Maternal Consumption of Milk or Dairy Products During Pregnancy and Birth Outcomes: A Systematic Review and Dose-Response Meta-Analysis
- 1Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China
- 3Key Laboratory of Precision Medical Research on Major Chronic Disease, Liaoning, China
Purpose: This study aimed to systematically review current evidence and quantitatively evaluate the associations between milk or dairy consumption during pregnancy and birth outcomes.
Methods: This systematic review had been reported in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A supplementary literature search in PubMed, Web of Science, Cochrane Library, and Embase was conducted on 30 March 2021. Studies that assessed the association of maternal consumption of milk or dairy with birth-related outcomes were identified. The dose-response meta-analyses of continuous data and categorical data were applied. One-stage approach and two-stage approach were used where appropriate.
Results: In total, 42 studies were eligible for the present systematic review, and 18 of them were included in the outcome-specific meta-analyses. The dose-response meta-analysis [Number of studies (N) = 9] predicted a maximum mean change in birthweight of 63.38 g [95% Confidence Interval (CI) = 0.08, 126.67] at 5.00 servings per day. Intake of dairy products had the greatest protective effect on small for gestational age at a maximum of 7.2 servings per day [Relative risk (RR) = 0.69, 95% CI = 0.56, 0.85] (N = 7). The risk of large for gestational age was predicted to be maximum at 7.20 servings per day of dairy consumption, with the RR and 95% CI of 1.30 (1.15, 1.46; N = 4). In addition, the relationship between dairy consumption and low birth weight (RR = 0.70, 95% CI = 0.33, 1.50; N = 5) and pre-mature birth (RR = 1.13, 95% CI = 0.87, 1.47; N = 5) was not significant, respectively.
Conclusions: Maternal consumption of dairy during pregnancy has a potential effect on fetal growth. Further well-designed studies are warranted to clarify the specific roles of individual dairy products.
Systematic Review Registration: identifier: PROSPERO 2020 CRD42020150608
Introduction
Adverse birth outcome is a global health issue. According to the Global Burden of Disease Study, pre-mature birth (PB) is one of ten leading causes of total years of life lost, and one of three leading global causes of death in children under-5 years (1). It is estimated that PB is responsible for 0.943 million neonatal deaths and 1.055 million under-five deaths per year (2). Besides, those pre-term survivors are also likely to suffer lifelong challenges from physical health (3), neurological development (4), and psychosocial deficits (5). Similarly, other adverse birth outcomes also result in lasting adverse consequences. As evidence suggests, birth characteristics have been linked to children's cardiometabolic risk (6), cancer prognosis (7), and age-related cognitive dysfunction (8). Furthermore, birth defects, the most serious of all adverse birth outcomes, increase the risk of cancer persisted into adulthood (9), and there remains a gradual decline in survival beyond 1 year of age that exceeded that of the general population (10).
In recent years, especially since the inception of the “developmental origins of health and disease” theory, the impact of maternal nutrition has been increasingly emphasized (11). Mounting evidence has indicated that maternal diet during pregnancy, a modifiable factor, is associated with birth outcomes (12, 13). Among these nutritious foods during pregnancy, milk and its derivatives receives certain attention (14). There are increasing reports of maternal dairy intake contributing to birth outcomes; however, some associations remain inconclusive with the growing body of evidence (15, 16).
Although a published systematic review and meta-analysis has assessed the effect of consuming dairy products over perinatal outcomes, the dose-response relationships are still unclear (17). In addition, the birth outcomes range widely from birth anthropometry, birth defects to other adverse birth events, these extensive outcomes need to be further summarized (16, 17). Therefore, the present study aimed to systematically review current evidence covering all birth outcomes, and quantitatively evaluate the dose-response associations of milk or dairy consumption during pregnancy with birth outcomes.
Methods
This systematic review had been reported in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. This systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42020150608.
Search Strategy
The first systematic literature search in PubMed, Web of Science, Cochrane Library, and Embase was conducted on May 23, 2019, and the supplementary search was performed on 30 March 2021. The search strategy was predefined according to the “PICO” principle: “P”-pregnant woman, “I”-milk or dairy consumption, “C”-no or low consumption of milk or dairy, “O”-birth outcomes. The complete search strategies are presented in Supplementary Material 1. In addition, there were no restrictions on publication date or language. Two authors (Huang DH and Ji C) independently conducted the literature search. Disagreements were resolved by discussion with a third investigator (Chang Q).
Study Selection: Inclusion and Exclusion Criteria
Retrieved references were exported to the EndNote reference manager for crude deduplication and organization. Four independent reviewers (Huang DH, Xu X, Gao SY, and Dai HX) screened the titles and abstracts of the studies for eligibility. Two reviewers (Xu X and Huang DH) further independently reviewed the full texts of potentially relevant articles based on the inclusion and exclusion criteria. Discrepancies were resolved by discussion, with another reviewer (Wu QJ) consulted as and when necessary. The studies were selected based on priori-determined eligibility criteria.
The inclusion criteria were as follows: (1) study design was interventional (dairy consumption as an intervention) or observational (cohort study, case-control study, and cross-sectional study with dairy consumption as an exposure); (2) exposure of interest was the consumption of milk and dairy products during pregnancy, including yogurt, cheese, butter, and ice cream; (3) outcomes of interest were birth-related outcomes, including birth anthropometric measurements, PB, spontaneous abortion (SA), and congenital abnormality; and (4) if the study had a different objective than the research question being addressed in the present review, it was considered eligible if the association between dairy intake during pregnancy and birth outcomes was adequately described (information on the above three criteria was required).
The exclusion criteria were as follows: (1) study without a control group; (2) study with non-human subjects; (3) exposure time examined was not during pregnancy; (4) exposure of interest was dietary pattern, or combined diet, or fortified dairy products; (5) exposure of interest was nutrients from dairy source; (6) outcome was not at birth, including outcomes of fetuses, infants, children, and pregnancy complications; (7) study was not an original article, namely a review, conference abstract, letter, or book chapter; and (8) studies were written in languages other than English or Chinese.
Data Extraction
The following information was extracted using a pre-designed data collection form: study characteristics (first author, year of publication, country, study design, and sample size), participant characteristics (maternal age and body mass index), exposure characteristics (types of dairy products, dietary assessment tools and whether validated, and time period covered), and outcomes characteristics (specific birth outcomes and main conclusions). Two reviewers (Li H and Huang DH) independently extracted and cross-checked the data. Inconsistencies were resolved by consensus with a third reviewer (Xia Y).
Quality Assessment
The National Institutes of Health Study Quality Assessment Tools were used to assess the quality of the included studies. The tools contained 12–14 questions applicable to each study design and aimed to evaluate all aspects of the included studies. In case of a cohort study design, the 14 criteria used were based on the research objective, study population, exposure, outcome of interest, and statistical analysis. Each item was scored as “Yes” (+1), “No” (+0), “Cannot Determine” (+0), “Not Applicable” (+0), and “Not Reported” (+0). The sum of each item was the study quality score. The score was then summarized into three grades: a score ≥10 was graded Good, that between 5 and 9 was graded Fair, and that ≤4 was graded Poor. Two authors (Xu X and Huang DH) independently assessed study quality, and any disagreement was resolved by consensus with the third author (Wu QJ).
Data Synthesis and Statistical Analysis
Crude estimates were calculated using given raw data where appropriate, although data synthesis preferred the most-adjusted results. Besides, if required to switch to a reference group, the hamling function in the “dosresmeta” package of R software was used to estimate an alternative comparison of the dose categories (18). The dose units for dairy consumption were unified to 200 ml or 200 g according to the average size of several portion sizes (19–21). The present study aimed to pool three or more sets of continuous or categorical data using dose-response meta-analyses proposed by Crippa et al. (22, 23). When the studies included in the meta-analysis all had more than two exposure groups, the two-stage method was used; when any of the included studies had fewer than three exposure groups, the one-stage method was used (23). The pooled dose-response curve was estimated by restricted cubic splines model with three knots at the 10th, 50th, and 90th percentiles of the distribution. Both restricted maximum likelihood (REML) and maximum likelihood (ML) were adopted and the better estimation method was selected based on the lowest Akaike information criterion and Bayesian information criterion values. Sensitivity analyses were performed with alternative knots. Publication bias were detected by Egger's test and Begg's test. Subgroup analyses were performed based on the presence or absence of a specific portion size. All analyses were conducted using R: A language and environment for statistical computing (version 3.6.2). A two-tailed P < 0.05 was considered statistically significant.
Results
Literature Search and Study Selection
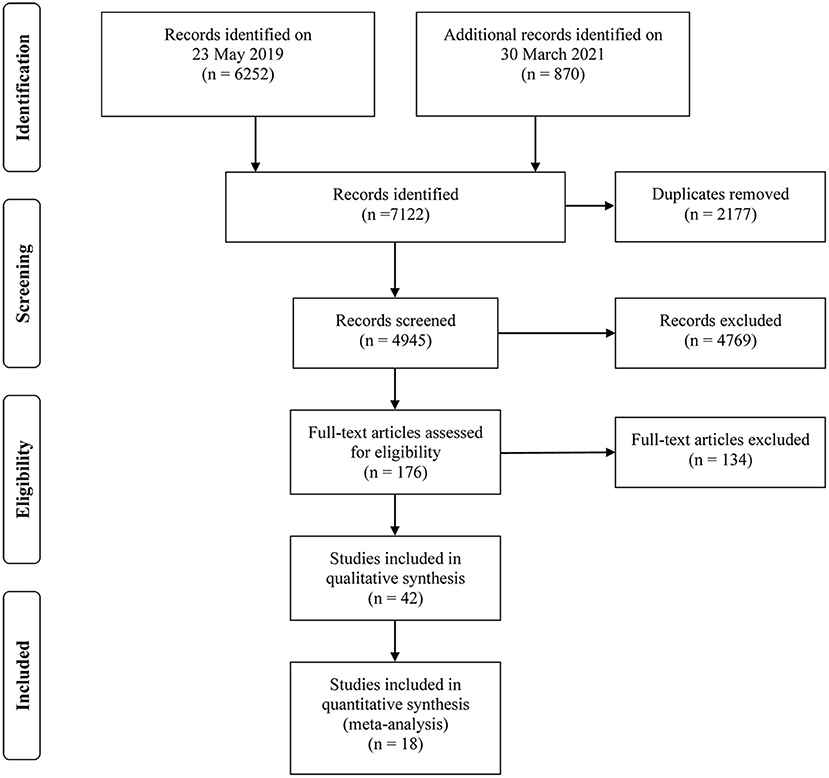
Of 7,122 studies, 2,177 duplicates were excluded, and 4,945 studies were screened by titles and abstracts. The remaining 176 relevant studies were reviewed in full text. Precisely 134 studies were further excluded, and the reasons for exclusion are presented in Supplementary Material 2. In total, 42 studies were eligible for the present systematic review (19–21, 24–62), and 18 of them were included in the outcome-specific dose-response meta-analyses (19–21, 27–31, 33, 37, 38, 43, 44, 49, 54, 58, 61, 62). The detailed PRISMA flow diagram showing the screening process is illustrated in Figure 1.
Study Characteristics
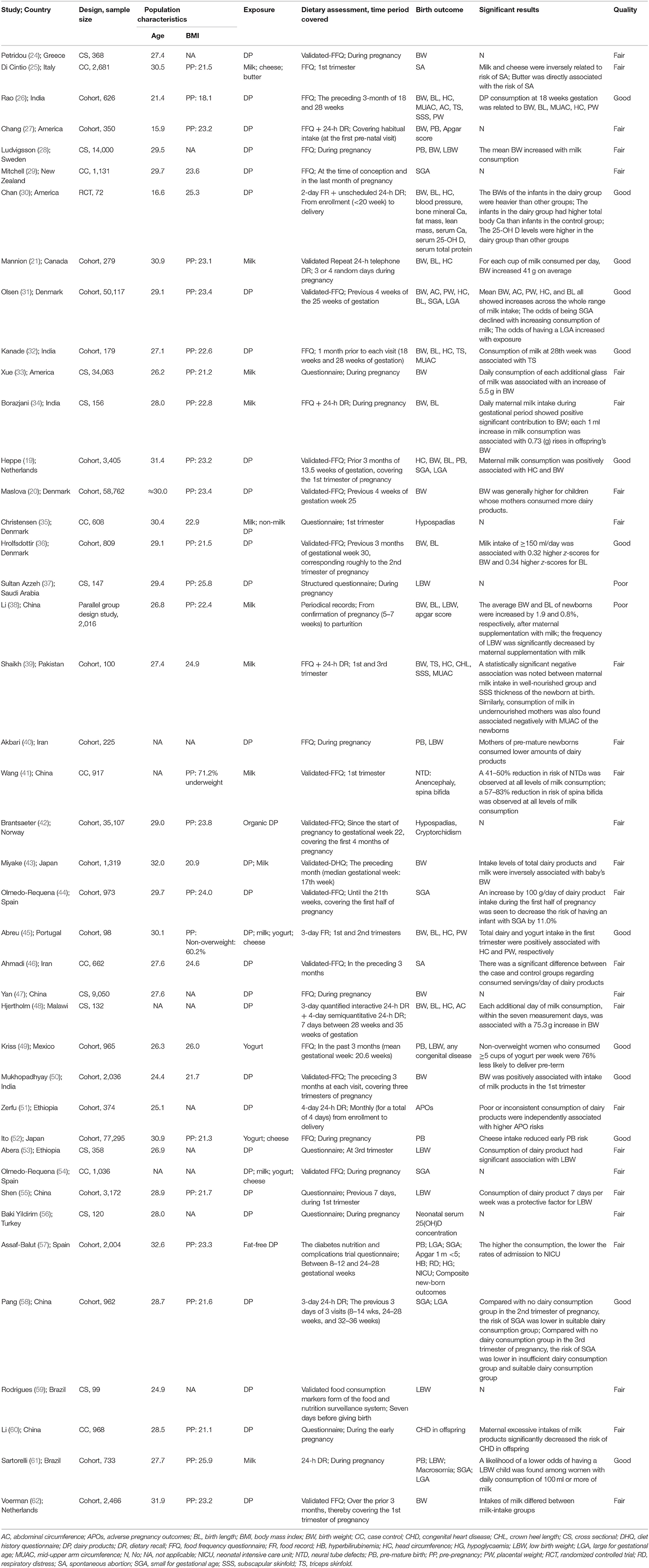
The detailed characteristics of the 42 included studies are shown in Table 1. There were 23 cohort studies (19–21, 26, 27, 31, 32, 36, 39, 40, 42–45, 49–52, 55, 57, 58, 61, 62), 10 cross-sectional studies (24, 28, 33, 34, 37, 47, 48, 53, 56, 59), seven case-control studies (25, 29, 35, 41, 46, 54, 60), and two interventional studies (30, 38). There were 13 studies rated as Good (19, 21, 26, 30–32, 36, 45, 49, 50, 52, 58, 61), 27 as Fair (20, 24, 25, 27–29, 33–35, 39–44, 46–48, 51, 53–57, 59, 60, 62), and two as Poor (37, 38). A summary of the quality assessment is presented in Supplementary Material 3. There were over 20 types of birth outcomes, including birth weight (BW) (19–21, 24, 26–28, 30–34, 36, 38, 39, 43, 45, 47, 48, 50, 62), birth length (BL) (19, 21, 26, 30–32, 34, 36, 38, 45, 48), head circumference (19, 21, 26, 30–32, 39, 45, 48), low birth weight (LBW) (28, 37, 38, 40, 49, 53, 55, 59, 61), PB (19, 27, 28, 40, 49, 52, 57, 61), small for gestational age (SGA) (19, 29, 31, 44, 54, 57, 58, 61), large for gestational age (LGA) (19, 31, 57, 58, 61), mid-upper arm circumference (26, 32, 39), triceps skinfold (26, 32, 39), abdominal circumference (26, 31, 48), placental weight (26, 31, 45), Apgar score (27, 38, 57), SA (25, 46), hypospadias (35, 42), subscapular skinfold (26, 39), serum 25-hydroxvitamin D (30, 56), crown heel length (39), neural tube defects (41), anencephaly (41), spina bifida (41), cryptorchidism (42), blood pressure (30), bone mineral Ca (30), fat mass (30), lean mass (30), serum Ca (30), serum total protein (30), hyperbilirubinemia (57), respiratory distress (57), hypoglycaemia (57), neonatal intensive care unit (57), composite new-born outcomes (57), congenital heart disease (60), macrosomia (61), any congenital disease (49), and adverse pregnancy outcomes (51). Outcome-specific findings of dose-response meta-analyses are described below.
Birth Weight
Nine studies were used for the one-stage dose-response meta-analysis (19–21, 27, 30, 33, 38, 43, 62). The overall pooled curve estimated by the REML method indicated a non-significant association between the mean differences in BW and increased consumption of dairy products (P = 0.0847) (Figure 2A). However, the first and second estimated coefficients of 33.1497 (P = 0.0296) and −32.4737 (P = 0.0263), respectively, suggested that the dose-response relationship tended to be positive. The pooled curve predicted a maximum mean change of 63.38 g (95% Confidence Interval [CI] = 0.08, 126.67) at 5.00 servings per day. The estimated dose to produce 50 and 80% of the predicted maximum effect was 1.02 and 1.97 servings/day, respectively, and the mean change in BW was 31.69 and 50.70 g, respectively. Sensitivity curves with alternative knots locations indicated the robustness of the pooled estimates (Supplementary Figure S1). Subgroup analysis showed the dose-response relationship was significant when the studies without specific portion size were excluded (P < 0.0001). In addition, there was no publication bias (P-linear = 0.3285; P-rank = 0.7139).
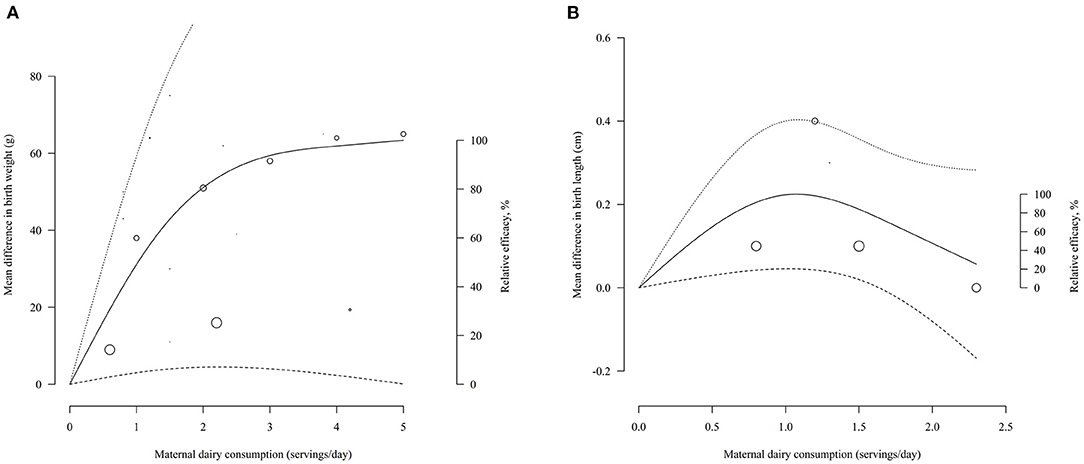
Figure 2. Pooled dose-response curves for mean change in birth-weight (A), birth-length (B) with maternal dairy consumption. Dashed lines represent the 95% confidence intervals. Circles indicate observed mean differences in individual studies, and the size of circles is proportional to the precision of the mean differences. The right axis represents the percentage of the maximum predicted effect.
Birth Length
Only three studies could be dose-response meta-analysis (19, 21, 38). Overall estimates fitted by the ML model suggested a marginal significant dose-response effect between dairy intake and BL (P = 0.0474) (Figure 2B). The first and second estimated coefficients were 0.3154 (P = 0.0143) and−0.2797 (P = 0.0306), respectively. The pooled curve predicted a maximum mean change of 0.22 cm (95% CI = 0.05, 0.40) at 1.07 servings per day. The estimated dose to produce 50 and 80% of the predicted maximum effect was 0.37 servings/day and 0.65 servings/day, respectively, and the mean change in BL was 0.11 and 0.18 cm, respectively. Sensitivity analysis with alternative knots indicated that the shapes of curves under different knots were unchanged (Supplementary Figure S2). Both Egger's test and Begg's test showed no publication bias (P-linear = 0.6; P-rank = 0.3).
Small for Gestational Age
A total of 10 sets of data from seven studies examined the dose-response relationship between dairy consumption and SGA risk (19, 29, 31, 44, 54, 58, 61). The pooled curve by ML model suggested that maternal dairy intake tended to prevent SGA (P = 0.0001) (Figure 3A), while the coefficients of −0.0920 (P = 0.2095) and 0.0449 (P = 0.5154) were not significant. It was predicted that the intake of dairy products had the greatest protective effect on SGA at a maximum of 7.2 servings per day [Relative risk (RR) = 0.69, 95% Confidence interval (CI) = 0.56, 0.85]. Sensitivity analysis showed that the relationship was robust (Supplementary Figure S3). The results of Egger's test and Begg's test were inconsistent, suggesting a potential publication bias (P-liner = 0.0409, P-rank = 0.7566). Subgroup analysis excluded the studies without specific portion size, and the pooled results were still significant (P < 0.0001).
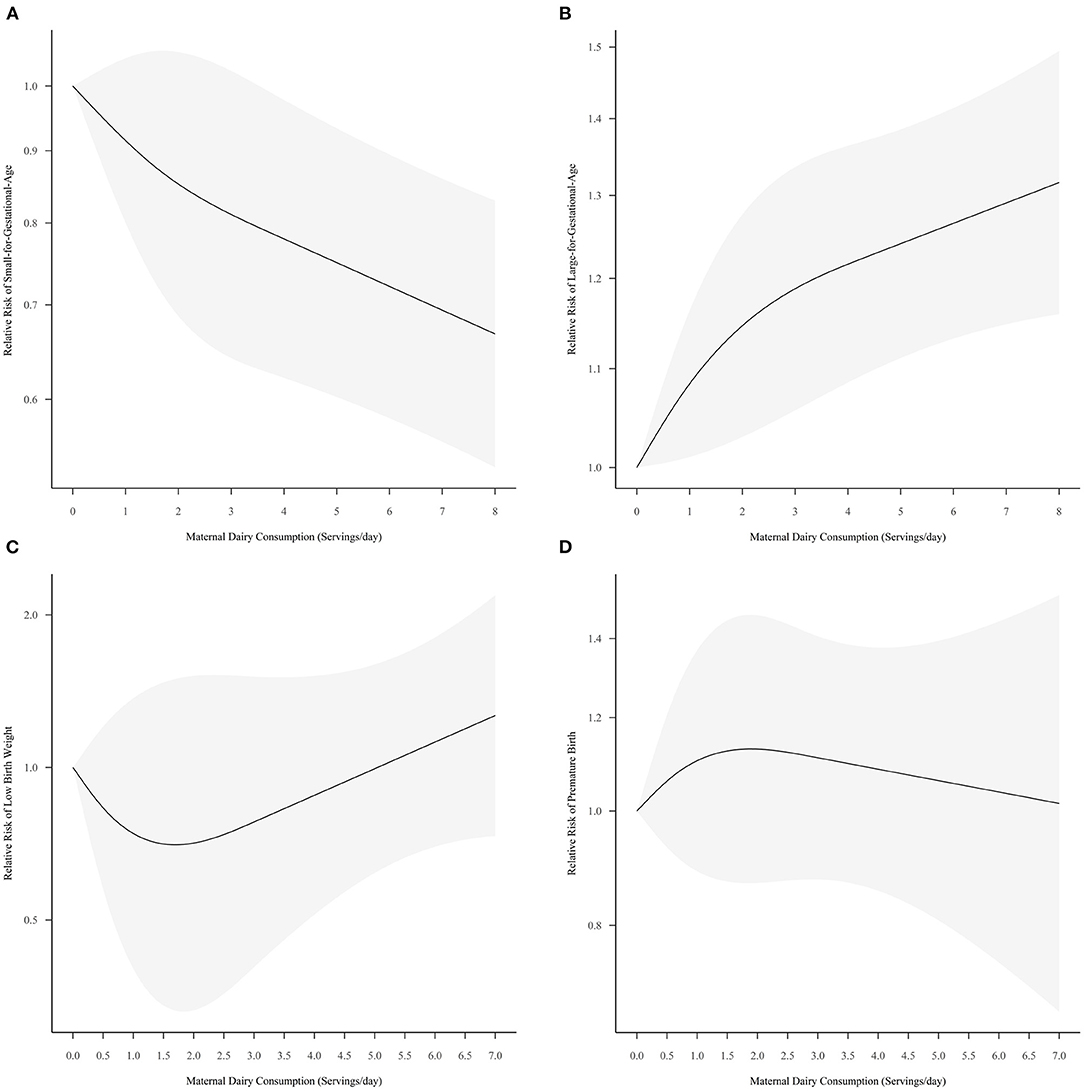
Figure 3. Pooled dose-response curves for the risk of small-for-gestational-age (A), large-for-gestational-age (B), low-birth-weight (C), and pre-mature-birth (D) with maternal dairy consumption. The gray part in this figure represents the 95% confidence intervals.
Large for Gestational Age
The pooled dose-response curve by six sets of data from four studies showed a significant upward trend for LGA risk with increased dairy consumption (P = 0.0001, Figure 3B) (19, 31, 58, 61). ML method estimated the first coefficient was 0.0870 (P = 0.0314), and the second coefficient was−0.1247 (P = 0.1662). The risk of LGA was predicted to be maximum at 7.20 servings/day of dairy consumption, with the RR and 95% CI of 1.30 (1.15, 1.46). There was a significant publication bias (P-liner = 0.0007, P-rank = 0.0169), and the sensitivity analysis showed a robust curve (Supplementary Figure S4).
Low Birth Weight
Five studies were included in the dose-response meta-analysis (28, 37, 38, 49, 61), and the pooled results showed a non-significant relationship (P = 0.4451). The two coefficients estimated by ML method were −0.3968 (P = 0.3272) and 1.0494 (P = 0.2918), respectively. The dose-response curve showed a visible but not significant downward trend first and then an upward trend for LBW with increasing dairy consumption (Figure 3C). Dairy consumption showed a potential protective effect on LBW at 1.70 servings/day (RR = 0.70, 95% CI = 0.33, 1.50). The RR and 95% CI for LBW were 1.24 (0.73, 2.10) when maternal dairy consumption reached a maximum of 6.80 servings/day. Sensitivity analysis with alternative knots was shown in (Supplementary Figure S5). The publication bias was not significant (P-liner = 0.5609, P-rank = 0.3223). The pooled results were still non-significant after excluding the studies without specific portion size (P = 0.4646).
Pre-mature Birth
A total of five studies were included in the one-stage dose-response meta-analysis (19, 27, 28, 49, 61). ML method estimated that this dose-response relationship was not significant (P = 0.6632) (Figure 3D), and the two coefficients of 0.1240 (P = 0.3780) and −0.2356 (P = 0.4107) were not statistically significant. It was predicted that the maximum risk of PB was 1.13 (95% CI: 0.87, 1.47) for 1.89 servings dairy products per day. Sensitivity analysis indicated a robust curve (Supplementary Figure S6). There was no publication bias in the included studies (P-liner = 0.7795, P-rank = 0.5858). Subgroup analysis showed the relationship was still not significant when the studies without specific portion size were excluded (P = 0.7439).
Spontaneous Abortion
The meta-analyses based on three sets of data from two studies found a non-significant protective effect of dairy intake against SA (P = 0.0842) (Supplementary Figure S7) (25, 46). The coefficients of −0.0564 (P = 0.8288) and −4.1686(P = 0.2784) estimated by REML method were not statistically significant. There was no publication bias (P-liner = 0.2403, P-rank = 0.5730). The sensitivity analysis was shown in (Supplementary Figure S8). The maximum predictive effect for SA was achieved at 2.5 servings/day of dairy consumption, with the RR and 95% CI of 0.00 (0.00, 150.40).
Hypospadias
In addition, we pooled three results from two studies and found that maternal rarely consumption of dairy products during pregnancy has a potential risk of hypospadias in offspring (RR = 1.27, 95% CI = 1.00, 1.60). (Supplementary Figure S9) (35, 42).
Discussion
The present systematic review and meta-analyses comprehensively summarized the current updated evidence on the associations between maternal consumption of milk or dairy during pregnancy and birth outcomes. There were more than 20 birth outcomes involved in the present systematic review, and eight outcomes that could be meta-analyzed, covering birth anthropometrics, congenital malformation, and other adverse birth events. Overall, the present findings suggested that maternal dairy consumption during pregnancy had a growth-promoting effect on offspring, including a marginal significant effect on birth anthropometrics, a significant protective effect on SGA, and a risk effect on LGA; in addition, low consumption of organic dairy products might have an adverse effect on hypospadias in offspring. Our findings still need to be constantly updated with respect to improved sample size because neither of them is particularly strong.
To the best of our knowledge, this is the first study to quantitatively evaluate such associations using dose-response meta-analysis methods. Although the outcomes of our study partially overlap with those of previous systematic reviews, the focuses of these reviews are not identical (16, 17). Furthermore, compared with the previous systematic reviews, we not only conducted meta-analyses, but also discussed the dose-response relationships between dairy consumption and birth outcomes (16, 17). Particularly, in addition to dose-response meta-analysis for categorical variables, the method of the dose-response meta-analysis of differences in means was also applied (22). Moreover, the present study used the one-stage approach that no longer excluded studies with fewer than three exposure groups, and thus more relevant studies were included for aggregating data (23).
Maternal Dairy Consumption and Birth Anthropometrics
BW-related studies accounted for a large proportion of all included birth outcomes. The present findings for BW are suggestive of growth-promoting effects of maternal dairy intake, which are largely consistent with those of previous systematic reviews (15–17). In addition, our findings further showed that BW increased but gradually slowed down with the increasing maternal dairy consumption, indicating that the BW-promoting effect of maternal dairy consumption was not linear. A significant dose-response relationship was also found with BL. Interestingly, this relationship curve increased first and then decreased, with BL peaking when dairy consumption reached one serving of dairy products per day. It suggests that there appears to be an optimal dose of dairy consumption during pregnancy for BL promotion, which may be a novel finding in contrast to prior studies (15–17). According to the global review of food-based dietary guidelines by Cámara et al., (63) the recommended intake of dairy products ranged from about two to four servings per day. At this dose range, our pooled curve shows a slow increase in BW, but the effect on BL cannot be estimated because the dose is outside the predicted limit of the BL curve. Nevertheless, our findings support the possible role of dairy consumption in promoting birth anthropometrics, and further determination of the optimal dose of maternal dairy consumption for offspring's growth and development is needed in the future.
Maternal Dairy Consumption and Adverse Birth Outcomes
As expected, we found a protective effect on SGA and a risk effect on LGA with increasing dairy consumption. However, our findings on LBW are inconsistent with the previous non-dose-response meta-analysis, which found a reduction in LBW risk associated with increased dairy consumption (17). Although our findings indicated that the dose-response relationship between maternal dairy consumption and LBW was not significant, the pooled curve showed a trend that dairy consumption gradually became a risk factor from a protective factor. It again supports our hypothesis that there is an optimal intake of dairy products for growth promotion.
The present results indicated that dairy consumption in pregnant women was not associated with PB. This finding updates the previous systematic reviews, one of which was prevented for drawing any conclusions due to lack of studies (16); the other was a non-dose-response meta-analysis of a small sample (17). To our knowledge, two studies have found a link between dairy intake and PB under certain conditions, suggesting that the association between dairy intake and PB may be related to the type of dairy products, classification of PB, and weight of the pregnant woman (49, 52).
Additionally, the potential protective effect of maternal dairy products consumed against SA was suggested by our synthesized data; however, this effect was based on a limited sample analysis and needed to be further determined. Surprisingly, although the primary studies were not statistically significant, our pooled results indicated a significant adverse effect of low maternal dairy intake on hypospadias in male offspring (35, 42). The exposures in both primary studies were organic dairy, so this finding suggests that the effects of organic dairy products may need to be treated differently from those of conventional dairy products.
Mechanisms
Mechanisms underlying the relationship of milk and dairy consumption with birth anthropometrics may be biologically plausible based on current evidence. A growing body of evidence supports the positive effect of milk proteins on growth and developments (64, 65). Besides, insulin-like growth factor-1 (IGF-1), which is associated with milk intake, has been proposed as a candidate for regulating the fetal growth (66, 67). Furthermore, nutrient-sensitive kinase mechanistic target of rapamycin complex 1 pathway (mTORC1) is another promising growth regulator (68, 69). As evidence suggested, milk provides all signals for mTORC1-activation, and the activated mTORC1 pathway can facilitate anabolism and growth (70). Despite these potential biological clues, more clear evidence is still needed to underlie the association between maternal dairy consumption and fetal growth.
The mechanism of low organic dairy consumption as a risk factor for hypospadias in offspring is ambiguous. Based on current evidence, there are following hypotheses. The first hypothesis is related to the nutritionally relevant composition differences between organically and conventionally produced foods (71), just as reported, organic dairy products may provide additional benefits for health (72). Another possible mechanism is related to the lower level of pesticide residues in organic dairy products (73). As evidence shows, pesticides can increase the risk of hypospadias (74, 75), and thus it is speculated that consumption of organic dairy products may reduce the risk of hypospadias.
Limitations
This large systematic review provides important insights into the effects of maternal dairy consumption on birth outcomes; nonetheless, several limitations should be considered. First, there are three limitations regarding the exposure: the examined exposure in the present systematic review was total dairy product rather than individual dairy products; dairy consumption was all during pregnancy but not the same “trimester;” the dose units for dairy consumption were unified according to the average size of several portion sizes. These issues are all attributed to the small number of homogeneous primary studies. Second, the limited number of studies and different data types prevented drawing any conclusions about other birth outcomes, such as head circumference, Apgar scores, and some congenital diseases. Third, in order to improve the sample size, we meta-analyzed the data regardless of the study design and study quality, which may increase the risk of bias for this systematic review. Therefore, our findings should be interpreted with caution and more well-designed studies are needed to update the conclusions of the present systematic review.
Conclusion
In conclusion, the present systematic review is the first to evaluate the dose-response associations between maternal milk or dairy consumption and birth outcomes. The present findings, including the non-linear effect of maternal dairy consumption on birth anthropometrics, the preventive effect on SGA, and the risk effect on LGA, suggest a growth-promoting effect of dairy consumption during pregnancy, and the optimal dosage of dairy consumption needs to be further determined. Despite a small number of studies, the findings that low consumption of organic dairy products may increase the risk of hypospadias in male offspring should be taken seriously, and the conclusion still needs to be further updated. Further well-designed studies are warranted to clarify the specific roles of individual dairy products.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
YZ: conceptualization, supervision, and funding acquisition. DH: conceptualization, resources, investigation, validation, methodology, software, formal analysis, writing—original draft, and writing—review and editing. QW: conceptualization, methodology, software, investigation, data curation, writing—review and editing, and project administration. XX: investigation, writing—review and editing, and project administration. CJ: resources, methodology, and formal analysis. YX: methodology, software, and validation. ZZ: methodology, software, and formal analysis. HD: investigation and formal analysis. HL: validation and visualization. SG: investigation and writing—original draft. QC: resources and data curation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China [grant number 2017YFC0907403, 2017].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the department of Library in Shengjing Hospital of China Medical University for the service platform.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.900529/full#supplementary-material
Abbreviations
PB, pre-mature birth; SA, spontaneous abortion; ML, maximum likelihood; BW, birth weight; BL, birth length; LBW, low birth weight; SGA, small for gestational age; LGA, large for gestational age; RR, Relative risk; CI, Confidence interval.
References
1. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1151–210. doi: 10.1016/S0140-6736(17)32152-9
2. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. (2016) 388:3027–35. doi: 10.1016/S0140-6736(16)31593-8
3. Markopoulou P, Papanikolaou E, Analytis A, Zoumakis E, Siahanidou T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta-analysis. J Pediatr. (2019) 210:69–80.e5. doi: 10.1016/j.jpeds.2019.02.041
4. Ream MA, Lehwald L. Neurologic consequences of preterm birth. Curr Neurol Neurosci Rep. (2018) 18:48. doi: 10.1007/s11910-018-0862-2
5. Eryigit-Madzwamuse S, Strauss V, Baumann N, Bartmann P, Wolke D. Personality of adults who were born very preterm. Arch Dis Child Fetal Neonatal Ed. (2015) 100:F524–9. doi: 10.1136/archdischild-2014-308007
6. Nordman H, Jääskeläinen J, Voutilainen R. Birth size as a determinant of cardiometabolic risk factors in children. Horm Res Paediatr. (2020) 93:144–53. doi: 10.1159/000509932
7. Sharma S, Kohli C, Johnson L, Bennet L, Brusselaers N, Nilsson PM. Birth size and cancer prognosis: a systematic review and meta-analysis. J Dev Orig Health Dis. (2020) 11:309–16. doi: 10.1017/S2040174419000631
8. Mosing MA, Lundholm C, Cnattingius S, Gatz M, Pedersen NL. Associations between birth characteristics and age-related cognitive impairment and dementia: a registry-based cohort study. PLoS Med. (2018) 15:e1002609. doi: 10.1371/journal.pmed.1002609
9. Daltveit DS, Klungsøyr K, Engeland A, Ekbom A, Gissler M, Glimelius I, et al. Cancer risk in individuals with major birth defects: large Nordic population based case-control study among children, adolescents, and adults. BMJ. (2020) 371:m4060. doi: 10.1136/bmj.m4060
10. Glinianaia SV, Morris JK, Best KE, Santoro M, Coi A, Armaroli A, et al. Long-term survival of children born with congenital anomalies: a systematic review and meta-analysis of population-based studies. PLoS Med. (2020) 17:e1003356. doi: 10.1371/journal.pmed.1003356
11. Barker DJ. The origins of the developmental origins theory. J Intern Med. (2007) 261:412–7. doi: 10.1111/j.1365-2796.2007.01809.x
12. Raghavan R, Dreibelbis C, Kingshipp BL, Wong YP, Abrams B, Gernand AD, et al. Dietary patterns before and during pregnancy and birth outcomes: a systematic review. Am J Clin Nutr. (2019) 109:729S−56S. doi: 10.1093/ajcn/nqy353
13. Abdollahi S, Soltani S, de Souza RJ, Forbes SC, Toupchian O, Salehi-Abargouei A. Associations between maternal dietary patterns and perinatal outcomes: a systematic review and meta-analysis of cohort studies. Adv Nutr. (2021) 12:1332–52. doi: 10.1093/advances/nmaa156
14. Willett WC, Ludwig DS. Milk and health. N Engl J Med. (2020) 382:644–54. doi: 10.1056/NEJMra1903547
15. Brantsæter AL, Olafsdottir AS, Forsum E, Olsen SF, Thorsdottir I. Does milk and dairy consumption during pregnancy influence fetal growth and infant birthweight? A systematic literature review. Food Nutr Res. (2012) 56:20050. doi: 10.3402/fnr.v56i0.20050
16. Achón M, Úbeda N, García-González Á, Partearroyo T, Varela-Moreiras G. Effects of milk and dairy product consumption on pregnancy and lactation outcomes: a systematic review. Adv Nutr. (2019) 10:S74–87. doi: 10.1093/advances/nmz009
17. Pérez-Roncero GR, López-Baena MT, Chedraui P, Pérez-López FR. The effect of consuming milk and related products during human pregnancy over birth weight and perinatal outcomes: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2020) 251:235–45. doi: 10.1016/j.ejogrb.2020.05.061
18. Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. (2008) 27:954–70. doi: 10.1002/sim.3013
19. Heppe DH, van Dam RM, Willemsen SP, den Breeijen H, Raat H, Hofman A, et al. Maternal milk consumption, fetal growth, and the risks of neonatal complications: the generation R study. Am J Clin Nutr. (2011) 94:501–9. doi: 10.3945/ajcn.111.013854
20. Maslova E, Halldorsson TI, Strøm M, Olsen SF. Low-fat yoghurt intake in pregnancy associated with increased child asthma and allergic rhinitis risk: a prospective cohort study. J Nutr Sci. (2012) 1:e5. doi: 10.1017/jns.2012.5
21. Mannion CA, Gray-Donald K, Koski KG. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. CMAJ. (2006) 174:1273–7. doi: 10.1503/cmaj.1041388
22. Crippa A, Orsini N. Dose-response meta-analysis of differences in means. BMC Med Res Methodol. (2016) 16:91. doi: 10.1186/s12874-016-0189-0
23. Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. (2019) 28:1579–96. doi: 10.1177/0962280218773122
24. Petridou E, Stoikidou M, Diamantopoulou M, Mera E, Dessypris N, Trichopoulos D. Diet during pregnancy in relation to birthweight in healthy singletons. Child Care Health Dev. (1998) 24:229–42. doi: 10.1046/j.1365-2214.1998.00068.x
25. Di Cintio E, Parazzini F, Chatenoud L, Surace M, Benzi G, Zanconato G, et al. Dietary factors and risk of spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. (2001) 95:132–6. doi: 10.1016/S0301-2115(00)00363-8
26. Rao S, Yajnik CS, Kanade A, Fall CH, Margetts BM, Jackson AA, et al. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune maternal nutrition study. J Nutr. (2001) 131:1217–24. doi: 10.1093/jn/131.4.1217
27. Chang SC, O'Brien KO, Nathanson MS, Caulfield LE, Mancini J, Witter FR. Fetal femur length is influenced by maternal dairy intake in pregnant African American adolescents. Am J Clin Nutr. (2003) 77:1248–54. doi: 10.1093/ajcn/77.5.1248
28. Ludvigsson JF, Ludvigsson J. Milk consumption during pregnancy and infant birthweight. Acta Paediatr. (2004) 93:1474–8. doi: 10.1080/08035250410018319
29. Mitchell EA, Robinson E, Clark PM, Becroft DM, Glavish N, Pattison NS, et al. Maternal nutritional risk factors for small for gestational age babies in a developed country: a case-control study. Arch Dis Child Fetal Neonatal Ed. (2004) 89:F431–5. doi: 10.1136/adc.2003.036970
30. Chan GM, McElligott K, McNaught T, Gill G. Effects of dietary calcium intervention on adolescent mothers and newborns: a randomized controlled trial. Obstet Gynecol. (2006) 108:565–71. doi: 10.1097/01.AOG.0000231721.42823.9e
31. Olsen SF, Halldorsson TI, Willett WC, Knudsen VK, Gillman MW, Mikkelsen TB, et al. Milk consumption during pregnancy is associated with increased infant size at birth: prospective cohort study. Am J Clin Nutr. (2007) 86:1104–10. doi: 10.1093/ajcn/86.4.1104
32. Kanade AN, Rao S, Kelkar RS, Gupte S. Maternal nutrition and birth size among urban affluent and rural women in India. J Am Coll Nutr. (2008) 27:137–45. doi: 10.1080/07315724.2008.10719685
33. Xue F, Willett WC, Rosner BA, Forman MR, Michels KB. Parental characteristics as predictors of birthweight. Hum Reprod. (2008) 23:168–77. doi: 10.1093/humrep/dem316
34. Borazjani F, Ahmadi KA, Shahri P. Relationship between maternal nutritional-social status and pregnancy outcomes. Pak J Nutr. (2011) 10:724–7. doi: 10.3923/pjn.2011.724.727
35. Christensen JS, Asklund C, Skakkebæk NE, Jørgensen N, Andersen HR, Jørgensen TM, et al. Association between organic dietary choice during pregnancy and hypospadias in offspring: a study of mothers of 306 boys operated on for hypospadias. J Urol. (2013) 189:1077–82. doi: 10.1016/j.juro.2012.09.116
36. Hrolfsdottir L, Rytter D, Hammer Bech B, Brink Henriksen T, Danielsen I, Steingrimsdottir L, et al. Maternal milk consumption, birth size and adult height of offspring: a prospective cohort study with 20 years of follow-up. Eur J Clin Nutr. (2013) 67:1036–41. doi: 10.1038/ejcn.2013.151
37. Sultan Azzeh F. Risk factors associated with delivering low birth weight infants among pregnant women: a preliminary study in Western Saudi Arabia. J Biol Sci. (2013) 13:417–21. doi: 10.3923/jbs.2013.417.421
38. Li YF, Hu NS, Tian XB, Li L, Wang SM, Xu XB, et al. Effect of daily milk supplementation on serum and umbilical cord blood folic acid concentrations in pregnant Han and Mongolian women and birth characteristics in China. Asia Pac J Clin Nutr. (2014) 23:567–74. doi: 10.6133/apjcn.2014.23.4.18
39. Shaikh F, Zeeshan F, Hakeem R, Basit A, Fawwad A, Hussain A. Maternal dietary intake and anthropometric measurements of newborn at birth. Open Diabetes J. (2014) 7:14–9. doi: 10.2174/1876524601407010014
40. Akbari Z, Mansourian M, Kelishadi R. Relationship of the intake of different food groups by pregnant mothers with the birth weight and gestational age: need for public and individual educational programs. J Edu Health Promot. (2015) 4:23. doi: 10.4103/2277-9531.154109
41. Wang M, Wang ZP, Gao LJ, Yang H, Zhao ZT. Maternal consumption of non-staple food in the first trimester and risk of neural tube defects in offspring. Nutrients. (2015) 7:3067–77. doi: 10.3390/nu7053067
42. Brantsæter AL, Torjusen H, Meltzer HM, Papadopoulou E, Hoppin JA, Alexander J, et al. Organic food consumption during pregnancy and hypospadias and cryptorchidism at birth: the Norwegian mother and child cohort study (MoBa). Environ Health Perspect. (2016) 124:357–64. doi: 10.1289/ehp.1409518
43. Miyake Y, Tanaka K, Okubo H, Sasaki S, Furukawa S, Arakawa M. Milk intake during pregnancy is inversely associated with the risk of post-partum depressive symptoms in Japan: the Kyushu Okinawa maternal and child health study. Nutr Res. (2016) 36:907–13. doi: 10.1016/j.nutres.2016.06.001
44. Olmedo-Requena R, Amezcua-Prieto C, Luna-Del-Castillo Jde D, Lewis-Mikhael AM, Mozas-Moreno J, Bueno-Cavanillas A, et al. Association between low dairy intake during pregnancy and risk of small-for-gestational-age infants. Matern Child Health J. (2016) 20:1296–304. doi: 10.1007/s10995-016-1931-2
45. Abreu S, Santos PC, Montenegro N, Mota J. Relationship between dairy product intake during pregnancy and neonatal and maternal outcomes among Portuguese women. Obes Res Clin Pract. (2017) 11:276–86. doi: 10.1016/j.orcp.2016.07.001
46. Ahmadi R, Ziaei S, Parsay S. Association between nutritional status with spontaneous abortion. Int J Fertil Steril. (2017) 10:337–42. doi: 10.22074/ijfs.2016.4577
47. Yan H, Dang SN, Mi BB, Qu PF, Zhang L, Wang HL, et al. Study on relationship between mother's animal sourced food intake during pregnancy and neonate birth weight. Zhonghua Liu Xing Bing Xue Za Zhi. (2017) 38:615–20. doi: 10.3760/cma.j.issn.0254-6450.2017.05.011
48. Hjertholm KG, Iversen PO, Holmboe-Ottesen G, Mdala I, Munthali A, Maleta K, et al. Maternal dietary intake during pregnancy and its association to birth size in rural Malawi: a cross-sectional study. Matern Child Nutr. (2018) 14:e12433. doi: 10.1111/mcn.12433
49. Kriss JL, Ramakrishnan U, Beauregard JL, Phadke VK, Stein AD, Rivera JA, et al. Yogurt consumption during pregnancy and preterm delivery in Mexican women: a prospective analysis of interaction with maternal overweight status. Matern Child Nutr. (2018) 14:e12522. doi: 10.1111/mcn.12522
50. Mukhopadhyay A, Dwarkanath P, Bhanji S, Devi S, Thomas A, Kurpad AV, et al. Maternal intake of milk and milk proteins is positively associated with birth weight: a prospective observational cohort study. Clin Nutr ESPEN. (2018) 25:103–9. doi: 10.1016/j.clnesp.2018.03.125
51. Zerfu TA, Pinto E, Baye K. Consumption of dairy, fruits and dark green leafy vegetables is associated with lower risk of adverse pregnancy outcomes (APO): a prospective cohort study in rural Ethiopia. Nutr Diabetes. (2018) 8:52. doi: 10.1038/s41387-018-0060-y
52. Ito M, Takamori A, Yoneda S, Shiozaki A, Tsuchida A, Matsumura K, et al. Fermented foods and preterm birth risk from a prospective large cohort study: the Japan environment and children's study. Environ Health Prev Med. (2019) 24:25. doi: 10.1186/s12199-019-0782-z
53. Abera Z, Ejara D, Gebremedhin S. Nutritional and non-nutritional factors associated with low birth weight in Sawula Town, Gamo Gofa Zone, Southern Ethiopia. BMC Res Notes. (2019) 12:540. doi: 10.1186/s13104-019-4529-0
54. Olmedo-Requena R, Martínez-Galiano JM, Amezcua-Prieto C, Cano-Ibáñez N, Salcedo-Bellido I, Barrios-Rodríguez R, et al. Association between low dairy intake during pregnancy and small for gestational age infants. Eur J Clin Nutr. (2019) 73:1642–5. doi: 10.1038/s41430-019-0513-y
55. Shen ZZ, Wang YW, Ma S, Zhan YL, Wu SS, Feng YH, et al. Risk factors for preterm birth, low birth weight and small for gestational age: a prospective cohort study. Zhonghua Liu Xing Bing Xue Za Zhi. (2019) 40:1125–9. doi: 10.3760/cma.j.issn.0254-6450.2019.09.020
56. Baki Yildirim S, Koşar Can Ö. An investigation of vitamin D deficiency in pregnant women and their infants in Giresun province located in the Black Sea region of Turkey. J Obstet Gynaecol. (2019) 39:498–503. doi: 10.1080/01443615.2018.1539469
57. Assaf-Balut C, Garcia de la Torre N, Bordiu E, Del Valle L, Valerio J, Jimenez I, et al. Consumption of fat-free dairy products is not associated with a lower risk of maternofetal adverse events. BMJ Open Diabetes Res Care. (2020) 8:e001145. doi: 10.1136/bmjdrc-2019-001145
58. Pang XX, Bai D, Cai CJ, Dong HL, Zhang YQ, Lan X, et al. Associations of dairy consumption during pregnancy and neonatal birth body mass: a prospective study. Sichuan Da Xue Xue Bao Yi Xue Ban. (2020) 51:680–4. doi: 10.12182/20200960105
59. Rodrigues B, Azeredo V, Silva A. Relationship between food consumption of pregnant women and birth weight of newborns. Rev Chil Nutr. (2020) 47:80–8. doi: 10.4067/S0717-75182020000100080
60. Li Y, Diao J, Li J, Luo L, Zhao L, Zhang S, et al. Association of maternal dietary intakes and CBS gene polymorphisms with congenital heart disease in offspring. Int J Cardiol. (2021) 322:121–8. doi: 10.1016/j.ijcard.2020.08.018
61. Sartorelli DS, Carvalho MR, da Silva Santos I, Crivellenti LC, Souza JP, Franco LJ. Dietary total antioxidant capacity during pregnancy and birth outcomes. Eur J Nutr. (2021) 60:357–67. doi: 10.1007/s00394-020-02251-y
62. Voerman E, Gaillard R, Geurtsen ML, Jaddoe VWV. Maternal first-trimester cow-milk intake is positively associated with childhood general and abdominal visceral fat mass and lean mass but not with other cardiometabolic risk factors at the age of 10 years. J Nutr. (2021) 151:1965–75. doi: 10.1093/jn/nxab047
63. Cámara M, Giner RM, González-Fandos E, López-García E, Mañes J, Portillo MP, et al. Food-based dietary guidelines around the world: a comparative analysis to update AESAN scientific committee dietary recommendations. Nutrients. (2021) 13:3131. doi: 10.3390/nu13093131
64. Yackobovitch-Gavan M, Phillip M, Gat-Yablonski G. How milk and its proteins affect growth, bone health, and weight. Horm Res Paediatr. (2017) 88:63–9. doi: 10.1159/000456662
65. Clark DC. Association of dairy protein intake during pregnancy with birth weight. Food Nutr Bull. (2018) 39:S54–9. doi: 10.1177/0379572118775824
66. Elhddad AS, Lashen H. Fetal growth in relation to maternal and fetal IGF-axes: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. (2013) 92:997–1006. doi: 10.1111/aogs.12192
67. Wiley AS, Lubree HG, Joshi SM, Bhat DS, Ramdas LV, Rao AS, et al. Cord IGF-I concentrations in Indian newborns: associations with neonatal body composition and maternal determinants. Pediatr Obes. (2016) 11:151–7. doi: 10.1111/ijpo.12038
68. Kim J, Guan KL. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol. (2019) 21:63–71. doi: 10.1038/s41556-018-0205-1
69. Melnik BC. Milk—a nutrient system of mammalian evolution promoting mTORC1-dependent translation. Int J Mol Sci. (2015) 16:17048–87. doi: 10.3390/ijms160817048
70. Melnik BC, John SM, Schmitz G. Milk consumption during pregnancy increases birth weight, a risk factor for the development of diseases of civilization. J Transl Med. (2015) 13:13. doi: 10.1186/s12967-014-0377-9
71. Barański M, Rempelos L, Iversen PO, Leifert C. Effects of organic food consumption on human health; the jury is still out! Food Nutr Res. (2017) 61:1287333. doi: 10.1080/16546628.2017.1287333
72. Tunick MH, Van Hekken DL. Dairy products and health: recent insights. J Agric Food Chem. (2015) 63:9381–8. doi: 10.1021/jf5042454
73. Crinnion WJ. Organic foods contain higher levels of certain nutrients, lower levels of pesticides, and may provide health benefits for the consumer. Altern Med Rev. (2010) 15:4–12.
74. Cognez N, Warembourg C, Zaros C, Metten MA, Bouvier G, Garlantézec R, et al. Residential sources of pesticide exposure during pregnancy and the risks of hypospadias and cryptorchidism: the French ELFE birth cohort. Occup Environ Med. (2019) 76:672–9. doi: 10.1136/oemed-2019-105801
Keywords: pregnant woman, milk, dairy, fetal growth, birth outcome, dose-response meta-analysis
Citation: Huang D, Wu Q, Xu X, Ji C, Xia Y, Zhao Z, Dai H, Li H, Gao S, Chang Q and Zhao Y (2022) Maternal Consumption of Milk or Dairy Products During Pregnancy and Birth Outcomes: A Systematic Review and Dose-Response Meta-Analysis. Front. Nutr. 9:900529. doi: 10.3389/fnut.2022.900529
Received: 20 March 2022; Accepted: 12 May 2022;
Published: 09 June 2022.
Edited by:
Aifric O'Sullivan, University College Dublin, IrelandReviewed by:
Olivia Engmann, University Hospital Jena, GermanyRosaura Leis, University of Santiago de Compostela, Spain
Copyright © 2022 Huang, Wu, Xu, Ji, Xia, Zhao, Dai, Li, Gao, Chang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhong Zhao, zhaoyuhong@sj-hospital.org
 Donghui Huang1
Donghui Huang1  Qijun Wu
Qijun Wu Chao Ji
Chao Ji Yang Xia
Yang Xia Qing Chang
Qing Chang Yuhong Zhao
Yuhong Zhao