to major complication, we performed random forest algorithm which is a conventional machine learning algorithm. The importance matrix plot revealed the importance of the eight clinical factors (Figure 4A). Then we used SHAP method to elaborate the specific role of each feature on the risk of major complication. As shown in the SHAP summary plot (Figure 4B), each dot corresponds to the SHAP value for each feature in a given patient. Dots are colored based on the values of features for individual patient. Red indicates higher feature values and blue indicates lower feature values. The X-axis coordinate of each dot was determined by the SHAP value, and the dots are stacked along each feature to show the density. Each SHAP value indicates how much each feature contributes, either positively or negatively, to the risk of major complication. The higher SHAP value of a feature is given, the higher risk of postoperative major complication the patient would have. For example, open surgery, low grip strength, low SMI and Child-Pugh class B are associated with a higher risk of major complication.
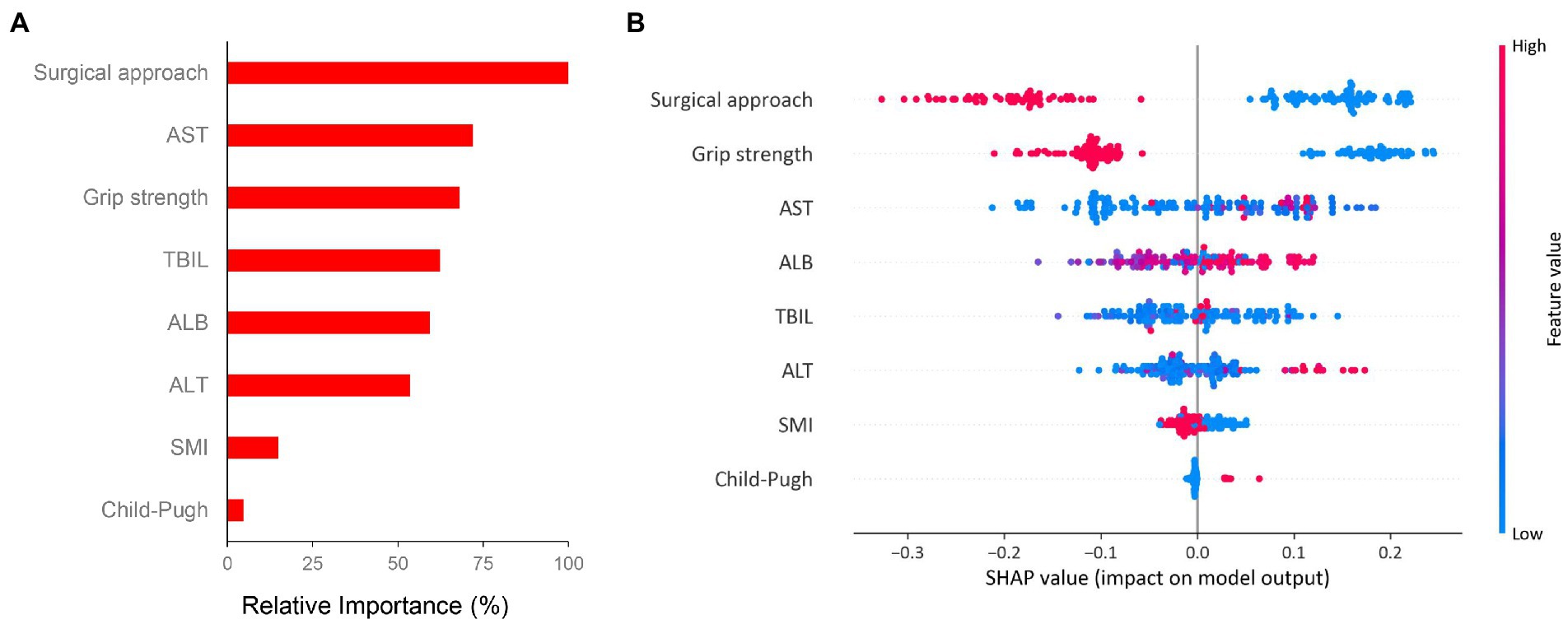
Figure 4. Importance matrix plot and SHAP summary plot of eight clinical features associated with major complication. (A) The importance ranking of eight clinical features using the random forest algorithm and (B) The SHAP summary plot of the eight feature clusters. Each patient is allocated one dot on the line for each feature and the dots are colored based on the values of features. Red indicates higher feature values and blue indicates lower feature values. A feature’s SHAp value (x-axis) represents the contribution of the specific feature to the risk of major complication. The higher SHAp value of a feature is given, the higher risk of postoperative major complication the patient would have. Abbreviations: SHAP, Shapley additive explanation; SMI, skeletal muscle index; TBIL, total bilirubin; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate transaminase.
4. Discussion
Liver resection remains the curative treatment for benign liver diseases, but postoperative complications seriously threaten the recovery and quality of life of patients. Preoperative identification of risk factors for complication is significantly important to optimize the treatment strategy and improve postoperative outcomes. In this study, we first conducted a prospective cohort study to evaluate the impact of sarcopenia on the short-term outcomes after hepatectomy in benign liver diseases. We comprehensively defined sarcopenia by muscle mass and muscle strength, and directly delineated the adverse impact of both muscle mass and muscle strength on postoperative outcomes. We propose that sarcopenia is a critical factor affecting the short-term outcomes in patients with benign liver diseases undergoing hepatectomy.
As a major component of malnutrition, sarcopenia has been widely investigated in various liver diseases, including hepatocellular carcinoma (HCC), cholangiocarcinoma, liver cirrhosis and non-alcoholic fatty liver disease (27–30). But the impact of sarcopenia on benign liver disease undergoing hepatectomy has never been investigated before. In our study, we evaluated the impact of sarcopenia on the short-term outcomes after hepatectomy and showed that sarcopenia is negatively associated with major complications, overall complications and high CCI score in patients with benign liver diseases following surgery. We also built valuable sarcopenia-based nomograms to predict major complications and overall complications, which showed favorable performance. In addition to sarcopenia, we also evaluated the role of other clinical indicators such as CONUT score, PNI score and ALBI score. CONUT score is a valuable biomarker which can reflect the patient’s immune-nutritional status. Previous studies have shown that CONUT score is closely associated with postoperative complications and survival prognosis in patients with hepatocellular carcinoma undergoing hepatectomy (31, 32). Spoletini et al. identified CONUT score as a predictor of morbidity after liver transplantation (33). Une et al. showed that CONUT score and sarcopenia are both valuable prognostic factors affecting the prognosis of patients with advanced urothelial carcinoma (34). Another study by Kodama et al. also confirmed the prognostic roles of CONUT score and skeletal muscle mass in patients with abdominal aortic aneurysm following open surgical repair (35). In our study, we found that postoperative complications were associated with a high CONUT score ≥ 2, a low PNI score < 50, and a high ALBI score ≥ −2.6, which were in accordance with previous studies. The clinicians should comprehensively assess the immune-nutritional status before surgery to minimize adverse postoperative outcomes.
There have been a variety of methods to evaluate sarcopenia, among which radiological evaluation is a most commonly used method (8, 36). The guidelines of EWGSOP propose that the definition of sarcopenia should be multidimensional, including both muscle mass and muscle strength (37). Muscle strength has been demonstrated to be a better predictor than muscle quantity, which can be assessed by handgrip strength, chair stand test and gait speed (23, 38). In accordance to the guidelines, we comprehensively assessed the muscle mass and strength to define sarcopenia in our study. We found that muscle mass and muscle strength are all significant predictors of postoperative complications. Patients with reduced muscle mass and strength experienced the worst short-term outcomes than patients with individual reduced muscle mass or patients with individual reduced muscle strength.
We also evaluated the impact of other clinical factors except muscle mass and muscle strength in our study. We found that surgical approach was an independent predictor of major complication and high CCI. In addition, age, surgical approach and operation time were independent risk factors of overall complications. Previous studies has demonstrated the favorable benefit of laparoscopic procedure versus open surgery for patients undergoing hepatectomy (39, 40). And operation time was also demonstrated to be associated with adverse outcomes after surgery (41–43). Interestingly, another study by Wijk et al. showed that older age, open surgery and longer operation time were associated with muscle quality loss, leading to a shorter OS in patients following liver resection (44).
Considering the adverse impact of sarcopenia on the short-term outcomes after hepatectomy, urgent efforts are needed to meliorate sarcopenia. According to our results, improving muscle mass and muscle strength are all important in revising sarcopenia. Deutz et al. found that adding leucine to high protein supplements could stimulate muscle protein synthesis and improve muscle mass in cancer patients (45). Smith et al. showed that resistance training was an effective method to improve muscle mass and muscle strength in clinical populations (46). The guidelines of EWGSOP suggested that supplementation of amino acids, vitamin D, testosterone, and growth hormone could improve muscle mass and muscle function (37). However, there has been no standard “pre-habilitation” strategy widely applied in clinical practice. A comprehensive understanding of molecular and metabolic mechanism of sarcopenia may provide new insights in finding novel therapeutic targets for sarcopenia in the future.
There are also some limitations in the study. Firstly, the sample size is relatively small, which may lead to some bias to the results. Prospective large-scale studies are needed to validate the results. Secondly, we only evaluate the impact of sarcopenia on postoperative complications after hepatectomy. It is still necessary to conduct further researches focusing on both short-term and long-term outcomes (e.g., recurrence and survival). Thirdly, the change in skeletal muscle after surgery has been identified as a significant predictor of outcomes in patients undergoing hepatectomy (47, 48). But the changes in sarcopenia-related factors have not been investigated in this study and we will pay more attention to the dynamic changes of sarcopenia in the further study. Lastly, although confirming the negative impact of sarcopenia, this study is an observational study without any interventions. Prospective interventional clinical trials are still necessary to find effective strategies for counteracting sarcopenia.
In conclusion, preoperative sarcopenia is closely associated with adverse short-term outcomes after hepatectomy in patients with benign liver diseases. Defining sarcopenia by muscle mass and muscle strength is more accurate and applicable to implement risk classification for patients following hepatectomy. In addition, valuable sarcopenia-based nomograms were built to predict major complications and overall complications for patients with benign liver diseases undergoing hepatectomy, which may provide new insights in clinical decision making.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the First Affiliated Hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZB, ZC, BC, JY, ZZ, QH, HY, CZ, and KC contribute to data acquisition. ZB, ZC, and ZZ contribute to draft of the manuscript. ZB, GC, and YW contribute to study concept and design. ZB, YY, JM, and BC contribute to data analysis. ZB, ZC, GC, and YW contribute to draft revising and study supervision. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by National Natural Science Foundation of China (82072685).
Acknowledgments
The authors thank our radiological colleagues for their assistance in collection and analysis of radiological information. The authors also thank all the nurses in hepatobiliary surgery for their cooperation and assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1040297/full#supplementary-material
References
1. Yang, T, Lau, WY, Lai, ECH, Yang, L-Q, Zhang, J, Yang, G-S, et al. Hepatectomy for bilateral primary Hepatolithiasis: a cohort study. Ann Surg. (2010) 251:84–90. doi: 10.1097/SLA.0b013e3181b2f374
2. Ariizumi, S-I, Katagiri, S, Kotera, Y, Yamashita, S, Omori, A, Kato, T, et al. Improved mortality, morbidity, and long-term outcome after anatomical hepatectomy with the Glissonean pedicle approach in patients with hepatocellular carcinoma: 30 Years’ experience at a single institute. Ann Surg. (2022) 275:947–54. doi: 10.1097/SLA.0000000000004311
3. Hobeika, C, Fuks, D, Cauchy, F, Goumard, C, Soubrane, O, Gayet, B, et al. Impact of cirrhosis in patients undergoing laparoscopic liver resection in a Nationwide multicentre survey. Br J Surg. (2020) 107:268–77. doi: 10.1002/bjs.11406
4. Hanna, L, Nguo, K, Furness, K, Porter, J, and Huggins, CE. Association between skeletal muscle mass and quality of life in adults with cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:839–57. doi: 10.1002/jcsm.12928
5. Arulananda, S, and Segelov, E. Sarcopenia and cancer-related inflammation measurements in advanced gastric and junctional cancers-ready for prime time? Ann Oncol. (2022) 33:669–71. doi: 10.1016/j.annonc.2022.04.008
6. Xiao, J, Caan, BJ, Cespedes Feliciano, EM, Meyerhardt, JA, Peng, PD, Baracos, VE, et al. Association of low Muscle Mass and low Muscle Radiodensity with morbidity and mortality for colon cancer surgery. JAMA Surg. (2020) 155:942–9. doi: 10.1001/jamasurg.2020.2497
7. Tantai, X, Liu, Y, Yeo, YH, Praktiknjo, M, Mauro, E, Hamaguchi, Y, et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. (2022) 76:588–99. doi: 10.1016/j.jhep.2021.11.006
8. Dasarathy, S, and Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. (2016) 65:1232–44. doi: 10.1016/j.jhep.2016.07.040
9. Kobayashi, A, Kaido, T, Hamaguchi, Y, Okumura, S, Shirai, H, Yao, S, et al. Impact of Sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg. (2019) 269:924–31. doi: 10.1097/SLA.0000000000002555
10. Voron, T, Tselikas, L, Pietrasz, D, Pigneur, F, Laurent, A, Compagnon, P, et al. Sarcopenia impacts on short-and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. (2015) 261:1173–83. doi: 10.1097/SLA.0000000000000743
11. Berardi, G, Antonelli, G, Colasanti, M, Meniconi, R, Guglielmo, N, Laurenzi, A, et al. Association of Sarcopenia and Body Composition with short-term outcomes after liver resection for malignant tumors. JAMA Surg. (2020) 155:e203336. doi: 10.1001/jamasurg.2020.3336
12. Kaido, T, Ogawa, K, Fujimoto, Y, Ogura, Y, Hata, K, Ito, T, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant. (2013) 13:1549–56. doi: 10.1111/ajt.12221
13. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
14. Yang, J, Chen, K, Zheng, C, Chen, K, Lin, J, Meng, Q, et al. Impact of sarcopenia on outcomes of patients undergoing liver resection for hepatocellular carcinoma. J Cachexia Sarcopenia Muscle. (2022) 13:2383–92. doi: 10.1002/jcsm.13040
15. Deng, L, Chen, B, Zhan, C, Yu, H, Zheng, J, Bao, W, et al. A novel clinical-Radiomics model based on sarcopenia and Radiomics for predicting the prognosis of intrahepatic cholangiocarcinoma after radical hepatectomy. Front Oncol. (2021) 11:744311. doi: 10.3389/fonc.2021.744311
16. Clavien, PA, Barkun, J, de Oliveira, ML, Vauthey, JN, Dindo, D, Schulick, RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
17. Slankamenac, K, Graf, R, Barkun, J, Puhan, MA, and Clavien, P-A. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. (2013) 258:1–7. doi: 10.1097/SLA.0b013e318296c732
18. Serenari, M, Han, K-H, Ravaioli, F, Kim, S-U, Cucchetti, A, Han, D-H, et al. A nomogram based on liver stiffness predicts postoperative complications in patients with hepatocellular carcinoma. J Hepatol. (2020) 73:855–62. doi: 10.1016/j.jhep.2020.04.032
19. Cloyd, JM, Mizuno, T, Kawaguchi, Y, Lillemoe, HA, Karagkounis, G, Omichi, K, et al. Comprehensive complication index validates improved outcomes over time despite increased complexity in 3707 consecutive Hepatectomies. Ann Surg. (2020) 271:724–31. doi: 10.1097/SLA.0000000000003043
20. Koch, M, Garden, OJ, Padbury, R, Rahbari, NN, Adam, R, Capussotti, L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the international study Group of Liver Surgery. Surgery. (2011) 149:680–8. doi: 10.1016/j.surg.2010.12.002
21. Mise, Y, Vauthey, J-N, Zimmitti, G, Parker, NH, Conrad, C, Aloia, TA, et al. Ninety-day postoperative mortality is a legitimate measure of Hepatopancreatobiliary surgical quality. Ann Surg. (2015) 262:1071–8. doi: 10.1097/SLA.0000000000001048
22. Roberts, HC, Denison, HJ, Martin, HJ, Patel, HP, Syddall, H, Cooper, C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. (2011) 40:423–9. doi: 10.1093/ageing/afr051
23. Chen, L-K, Woo, J, Assantachai, P, Auyeung, T-W, Chou, M-Y, Iijima, K, et al. Asian working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012
24. Kuroda, D, Sawayama, H, Kurashige, J, Iwatsuki, M, Eto, T, Tokunaga, R, et al. Controlling nutritional status (Conut) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. (2018) 21:204–12. doi: 10.1007/s10120-017-0744-3
25. Matsuda, T, Umeda, Y, Matsuda, T, Endo, Y, Sato, D, Kojima, T, et al. Preoperative prognostic nutritional index predicts postoperative infectious complications and oncological outcomes after hepatectomy in intrahepatic cholangiocarcinoma. BMC Cancer. (2021) 21:708. doi: 10.1186/s12885-021-08424-0
26. Johnson, PJ, Berhane, S, Kagebayashi, C, Satomura, S, Teng, M, Reeves, HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the Albi grade. J Clin Oncol. (2015) 33:550–8. doi: 10.1200/JCO.2014.57.9151
27. Fujiwara, N, Nakagawa, H, Kudo, Y, Tateishi, R, Taguri, M, Watadani, T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. (2015) 63:131–40. doi: 10.1016/j.jhep.2015.02.031
28. Moon, JH, Koo, BK, and Kim, W. Non-alcoholic fatty liver disease and sarcopenia additively increase mortality: a Korean Nationwide survey. J Cachexia Sarcopenia Muscle. (2021) 12:964–72. doi: 10.1002/jcsm.12719
29. Li, H, Dai, J, Lan, T, Liu, H, Wang, J, Cai, B, et al. Combination of albumin-globulin score and skeletal muscle index predicts long-term outcomes of intrahepatic cholangiocarcinoma patients after curative resection. Clin Nutr. (2021) 40:3891–900. doi: 10.1016/j.clnu.2021.04.038
30. Tandon, P, Montano-Loza, AJ, Lai, JC, Dasarathy, S, and Merli, M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. (2021) 75:S147–62. doi: 10.1016/j.jhep.2021.01.025
31. Li, L, Liu, C, Yang, J, Wu, H, Wen, T, Wang, W, et al. Early postoperative controlling nutritional status (Conut) score is associated with complication iii-V after hepatectomy in hepatocellular carcinoma: a retrospective cohort study of 1, 334 patients. Sci Rep. (2018) 8:13406. doi: 10.1038/s41598-018-31714-w
32. Harimoto, N, Yoshizumi, T, Inokuchi, S, Itoh, S, Adachi, E, Ikeda, Y, et al. Prognostic significance of preoperative controlling nutritional status (Conut) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol. (2018) 25:3316–23. doi: 10.1245/s10434-018-6672-6
33. Spoletini, G, Ferri, F, Mauro, A, Mennini, G, Bianco, G, Cardinale, V, et al. Conut score predicts early morbidity after liver transplantation: a collaborative study. Front Nutr. (2021) 8:793885. doi: 10.3389/fnut.2021.793885
34. Une, M, Ito, M, Suzuki, H, Toide, M, Kobayashi, S, Fukushima, H, et al. Controlling nutritional status (CONUT) score and sarcopenia as mutually independent prognostic biomarkers in advanced urothelial carcinoma. Cancers (Basel) (2022) 14. doi: 10.3390/cancers14205075
35. Kodama, A, Takahashi, N, Sugimoto, M, Niimi, K, Banno, H, and Komori, K. Associations of nutritional status and muscle size with mortality after open aortic aneurysm repair. J Vasc Surg. (2019) 70:1585–93. doi: 10.1016/j.jvs.2019.01.049
36. Durand, F, Buyse, S, Francoz, C, Laouénan, C, Bruno, O, Belghiti, J, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. (2014) 60:1151–7. doi: 10.1016/j.jhep.2014.02.026
37. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
38. Leong, DP, Teo, KK, Rangarajan, S, Lopez-Jaramillo, P, Avezum, A, Orlandini, A, et al. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (pure) study. Lancet. (2015) 386:266–73. doi: 10.1016/S0140-6736(14)62000-6
39. Liu, X, Min, X, Ma, Z, He, X, and Du, Z. Laparoscopic hepatectomy produces better outcomes for Hepatolithiasis than open hepatectomy: an updated systematic review and meta-analysis. Int J Surg. (2018) 51:151–63. doi: 10.1016/j.ijsu.2018.01.016
40. Macacari, RL, Coelho, FF, Bernardo, WM, Kruger, JAP, Jeismann, VB, Fonseca, GM, et al. Laparoscopic vs. open left lateral Sectionectomy: an update meta-analysis of randomized and non-randomized controlled trials. Int J Surg. (2019) 61:1–10. doi: 10.1016/j.ijsu.2018.11.021
41. Song, KB, Hong, S, Kim, HJ, Park, Y, Kwon, J, Lee, W, et al. Predictive factors associated with complications after laparoscopic distal pancreatectomy. J Clin Med. (2020) 9:2766. doi: 10.3390/jcm9092766
42. Surace, P, Sultan, AA, George, J, Samuel, LT, Khlopas, A, Molloy, RM, et al. The association between operative time and short-term complications in Total hip arthroplasty: an analysis of 89,802 surgeries. J Arthroplast. (2019) 34:426–32. doi: 10.1016/j.arth.2018.11.015
43. Cregar, WM, Goodloe, JB, Lu, Y, and Gerlinger, TL. Increased operative time impacts rates of short-term complications after Unicompartmental knee arthroplasty. J Arthroplast. (2021) 36:488–94. doi: 10.1016/j.arth.2020.08.032
44. van Wijk, L, van Duinhoven, S, Liem, MSL, Bouman, DE, Viddeleer, AR, and Klaase, JM. Risk factors for surgery-related muscle quantity and muscle quality loss and their impact on outcome. Eur J Med Res. (2021) 26:36. doi: 10.1186/s40001-021-00507-9
45. Deutz, NEP, Safar, A, Schutzler, S, Memelink, R, Ferrando, A, Spencer, H, et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. (2011) 30:759–68. doi: 10.1016/j.clnu.2011.05.008
46. Smith, C, Woessner, MN, Sim, M, and Levinger, I. Sarcopenia definition: does it really matter? Implications for resistance training. Ageing Res Rev. (2022) 78:101617. doi: 10.1016/j.arr.2022.101617
47. Kobayashi, A, Kaido, T, Hamaguchi, Y, Okumura, S, Taura, K, Hatano, E, et al. Impact of postoperative changes in Sarcopenic factors on outcomes after hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. (2016) 23:57–64. doi: 10.1002/jhbp.302
Keywords: sarcopenia, hepatectomy, nutrition, benign liver disease, complication
Citation: Bo Z, Chen Z, Chen B, Yang J, Zhao Z, Yang Y, Ma J, He Q, Yu H, Zheng C, Chen K, Wang Y and Chen G (2023) Development of sarcopenia-based nomograms predicting postoperative complications of benign liver diseases undergoing hepatectomy: A multicenter cohort study. Front. Nutr. 10:1040297. doi: 10.3389/fnut.2023.1040297
Edited by:
Guohao Wu, Fudan University, ChinaReviewed by:
Mohammad Waseem, University of Maryland Eastern Shore, United StatesJeroen Van Vugt, Erasmus Medical Center, Netherlands
Copyright © 2023 Bo, Chen, Chen, Yang, Zhao, Yang, Ma, He, Yu, Zheng, Chen, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Wang, ✉ wang.yi@wmu.edu.cn; Gang Chen, ✉ chen.gang@wmu.edu.cn
†These authors have contributed equally to this work
 Zhiyuan Bo1,2†
Zhiyuan Bo1,2†  Bo Chen
Bo Chen Jinhuan Yang
Jinhuan Yang Yi Yang
Yi Yang Jun Ma
Jun Ma Qikuan He
Qikuan He Haitao Yu
Haitao Yu Chongming Zheng
Chongming Zheng Yi Wang
Yi Wang Gang Chen
Gang Chen