- Department of Neurology, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
A serious complication of acute ischemic stroke (AIS) after mechanical thrombectomy (MT) is hemorrhagic transformation (HT), which is potentially associated with clinical deterioration. This study examined predictors of HT following MT in AIS patients. Patients with AIS due to large artery occlusion in the anterior circulation, treated with MT and successfully recanalized (modified Thrombolysis in Cerebral Infarction score 2b/3), were studied retrospectively. HT was evaluated by computed tomography (CT) 24 h after MT and was diagnosed and classified into parenchymal hematoma (PH) and hemorrhagic infarction (HI). Multivariate logistic regression models were used to determine the risk factors for HT. Receiver operating characteristic (ROC) curve analysis was performed to determine the predictive utility of risk factors for HT. We enrolled 135 patients: 49 in the HT group and 86 in the non-HT group. The two groups differed significantly in baseline fibrinogen levels (p = 0.003) and platelet counts (p = 0.006). Multivariate logistic regression analyses showed that lower fibrinogen levels [odds ratio (OR), 0.41; 95% CI, 0.23–0.72; p = 0.002] and platelet counts (OR, 0.58; 95% CI, 0.33–0.99; p = 0.048) were independently associated with a higher risk of HT. Together, the binary variates fibrinogen and platelets well-predicted HT (area under the curve, 0.703; specificity, 77.9%; sensitivity, 55.1%). The combination of fibrinogen <2.165 g/L and platelets <171.5 × 109/L was the strongest predictor of HT (OR, 23.17; 95% CI, 5.75–126.80; p < 0.0001). Our study suggests that lower baseline fibrinogen levels and platelet counts may be risk factors for HT in AIS patients following MT and reperfusion. Specifically, the combination of fibrinogen level and platelet count may predict the risk of HT after MT in these patients.
Introduction
Acute ischemic stroke (AIS) is the leading cause of long-term disability in developed countries and the leading cause of mortality worldwide (1). Mechanical thrombectomy (MT) has become the standard of care for patients with acute intracranial large-vessel occlusion. With the DIFFUSE 3 and DAWN trials extending the time window to up to 24 h, more AIS patients are now eligible for MT (2, 3). Hemorrhagic transformation (HT), a common and severe complication, is usually associated with a poor functional outcome, or even death, after MT and has a reported incidence of up to 46.1% in clinical MT trials (4). Therefore, identifying risk factors for HT could help guide patient selection for MT, which will improve procedural safety and clinical outcomes.
Studies have examined possible risk factors for HT in the setting of MT in AIS patients. Li et al. (5) found that a higher National Institutes of Health Stroke Scale (NIHSS) score, increased systolic blood pressure, history of coronary heart disease, and use of intravenous thrombolysis or oral anti-platelet or anticoagulation drugs were associated with HT in patients undergoing MT. Moreover, ischemic volume, cerebral collateral circulation, baseline Alberta Stroke Program Early CT Score (ASPECTS), and delayed endovascular treatment are associated with an increased risk of HT after MT (6–8). However, most of these risk factors are assessed using clinical and imaging data (9) that are complex and subjective. Hence, it is necessary to identify blood biomarkers that can accurately predict HT after MT.
Studies of blood biomarkers have shown that blood glucose, lipid profiles, bilirubin, aminotransferase, alkaline phosphatase, globulin, biomarkers of disruption of the blood–brain barrier (BBB) (10), inflammation and oxidative stress (11), vasoreactivity (12), and coagulation/fibrinolysis disorder (13–15) are associated with HT in AIS patients (16). These biomarkers may reflect the pathophysiology of HT. However, most of these studies are on thrombolysis treatments, and there are limited data on blood biomarkers and the clinical relevance of HT in the setting of MT.
Platelet and fibrinogen are well-known biomarkers of the coagulation system. Fibrinogen level and platelet counts are proven to be associated with HT in AIS patients after thrombolysis (14, 17, 18). However, research about biomarkers and HT after AIS in the setting of MT is relatively less. Therefore, this study examined blood biomarkers that predict HT in AIS patients after reperfusion to provide reference data facilitating patient selection for MT.
Materials and Methods
Study Population
This study recruited 135 AIS patients who had undergone MT and recanalization at the Department of Neurology, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine between October 2012 and May 2018. The inclusion criteria were a diagnosis of AIS confirmed by computed tomography (CT) or diffusion-weighted imaging (DWI), acute anterior circulation occlusion determined by CT angiography (CTA) or digital subtraction angiography (DSA), MT performed within 24 h of symptom onset following reperfusion graded using the modified Thrombolysis in Cerebral Infarction (mTICI) scale (2b/3) with or without intravenous thrombolysis, and routine blood tests before MT and follow-up CT 24 h after MT. Patients were excluded if they had no clinical or laboratory information or follow-up CT imaging.
Data Collection
Data on sex, age, history of stroke or transient ischemic attack (TIA), and vascular disease risk factors (e.g., smoking, drinking, hypertension, diabetes mellitus, coronary heart disease, and atrial fibrillation) were recorded. Stroke subtypes were based on the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification and included large-artery atherosclerosis (LAA), cardioembolism (CE), stroke of other determined cause (ODC), and stroke of undetermined etiology (SUE) (19). Blood pressure was recorded on admission. Neurological deficits were evaluated at baseline, i.e., preoperatively, using the NIHSS. Disability was assessed by the modified Rankin Scale (mRS) score at 90 days after MT. mRS ≤2 was considered a good clinical outcome, while mRS ≥3 was considered a bad clinical outcome.
Imaging parameters included the baseline ASPECTS (20). All patients underwent non-contrast CT and CTA before MT. HT was determined based on 24-h post-interventional non-contrast CT. The CT reading process was performed by two neurologists with more than 2-year working experience in our department. HT was diagnosed and classified into parenchymal hematoma (PH) and hemorrhagic infarction (HI) according to the recommendations of the European Cooperative Acute Stroke Study (ECASS)II classification (21). We had a uniform standard distinction between contrast extravasation after MT and HT. Hyperdensity meeting the following standards was considered to be HT: 1. Hyperdensity with Hounsfield units (HU) <90; 2. Hyperdensity persisting longer than 24 h and/or create mass effect with a hypoattenuation rim; 3. After 24 h, there was still visible hyperdensity on CT (22). Blood samples were obtained before treatments including MT and intravenous thrombolysis and included glucose level, routine blood counts, coagulation function, renal function, electrolytes, and myocardial enzymes. Treatments included intravenous thrombolysis and anticoagulant agents. The procedure time was recorded.
Endovascular Therapy
Patients were eligible for MT if acute occlusion of the anterior circulation was diagnosed by CTA. Some of the patients within the time window for intravenous thrombolysis, and without contraindications, were given intravenous alteplase as bridging therapy. Patients were treated directly with MT if there was any contraindication to thrombolysis or a heavy thrombus burden. After successful local anesthesia, the patient's femoral artery was punctured to determine the occlusion site. Using a coaxial catheter, the tip of a microcatheter (Rebar™ 21/27; EV3, USA) was placed at the distal end of the occluded artery under micro-guidewire guidance. Solitaire™ AB embolization stents (EV3) 4–6 mm in diameter and 15–30 mm long were selected, according to the diameter of the occluded blood vessels. The stent was introduced into the distal end of the occlusion and then released. Then, contrast agent was injected for visualization, and negative pressure was applied to the catheter to withdraw the stent slowly and remove the thrombus. Recanalization of the main arteries was confirmed by reexamination showing thrombus removal. Evaluation of the grade of recanalization was based on mTICI grade, which is defined as: 0, No perfusion; 1, Minimal flow past the occlusion with little to no perfusion; 2a, Antegrade partial perfusion of less than half of the downstream ischemic territory; 2b, Antegrade partial perfusion of half or greater of the downstream ischemic territory; 3, Antegrade complete perfusion of the downstream ischemic territory. The mTICI scores of 2b/3 were considered recanalization after MT (23).
Statistical Analyses
Quantitative data were provided as medians and interquartile range (IQR) and categorical variables as frequencies and percentages. Differences in baseline characteristics between the non-HT and HT groups were compared using the Mann–Whitney U-test for quantitative data and Pearson chi-square test for categorical variables. Univariate and subsequent multivariate logistic regression analyses were performed to assess the independent risk factors for HT, with adjustment for potential confounders. Potential risk factors of HT including clinical characteristics, vascular disease history, blood biomarkers, intravenous thrombolysis, and treatment time were selected from baseline characteristics as variables in univariate logistic regression analysis. After adjusting for potential confounders, including sex, age, history of smoking and drinking, disease history of hypertension, diabetes mellitus, stroke, coronary artery disease, and atrial fibrillation, all the other factors were selected to perform multivariate logistic regression analysis. Receiver operating characteristic (ROC) curve analyses were performed to investigate the HT prediction efficacy of combinations of binary variates. The cutoff values for binary variates were determined by Youden's index (J) = (Sensitivity + specificity – 1). The point corresponding to the maximum Youden's index was considered a cutoff value. Statistical analyses were performed using SPSS software (ver. 22.0; IBM Corp., Armonk, NY, USA). p-values <0.05 were considered statistically significant.
Results
Baseline Characteristics and Clinical Outcome of the Study Population
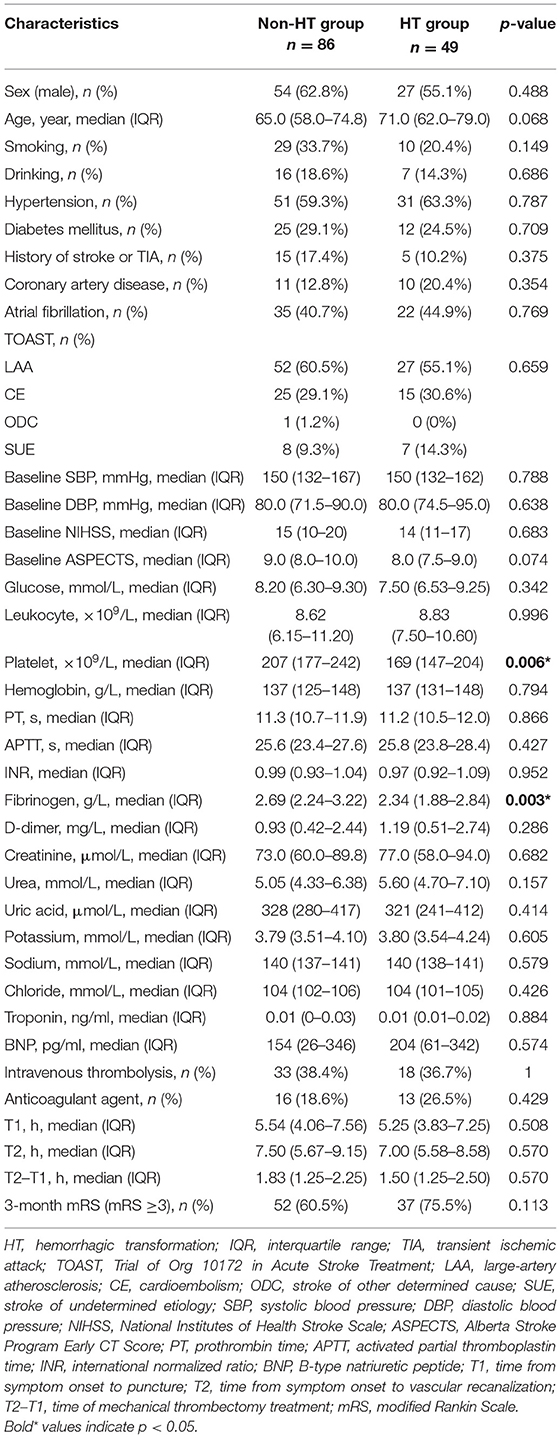
Ultimately, this study enrolled 135 AIS patients treated with MT following reperfusion. HT was diagnosed in 49 patients (36.3%) within 24 h after MT. Table 1 compared the baseline characteristics and clinical outcome of the HT and non-HT groups. The patients with HT had significantly lower fibrinogen levels (p = 0.003) and platelet counts (p = 0.006), but there were no group differences in the other baseline characteristics and clinical outcome. Table 2 compared the baseline characteristics and clinical outcome of the HI and PH subgroups. Among the 49 HT patients, 29 patients were diagnosed as HI and 20 patients as PH. There was no significant difference in baseline characteristics of the HI and PH groups. But the clinical outcome of the PH group was significantly worse than that of the HI group.
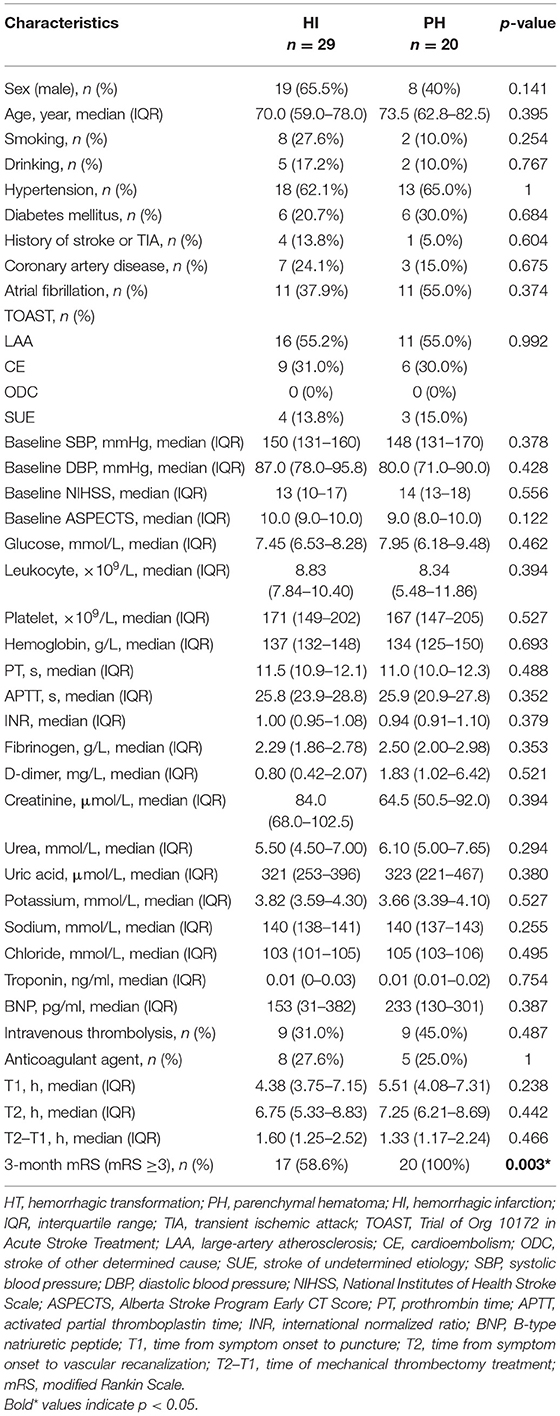
Table 2. Comparison of the baseline characteristics and clinical outcome according to the subcategorized groups of HT.
Risk Factors for Hemorrhagic Transformation in Acute Ischemic Stroke Patients Treated With Mechanical Thrombectomy
To identify the risk factors for HT in AIS patients after MT, we conducted univariate and multivariate logistic regression analyses (shown in Table 3). After adjusting for potential confounders, including sex, age, and disease history, lower baseline fibrinogen level (OR, 0.41; 95% CI, 0.23–0.72; p = 0.002) and platelet count (OR, 0.58; 95% CI, 0.33–0.99; p = 0.048) were independently associated with higher odds of HT.
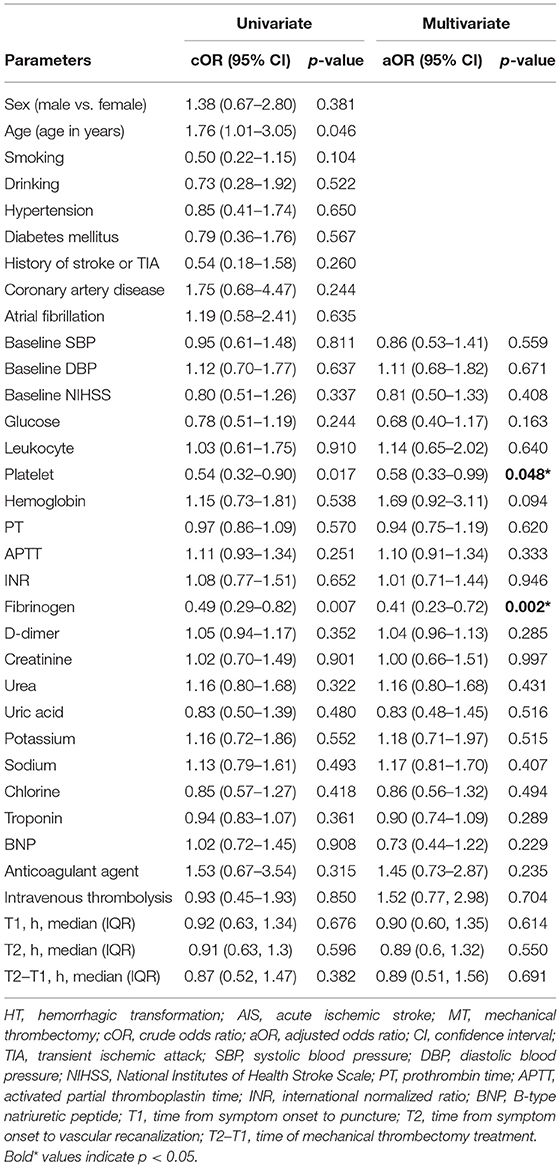
Table 3. Univariate and multivariate logistic regression analyses of risk factors for HT in AIS patients with MT.
Predictors of Hemorrhagic Transformation in Acute Ischemic Stroke Patients Treated With Mechanical Thrombectomy
Following logistic regression analysis of HT, we performed ROC curve analysis to evaluate the predictive utility of fibrinogen and platelets. We found that the area under the curve (AUC) for fibrinogen was 0.654 (95% CI, 0.557–0.751), and the cutoff value was 2.165 g/L. The AUC for platelets was 0.643 (95% CI, 0.544–0.743), and the cutoff value was 171.5 × 109/L. The fibrinogen and platelet cutoffs together predicted HT with an AUC of 0.703, specificity of 77.9%, and sensitivity of 55.1%. The positive predictive value was 58.7%, and the negative predictive value was 75.3% (shown in Figure 1).
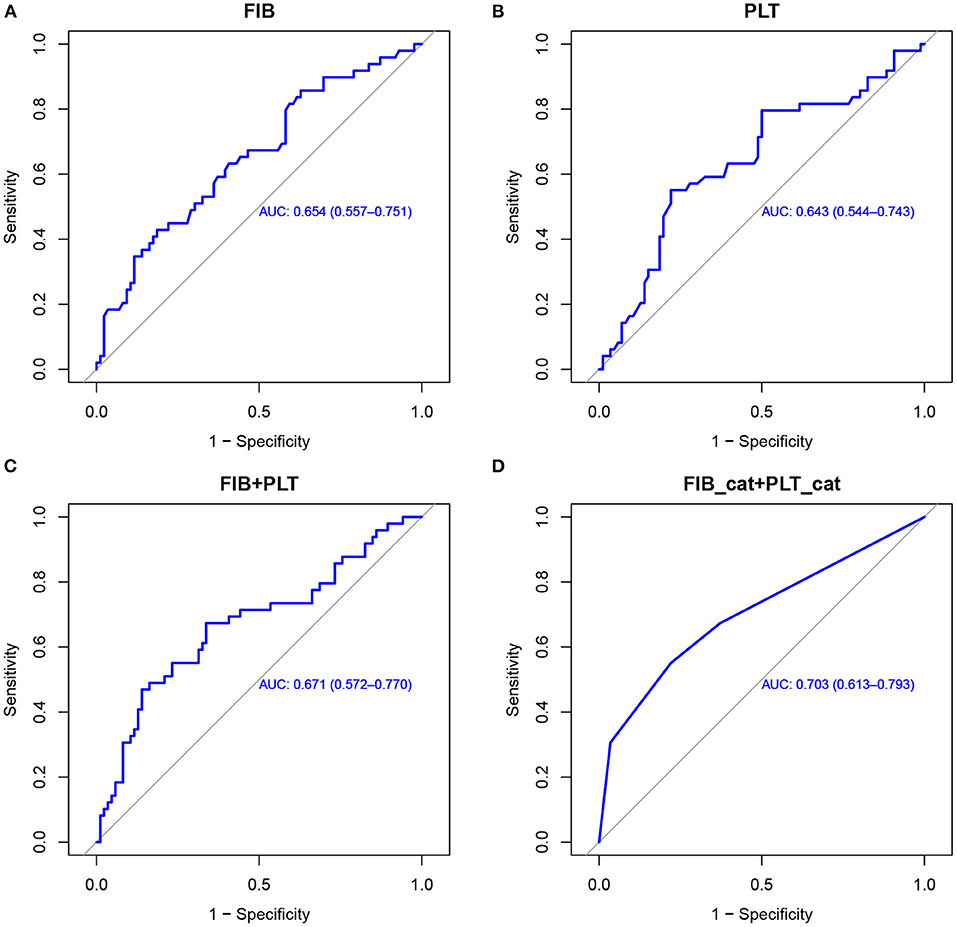
Figure 1. ROC curves used to evaluate the predictive utility of risk factors for HT in AIS patients with MT. Utility of fibrinogen levels (A), platelet counts (B), fibrinogen combined with platelets (C), and a combination of the binary variates fibrinogen (2.165 g/L) and platelets (171.5 × 109/L) (D) for predicting HT. ROC, receiver operating characteristic; HT, hemorrhagic transformation; AIS, acute ischemic stroke; MT, mechanical thrombectomy; FIB, fibrinogen; PLT, platelets.
Figure 2 and Table 4 showed the predictive power of the binary variates fibrinogen and platelets for HT after MT. Setting fibrinogen ≥2.165 g/L and platelets ≥171.5 × 109/L as references, the combination of fibrinogen ≥2.165 g/L and platelets ≥171.5 × 109/L predicted the lowest risk for HT, while the combination of fibrinogen <2.165 g/L and platelets <171.5 × 109/L was the strongest predictor of HT (OR, 23.17; 95% CI, 5.75–126.80; p < 0.0001) (shown in Figure 2; Table 4).
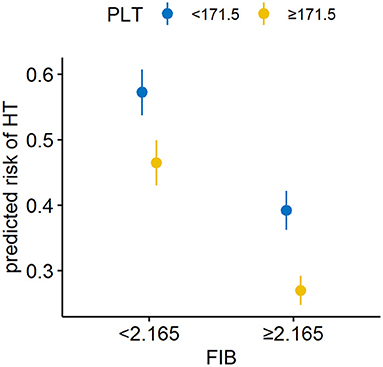
Figure 2. Combined utility of the binary variates fibrinogen and platelets for predicting HT in AIS patients with MT. The combination of a fibrinogen level <2.165 g/L and platelet count <171.5 × 109/L was the strongest predictor of HT. HT, hemorrhagic transformation; AIS, acute ischemic stroke; MT, mechanical thrombectomy; FIB, fibrinogen; PLT, platelets.
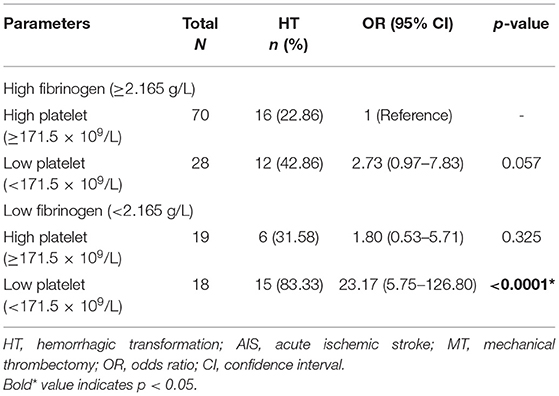
Table 4. Combined utility of the binary variates fibrinogen and platelets for predicting HT in AIS patients with MT.
Discussion
In 2015, five randomized controlled trials demonstrated the superiority of MT for AIS caused by large-vessel occlusion in the anterior circulation (24). However, the incidence of HT after endovascular treatment varies from 46.0 to 49.5% (4, 8). Based on the radiological appearance, HT is roughly categorized as HI or PH (21). Symptomatic intracerebral hemorrhage (sICH) is defined as a PH2 hematoma with significant clinical deterioration caused by bleeding (25). The rate of sICH in the MT group was 7% in the DIFFUSE 3 trial and 6% in the DAWN trial (2, 3). In our study, HT occurred in 36.3% of the enrolled patients, similar to other studies (4, 8). We roughly categorize HT subtypes into HI and PH. However, we did not differentiate sICH from asymptomatic intracerebral hemorrhage or PH2 from other subtypes of HT since sICH and PH2 may have more clinical implications. Further study should identify more detailed classification to improve clinical utility.
An important finding of this study was the independent effects of decreased fibrinogen levels and platelet counts on hemorrhagic complications in AIS patients who underwent MT following reperfusion. Fibrinogen and platelets are important for hemostatic function, which indicates that there is a relationship between HT and coagulation/fibrinolysis disorder. Bleeding is more likely to occur in conjunction with a coagulation/fibrinolysis disorder; therefore, biomarkers of this system may serve as early predictors of HT incidence in AIS patients with MT (16).
Our study found that lower baseline fibrinogen levels were related to a higher risk of HT after MT. There are limited data on the relationship between fibrinogen and HT in the setting of MT, and most were obtained in the setting of thrombolysis. Wang et al. (17) found that fibrinogen <1.50 g/L was a risk factor for HT after thrombolysis. Conversely, in most studies, a higher baseline fibrinogen level was related to a higher likelihood of HT in AIS patients undergoing thrombolysis (14). Another study found that pre- and post-thrombolysis variation of fibrinogen >200 mg/dl was an independent predictor of sICH (26). Vandelli et al. (27) suggested that a decrease in post-thrombolysis fibrinogen levels of <2 g/L, or of ≥25%, was a risk factor for intracerebral hemorrhage. Yan et al. (28) found that an early decrease in fibrinogen levels was related to sICH after reperfusion therapy with thrombolysis, with or without endovascular thrombectomy. The above studies were based on thrombolysis treatments. Thrombolytic drugs themselves may affect fibrinogen levels (29). Our results indicated that lower baseline fibrinogen levels were associated with a higher risk of HT after MT, which may be attributed to the effect of thrombectomy. Lower preoperative fibrinogen plasma concentration was proven to be associated with excessive bleeding (usually defined as the amount of chest tube drainage after surgery) after cardiac operations in multiple studies (30–32). Fibrinogen is a key protein in the coagulation cascade and thus a potential biomarker for bleeding (33). Under normal pathological conditions, stable blood clot formation is the result of thrombin cleavage of fibrinogen into fibrin. As the amount of insoluble fibrin increases, factor XIII cross-links fibrin monomers forming the matrix for blood clot formation. During the coagulation cascade, fibrinogen concentration is depleted as it is cleaved into fibrin. A lower fibrinogen level may not ensure an appropriate coagulation during and after major surgical procedures (32). To our knowledge, this is the first study of the relationship between preoperative fibrinogen levels and HT in the setting of MT. The patients enrolled in our study underwent MT with or without thrombolysis. Therefore, future study should divide the patients receiving MT into thrombolysis and non-thrombolysis subgroups. It is important to obtain baseline fibrinogen levels and then monitor them during follow-up.
Our study also showed that lower baseline platelet counts were associated with a higher risk of HT after MT. The role of platelet count as a predictor of HT after thrombolysis has been investigated in studies with conflicting results. Some indicated that a lower platelet count does not significantly increase the risk of HT (34, 35). On the contrary, a lower baseline platelet count was suggested to be associated with an increased risk of HT after thrombolysis in another study (18), and clinical guidelines from the American Heart Association/American Stroke Association published in 2018 did not recommend reperfusion therapy in patients with platelets <100,000/mm3 (36). However, only a few studies have mentioned HT in the AIS patients treated with MT. Monch et al. (37) were the first ones to reveal that there was no clear association between initial thrombocytopenia (TP), a decline of platelet counts (DPC), and sICH. A recent study also showed no association between pre-procedural platelet count and sICH after thrombectomy (38). Thus, the results of the two studies are contradictory to ours. However, it is difficult to compare these studies due to variations in patient selection, study design, and complicated situation of thrombectomy procedure. Therefore, more research on platelet and HT in the setting of MT will be necessary. Also, considering that platelets have a high turnover during MT, therefore, follow-up of platelet count drop will be needed in future study. Platelets are small blood cells traditionally known for their role in hemostasis. Except for its hemostatic function, platelet also plays an important role in inflammation, angiogenesis, and controlled apoptosis following tissue damage. The term “platelet activation” is used to describe numerous processes, including changes in shape, upregulation of distinct surface molecules, protein synthesis from mRNA and exocytosis, and the release of granule contents. Upregulation of large receptor allows platelets to interact with almost every type of immune cell to mediate immune responses. Activated platelets also secrete a vast range of pro- and anti-inflammatory mediators. Platelets are an abundant source of growth factors, which recruit progenitor cells to the sites of injury to promote angiogenesis, specific regeneration, and tissue remodeling (39). FasL-induced apoptosis has been shown to serve as a controlled way to eliminate inflammation and prevent spreading of inflammatory responses within the eye (40). Blocking of FasL AND platelet depletion led to a decrease in apoptosis of neuronal tissue in models of stroke, suggesting that platelets are an important contributor to the prevention of uncontrolled cell death across tissues including the brain (41). Therefore, we supposed that platelet depletion may play an important role in HT in AIS undergoing MT by decreasing the “platelet activation” process, thus reducing the abilities of hemostasis, inflammatory response, angiogenesis, and tissue repair.
We also derived cutoff points for fibrinogen and platelets, which accurately predicted the risk of HT in AIS after MT. The specificity (77.9%) and sensitivity (55.1%) were best with a cutoff of <2.165 g/L for fibrinogen and <171.5 × 109/L for platelets.
Some limitations of this study must be acknowledged. First, this was a single-center retrospective study with a relatively small sample size, and cause–effect relationships could not be inferred. Multicenter prospective studies are necessary to establish causality and provide more reliable long-term prognostic information. Second, our study roughly categorized HT subtypes into HI and PH; more detailed classification should be applied in future study. Third, due to the effect of fibrinogen depletion, further study is needed to obtain baseline and follow-up fibrinogen data. Fourth, since patients were treated between 2012 and 2018, processes and devices deployed after evidence in favor of MT published in 2015, the patients presumably contained heterogeneity. Therefore, future study will not include patients undergoing MT before 2015. Fifth, the relatively low AUC, specificity, sensitivity, and positive and negative predictive values of fibrinogen and platelet cutoffs together were detected, which may be due to the small sample size. Therefore, larger sample-size studies are needed to validate the result of the present study. Last but not least, we did not conduct further external validation of the model, which leaves this study remaining an exploratory approach on the descriptive level. The patients were enrolled independently from the two hospital districts of our department, which is under independent operation and management from the aspect of doctors, treatments, and laboratory equipment. Therefore, this study was a multicenter analysis theoretically to some extent. However, lacking external validation of the model is one of the greatest limitations of our present study. Therefore, further multicenter prospective research is needed to validate the model in this study.
Conclusion
Lower baseline fibrinogen levels and platelet counts are associated with HT in AIS patients with anterior circulation large-vessel occlusion after MT. The risk of HT after MT can be predicted by a fibrinogen level <2.165 g/L together with a platelet count of <171.5 × 109/L. Platelet counts and fibrinogen levels as circulatory biomarkers are commonly checked before MT procedure in the emergency room. Therefore, it is feasible that the pre-procedural fibrinogen level and platelet counts may be used as biomarkers to identify patients with an increased risk of HT after MT in AIS patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Independent Ethics Committee of Shanghai Ninth People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JL conceived the study. JS designed the study and helped revise the manuscript. CL and HP analyzed all data and prepared the drafting of the article. YQ and PH contributed to the acquisition of clinical data. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by grants from the National Natural Science Foundation of China (81271302 to JL; 82071282 to JS); the research-oriented physicians project II from Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20161422 to JL); the Clinical Research Project from Shanghai Jiao Tong University School of Medicine (DLY201614 to JL); the Biomedicine Key program from Shanghai Municipal Science and Technology Commission (16411953100 to JL); the Rare Disease Registration Platform of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine (JYHJB08 to JS); and the Horizontal Research Project from Shanghai Ninth People's Hospital (JYHX2021001 to JS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge the specialist editors with suitable professional knowledge who provided professional services for reviewing this manuscript.
References
1. Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ. (2020) 368:l6983. doi: 10.1136/bmj.l6983
2. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New Eng J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
3. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. New Eng J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
4. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. (2016) 15:1138–47. doi: 10.1016/S1474-4422(16)30177-6
5. Li W, Xing X, Wen C, Liu H. Risk factors and functional outcome were associated with hemorrhagic transformation after mechanical thrombectomy for acute large vessel occlusion stroke. J Neurosurg Sci. (2020). doi: 10.23736/S0390-5616.20.05141-3. [Epub ahead of print].
6. Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. (2011) 42:2235–9. doi: 10.1161/STROKEAHA.110.604603
7. Raychev R, Saver JL, Jahan R, Nogueira RG, Goyal M, Pereira VM, et al. The impact of general anesthesia, baseline ASPECTS, time to treatment, and IV tPA on intracranial hemorrhage after neurothrombectomy: pooled analysis of the SWIFT PRIME. SWIFT, and STAR trials. J Neurointerv Surg. (2020) 12:2–6. doi: 10.1136/neurintsurg-2019-014898
8. Hao Y, Yang D, Wang H, Zi W, Zhang M, Geng Y, et al. Predictors for symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke. Stroke. (2017) 48:1203–9. doi: 10.1161/STROKEAHA.116.016368
9. Álvarez-Sabín J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol. (2013) 12:689–705. doi: 10.1016/S1474-4422(13)70055-3
10. Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. (2004) 56:468–77. doi: 10.1002/ana.20199
11. Wang W, Li M, Chen Q, Wang J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: mechanisms, models, and biomarkers. Mol Neurobiol. (2015) 52:1572–9. doi: 10.1007/s12035-014-8952-x
12. Kazmierski R, Michalak S, Wencel-Warot A, Nowinski WL. Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology. (2012) 79:1677–85. doi: 10.1212/WNL.0b013e31826e9a83
13. Prodan CI, Stoner JA, Cowan LD, Dale GL. Lower coated-platelet levels are associated with early hemorrhagic transformation in patients with non-lacunar brain infarction. J Thromb Haemost. (2010) 8:1185–90. doi: 10.1111/j.1538-7836.2010.03851.x
14. Xu X, Li C, Wan T, Gu X, Zhu W, Hao J, et al. Risk factors for hemorrhagic transformation after intravenous thrombolysis in acute cerebral infarction: a retrospective single-center study. World Neurosurg. (2017) 101:155–60. doi: 10.1016/j.wneu.2017.01.091
15. Christoforidis GA, Karakasis C, Mohammad Y, Caragine LP, Yang M, Slivka AP. Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: the role of pial collateral formation. Am J Neuroradiol. (2009) 30:165–70. doi: 10.3174/ajnr.A1276
16. Lu G, He Q, Shen Y, Cao F. Potential biomarkers for predicting hemorrhagic transformation of ischemic stroke. Int J Neurosci. (2018) 128:79–89. doi: 10.1080/00207454.2017.1349766
17. Wang R, Zeng J, Wang F, Zhuang X, Chen X, Miao J. Risk factors of hemorrhagic transformation after intravenous thrombolysis with rt-PA in acute cerebral infarction. QJM. (2019) 112:323–6. doi: 10.1093/qjmed/hcy292
18. Gensicke H, Al Sultan AS, Strbian D, Hametner C, Zinkstok SM, Moulin S, et al. Intravenous thrombolysis and platelet count. Neurology. (2018) 90:e690–e7. doi: 10.1212/WNL.0000000000004982
19. Ornello R, Degan D, Tiseo C, Di Carmine C, Perciballi L, Pistoia F, et al. Distribution and temporal trends from 1993 to 2015 of ischemic stroke subtypes: a systematic review and meta-analysis. Stroke. (2018) 49:814–9. doi: 10.1161/STROKEAHA.117.020031
20. Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. (2005) 36:2400–3. doi: 10.1161/01.STR.0000185698.45720.58
21. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet (London, England). (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
22. Yedavalli V, Sammet S. Contrast extravasation versus hemorrhage after thrombectomy in patients with acute stroke. J Neuroimag. (2017) 27:570–6. doi: 10.1111/jon.12446
23. Yoo AJ, Simonsen CZ, Prabhakaran S, Chaudhry ZA, Issa MA, Fugate JE, et al. Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke. (2013) 44:2509–12. doi: 10.1161/STROKEAHA.113.001990
24. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet (London, England). (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
25. Charbonnier G, Bonnet L, Biondi A, Moulin T. Intracranial bleeding after reperfusion therapy in acute ischemic stroke. Front Neurol. (2020) 11:629920. doi: 10.3389/fneur.2020.629920
26. Matosevic B, Knoflach M, Werner P, Pechlaner R, Zangerle A, Ruecker M, et al. Fibrinogen degradation coagulopathy and bleeding complications after stroke thrombolysis. Neurology. (2013) 80:1216–24. doi: 10.1212/WNL.0b013e3182897015
27. Vandelli L, Marietta M, Gambini M, Cavazzuti M, Trenti T, Cenci MA, et al. Fibrinogen decrease after intravenous thrombolysis in ischemic stroke patients is a risk factor for intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2015) 24:394–400. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.005
28. Yan S, Zhang X, Zhang R, Xu J, Lou M. Early fibrinogen depletion and symptomatic intracranial hemorrhage after reperfusion therapy. Stroke. (2019) 50:2716–21. doi: 10.1161/STROKEAHA.119.025711
29. Rao AK, Pratt C, Berke A, Jaffe A, Ockene I, Schreiber TL, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial–phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. (1988) 11:1–11. doi: 10.1016/0735-1097(88)90158-1
30. Waldén K, Jeppsson A, Nasic S, Backlund E, Karlsson M. Low preoperative fibrinogen plasma concentration is associated with excessive bleeding after cardiac operations. Ann Thorac Surg. (2014) 97:1199–206. doi: 10.1016/j.athoracsur.2013.11.064
31. Alagha S, Songur M, Avci T, Vural K, Kaplan S. Association of preoperative plasma fibrinogen level with postoperative bleeding after on-pump coronary bypass surgery: does plasma fibrinogen level affect the amount of postoperative bleeding? Interact Cardiovasc Thorac Surg. (2018) 27:671–6. doi: 10.1093/icvts/ivy132
32. Karlsson M, Ternstrom L, Hyllner M, Baghaei F, Nilsson S, Jeppsson A. Plasma fibrinogen level, bleeding, and transfusion after on-pump coronary artery bypass grafting surgery: a prospective observational study. Transfusion. (2008) 48:2152–8. doi: 10.1111/j.1537-2995.2008.01827.x
33. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. (2005) 3:1894–904. doi: 10.1111/j.1538-7836.2005.01365.x
34. Breuer L, Huttner HB, Kiphuth IC, Ringwald J, Hilz MJ, Schwab S, et al. Waiting for platelet counts causes unsubstantiated delay of thrombolysis therapy. Eur Neurol. (2013) 69:317–20. doi: 10.1159/000345702
35. Cucchiara BL, Jackson B, Weiner M, Messe SR. Usefulness of checking platelet count before thrombolysis in acute ischemic stroke. Stroke. (2007) 38:1639–40. doi: 10.1161/STROKEAHA.106.480889
36. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–e418. doi: 10.1161/STR.0000000000000211
37. Monch S, Boeckh-Behrens T, Kreiser K, Blum P, Hedderich D, Maegerlein C, et al. Thrombocytopenia and declines in platelet counts: predictors of mortality and outcome after mechanical thrombectomy. J Neurol. (2019) 266:1588–95. doi: 10.1007/s00415-019-09295-z
38. Venditti L, Chassin O, Ancelet C, Legris N, Sarov M, Lapergue B, et al. Pre-procedural predictive factors of symptomatic intracranial hemorrhage after thrombectomy in stroke. J Neurol. (2021) 268:1867–75. doi: 10.1007/s00415-020-10364-x
39. Leiter O, Walker TL. Platelets: the missing link between the blood and brain? Prog Neurobiol. (2019) 183:101695. doi: 10.1016/j.pneurobio.2019.101695
40. Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. (1995) 270:1189–92. doi: 10.1126/science.270.5239.1189
Keywords: hemorrhagic transformation, acute ischemic stroke, mechanical thrombectomy, fibrinogen, platelets
Citation: Lin C, Pan H, Qiao Y, Huang P, Su J and Liu J (2021) Fibrinogen Level Combined With Platelet Count for Predicting Hemorrhagic Transformation in Acute Ischemic Stroke Patients Treated With Mechanical Thrombectomy. Front. Neurol. 12:716020. doi: 10.3389/fneur.2021.716020
Received: 28 May 2021; Accepted: 04 August 2021;
Published: 31 August 2021.
Edited by:
Steffen Tiedt, LMU Munich University Hospital, GermanyReviewed by:
Paul Reidler, LMU Munich University Hospital, GermanySarah Zweynert, Charité – Universitätsmedizin Berlin, Germany
Copyright © 2021 Lin, Pan, Qiao, Huang, Su and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianren Liu, liujr021@sjtu.edu.cn; Jingjing Su, jingjingsu2000@163.com
†These authors have contributed equally to this work and share first authorship
 Changchun Lin
Changchun Lin Hui Pan†
Hui Pan† Yuan Qiao
Yuan Qiao Jingjing Su
Jingjing Su Jianren Liu
Jianren Liu