A new role for P2X4 receptors as modulators of lung surfactant secretion
- Institute of General Physiology, University of Ulm, Ulm, Germany
In recent years, P2X receptors have attracted increasing attention as regulators of exocytosis and cellular secretion. In various cell types, P2X receptors have been found to stimulate vesicle exocytosis directly via Ca2+ influx and elevation of the intracellular Ca2+ concentration. Recently, a new role for P2X4 receptors as regulators of secretion emerged. Exocytosis of lamellar bodies (LBs), large storage organelles for lung surfactant, results in a local, fusion-activated Ca2+ entry (FACE) in alveolar type II epithelial cells. FACE is mediated via P2X4 receptors that are located on the limiting membrane of LBs and inserted into the plasma membrane upon exocytosis of LBs. The localized Ca2+ influx at the site of vesicle fusion promotes fusion pore expansion and facilitates surfactant release. In addition, this inward-rectifying cation current across P2X4 receptors mediates fluid resorption from lung alveoli. It is hypothesized that the concomitant reduction in the alveolar lining fluid facilitates insertion of surfactant into the air–liquid interphase thereby “activating” it. These findings constitute a novel role for P2X4 receptors in regulating vesicle content secretion as modulators of the secretory output during the exocytic post-fusion phase.
Introduction
In recent years, P2X receptors have attracted increasing attention as regulators of exocytosis and cellular secretion in a wide variety of organs including the lungs (Burnstock et al., 2012). P2X receptors are membrane cation channels that are activated by extracellular adenosine triphosphate (ATP), the molecular and functional properties of which have been reviewed in detail elsewhere (Surprenant, 1996; North, 2002; Khakh and North, 2006; Burnstock and Kennedy, 2011; Kaczmarek-Hajek et al., 2012). ATP has been known to stimulate cellular secretion for several decades (Rodriguez Candela and Garcia-Fernandez, 1963; Diamant and Kruger, 1967). One of the earliest indications for involvement of P2X receptors in stimulating secretion came from the studies of Cockcroft and Gomperts (1979a,b, 1980). They found that ATP triggers degranulation and histamine release in mast cells via activation of P2Z (Cockcroft and Gomperts, 1980), which later turned out to be P2X7 (Surprenant et al., 1996). Since the first cloning of P2X receptor subunits in 1994 (Brake et al., 1994; Valera et al., 1994), P2X receptors have been found to stimulate and modulate various cellular secretion pathways, including fluid secretion in exocrine glands and epithelia (Novak, 2011), secretion of cytokines via release of plasma-derived microvesicles (Solini et al., 1999; MacKenzie et al., 2001) or exosomes (Qu et al., 2007; Qu and Dubyak, 2009).
Moreover, several members of the P2X family have been implicated in regulating exocytosis of secretory organelles in a variety of cell types (Gu and MacDermott, 1997; Ulmann et al., 2008; Jacques-Silva et al., 2010; Gutierrez-Martin et al., 2011; Huang et al., 2011). Substantial evidence suggests that P2X receptor activation stimulates exocytosis directly via influx of Ca2+ from the extracellular space and elevation of the cytoplasmic Ca2+ concentration ([Ca2+]c; Kim et al., 2004; Shigetomi and Kato, 2004; Jacques-Silva et al., 2010; Hayoz et al., 2012). It is well established that a series of Ca2+-dependent steps during the exocytic pre-fusion stage is essential for fusion of exocytic vesicles with the plasma membrane (Burgoyne and Morgan, 1998; Sudhof, 2004; Neher and Sakaba, 2008). Ca2+ can either enter through P2X receptor pores themselves or through voltage-gated Ca2+ channels, which are activated as a consequence of the P2X receptor-mediated membrane depolarization (Khakh and North, 2006). In line with these findings, several studies proposed a role for P2X4 receptors in exocytosis that is mediated via an increase in the intracellular Ca2+ concentration. P2X4 receptors have a relatively slow desensitization (5–10 s) and a high Ca2+ permeability, Ca2+ contributes 8% of the whole current in human P2X4 (Wang et al., 1996; Garcia-Guzman et al., 1997; North, 2002; Egan and Khakh, 2004). Hence, activation of P2X4 receptors can generate sufficient increases in [Ca2+]c to stimulate regulated exocytosis. Indeed, insulin secretion from pancreatic islets (Ohtani et al., 2011) and exocytic response in parotid acinar cells (Bhattacharya et al., 2012) following stimulation with ATP were augmented in the presence of ivermectin, a selective potentiator of P2X4 receptor currents (Khakh et al., 1999). P2X4 activation was also found to modulate glutamate and gamma-aminobutyric acid (GABA) release in hypothalamic neurons (Vavra et al., 2011) and brain-derived neurotrophic factor (BDNF) in microglial cells (Trang et al., 2009).
In all of these systems, activation of P2X receptors adjusts the secretory output predominantly by modulating the number of vesicles that fuse with the plasma membrane. Depending on the cell type and the shape of the Ca2+ signal, the rise in [Ca2+]c triggers fusion of secretory vesicles with the plasma membrane, but also affects maturation and trafficking of secretory vesicles to the plasma membrane (Neher and Sakaba, 2008; Dolensek et al., 2011; Gutierrez-Martin et al., 2011).
Vesicular P2X4 Receptors Promote Surfactant Secretion Via Face – “Fusion-Activated Ca2+-Entry”
Apart from regulating secretion via adjusting the number of fusing organelles the amount and composition of the secretory output is – at least for exocytosis of large secretory granules and secretion of bulky vesicle contents – modulated following fusion of the vesicle with the plasma membrane during the so-called exocytic “post-fusion” phase. Recent evidence also suggests a role for P2X4 receptors therein. It has been demonstrated that activation of P2X4 receptors following vesicle–plasma membrane fusion modulates the secretion and activation of pulmonary surfactant (Miklavc et al., 2011; Dietl et al., 2012; Thompson et al., 2013).
Pulmonary surfactant is secreted via exocytosis of lamellar bodies (LBs), large lysosome-related storage organelles in alveolar type II (ATII) epithelial cells. Surfactant is stored in LBs as densely packed membranous structures that do not readily diffuse out of fused LBs following opening of the exocytic fusion pore. Rather, surfactant is so insoluble, that it may remain entrapped within the fused vesicle for many minutes and the slowly expanding fusion pore acts as a mechanical barrier for the release (Dietl et al., 2001; Haller et al., 2001; Singer et al., 2003; Dietl and Haller, 2005; Miklavc et al., 2012).
Miklavc et al. (2010) initially discovered that exocytosis of LBs results in localized Ca2+ influx at the site of vesicle fusion which they termed “FACE” for “fusion-activated Ca2+-entry”. Subsequently, they found that FACE is mediated via activation of P2X4 receptors expressed on the limiting membranes of LBs (Miklavc et al., 2011). Upon exocytosis of LBs, the P2X4 receptor is readily part of the apical membrane as soon as membrane fusion is completed (Miklavc et al., 2009). Activation of P2X4 in the presence of extracellular ATP then results in a transient, non-selective, inward-rectifying, cation current at the site of the fused vesicle (Miklavc et al., 2011; Thompson et al., 2013) (Figure 1). The relatively high Ca2+ permeability of P2X4 receptors (North, 2002) causes a local, transient rise of [Ca2+]c around the fused vesicle which promotes fusion pore expansion (Miklavc et al., 2011). In ATII cells, vesicle content (i.e., surfactant) release is tightly regulated via Ca2+-dependent fusion pore expansion (Haller et al., 2001) and it has been demonstrated that FACE via P2X4 receptors on LBs directly facilitates surfactant release in the alveolus (Miklavc et al., 2011).
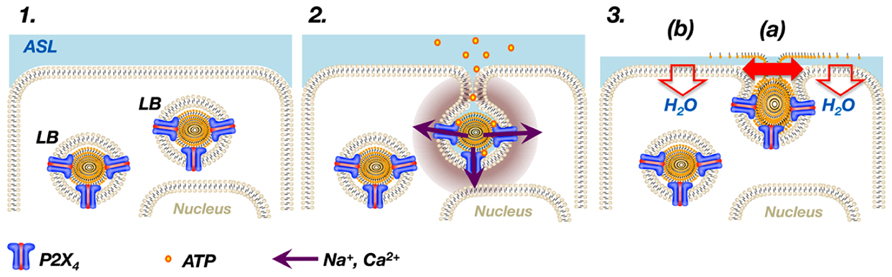
FIGURE 1. P2X4 receptors on LBs modulate surfactant secretion. P2X4 receptors are expressed on LBs, large storage organelles for pulmonary surfactant in ATII cells (1). Upon exocytosis of LBs, P2X4 receptors readily become part of the apical membrane and activation of P2X4 by extracellular ATP results in a transient, non-selective, inward-rectifying, cation current at the site of the fused vesicle causing a local increase in Ca2+ around the fused vesicle (2). The local increase in Ca2+ promotes fusion pore expansion (3a). In addition, the inward-rectifying cation current on the apical side results in vectorial ion transport across ATII cells, which in turn promotes fluid resorption and thereby facilitates adsorption of newly released surfactant into the air–liquid interface (3b). ASL = alveolar surface liquid.
Ca2+ channels localized in the membranes of the secretory vesicles that respond to changes in the membrane potential or extracellular agonists upon fusion are ideally suited for generating a localized rise in Ca2+ and selectively affect the individual fused vesicle. Yet, so far such mechanisms have only been known in invertebrates (Smith et al., 2000; Yao et al., 2009; Miklavc and Frick, 2011) and P2X4 receptors on LBs resemble the first analog mechanism in mammals. It will be interesting to see whether a similar role for P2X receptors is present in other secretory cells. Similar to LBs in ATII cells, many different cell types harbor secretory lysosomes or lysosome-related organelles to store for secretory products that are released via exocytosis of these organelles (Dell’Angelica et al., 2000; Blott and Griffiths, 2002; Luzio et al., 2007). Many of these contain rather bulky, macromolecular vesicle contents and release is modulated via the exocytic post-fusion phase (Thorn, 2009). In addition, it is well established that P2X receptors, in particular P2X4, are predominantly located within lysosomal compartments and inserted into the cell surface upon exocytosis (Qureshi et al., 2007; Toyomitsu et al., 2012).
Vesicular P2X4 Receptors Facilitate “Activation” of Surfactant
Following release into the alveolar hypophase surfactant maintains its compact organization, constituting lamellar body-like particles (LBPs; Haller et al., 2004). To gain its vital function of reducing the surface tension within alveoli, it needs to be inserted into the air–liquid interface. Freshly released LBPs disintegrate when they contact an air–liquid interface, leading to instantaneous spreading and insertion of surfactant material at this interface (Dietl and Haller, 2005). Thompson et al. (2013) demonstrated that, in addition to facilitating fusion pore dilation, FACE via P2X4 also drives fluid resorption from the alveolar lumen. The P2X4 mediated inward-rectifying cation current on the apical side results in vectorial ion transport across ATII cells, which in turn promotes apical to basolateral fluid transport (Thompson et al., 2013) (Figure 1). FACE-dependent transepithelial fluid resorption is a rather transient process which requires the presence of luminal ATP or other P2X4 agonists and hence it is unlikely that it is a major contributor to regulation of alveolar liquid homeostasis under physiological conditions (Folkesson and Matthay, 2006). However, it has been suggested that this localized alveolar fluid resorption results in temporary thinning of the alveolar hypophase which in turn promotes contact between LBPs and the interphase and facilitates adsorption of newly released surfactant into the air–liquid interface (Thompson et al., 2013). Hence, activation of P2X4 and FACE (which in order to embrace the true nature of FACE should now be referred to as “fusion-activated cation entry”) facilitates surfactant release via fusion pore opening and contributes to “activation” or “functionalising” of surfactant. Such a temporal and local coordination of surfactant secretion and reduction of alveolar lining fluid could constitute a powerful mechanism for fine-tuning surfactant replenishment – the integrators being vesicular P2X4 receptors and extracellular ATP.
ATP as Integrator for Surfactant Secretion and “Activation”
It is intriguing that extracellular ATP plays multiple functions for surfactant secretion in the alveolus. Apart from P2X4 receptors, ATII cells also express P2Y2 receptors (Garcia-Verdugo et al., 2008; Burnstock et al., 2012) and activation thereof is one of the most potent stimuli for LB exocytosis and surfactant secretion (Rice and Singleton, 1987; Frick et al., 2001; Andreeva et al., 2007; Dietl et al., 2010). Hence, ATP is integrating the entire secretion process from stimulating LB exocytosis to facilitating surfactant release and “activating” surfactant during the post-fusion phase.
Despite this importance of ATP for lung function, the origins of ATP in the alveoli are still elusive. It has been reported that ATP is present in the pulmonary hypophase (Patel et al., 2005), however, the estimated concentration under resting conditions is in the low nM range (Bove et al., 2010), well below the EC50 values for P2X4 activation (North, 2002) or P2Y2 activation (Lazarowski et al., 1995; Brunschweiger and Muller, 2006).
Cell stretch during deep inflation is considered the most potent if not only physiologically relevant stimulus for surfactant secretion (Wirtz and Dobbs, 2000; Dietl et al., 2004, 2010; Frick et al., 2004) and stretch-induced ATP release from alveolar epithelial cells (Patel et al., 2005; Mishra et al., 2011) could represent a key regulatory element (Dietl et al., 2010). Several possible pathways for ATP release have been described in the respiratory epithelia. ATP can either be released into the hypophase via regulated exocytosis from secretory cells (Kreda et al., 2010; Okada et al., 2011), or in a conductive way via pannexin hemichannels (Ransford et al., 2009; Seminario-Vidal et al., 2011) or P2X7 receptors (Mishra et al., 2011). In particular, local ATP release within individual alveoli may provide an ideal mechanism to gradually adapt local surfactant secretion to local demands. The alveolar epithelium consists of only two cell types; besides surfactant secreting ATII cells, flat alveolar type I (ATI) cells cover most of the alveolar surface. In contrast to primary ATII cells that only express P2X4 receptors (Miklavc et al., 2011) ATI cells express P2X4 and P2X7 receptors (Weinhold et al., 2010; Burnstock et al., 2012). P2X7 knock-out mice fail to increase surfactant secretion in response to hyperventilation and substantial evidence suggests that ATP release via P2X7 receptors on ATI cells maintains alveolar surfactant homeostasis in response to increased alveolar distension by stimulating P2Y2 receptors on ATII cells (Mishra et al., 2011) and, in light of our recent findings, possible activation of P2X4 (Miklavc et al., 2011; Thompson et al., 2013). In addition to responding to mechanical distension of alveoli, alveolar epithelial cells also respond to increased tension forces at the air–liquid interphase with exocytic release of ATP (e.g., upon local depletion of surfactant or when coming in close proximity to the air–liquid interphase following a decrease in alveolar hypophase height; Ramsingh et al., 2011).
Whether ATII cells also release ATP, to act in an autocrine feedback loop, is still unknown. Many secretory vesicles, including lysosome-related organelles, have been found to contain significant amounts of ATP (Bodin and Burnstock, 2001; Praetorius and Leipziger, 2009; Lazarowski et al., 2011) and it has been reported that ATP is released from ATII-like A549 cells, likely via exocytosis (Tatur et al., 2008; Ramsingh et al., 2011). It is tempting to speculate that LBs contain ATP and hence provide the ligand for the P2X4 receptors themselves. In such a scenario, the high degree of pH sensitivity of this receptor (Clarke et al., 2000; Zsembery et al., 2003; Coddou et al., 2011) could prevent intravesicular activation of the receptor in the presence of vesicular ATP (pH of LB is <6.1; Chander et al., 1986).
Also, under pathophysiological conditions resulting from many chronic lung diseases, release of purine nucleotides from respiratory epithelia is significantly increased (Adriaensen and Timmermans, 2004; Lommatzsch et al., 2010). It has been demonstrated that trauma-induced damage of the alveolus leads to substantial ATP release and that extracellular ATP is a key player to rescue alveolar function following damage, including regulation of surfactant secretion (Riteau et al., 2010; Belete et al., 2011). In addition, several studies have demonstrated up-regulation of P2X receptors in various cell types during pathological conditions including inflammation, tumor growth, and injury (Burnstock and Kennedy, 2011) and it has been hypothesized that chronic extracellular ATP may be responsible (Geisler et al., 2013). Such a mechanism could be particularly relevant in the lung, and P2X receptors may play an even greater role in many pathological conditions with chronically increased extracellular ATP levels. Initial evidence came from studies indicating that smoke-induced lung inflammation leads to increased levels of ATP in broncho-alveolar fluid and up-regulation of P2X7 expression (Lommatzsch et al., 2010; Lucattelli et al., 2011). A similar role for P2X4 receptors under pathophysiological conditions is still to be confirmed. However, it is becoming increasingly evident that purinergic signaling is taking center stage in regulating secretion of pulmonary surfactant and adapting it to local demands under physiological and diseased conditions. P2X4 receptors on LBs provide an ideal mechanism for fine-tuning surfactant secretion via ATP levels in the alveolar hypophase.
Despite recent advances in our understanding how purinergic signaling in the alveolus, in particular activation of vesicular P2X4 receptors, modulates LB exocytosis, surfactant secretion and activation of released surfactant, several important questions still remain to be answered: What is the physiological relevance of such a complex regulatory mechanism? What are the sources of ATP under physiological and more importantly pathophysiological conditions? And – extending the scope from the lung – is purinergic signaling a general mechanism for secretion of large, macromolecular vesicle contents or is it unique to LB exocytosis and surfactant secretion? The answers to these questions warrant further research and certainly promise an increased understanding of the role of P2X receptors in regulating exocytosis and cellular secretion.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adriaensen, D., and Timmermans, J. P. (2004). Purinergic signalling in the lung: important in asthma and COPD? Curr. Opin. Pharmacol. 4, 207–214. doi: 10.1016/j.coph.2004.01.010
Andreeva, A. V., Kutuzov, M. A., and Voyno-Yasenetskaya, T. A. (2007). Regulation of surfactant secretion in alveolar type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L259–L271. doi: 10.1152/ajplung.00112.2007
Belete, H. A., Hubmayr, R. D., Wang, S., and Singh, R. D. (2011). The role of purinergic signaling on deformation induced injury and repair responses of alveolar epithelial cells. PLoS ONE 6:e27469. doi: 10.1371/journal.pone.0027469
Bhattacharya, S., Verrill, D. S., Carbone, K. M., Brown, S., Yule, D. I., and Giovannucci, D. R. (2012). Distinct contributions by ionotropic purinoceptor subtypes to ATP-evoked calcium signals in mouse parotid acinar cells. J. Physiol. 590, 2721–2737. doi: 10.1113/jphysiol.2012.228148
Blott, E. J., and Griffiths, G. M. (2002). Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 3, 122–131. doi: 10.1038/nrm732
Bodin, P., and Burnstock, G. (2001). Purinergic signalling: ATP release. Neurochem. Res. 26, 959–969. doi: 10.1023/A:1012388618693
Bove, P. F., Grubb, B. R., Okada, S. F., Ribeiro, C. M., Rogers, T. D., Randell, S. H., et al. (2010). Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J. Biol. Chem. 285, 34939–34949. doi: 10.1074/jbc.M110.162933
Brake, A. J., Wagenbach, M. J., and Julius, D. (1994). New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 371, 519–523. doi: 10.1038/371519a0
Brunschweiger, A., and Muller, C. E. (2006). P2 receptors activated by uracil nucleotides – an update. Curr. Med. Chem. 13, 289–312. doi: 10.2174/092986706775476052
Burgoyne, R. D., and Morgan, A. (1998). Calcium sensors in regulated exocytosis. Cell Calcium 24, 367–376. doi: 10.1016/S0143-4160(98)90060-4
Burnstock, G., Brouns, I., Adriaensen, D., and Timmermans, J. P. (2012). Purinergic signaling in the airways. Pharmacol. Rev. 64, 834–868. doi: 10.1124/pr.111.005389
Burnstock, G., and Kennedy, C. (2011). P2X receptors in health and disease. Adv. Pharmacol. 61, 333–372. doi: 10.1016/B978-0-12-385526-8.00011-4
Chander, A., Johnson, R. G., Reicherter, J., and Fisher, A. B. (1986). Lung lamellar bodies maintain an acidic internal pH. J. Biol. Chem. 261, 6126–6131.
Clarke, C. E., Benham, C. D., Bridges, A., George, A. R., and Meadows, H. J. (2000). Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J. Physiol. 523(Pt 3), 697–703. doi: 10.1111/j.1469-7793.2000.00697.x
Cockcroft, S., and Gomperts, B. D. (1979a). Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J. Physiol. 296, 229–243.
Cockcroft, S., and Gomperts, B. D. (1979b). ATP induces nucleotide permeability in rat mast cells. Nature 279, 541–542. doi: 10.1038/279541a0
Cockcroft, S., and Gomperts, B. D. (1980). The ATP4-receptor of rat mast cells. Biochem. J. 188, 789–798.
Coddou, C., Yan, Z., Obsil, T., Huidobro-Toro, J. P., and Stojilkovic, S. S. (2011). Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 63, 641–683. doi: 10.1124/pr.110.003129
Dell’Angelica, E. C., Mullins, C., Caplan, S., and Bonifacino, J. S. (2000). Lysosome-related organelles. FASEB J. 14, 1265–1278. doi: 10.1096/fj.14.10.1265
Diamant, B., and Kruger, P. G. (1967). Histamine release from isolated rat peritoneal mast cells induced by adenosine-5′-triphosphate. Acta Physiol. Scand. 71, 291–302. doi: 10.1111/j.1748-1716.1967.tb03736.x
Dietl, P., Frick, M., Mair, N., Bertocchi, C., and Haller, T. (2004). Pulmonary consequences of a deep breath revisited. Biol. Neonate 85, 299–304. doi: 10.1159/000078176
Dietl, P., and Haller, T. (2005). Exocytosis of lung surfactant: from the secretory vesicle to the air–liquid interface. Annu. Rev. Physiol. 67, 595–621. doi: 10.1146/annurev.physiol.67.040403.102553
Dietl, P., Haller, T., and Frick, M. (2012). Spatio-temporal aspects, pathways and actions of Ca(2+) in surfactant secreting pulmonary alveolar type II pneumocytes. Cell Calcium 52, 296–302. doi: 10.1016/j.ceca.2012.04.010
Dietl, P., Haller, T., Mair, N., and Frick, M. (2001). Mechanisms of surfactant exocytosis in alveolar type II cells in vitro and in vivo. News Physiol. Sci. 16, 239–243.
Dietl, P., Liss, B., Felder, E., Miklavc, P., and Wirtz, H. (2010). Lamellar body exocytosis by cell stretch or purinergic stimulation: possible physiological roles, messengers and mechanisms. Cell. Physiol. Biochem. 25, 1–12. doi: 10.1159/000272046
Dolensek, J., Skelin, M., and Rupnik, M. S. (2011). Calcium dependencies of regulated exocytosis in different endocrine cells. Physiol. Res. 60(Suppl. 1), S29–S38.
Egan, T. M., and Khakh, B. S. (2004). Contribution of calcium ions to P2X channel responses. J. Neurosci. 24, 3413–3420. doi: 10.1523/JNEUROSCI.5429-03.2004
Folkesson, H. G., and Matthay, M. A. (2006). Alveolar epithelial ion and fluid transport: recent progress. Am. J. Respir. Cell Mol. Biol. 35, 10–19. doi: 10.1165/rcmb.2006-0080SF
Frick, M., Bertocchi, C., Jennings, P., Haller, T., Mair, N., Singer, W., et al. (2004). Ca2+ entry is essential for cell strain-induced lamellar body fusion in isolated rat type II pneumocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L210–L220. doi: 10.1152/ajplung.00332.2003
Frick, M., Eschertzhuber, S., Haller, T., Mair, N., and Dietl, P. (2001). Secretion in alveolar type II cells at the interface of constitutive and regulated exocytosis. Am. J. Respir. Cell Mol. Biol. 25, 306–315. doi: 10.1165/ajrcmb.25.3.4493
Garcia-Guzman, M., Soto, F., Gomez-Hernandez, J. M., Lund, P. E., and Stuhmer, W. (1997). Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol. Pharmacol. 51, 109–118.
Garcia-Verdugo, I., Ravasio, A., De Paco, E. G., Synguelakis, M., Ivanova, N., Kanellopoulos, J., et al. (2008). Long-term exposure to LPS enhances the rate of stimulated exocytosis and surfactant secretion in alveolar type II cells and upregulates P2Y2 receptor expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L708–L717. doi: 10.1152/ajplung.00536.2007
Geisler, J. C., Corbin, K. L., Li, Q., Feranchak, A. P., Nunemaker, C. S., and Li, C. (2013). Vesicular nucleotide transporter-mediated ATP release regulates insulin secretion. Endocrinology 154, 675–684. doi: 10.1210/en.2012-1818
Gu, J. G., and MacDermott, A. B. (1997). Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature 389, 749–753. doi: 10.1038/39639
Gutierrez-Martin, Y., Bustillo, D., Gomez-Villafuertes, R., Sanchez-Nogueiro, J., Torregrosa-Hetland, C., Binz, T., et al. (2011). P2X7 receptors trigger ATP exocytosis and modify secretory vesicle dynamics in neuroblastoma cells. J. Biol. Chem. 286, 11370–11381. doi: 10.1074/jbc.M110.139410
Haller, T., Dietl, P., Pfaller, K., Frick, M., Mair, N., Paulmichl, M., et al. (2001). Fusion pore expansion is a slow, discontinuous, and Ca2+-dependent process regulating secretion from alveolar type II cells. J. Cell Biol. 155, 279–289. doi: 10.1083/jcb.200102106
Haller, T., Dietl, P., Stockner, H., Frick, M., Mair, N., Tinhofer, I., et al. (2004). Tracing surfactant transformation from cellular release to insertion into an air–liquid interface. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L1009–L1015. doi: 10.1152/ajplung.00342.2003
Hayoz, S., Jia, C., and Hegg, C. (2012). Mechanisms of constitutive and ATP-evoked ATP release in neonatal mouse olfactory epithelium. BMC Neurosci. 13:53. doi: 10.1186/1471-2202-13-53
Huang, Y. A., Stone, L. M., Pereira, E., Yang, R., Kinnamon, J. C., Dvoryanchikov, G., et al. (2011). Knocking out P2X receptors reduces transmitter secretion in taste buds. J. Neurosci. 31, 13654–13661. doi: 10.1523/JNEUROSCI.3356-11.2011
Jacques-Silva, M. C., Correa-Medina, M., Cabrera, O., Rodriguez-Diaz, R., Makeeva, N., Fachado, A., et al. (2010). ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc. Natl. Acad. Sci. U.S.A. 107, 6465–6470. doi: 10.1073/pnas.0908935107
Kaczmarek-Hajek, K., Lorinczi, E., Hausmann, R., and Nicke, A. (2012). Molecular and functional properties of P2X receptors – recent progress and persisting challenges. Purinergic Signal. 8, 375–417. doi: 10.1007/s11302-012-9314-7
Khakh, B. S., and North, R. A. (2006). P2X receptors as cell-surface ATP sensors in health and disease. Nature 442, 527–532. doi: 10.1038/nature04886
Khakh, B. S., Proctor, W. R., Dunwiddie, T. V., Labarca, C., and Lester, H. A. (1999). Allosteric control of gating and kinetics at P2X(4) receptor channels. J. Neurosci. 19, 7289–7299.
Kim, J. H., Nam, J. H., Kim, M. H., Koh, D. S., Choi, S. J., Kim, S. J., et al. (2004). Purinergic receptors coupled to intracellular Ca2+ signals and exocytosis in rat prostate neuroendocrine cells. J. Biol. Chem. 279, 27345–27356. doi: 10.1074/jbc.M313575200
Kreda, S. M., Seminario-Vidal, L., Van Heusden, C. A., O’Neal, W., Jones, L., Boucher, R. C., et al. (2010). Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J. Physiol. 588, 2255–2267. doi: 10.1113/jphysiol.2009.186643
Lazarowski, E. R., Sesma, J. I., Seminario-Vidal, L., and Kreda, S. M. (2011). Molecular mechanisms of purine and pyrimidine nucleotide release. Adv. Pharmacol. 61, 221–261. doi: 10.1016/B978-0-12-385526-8.00008-4
Lazarowski, E. R., Watt, W. C., Stutts, M. J., Boucher, R. C., and Harden, T. K. (1995). Pharmacological selectivity of the cloned human P2U-purinoceptor: potent activation by diadenosine tetraphosphate. Br. J. Pharmacol. 116, 1619–1627. doi: 10.1111/j.1476-5381.1995.tb16382.x
Lommatzsch, M., Cicko, S., Muller, T., Lucattelli, M., Bratke, K., Stoll, P., et al. (2010). Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 181, 928–934. doi: 10.1164/rccm.200910-1506OC
Lucattelli, M., Cicko, S., Muller, T., Lommatzsch, M., De Cunto, G., Cardini, S., et al. (2011). P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am. J. Respir. Cell Mol. Biol. 44, 423–429. doi: 10.1165/rcmb.2010-0038OC
Luzio, J. P., Pryor, P. R., and Bright, N. A. (2007). Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632. doi: 10.1038/nrm2217
MacKenzie, A., Wilson, H. L., Kiss-Toth, E., Dower, S. K., North, R. A., and Surprenant, A. (2001). Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15, 825–835. doi: 10.1016/S1074-7613(01)00229-1
Miklavc, P., Albrecht, S., Wittekindt, O. H., Schullian, P., Haller, T., and Dietl, P. (2009). Existence of exocytotic hemifusion intermediates with a lifetime of up to seconds in type II pneumocytes. Biochem. J. 424, 7–14. doi: 10.1042/BJ20091094
Miklavc, P., and Frick, M. (2011). Vesicular calcium channels as regulators of the exocytotic post-fusion phase. Commun. Integr. Biol. 4, 796–798.
Miklavc, P., Frick, M., Wittekindt, O. H., Haller, T., and Dietl, P. (2010). Fusion-activated Ca(2+) entry: an “active zone” of elevated Ca(2+) during the post-fusion stage of lamellar body exocytosis in rat type II pneumocytes. PLoS ONE 5:e10982. doi: 10.1371/journal.pone.0010982
Miklavc, P., Hecht, E., Hobi, N., Wittekindt, O. H., Dietl, P., Kranz, C., et al. (2012). Actin coating and compression of fused secretory vesicles are essential for surfactant secretion – a role for Rho, formins and myosin II. J. Cell Sci. 125, 2765–2774. doi: 10.1242/jcs.105262
Miklavc, P., Mair, N., Wittekindt, O. H., Haller, T., Dietl, P., Felder, E., et al. (2011). Fusion-activated Ca2+ entry via vesicular P2X4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc. Natl. Acad. Sci. U.S.A. 108, 14503–14508. doi: 10.1073/pnas.1101039108
Mishra, A., Chintagari, N. R., Guo, Y., Weng, T., Su, L., and Liu, L. (2011). Purinergic P2X7 receptor regulates lung surfactant secretion in a paracrine manner. J. Cell Sci. 124, 657–668. doi: 10.1242/jcs.066977
Neher, E., and Sakaba, T. (2008). Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59, 861–872. doi: 10.1016/j.neuron.2008.08.019
Novak, I. (2011). Purinergic signalling in epithelial ion transport: regulation of secretion and absorption. Acta Physiol. (Oxf.) 202, 501–522. doi: 10.1111/j.1748-1716.2010.02225.x
Ohtani, M., Ohura, K., and Oka, T. (2011). Involvement of P2X receptors in the regulation of insulin secretion, proliferation and survival in mouse pancreatic beta-cells. Cell. Physiol. Biochem. 28, 355–366. doi: 10.1159/000331752
Okada, S. F., Zhang, L., Kreda, S. M., Abdullah, L. H., Davis, C. W., Pickles, R. J., et al. (2011). Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium. Am. J. Respir. Cell Mol. Biol. 45, 253–260. doi: 10.1165/rcmb.2010-0253OC
Patel, A. S., Reigada, D., Mitchell, C. H., Bates, S. R., Margulies, S. S., and Koval, M. (2005). Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L489–L496. doi: 10.1152/ajplung.00074.2005
Praetorius, H. A., and Leipziger, J. (2009). ATP release from non-excitable cells. Purinergic Signal. 5, 433–446. doi: 10.1007/s11302-009-9146-2
Qu, Y., and Dubyak, G. R. (2009). P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal. 5, 163–173. doi: 10.1007/s11302-009-9132-8
Qu, Y., Franchi, L., Nunez, G., and Dubyak, G. R. (2007). Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179, 1913–1925.
Qureshi, O. S., Paramasivam, A., Yu, J. C., and Murrell-Lagnado, R. D. (2007). Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 120, 3838–3849. doi: 10.1242/jcs.010348
Ramsingh, R., Grygorczyk, A., Solecki, A., Cherkaoui, L. S., Berthiaume, Y., and Grygorczyk, R. (2011). Cell deformation at the air-liquid interface induces Ca2+-dependent ATP release from lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L587–L595. doi: 10.1152/ajplung.00345.2010
Ransford, G. A., Fregien, N., Qiu, F., Dahl, G., Conner, G. E., and Salathe, M. (2009). Pannexin 1 contributes to ATP release in airway epithelia. Am. J. Respir. Cell Mol. Biol. 41, 525–534. doi: 10.1165/rcmb.2008-0367OC
Rice, W. R., and Singleton, F. M. (1987). P2Y-purinoceptor regulation of surfactant secretion from rat isolated alveolar type II cells is associated with mobilization of intracellular calcium. Br. J. Pharmacol. 91, 833–838. doi: 10.1111/j.1476-5381.1987.tb11282.x
Riteau, N., Gasse, P., Fauconnier, L., Gombault, A., Couegnat, M., Fick, L., et al. (2010). Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 182, 774–783. doi: 10.1164/rccm.201003-0359OC
Rodriguez Candela, J. L., and Garcia-Fernandez, M. C. (1963). Stimulation of secretion of insulin by adenosine-triphosphate. Nature 197, 1210. doi: 10.1038/1971210a0
Seminario-Vidal, L., Okada, S. F., Sesma, J. I., Kreda, S. M., Van Heusden, C. A., Zhu, Y., et al. (2011). Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J. Biol. Chem. 286, 26277–26286. doi: 10.1074/jbc.M111.260562
Shigetomi, E., and Kato, F. (2004). Action potential-independent release of glutamate by Ca2+ entry through presynaptic P2X receptors elicits postsynaptic firing in the brainstem autonomic network. J. Neurosci. 24, 3125–3135. doi: 10.1523/JNEUROSCI.0090-04.2004
Singer, W., Frick, M., Haller, T., Bernet, S., Ritsch-Marte, M., and Dietl, P. (2003). Mechanical forces impeding exocytotic surfactant release revealed by optical tweezers. Biophys. J. 84, 1344–1351. doi: 10.1016/S0006-3495(03)74950-9
Smith, R. M., Baibakov, B., Ikebuchi, Y., White, B. H., Lambert, N. A., Kaczmarek, L. K., et al. (2000). Exocytotic insertion of calcium channels constrains compensatory endocytosis to sites of exocytosis. J. Cell Biol. 148, 755–767. doi: 10.1083/jcb.148.4.755
Solini, A., Chiozzi, P., Morelli, A., Fellin, R., and Di Virgilio, F. (1999). Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J. Cell Sci. 112(Pt 3), 297–305.
Sudhof, T. C. (2004). The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547. doi: 10.1146/annurev.neuro.26.041002.131412
Surprenant, A. (1996). Functional properties of native and cloned P2X receptors. Ciba Found. Symp. 198, 208–219; discussion 219–222.
Surprenant, A., Rassendren, F., Kawashima, E., North, R. A., and Buell, G. (1996). The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272, 735–738. doi: 10.1126/science.272.5262.735
Tatur, S., Kreda, S., Lazarowski, E., and Grygorczyk, R. (2008). Calcium-dependent release of adenosine and uridine nucleotides from A549 cells. Purinergic Signal. 4, 139–146. doi: 10.1007/s11302-007-9059-x
Thompson, K. E., Korbmacher, J. P., Hecht, E., Hobi, N., Wittekindt, O. H., Dietl, P., et al. (2013). Fusion-activated cation entry (FACE) via P2X(4) couples surfactant secretion and alveolar fluid transport. FASEB J. 27, 1772–1783. doi: 10.1096/fj.12-220533
Thorn, P. (2009). New insights into the control of secretion. Commun. Integr. Biol. 2, 315–317. doi: 10.4161/cib.2.4.8262
Toyomitsu, E., Tsuda, M., Yamashita, T., Tozaki-Saitoh, H., Tanaka, Y., and Inoue, K. (2012). CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal. 8, 301–310. doi: 10.1007/s11302-011-9288-x
Trang, T., Beggs, S., Wan, X., and Salter, M. W. (2009). P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J. Neurosci. 29, 3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009
Ulmann, L., Hatcher, J. P., Hughes, J. P., Chaumont, S., Green, P. J., Conquet, F., et al. (2008). Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J. Neurosci. 28, 11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008
Valera, S., Hussy, N., Evans, R. J., Adami, N., North, R. A., Surprenant, A., et al. (1994). A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature 371, 516–519. doi: 10.1038/371516a0
Vavra, V., Bhattacharya, A., and Zemkova, H. (2011). Facilitation of glutamate and GABA release by P2X receptor activation in supraoptic neurons from freshly isolated rat brain slices. Neuroscience 188, 1–12. doi: 10.1016/j.neuroscience.2011.04.067
Wang, C. Z., Namba, N., Gonoi, T., Inagaki, N., and Seino, S. (1996). Cloning and pharmacological characterization of a fourth P2X receptor subtype widely expressed in brain and peripheral tissues including various endocrine tissues. Biochem. Biophys. Res. Commun. 220, 196–202. doi: 10.1006/bbrc.1996.0380
Weinhold, K., Krause-Buchholz, U., Rodel, G., Kasper, M., and Barth, K. (2010). Interaction and interrelation of P2X7 and P2X4 receptor complexes in mouse lung epithelial cells. Cell. Mol. Life Sci. 67, 2631–2642. doi: 10.1007/s00018-010-0355-1
Wirtz, H. R., and Dobbs, L. G. (2000). The effects of mechanical forces on lung functions. Respir. Physiol. 119, 1–17. doi: 10.1016/S0034-5687(99)00092-4
Yao, C. K., Lin, Y. Q., Ly, C. V., Ohyama, T., Haueter, C. M., Moiseenkova-Bell, V. Y., et al. (2009). A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell 138, 947–960. doi: 10.1016/j.cell.2009.06.033
Keywords: P2X4 receptor, lamellar body, alveolar epithelial cell, exocytosis, calcium, cellular secretion, pulmonary surfactant
Citation: Miklavc P, Thompson KE and Frick M (2013) A new role for P2X4 receptors as modulators of lung surfactant secretion. Front. Cell. Neurosci. 7:171. doi: 10.3389/fncel.2013.00171
Received: 05 August 2013; Paper pending published: 02 September 2013;
Accepted: 12 September 2013; Published online: 08 October 2013.
Edited by:
Geoffrey Burnstock, University College Medical School, UKReviewed by:
Jean-Pierre Timmermans, University of Antwerp (State University Centre Antwerp), BelgiumRuth Dorothy Murrell-Lagnado, University of Cambridge, UK
Copyright © 2013 Miklavc, Thompson and Frick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manfred Frick, Institute of General Physiology, University of Ulm, Albert-Einstein Allee 11, 89081 Ulm, Germany e-mail: manfred.frick@uni-ulm.de
 Pika Miklavc
Pika Miklavc Kristin E. Thompson
Kristin E. Thompson  Manfred Frick
Manfred Frick