- 1Neuroimaging Research Branch, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD, United States
- 2Department of Psychiatry and Psychology, Mayo Clinic, Phoenix, AZ, United States
- 3Department of Psychology, College of Liberal Arts, Temple University, Philadelphia, PA, United States
The positive treatment outcomes of low frequency (LF) repetitive transcranial magnetic stimulation (rTMS) when applied over the right dorsolateral prefrontal cortex (DLPFC) in treatment-refractory depression has been verified. However, the mechanism of action behind these results have not been well-explored. In this work we used simultaneous functional magnetic resonance imaging (fMRI) during TMS to explore the effect of LF rTMS on brain activity when applied to the right [RDLPFC1 (MNI: 50, 30, 36)] and left DLPFC sites [LDLPFC1 (MNI: -50, 30, 36), LDLPFC2 (MNI: -41, 16, 54)]. Seventeen healthy adult volunteers participated in this study. To identify brain areas affected by rTMS, an independent component analysis and a general linear model were used. Our results showed an important laterality effect when contrasting rTMS over the left and right sites. Specifically, LF rTMS increased brain activity at the striatum, thalamus, and areas of the default mode network when applied to the right, but not to the contralateral left DLPFC. In contrast, no site differences were observed when evaluating the effect of LF rTMS over the two left sites. These findings demonstrate that LF rTMS to the right DLPFC was able to stimulate the cortico-striato-thalamo-cortical pathway, which is dysregulated in patients with major depressive disorder; therefore, possibly providing some neurobiological justification for the successful outcomes found thus far for LF rTMS in the treatment of depression.
Introduction
The efficacy of repetitive transcranial magnetic stimulation (rTMS) in treating certain neuropsychiatric disorders, specifically treatment-refractory depression (TRD) has been well-documented (Anand and Hotson, 2002; Gaynes et al., 2014; Wei et al., 2017). The dorsolateral prefrontal cortex (DLPFC) has conventionally been the preferred stimulation site for treatment of TRD (George et al., 1995). The cortico-striato-thalamo-cortical (CSTC) loop is a key network involved in emotional regulation (Peters et al., 2016) through heavy dopaminergic projections at individual nodes (Gerfen, 2000; Sanchez-Gonzalez et al., 2005). Major depression is associated with a state of reduced dopaminergic transmission along the CSTC loop (Dunlop and Nemeroff, 2007; Grace, 2016) and it is hypothesized that TMS over the DLPFC stimulates the CSTC pathway, improving the dopamine state (Strafella et al., 2001), therefore normalizing some affective and cognitive functions (Nitschke et al., 2004; Grimm et al., 2008; Goldstein and Volkow, 2011; da Silva et al., 2013).
In general, depression treatment protocols employ high frequency (HF—10 to 20 Hz) rTMS to the left DLPFC (Polley et al., 2011; McClintock et al., 2018). While the efficacy of this approach has been verified (George et al., 1995, 1997) and its mechanism explored (Strafella et al., 2001; Cho and Strafella, 2009; Caparelli et al., 2022), more recently it has been demonstrated that low frequency (LF) rTMS, which refers to a stimulation rate of ≤1 Hz (Wassermann, 1998), to the right DLPFC is as effective as HF rTMS to the left DLPFC in attenuating the severity of TRD (Chen et al., 2013; Garnaat et al., 2018). However, whether LF TMS activates the CSTC network, or other key limbic structures remains largely unknown.
In this study we aimed to investigate the differences in the mechanism of action for LF rTMS when applied to the right DLPFC and to the left DLPFC, since, clinically, the left DLPFC is the most commonly used stimulation target followed by the right DLPFC. Following the approach of our previous work (Caparelli et al., 2022), where the effect of HF rTMS was evaluated on three different DLPFC sites, we evaluated LF rTMS for the same three sites, two sites on the left: (1) Montreal Neurological Institute (MNI) coordinates = -50, 30, 36 [chosen because it has previously shown to have promising treatment results for cocaine addiction, such as, reduction of craving and cocaine use, as well as improvement of their depressive symptoms (Terraneo et al., 2016)], (2) MNI coordinates = –41, 16, 54 [chosen as a standardized metric, the “5 cm rule” (Fox et al., 2012)], and one on the right: MNI coordinates = 50, 30, 36 (the contralateral side of site #1), since the functional asymmetric aspect of the DLPFC has been previously reported (Chen et al., 2018). Here we aspire to verify the top-down mechanism of LF (0.4 Hz) rTMS on these three different DLPFC sites and to understand the site and laterality differences of LF rTMS.
Materials and methods
The current study utilized LF (0.4 Hz) rTMS targeting the DLPFC, combined with the simultaneous fMRI recording. The study consisted of four study visits for each of the participants.
Participants
Seventeen healthy adult volunteers (nine males, eight females, age: 37.1 ± 11.2 years old) participated in the study, among which fifteen were completers (participants who completed all four study visits) and two non-completers [one lost contact, and the other withdrew from the study (Caparelli et al., 2022)]. Participants with any neuropsychiatric disorder and/or any contraindication to fMRI scanning or rTMS administration were excluded (Caparelli et al., 2022). The study was approved by the Institutional Review Board panel of the National Institute on Drug Abuse and written informed consent was obtained from each participant.
Experiment design and data acquisition
As previously described (Caparelli et al., 2022), this study was composed of four study visits (days). On the first day the three stimulation sites, LDLPFC1 (MNI: -50, 30, 36), LDLPFC2 (MNI: -41, 16, 54) and RDLPFC1 (MNI: 50, 30, 36) were localized and the resting motor threshold (rMT) was determined outside the MRI scanner. On the following three study days the simultaneous TMS-fMRI sessions (one stimulation site per day) were carried out using a TMS intensity of 100% of the rMT. The LF (0.4 Hz) rTMS sessions were carried out in the morning, and the HF (10 Hz) rTMS sessions (Caparelli et al., 2022) were acquired in the afternoon, with a minimum of 1.5 h between sessions.
Transcranial magnetic stimulation
The MagVenture system (MagPro X100 with MagOption stimulator, C-B60 coil, MRi-B91 Air Cooled coil–MagVenture Inc., Alpharetta, GA, USA) and the TMS Neuronavigation system (Brainsight–Rogue Resolutions Ltd, Montreal, QC, Canada) were used in this study (Caparelli et al., 2022). The neuronavigation system was used only for the rMT determination and localization of the stimulation sites. The identified stimulation sites were marked on the participant’s cap to guide the application of TMS stimulation on the following concurrent TMS-fMRI experiment days. For each TMS-fMRI session, the TMS coil was centered over the indicator for the stimulation site assigned for that day, with the order of the stimulation sites varied among subjects. Foam pads were used to minimize head motions (Caparelli et al., 2022).
Magnetic resonance imaging
A 3 Tesla Prisma-fit Siemens scanner and a transmit-receive (Tx/Rx) single-channel birdcage head radio-frequency coil were used to acquire the fMRI data using a gradient-echo echo-planar imaging sequence (EPI–TE/TR of 27/2,500 ms, TR delay of 500 ms, spatial resolution of 3.4 × 3.4 × 4.4 mm3, 36 slices per volume, 280 volumes, axial orientation, flip angle of 78°) and the T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) images (Caparelli et al., 2022). Same as our previous work (Caparelli et al., 2022), each TMS-fMRI session was composed of two fMRI scans and each scan started with 10 baseline volumes, followed by six alternating blocks, three blocks of stimulation “ON” (30 volumes with TMS on) and three blocks of stimulation “OFF” (60 volumes with TMS off). During the stimulation block, the TMS pulses were applied in an interleaved fashion with the fMRI acquisition, and for every TR, the TMS pulse was applied 100 ms before the fMRI volume acquisition (Figure 1). Thus, the rTMS was applied at frequency of 0.4 Hz, i.e., one pulse every 2.5 s.
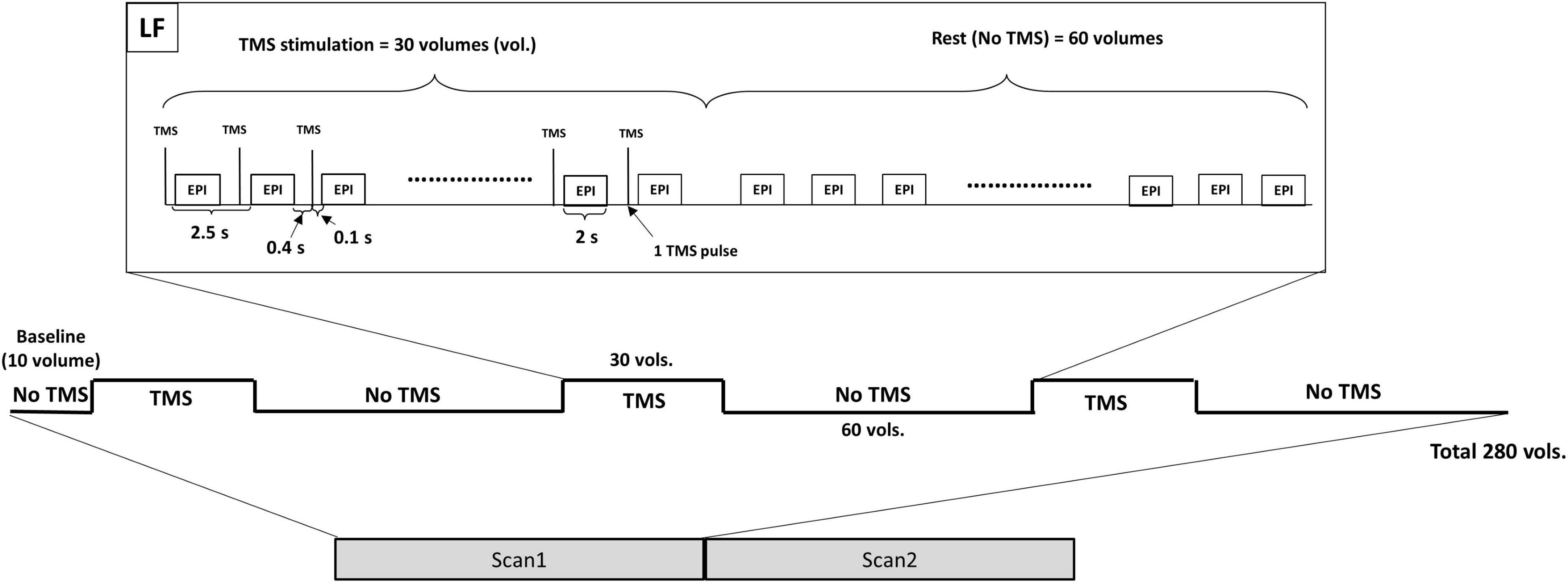
Figure 1. The TMS-fMRI session is composed of two fMRI scans. Each scan starts with a baseline followed by three stimulation blocks alternated by three resting blocks. During the stimulation block, each TMS pulse is applied 100 ms before the next EPI acquisition (400 ms after the previous EPI acquisition). TMS, transcranial magnetic stimulation; LF, low frequency; EPI, echo-planar imaging.
Data analysis
Two approaches were considered for the analysis of the fMRI data, the Independent Component Analysis (ICA) and the General Linear Model (GLM) analysis. The analytical methods for both approaches have been detailed in our previous work (Caparelli et al., 2022), and summarized below. All analyses were carried out using FSL (the FMRIB Software Library, Oxford, UK) (Jenkinson et al., 2012), AFNI (Cox, 1996), or RStudio (RStudio Team, 2020).
Independent component analysis approach
Preprocessing, independent component analysis, and spatial regression
The raw fMRI data were preprocessed (slice-timing and motion correction, spatial normalization to the MNI space, spatial smoothing, detrending, scaling); bad fMRI scans [with excessive motion (more than 30% of the volumes with motion above 0.3 mm of Euclidean distance) and/or any imaging artifact] were discarded whenever they could not be repeated (for final data set, see Table 1). Preprocessed data was then fed into the group ICA, which was carried out using MELODIC in FSL with the component number set at 40. Next, fifteen Independent Components (ICs) were chosen (Figure 2 and Table 2), after excluding some meaningless ICs [e.g., components showing motion artifacts, scattered small clusters across the brain, cerebral spinal fluid (CSF), white matter (WM), or components showing only either occipital or cerebellum which is considered to be minimally affected by TMS delivered over the DLPFC (Vink et al., 2018)—Supplementary Figure 2]. Following, a spatial regression analysis was conducted to extract the time courses for each IC, participant, scan, and stimulation site. The time courses of the six motion parameters and the average time courses extracted from the CSF and WM regions were regressed out from the IC time courses as nuisance regressors (Caparelli et al., 2022).

Table 1. Number of available data sets per functional magnetic resonance imaging (fMRI) scan and stimulation site.
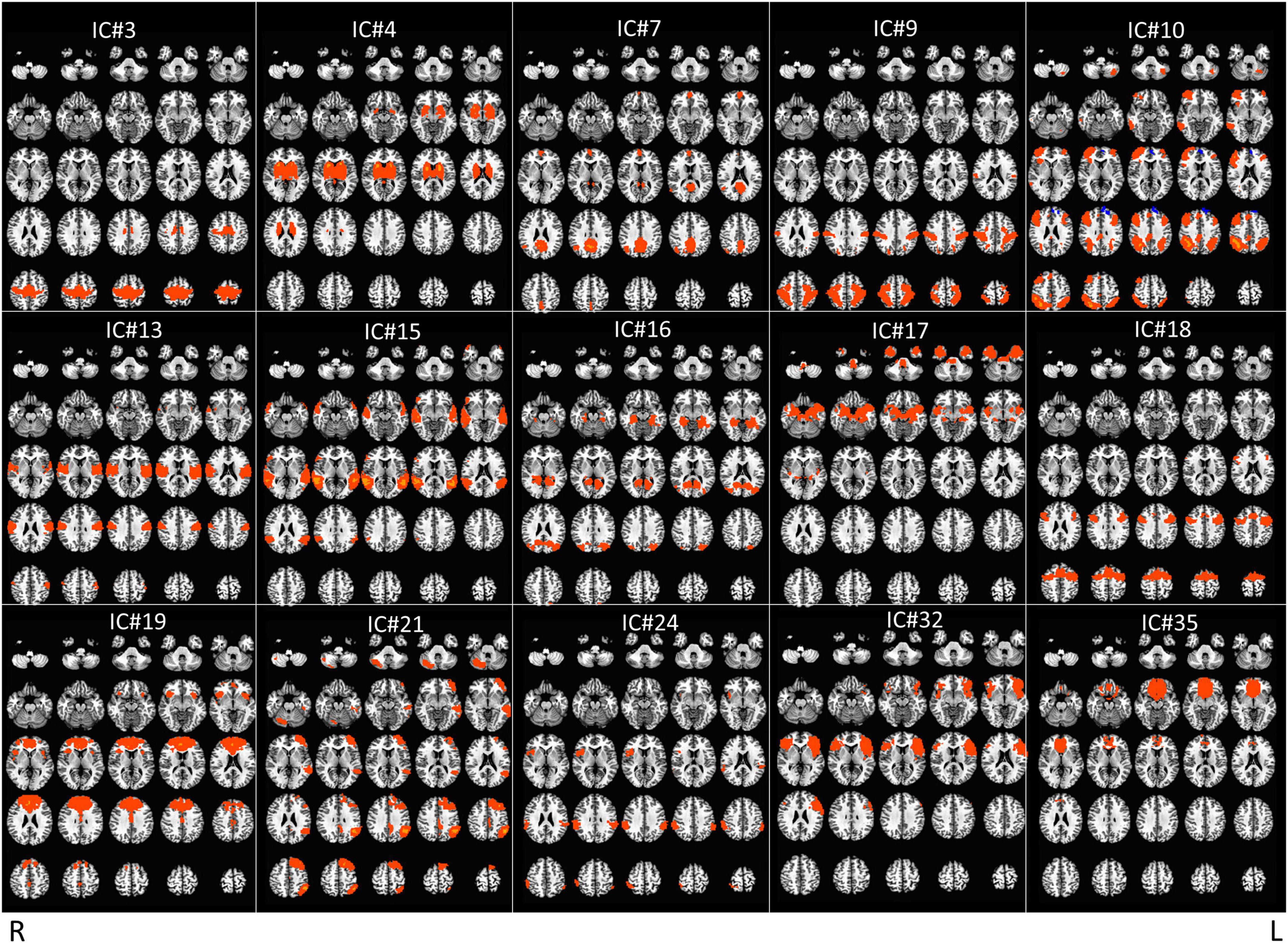
Figure 2. Display of the 15 meaningful ICs from group independent component analysis (ICA). Radiological convention.
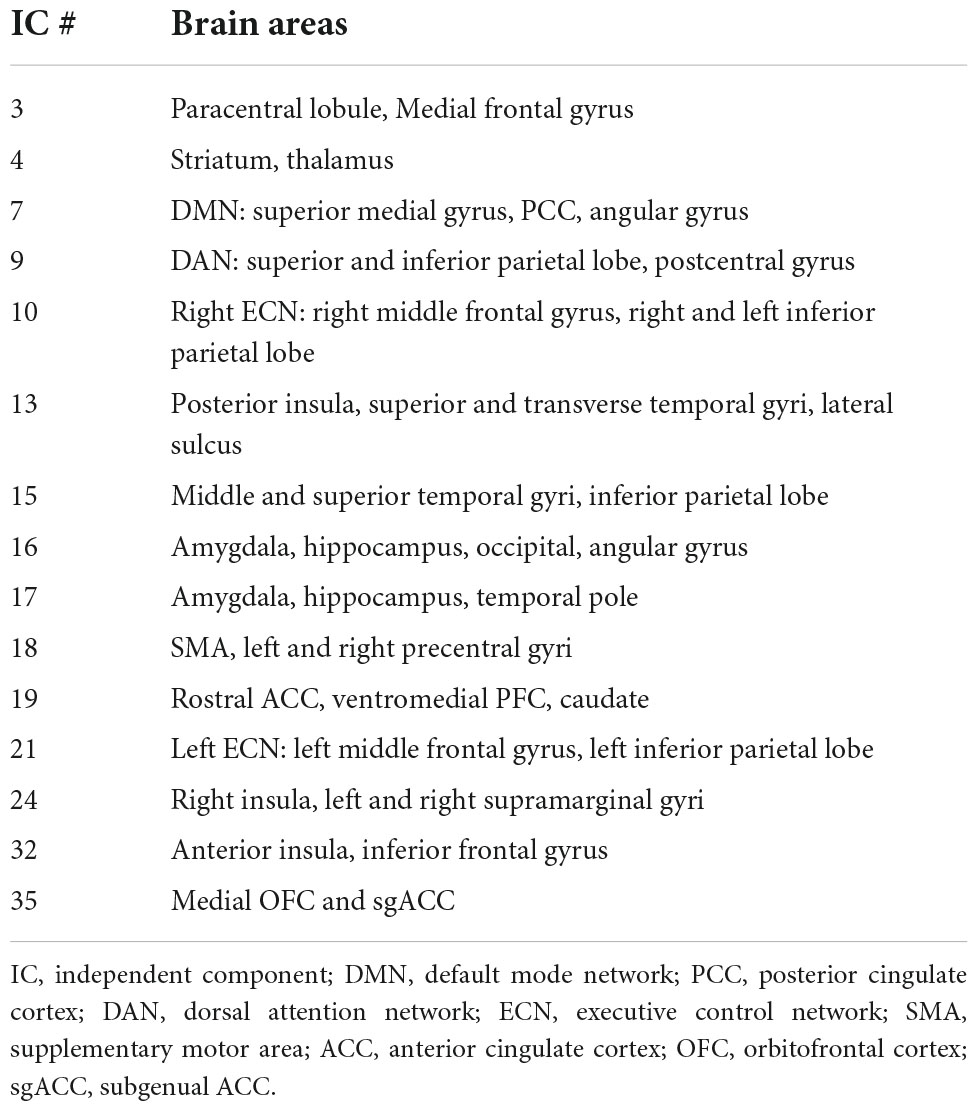
Table 2. Brain areas for the 15 independent components (ICs) presented in Figure 2.
Deconvolution
The IC time courses, for each IC, scan and stimulation site, were averaged across participants. Next, for each averaged IC time course, the hemodynamic response function (HRF) of the rTMS with duration of one stimulation-rest epoch [one stimulation block (TMS “ON”–30 images) + one resting block (TMS “OFF”–60 images)] was estimated through a linear regression with TENT function, which extracts the impulse response function for the averaged stimulation-rest epochs from the data. Following, the estimated HRF was smoothed and concatenated three times along with 10 time points of zeros values at the beginning of the vector, to generate the TMS response vector (TRV) for each IC, scan and stimulation site. Then, the TRVs, for each IC and stimulation site, were averaged across scans and correlated with the TMS paradigm (block design vector), just to determine the TRVs orientation. Finally, the signs of TRVs (from scan 1 and scan 2) were flipped only if correlation value was negative (Caparelli et al., 2022).
Correlation and T-tests
For each IC and stimulation site, the final TRV derived from one scan was correlated with the IC time course from the other scan for each participant [i.e., TRV (scan 1) correlated with IC time course (scan 2) and TRV (scan 2) correlated with IC time course (scan 1)]; finally, for each participant, the two correlation values were averaged and transformed to z-values through Fisher r-to-z-transformation.
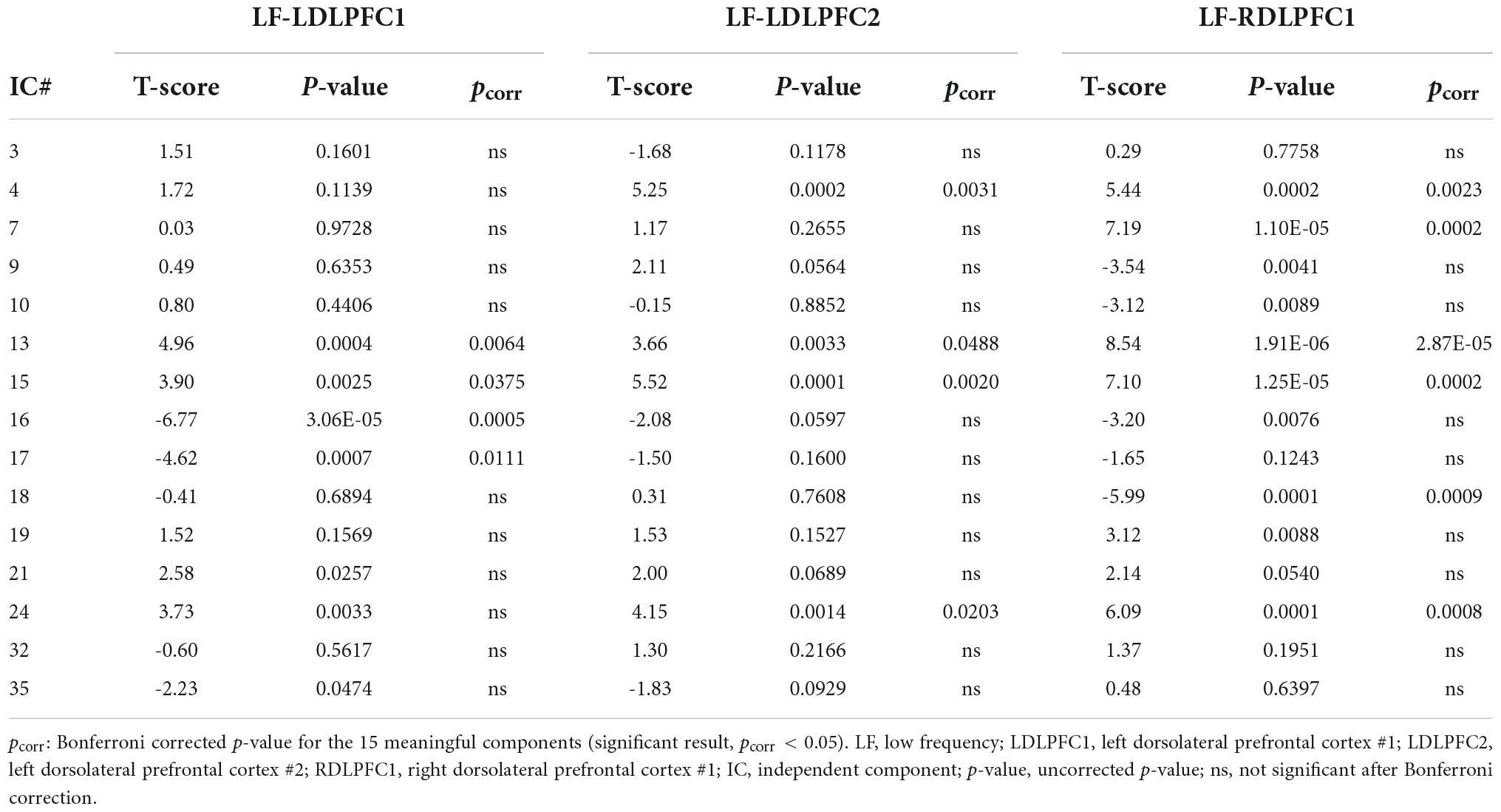
Statistical analyses were carried out to evaluate the significant results within and between sites. One-sample T-tests were conducted for each IC and stimulation site, and the results were Bonferroni corrected for the 15 ICs. Subsequently, the site (LDLPFC1 vs. LDLPFC2) and laterality (LDLPFC1 vs. RDLPFC1) differences were evaluated using two-sample T-tests that only included ICs with significant results from the one-sample T-tests for at least one of the sites (six ICs were included for site differences and eight for laterality differences). The results were then Bonferroni corrected based on the respective numbers of ICs.
General linear model analysis
For comparison, a traditional GLM analysis was performed on the preprocessed fMRI data (slice-timing and motion correction, image spatial normalization to the MNI space, spatial smoothing and scaling) using the estimated time course constructed by convolving the block-design paradigm (TMS “ON” contrasting with TMS “OFF”) with the conventional HRF. Head motion parameters and average WM and CSF time courses were used as nuisance regressors in the first-level analysis, and volumes with excessive motion were censored out. For statistical analyses, one sample T-tests were conducted for each stimulation site, and two-sample T-tests were carried out to evaluate site and laterality differences.
Results
Independent component analysis approach
Results from the one sample T-test (Table 3) revealed a significant positive TMS effect at the posterior insula, superior and transverse temporal gyri, lateral sulcus (IC#13), and at the middle and superior temporal gyri and the inferior parietal lobe (IC#15) for all stimulation sites. A significant positive effect was also observed at the striatum, and thalamus (IC#4); and at the right insula and left and right supramarginal gyri (IC#24) when TMS was applied over the LDLPFC2 and RDLPFC1.
An evident negative TMS effect was observed at the amygdala, hippocampus, occipital lobe, angular gyrus (IC# 16), and at the amygdala, hippocampus, and temporal pole (IC#17) when TMS was applied over the LDLPFC1. Negative TMS effects were also observed at the supplementary motor area (SMA), and the left and right precentral gyri (IC#18) when TMS was applied over the RDLPFC1. Significant positive TMS effect was observed at DMN (IC#7) only when TMS was applied over the RDLPFC1.
Results from the two-sample T-tests (Table 4 and Figure 3) showed no site difference when contrasting the LDLPFC1 stimulation affected networks with the LDLPFC2 stimulation affected networks. However, laterality differences were observed at the DMN (IC#7) and at the striatum and thalamus (IC#4) when contrasting the TMS effect over the left and right DLPFC1.
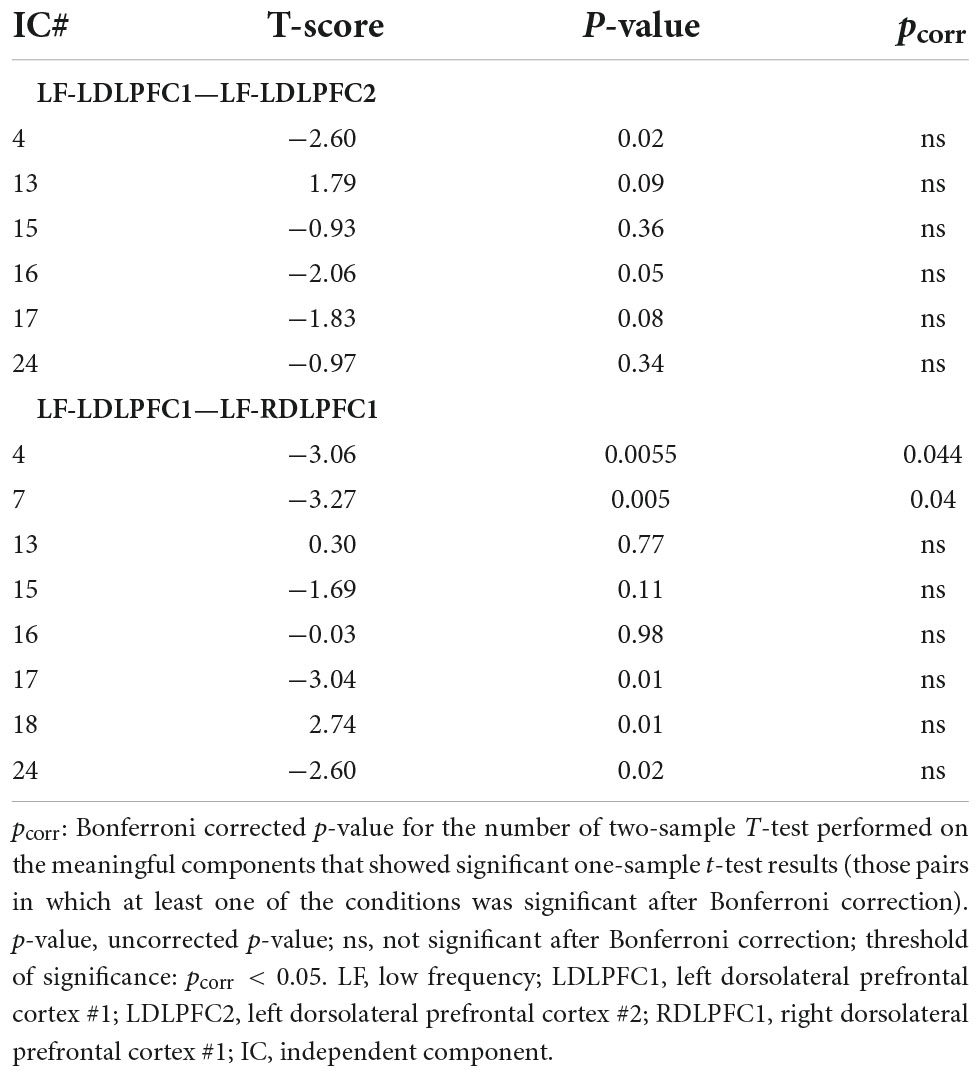
Table 4. Two-sample T-test results for site (LF-LDLPFC1—LF-LDLPFC2) and laterality (LF-LDLPFC1—LF-RDLPFC1) differences.
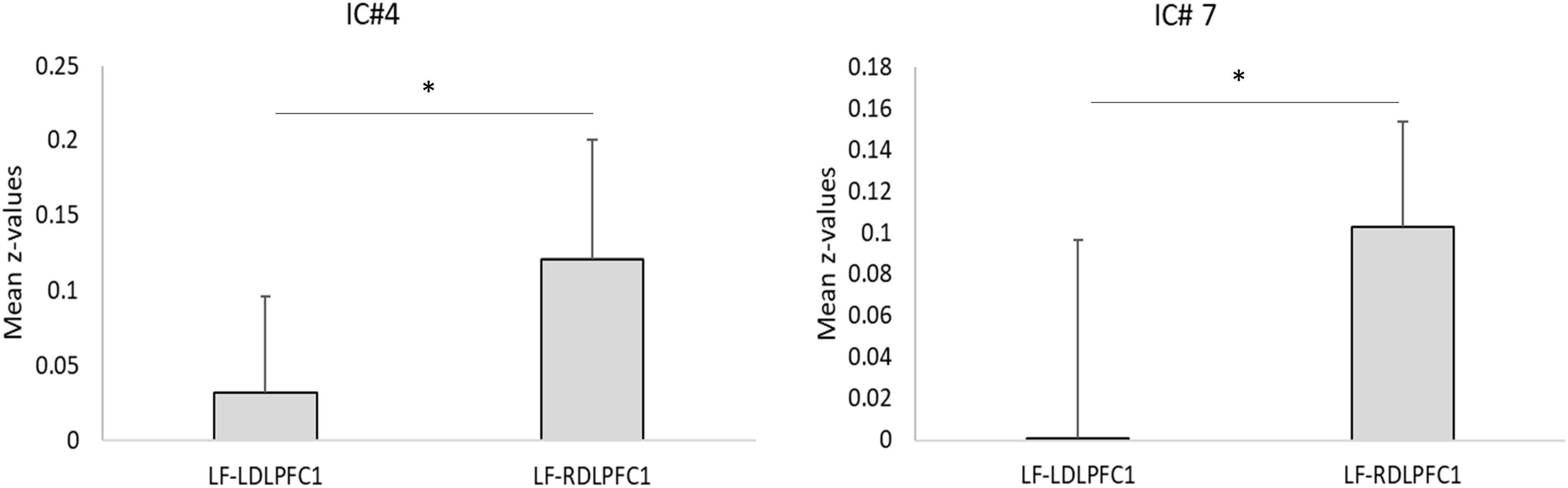
Figure 3. Laterality test. Plots for the mean correlation values (z-values) for ICs with significant laterality effects. *Two-sample t-test significant after Bonferroni correction as shown on Table 4 (*pcorr < 0.05). Error bars: standard deviation. LF, low frequency; LDLPFC1, left dorsolateral prefrontal cortex #1; RDLPFC1, right dorsolateral prefrontal cortex #1.
General linear model analysis
Results for the traditional GLM-based analysis are shown on Figure 4. It revealed significant activation at the left and the right superior temporal gyri when TMS was applied over both left DLPFC sites (LDLPFC1 and LDLPFC2). Additionally, significant positive activation was observed at the left superior and middle temporal gyri when TMS was applied over the right DLPFC site (RDLPFC1).

Figure 4. Results of general linear model (GLM) based analysis, one sample T-test for each stimulation site. Significance: uncorrected p-value (p = 0.001), corrected p-value (pcorr < 0.05). Radiological convention. LDLPFC1, left dorsolateral prefrontal cortex #1; RDLPFC1, right dorsolateral prefrontal cortex #1; LDLPFC2, left dorsolateral prefrontal cortex #2; LF, Low frequency; R, right; L, left.
However, positive activation at the right insula and precentral gyrus was observed only when TMS was applied over the LDLPFC1, and activation at right putamen and caudate was observed only when it was applied over the LDLPFC2. Conversely, significant de-activation was observed at the right superior frontal gyrus when TMS was applied over the RDLPFC1.
Neither site differences, contrasting between the two left DLPFC sites (LDLPFC1—LDLPFC2), nor laterality differences, contrasting between the left and right DLPFC1 sites (LDLPFC1—RDLPFC1), were observed when using the GLM analysis method.
Discussion
The results of this study reveal an important mechanistic aspect of LF rTMS over the right DLPFC through activating the striatum and thalamus (CSTC loop) and the DMN. Our results suggest a potential mechanism of action of LF rTMS to the right DLPFC that has not been explored before, which may explain the positive outcome of LF rTMS when treating depression.
Low frequency repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex—treatment
The role of CSTC dysregulation in depression has been well-studied (Peters et al., 2016) and a recent meta-analysis revealed that an antidepressant response from rTMS could be predicted by baseline DMN network connectivity (Long et al., 2020). In regard to the effect of depression on relevant network connectivity, there is evidence for enhanced connectivity (Rubart et al., 2018) associated with increased stimulation-induced activation (Sheline et al., 2009) within the DMN. These changes within the DMN correlated with depression severity and feelings of hopelessness in patients with TRD (Grimm et al., 2009); besides, one study showed TMS successfully induced anticorrelated connectivity between the DLPFC and medial prefrontal DMN nodes (Liston et al., 2014).
The efficacy of LF rTMS applied to the right DLPFC has also been documented in patients with TRD. In a meta-analysis of eight studies (n = 263), 34.6% of patients receiving active stimulation were classified as remitters (resolution of symptoms) compared to 9.7% receiving sham stimulation (P < 0.0001) (Berlim et al., 2013). Similarly, in another study, LF (1 Hz) rTMS applied over the right DLPFC produced a clinically relevant reduction in depressive symptomatology for TRD patients comparable to venlafaxine ER, an oral antidepressant medication, in a 4-week, double-blind study (rTMS with placebo vs. venlafaxine ER with sham rTMS) (Bares et al., 2009).
In contrast, LF (1 Hz) stimulation [contrasted with high (17 Hz) frequency and sham stimulations] targeting the left DLPFC in TRD patients (n = 72) did not show significant differences in depression severity, when compared active vs. sham rTMS (Miniussi et al., 2005). However, in another study LF TMS (1 Hz), applied over the right DLPFC, had the same effect as HF TMS (20 Hz), applied over the left DLPFC (Isenberg et al., 2005). Moreover, both LF TMS to the right DLPFC and HF TMS to the left DLPFC reduced depression severity, as assessed by the Beck Depression Inventory (BDI-II) (Beck et al., 1961) and the Clinical Global Impression (Busner and Targum, 2007) of Change (CGIC) scores, suggesting that rTMS applied at LF over the right frontal cortex appears to be as effective for treatment of refractory depression as HF rTMS over the left frontal cortex.
Additionally, 1 Hz rTMS over the right DLPFC decreased total scores on the Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960) and the Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959), and was effective in 42.9% of TRD subjects in the sample (Pallanti et al., 2012). Furthermore, 1 Hz rTMS over the right DLPFC improved health-related quality of life in unipolar and bipolar TRD patients (Dumas et al., 2014), and also improved generalized anxiety disorder symptoms, such as excessive worry and depressive symptoms (Diefenbach et al., 2016).
Finally, a meta-analysis has shown that LF (≤1 Hz) rTMS applied to the right DLPFC was as effective as HF (10–20 Hz) rTMS applied to the left DLPFC, on treating major depressive disorder (MDD), however LF right-sided rTMS produced fewer side effects and had less risk for seizures; suggesting that its clinical applicability is more promising and should be explored further (Chen et al., 2013). Taken together, the efficacy of low frequency stimulation at the right DLPFC is evident and comparable to the standard high frequency stimulation at the left DLPFC. However, the neurobiological underpinnings of LF- right DLPFC TMS remain under investigation.
Implications of main findings
Our current study provides mechanistic understandings underlying the effectiveness of LF rTMS over the right DLPFC. The increased activity at the striatum and thalamus after LF rTMS to the right DLPFC, observed in this current work, was also observed in previous studies, after HF rTMS was applied to the left DLPFC (Strafella et al., 2001; Caparelli et al., 2022). These findings, in healthy volunteers, demonstrate the ability of TMS to stimulate the CSTC loop, either through LF to the right or the HF to left DLPFC, thus suggesting the modulation of the dysregulated dopamine reward circuitry in patients with MDD (Ko et al., 2008; Dunlop et al., 2016). It, therefore, justifies, at least in part, the significant efficacy of rTMS in attenuating symptom severity in patients with TRD. In addition, an increase in activity at the DMN was observed when LF rTMS was applied to the right DLPFC. Because DMN activity is considered a core component of pathological network dysfunction, since it compromises the ability for dynamic network change in response to changing demands in an otherwise healthy brain (Garnaat et al., 2018), our finding are in agreement with other studies that have reported that the positive outcome in treating depression through LF-TMS applied to the right DLPFC, is associated with the regulation of DMN activity by TMS (Sheline et al., 2009; Liston et al., 2014).
The observed increase in brain activity after LF rTMS to the right DLPFC is also in line with previous work, in which 1 Hz stimulation to the right DLPFC increased brain activation at the right DLPFC, during a decision-making gambling task, and improved anxiety, worry and depressive symptoms (Diefenbach et al., 2016). These, findings not only support the therapeutic effect of LF rTMS, but also demonstrate that LF rTMS does not always induce an inhibitory effect (Chen et al., 1997; Romero et al., 2002). In contrary, these results suggest that LF rTMS to the right DLPFC may have the effect of normalizing the imbalance of right and left prefrontal activity (Noda, 2021).
Secondary findings
Although no site effect was observed when contrasting the results of LF rTMS over the two sites for the left DLPFC, some results from the one-sample T-test analysis may indicate specific LF rTMS effects for each stimulation site. For example, LF rTMS over the LDLPFC1 induced a decrease in activity at amygdala, hippocampus, occipital cortex, angular gyrus, and temporal pole (ICs #16 and #17). This finding is supported by previous work that showed that 1 Hz rTMS over the left PFC at 100% of the MT decreased regional cerebral blood flow (rCBF) at right prefrontal cortex, left medial temporal cortex, left basal ganglia, and left amygdala (Speer et al., 2000). On the other hand, the increased activity at insula (IC#24) observed when LF rTMS was applied to the RDLPFC1 and LDLPFC2, seems to contradict previous finding, where in a SPECT study 1 Hz rTMS over the right PFC, in TRD patients, showed significant decreases in rCBF at the in the PFC, OFC, sgACC, globus pallidus, thalamus, anterior and posterior insula, and midbrain in the right hemisphere (Kito et al., 2011). In addition, our finding shows a decrease in brain activity at the SMA and motor areas (IC#18) when LF rTMS was applied to the RDLPFC1, which opposes previous finding that have shown a decrease in the rMT after patients received 1 Hz rTMS over the right DLPFC (Pallanti et al., 2012). Several factors may contribute to these seemingly contradicting results, for example, those findings were reported in TRD patients, and ours are in healthy controls. In addition, the stimulation site on the SPECT study was at the PFC at large, without specifically targeting the DLPFC. Furthermore, it should be noted that most of the LF rTMS studies cited here used 1 Hz rTMS and ours used 0.4 Hz rTMS, which may introduce some divergences on the findings. Further research is needed to delineate the effect of LF (0.4 Hz) rTMS over the right DLPFC on regional brain activation and network functional connectivity in TRD patients.
General linear model results
The GLM findings, although showing very few significant clusters, still reproduced the ICA findings in a shorter scale. For example, the activation observed at right putamen and caudate when rTMS was applied over the LDLPFC2 overlapped with the significant finding at IC#4, and the significant negative activation observed at the right superior frontal gyrus when rTMS was applied over the RDLPFC1 overlapped with the significant finding at IC#18. Overall, the complete overlap of the results between the two analysis methods strongly supports our findings.
Non-transcranial magnetic stimulation related findings
We observed increased activity in the auditory cortex for all three sites (IC#13 and #15). This effect, although weaker, was also observed in the GLM findings (Figure 4 and Supplementary Figure 1). This activity at the auditory cortex is probably induced by the acoustic noise produced by the TMS coil, when delivering pulses, not necessarily related to magnetic stimulation. Although these results show that the TMS pulse was not masked and therefore likely created an anticipatory effect since the pulses came at predictable times, these effects were eliminated when contrasting each pair of stimulation sites (two sample T-test), showing that the effect of auditory stimulation and its anticipation were removed when the sites were contrasted.
Limitations
The current study carries few limitations as described below.
(1) The lack of sham. The lack of sham is a limitation in this study, however, most of the non-magnetic stimulation sources that the sham stimulation intends to control, such as, TMS “tap” sensations and acoustic noise, were either mostly eliminated, when contrasting the brain activation from each pair of sites, or not significant, due to the study design. For example, the TMS acoustic noise, which mostly depend on the TMS frequency and intensity, were fixed within subjects. Consequently, the results for the IC# 15, which includes the auditory cortex, were not significant when contrasting two stimulation sites. The “tap” sensation, for the very low frequency (0.4 Hz), may have been subtle, since there were no major complaints about pain or discomfort during these low frequency sessions.
(2) Small sample size. Although, small sample size on simultaneous TMS-fMRI studies is not uncommon, and have proofed to find meaningful findings (Ruff et al., 2008; de Weijer et al., 2014; Hawco et al., 2017; Navarro de Lara et al., 2017; Dowdle et al., 2018; Vink et al., 2018; Luber et al., 2022), it still may represent a limitation on this study. Therefore, besides choosing an analysis streamline that minimize, as much as possible, the excessive use of the data, we focus our discussion on the results from ROI analysis defined by ICA, instead of the GLM (voxel-wise analysis) results, which provided very few significant activation clusters as shown on Figure 4. Consequently, while findings from this work should be considered preliminary, given the small to moderate sample size, some interesting hypotheses (as described above) were generated based on our current findings.
General linear model vs. independent component analysis
Traditional GLM analysis on block-design fMRI data is performed by convolving the boxcar stimulus with the canonical HRF to construct the expected brain response function. However, the canonical HRF was estimated based on task-induced fMRI signal changes and typically assumed spatially invariance across the brain, which might not be suitable to analyze TMS-induced brain response. Therefore, the analysis framework, developed in our previous work (Caparelli et al., 2022), was employed here to estimate the brain responses induced by the TMS “ON” blocks in different brain networks. The brain responses were estimated assuming the brain as a time-invariant system by averaging the fMRI time series across the TMS ON-OFF periods (stimulus-rest epochs). More specifically, the HRF for TMS stimulation was estimated using the TENT function, which extracted the impulse response function for the averaged stimulus-rest epoch from the data.
Compared with traditional GLM analysis, the ICA-based analysis used in this study, which employs the data-driven estimated HRF, was more sensitive on detecting TMS-induced brain activity changes by estimating the TMS-induced brain responses directly from the data and not assuming fixed responses across the brain networks.
Overall conclusion
In conclusion, here we showed that LF (0.4 Hz) rTMS to the right DLPFC has a similar top-down mechanism to, the previously reported, HF rTMS stimulation to the left DLPFC, which has shown to activate brain circuitry that are dysfunctional in MDD. This finding brings new insights into the mechanism behind LF rTMS to the right DLPFC and justifies its positive clinical outcomes. Finally, given the fewer side effects of LF rTMS when compared with HF rTMS, its clinical applicability is more promising.
Data availability statement
The datasets presented in this article are not readily available because the availability of any human data will follow local institutional regulations, where data was generated. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board panel of the National Institute on Drug Abuse. The patients/participants provided their written informed consent to participate in this study.
Author contributions
EC designed the study, carried the experimental setup, participated in data collection, data analysis, and manuscript preparation. OA participated in data collection and provided relevant inputs for the introduction and discussion. HG provided input in data analysis and relevant inputs on the interpretation of the results. TZ provided input in the study design and data analysis and participated in the experimental setup and some data collection. BS participated in data collection. YY provided input in the study design and data analysis and provided the financial support. All authors contributed to manuscript revision and approved the submitted version.
Funding
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA-IRP fund #: 1ZIA DA000469), the National Institutes of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.997259/full#supplementary-material
References
Anand, S., and Hotson, J. (2002). Transcranial magnetic stimulation: Neurophysiological applications and safety. Brain Cogn 50, 366–386.
Bares, M., Kopecek, M., Novak, T., Stopkova, P., Sos, P., Kozeny, J., et al. (2009). Low frequency (1-Hz), right prefrontal repetitive transcranial magnetic stimulation (rTMS) compared with venlafaxine ER in the treatment of resistant depression: A double-blind, single-centre, randomized study. J. Affect. Disord. 118, 94–100. doi: 10.1016/j.jad.2009.01.032
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., and Erbaugh, J. (1961). An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571. doi: 10.1001/archpsyc.1961.01710120031004
Berlim, M. T., Van den Eynde, F., and Jeff Daskalakis, Z. (2013). Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: A meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology 38, 543–551. doi: 10.1038/npp.2012.237
Busner, J., and Targum, S. D. (2007). The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry 4, 28–37.
Caparelli, E. C., Schleyer, B., Zhai, T., Gu, H., Abulseoud, O. A., and Yang, Y. (2022). High-frequency transcranial magnetic stimulation combined with functional magnetic resonance imaging reveals distinct activation patterns associated with different dorsolateral prefrontal cortex stimulation sites. Neuromodulation 25, 633–643. doi: 10.1016/j.neurom.2022.03.002
Chen, J., Zhou, C., Wu, B., Wang, Y., Li, Q., Wei, Y., et al. (2013). Left versus right repetitive transcranial magnetic stimulation in treating major depression: A meta-analysis of randomised controlled trials. Psychiatry Res. 210, 1260–1264. doi: 10.1016/j.psychres.2013.09.007
Chen, R., Classen, J., Gerloff, C., Celnik, P., Wassermann, E. M., and Hallett, M. (1997). Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48, 1398–1403. doi: 10.1212/wnl.48.5.1398
Chen, Z., Zhao, X., Fan, J., and Chen, A. (2018). Functional cerebral asymmetry analyses reveal how the control system implements its flexibility. Hum. Brain Mapp. 39, 4678–4688. doi: 10.1002/hbm.24313
Cho, S. S., and Strafella, A. P. (2009). rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PloS One 4:e6725. doi: 10.1371/journal.pone.0006725
Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173.
da Silva, M. C., Conti, C. L., Klauss, J., Alves, L. G., do Nascimento Cavalcante, H. M., Fregni, F., et al. (2013). Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J. Physiol. Paris 107, 493–502. doi: 10.1016/j.jphysparis.2013.07.003
de Weijer, A. D., Sommer, I. E., Bakker, E. J., Bloemendaal, M., Bakker, C. J., Klomp, D. W., et al. (2014). A setup for administering TMS to medial and lateral cortical areas during whole-brain FMRI recording. J. Clin. Neurophysiol. 31, 474–487. doi: 10.1097/wnp.0000000000000075
Diefenbach, G. J., Bragdon, L. B., Zertuche, L., Hyatt, C. J., Hallion, L. S., Tolin, D. F., et al. (2016). Repetitive transcranial magnetic stimulation for generalised anxiety disorder: A pilot randomised, double-blind, sham-controlled trial. Br. J. Psychiatry 209, 222–228. doi: 10.1192/bjp.bp.115.168203
Dowdle, L. T., Brown, T. R., George, M. S., and Hanlon, C. A. (2018). Single pulse TMS to the DLPFC, compared to a matched sham control, induces a direct, causal increase in caudate, cingulate, and thalamic BOLD signal. Brain Stimul. 11, 789–796. doi: 10.1016/j.brs.2018.02.014
Dumas, R., Boyer, L., Richieri, R., Guedj, E., Auquier, P., and Lançon, C. (2014). [Health-related quality of life assessment in depression after low-frequency transcranial magnetic stimulation]. Encephale 40, 74–80. doi: 10.1016/j.encep.2013.04.004
Dunlop, B. W., and Nemeroff, C. B. (2007). The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 64, 327–337. doi: 10.1001/archpsyc.64.3.327
Dunlop, K., Hanlon, C. A., and Downar, J. (2016). Noninvasive brain stimulation treatments for addiction and major depression. Ann. N. Y. Acad. Sci. 1394, 31–54. doi: 10.1111/nyas.12985
Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D., and Pascual-Leone, A. (2012). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry 72, 595–603. doi: 10.1016/j.biopsych.2012.04.028
Garnaat, S. L., Yuan, S., Wang, H., Philip, N. S., and Carpenter, L. L. (2018). Updates on Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder. Psychiatr. Clin. North Am. 41, 419–431. doi: 10.1016/j.psc.2018.04.006
Gaynes, B. N., Lloyd, S. W., Lux, L., Gartlehner, G., Hansen, R. A., Brode, S., et al. (2014). Repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis. J. Clin. Psychiatry 75:477–489;quiz489. doi: 10.4088/JCP.13r08815
George, M. S., Wassermann, E. M., Kimbrell, T. A., Little, J. T., Williams, W. E., Danielson, A. L., et al. (1997). Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: A placebo-controlled crossover trial. Am. J. Psychiatry 154, 1752–1756.
George, M. S., Wassermann, E. M., Williams, W. A., Callahan, A., Ketter, T. A., Basser, P., et al. (1995). Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 6, 1853–1856.
Gerfen, C. R. (2000). Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 23:S64–S70. doi: 10.1016/s1471-1931(00)00019-7
Goldstein, R. Z., and Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 12, 652–669. doi: 10.1038/nrn3119
Grace, A. A. (2016). Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17, 524–532. doi: 10.1038/nrn.2016.57
Grimm, S., Beck, J., Schuepbach, D., Hell, D., Boesiger, P., Bermpohl, F., et al. (2008). Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol. Psychiatry 63, 369–376. doi: 10.1016/j.biopsych.2007.05.033
Grimm, S., Boesiger, P., Beck, J., Schuepbach, D., Bermpohl, F., Walter, M., et al. (2009). Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology 34, 932–943. doi: 10.1038/npp.2008.81
Hamilton, M. (1959). The assessment of anxiety states by rating. Br. J. Med. Psychol. 32, 50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x
Hamilton, M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. doi: 10.1136/jnnp.23.1.56
Hawco, C., Armony, J. L., Daskalakis, Z. J., Berlim, M. T., Chakravarty, M. M., Pike, G. B., et al. (2017). Differing time of onset of concurrent tms-fmri during associative memory encoding: A measure of dynamic connectivity. Front. Hum. Neurosci. 11:404. doi: 10.3389/fnhum.2017.00404
Isenberg, K., Downs, D., Pierce, K., Svarakic, D., Garcia, K., Jarvis, M., et al. (2005). Low frequency rTMS stimulation of the right frontal cortex is as effective as high frequency rTMS stimulation of the left frontal cortex for antidepressant-free, treatment-resistant depressed patients. Ann. Clin. Psychiatry 17, 153–159. doi: 10.1080/10401230591002110
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., and Smith, S. M. (2012). FSL. Neuroimage 62, 782–790. doi: 10.1016/j.neuroimage.2011.09.015
Kito, S., Hasegawa, T., and Koga, Y. (2011). Neuroanatomical correlates of therapeutic efficacy of low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry Clin. Neurosci. 65, 175–182. doi: 10.1111/j.1440-1819.2010.02183.x
Ko, J. H., Monchi, O., Ptito, A., Bloomfield, P., Houle, S., and Strafella, A. P. (2008). Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task: A TMS-[(11)C]raclopride PET study. Eur. J. Neurosci. 28, 2147–2155. doi: 10.1111/j.1460-9568.2008.06501.x
Liston, C., Chen, A. C., Zebley, B. D., Drysdale, A. T., Gordon, R., Leuchter, B., et al. (2014). Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry 76, 517–526. doi: 10.1016/j.biopsych.2014.01.023
Long, Z., Du, L., Zhao, J., Wu, S., Zheng, Q., and Lei, X. (2020). Prediction on treatment improvement in depression with resting state connectivity: A coordinate-based meta-analysis. J. Affect. Disord. 276, 62–68. doi: 10.1016/j.jad.2020.06.072
Luber, B., Davis, S. W., Deng, Z.-D., Murphy, D., Martella, A., Peterchev, A. V., et al. (2022). Using diffusion tensor imaging to effectively target TMS to deep brain structures. NeuroImage 249:118863. doi: 10.1016/j.neuroimage.2021.118863
McClintock, S. M., Reti, I. M., Carpenter, L. L., McDonald, W. M., Dubin, M., Taylor, S. F., et al. (2018). Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J. Clin. Psychiatry 79:16cs10905. doi: 10.4088/JCP.16cs10905
Miniussi, C., Bonato, C., Bignotti, S., Gazzoli, A., Gennarelli, M., Pasqualetti, P., et al. (2005). Repetitive transcranial magnetic stimulation (rTMS) at high and low frequency: An efficacious therapy for major drug-resistant depression? Clin. Neurophysiol. 116, 1062–1071. doi: 10.1016/j.clinph.2005.01.002
Navarro de Lara, L. I., Tik, M., Woletz, M., Frass-Kriegl, R., Moser, E., Laistler, E., et al. (2017). High-sensitivity tms/fmri of the human motor cortex using a dedicated multichannel mr coil. Neuroimage 150, 262–269. doi: 10.1016/j.neuroimage.2017.02.062
Nitschke, J. B., Heller, W., Etienne, M. A., and Miller, G. A. (2004). Prefrontal cortex activity differentiates processes affecting memory in depression. Biol. Psychol. 67, 125–143. doi: 10.1016/j.biopsycho.2004.03.004
Noda, Y. (2021). Potential neurophysiological mechanisms of 1hz-tms to the right prefrontal cortex for depression: an exploratory tms-eeg study in healthy participants. J. Pers. Med. 11:68. doi: 10.3390/jpm11020068
Pallanti, S., Di Rollo, A., Antonini, S., Cauli, G., Hollander, E., and Quercioli, L. (2012). Low-frequency rtms over right dorsolateral prefrontal cortex in the treatment of resistant depression: cognitive improvement is independent from clinical response, resting motor threshold is related to clinical response. Neuropsychobiology 65, 227–235. doi: 10.1159/000336999
Peters, S. K., Dunlop, K., and Downar, J. (2016). Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front. Syst. Neurosci. 10:104. doi: 10.3389/fnsys.2016.00104
Polley, K. H., Navarro, R., Avery, D. H., George, M. S., and Holtzheimer, P. E. (2011). 2010 Updated avery-george-holtzheimer database of rtms depression studies. Brain Stimul. 4, 115–116. doi: 10.1016/j.brs.2010.09.001
Romero, J. R., Anschel, D., Sparing, R., Gangitano, M., and Pascual-Leone, A. (2002). Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin. Neurophysiol. 113, 101–107. doi: 10.1016/s1388-2457(01)00693-9
Rubart, A., Hohagen, F., and Zurowski, B. (2018). [Psychotherapy of depression as neurobiological process - evidence from neuroimaging]. Psychother. Psychosom. Med. Psychol. 68, 258–271. doi: 10.1055/a-0598-4972
Ruff, C. C., Bestmann, S., Blankenburg, F., Bjoertomt, O., Josephs, O., Weiskopf, N., et al. (2008). Distinct causal influences of parietal versus frontal areas on human visual cortex: Evidence from concurrent TMS-fMRI. Cereb. Cortex 18, 817–827. doi: 10.1093/cercor/bhm128
Sanchez-Gonzalez, M. A., Garcia-Cabezas, M. A., Rico, B., and Cavada, C. (2005). The primate thalamus is a key target for brain dopamine. J. Neurosci. 25, 6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005
Sheline, Y. I., Barch, D. M., Price, J. L., Rundle, M. M., Vaishnavi, S. N., Snyder, A. Z., et al. (2009). The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U.S.A. 106, 1942–1947. doi: 10.1073/pnas.0812686106
Speer, A. M., Kimbrell, T. A., Wassermann, E. M., Repella, D., Willis, M. W., Herscovitch, P., et al. (2000). Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol. Psychiatry 48, 1133–1141. doi: 10.1016/S0006-3223(00)01065-9
Strafella, A. P., Paus, T., Barrett, J., and Dagher, A. (2001). Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 21:RC157.
Terraneo, A., Leggio, L., Saladini, M., Ermani, M., Bonci, A., and Gallimberti, L. (2016). Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur. Neuropsychopharmacol. 26, 37–44. doi: 10.1016/j.euroneuro.2015.11.011
Vink, J. J. T., Mandija, S., Petrov, P. I., van den Berg, C. A. T., Sommer, I. E. C., and Neggers, S. F. W. (2018). A novel concurrent TMS-fMRI method to reveal propagation patterns of prefrontal magnetic brain stimulation. Hum. Brain Mapp. 39, 4580–4592. doi: 10.1002/hbm.24307
Wassermann, E. M. (1998). Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, june 5-7, 1996. Electroencephalogr. Clin. Neurophysiol. 108, 1–16. doi: 10.1016/s0168-5597(97)00096-8
Keywords: DLPFC, fMRI, low frequency, multimodality, TMS
Citation: Caparelli EdC, Abulseoud OA, Gu H, Zhai T, Schleyer B and Yang Y (2022) Low frequency repetitive transcranial magnetic stimulation to the right dorsolateral prefrontal cortex engages thalamus, striatum, and the default mode network. Front. Neurosci. 16:997259. doi: 10.3389/fnins.2022.997259
Received: 18 July 2022; Accepted: 16 September 2022;
Published: 30 September 2022.
Edited by:
Oscar Arias-Carrion, Hospital General Dr. Manuel Gea González, MexicoReviewed by:
Jonas Persson, Uppsala University, SwedenColleen Mills-Finnerty, United States Department of Veterans Affairs, United States
Copyright © 2022 Caparelli, Abulseoud, Gu, Zhai, Schleyer and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabeth de Castro Caparelli, elisabeth.caparelli@nih.gov
 Elisabeth de Castro Caparelli
Elisabeth de Castro Caparelli Osama A. Abulseoud
Osama A. Abulseoud Hong Gu
Hong Gu Tianye Zhai
Tianye Zhai Brooke Schleyer
Brooke Schleyer Yihong Yang
Yihong Yang