- 1College of Horticulture, Shenyang Agricultural University, Shenyang, China
- 2Institute of Grassland Research, Chinese Academy of Agricultural Sciences, Hohhot, China
- 3College of Grassland Science and Technology, China Agricultural University, Beijing, China
- 4Inner Mongolia Engineering Research Center of Development and Utilization of Microbial Resources in Silage, Inner Mongolia Academy of Agricultural and Animal Husbandry Science, Hohhot, China
- 5Inner Mongolia Key Laboratory of Microbial Ecology of Silage, Inner Mongolia Academy of Agricultural and Animal Husbandry Science, Hohhot, China
This study aimed to evaluate the effects of Bacillus subtilis or Lentilactobacillus buchneri on the fermentation quality, aerobic stability, and bacterial and fungal communities of whole plant corn silage during aerobic exposure. Whole plant corn was harvested at the wax maturity stage, which chopped to a length of approximately 1 cm, and treated with the following: distilled sterile water control, 2.0 × 105 CFU/g of Lentilactobacillus buchneri (LB) or 2.0 × 105 CFU/g of Bacillus subtilis (BS) for 42 days silage. Then, the samples were exposed to air (23–28°C) after opening and sampled at 0, 18 and 60 h, to investigate fermentation quality, bacterial and fungal communities, and aerobic stability. Inoculation with LB or BS increased the pH value, acetic acid, and ammonia nitrogen content of silage (P < 0.05), but it was still far below the threshold of inferior silage, the yield of ethanol was reduced (P < 0.05), and satisfactory fermentation quality was achieved. With the extension of the aerobic exposure time, inoculation with LB or BS prolonged the aerobic stabilization time of silage, attenuated the trend of pH increase during aerobic exposure, and increased the residues of lactic acid and acetic acid. The bacterial and fungal alpha diversity indices gradually declined, and the relative abundance of Basidiomycota and Kazachstania gradually increased. The relative abundance of Weissella and unclassified_f_Enterobacteria was higher and the relative abundance of Kazachstania was lower after inoculation with BS compared to the CK group. According to the correlation analysis, Bacillus and Kazachstania are bacteria and fungi that are more closely related to aerobic spoilage and inoculation with LB or BS could inhibit spoilage. The FUNGuild predictive analysis indicated that the higher relative abundance of fungal parasite-undefined saprotroph in the LB or BS groups at AS2, may account for its good aerobic stability. In conclusion, silage inoculated with LB or BS had better fermentation quality and improved aerobic stability by effectively inhibiting the microorganisms that induce aerobic spoilage.
1. Introduction
Whole plant corn silage is a high-quality source of roughage and is widely used in ruminant diets (Gallo et al., 2022) because of the rich nutrition and high feeding value that can effectively meet the production requirements of ruminants (Chen et al., 2014; Cui et al., 2022). However, whole plant corn silage has high levels of residual soluble carbohydrates and lactic acid, and low concentrations of natural antifungal compounds, making it more prone to aerobic spoilage after opening during production (Lara et al., 2016; Kung et al., 2018). Thus, improvement in the aerobic stability of corn silage is a conventional but constant concern for making high-quality and safe silage (Huang et al., 2021).
Aerobic spoilage is caused by the growth of aerobic microorganisms such as yeasts and filamentous fungi, which can metabolize lactic acid under aerobic conditions, thereby leading to its deterioration (Mendonça et al., 2020; Ferrero et al., 2021a). The use of microbial inoculants, such as lactic acid bacteria (LAB), is an effective means of reducing aerobic spoilage and the accumulation of toxic matter during the ensiling process (Bernardi et al., 2019; Liu et al., 2020). LAB fermentation types are divided into homofermentative and heterofermentative types, Lentilactobacillus buchneri is the most commonly used heterofermentative bacterial species (Benjamim da Silva et al., 2021); it is capable of producing a mix of lactic and acetic acids and has been used to improve the aerobic stability of silages (Basso et al., 2012; Mangwe et al., 2016). The addition of L. buchneri to silage improved aerobic stability by fermenting lactic acid to acetic acid, which inhibits filamentous fungi growth, thereby prolonging the duration that silage can be exposed to air and slowing its deterioration (Kung and Ranjit, 2001; Zhang et al., 2021). However, the heterofermentative pathway is less efficient in terms of both acidification and preservation of nutrients during the anaerobic storage phase than the homofermentative pathway (Wilkinson and Davies, 2013; Guan et al., 2021). Therefore, our goal is search for silage inoculants that can not only ensure the quality of silage fermentation, but also improve aerobic stability.
Bacillus subtilis can inhibit the growth of pathogenic bacteria and strengthen intestinal barrier function, which is beneficial to ruminant growth performance, and has a good capacity for ruminants to maintain the microbial balance of the gastrointestinal tract (Zhang et al., 2017). Studies have confirmed that inoculation of B. subtilis in silage can improve silage fermentation (Guo et al., 2022), by producing cellulases, converting water soluble carbohydrates into lactic acid and decreasing pH, thereby inhibiting harmful microbial activity, and has the potential to be a silage inoculant (Li et al., 2018). In addition, researchers have confirmed that B. subtilis can produce antifungal and antibacterial substances, that can inhibit the growth of undesirable microorganisms such as yeast and filamentous fungi, and prevent silage spoilage and mycotoxin production (Bonaldi et al., 2021), which may be applicable to improving aerobic stability. Inoculation with B. subtilis can improve the aerobic stability of silage (Bumbieris et al., 2017; Bai et al., 2020), but studies on its role in whole plant corn silage are inadequate. Gandra et al. (2017) observed that co-inoculation of L. buchneri and B. subtilis improved the aerobic stability of sunflower silage by reducing the number of aerobic and spoilage microorganisms and increasing the number of lactic acid bacteria. However, the mechanism of the influence of B. subtilis on aerobic stability has not been explored in sufficient depth in previous studies.
The next generation sequencing method enabled us to identify the microbial taxa associated with silage (Bai et al., 2022b). We hypothesized that inoculation with L. buchneri or B. subtilis could improve aerobic stability by altering the microbial community structure. Therefore, the aim of this study was to investigate the effects of B. subtilis or L. buchneri on the fermentation quality, aerobic stability, and bacterial and fungal communities of whole plant corn silage during aerobic exposure.
2. Materials and methods
2.1. Silage preparation and aerobic stability
The experimental planting area is located in Shenhe District, Shenyang City, Liaoning Province, Baicaoyuan Scientific Research Base of Shenyang Agricultural University (41°50’N, 123°34’E). The annual average temperature is 8.1°C, the annual average precipitation is 721.9 mm, the annual frost-free period is 150–170 days, and the soil type is brown earth loam containing 48 sand, 29% silt and 23% clay in the depth of 0–20 cm. The whole plant corn variety is Dongdan60, which is harvested at the wax maturity stage, and the stubble height is 15 cm. Whole plant corn was chopped to a length of approximately 1 cm by a grass shredding machine (Donghong No. 1, Donghong Mechanical Equipment Co., Ltd., Donggang, China), and 600 g of chopped material was applied to the (1) distilled sterile water control (CK), (2) Lentilactobacillus buchneri (Lallemand Animal Nutrition, Milwaukee, WI) (LB, 2 × 105 cfu/g), and (3) Bacillus subtilis groups (Shandong Vland Biological Technology Co. LTD) (BS, 2 × 105 cfu/g). The inoculant was dissolved in 3 mL sterile water and sprayed evenly. After thoroughly mixing, the materials were ensiled in vacuum-sealed silage bags (35 × 24 cm) and fermented at room temperature (23–28°C).
After 42 days of ensiling, silage bags were opened, removed approximately 200 g of the silage sample from the silage bag and add it to a beaker with a 250 mL volume, and aerobic stability was observed for 100 h. Beakers were then placed inside a Styrofoam block, and the silage samples were exposed to air at ambient temperature. Samples were taked at three time points of 0 h (AS0), 18 h (AS1), and 60 h (AS2) to analyse the fermentation quality and aerobic stability. The temperature during aerobic exposure was measured by a 64-well thermograph with a probe placed in the centre of the silage, with temperature changes recorded every 10 min (Zhao et al., 2021).
2.2. Analyses of chemical components and fermentation characteristics
Ten grams of silage sample was added to 90 mL of distilled water, and placed in a refrigerator at 4°C for 12 h. A pH metre with a glass electrode was used (PB-10, Sartorius Group, Göttingen, Germany) to measure the pH of the extract (Han et al., 2015). The leaching solution was filtered through a 0.22 μm filter membrane, and the contents of lactic acid (LA), acetic acid (AA), butyric acid (BA) and ethanol in the leaching solution were determined by high-performance liquid chromatography (HPLC; Waters1525, Waters Corporation, Milford, MA, United States). The analysis conditions were as follows: chromatographic column, Carbomix H-NP5 (Sepax Technologies, Inc., Newark, DE, United States); detector, refractive index detector (Waters2414, Waters Corporation, Milford, MA, United States); mobile phase, 2.5 mmol/L H2SO4; flow rate, 0.6 ml/min; column temperature, 55°C; sample volume, 10 μL (Wang et al., 2021). The organic acid concentrations were obtained by comparing the curves of the silage extracts with the curves of experimental standard. Ammonia nitrogen (AN) was determined by the phenol-sodium hypochlorite colorimetric method (Ke et al., 2017).
Approximately 100 g of silage was weighed, placed in a paper bag and dried in an oven for 48 h. The weight loss of the silage sample was recorded during the process, and the dry matter (DM) content of the sample was calculated. Water-soluble carbohydrates (WSC) were determined by anthrone-sulfuric acid colorimetry (Thomas, 1977), starch was determined by acid hydrolysis (Bai et al., 2022b), and crude protein (CP) was determined by the Kjeldahl method (Huang et al., 2021) using an autoanalyzer (Kjeltec 8400; FOSS Co. Ltd., Hillerød, Denmark). NDF (neutral detergent fiber assayed with a heat stable amylase; aNDF) and ADF (acid detergent fiber) were measured according to the Van Soest method (Van Soest et al., 1991) using an Ankom 2000 fiber analyser (Ankom, Macedon, NY, United States). The quantities of lactic acid bacteria, coliform bacteria, yeasts and filamentous fungi in silage were determined by culturing on MRS agar, violet-red bile agar and rose Bengal medium, respectively (Bai et al., 2022a).
2.3. Bacterial and fungal communities
Fresh samples (10 g) were put into 90 mL of sterile distilled water, shaken using a cryogenic oscillator (THZ-98C, Shanghai Yiheng Scientific Instrument Co. Ltd., Shanghai, China) at 4°C and 180 rpm for 30 min, and then filtered through three layers of sterile gauze. The filtrate was centrifuged in a cryogenic centrifuge (ST 16R, Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 13,000 × g for 15 min at 4°C to enrich the sediment. Sediments were used for high-throughput sequencing (Wang et al., 2018).
Total DNA was extracted from bacteria and fungi in the silage samples with an E.Z.N.A. ® Stool DNA Kit (Omega Bio-Tek, United States). The hypervariable region V3-V4 of full-length bacterial 16S rRNA genes were amplified by PCR with specific forward 341F (CCTAYGGGRBGCASCAG) and reverse 806R (GGACTACNNGGGTATCTAAT) primers and full-length fungal internal transcribed spacers (ITS) were amplified with specific forward ITS1F (CTTGGTCATTTAGAGGAAGTAA) and reverse ITS2R (GCTGCGTTCTTCATCGATGC) primers (Bai et al., 2021). The AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States) was used to purify the PCR products, and a Quantus™ Fluorometer (Promega, United States) was used for quantitative determination, using the MiSeq PE300 platform of the Illumina Company for sequencing (Shanghai Majorbio Bio-Pharm Technology Co. Ltd.). Next fastp and FLASH software programs were used to control the quality and splice raw sequences, respectively. Furthermore, UPARSE software was used to cluster sequences with 97% similarity in operational taxonomic units (OTUs). Using mothur software1 to calculate the alpha diversity, Chao, Shannon indices, etc., PCoA analysis (principal coordinate analysis) based on the Bray-Curtis distance algorithm was used to test the similarity of microbial community structure among samples. Species were selected for correlation heatmap graph analysis based on Spearman correlation | r| > 0.6 P < 0.05 (Wang et al., 2021). The sequencing data were submitted to the NCBI Sequence Read Archive database (accession: PRJNA890780).
2.4. Data statistics and analysis
Microbiological data were log10-transformed on a fresh basis. Two-way ANOVA was performed on the data using SPSS 22.0 to determine the effect of silage time and mixed silage ratio. The data for pH and organic acids were analysed using the general linear model procedure of the Statistical Package for Social Science (SPSS 21.0, SPSS, Inc., Chicago, IL, USA) according to the model:
where Yij represents the response variable, μ is the overall mean, Ti is the effect of inoculants, Dj is the effect of ensiling time, (T × D)ij is the effect of the interaction between the inoculants and ensiling time, and εij is the random residual error. Multiple comparisons were performed using Tukey’s method, and P < 0.05 indicated a significant difference.
3. Results
3.1. Characteristics of whole plant corn before ensiling
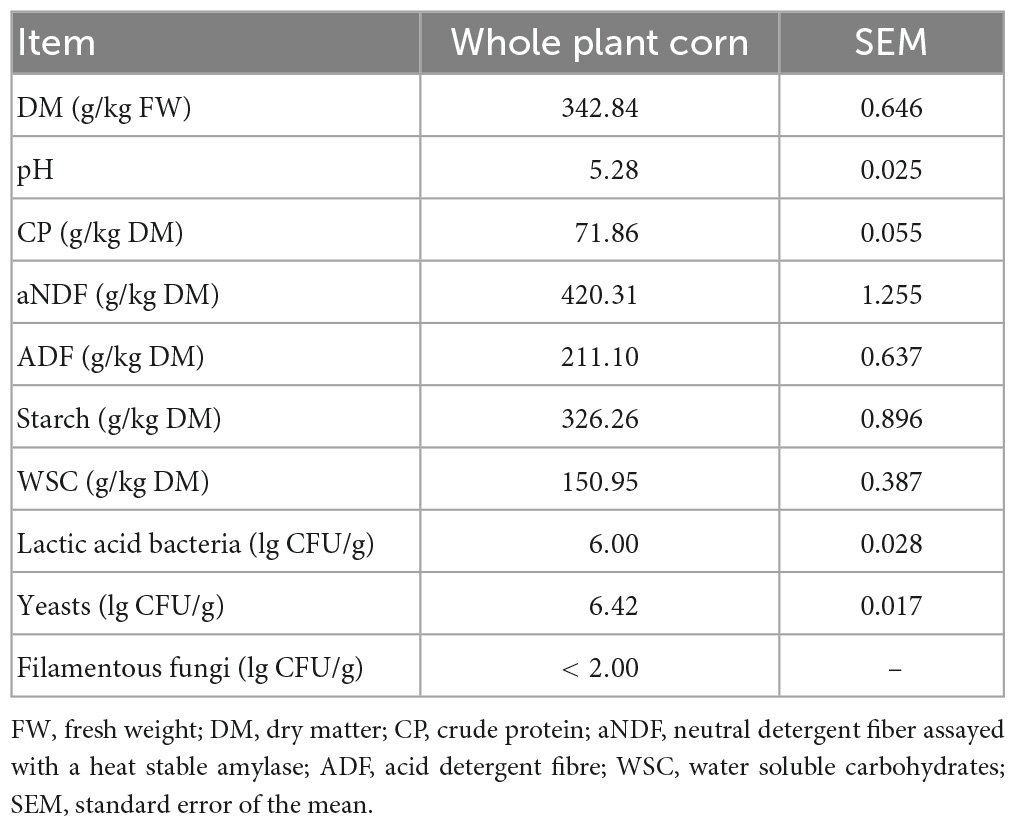
The contents of DM, pH, CP, WSC, and starch in whole plant corn fresh forage were 342.84 g/kg FW, 5.28, 71.86, 150.95, and 326.26 g/kg DM, respectively, and the coliform bacteria, lactic acid bacteria, and yeasts were 5.55, 6.00, and 6.42 lg CFU/g, respectively. The number of filamentous fungi was lower than the detection level (Table 1).
3.2. Fermentation quality, nutrient composition and microbial counts of silage
The pH, AA and AN contents of the BS and LB groups were higher than those of the CK group (P < 0.05; Table 2), the LA concentration of the LB group was higher than that of the other two groups (P < 0.05), and BA was not detected in any of the groups (the minimum detectable limit is approximately 0.1 μg/mL). The CP content of the BS group was lower than that of the CK group (P < 0.05), the WSC content in the LB group was higher than those in the other two groups (P < 0.05), and there was no significant difference in the contents of aNDF and ADF among the groups (P > 0.05). The numbers of lactic acid bacteria in the BS group were significantly higher than those in the CK and LB groups (P < 0.05). The number of filamentous fungi in each group was lower than the detection level (<2.00 lg CFU/g).
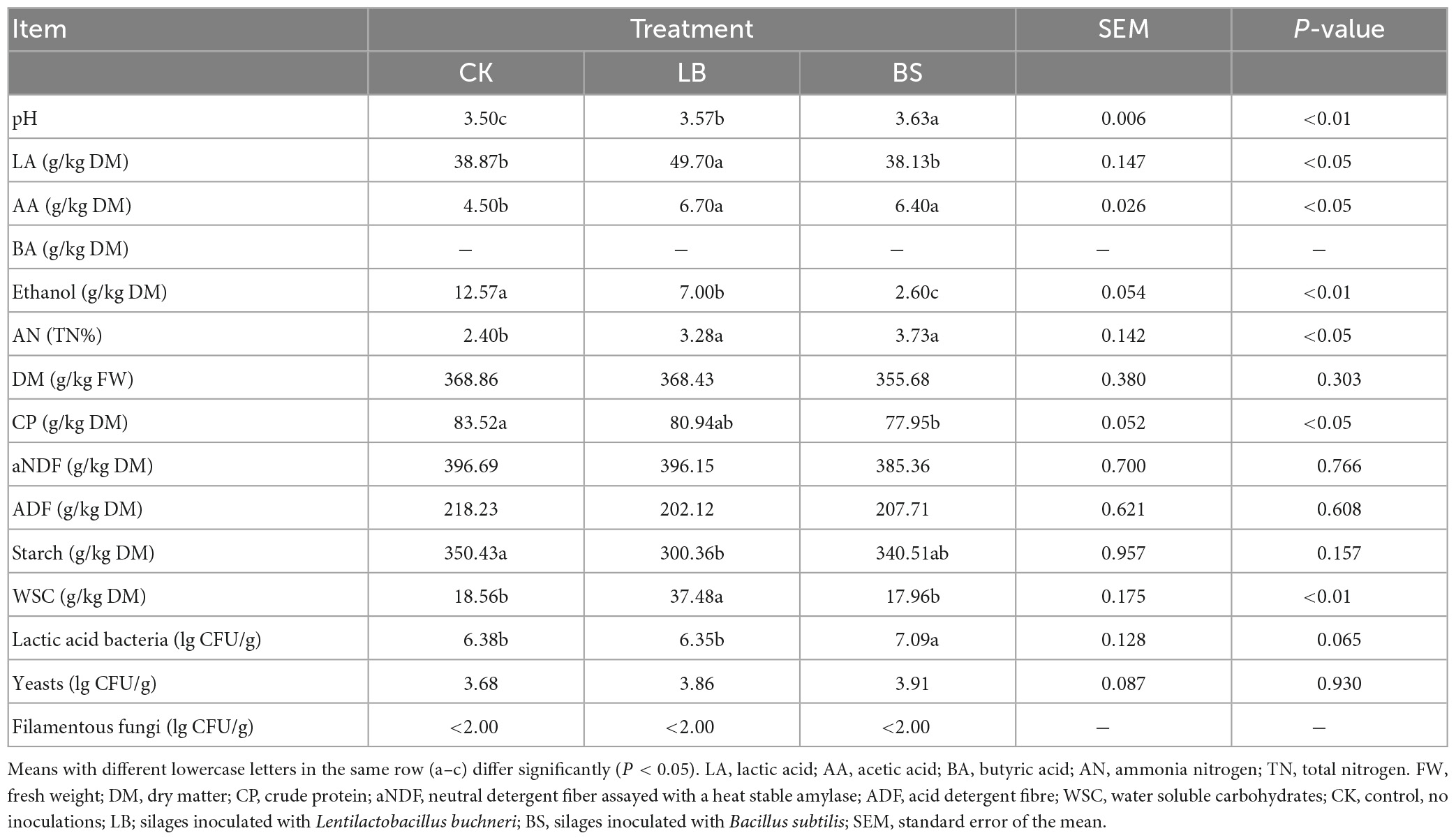
Table 2. Fermentation quality, nutrient composition and microbial counts of whole plant corn silage after 42 days of ensiling.
3.3. Dynamics of temperature and fermentation quality during aerobic exposure
The aerobic stability levels of the LB and BS treatment groups were better than that of the CK group (Figure 1). Aerobic exposure time and inoculant treatment affected pH, LA, AA, and ethanol content (P < 0.05; Figure 2), and their interactions affected pH and ethanol content (P < 0.05). With the prolongation of aerobic exposure time, the pH value of each group gradually increased, the contents of LA, AA, and ethanol gradually decreased, and the pH of CK increased the most, from 3.52 to 4.90. LA and AA in the LB and BS groups were higher than those in the CK group at AS2 (P < 0.05).
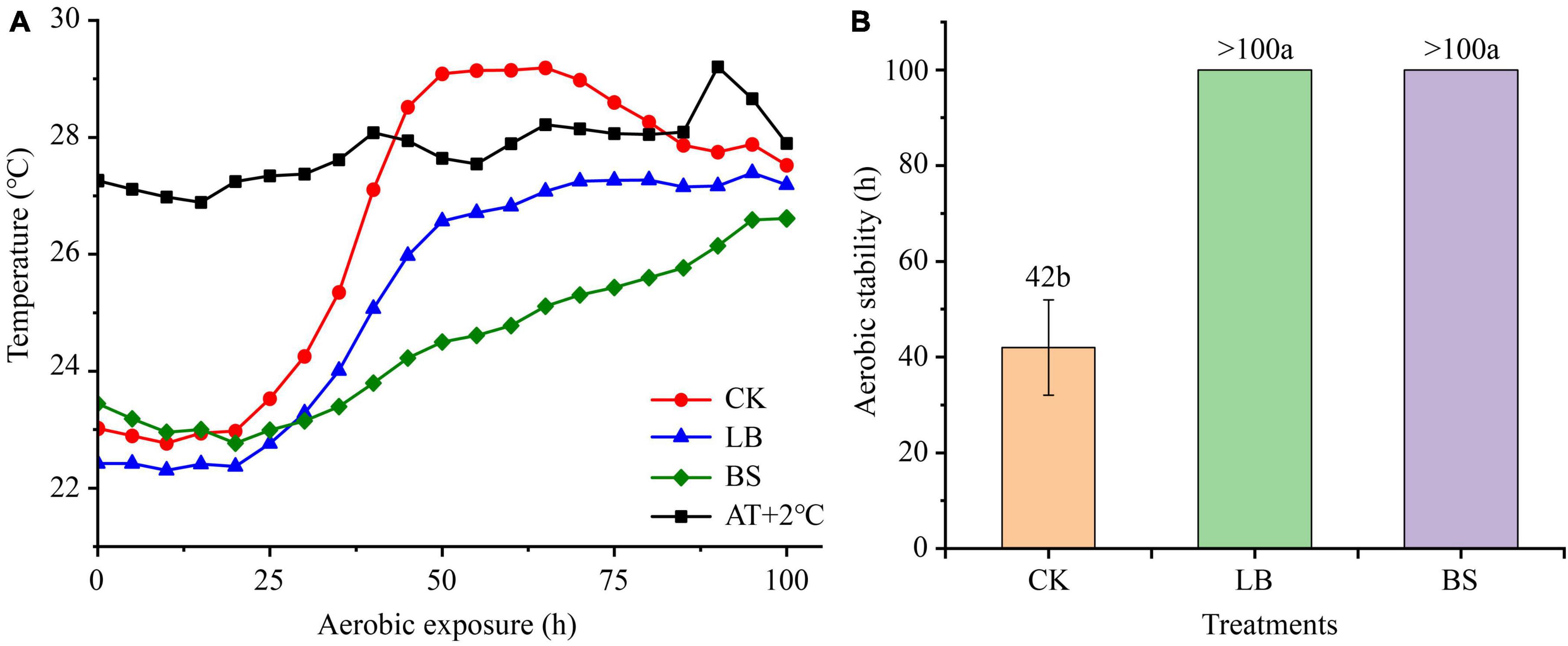
Figure 1. Dynamics of whole plant corn silage temperature (°C) versus ambient temperature during aerobic exposure (A) and the time when silage temperature was 2°C below ambient temperature (B). Means with different lowercase letters (a–b) indicate significant differences in aerobic stability time (P < 0.05). AT + 2°C, ambient temperature +2°C. CK, control, no inoculations; LB, silages inoculated with Lentilactobacillus buchneri; BS, silages inoculated with Bacillus subtilis.
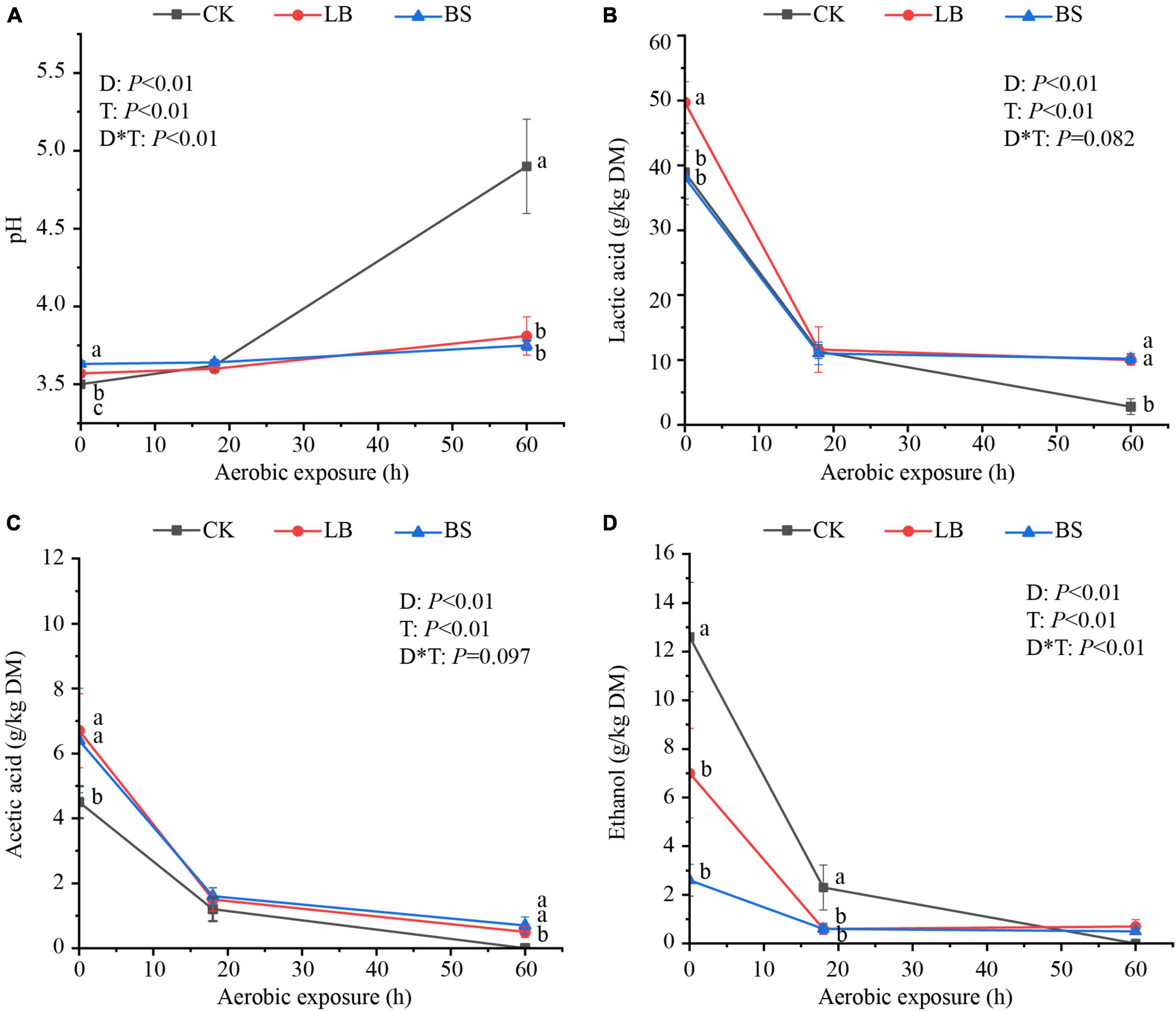
Figure 2. Fermentation quality (A, pH; B, lactic acid; C, acetic acid; D, ethanol) of whole plant corn silage during aerobic exposure. Means with different lowercase letters (a–c) indicate significant differences in different treatments at the same time (P < 0.05). D, Aerobic exposure time; T, treatment; D*T, interaction between aerobic exposure time and treatment; CK, control; no inoculations; LB, silages inoculated with Lentilactobacillus buchneri; BS, silages inoculated with Bacillus subtilis.
3.4. Microbial community during aerobic exposure
The Chao index of the CK group of bacteria decreased with increasing aerobic exposure time (P < 0.05; Figures 3A–C), and the Shannon and Simpson indices were not significantly different (P > 0.05). At AS2, the Chao index was higher in the LB group than in the CK group (P < 0.05), and the Chao and Shannon indices were higher in the BS group than in the CK group (P < 0.05). The Shannon and Chao indices of the fungal communities gradually decreased with increasing aerobic exposure time (P < 0.05 Figures 3D–F). The Shannon and Chao indices of the BS group were higher than those of the other two groups at AS1 and AS2 (P < 0.05), and the Simpson index was lower than those of the other two groups at AS1 (P < 0.05).
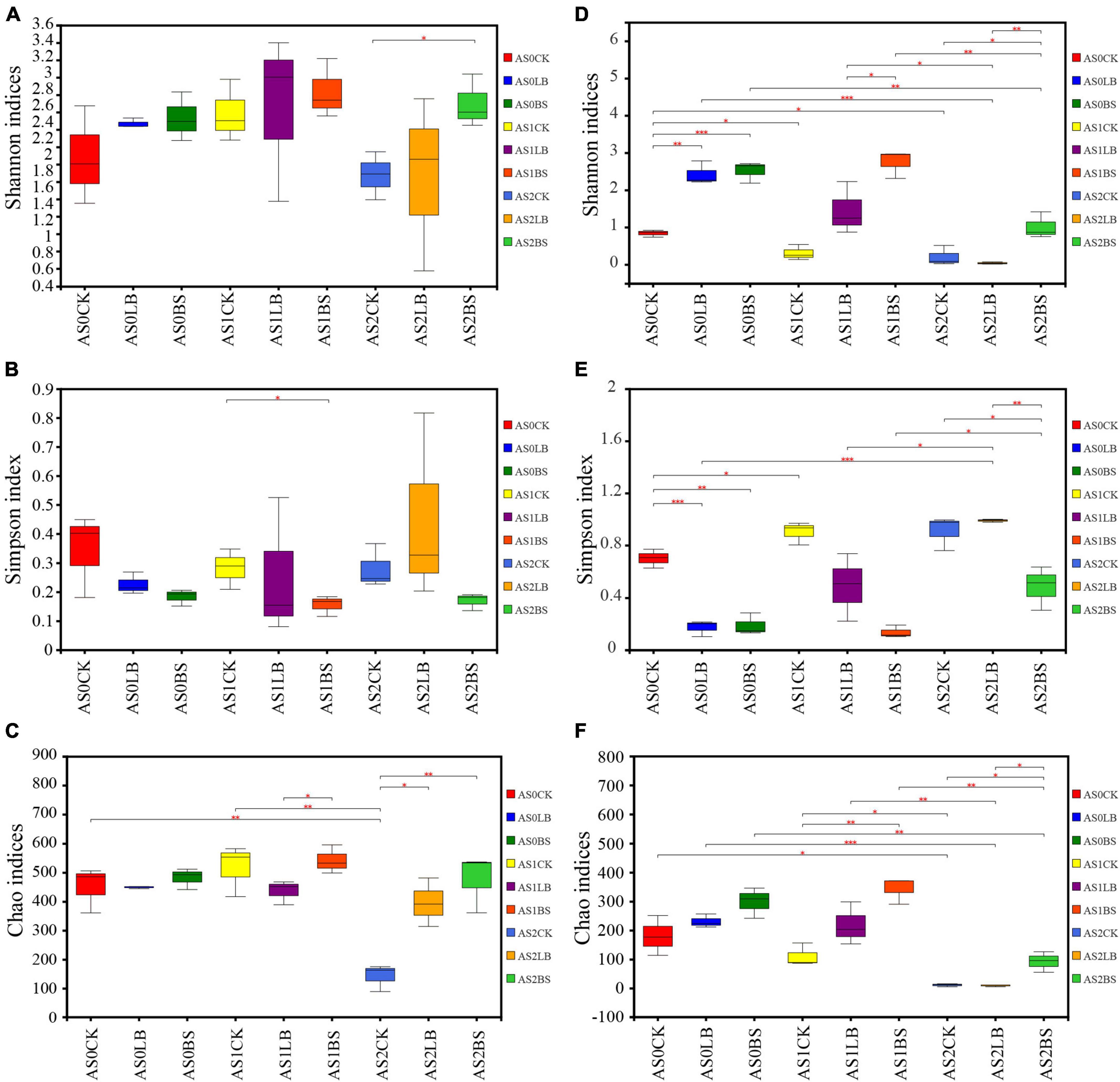
Figure 3. Differences in bacterial (A–C) and fungal (D–F) community diversity and richness in whole plant corn silage during aerobic exposure. (A) Shannon index. (B) Simpson index. (C) Chao index. (D) Shannon index. (E) Simpson index. (F) Chao index. *Significance at P < 0.05; **significance at P < 0.01; ***significance at P < 0.001. AS0CK, not inoculated silage at 0 h of aerobic exposure; AS0LB, Lentilactobacillus buchneri inoculated silage at 0 h of aerobic exposure; AS0BS, inoculated Bacillus subtilis silage at 0 h of aerobic exposure; AS1CK, not inoculated silage at 18 h of aerobic exposure; AS1LB, Lentilactobacillus buchneri inoculated silage at 18 h of aerobic exposure; AS1BS, inoculated Bacillus subtilis silage at 18 h of aerobic exposure; AS2CK, not inoculated silage at 60 h of aerobic exposure; AS2LB, Lentilactobacillus buchneri inoculated silage at 60 h of aerobic exposure; AS2BS, inoculated Bacillus subtilis silage at 60 h of aerobic exposure.
operational taxonomic unit clustering of the sequences according to a similarity of 97% (Figure 4), revealed 124 shared OTUs among the treated bacterial samples, and there were more OTUs unique to BS under aerobic exposure to AS1 (Figure 4A). There were 6 shared OTUs in all treated sample fungi, and aerobic exposure to the AS0, AS1, and BS groups had more OTUs during aerobic exposure, up to 105 at AS1 (Figure 4B).
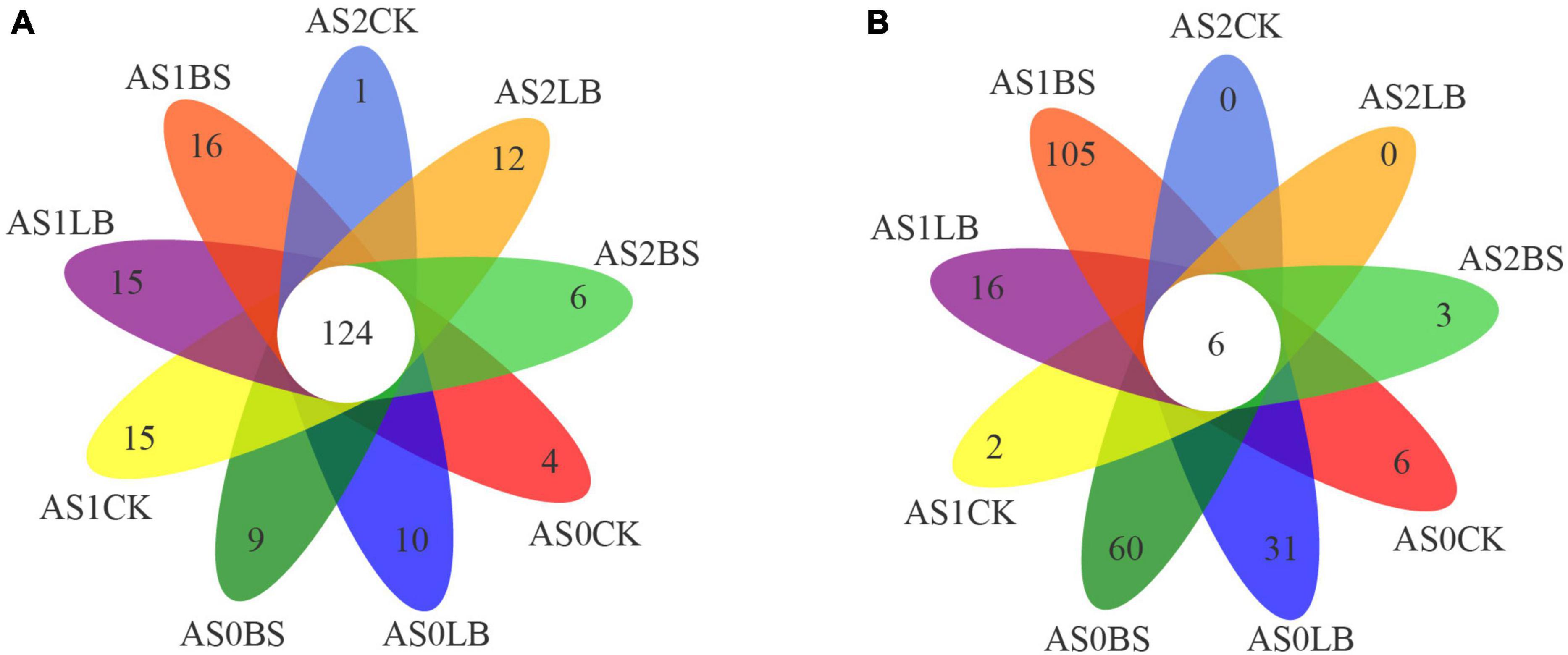
Figure 4. Venn diagram of bacterial (A) and fungal (B) operational taxonomic units (OTUs) in whole plant corn silage during aerobic exposure. AS0CK, not inoculated silage at 0 h of aerobic exposure; AS0LB, Lentilactobacillus buchneri inoculated silage at 0 h of aerobic exposure; AS0BS, inoculated Bacillus subtilis silage at 0 h of aerobic exposure; AS1CK, not inoculated silage at 18 h of aerobic exposure; AS1LB, Lentilactobacillus buchneri inoculated silage at 18 h of aerobic exposure; AS1BS, inoculated Bacillus subtilis silage at 18 h of aerobic exposure; AS2CK, not inoculated silage at 60 h of aerobic exposure; AS2LB, Lentilactobacillus buchneri inoculated silage at 60 h of aerobic exposure; AS2BS, inoculated Bacillus subtilis silage at 60 h of aerobic exposure.
The LB and BS groups were separated from the CK group in the bacterial community at AS0 and AS1 (Figure 5A). Components 1 and 2 could be well-explained in fungal community structure, and the LB and BS groups were separated from the CK group at AS0 (Figure 5B).
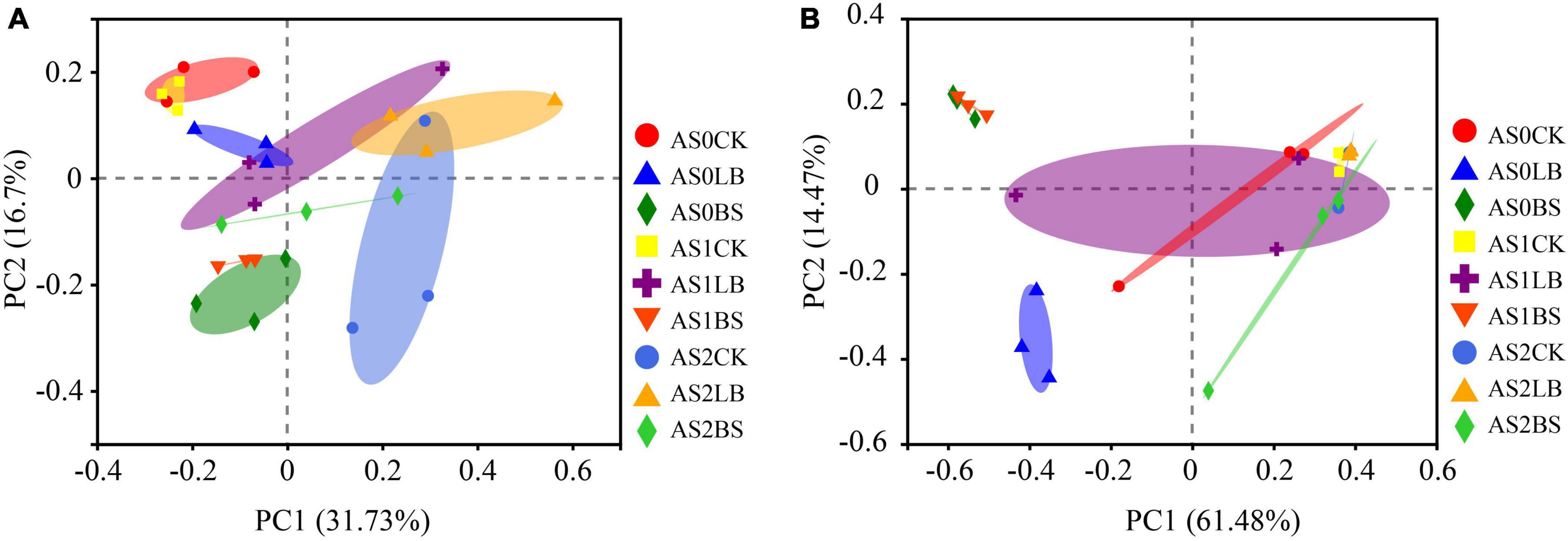
Figure 5. Principal component analysis of bacterial communities (A) and fungal (B) operational taxonomic units (OTUs) in whole plant corn silage during aerobic exposure. AS0CK, not inoculated silage at 0 h of aerobic exposure; AS0LB, Lentilactobacillus buchneri inoculated silage at 0 h of aerobic exposure; AS0BS, inoculated Bacillus subtilis silage at 0 h of aerobic exposure; AS1CK, not inoculated silage at 18 h of aerobic exposure; AS1LB, Lentilactobacillus buchneri inoculated silage at 18 h of aerobic exposure; AS1BS, inoculated Bacillus subtilis silage at 18 h of aerobic exposure; AS2CK, not inoculated silage at 60 h of aerobic exposure; AS2LB, Lentilactobacillus buchneri inoculated silage at 60 h of aerobic exposure; AS2BS, inoculated Bacillus subtilis silage at 60 h of aerobic exposure.
Whole plant corn silage was mainly regulated by Firmicutes and Proteobacteria during aerobic exposure, and Firmicutes had the highest relative abundance (Figure 6A). From AS0 to AS1 of aerobic exposure, the relative abundance of Proteobacteria increased and that of Firmicutes decreased in each treatment. At AS2, the relative abundances of Proteobacteria in the CK and LB groups decreased, but the BS group remained unchanged. The dominant genera were Levilactobacillus, Lentilactobacillus and Lactiplantibacillus (Figure 6B). During the aerobic exposure, differences were observed among treatments in Levilactobacillus, Lentilactobacillus, Lactiplantibacillus, unclassified_f_Enterobacteriaceae, Serratia, Weissella, etc., (P < 0.05; Figure 6C). At AS0, the relative abundance of Serratia in the BS group was higher than those in the CK and LB groups (P < 0.05), and the relative abundance of Lentilactobacillus in the LB and BS group were lower than that in the CK group at AS2 (P < 0.05).
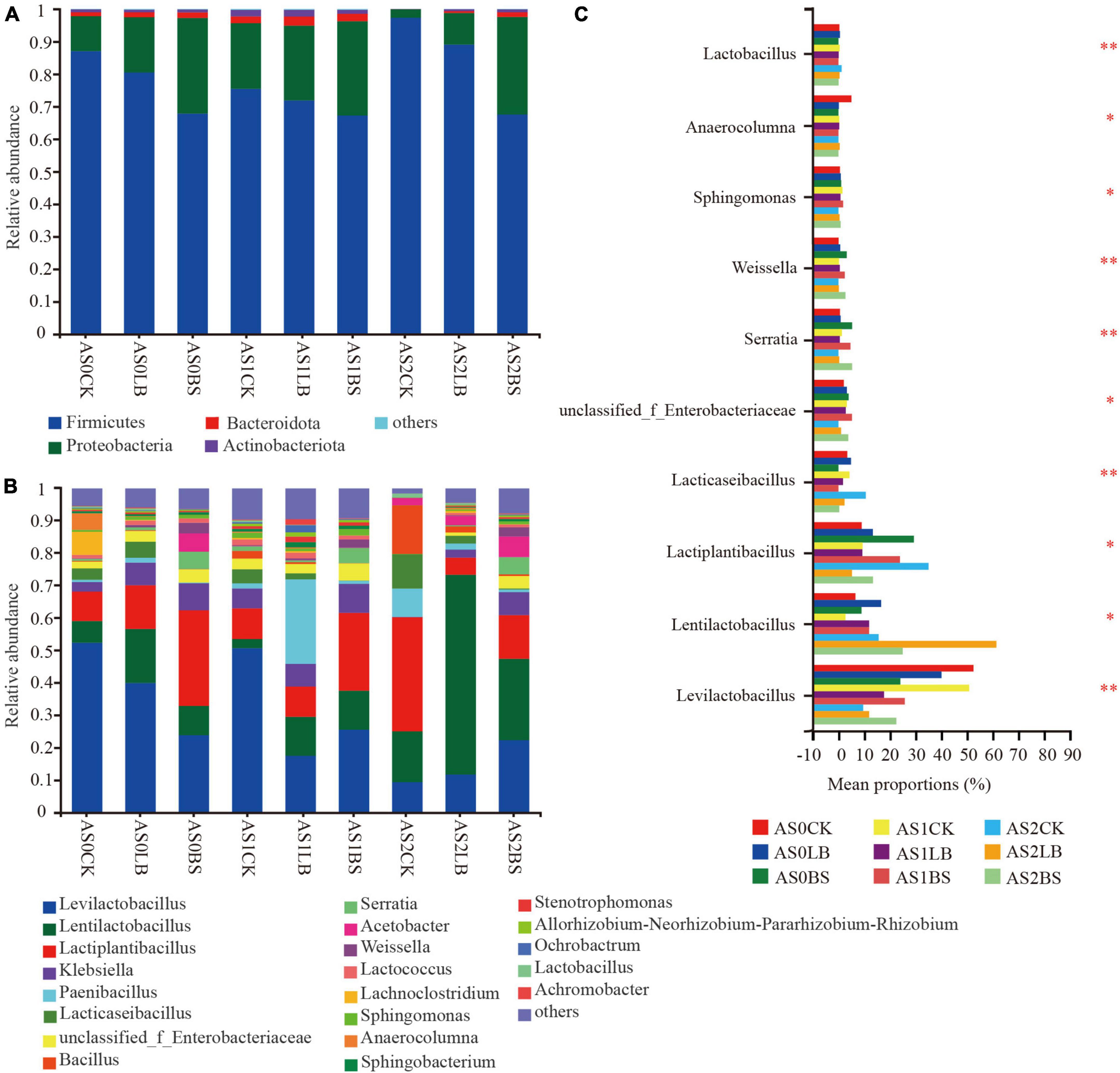
Figure 6. Dynamic changes in relative abundance of bacteria at the phylum level (A) and genus level (B) during aerobic exposure and the differences in genus level (C). *Significance at P < 0.05; **significance at P < 0.01. AS0CK, not inoculated silage at 0 h of aerobic exposure; AS0LB, Lentilactobacillus buchneri inoculated silage at 0 h of aerobic exposure; AS0BS, inoculated Bacillus subtilis silage at 0 h of aerobic exposure; AS1CK, not inoculated silage at 18 h of aerobic exposure; AS1LB, Lentilactobacillus buchneri inoculated silage at 18 h of aerobic exposure; AS1BS, inoculated Bacillus subtilis silage at 18 h of aerobic exposure; AS2CK, not inoculated silage at 60 h of aerobic exposure; AS2LB, Lentilactobacillus buchneri inoculated silage at 60 h of aerobic exposure; AS2BS, inoculated Bacillus subtilis silage at 60 h of aerobic exposure.
The dominant fungi during aerobic exposure of whole plant corn silage were Ascomycota and Basidiomycota (Figure 7A). The relative abundance of Basidiomycota in each treatment increased with prolonged aerobic exposure, but the relative abundance of Basidiomycota in the LB and BS groups were lower than those in the CK group at AS0 and AS1, delaying the trend of increasing Basidiomycota relative abundance. The composition of fungal species at the genus level is shown in Figure 7B. The relative abundance of Kazachstania gradually increased during aerobic exposure, but the relative abundance of Kazachstania in the LB and BS groups were lower than those in the CK group at AS0 and AS1. At AS0, the CK group was dominated by fungi such as Kazachstania and Pichia, Candida accounted for a large proportion in the LB treatment, the relative abundances of Papiliotrema and Hannaella in the BS group accounted for the largest proportions, and the relative abundance of Issatchenkia in the BS group was higher than those in the other treatments at AS2 (P < 0.05; Figure 7C).
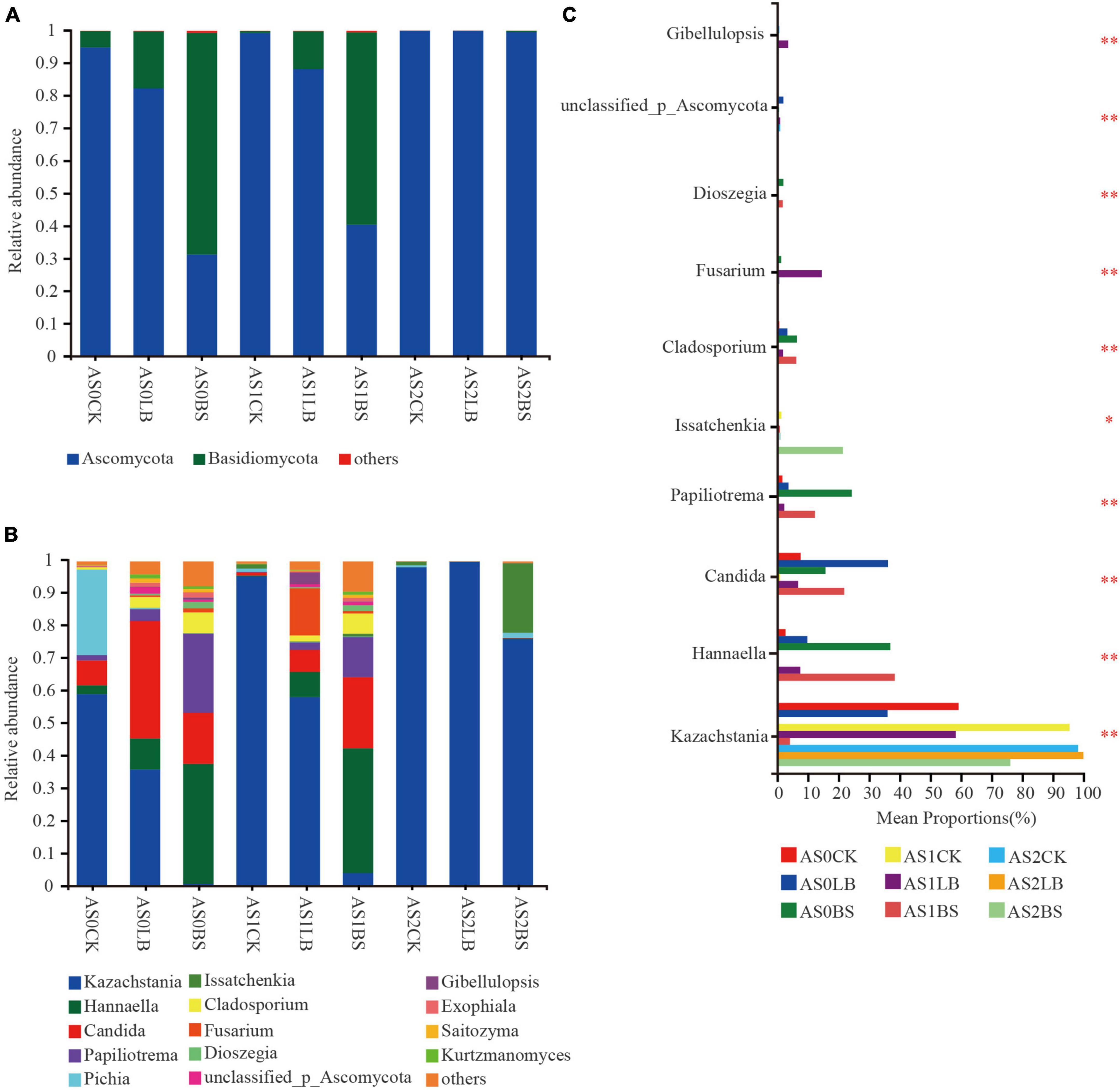
Figure 7. Dynamic changes in relative abundance of fungi at the phylum level (A) and genus level (B) during aerobic exposure and the differences in genus level (C). *Significance at P < 0.05; **significance at P < 0.01. AS0CK, not inoculated silage at 0 h of aerobic exposure; AS0LB, Lentilactobacillus buchneri inoculated silage at 0 h of aerobic exposure; AS0BS, inoculated Bacillus subtilis silage at 0 h of aerobic exposure; AS1CK, not inoculated silage at 18 h of aerobic exposure; AS1LB, Lentilactobacillus buchneri inoculated silage at 18 h of aerobic exposure; AS1BS, inoculated Bacillus subtilis silage at 18 h of aerobic exposure; AS2CK, not inoculated silage at 60 h of aerobic exposure; AS2LB, Lentilactobacillus buchneri inoculated silage at 60 h of aerobic exposure; AS2BS, inoculated Bacillus subtilis silage at 60 h of aerobic exposure.
Silage pH was negatively correlated with the abundances of Levilactobacillus, Lactococcus, etc., in the bacterial community (P < 0.05; Figure 8A). The contents of LA and AA were positively correlated with the abundance of Levilactobacillus (P < 0.05) but negatively correlated with the abundance of Bacillus (P < 0.05). From the correlation analysis of fungal and fermentation characteristics during aerobic exposure, the pH value was negatively correlated with the abundance of Hannaella, Candida, etc., in the fungal community (P < 0.01; Figure 8B), but positively correlated with the abundance of Kazachstania (P < 0.05). The contents of LA and AA were positively correlated with the abundance of Hannaella, Candida, etc., (P < 0.01), but negatively correlated with the abundance of Kazachstania (P < 0.01).
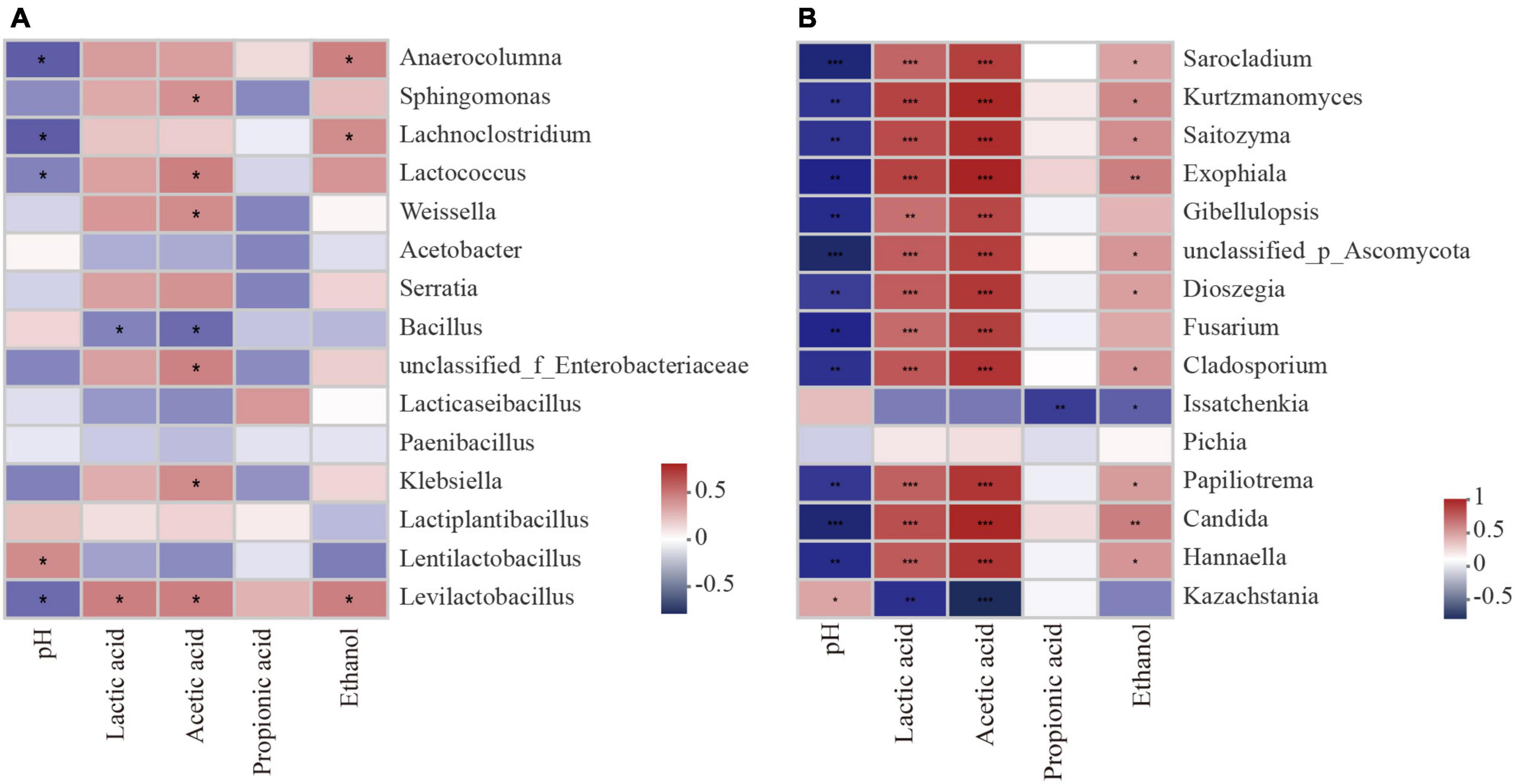
Figure 8. Spearman analysis between silage parameters and bacterial species (A) fungal species (B) during aerobic exposure. *Significance at P < 0.05; **significance at P < 0.01; ***significance at P < 0.001.
Functional predictions of fungal communities were based on FUNGuild (Figure 9) determined that the undefined saprotroph was inhibited in the LB and BS groups compared to AS1CK, which became dominant in all treatments at AS2.
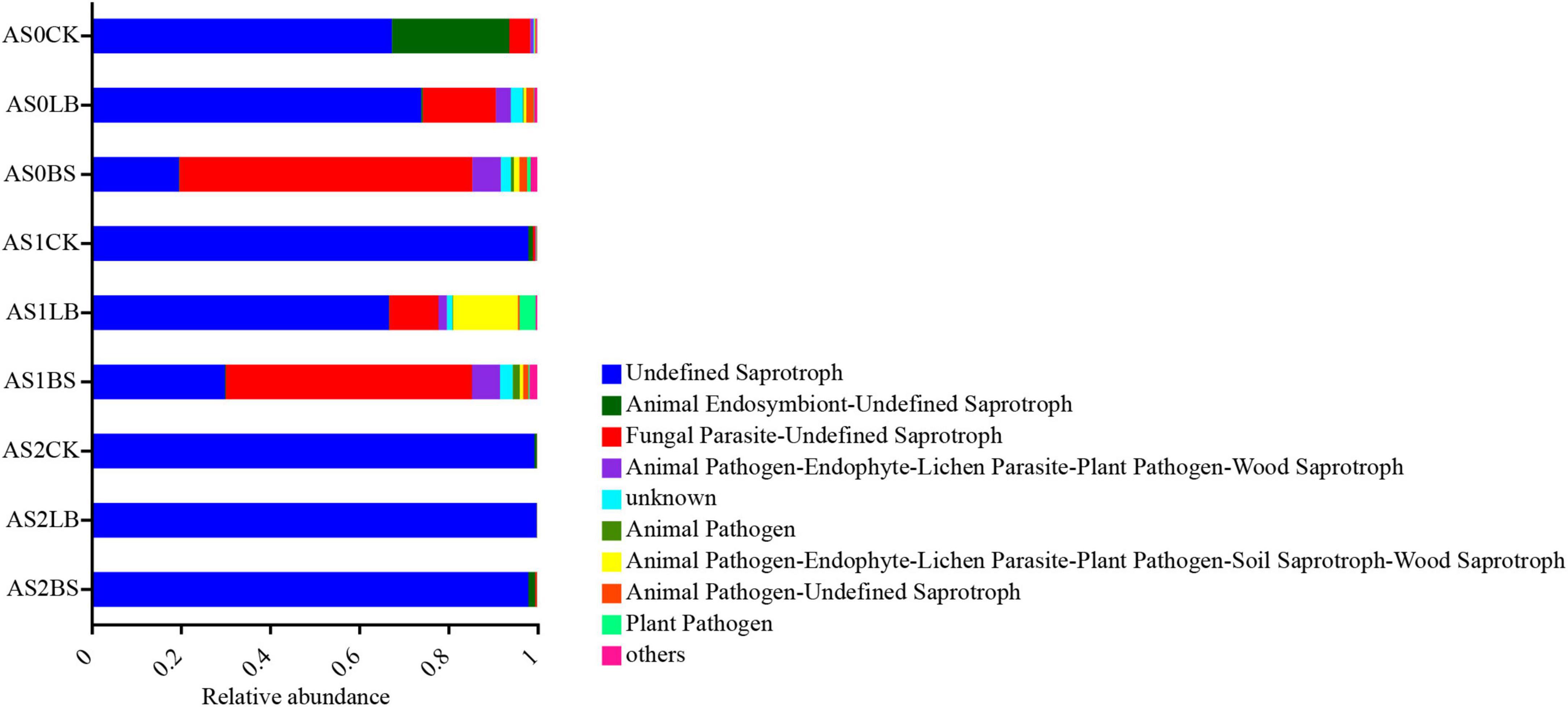
Figure 9. Variations in composition of fungal functional groups inferred by FUNGuild during aerobic exposure. AS0CK, not inoculated silage at 0 h of aerobic exposure; AS0LB, Lentilactobacillus buchneri inoculated silage at 0 h of aerobic exposure; AS0BS, inoculated Bacillus subtilis silage at 0 h of aerobic exposure; AS1CK, not inoculated silage at 18 h of aerobic exposure; AS1LB, Lentilactobacillus buchneri inoculated silage at 18 h of aerobic exposure; AS1BS, inoculated Bacillus subtilis silage at 18 h of aerobic exposure; AS2CK, not inoculated silage at 60 h of aerobic exposure; AS2LB, Lentilactobacillus buchneri inoculated silage at 60 h of aerobic exposure; AS2BS, inoculated Bacillus subtilis silage at 60 h of aerobic exposure.
4. Discussion
4.1. Material characteristics and silage quality
The composition of the fresh forage in this experiment was within the expected range for whole plant corn (Ranjit and Kung, 2000; Ferraretto and Shaver, 2015). It is generally believed that pH and AN contents lower than 4.2 and 10% TN indicate good silage quality (Ahmadi et al., 2019). In the quality analysis of silage after 42 days of fermentation, the pH value and AN of the LB and BS treatments were higher than the CK group, but both were within a good range, and the ethanol content was low with no BA production (Sifeeldein et al., 2019), indicating that the fermentation quality of all treatments was in good condition. The LA content of the LB group was higher than that of the CK group, presumably because of the higher abundance of Lentilactobacillus in the LB group (Wu et al., 2022b). Lentilactobacillus buchneri can convert part of the lactic acid from the silage to acetic acid, increasing the acetic acid concentration of silage and causing small increases in pH (Schmidt and Kung, 2010; Benjamim da Silva et al., 2021; Ferrero et al., 2021b). In this trial, LB inoculation increased the AA content, resulting in an increase in pH value. The AA content of the BS group was significantly higher than that of the CK group, which may account for the better aerobic stability of the BS group (Basso et al., 2012; Bai et al., 2022a). Acetic acid has good antifungal properties and can inhibit the growth of fungi, thereby inhibiting the process of aerobic spoilage of silage (Santos et al., 2020). There was no significant difference in the number of yeasts among treatments, and the number of filamentous fungi was below the detection limit, because the lower pH maintains an acidic environment and inhibits the growth of yeasts and filamentous fungi (Sun et al., 2021).
The relative abundances of unclassified_f_Enterobacteriaceae and Klebsiella in the BS treatment were higher, with a higher pH, presumably explaining the higher protease activity during silage, which may account for the higher CP degradation (Chen et al., 2014). The study by Bernardi et al. (2019) in the literature also observed a decrease in the CP content of silage after the addition of BS with an increasing concentration of addition, similar to the results of this experiment. The content of aNDF and ADF in BS group did not differ, indicating that B. subtilis has the low fiber dissolving activity in this experiment (Guo et al., 2022). In the LB group, the WSC content was higher and the starch content was lower than in the CK group, which is similar to the results of Comino et al. (2014).
4.2. Aerobic stability of silage
Usually, the core temperature of silage is 2°C higher than the ambient temperature to measure whether aerobic deterioration occurs (Ranjit and Kung, 2000). In this study, the aerobic stabilization times were higher in the LB and BS groups than in the CK group, suggesting that inoculation with LB or BS improves aerobic stability. This is due to the higher AA concentrations in the LB and BS groups at AS0, inhibiting the growth of aerobic microorganisms (Basso et al., 2012). In addition, BS can produce antifungal and antibacterial substances that inhibit the growth of undesirable microorganisms such as yeast (Bonaldi et al., 2021).
In the present study, all treatments of whole plant corn silage significantly increased the pH and decreased the LA and AA contents with prolonged aerobic exposure, because in the process of aerobic exposure, aerobic microorganisms decompose the organic acids such as lactic acid and acetic acid produced in the process of ensilage, and produced carbon dioxide (CO2) and water, which leads to an increase in the pH value (Tabacco et al., 2011; Li et al., 2017; Guo et al., 2022). Compared to the CK group, the LB or BS groups showed slower increases in pH and high LA and AA residues, indicating that the addition of LB and BS retarded the loss of material from the silage during aerobic exposure, in line with the findings of Lara et al. (2016). In the study of Yuan et al. (2018), ethanol as an exogenous addition (25 ml/kg FW) had the potential to inhibit aerobic spoilage. The ethanol in this experiment was a product of natural fermentation. The ethanol content of the CK group, although higher than those of the LB and BS groups, was well below the level of exogenous addition, which may account for the lack of effect on aerobic stability (Huisden et al., 2009).
4.3. Microbial community in silage
Our results showed that the duration of aerobic exposure had no significant effect on the Shannon and Simpson indices of the whole plant corn silage bacterial community, indicating that bacterial diversity was maintained in silage during the aerobic exposure phase (Drouin et al., 2019). In contrast, bacterial richness was reduced in the CK group in terms of the Chao index; presumably, the higher pH in the later stages of aerobic exposure provided conditions for the proliferation of spoilage microorganisms, which in turn reduced bacterial richness (Wu et al., 2022a). Inoculation with LB or BS maintained bacterial diversity and richness better during aerobic exposure. Principal component analysis clearly showed the differences between groups of bacterial and fungal communities, respectively, indicating that inoculation with LB or BS altered bacterial and fungal communities during aerobic exposure. Fungal diversity decreased in diversity and abundance with increasing exposure time, indicating that the fungal community composition gradually simplified (Zhang et al., 2019). The fungal community diversities of the LB and BS treatments were higher than that of the CK group, and the fungal abundance and special OTU numbers were higher in the BS of the silage inoculation group than in the CK and LB groups, which may account for the higher aerobic stability of LB or BS (Bai et al., 2020).
Similar to most studies, the bacterial communities of whole plant corn silage in all treatments were mainly regulated by Firmicutes and Proteobacteria during aerobic exposure in this experiment (Drouin et al., 2019; Liu et al., 2020). The inoculation of LB or BS significantly affected the relative abundances of Levilactobacillus, Lentilactobacillus, Lactiplantibacillus, Lacticaseibacillus, Enterobacteria and Weissella, etc. Regarding the genus Lactobacillus, to which Lactiplantibacillus, Lentilactobacillus, and Lacticaseibacillus originally belonged (Zheng et al., 2020), in the study of Wu et al. (2022a), inoculation with L. buchneri improved aerobic stability and maintained the high relative abundance of Lactobacillus during aerobic exposure. In this experiment, the relative abundance of Lentilactobacillus at AS2 was higher in the LB and BS groups than in the CK group, which may be one of the reasons for the improved aerobic stability of the LB and BS groups. Research has reported that Enterobacteria are able to improve aerobic stability (Wilkinson and Davies, 2013). We found that the relative abundance of Enterobacteria was significantly different among BS groups at AS2, which may be one of the reasons for its better aerobic stability. During aerobic exposure, the relative abundance of Weissella in the BS group was higher than those in the other two groups. Weissella, another major microorganism in all silage throughout the fermentation process (Guan et al., 2018), was able to produce lactate (Ni et al., 2018). In the significant differences in bacterial relative abundance analysis, Serratia was the most abundant genus in the BS group, Serratia can produce prodigiosin, a secondary metabolite that might inhibit the proliferation of fungi (Zhang et al., 2019), which may be one of the reasons for the higher aerobic stability in the BS group. Bacillus caused aerobic spoilage in a previous study (Liu et al., 2020). In this experiment, the relative abundance of Bacillus in the LB and BS groups were lower than that in the CK group at AS2, indicating that the addition of LB and BS could inhibit the growth of Bacillus and improve aerobic stability.
Ascomycota and Basidiomycota were the dominant fungal phyla of whole plant corn silage, which was similar to the findings of Liu et al. (2019). During aerobic exposure, LB or BS inoculation delayed the increasing trend of the relative abundance of Basidiomycota, indicating that inoculation with LB or BS significantly altered the structure of the whole plant corn silage fungal population during aerobic exposure (Zhang et al., 2019). Wang et al. (2021) found that Kazachstania was dominant in corn silage with poor aerobic stability. In the early stage of aerobic exposure, Kazachstania, Hannaella, and Candida possessed dominant positions in the fungal community; as time progressed, the relative abundance of Kazachstania gradually increased, becoming all the dominant fungi in AS2. The inoculation of LB or BS delayed fungal succession and inhibited Kazachstania, thus resulting in higher aerobic stability. At AS0, the relative abundance of Candida was significantly higher in the LB group than in the other two groups, and the relative abundances of Hannaella were significantly higher in the BS groups of AS1 and AS2. Candida and Hannaella were the fungal genera related to aerobic spoilage (Drouin et al., 2019; Wang et al., 2020), which may play a role in the early stage of aerobic exposure in this experiment but is not the decisive factor triggering aerobic spoilage. The community composition of the BS group did not change much at AS1 and AS2, but the relative abundance of Issatchenkia was higher in BS at AS2, and Issatchenkia also dominated after aerobic exposure of barley silage, as reported by Liu et al. (2020).
The FUNGuild prediction results showed that, inoculation with LB and BS reduced the relative abundance of undefined saprotroph and increased the relative abundance of fungal parasite-undefined saprotroph during the aerobic exposure phase, presumably associated with increased aerobic stability, undefined saprotroph was dominated by the highest relative abundance of undefined saprotroph in each treatment after aerobic decay (Wu et al., 2022c).
5. Conclusion
Compared to the CK group, LB or BS inoculated in whole plant corn silage maintained satisfactory fermentation quality, improved aerobic stability, and effectively inhibited aerobic spoilage. In combination with the analysis of the microbial community structure, it was found that the delay in aerobic spoilage may be related to the Bacillus and Kazachstania inhibited and higher relative abundance of fungal parasite-undefined saprotroph by inoculated of LB or BS.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
HY, JS, ZY, CB, and YX designed the study and wrote the manuscript. HY, MZ, GP, HZ, and RY performed the experiments. JS, ZY, CB, and YX reviewed and edited the manuscript. HY, MZ, and GP analyzed the data. ZY, CB, and YX funded and supervised the experiments. All authors reviewed the manuscript and approved the submitted version.
Funding
This work was funded by Project of Education Department of Liaoning Province (LJKMZ20221021), the National Natural Science Foundation of China (31872422), and the Project of Central Government Guiding Fund for Developing Local Science and Technology – Free Exploration Basic Research Project (2022ZY0152).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Ahmadi, F., Lee, Y. H., Lee, W. H., Oh, Y. K., Park, K., and Kwak, W. S. (2019). Long-term anaerobic conservation of fruit and vegetable discards without or with moisture adjustment after aerobic preservation with sodium metabisulfite. Waste Manage. 87, 258–267. doi: 10.1016/j.wasman.2019.02.010
Bai, C., Pan, G., Leng, R., Ni, W., Yang, J., Sun, J., et al. (2022a). Effect of ensiling density and storage temperature on fermentation quality, bacterial community, and nitrate concentration of sorghum-sudangrass silage. Front. Microbiol. 13:828320. doi: 10.3389/fmicb.2022.828320
Bai, J., Franco, M., Ding, Z., Hao, L., Ke, W., Wang, M., et al. (2022b). Effect of Bacillus amyloliquefaciens and Bacillus subtilis on fermentation, dynamics of bacterial community and their functional shifts of whole-plant corn silage. J. Anim. Sci. Biotechnol. 13:7. doi: 10.1186/s40104-021-00649-0
Bai, C., Wang, C., Sun, L., Xu, H., Jiang, Y., Na, N., et al. (2021). Dynamics of bacterial and fungal communities and metabolites during aerobic exposure in whole-plant corn silages with two different moisture levels. Front. Microbiol. 12:663895. doi: 10.3389/fmicb.2021.663895
Bai, J., Xu, D., Xie, D., Wang, M., Li, Z., and Guo, X. (2020). Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bioresour. Technol. 315:123881. doi: 10.1016/j.biortech.2020.123881
Basso, F. C., Bernardes, T. F., de Toledo Piza Roth, A. P., Lodo, B. N., Berchielli, T. T., and Reis, R. A. (2012). Fermentation and aerobic stability of corn silage inoculated with Lactobacillus buchneri. Rev. Bras. Zootecn. 41, 1789–1794. doi: 10.1590/S1516-35982012000700032
Benjamim da Silva, É., Costa, D. M., Santos, E. M., Moyer, K., Hellings, E., and Kung, L. (2021). The effects of Lactobacillus hilgardii 4785 and Lactobacillus buchneri 40788 on the microbiome, fermentation, and aerobic stability of corn silage ensiled for various times. J. Dairy Sci. 104, 10678–10698. doi: 10.3168/jds.2020-20111
Bernardi, A., Härter, C. J., Silva, A. W. L., Reis, R. A., and Rabelo, C. H. S. (2019). A meta-analysis examining lactic acid bacteria inoculants for maize silage: Effects on fermentation, aerobic stability, nutritive value and livestock production. Grass Forage Sci. 74, 596–612. doi: 10.1111/gfs.12452
Bonaldi, D. S., Carvalho, B. F., Ávila, C. L. D. S., and Silva, C. F. (2021). Effects of Bacillus subtilis and its metabolites on corn silage quality. Appl. Microbiol. Biotechnol. 73, 46–53. doi: 10.1111/lam.13474
Bumbieris, V. H. Jr., Guimarães, V. A. D. P., Fortaleza, A. P. D. S., Massaro, F. L. Jr., Moraes, G. J. D., and Meza, D. A. R. (2017). Aerobic stability in corn silage (Zea mays L.) ensiled with different microbial additives. Acta. Sci. Anim. Sci. 39, 357–362. doi: 10.4025/actascianimsci.v39i4.34426
Chen, L., Guo, G., Yuan, X., Shimojo, M., Yu, C., and Shao, T. (2014). Effect of applying molasses and propionic acid on fermentation quality and aerobic stability of total mixed ration silage prepared with whole-plant corn in Tibet. Asian-Australas J. Anim. Sci. 27, 349–356. doi: 10.5713/ajas.2013.13378
Comino, L., Tabacco, E., Righi, F., Revello-Chion, A., Quarantelli, A., and Borreani, G. (2014). Effects of an inoculant containing a Lactobacillus buchneri that produces ferulate-esterase on fermentation products, aerobic stability, and fibre digestibility of maize silage harvested at different stages of maturity. Anim. Feed Sci. Technol. 198, 94–106. doi: 10.1016/j.anifeedsci.2014.10.001
Cui, Y., Liu, H., Gao, Z., Xu, J., Liu, B., Guo, M., et al. (2022). Whole-plant corn silage improves rumen fermentation and growth performance of beef cattle by altering rumen microbiota. Appl. Microbiol. Biotechnol. 106, 4187–4198. doi: 10.1007/s00253-022-11956-5
Drouin, P., Tremblay, J., and Chaucheyras-Durand, F. (2019). Dynamic succession of microbiota during ensiling of whole plant corn following inoculation with Lactobacillus buchneri and Lactobacillus hilgardii alone or in combination. Microorganisms 7:595. doi: 10.3390/microorganisms7120595
Ferraretto, L. F., and Shaver, R. D. (2015). Effects of whole-plant corn silage hybrid type on intake, digestion, ruminal fermentation, and lactation performance by dairy cows through a meta-analysis. J. Dairy Sci. 98, 2662–2675. doi: 10.3168/jds.2014-9045
Ferrero, F., Tabacco, E., and Borreani, G. (2021a). Lentilactobacillus hilgardii inoculum, dry matter contents at harvest and length of conservation affect fermentation characteristics and aerobic stability of corn silage. Front. Microbiol. 12:675563. doi: 10.3389/fmicb.2021.675563
Ferrero, F., Tabacco, E., Piano, S., Casale, M., and Borreani, G. (2021b). Temperature during conservation in laboratory silos affects fermentation profile and aerobic stability of corn silage treated with Lactobacillus buchneri, Lactobacillus hilgardii, and their combination. J. Dairy Sci. 104, 1696–1713. doi: 10.3168/jds.2020-18733
Gallo, A., Fancello, F., Ghilardelli, F., Zara, S., and Spanghero, M. (2022). Effects of several commercial or pure lactic acid bacteria inoculants on fermentation and mycotoxin levels in high-moisture corn silage. Anim. Feed Sci. Technol. 286:115256. doi: 10.1016/j.anifeedsci.2022.115256
Gandra, J. R., Oliveira, E. R., de Sena Gandra, E. R., Takiya, C. S., Tonissi Buschineli de Goes, R. H., Pires Oliveira, K. M., et al. (2017). Inoculation of Lactobacillus buchneri alone or with Bacillus subtilis and total losses, aerobic stability, and microbiological quality of sunflower silage. J. Appl. Anim. Res. 45, 609–614. doi: 10.1080/09712119.2016.1249874
Guan, H., Ran, Q., Li, H., and Zhang, X. (2021). Succession of microbial communities of corn silage inoculated with heterofermentative lactic acid bacteria from ensiling to aerobic exposure. Fermentation. 7:258. doi: 10.3390/fermentation7040258
Guan, H., Yan, Y., Li, X., Li, X., Shuai, Y., Feng, G., et al. (2018). Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 265, 282–290. doi: 10.1016/j.biortech.2018.06.018
Guo, X., Guo, W., Yang, M., Sun, Y., Wang, Y., Yan, Y., et al. (2022). Effect of Bacillus additives on fermentation quality and bacterial community during the ensiling process of whole-plant corn silage. Processes 10:978. doi: 10.3390/pr10050978
Han, L. Y., Li, J., Na, R. S., Yu, Z., and Zhou, H. (2015). Effect of two additives on the fermentation, in vitro digestibility and aerobic security of Sorghum–sudangrass hybrid silages. Grass Forage Sci. 70, 185–194. doi: 10.1111/gfs.12092
Huang, Z., Wang, M., Ke, W., and Guo, X. (2021). Screening of high 1,2-propanediol production by Lactobacillus buchneri strains and their effects on fermentation characteristics and aerobic stability of whole-plant corn silage. Agriculture 11:590. doi: 10.3390/agriculture11070590
Huisden, C. M., Adesogan, A. T., Kim, S. C., and Ososanya, T. (2009). Effect of applying molasses or inoculants containing homofermentative or heterofermentative bacteria at two rates on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 92, 690–697. doi: 10.3168/jds.2008-1546
Ke, W. C., Ding, W. R., Xu, D. M., Ding, L. M., Zhang, P., Li, F. D., et al. (2017). Effects of addition of malic or citric acids on fermentation quality and chemical characteristics of alfalfa silage. J. Dairy Sci. 100, 8958–8966. doi: 10.3168/jds.2017-12875
Kung, L., and Ranjit, N. K. (2001). The effect of Lactobacillus buchneri and other additives on the fermentation and aerobic stability of barley silage. J. Dairy Sci. 84, 1149–1155. doi: 10.3168/jds.S0022-0302(01)74575-4
Kung, L., Smith, M. L., Benjamim da Silva, E., Windle, M. C., da Silva, T. C., and Polukis, S. A. (2018). An evaluation of the effectiveness of a chemical additive based on sodium benzoate, potassium sorbate, and sodium nitrite on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 101, 5949–5960. doi: 10.3168/jds.2017-14006
Lara, E. C., Basso, F. C., de Assis, F. B., Souza, F. A., Berchielli, T. T., and Reis, R. A. (2016). Changes in the nutritive value and aerobic stability of corn silages inoculated with Bacillus subtilis alone or combined with Lactobacillus plantarum. Anim. Prod. Sci. 56, 1867–1874. doi: 10.1071/AN14686
Li, D. X., Ni, K. K., Zhang, Y. C., Lin, Y. L., and Yang, F. Y. (2018). Influence of lactic acid bacteria, cellulase, cellulase-producing Bacillus pumilus and their combinations on alfalfa silage quality. J. Integr. Agric. 17, 2768–2782. doi: 10.1016/S2095-3119(18)62060-X
Li, M., Shan, G., Zhou, H., Buescher, W., Maack, C., Jungbluth, K. H., et al. (2017). CO2 production, dissolution and pressure dynamics during silage production: Multi-sensor-based insight into parameter interactions. Sci. Rep. 7:14721. doi: 10.1038/s41598-017-14187-1
Liu, B., Huan, H., Gu, H., Xu, N., Shen, Q., and Ding, C. (2019). Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 273, 212–219. doi: 10.1016/j.biortech.2018.10.041
Liu, B., Yang, Z., Huan, H., Gu, H., Xu, N., and Ding, C. (2020). Impact of molasses and microbial inoculants on fermentation quality, aerobic stability, and bacterial and fungal microbiomes of barley silage. Sci. Rep. 10:5342. doi: 10.1038/s41598-020-62290-7
Mangwe, M. C., Rangubhet, K. T., Mlambo, V., Yu, B., and Chiang, H. I. (2016). Effects of Lactobacillus formosensis S215T and Lactobacillus buchneri on quality and in vitro ruminal biological activity of condensed tannins in sweet potato vines silage. J. Appl. Microbiol. 121, 1242–1253. doi: 10.1111/jam.13260
Mendonça, R. D. C. A. D., Cardoso, M. V. S. B., Pantoja, S. O. S., Souza, M. S. D., Domingues, F. N., Faturi, C., et al. (2020). Effects of cutting height and bacterial inoculant on corn silage aerobic stability and nutrient digestibility by sheep. Rev. Bras. Zootecn. 49:e20190231. doi: 10.37496/rbz4920190231
Ni, K., Zhao, J., Zhu, B., Su, R., Pan, Y., Ma, J., et al. (2018). Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 265, 563–567. doi: 10.1016/j.biortech.2018.05.097
Ranjit, N. K., and Kung, L. (2000). The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 83, 526–535. doi: 10.3168/jds.S0022-0302(00)74912-5
Santos, A. P. M. D., Santos, E. M., Araújo, G. G. L. D., Oliveira, J. S. D., Zanine, A. D. M., Pinho, R. M. A., et al. (2020). Effect of inoculation with preactivated Lactobacillus Buchneri and urea on fermentative profile, aerobic stability and nutritive value in corn silage. Agriculture 10:335. doi: 10.3390/agriculture10080335
Schmidt, R. J., and Kung, L. (2010). The effects of Lactobacillus buchneri with or without a homolactic bacterium on the fermentation and aerobic stability of corn silages made at different locations. J. Dairy Sci. 93, 1616–1624. doi: 10.3168/jds.2009-2555
Sifeeldein, A., Wang, S., Li, J., Dong, Z., Chen, L., Kaka, N. A., et al. (2019). Phylogenetic identification of lactic acid bacteria isolates and their effects on the fermentation quality of sweet sorghum (Sorghum bicolor) silage. J. Appl. Anim. Res. 126, 718–729. doi: 10.1111/jam.14123
Sun, L., Bai, C., Xu, H., Na, N., Jiang, Y., Yin, G., et al. (2021). Succession of bacterial community during the initial aerobic, intense fermentation, and stable phases of whole-plant corn silages treated with lactic acid bacteria suspensions prepared from other silages. Front. Microbiol. 12:655095. doi: 10.3389/fmicb.2021.655095
Tabacco, E., Piano, S., Revello-Chion, A., and Borreani, G. (2011). Effect of Lactobacillus buchneri LN4637 and Lactobacillus buchneri LN40177 on the aerobic stability, fermentation products, and microbial populations of corn silage under farm conditions. J. Dairy Sci. 94, 5589–5598. doi: 10.3168/jds.2011-4286
Thomas, T. A. (1977). An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 28, 639–642. doi: 10.1002/jsfa.2740280711
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wang, C., Han, H., Sun, L., Na, N., Xu, H., Chang, S., et al. (2021). Bacterial succession pattern during the fermentation process in whole-plant corn silage processed in different geographical areas of northern China. Processes 9:900. doi: 10.3390/pr9050900
Wang, M., Xu, S., Wang, T., Jia, T., Xu, Z., Wang, X., et al. (2018). Effect of inoculants and storage temperature on the microbial, chemical and mycotoxin composition of corn silage. Asian-Australas J. Anim. Sci. 31, 1903–1912. doi: 10.5713/ajas.17.0801
Wang, T., Teng, K., Cao, Y., Shi, W., Xuan, Z., Zhou, J., et al. (2020). Effects of Lactobacillus hilgardii 60TS-2, with or without homofermentative Lactobacillus plantarum B90, on the aerobic stability, fermentation quality and microbial community dynamics in sugarcane top silage. Bioresour. Technol. 312:123600. doi: 10.1016/j.biortech.2020.123600
Wilkinson, J. M., and Davies, D. R. (2013). The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 68, 1–19. doi: 10.1111/j.1365-2494.2012.00891
Wu, B., Hu, Z., Wei, M., Yong, M., and Niu, H. (2022a). Effects of inoculation of Lactiplantibacillus plantarum and Lentilactobacillus buchneri on fermentation quality, aerobic stability, and microbial community dynamics of wilted Leymus chinensis silage. Front. Microbiol. 13:928731. doi: 10.3389/fmicb.2022.928731
Wu, C., Sun, W., Huang, Y., Dai, S., Peng, C., Zheng, Y., et al. (2022b). Effects of different additives on the bacterial community and fermentation mode of whole-plant paper mulberry silage. Front. Microbiol. 13:904193. doi: 10.3389/fmicb.2022.904193
Wu, X., Amanze, C., Yu, Z., Li, J., Liu, Y., Shen, L., et al. (2022c). Evaluation of fungal community assembly and function during food waste composting with Aneurinibacillus sp. LD3 inoculant. Bioresour. Technol. 363:127923. doi: 10.1016/j.biortech.2022.127923
Yuan, X., Wen, A., Wang, J., Li, J., Desta, S. T., Undersander, D. J., et al. (2018). Fermentation quality, in vitro digestibility and aerobic stability of total mixed ration silages prepared with whole-plant corn (Zea mays L.) and hulless barley (Hordeum vulgare L.) straw. Anim. Prod. Sci. 58, 1860–1868. doi: 10.1071/AN15874
Zhang, F., Miao, F., Wang, X., Lu, W., and Ma, C. (2021). Effects of homo- and hetero-fermentative lactic acid bacteria on the quality and aerobic stability of corn silage. Toxins 101, 761–770. doi: 10.1139/cjas-2019-0170
Zhang, L., Ma, Q., Ma, S., Zhang, J., Jia, R., Ji, C., et al. (2017). Ameliorating effects of Bacillus subtilis ANSB060 on growth performance, antioxidant functions, and aflatoxin residues in ducks fed diets contaminated with aflatoxins. Toxins 9:1. doi: 10.3390/toxins9010001
Zhang, L., Zhou, X., Gu, Q., Liang, M., Mu, S., Zhou, B., et al. (2019). Analysis of the correlation between bacteria and fungi in sugarcane tops silage prior to and after aerobic exposure. Bioresour. Technol. 291:121835. doi: 10.1016/j.biortech.2019.121835
Zhao, J., Wang, S., Dong, Z., Chen, L., and Shao, T. (2021). Partial substitution of whole-crop corn with bamboo shoot shell improves aerobic stability of total mixed ration silage without affecting in vitro digestibility. J. Anim. Physiol. Anim. Nutr. 105, 431–441. doi: 10.1111/jpn.13476
Zheng, J., Wittouck, S., Salvetti, E., Franz, C. M. A. P., Harris, H. M. B., Mattarelli, P., et al. (2020). A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 70, 2782–2858. doi: 10.1099/ijsem.0.004107
Keywords: silage, aerobic exposure, fermentation quality, bacterial community, fungal community
Citation: Yin H, Zhao M, Pan G, Zhang H, Yang R, Sun J, Yu Z, Bai C and Xue Y (2023) Effects of Bacillus subtilis or Lentilactobacillus buchneri on aerobic stability, and the microbial community in aerobic exposure of whole plant corn silage. Front. Microbiol. 14:1177031. doi: 10.3389/fmicb.2023.1177031
Received: 01 March 2023; Accepted: 31 March 2023;
Published: 17 April 2023.
Edited by:
Wenyi Zhang, Inner Mongolia Agricultural University, ChinaReviewed by:
Paul James Weimer, University of Wisconsin-Madison, United StatesCláudia Braga Pereira Bento, Universidade Federal dos Vales do Jequitinhonha e Mucuri, Brazil
Copyright © 2023 Yin, Zhao, Pan, Zhang, Yang, Sun, Yu, Bai and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunsheng Bai, bsc@syau.edu.cn; Yanlin Xue, xueyanlin_1979@163.com
 Hang Yin1
Hang Yin1 Zhu Yu
Zhu Yu Chunsheng Bai
Chunsheng Bai Yanlin Xue
Yanlin Xue