- Department of Molecular and Cell Biology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan
The microRNA pathways govern complex interactions of the host and virus at the transcripts level that regulate cellular responses, viral replication and viral pathogenesis. As a group of single-stranded short non-coding ribonucleotides (ncRNAs), the microRNAs complement their messenger RNA (mRNA) targets to effect post-transcriptional or translational gene silencing. Previous studies showed the ability of human immunodeficiency virus 1 (HIV-1) to encode microRNAs which modify cellular defence mechanisms thus creating an environment favorable for viral invasion and replication. In corollary, cellular microRNAs were linked to the alteration of HIV-1 infection at different stages of replication and latency. As evidences further establish the regulatory involvement of both cellular and viral microRNA in HIV-1-host interactions, there is a necessity to organize this information. This paper would present current and emerging knowledge on these multi-dimensional interactions that may facilitate the design of microRNAs as effective antiretroviral reagents.
Introduction
The human immunodeficiency virus 1 (HIV-1) is the retroviral agent causing acquired immunodeficiency syndrome (AIDS), a disease leading to systemic failure of the immune system with life threatening consequences. The decades old magnanimous problem of HIV-1 infection has challenged researchers to address its control and eradication. One of the most recent strategies introduced is the use of small non-coding ribonucleotides (ncRNAs) which includes microRNAs (Arbuthnot, 2011). The microRNAs are ubiquitous ~22–25 nt endogenously expressed ncRNAs targeting specific messenger RNA (mRNA) sequences, thus inducing its degradation or effecting translational inhibition. As proven vital regulatory components of viral infection and immunity (Huang et al., 2011), microRNAs can be directed to target viral and cellular transcripts to suppress infection. In fact, numerous studies have been proposed to integrate cellular microRNAs as nucleotide-based therapy for HIV (Boden et al., 2004; Lo et al., 2007; Aagaard et al., 2008). However, HIV-1 is a fastidious mutant consequently making cellular microRNAs prone to losing its viral transcript target efficacy as constant genome revisions occur in the course of viral evolution. Thus, simultaneous expression of microRNAs aimed to repress multiple HIV-1 targets may deter the effects of escape mutants. In another scenario, the cellular microRNAs can target host gene products that regulate cell defense responses. Once the issues of microRNA off-target effects, cell toxicity and delivery systems are addressed, the development of microRNAs as an anti-HIV-1 therapeutic strategy becomes more realistic (Boden et al., 2007; Liu et al., 2011). Now, the greater challenge is to determine the specific roles of the current inventory of 1921 human and three HIV-1 microRNAs (Kozomara and Griffiths-Jones, 2011) in HIV-1 infection. This difficult task of functional assignments correlated to microRNA-mRNA interactions has been made easier with genomics-based predictive tools in the recent years (Tan Gana et al., 2012). In addition, significant improvements on techniques for microRNA discovery and functional elucidation are likely to further expand these emerging interactive networks.
Whereas the current knowledge on cellular and viral microRNA functions involved in HIV-1 infection is still considerably few, consensus evidences suggest complex interactions (Chiang and Rice, 2011; Sanchez-Del Cojo et al., 2011). Figure 1 implies that microRNA regulation is anchored on genomic information processing on four scenarios that may possibly explain the confounded nature of their effects in virus-infected host systems. First, HIV-1 infection alters host microRNA networks to initiate successful viral invasion and latency, thus, affecting global host microRNA regulome. Second, HIV-1 microRNAs are produced from both sense and antisense transcripts to target either its own viral transcript or host genes for immune compromise. Third, the host microRNA systems may consequentially target the HIV-1 genomic elements or its genes to innate immune responses. Fourth, the interplay of microRNA and target mRNA between host and HIV-1 can be organized into regulatory modules (cis- and trans- regulation) of essential biochemical pathways as critical determinants of host cell fate and survival. This framework would be the basis of our paper discussion covering an update on the current information on microRNA biogenesis and mechanisms involved in host-virus interactions. Also the paper would contain recently elucidated cellular and viral microRNA functions in HIV-1 infection from computational and experimental literature. Lastly, the integration of information would define future roles of microRNAs in HIV-1 control.
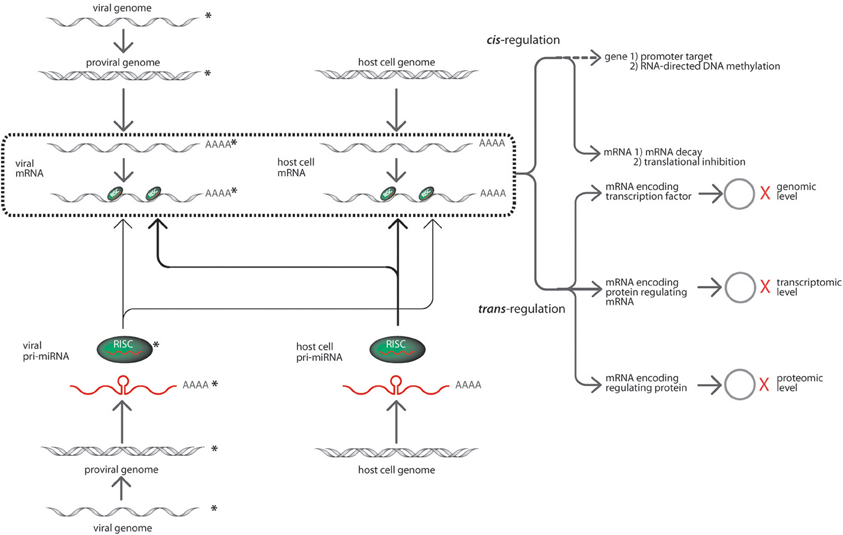
Figure 1. The prospective targeting interactions of HIV-1 and cellular microRNAs (modified from Cullen, 2006). The interactions among HIV-1 and cellular microRNAs with their corresponding targets may occur in several modules. For instance, HIV-1 encoded microRNAs processed via the host RNAi machinery and incorporated into RISC (in green with the mature microRNA in red) are sourced from HIV-1 pro-viral strands (grey double helix) initially from precursor microRNAs (red lines with poly adenines). These HIV-1 microRNAs can target its viral transcripts or the cellular transcripts. The targeting interactions of microRNAs are shown in solid light arrow lines. In corollary, cellular microRNAs derived from precursor microRNAs (red lines with poly adenines) generated by the host cell genome (grey double helix). The host cellular microRNAs are encoded in the same manner and can target both viral and cellular transcripts where the targeting interactions are shown in solid bold arrow lines. The targeting of mRNA transcripts happens in a highly specific Watson and Crick base-pairing with either complete complementation or seed region complementarity. The box in bold broken lines consolidates all targeting events of the various microRNA-initiated regulatory activities within the systems biology of host-virus interaction. The type of microRNA silencing mechanisms may be grouped as a cis- and trans-regulation event. The cis-regulation event involves microRNA targeting of mRNAs initiating post-transcriptional regulatory responses via mRNA degradation and translational inhibition. Whereas trans-regulation is a tripartite regulatory event which include expression variation of microRNA target genes regulating various viral and cellular activities such as transcription factors, RNA regulatory proteins, interactive genes. The cascades of events cause changes in viral and cellular activities inducing transcriptional regulation, transcriptional variation and protein translational modifications as indicated by the hollow circles = protein products; X (in red) = regulation of expression. The HIV-1 components are distinguished from host cell components with asterisks beside the drawings.
MicroRNA Biogenesis Pathways and Mechanisms
The genomic locations of the microRNA gene progenitors of the ~100 nt, 5′ methyl-7G capped and 3′ poly-adenylated primary-microRNAs (pri-microRNA) transcribed by either RNA II or III polymerase determine the mode of microRNA biogenesis (Figure 2). The canonical pathway utilizes the microprocessor complex, an interaction between Drosha RNase III enzyme (Faller and Guo, 2008) and DiGeorge critical region gene 8 (DGCR8) (Faller et al., 2010), a ribonuclease binding protein (RBP) to cleave the pri-microRNA into 70 nt preliminary-microRNAs (pre-microRNAs). While, a non-canonical pathway is followed by mirtrons, a group of intron-derived pre-microRNAs utilizing spliceosomes (Okamura et al., 2007, 2008; Berezikov et al., 2010). Recently, an emerging mode of biogenic pathway has been proposed for a set of splicing-independent mirtrons called simtrons which neither utilize DGCR8, Dicer, Exportin-5, or Argonaute 2 (Ago2) in their biogenesis (Havens et al., 2012).
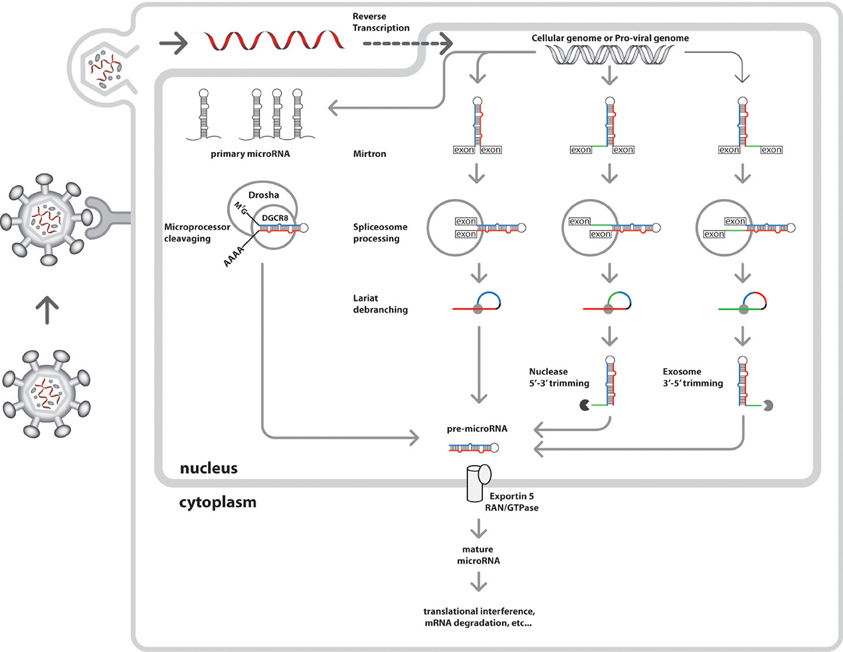
Figure 2. Nuclear events of the integrated cellular and HIV-1 microRNA biogenic pathways. The nucleus of the host cell is the central site of both cellular and viral microRNA biogenesis. Initially, HIV-1 virion particles attach to host cells via CD4 receptors signaling viral attack. This would be followed by HIV-1 particle fusion with the cell membrane and uncoating to load its RNA genome into the cytoplasm. The viral replicase enzyme facilitates production of more RNA genome later to be shuttled into the nucleus for viral transcription and integration into cellular genome. HIV-1 microRNA biogenesis is synchronous to cellular microRNA production in the host cell nucleus where other microRNA biogenic enzymes are present. Independent nuclear events of miRNA biogenesis have several modes of pre-microRNA generation (viral and cellular), initially from primary microRNA transcribed by either RNA polymerase II or III. he canonical pathway is undertaken by intergenic microRNAs resulting from microprocessor cleavage (Drosha and DGCR8) of pri-microRNA transcripts into pre-microRNAs, An alternative pathway for intron-coded microRNAs called mirtrons produce pre-microRNAs via splicing by spliceosomes and debranching by lariat debranching enzyme (Ldbr). There are three possible variants of mirtron processing, namely regular, the 5′ tailed mirtrons (subject to nuclease processing) and 3′ tailed mirtons (subject to exosome processing) (Westholm and Lai, 2011; Westholm et al., 2012). The sections of these pre-microRNA variants are shown in different colors, which the future main mature microRNAs are in blue, the secondary mature microRNAs are in red, the loops in black, and the branches are in green. Once generated, the pre-miRNAs are ready cytoplasmic shuttling, where further processing into mature microRNAs are achieved. Lately, a new biogenic pathway has been proposed for a set of splicing-independent mirtrons called simtrons which independent from DGCR8, Dicer, Exportin-5, or Argonaute 2 (Ago2) (Havens et al., 2012) (not shown).
Then, the pre-microRNA associates with Ran/GTPase (Bohnsack et al., 2004; Okada et al., 2009) and exportins for cytoplasmic translocation from nucleus (Bohnsack et al., 2004). In the cytoplasm (Figure 3), catalytic hydrolysis of Ran/GTPase allows the dissociation of pre-microRNA and transporter proteins (Kim, 2004). Another enzyme called Dicer, splices the pre-microRNA into the mature ~22 nt microRNA capable of mRNA duplexing (Carmell and Hannon, 2004; Cullen, 2004; Hammond, 2005; Harvey et al., 2008; Flores-Jasso et al., 2009). A complement strand from the mature double stranded microRNA is integrated into the RNA-induced silencing complex (RISC) which would be attached to the target transcript to elicit the regulatory processes (Kawamata and Tomari, 2010).
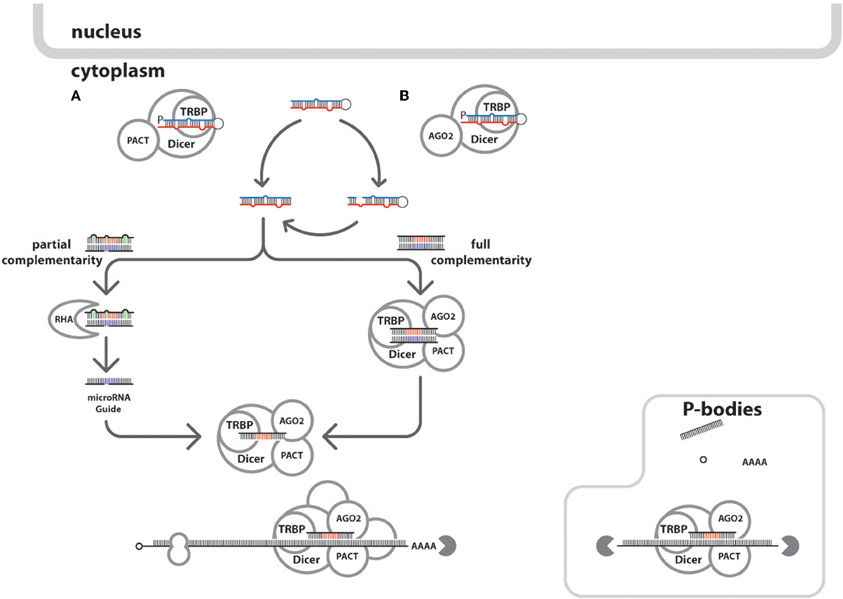
Figure 3. Cytoplasmic events of the integrated cellular and HIV-1 microRNA biogenic pathways. Mature microRNAs are processed in the host cytoplasm after the pre-microRNAs are shuttled from the nucleus. There are two ways the processing can happen: (A) by the Dicer/TRBPorTARBP or TARBP/PACT leading to the generation of microRNA duplexes, or (B) via the Ago proteins-assisted generation of pre-cursor microRNA which in the end generate the microRNA duplexes via Dicer action. The circular arrows show the formation microRNA duplex produced after the circuitous cleavage reactions of either Dicer, Ago2 proteins. Also shown in the microRNAs duplexes are the representative seed sites in red and blue and green indicates mismatches and bulges. Depending on the degree of complementation of the guide strands in the microRNA:RISC assembly to its target mRNA, would either cause mRNA degradation or translational repression. Furthermore, microRNA duplexes undergoing asymmetric unwinding would be assembled into the RISC loading complex after the duplex unwinding by RNA helicase A (RHA, gray crescent), through a bypass mechanism. Once the microRNA guide is loaded into the RISC associated proteins (Dicer; TRBP; Ago2; PACT; GW182, not shown) forms the activated microRNA:RISC to seek and bind targets within mRNA transcript. The mRNA transcripts are shown with other components namely: methyl cap (in hollow small circle), ribosome (hollow figure eight), and poly-A tail (AAAA). In HIV-1, it was demonstrated that P-bodies modulate microRNA processing mechanisms (Nathans et al., 2009). The solid gray pac-man indicates mRNA degradation events.
The gene regulatory effects caused by the microRNA and mRNA target interaction dictated by highly stringent base-complementation of the binding sites have been demonstrated extensively (Long et al., 2008; Brodersen and Voinnet, 2009; Ajay et al., 2010). Perfect complementation of the microRNA and mRNA causes endonucleolytic cleavage-induced gene silencing while non-perfect complementation initiate translational inhibition of proteins (Jackson and Standart, 2007; Seitz, 2009; Pan et al., 2010). Other factors shown to enhance these regulatory interactions include the presence of several binding sites within the target mRNA and CTG repeats extension (Hon and Zhang, 2007), deadenylation and decapping-mediated conformational changes within the microRNA-RISC complex (Lin et al., 2005; Eulalio et al., 2007), the A-U bias (Frank et al., 2010) and single nucleotide polymorphisms (SNPs) within the seed regions (Landi et al., 2011).
Since, the viral genomic elements intersperse within the host genome during invasion, it is possible that these viral genomic fragments are processed into microRNAs by of the host microRNA machinery (Figures 2, 3). As consequence, these viral sequences will follow several pathways of microRNA biogenesis similar to its host. As example are the observed non-significant differences among microRNA profiles in normalized Dicer and Drosha expressions in HIV-1 infected CD4+ cells for both in vitro and in vivo studies (Bignami et al., 2012). In contrast, it was confirmed that microRNA expression among monocytes could happen in the absence of the Dicer enzyme, thus, implying an alternative mode of viral microRNA production (Coley et al., 2010). Another possible consequence of repeated integration and recombination of the HIV-1 into the host genome is the generation of orthologous microRNAs. This is the case of hiv1-miR-N367 and hsa-miR-192 with identical seed sequences shown to down regulate similar functional targets in a dual fluorescent reporter study, thus, making them functional orthologs (You et al., 2012).
Alterations in the Cellular MicroRNA Pathways During HIV-1 Infection
HIV-1 infection of host cells modifies the global RNA interference machinery which in effect changes the microRNA-regulated pathways via several bio-molecular interactions (Sanghvi and Steel, 2011b). Experiments confirmed the RNA silencing suppressor (RSS) activity of HIV-1 transactivator of transcription (Tat) (Bivalkar-Mehla et al., 2011). RSS is defined as a molecule encoded by a virus which can counter the effect of host cell microRNA-mediated antiviral defense pathways, or natural immunity (Houzet and Jeang, 2011). The HIV-1 Tat protein via association with the trans-activation response (TAR) element at the terminal 5′end of HIV-1 transcripts, promotes viral transcription by recruiting and increasing the processivity of RNA polymerase II (Hayes et al., 2011). The molecular complex creates a stabilizing effect on transcriptional elongation elicited by a cyclin-dependent kinase (CDK9), another subunit of the positive transcription elongation factor b (P-TEFb) together with Cyclin T1 (CCNT1), which functions to phosphorylate the C-terminus of RNA polymerase II (Sanghvi and Steel, 2011a). Also the binding between Tat protein to the TAR element blocks the TAR element interaction with the Dicer protein thus influencing cellular silencing mechanism (Bennasser and Jeang, 2006; Qian et al., 2009). Further characterization of Tat protein exhibited two most essential prerequisites for RSS activity, namely by harboring dsRNA binding domain (RKKRRQRR) and GW/WG motif essential in sequestering Ago proteins thus preventing RISC formation (Qian et al., 2009; Bivalkar-Mehla et al., 2011; Houzet and Jeang, 2011). In another comparative gene expression profile analysis between HIV-1 infected and non-infected macrophages, it was showed that HIV-1-encoded Vpr (Viral Protein R) protein similarly suppresses Dicer function (Coley et al., 2010). Table 1 includes the RNAi pathway related gene products that are targeted by HIV-1 microRNAs. With the inherent small size of microRNAs, bi-target co-regulation is a prospective occurrence when a microRNA seed sequence would complement both viral and cellular mRNAs simultaneously due to sequence similarities. Though, the mechanisms of these interactivities are not thoroughly explainable at the moment, computational analyses suggest the probabilities of their existence, in particular during viral infection (Veksler-Lublinsky et al., 2010).
Alterations in Cellular MicroRNA Expression During HIV-1 Infection
HIV-1 infection induced changes in cellular microRNA expressions result from combinatorial molecular interactions among proteins, transcripts, and genomes. These manifest as circuitous microRNA attenuation of the different cellular host metabolic processes. At this point, the current knowledge of the exact mechanisms on how HIV-1 infection remains to be understood fully. The current data available are mostly derived from microarray data comparing non-infected and HIV-1 infected cell lines. Over expression analyses of microRNAs among various cell lines simulated with HIV-1 infection are usually used to validate these differences in an attempt to explain the possible regulatory mechanisms behind the miroRNA interactions. Examples include the (Houzet et al., 2008) investigation which reported 59 simultaneously down-regulated cellular microRNAs of HIV-1 infected individuals. Prior studies indicated that HIV-1 infection can down-regulate as much as 43% of the 312 microRNA gene arrays (Yeung et al., 2005). In addition, unique and variable global modifications in microRNA expressions were exhibited by different host cell types and lines in reaction to HIV-1 infection (Yeung et al., 2005; Bennasser et al., 2006; Noorbakhsh et al., 2010; Gupta et al., 2011). A most recent example is the notable differences among the 21 microRNA profiles between the elite long-term non-progressors where viral replication is continuously suppressed against multiple uninfected individuals from 377 microRNAs changes in HIV-1 infected CD4+ lymphocytes (Bignami et al., 2012). Determining the alterations in cellular microRNA expression patterns among various types of HIV-1 infected cell lines may account factors such as productive infection and constant exposure to HIV-1 that drive these changes. Also modifications in microRNA profiles may be attributed to temporal variability of immune responses during the course of HIV-1 infection. When fully elucidated, these patterned variations of microRNA expressions may reflect vital information in HIV-1 disease diagnostics and progression. Currently, microarray data has been the greatest source of these analyses of microRNA expression pattern changes, aided by complex algorithms to detect actual variation.
An in depth analyses of these global changes confirm the existence of clustered microRNA expression signatures in HIV-1 infected cells. For example, downregulation of polycistronic microRNA hsa-miR-17/92 consistently suppressed viral production as observed among various HIV-1 infected cells (Triboulet et al., 2007). While, hsa-miR-27b, hsa-miR-29b, hsa-miR-150, and hsa-miR-223 were identified as significantly down-regulated upon CD4(+) T cell activation (Chiang et al., 2012). In contrast, hsa-miR-28, hsa-miR-125b, hsa-miR-150, hsa-miR-223, and hsa-miR-382, which were enriched in resting CD4+ T cells against the activated CD4+ T cells (Huang et al., 2007). Moreover, the T-cell-specific microRNAs, namely hsa-miR-150, hsa-miR-191, hsa-miR-223, hsa-miR-16, and hsa-miR-146b, showed variable expression patterns at various stages of HIV-1 infection. Recently, gene expression profile analyses of cellular microRNAs in HIV-1 infected CD4+ T cells demonstrated the down-regulation of hsa-miR-21, hsa-miR-26a, hsa-miR-155, hsa-miR-29a, hsa-miR-29b, and hsa-miR-29c, contrary to the observed upregulation of hsa-miR-223 (Sun et al., 2012). The identification of microRNA families may hold significance in correlating the targets as orthologous modules as previously mentioned.
HIV-1 Encoded MicroRNAs and their Interactions
The low number of verified HIV-1 encoded microRNAs (Table 1) in the miRBase (2012) confirm the difficulty of their identification thus making them among the least characterized of RNA virus-generated microRNAs (Grundhoff and Sullivan, 2011). This scarcity may be due to their inherent low number because of the small genome size or low levels of expression currently undetectable by conventional biochemical techniques thus may require enrichment processes to be detected (Althaus et al., 2012). Previously, Lin and Cullen (2007) estimated that retroviral microRNAs comprise only 0.5% of the total microRNAs detectable in HIV-1 infected cells. In addition, the limited access of viruses to nuclear microRNA processing machinery and the natural destabilization effects of microRNA processing may also limit their biogenesis (Grundhoff and Sullivan, 2011). Also, several reports confirmed the endonucleolytic effects of Dicer or Drosha against viral RNA genomes thus reducing viral mRNA production (Ouellet and Provost, 2010).
However, the advent of highly sensitive technologies like next generation sequencing and RNAse protection assays (RPA), as well as improved computational prediction may contribute to the discovery of new HIV-1 microRNA species. Recent pyrosequencing results estimated at least 40% or 125 of the candidates as putative HIV-1 microRNAs originating from the TAR, RRE and nef region, and major components of non-coding RNAs in HIV-1 infected cells (Yeung et al., 2009). The deep sequencing report of (Schopman et al., 2012) further supports these observations, as HIV-1 microRNAs are suggested to arise from structured regions of the genome which facilitate Drosha and Dicer mediated RNA processing. Hence, with the increased possibilities that many putative HIV-1 microRNAs identified by these breakthrough procedures, they require further investigations on their isolation and functional characterization.
In general, the target interactions of HIV-1 microRNAs with its mRNA seem to function as viral genome regulators (Table 1). However, current experiments open this into a subject of debate and further investigation. Although, functional studies suggest auxiliary functions of HIV-1 microRNAs which target host cellular transcripts mainly for immune evasion (Boss and Renne, 2011). These observations are explained further in succeeding discussions below.
HIV-1 TAR MicroRNA
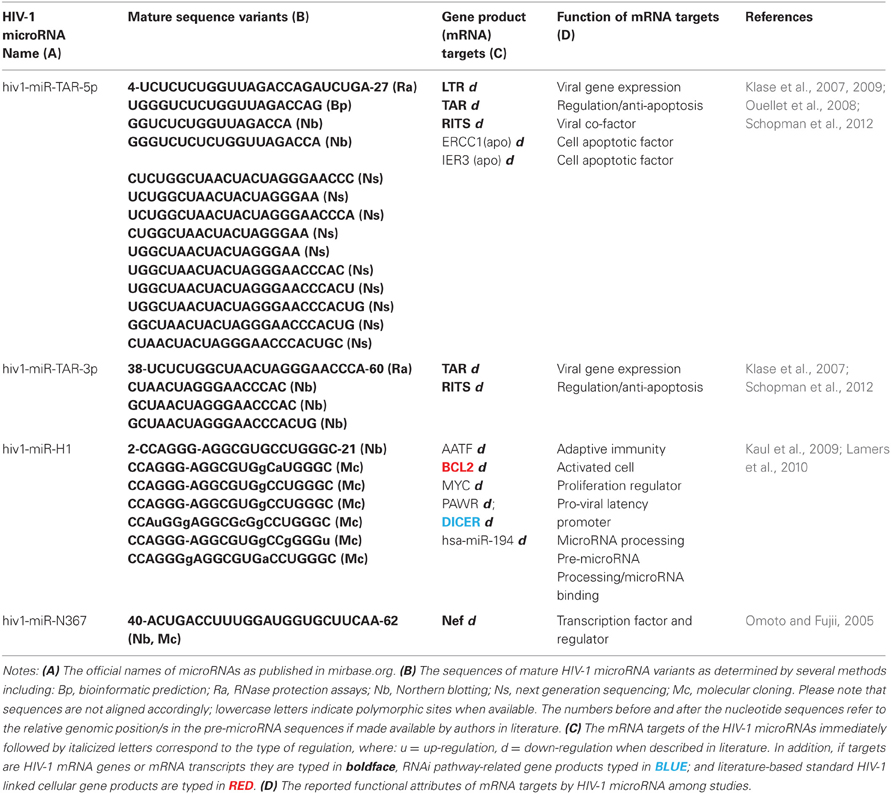
The 50 nt HIV-1 TAR element within the 5′ region of the viral RNA serves as the progenitor of hiv1-miR-TAR via asymmetrical processing of the transcript (Ouellet et al., 2008). Experiments confirmed hiv1-miR TAR to target host cell microRNA-related proteins, namely, Dicer, trans-activation responsive RNA binding protein (TARBP2, TRBP), and the RNA induced transcriptional silencing (RITS) complex. Recent functional studies showed that HIV-1 TAR microRNA down-regulates the DNA excision repair (ERCC1) and radiation-inducible immediate-early gene IEX-1 proteins (IER3) thus exerting its anti-apoptotic effect in infected cells (Klase et al., 2009).
Computer simulation studies established the HIV-1 TAR element as a potential microRNA rich region because of the following evidences (Narayanan et al., 2011): (a) the hairpin formation of the TAR element concurs with Dicer substrate specifications, allowing the complement fit to five distinct Dicer element, (b) TAR binds to important microRNA proteins, Dicer, and TARBP2, thus singling out its essential role in the microRNA-mediated gene regulatory processes. Moreover, the TARBP2 association with Dicer is necessary for efficient loading of microRNAs into the RISC, the consequential loss of TARBP2 function culminates in the loss of RNA silencing ability (Sanghvi and Steel, 2011a). The TARBP2 sequestration is known to restrict the availability of Dicer enzyme leading to modification of microRNA processing (Haase et al., 2005). Furthermore, TARBP2 and TAR element association suppresses interferon (IFN)-induced protein kinase R function (Gatignol et al., 2005).
Cloning studies of TAR-related microRNAs demonstrated a greater abundance of the 3′ mature sequence over the 5′ mature sequences involved in microRNA-derived silencing (Lamers et al., 2010). These observations collectively suggest that, in infected cells, hiv1-miR-TAR-3p is superior to the hiv1-miR-TAR-5p in suppressing gene expression, supporting speculations that there are preferential releases of these microRNA species. This also corroborates to the evident accumulation of the 3′ HIV-1 TAR RNAs in vivo (Ouellet et al., 2008).
HIV-1 H1 MicroRNA
The 81bp stem loop of HIV-1 transcript formed in the 3′-U3 (LTR) region known as the binding sites of the two nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) is the origin of hiv1-miR-H1 (MI0006106). It was shown to degrade apoptosis antagonizing factor (AATF) which decreases cell viability and reduced expression of cellular factors, Bcl-2, c-myc, Par-4 as well as the microRNA Dicer protein (Kaul, 2007; Kaul et al., 2009). The report also indicated hiv1-miR-H1 interaction with hsa-mir-149, affecting the latter's target HIV-1 encoded Vpr protein (Kaul et al., 2009). It is assumed that hiv1-miR-H1 and hiv1-miR-TAR are antagonistic to one another as they have contrasted activities against apoptotic elements. Further functional studies demonstrated deletion-driven evolution patterns in hiv1-miR-H1 among various AIDS patients. In addition, causal association was suggested between the appearance of a less stable hiv1-miR-H1 variant and induction of AIDS-related lymphoma (Lamers et al., 2010).
HIV-1 Nef MicroRNA
Nef protein has been shown to downregulate cell surface CD4 and MHC class I molecules through the clathrin-mediated endocytic pathway (Lubben et al., 2007; Schaefer et al., 2008). It is also involved in cellular signal transduction pathway through interaction with non-receptor type Tyr kinase molecules such as, Fyn and Lyn. Since, Nef functions in favor of HIV-1 replication and it is relatively conserved among various HIV-1 variants, miRNA-mediated control of Nef could have a great effect on the viral life cycle and its pathogenesis (Arien and Verhasselt, 2008; Foster and Garcia, 2008; Malim and Emerman, 2008). Although, HIV-1 3′ LTR is partially overlapping with nef microRNA (hiv1-miR-N367), as proposed previously (Omoto and Fujii, 2005, 2006), it remained controversial due to its non-duplicability. These reports may support a hypothesis of hyper-evolution of HIV-1 genome as a consequence of peptide-based immunity and RNA interference mechanisms (Narayanan et al., 2011).
HIV-1 Antisense MicroRNAs
Recent reports have indicated the ability of HIV-1 utilizing antisense transcripts in infected cells leading to discoveries of new viral microRNAs. In the RACE analyses of HIV-1 infected 293T and Jurkat cells, it was shown that cryptic transcription initiation sites in the 5′ border of the 3′ LTR and a new poly A signal within this LTR were present; also indicated was the possible role of the Tat protein as the modulator of transcription of this antisense RNA (Landry et al., 2007). In another study, an antisense peptide open reading frame (ORF) called “asp” coding for a hydrophobic protein was derived from Jurkat cells infected with HIV-1 although its origin, generation or the function is not yet clarified (Clerc et al., 2011). As the existence of antisense HIV-1 microRNAs remains to be proven, this concept opens a possibility where long antisense transcripts can complement with the sense transcripts within the viral genome. These sites in double stranded configuration can provide biogenic zones of Dicer-mediated microRNAs (Houzet and Jeang, 2011).
Cellular MicroRNAs Involved in HIV-1 Infection
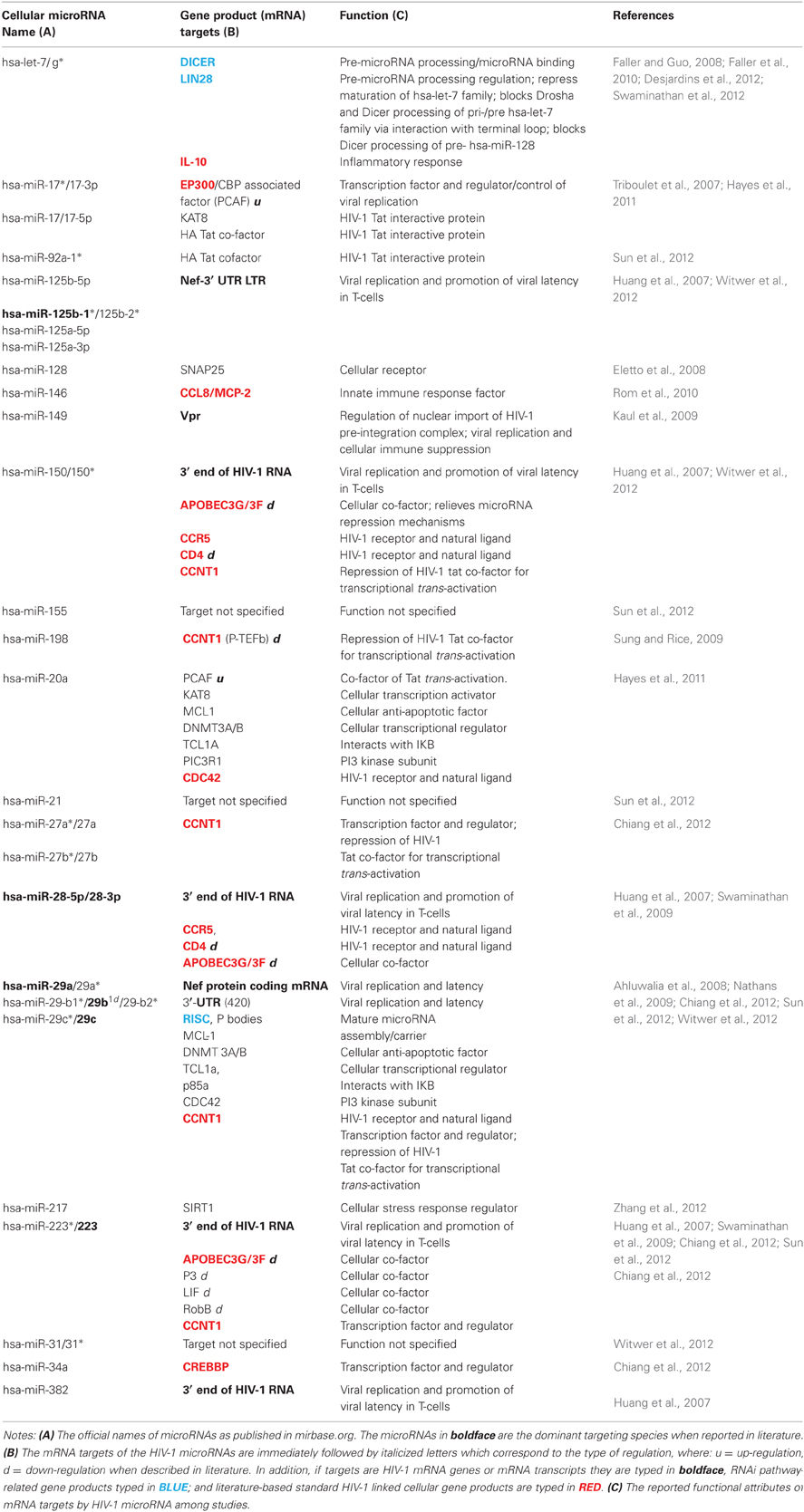
HIV-1 infection triggers multi-modal cascades of host cell microRNA targeting interactions that either activate or inhibit viral invasion and replication as shown in Figure 1. These microRNA targeting scenarios are likely to occur on at least two fronts. First, the cellular microRNA might directly target the HIV-1 genome, either in sense or antisense orientation, to suppress the production of viral proteins. An outstanding example is hsa-miR-29 which targets the HIV-1 nef transcript (Hariharan et al., 2005; Ahluwalia et al., 2008). Since, the HIV-1 nef gene is located at the proviral DNA 3′ portion, cellular microRNA targeting of this region would have serious implications in the viral life cycle. The group of (Nathans et al., 2009) proved that ectopic expression of hsa-miR-29 can repress production of nef protein resulting to suppressed viral replication and infectivity. The study also reported that hsa-mir-29a/ HIV-1 interactions enhance viral mRNA associations with RISC and P-body structures, thus suggesting prospective roles of P-bodies to viral latency regulation. In another study, a set of microRNAs namely, hsa-mir-28, hsa-miR-125b, has-miR-150, hsa-miR-223, and hsa-miR-382 were shown to bind in the 3′ position of HIV-1 transcripts which triggers viral latency (Huang et al., 2007). Recently, Sun et al identified another set of cellular microRNA, namely hsa-miR-15a, hsa-miR-15b, hsa-miR-16, hsa-miR-24, hsa-miR-29a, hsa-miR-29b, hsa-miR-150, and hsa-miR-223 that are directly targeting HIV-1 3′-UTR, and exhibiting weak but significant inhibitory effects on HIV-1 replication (Sun et al., 2012). In a review by Sun and Rossi, using the PITA software, 256 seed-match sites were identified to complement Nef-3′ LTR sequence (Sun and Rossi, 2011).
In the second scenario, the host cell as triggered by HIV-1 infection would initiate cellular microRNA production to attenuate cellular factors involved in antiviral responses against HIV-1. Table 2 summarizes the list of cellular microRNAs with their validated host cellular protein targets and their corresponding cellular functions. As likely initial repercussions, these microRNAs may target the genes involved in immune responses for innate and adaptive immunity (Kulpa and Collins, 2011). This bipartite defense system initially triggers natural killer (NK) cell activities as elicited by partial detection of HIV-1 components. In a later reaction, adaptive immunity is induced through production of antigen-specific antibodies by B-cells and eliciting cell-mediated immunity through antigen-specific cytotoxic T lymphocytes, of which microRNAs were found to target various cellular receptors (Cobos-Jimenez et al., 2011). As examples are cellular microRNA interactions with chemokines (Zhou et al., 2010).
Transcriptional control is vital to the HIV- 1 proliferation, thus determining microRNA interactions among host transcription factors and regulators is a necessity (Victoriano and Okamoto, 2012). Among examples are reporter assays suggesting hsa-miR-223 bi-functional effects in HIV-1 replication are targets were varied in two different cell lines namely, Sp3 and LIF in NB4 cells, while RhoB and NF-1A in HEK293 cells (Sun et al., 2012). Next is the hsa-miR-29 family which also targets cellular proteins Mcl-1, DNMT 3A/B, Tcl1, p85, and CDC42, further establishes its diverse roles in HIV-1 latency (Sun et al., 2012; Witwer et al., 2012). Another is hsa-miR-198 targeting CCNT1 (Kaul et al., 2009), a key component in the Tat-mediated transcription of the virus, when suppressed can impair HIV-1 replication (Imai et al., 2009).
Cellular microRNAs linked to chromatin regulation show proof that microRNAs are critical elements of epigenetic control in HIV-1 infection (Obbard et al., 2009; Easley et al., 2010). Recent evidences support that chromatin modification may explain mechanisms of HIV-1 transcription and thus the maintenance of latency. Some microRNA species are considered involved in gene silencing by modulating methylation and deactylation of histone proteins (Triboulet et al., 2007).
The above mentioned functional gene product clusters are just few focal points of cellular microRNA interactivities related to HIV-1 infection. It is expected that as more interactions are validated, the complex nature of cellular microRNA regulation linked to HIV-1 infection and host response would be further characterized. However, the scope of cellular microRNA interactions may involve other non-listed prospective gene targets which may also influence HIV-1 infection.
Beyond Crosstalks Among Cellular and HIV-1 MicroRNA Machineries
Preceding discussions on microRNA interactions in host-HIV-1 infection further confirm their inherent complexity. It perfectly illustrates the constant attenuation of gene regulatory networks to maintain homeostasis in the HIV-1 infected cells. However, as HIV-1 remains an incurable disease among humans, it is implied that it can successfully compromise host immune and defense reactions wherein microRNA regulation might play pivotal roles. Thus, future studies must focus on how to reprogram microRNAs to favorably initiate the cellular anti-HIV-1 defense response. To realize such goal, it becomes necessary to organize succeeding investigations as follows: First is to globally account cellular and viral microRNA interrelationships affecting biomolecular pathways in HIV-1 infection. This allows the possibility of unlocking the combination of molecular switches that would allow the host cell successfully defend itself against HIV-1. Second is to determine the simultaneous targets of viral and cellular microRNAs. These bi-targets may reveal signatures of gene families or microRNA clusters characterizing HIV-1 infection patterns. Third is to capture temporal changes among microRNA expression patterns during HIV-1 disease progression. In assessing the current amount of information on hand, there remains much work to be done in unlocking the ultimate roles of microRNAs in HIV-1 pathogencity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was partially supported by the Ministries of Education, Culture, Sports, Science and Technology, and Health, Labor, and Welfare of Japan. We sincerely express our gratitude to the invaluable support of Drs. Kaori Asamitsu, Satoshi Kanazawa, and Hiroaki Uranishi of the Department of Cell and Molecular Biology, Nagoya City University Graduate School of Medical Sciences. We also express our sincerest gratitude for Mr. Issey Takahashi of the Nagoya City University Graduate School of Design and Architecture for rendering the scientific illustrations.
References
Aagaard, L. A., Zhang, J., Von Eije, K. J., Li, H., Saetrom, P., Amarzguioui, M., and Rossi, J. J. (2008). Engineering and optimization of the miR-106b cluster for ectopic expression of multiplexed anti-HIV RNAs. Gene Ther. 15, 1536–1549.
Ahluwalia, J. K., Khan, S. Z., Soni, K., Rawat, P., Gupta, A., Hariharan, M., Scaria, V., Lalwani, M., Pillai, B., Mitra, D., and Brahmachari, S. K. (2008). Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication. Retrovirology 5, 117.
Ajay, S. S., Athey, B. D., and Lee, I. (2010). Unified translation repression mechanism for microRNAs and upstream AUGs. BMC Genomics 11, 155.
Althaus, C. F., Vongrad, V., Niederost, B., Joos, B., Di Giallonardo, F., Rieder, P., Pavlovic, J., Trkola, A., Gunthard, H. F., Metzner, K. J., and Fischer, M. (2012). Tailored enrichment strategy detects low abundant small noncoding RNAs in HIV-1 infected cells. Retrovirology 9, 27.
Arien, K. K., and Verhasselt, B. (2008). HIV Nef: role in pathogenesis and viral fitness. Curr. HIV Res. 6, 200–208.
Bennasser, Y., and Jeang, K. T. (2006). HIV-1 Tat interaction with Dicer: requirement for RNA. Retrovirology 3, 95.
Bennasser, Y., Le, S. Y., Yeung, M. L., and Jeang, K. T. (2006). MicroRNAs in human immunodeficiency virus-1 infection. Methods Mol. Biol. 342, 241–253.
Berezikov, E., Liu, N., Flynt, A. S., Hodges, E., Rooks, M., Hannon, G. J., and Lai, E. C. (2010). Evolutionary flux of canonical microRNAs and mirtrons in Drosophila. Nat. Genet. 42, 6–9; Author reply 9–10.
Bignami, F., Pilotti, E., Bertoncelli, L., Ronzi, P., Gulli, M., Marmiroli, N., Magnani, G., Pinti, M., Lopalco, L., Mussini, C., Ruotolo, R., Galli, M., Cossarizza, A., and Casoli, C. (2012). Stable changes in CD4+ T lymphocyte miRNA expression after exposure to HIV-1. Blood 119, 6259–6267.
Bivalkar-Mehla, S., Vakharia, J., Mehla, R., Abreha, M., Kanwar, J. R., Tikoo, A., and Chauhan, A. (2011). Viral RNA silencing suppressors (RSS): novel strategy of viruses to ablate the host RNA interference (RNAi) defense system. Virus Res. 155, 1–9.
Boden, D., Pusch, O., and Ramratnam, B. (2007). Overcoming HIV-1 resistance to RNA interference. Front. Biosci. 12, 3104–3116.
Boden, D., Pusch, O., Silbermann, R., Lee, F., Tucker, L., and Ramratnam, B. (2004). Enhanced gene silencing of HIV-1 specific siRNA using microRNA designed hairpins. Nucleic Acids Res. 32, 1154–1158.
Bohnsack, M. T., Czaplinski, K., and Gorlich, D. (2004). Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 10, 185–191.
Boss, I. W., and Renne, R. (2011). Viral miRNAs and immune evasion. Biochim. Biophys. Acta 1809, 708–714.
Brodersen, P., and Voinnet, O. (2009). Revisiting the principles of microRNA target recognition and mode of action. Nat. Rev. Mol. Cell Biol. 10, 141–148.
Carmell, M. A., and Hannon, G. J. (2004). RNase III enzymes and the initiation of gene silencing. Nat. Struct. Mol. Biol. 11, 214–218.
Chiang, K., and Rice, A. P. (2011). Mini ways to stop a virus: microRNAs and HIV-1 replication. Future Virol. 6, 209–221.
Chiang, K., Sung, T.-L., and Rice, A. P. (2012). Regulation of Cyclin T1 and HIV-1 Replication by MicroRNAs in Resting CD4+ T Lymphocytes. J. Virol. 86, 3244–3252.
Clerc, I., Laverdure, S., Torresilla, C., Landry, S., Borel, S., Vargas, A., Arpin-Andre, C., Gay, B., Briant, L., Gross, A., Barbeau, B., and Mesnard, J. M. (2011). Polarized expression of the membrane ASP protein derived from HIV-1 antisense transcription in T cells. Retrovirology 8, 74.
Cobos-Jimenez, V., Booiman, T., Hamann, J., and Kootstra, N. A. (2011). Macrophages and HIV-1. Curr. Opin. HIV AIDS 6, 385–390.
Coley, W., Van Duyne, R., Carpio, L., Guendel, I., Kehn-Hall, K., Chevalier, S., Narayanan, A., Luu, T., Lee, N., Klase, Z., and Kashanchi, F. (2010). Absence of DICER in monocytes and its regulation by HIV-1. J. Biol. Chem. 285, 31930–31943.
Cullen, B. R. (2004). Derivation and function of small interfering RNAs and microRNAs. Virus Res. 102, 3–9.
Desjardins, A., Yang, A., Bouvette, J., Omichinski, J. G., and Legault, P. (2012). Importance of the NCp7-like domain in the recognition of pre-let-7g by the pluripotency factor Lin28. Nucleic Acids Res. 40, 1767–1777.
Easley, R., Van Duyne, R., Coley, W., Guendel, I., Dadgar, S., Kehn-Hall, K., and Kashanchi, F. (2010). Chromatin dynamics associated with HIV-1 Tat-activated transcription. Biochim. Biophys. Acta 1799, 275–285.
Eletto, D., Russo, G., Passiatore, G., Del Valle, L., Giordano, A., Khalili, K., Gualco, E., and Peruzzi, F. (2008). Inhibition of SNAP25 expression by HIV-1 Tat involves the activity of mir-128a. J. Cell. Physiol. 216, 764–770.
Eulalio, A., Rehwinkel, J., Stricker, M., Huntzinger, E., Yang, S. F., Doerks, T., Dorner, S., Bork, P., Boutros, M., and Izaurralde, E. (2007). Target-specific requirements for enhancers of decapping in miRNA-mediated gene silencing. Genes Dev. 21, 2558–2570.
Faller, M., and Guo, F. (2008). MicroRNA biogenesis: there's more than one way to skin a cat. Biochim. Biophys. Acta 1779, 663–667.
Faller, M., Toso, D., Matsunaga, M., Atanasov, I., Senturia, R., Chen, Y., Zhou, Z. H., and Guo, F. (2010). DGCR8 recognizes primary transcripts of microRNAs through highly cooperative binding and formation of higher-order structures. RNA 16, 1570–1583.
Flores-Jasso, C. F., Arenas-Huertero, C., Reyes, J. L., Contreras-Cubas, C., Covarrubias, A., and Vaca, L. (2009). First step in pre-miRNAs processing by human Dicer. Acta Pharmacol. Sin. 30, 1177–1185.
Frank, F., Sonenberg, N., and Nagar, B. (2010). Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature 465, 818–822.
Gatignol, A., Laine, S., and Clerzius, G. (2005). Dual role of TRBP in HIV replication and RNA interference: viral diversion of a cellular pathway or evasion from antiviral immunity? Retrovirology 2, 65.
Gupta, A., Nagilla, P., Le, H. S., Bunney, C., Zych, C., Thalamuthu, A., Bar-Joseph, Z., Mathavan, S., and Ayyavoo, V. (2011). Comparative expression profile of miRNA and mRNA in primary peripheral blood mononuclear cells infected with human immunodeficiency virus (HIV-1). PLoS ONE 6:e22730. doi: 10.1371/journal.pone.0022730
Haase, A. D., Jaskiewicz, L., Zhang, H., Laine, S., Sack, R., Gatignol, A., and Filipowicz, W. (2005). TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 6, 961–967.
Hammond, S. M. (2005). Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 579, 5822–5829.
Hariharan, M., Scaria, V., Pillai, B., and Brahmachari, S. K. (2005). Targets for human encoded microRNAs in HIV genes. Biochem. Biophys. Res. Commun. 337, 1214–1218.
Harvey, S. J., Jarad, G., Cunningham, J., Goldberg, S., Schermer, B., Harfe, B. D., McManus, M. T., Benzing, T., and Miner, J. H. (2008). Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J. Am. Soc. Nephrol. 19, 2150–2158.
Havens, M. A., Reich, A. A., Duelli, D. M., and Hastings, M. L. (2012). Biogenesis of mammalian microRNAs by a non-canonical processing pathway. Nucleic Acids Res. 40, 4626–4640.
Hayes, A. M., Qian, S., Yu, L., and Boris-Lawrie, K. (2011). Tat RNA silencing suppressor activity contributes to perturbation of lymphocyte miRNA by HIV-1. Retrovirology 8, 36.
Hon, L. S., and Zhang, Z. (2007). The roles of binding site arrangement and combinatorial targeting in microRNA repression of gene expression. Genome Biol. 8, R166.
Houzet, L., and Jeang, K. T. (2011). MicroRNAs and human retroviruses. Biochim. Biophys. Acta 1809, 686–693.
Houzet, L., Yeung, M. L., De Lame, V., Desai, D., Smith, S. M., and Jeang, K. T. (2008). MicroRNA profile changes in human immunodeficiency virus type 1 (HIV-1) seropositive individuals. Retrovirology 5, 118.
Huang, J., Wang, F., Argyris, E., Chen, K., Liang, Z., Tian, H., Huang, W., Squires, K., Verlinghieri, G., and Zhang, H. (2007). Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat. Med. 13, 1241–1247.
Huang, Y., Shen, X., Zou, Q., Wang, S., Tang, S., and Zhang, G. (2011). Biological functions of microRNAs: a review. J. Physiol. Biochem. 67, 129–139.
Imai, K., Asamitsu, K., Victoriano, A. F., Cueno, M. E., Fujinaga, K., and Okamoto, T. (2009). Cyclin T1 stabilizes expression levels of HIV-1 Tat in cells. FEBS J. 276, 7124–7133.
Jackson, R. J., and Standart, N. (2007). How do microRNAs regulate gene expression? Sci. STKE 2007, re1.
Kaul, D., Ahlawat, A., and Gupta, S. D. (2009). HIV-1 genome-encoded hiv1-mir-H1 impairs cellular responses to infection. Mol. Cell. Biochem. 323, 143–148.
Kim, V. N. (2004). MicroRNA precursors in motion: exportin-5 mediates their nuclear export. Trends Cell Biol. 14, 156–159.
Klase, Z., Kale, P., Winograd, R., Gupta, M. V., Heydarian, M., Berro, R., McCaffrey, T., and Kashanchi, F. (2007). HIV-1 TAR element is processed by Dicer to yield a viral micro-RNA involved in chromatin remodeling of the viral LTR. BMC Mol. Biol. 8:63. doi: 10.1186/1471-2199-8-63
Klase, Z., Winograd, R., Davis, J., Carpio, L., Hildreth, R., Heydarian, M., Fu, S., McCaffrey, T., Meiri, E., Ayash-Rashkovsky, M., Gilad, S., Bentwich, Z., and Kashanchi, F. (2009). HIV-1 TAR miRNA protects against apoptosis by altering cellular gene expression. Retrovirology 6, 18.
Kozomara, A., and Griffiths-Jones, S. (2011). miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 39, D152–D157.
Kulpa, D. A., and Collins, K. L. (2011). The emerging role of HLA-C in HIV-1 infection. Immunology 134, 116–122.
Lamers, S. L., Fogel, G. B., and McGrath, M. S. (2010). HIV-miR-H1 evolvability during HIV pathogenesis. BioSystems 101, 88–96.
Landi, D., Barale, R., Gemignani, F., and Landi, S. (2011). Prediction of the biological effect of polymorphisms within microRNA binding sites. Methods Mol. Biol. 676, 197–210.
Landry, S., Halin, M., Lefort, S., Audet, B., Vaquero, C., Mesnard, J. M., and Barbeau, B. (2007). Detection, characterization and regulation of antisense transcripts in HIV-1. Retrovirology 4, 71.
Lin, J., and Cullen, B. R. (2007). Analysis of the interaction of primate retroviruses with the human RNA interference machinery. J. Virol. 81, 12218–12226.
Lin, S. L., Chang, D., and Ying, S. Y. (2005). Asymmetry of intronic pre-miRNA structures in functional RISC assembly. Gene 356, 32–38.
Liu, Y. P., Westerink, J. T., Ter Brake, O., and Berkhout, B. (2011). RNAi-inducing lentiviral vectors for anti-HIV-1 gene therapy. Methods Mol. Biol. 721, 293–311.
Lo, H. L., Chang, T., Yam, P., Marcovecchio, P. M., Li, S., Zaia, J. A., and Yee, J. K. (2007). Inhibition of HIV-1 replication with designed miRNAs expressed from RNA polymerase II promoters. Gene Ther. 14, 1503–1512.
Long, D., Chan, C. Y., and Ding, Y. (2008). Analysis of microRNA-target interactions by a target structure based hybridization model. Pac. Symp. Biocomput. 2008, 64–74.
Lubben, N. B., Sahlender, D. A., Motley, A. M., Lehner, P. J., Benaroch, P., and Robinson, M. S. (2007). HIV-1 Nef-induced down-regulation of MHC class I requires AP-1 and clathrin but not PACS-1 and is impeded by AP-2. Mol. Biol. Cell 18, 3351–3365.
Malim, M. H., and Emerman, M. (2008). HIV-1 accessory proteins–ensuring viral survival in a hostile environment. Cell Host Microbe 3, 388–398.
Narayanan, A., Kehn-Hall, K., Bailey, C., and Kashanchi, F. (2011). Analysis of the roles of HIV-derived microRNAs. Expert Opin. Biol. Ther. 11, 17–29.
Nathans, R., Chu, C. Y., Serquina, A. K., Lu, C. C., Cao, H., and Rana, T. M. (2009). Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol. Cell 34, 696–709.
Noorbakhsh, F., Ramachandran, R., Barsby, N., Ellestad, K. K., Leblanc, A., Dickie, P., Baker, G., Hollenberg, M. D., Cohen, E. A., and Power, C. (2010). MicroRNA profiling reveals new aspects of HIV neurodegeneration: caspase-6 regulates astrocyte survival. FASEB J. 24, 1799–1812.
Obbard, D. J., Gordon, K. H., Buck, A. H., and Jiggins, F. M. (2009). The evolution of RNAi as a defence against viruses and transposable elements. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 99–115.
Okada, C., Yamashita, E., Lee, S. J., Shibata, S., Katahira, J., Nakagawa, A., Yoneda, Y., and Tsukihara, T. (2009). A high-resolution structure of the pre-microRNA nuclear export machinery. Science 326, 1275–1279.
Okamura, K., Chung, W. J., and Lai, E. C. (2008). The long and short of inverted repeat genes in animals: microRNAs, mirtrons and hairpin RNAs. Cell Cycle 7, 2840–2845.
Okamura, K., Hagen, J. W., Duan, H., Tyler, D. M., and Lai, E. C. (2007). The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 130, 89–100.
Omoto, S., and Fujii, Y. R. (2005). Regulation of human immunodeficiency virus 1 transcription by nef microRNA. J. Gen. Virol. 86, 751–755.
Omoto, S., and Fujii, Y. R. (2006). Cloning and detection of HIV-1-encoded microRNA. Methods Mol. Biol. 342, 255–265.
Ouellet, D. L., Plante, I., Landry, P., Barat, C., Janelle, M. E., Flamand, L., Tremblay, M. J., and Provost, P. (2008). Identification of functional microRNAs released through asymmetrical processing of HIV-1 TAR element. Nucleic Acids Res. 36, 2353–2365.
Ouellet, D. L., and Provost, P. (2010). Current knowledge of MicroRNAs and noncoding RNAs in virus-infected cells. Methods Mol. Biol. 623, 35–65.
Pan, W., Xin, P., and Clawson, G. A. (2010). MicroRNAs align with accessible sites in target mRNAs. J. Cell. Biochem. 109, 509–518.
Qian, S., Zhong, X., Yu, L., Ding, B., De Haan, P., and Boris-Lawrie, K. (2009). HIV-1 Tat RNA silencing suppressor activity is conserved across kingdoms and counteracts translational repression of HIV-1. Proc. Natl. Acad. Sci. U.S.A. 106, 605–610.
Rom, S., Rom, I., Passiatore, G., Pacifici, M., Radhakrishnan, S., Del Valle, L., Pina-Oviedo, S., Khalili, K., Eletto, D., and Peruzzi, F. (2010). CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells. FASEB J. 24, 2292–2300.
Sanchez-Del Cojo, M., Lopez-Huertas, M. R., Mateos, E., Alcami, J., and Coiras, M. (2011). Mechanisms of RNA interference in the HIV-1-host cell interplay. AIDS Rev. 13, 149–160.
Sanghvi, V. R., and Steel, L. F. (2011a). The cellular TAR RNA binding protein, TRBP, promotes HIV-1 replication primarily by inhibiting the activation of double-stranded RNA-dependent kinase PKR. J. Virol. 85, 12614–12621.
Sanghvi, V. R., and Steel, L. F. (2011b). A re-examination of global suppression of RNA interference by HIV-1. PLoS ONE 6:e17246. doi: 10.1371/journal.pone.0017246
Schaefer, M. R., Wonderlich, E. R., Roeth, J. F., Leonard, J. A., and Collins, K. L. (2008). HIV-1 Nef targets MHC-I and CD4 for degradation via a final common beta-COP-dependent pathway in T cells. PLoS Pathog. 4:e1000131. doi: 10.1371/journal.ppat.1000131
Schopman, N. C., Willemsen, M., Liu, Y. P., Bradley, T., Van Kampen, A., Baas, F., Berkhout, B., and Haasnoot, J. (2012). Deep sequencing of virus-infected cells reveals HIV-encoded small RNAs. Nucleic Acids Res. 40, 414–427.
Sun, G., Li, H., Wu, X., Covarrubias, M., Scherer, L., Meinking, K., Luk, B., Chomchan, P., Alluin, J., Gombart, A. F., and Rossi, J. J. (2012). Interplay between HIV-1 infection and host microRNAs. Nucleic Acids Res. 40, 2181–2196.
Sun, G., and Rossi, J. J. (2011). MicroRNAs and their potential involvement in HIV infection. Trends Pharmacol. Sci. 32, 675–681.
Sung, T. L., and Rice, A. P. (2009). miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog. 5:e1000263. doi: 10.1371/journal.ppat.1000263
Swaminathan, S., Suzuki, K., Seddiki, N., Kaplan, W., Cowley, M. J., Hood, C. L., Clancy, J. L., Murray, D. D., Méndez, C., Gelgor, L., Anderson, B., Roth, N., Cooper, D. A., and Kelleher, A. D. (2012). Differential regulation of the Let-7 family of microRNAs in CD4+ T cells alters IL-10 expression. J. Immunol. 188, 6238–6246.
Swaminathan, S., Zaunders, J., Wilkinson, J., Suzuki, K., and Kelleher, A. D. (2009). Does the presence of anti-HIV miRNAs in monocytes explain their resistance to HIV-1 infection? Blood 113, 5029–5030; Author reply 5030–5031.
Tan Gana, N. H., Victoriano, A. F., and Okamoto, T. (2012). Evaluation of online miRNA resources for biomedical applications. Genes Cells 17, 11–27.
Triboulet, R., Mari, B., Lin, Y. L., Chable-Bessia, C., Bennasser, Y., Lebrigand, K., Cardinaud, B., Maurin, T., Barbry, P., Baillat, V., Reynes, J., Corbeau, P., Jeang, K. T., and Benkirane, M. (2007). Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 315, 1579–1582.
Veksler-Lublinsky, I., Shemer-Avni, Y., Kedem, K., and Ziv-Ukelson, M. (2010). Gene bi-targeting by viral and human miRNAs. BMC Bioinformatics 11, 249.
Victoriano, A. F., and Okamoto, T. (2012). Transcriptional control of HIV replication by multiple modulators and their implication for a novel antiviral therapy. AIDS Res. Hum. Retroviruses 28, 125–138.
Westholm, J. O., Ladewig, E., Okamura, K., Robine, N., and Lai, E. C. (2012). Common and distinct patterns of terminal modifications to mirtrons and canonical microRNAs. RNA 18, 177–192.
Westholm, J. O., and Lai, E. C. (2011). Mirtrons: microRNA biogenesis via splicing. Biochimie 93, 1897–1904.
Witwer, K. W., Watson, A. K., Blankson, J. N., and Clements, J. E. (2012). Relationships of PBMC microRNA expression, plasma viral load, and CD4+ T-cell count in HIV-1-infected elite suppressors and viremic patients. Retrovirology 9, 5.
Yeung, M. L., Bennasser, Y., Myers, T. G., Jiang, G., Benkirane, M., and Jeang, K. T. (2005). Changes in microRNA expression profiles in HIV-1-transfected human cells. Retrovirology 2, 81.
Yeung, M. L., Bennasser, Y., Watashi, K., Le, S. Y., Houzet, L., and Jeang, K. T. (2009). Pyrosequencing of small non-coding RNAs in HIV-1 infected cells: evidence for the processing of a viral-cellular double-stranded RNA hybrid. Nucleic Acids Res. 37, 6575–6586.
You, X., Zhang, Z., Fan, J., Cui, Z., and Zhang, X. E. (2012). Functionally orthologous viral and cellular microRNAs studied by a novel dual-fluorescent reporter system. PLoS ONE 7:e36157. doi: 10.1371/journal.pone.0036157
Zhang, H. S., Wu, T. C., Sang, W. W., and Ruan, Z. (2012). MiR-217 is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation by down-regulation of SIRT1. Biochim. Biophys. Acta 1823, 1017–1023.
Keywords: microRNA, HIV-1 mechanisms, transcription factors, targets
Citation: Tan Gana NH, Onuki T, Victoriano AFB and Okamoto T (2012) MicroRNAs in HIV-1 infection: an integration of viral and cellular interaction at the genomic level. Front. Microbio. 3:306. doi: 10.3389/fmicb.2012.00306
Received: 06 June 2012; Paper pending published: 17 June 2012;
Accepted: 01 August 2012; Published online: 24 August 2012.
Edited by:
Hironori Sato, National Institute of Infectious Diseases, JapanCopyright © 2012 Tan Gana, Onuki, Victoriano and Okamoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Takashi Okamoto, Department of Molecular and Cell Biology, Nagoya City University Graduate School of Medical Sciences, 1-Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan. e-mail: tokamoto@med.nagoya-cu.ac.jp