Indications and hemoglobin thresholds for red blood cell transfusion and iron replacement in adults with gastrointestinal bleeding: An algorithm proposed by gastroenterologists and patient blood management experts
- 1Unidad de Gastroenterología, Hepatología y Nutrición, Hospital Universitario San Jorge, Huesca, Spain
- 2Departamento de Medicina, Universidad de Zaragoza, Zaragoza, Spain
- 3Instituto Aragonés de Ciencias de la Salud (IACS), Zaragoza, Spain
- 4Instituto de Investigación Sanitaria Aragón (IIS), Zaragoza, Spain
- 5Vifor Pharma, Barcelona, Spain
- 6Servicio de Aparato Digestivo, Hospital Clínico Universitario “Lozano Blesa”, Zaragoza, Spain
- 7Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Madrid, Spain
- 8Servei de Digestiu, Hospital de la Santa Creu y Sant Pau, Universidad Autónoma de Barcelona, Barcelona, Spain
- 9Unidad de Gestión Clínica de Aparato Digestivo, Hospital Universitario Reina Sofía de Córdoba, Córdoba, Spain
- 10Servicio de Aparato Digestivo, Hospital Universitario Miguel Servet, Zaragoza, Spain
- 11Servicio de Aparato Digestivo, Hospital Universitario de La Princesa, Madrid, Spain
- 12Instituto de Investigación Sanitaria Princesa (IIS-IP), Universidad Autónoma de Madrid (UAM), Madrid, Spain
- 13Servicio de Aparato Digestivo, Hospital Universitario Costa del Sol, Marbella, Spain
- 14Servicio de Aparato Digestivo, Hospital Universitario Donostia, Donostia, Spain
- 15Instituto de Investigación Sanitaria Biodonostia, Universidad del País Vasco (UPV/EHU), Donostia, Spain
- 16Servei de Digestiu, Corporació Sanitaria Park Taulí, Sabadell, Spain
- 17Department of Medicine, Universidad Autónoma de Barcelona, Barcelona, Spain
- 18Servicio de Aparato Digestivo, Centro Médico Teknon, Barcelona, Spain
- 19Servicio de Aparato Digestivo, Hospital General Universitario de Alicante, Alicante, Spain
- 20Servicio de Aparato Digestivo, Hospital General Universitario de Valencia, Valencia, Spain
- 21Servicio de Aparato Digestivo, Hospital Universitario Ramón y Cajal, Madrid, Spain
- 22Servei de Digestiu, Hospital Universitario Arnau de Vilanova, Lleida, Spain
- 23Department of Medicine, Universidad de Lleida, Lleida, Spain
- 24Servicio a Medicina Intensiva, Hospital Universitario La Paz (IdiPAZ), Madrid, Spain
- 25PBM Group, Hospital La Paz Institute for Health Research (IdiPAZ), Madrid, Spain
- 26Servicio de Medicina Interna, Complex Hospitalari Moisès Broggi, Sant Joan Despí, Barcelona, Spain
- 27Grupo Español de Rehabilitación Multimodal (GERM), Zaragoza, Spain
- 28Banco de Sangre y Tejidos de Navarra, Servicio Navarro de Salud, Osasunbidea, Pamplona, Spain
- 29Instituto Aragonés de Ciencias de la Salud, Universidad de Zaragoza, Zaragoza, Spain
Gastrointestinal (GI) bleeding is associated with considerable morbidity and mortality. Red blood cell (RBC) transfusion has long been the cornerstone of treatment for anemia due to GI bleeding. However, blood is not devoid of potential adverse effects, and it is also a precious resource, with limited supplies in blood banks. Nowadays, all patients should benefit from a patient blood management (PBM) program that aims to minimize blood loss, optimize hematopoiesis (mainly by using iron replacement therapy), maximize tolerance of anemia, and avoid unnecessary transfusions. Integration of PBM into healthcare management reduces patient mortality and morbidity and supports a restrictive RBC transfusion approach by reducing transfusion rates. The European Commission has outlined strategies to support hospitals with the implementation of PBM, but it is vital that these initiatives are translated into clinical practice. To help optimize management of anemia and iron deficiency in adults with acute or chronic GI bleeding, we developed a protocol under the auspices of the Spanish Association of Gastroenterology, in collaboration with healthcare professionals from 16 hospitals across Spain, including expert advice from different specialties involved in PBM strategies, such as internal medicine physicians, intensive care specialists, and hematologists. Recommendations include how to identify patients who have anemia (or iron deficiency) requiring oral/intravenous iron replacement therapy and/or RBC transfusion (using a restrictive approach to transfusion), and transfusing RBC units 1 unit at a time, with assessment of patients after each given unit (i.e., “don’t give two without review”). The advantages and limitations of oral versus intravenous iron and guidance on the safe and effective use of intravenous iron are also described. Implementation of a PBM strategy and clinical decision-making support, including early treatment of anemia with iron supplementation in patients with GI bleeding, may improve patient outcomes and lower hospital costs.
Introduction
Acute or chronic gastrointestinal (GI) bleeding affects 47 in 100,000 people worldwide (2) and is one of the most significant clinical problems observed by gastroenterologists, hepatologists, internal medicine physicians, and surgeons (3–6). Upper GI bleeding is associated with mortality rates of 3–14% (4), while a mortality rate of approximately 3% has been reported in patients with lower GI bleeding (7). However, rapid correction of anemia and hypotension may reduce bleeding-associated mortality by preventing cardiovascular decompensation (8–10).
Red blood cell (RBC) transfusion has long been the cornerstone of treatment for anemia due to GI bleeding although the rate of transfusions vary, with 6% of RBC transfusions in Northern Spain (11) and 11–14% of RBC transfusions in England (12, 13) being given due to GI bleeding. However, evidence is accumulating of a dose-response relationship between transfusion and increased patient morbidity, mortality, and length of hospital stay (14–18). Therefore, there has recently been a shift toward a more restrictive use of RBC transfusion on the basis that outcomes are similar or better than when a more liberal strategy is used (19–29). Reduced rates of rebleeding (21, 22), reduced transfusion-associated risks (30, 31), lower all-cause mortality (21), and shorter hospital stays (22) have all been reported when a restrictive rather than liberal transfusion strategy had been implemented. Furthermore, no differences in the risk of ischemic events (21) or major adverse cardiovascular events (25) have been noted between liberal and restrictive strategies. A restrictive transfusion strategy can also reduce healthcare resource utilization and costs (32–34). For example, the United Kingdom’s National Health Service (NHS) saved £3.3 million in the year following the introduction of a restrictive transfusion policy for lower GI bleeding (34). Cochrane meta-analyses support the use of restrictive RBC transfusion strategies across a broad range of clinical indications, including in hemodynamically stable patients with GI bleeding (35). A restrictive approach is now commonly used, resulting in a global reduction in RBC transfusions (36) and transfusion-associated risks. Importantly, reduced demand for blood supplies helps to ensure that blood is available for those who need it most, which is important given that currently approximately 25 million units of blood are transfused to more than 5 million patients each year in Europe (37). With an aging population and a decreasing number of blood donors, it is expected that more and more countries will experience challenges in ensuring that blood supplies are adequate (38). Indeed, blood shortages have been reported in several European countries during health emergencies in recent years (39). Despite the continued efforts of the European Commission to ensure the optimum use of blood components (40), data from the NHS suggest that approximately 15–20% of RBC transfusions are used inappropriately (41). Moreover, the persistent variation in blood utilization across European Union member states indicates that the inappropriate use of blood supplies is widespread (42). Despite the benefits of a restrictive approach to transfusion, consensus recommendations are lacking, and major shortcomings have been identified in many clinical practice guidelines for transfusion practice (43). Applying consistent criteria to clinical decision-making regarding the eligibility and timing of transfusion in patients with GI bleeding is crucial (30).
Patient blood management (PBM) is an evidence-based bundle of care that aims to optimize outcomes for all patients with bleeding potential by managing and preserving blood. The concept of PBM is built on 3 pillars: (1) optimization of RBC mass, including the use of iron replacement therapy and/or erythropoiesis-stimulating agents where needed; (2) minimization of blood loss/bleeding; and (3) optimization of the patient’s tolerance of anemia (36, 44, 45). PBM can reduce patient mortality and morbidity (46) and its incorporation into healthcare management has the potential to bring benefits to many patients and healthcare institutions (47–50). It is also crucial to raise awareness about the importance of preserving and managing the patient’s own blood and maintaining well-functioning bone marrow erythropoiesis, rather than routinely resorting to the use of donor blood. This approach should improve patient outcomes as well as reduce the rate of over-transfusion, thereby preventing many transfusions that would otherwise have been deemed appropriate.
We therefore developed the current protocol, based on the three pillars of PBM, with the aim of optimizing the management of patients with anemia and iron deficiency due to GI bleeding in clinical practice. For this purpose, it was deemed very important to include the expert opinion of PBM experts, because of their high awareness of the need to save blood and minimize healthcare costs.
Aims of the protocol
This protocol was commissioned and approved by the Spanish Association of Gastroenterology (Asociación Española de Gastroenterología [AEG]), after an expert review was performed by professionals from 16 Spanish hospitals, who were also part of the Working Group on “Esophagus, Stomach, and Duodenum” for the AEG. The review was conducted in collaboration with internal medicine physicians, intensive care specialists, and hematologists with advanced knowledge of PBM programs. This was not a formal systematic literature review but was based on a review of the literature to provide best practice advice statements. No formal rating of the quality of evidence or strength of recommendation was performed.
All the authors were invited for their experience, prestige, academic recognition, and representation in their respective societies. Most of the authors worked on the initial reference document, which was presented at a Congress of the AEG. Subsequently, it was disseminated among the members of the AEG and underwent internal and external peer-review through the standard procedures of Clinical Gastroenterology and Hepatology. Several years later, the document was updated with the comments and criticism received and presented for additional rounds of review and discussion; it was unanimously approved 6 months later. A shorter online version of the protocol is also available on the AEG website: https://www.aegastro.es/documents/prodiggest/Prodiggest-Management-of-anaemia-and-iron-deficiency-in-gastrointestinal-bleeding.pdf.
The primary goal of the project was to develop a healthcare protocol for the management of anemia and iron deficiency associated with GI bleeding. Therefore, current indications for RBC transfusion were reviewed in the context of patients with GI bleeding. The protocol aimed to provide guidance on achieving an adequate balance between the use and overuse of RBC transfusion according to a restrictive model, promoting the use of restrictive rather than liberal criteria and a 1 blood unit policy (i.e., “don’t give two without review”). Other aims of the protocol were to provide information on the advantages and limitations of oral versus intravenous iron in patients with GI bleeding and to provide guidance on the safe and effective use of intravenous iron for the treatment of blood loss associated with GI bleeding.
This protocol focuses on the management of acute anemia or iron deficiency in patients with acute GI bleeding (with or without portal hypertension) and on the management of chronic anemia or iron deficiency due to fecal occult blood loss. The protocol is not intended for use in pediatric patients or for patients with anemia or iron deficiency due to causes other than GI bleeding such as inflammatory bowel disease. The management of other aspects of GI bleeding are also out of the scope of the protocol (e.g., evaluating the extent of blood loss, resuscitation measures, identifying and treating the sources of bleeding).
This protocol is relevant to clinicians and nurses who treat adults with acute or chronic GI bleeding, and it is intended for use in general clinical practice in both the primary and secondary care settings. Management of acute GI bleeding currently requires an interdisciplinary approach, which may involve general practitioners, emergency and internal medicine physicians, gastroenterologists, hepatologists, endoscopists, surgeons, critical care practitioners, hematologists, biopathologists, intensive care specialists, nurses, and interventional radiologists, depending on local practice.
Treatment of anemia
Preliminary considerations
Acute GI bleeding results in blood loss, which, in extreme cases, can lead to hypovolemic shock and death. Although several international consensus/guidelines were published between 2012 and 2021 by the American Society for Gastrointestinal Endoscopy, the European Society of Gastrointestinal Endoscopy, and the American College of Gastroenterology (27, 51–55), the importance of correcting anemia and iron deficiency often remains undervalued or underestimated within the complexities of managing GI bleeding.
Until recently, RBC transfusion was considered a key treatment for acute blood loss anemia for many patients. However, its use in this setting is subject to substantial variability, with observational studies suggesting that RBC transfusion is used in 25–43% of cases of GI bleeding overall and that over 40% and 60% of transfusions in patients with upper and lower GI bleeding, respectively, are given in the absence of clinically significant anemia (hemoglobin ≥ 8 g/dL) (5, 56–58). More recently, goal-directed fluid therapy with restrictive volume restitution (59–61) has started to be used to correct hemodynamic instability, achieve hemodynamic control, and avoid hypovolemic shock, with RBC transfusions only recommended for patients with symptomatic anemia who do not respond to fluid therapy. Adaptation of transfusion criteria to align with clinical practice guidelines and expert reviews (26, 29, 57, 62–65) is therefore required to help optimize the use of transfusion resources and improve patient outcomes and clinical results.
Before determining what, when, and how to transfuse, certain considerations must be highlighted. First, it should be noted that hemoglobin levels are not always indicative of the full extent of blood loss in patients with GI bleeding. For example, decreased hemoglobin levels can be caused by fluid movement from the interstitial space to the vascular compartment, and infusion of intravenous fluids or overhydration may also lead to false low-concentration readings. A “normal” initial hemoglobin level, therefore, does not exclude the possibility of severe bleeding. Hemodynamic parameters may provide a better overview of the extent of blood loss together with regular monitoring of hemoglobin levels in the event of potentially severely bleeding lesions.
Second, excessive blood volume replacement can lead to an increased risk of rebleeding. This is because vasodilation and the subsequent increase in blood pressure can result in erosion of newly formed hemostatic plugs and dilution of clotting factors. Furthermore, the impact of hypothermia on in vivo coagulation must be considered. Overexpansion of plasma volume in patients bleeding because of portal hypertension may also induce an increase in portal pressure that may favor recurrent bleeding. A restrictive transfusion model that precludes the use of blood to increase blood volume is therefore recommended (29, 57), and RBC units should be transfused 1 unit at a time (66, 67), except in severe cases or in the case of uncontrolled active bleeding.
Third, the source of bleeding should be identified and the management of clotting disorders in patients with GI bleeding should also be considered. Thrombocytopenia is uncommon and is observed in only 5% of patients with upper GI bleeding and 1% of those with lower GI bleeding (68, 69). International normalized ratio (INR) levels > 1.5 are detected in 15% and 11% of patients with upper and lower GI bleeding, respectively, due primarily to the presence of liver failure or the use of oral anticoagulants such as warfarin or acenocoumarol (7, 68). Platelet transfusions are recommended for patients who have significant bleeding and a platelet count < 30 × 109/L (66), particularly in cases of acute bleeding related to portal hypertension. It is not recommended to correct slight coagulation abnormalities with fresh frozen plasma transfusions in patients who have been receiving vitamin K antagonist treatment (66). The use of prothrombin complex concentrates and vitamin K to reverse the effects of oral anticoagulants is usually preferred.
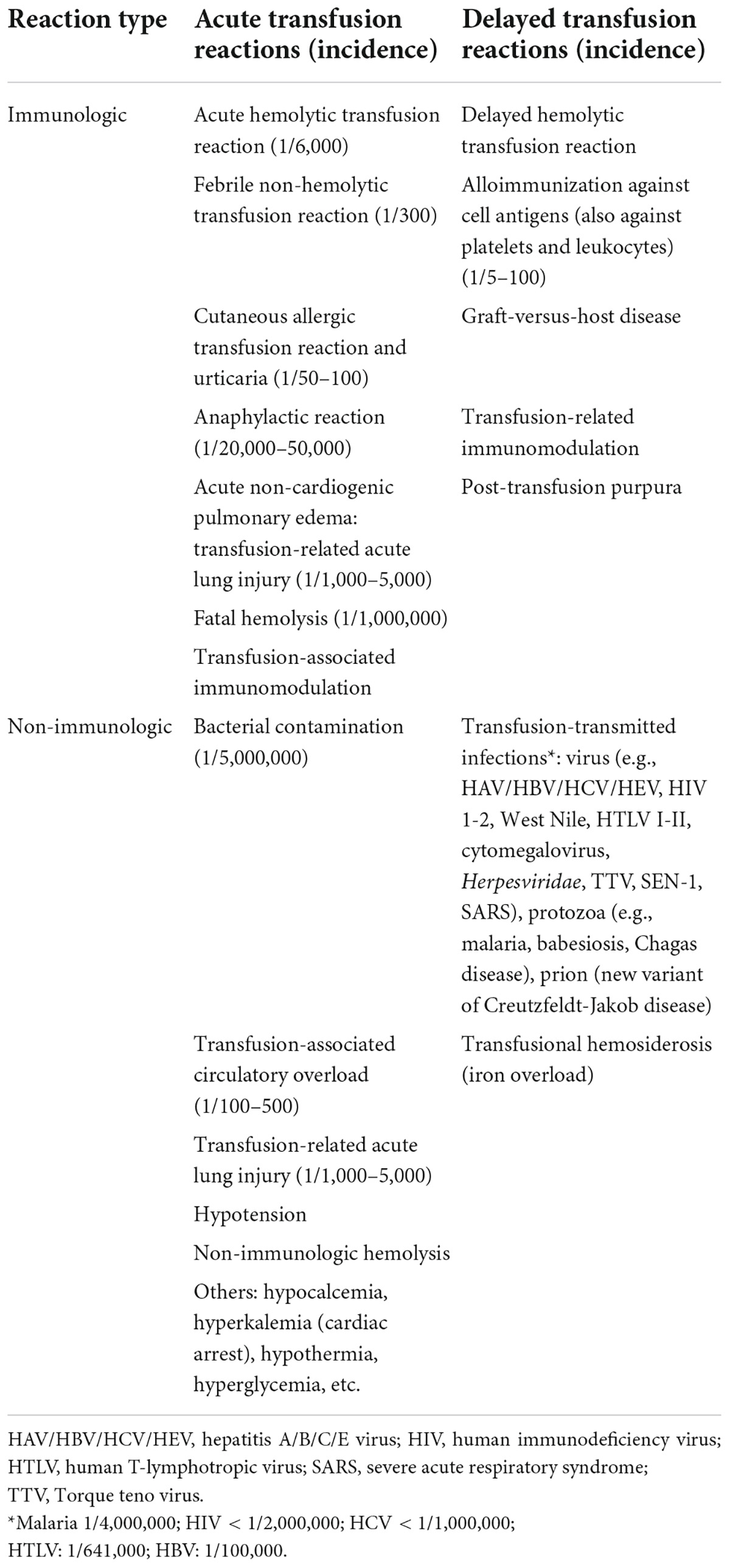
Finally, consideration should be given to the adverse consequences that can accompany transfusion (Table 1) and the associated costs [e.g., in one European analysis, the estimated cost of a 2-unit RBC transfusion was €878 (70)].
Management of anemia due to acute gastrointestinal blood loss
The GI bleeding protocol presented here should be integrated into a hospital’s wider PBM program. Many factors should be considered when deciding whether to give an RBC transfusion to patients with anemia. The criteria used in PBM to decide what, who, and when to transfuse are broadly based on the severity of the bleeding, the impact on hemodynamic stability, the source and activity of the bleeding, the likelihood of rebleeding, and the presence of comorbidities (71) that may affect bleeding control or increase the risk of tissue hypoxia (Figure 1). Blood transfusion is an early example of “personalized medicine” as treatment decisions should be individualized and guidelines should not supersede the clinical judgment of the treating physician when deciding which patients should undergo transfusion. Adequate replenishment of blood volume is essential to maintain and optimize organ perfusion. However, this should generally involve crystalloid- and colloid-based fluid therapy rather than blood transfusion. The following section includes some important considerations in the management of patients with anemia due to acute blood loss.

Figure 1. Factors influencing the decision to give transfusions. COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident.
1. Patients receiving anticoagulant treatment are at increased risk of severe or persistent bleeding; therefore, this should firstly be reversed. In contrast, antiplatelet drugs should only be discontinued if absolutely necessary (6, 62).
2. Dyspnea, chest pain, tachycardia, hypotension that is refractory to initial blood volume replacement, obnubilation, and oliguria are warning signs or symptoms of acute blood loss (27).
3. Active bleeding or identification of a visible vessel during endoscopy indicates an increased risk of rebleeding (27).
4. The presence of gastroesophageal varices also increases the risk of rebleeding if the plasma volume is overexpanded following transfusion (19, 21, 29).
5. Factors that increase the risk of a vascular event (e.g., history of coronary artery disease, arrhythmias, and/or heart failure – see section below) add to the complexity of the decision-making process (55).
Clinical evaluations used within the patient blood management protocol
It is necessary to consider and record any variables that may influence the decision whether to administer a RBC transfusion, as well as variables that have an impact on the rate of transfusion or route of administration of iron replacement therapy (oral vs. intravenous) (66). This includes:
• The patient’s medical history, especially any recent history of ischemic or thrombotic events, cardiopulmonary disease, chronic kidney disease, imminent surgery (within < 30 days), severity of bleeding and associated anemia, or any clinical condition that may interfere with oral iron availability or absorption (e.g., iron tolerance or refractivity).
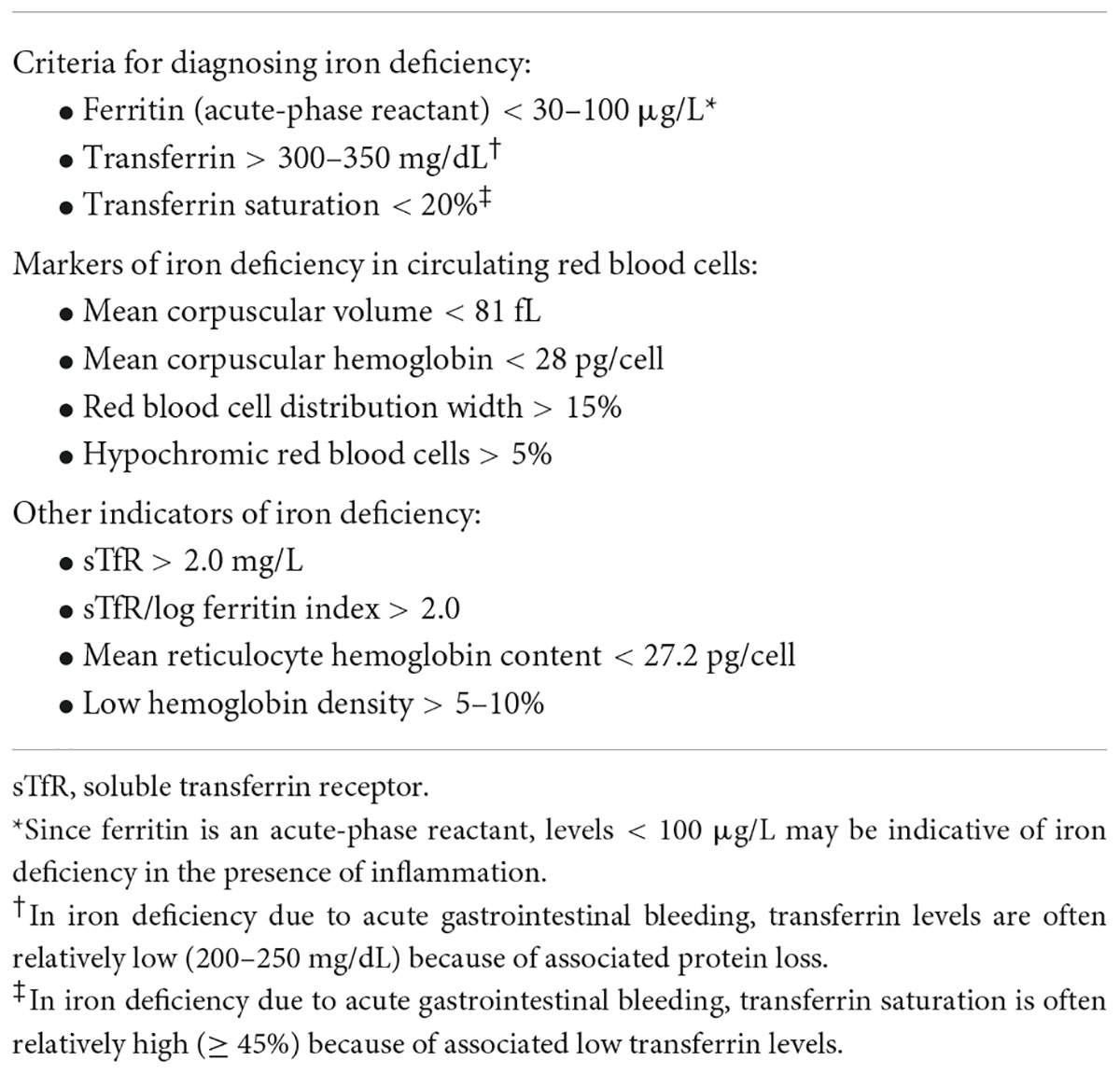
• As a minimum, the following laboratory tests that could indicate the presence or absence of iron deficiency should also be performed: hemoglobin level; numbers of RBC and reticulocytes; RBC distribution width; mean corpuscular hemoglobin; mean corpuscular volume; serum ferritin and transferrin saturation (TSAT) levels; and measurement of C-reactive protein, creatinine, and urea levels.
• Additional complementary tests may be performed depending on the clinical scenario, such as more extensive laboratory tests including the measurement of serum vitamin B12, folate, haptoglobin, soluble transferrin receptor, or lactate dehydrogenase levels, and reticulocyte hemoglobin content.
• A GI endoscopy or computed tomography angiogram may also be performed to identify the cause and extent of the bleeding as well as the presence of endoscopic warning signs of rebleeding (according to the Forrest Classification System), which could reduce the risk of rebleeding and transfusion. Both endoscopy and angiography can offer additional and effective therapeutic resources in the control and cessation of bleeding.
Restrictive red blood cell transfusion within patient blood management
Various measures for avoiding transfusion are proposed by the PBM strategy. These include testing for and evaluating any anemia (and its origin) or coagulopathy issues, and providing specific treatment (e.g., intravenous iron replacement therapy in the case of iron deficiency anemia, reversal of anticoagulant/antiplatelet therapy, prohemostatic drugs). Strategies to reduce the risk of bleeding associated with invasive procedures should also be employed. Iatrogenic blood loss due to the taking of excessive analytical samples should be avoided by using pediatric tubes and non-invasive point-of-care monitoring for hemoglobin levels, INR, hypoxia, etc. Moreover, the minimum clinically effective transfusion volume should be used (where feasible) (72).
Transfusion thresholds
An algorithm depicting the indications for RBC transfusion in patients with acute GI bleeding is shown in Figure 2. The protocol establishes the following guidelines:
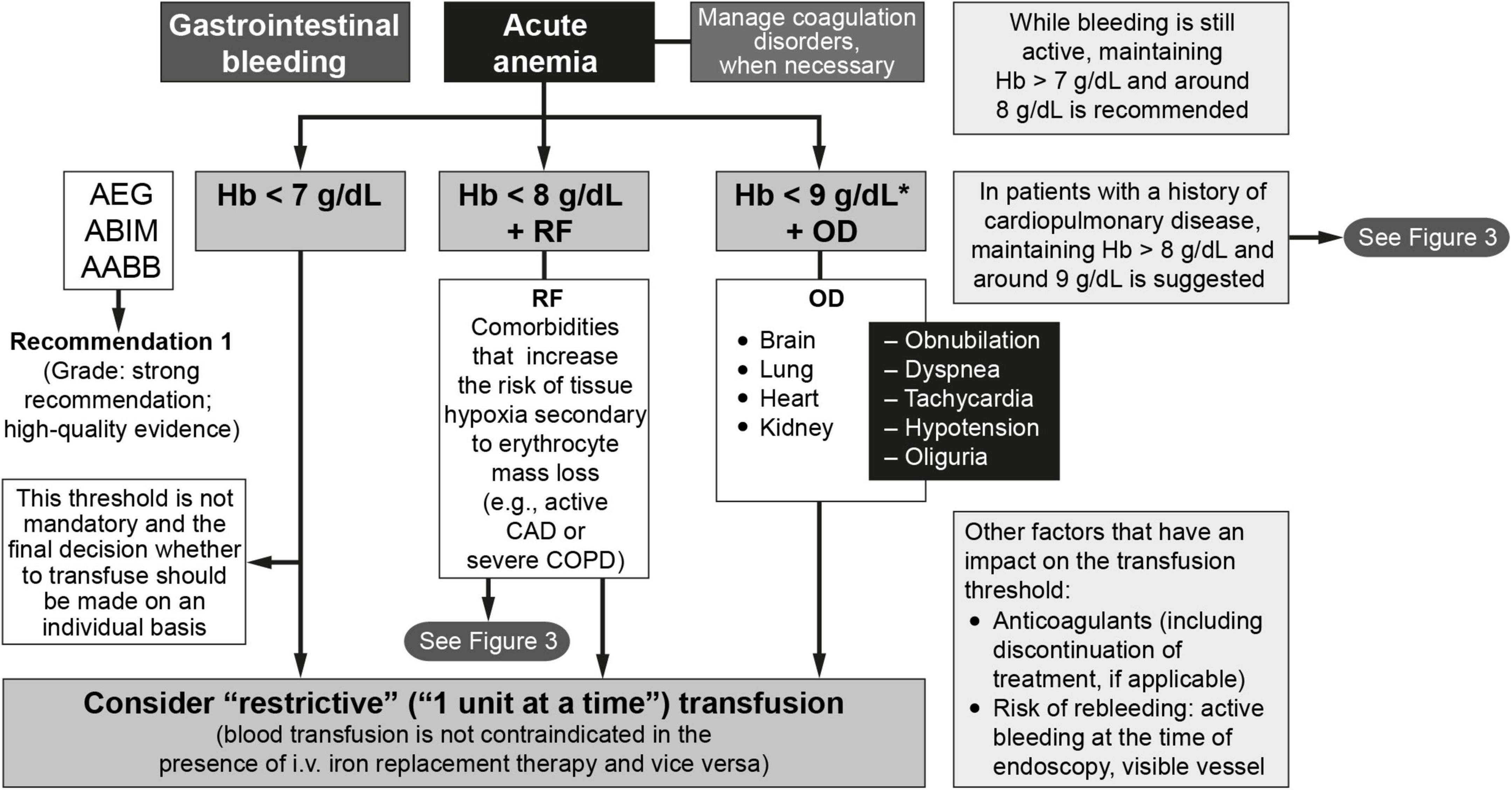
Figure 2. The management of anemia and iron deficiency in patients with acute gastrointestinal bleeding (64, 81). AABB, American Association of Blood Banks; ABIM, American Board of Internal Medicine; AEG, Asociación Española de Gastroenterología; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; Hb, hemoglobin; i.v., intravenous; OD, organ dysfunction; RF, risk factors. * < 10 g/dL if severe bleeding.
1. In the absence of risk factors and warning signs or organ dysfunction, a hemoglobin level of < 7 g/dL is often used as the cutoff for transfusion when a restrictive approach is being used (16, 17, 20, 73, 74). This threshold is not mandatory and the final decision whether to transfuse should be made on an individual basis. Close monitoring alone without transfusion is an option for patients with hemoglobin levels below this threshold who have no comorbidities or symptoms, are hemodynamically stable, have inactive bleeding, and have a low risk of rebleeding.
2. The question of when to transfuse the patient who has ischemic heart disease and/or heart failure remains controversial. In fact, taken together, the short- and long-term findings from available clinical trials indicate that the question of non-inferiority and/or superiority of the two transfusion approaches (restrictive vs. liberal) in these patients remains unanswered (25, 75–81). The greatest difficulty lies in the large number of possible scenarios. An algorithm to determine if transfusion is indicated in patients with cardiovascular risk can be found in Figure 3 (81). For most patients in this category, the recommendation is to transfuse to maintain hemoglobin levels > 8 g/dL but no more than 9 g/dL. Therefore, following the recommendations of the American Association of Blood Banks (64) and based on the clinical trial results available to date, restrictive RBC transfusion should be considered for patients with a hemoglobin level of < 8 g/dL in the following cases (25, 75–81):
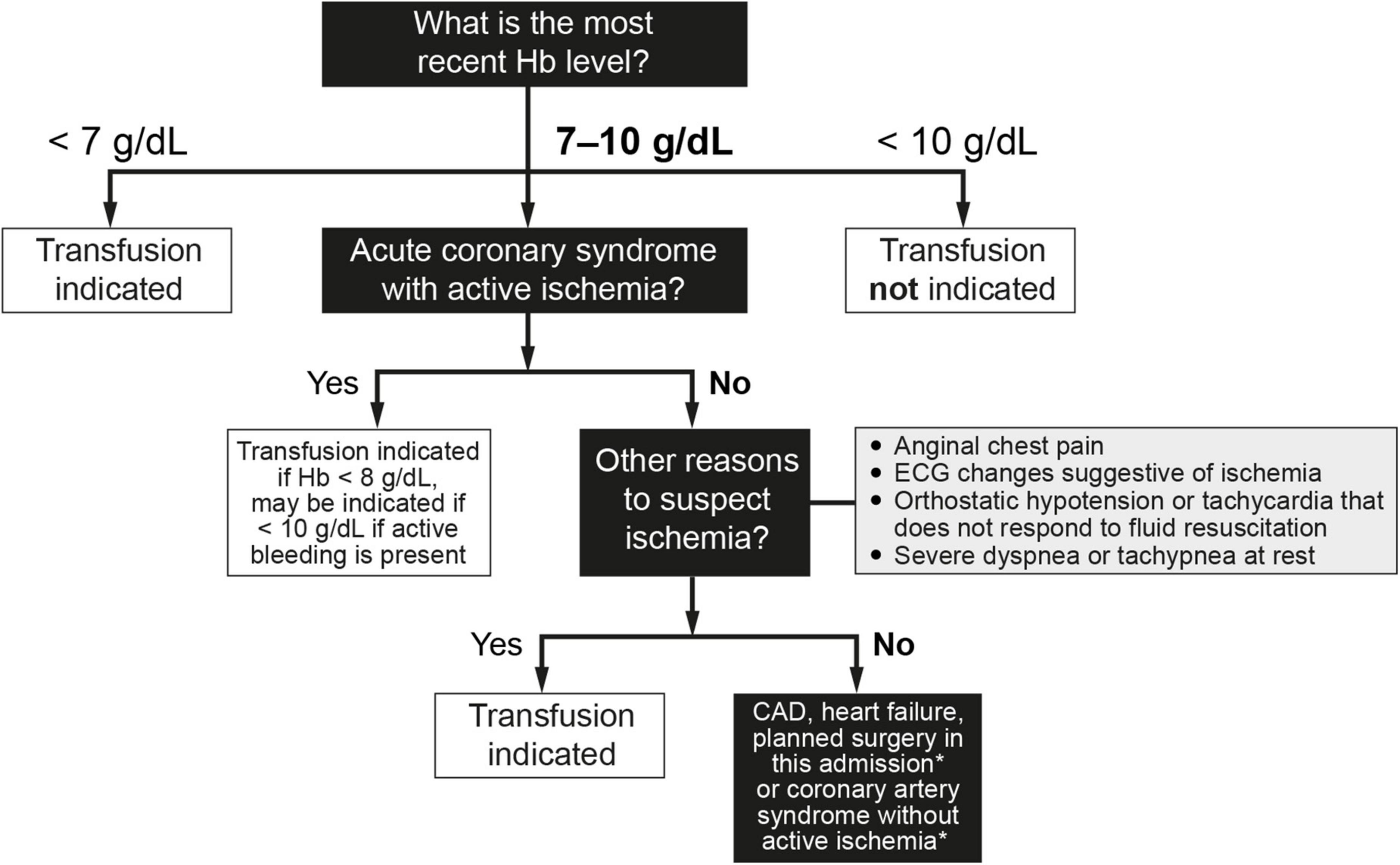
Figure 3. Indications for red blood cell transfusion in the presence of cardiovascular risk factors (64, 77–81). In cases of both acute hemorrhage and chronic blood loss, the decision to transfuse red blood cell concentrates does not exempt the indication to replenish iron stores, because 1 red blood cell unit only provides 200 mg of iron. This consideration is especially important in patients with CAD and/or heart failure. CAD, coronary artery disease; ECG, electrocardiographic; Hb, hemoglobin. *Consider transfusion if Hb < 7.5 g/dL.
• Patients with an acute coronary syndrome (e.g., acute myocardial infarction or with known coronary artery disease and unstable angina) and who have ongoing ischemia despite anti-ischemic therapy, such as medical therapy or angioplasty. Lower target hemoglobin levels are often used for patients whose signs and/or symptoms of ischemia resolve with anti-ischemic therapy.
• Patients with other findings suggestive of active ischemia, including anginal chest pain, electrocardiographic changes suggestive of ischemia, orthostatic hypotension or tachycardia that does not respond to fluid resuscitation, or severe dyspnea or tachypnea at rest. Clinical judgment is required to determine if a patient’s symptoms signify active ongoing ischemia (i.e., transfusion is indicated) or merely reduced oxygen carrying capacity (which may be treated without transfusion). Signs or symptoms that do not necessarily warrant transfusion include irritability, weakness, tiredness, or exertional dyspnea. Conversely, these signs/symptoms may not always be present in a patient with ischemia (e.g., a patient receiving a beta-blocker may not have tachycardia).
• Patients with chronic obstructive pulmonary disease (COPD).
• The presence of vascular risk factors for arteriosclerosis (e.g., diabetes, hypertension, dyslipidemia, smoking) alone are not sufficient to warrant transfusion, unless the patient has signs of active bleeding and/or hemodynamic instability that is not corrected with fluid replacement.
• The threshold for patients with a history of heart failure is less clear, but a hemoglobin threshold of between 7 and 8 g/dL (70–80 g/L) is likely to be appropriate for most patients. Hospitalized patients with heart failure are especially challenging to manage, and the improvement in oxygenation from transfusion must be balanced against the risks of worsening heart failure due to the volume of transfused blood. When RBC transfusion is required in a patient with heart failure, careful attention to volume status is recommended, including adjustment of transfusion rate and use of supplemental diuretics as needed to avoid volume overload.
3. RBC transfusion should only be considered for patients with hemoglobin levels > 8 g/dL but < 9 g/dL in the presence of organ (heart, brain, lung, or liver) dysfunction. In this situation, clinicians should consider the advantages and disadvantages of use/overuse of transfusion, regardless of any laboratory test results. The volume of blood transfused should not exceed the amount required to relieve symptoms of anemia or to achieve a hemoglobin level of 7–8 g/dL (which is also considered safe for stable, non-cardiac patients). An important point to consider is that RBC transfusion does not exempt the need for intravenous iron replacement therapy, since 1 RBC unit provides no more than 200 mg of iron.
Management of chronic anemia due to occult blood loss
Hemoglobin thresholds for transfusion in the case of chronic anemia associated with occult GI bleeding (Figure 4) differ from those used in the setting of acute bleeding (Figure 2) (73, 74, 82). RBC transfusion should be considered for patients with hemoglobin levels < 5 g/dL and for those with hemoglobin levels < 6 g/dL in the presence of risk factors such as cardiopulmonary failure, ischemic heart disease, cardiac arrhythmia, and COPD. These hemoglobin thresholds are supported by studies showing how in patients with severe chronic anemia (hemoglobin < 6 g/dL), and even extreme anemia (hemoglobin ≤ 5 g/dL) due to digestive or gynecological blood loss, third-generation intravenous iron is effective and safe for the rapid correction of anemia (29, 73). This policy helps to avoid unnecessary blood transfusions. Transfusion should also be considered for patients with hemoglobin levels < 7 g/dL with associated warning signs and symptoms of organ dysfunction, such as dyspnea, precordial pain, tachycardia, hypoxia, or orthostatic hypotension. Patients with hemoglobin levels > 7 g/dL and no symptoms of organ dysfunction should undergo observation and correction of any iron deficiency. As mentioned previously, if a patient receives a transfusion, this does not exempt the need for intravenous iron replacement therapy, and vice versa. RBC transfusions should not be administered to patients who only have iron deficiency, except in those who are hemodynamically unstable.

Figure 4. Algorithm for the management of chronic anemia associated with gastrointestinal blood loss. CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EPO, erythropoietin; Hb, hemoglobin; i.v., intravenous; OD, organ dysfunction; RBC, red blood cell; RF, risk factors; WS, warning signs. *RBC transfusion is not enough to replenish iron stores; 1 RBC unit supplies 200 mg of iron; **Warning signs in patients with Hb levels < 7 g/dL could be triggered by events such as atrial fibrillation, sepsis with a systemic inflammatory response, or an “acute on chronic” bleeding episode.
Treatment of iron deficiency
Preliminary considerations
Iron is crucial for erythropoiesis and oxygen transport, and it also serves important roles in cell energy production, maturation of the immune system, and efficient organ function (83–86). Although iron deficiency anemia is the most prevalent cause of anemia worldwide, not all anemias are due to an iron deficit. Furthermore, many patients have been found to have iron deficiency who do not have anemia. The criteria used to diagnose iron deficiency are summarized in Table 2. It should be noted that the terms iron deficiency and iron deficiency anemia are often confused in publications and these terms are not necessarily synonymous (87–89). GI-related issues that may lead to the onset of iron deficiency and iron deficiency anemia are shown in Table 3.
Acute GI bleeding leads to a reduction in RBC volume resulting in a need for RBC regeneration, which subsequently can lead to iron deficiency and iron deficiency anemia; hence, it is important to consider the use of iron replacement therapy in these patients. Although the anemia in acute GI bleeding is initially due to blood loss, if iron stores are reduced or iron absorption is disrupted, the iron deficit will continue to be observed over time. For example, > 60% of patients enrolled in a Spanish study developed iron deficiency anemia within 1 month of non-variceal upper GI bleeding (90). Risk factors for the development of iron deficiency anemia were age > 75 years, initial ferritinemia < 65 μg/L, initial hemoglobin levels < 10 g/dL, and TSAT < 10% at day 5. After an episode of upper GI bleeding, blood urea levels temporarily rise due to the absorption of extravasated urea nitrogen in the intestinal lumen, and urea levels may therefore be higher than creatinine levels. Consequently, blood urea levels > 80 mg/dL at admission may indicate significant bleeding and an increased risk of anemia. The use of iron replacement therapy in patients with chronic iron deficiency anemia can lead to a reduction in RBC transfusions (91) and a consequent reduction in the associated risks. Iron replacement therapy should be started as soon as iron deficiency is detected, to replenish iron stores and restore normal erythropoiesis as soon as possible (92, 93).
Advantages and limitations of oral and intravenous iron administration
The choice between oral and intravenous iron replacement therapy depends on various factors, such as the severity and speed of the onset of anemia, cost, the availability of existing formulations, patient tolerance to oral iron, patient preferences, and the existence of other limiting factors (e.g., malabsorption of oral iron, inflammation, or allergies to intravenous iron) (86, 94–96). Under suitable conditions, oral iron is effective, readily available, inexpensive, and well tolerated (97–99). For example, many patients who have mild iron deficiency anemia associated with chronic fecal occult blood loss may be effectively managed with oral iron. However, treatment with oral iron (especially iron sulfate) is associated with GI side effects in many patients (100). Other limitations to oral iron therapy are low levels of adherence, lack of suitability for patients with severe bleeding or continuous occult blood loss, the excessive time (months) required to replenish iron stores, and the lack of clarity regarding total costs, which may be higher than initially expected when absenteeism/presenteeism considerations are also taken into consideration. Newer intravenous iron compounds are considered safer than blood transfusion, have fewer GI side effects than oral iron (100, 101), and are also associated with improved/guaranteed adherence (67, 91, 94, 101, 102). These compounds also provide the total iron dose required (often in a single infusion), quickly increase hemoglobin levels, and promote more effective replenishment of iron stores in comparison with oral iron (67, 91, 94, 101–104). Much of the data supporting the efficacy and safety of intravenous iron in patients with GI bleeding come from studies of ferric carboxymaltose (FCM) (33, 91, 101, 102). A prospective study showed that 1,500/2,000 mg of intravenous FCM (given over 2 infusions) increased hemoglobin and iron levels faster and more effectively when compared with 6 weeks of oral ferrous sulfate treatment in patients with anemia due to acute GI bleeding (102). FCM was also better tolerated than oral iron and associated with significantly improved quality of life (102). Furthermore, a retrospective study showed that a single 1,000-mg FCM infusion effectively increased hemoglobin levels in elderly patients who had comorbidities and acute GI bleeding (including some who were hemodynamically unstable), thereby supporting the use of a restrictive transfusion policy (33). Hemoglobin levels were also increased, and transfusion rates significantly reduced in a retrospective study of FCM in patients with chronic GI bleeding who had previously been receiving chronic transfusion support (91). Similar effects on iron parameters have been reported for other intravenous iron formulations (105–107).
There are, however, limitations to the use of intravenous iron. These include the requirement for monitoring during infusion, the risk of infusion-related reactions (although these are extremely uncommon), the requirement for equipment and staff training, and the potential for increased costs (67, 91, 94, 101, 102). Furthermore, although increases in hemoglobin levels occur more quickly than with oral iron, it may still take 3–5 days for levels to improve following infusion. Therefore, blood transfusion is a necessary therapeutic option to maintain the transport of oxygen to the tissues in cases of severe anemia where alternative treatments are not available or where it is not possible to wait for these to take effect. Nonetheless, intravenous iron can be complementary to blood transfusion to treat any underlying iron deficiency (i.e., blood transfusion does not contraindicate intravenous iron treatment or vice versa). The decision about when intravenous iron should be administered (before, during, or after blood transfusion) is related to the patient’s hemodynamic status. In patients with hemodynamic instability, alarm signs, or organ dysfunction, transfusion should be prioritized, and transfusion should not coincide with the intravenous iron infusion, i.e, it should not be administered in the same line nor at the same time. Intravenous iron should be administered once the transfusion of the first unit has been completed, after clinical and analytical evaluation, and in the absence of any adverse reactions. However, in the event of hemodynamic stability, with stabilized and controlled bleeding, and in the absence of clinical signs or symptoms suggesting that an urgent transfusion is required, intravenous iron can be administered (over 15–30 min), while the pre-transfusion compatibility tests are being performed by the transfusion service and the red blood cell concentrates are awaited. In general, we must aim to transfuse the minimum number of blood units possible to achieve the required clinical effect alongside treatment specific to the cause of the anemia. Adverse effects associated with intravenous iron infusion can include nausea, headache, dizziness, hypertension, skin rash, injection-site reactions, hypophosphatemia, increased alanine aminotransferase levels, and (extremely rarely) hypersensitivity reactions. In general, the third-generation compounds have the safest profiles. These agents are better tolerated and are associated with a lower risk of infusion-related/anaphylactoid reactions, and a reduced generation of non-transferrin bound free iron when compared with earlier compounds (108, 109). However, each intravenous iron product is unique and thus it is not possible to extrapolate data from one product to compare with that of another (110). The use of FCM also permits a higher iron dose to be given over fewer, shorter infusions (only 1 or 2 doses of 1,000 mg, each given over a 15-min infusion, are usually required in comparison with, for example, multiple, longer infusions for iron sucrose) (103, 109, 111, 112).
Indications for the use of intravenous iron
The indications for the use of intravenous iron in patients with GI bleeding are shown in Table 4. In patients with acute GI bleeding and uncontrolled hypertension or hemodynamic instability (as indicated by a systolic blood pressure < 90 mmHg or heart rate > 100 bpm), blood volume should initially be replenished with fluids and transfusion is not needed. However, in the case of acute anemia due to severe GI bleeding, RBC transfusion is recommended.
None of the scenarios that support the use of RBC transfusion preclude the use of intravenous iron for the treatment of any associated iron deficiency. It should be remembered that 1 unit of RBC concentrate provides approximately 200 mg of iron and, therefore, is not enough to replenish iron stores (estimated at up to 2 g in patients with hemoglobin levels < 10 g/dL post-bleeding and a body weight of 75 kg; Table 5) (111, 113).
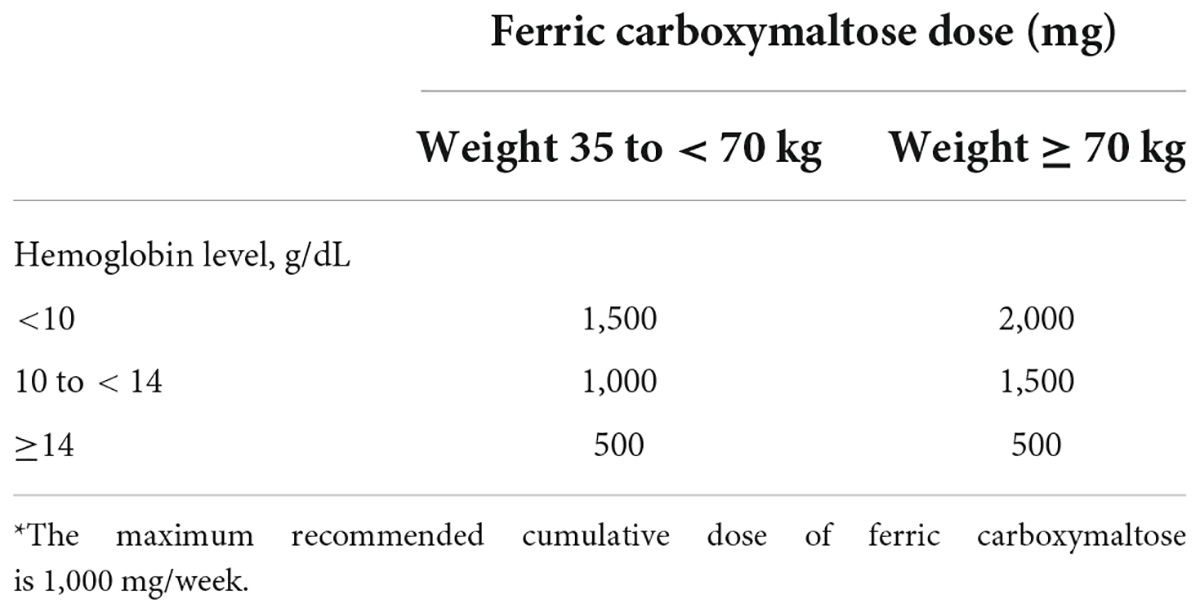
Table 5. A simplified formula for calculating the required dose of ferric carboxymaltose, based on patient body weight and hemoglobin levels* (110, 112).
In patients with iron deficiency/iron deficiency anemia due to chronic GI bleeding, treatment with intravenous iron can be a very good alternative to RBC transfusion, thereby reducing or avoiding the need for transfusions (73). However, transfusion should be used in these patients when pharmacologic treatment of anemia has failed or in cases of severe anemia.
Contraindications to intravenous iron include persistent bacteremia, serious known allergy, hypersensitivity to other parenteral iron-containing products, TSAT > 45% (or ferritin levels > 500 μg/L with TSAT > 25% in patients with inflammatory conditions), or hemochromatosis/hemosiderosis/porphyria cutanea tarda. A history of bronchial asthma or severe eczema also increases the risk of a hypersensitivity reaction after administration of intravenous iron, and this aspect should be considered before establishing the need for intravenous iron treatment in such patients. Intravenous iron should also generally not be used during the first trimester of pregnancy.
Until recently, intravenous doses of iron were often calculated using the Ganzoni formula (114). This formula is based on body weight, actual compared with target hemoglobin levels, and iron stores. A limitation of this formula is that it underestimates the dose of iron required in patients with acute bleeding and is only reliable in patients with pure iron deficiency. In patients with mixed anemia (where anemia is partly due to other causes), use of the Ganzoni formula may lead to excessive iron replenishment and subsequent iron overload. A simple and efficient method for calculating the dose of intravenous iron based on body weight and hemoglobin levels has been developed based on information included in the summary of product characteristics for FCM (Table 5) (111, 113). This is commonly used in hospital settings in the context of GI bleeding, especially in severe cases. In a randomized, placebo-controlled clinical trial of oral vs. intravenous iron in patients who had experienced an upper gastrointestinal hemorrhage, the authors suggested that treatment with intravenous FCM, even when given at lower than standard doses, was significantly beneficial compared with oral ferrous sulphate in patients with lower body weight (115), although this finding needs to be validated in studies involving a larger number of patients.
Summary
GI bleeding is common in hospital settings and requires management by an interdisciplinary team (3). Blood volume depletion, loss of RBC mass, and associated clotting disorders require support and replacement strategies. Although transfusions undoubtedly save lives and are a fundamental pillar of the management of severe GI bleeding, they remain one of the most overused medical procedures and are associated with many “Do Not Do Recommendations” (66). The decision to give an RBC transfusion is challenging and should take into account individual patient characteristics, together with the source, activity, and extent of bleeding and the patient’s clinical tolerance of anemia. Several studies support the use of clinical decision-making tools to promote PBM and restrictive transfusion practices, and to improve RBC utilization, even in high-risk patients (33, 50, 74, 91, 116–118). In some patient groups, a restrictive strategy can reduce unnecessary use of allogeneic transfusions and is associated with equivalent or better outcomes than a liberal strategy.
The decision to administer oral or intravenous iron to a patient with bleeding depends on multiple factors, including the severity of anemia, the presence of inflammation, whether a rapid increase in hemoglobin levels and replenishment of iron stores are needed to benefit patient symptoms and quality of life, costs, and patient adherence to treatment with oral iron (94, 97, 103, 119, 120). The protocol presented here is the result of a thorough review performed by gastroenterologists, hepatologists, hematologists, and PBM experts with solid training in the implementation of PBM-based policies. It also reflects current clinical practice in Spain regarding the management of anemia and iron deficiency in patients with acute or chronic GI bleeding. The protocol has been designed to maintain a balance between the use and overuse of transfusion and advises on the safe use of oral versus intravenous iron in these settings. This has permitted the introduction of a prospective database – AEG-REDCap (Research Electronic Data Capture Service of the AEG) – in many Spanish hospitals, in which the clinical characteristics of patients who are admitted because of GI bleeding can be recorded. Having a prospective registry of cases should help avoid the confounding bias that may result from inadequate variability in clinical practice.
In summary, collaboration between physicians in direct contact with patients who have GI bleeding and hematologists and other specialists involved in PBM is important and should contribute to the improved future management and correction of iron deficiency anemia. While blood is a costly resource, which is dependent on the participation of donors, replenishment of iron stores in patients contributes to an improvement in health-related quality of life and an overall reduction in healthcare costs.
Author contributions
MM and JAGE developed the concept for the manuscript and wrote the main body of the article. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study received funding from an unrestricted grant from Vifor Pharma. The funder was not involved in the study design, collection, analysis, and interpretation of data or the decision to submit for publication.
Acknowledgments
Medical writing support (including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables and figures, collating author comments, copyediting, fact checking, and referencing) was provided by Dawn Batty, PhD, of Aspire Scientific (Bollington, United Kingdom) and was funded by an unrestricted grant from Vifor Pharma. The authors thank Eloísa Urrechaga, Biocruces Bizkaia Research Institute, Bilbao, Spain, for reviewing the sections on laboratory tests.
Conflict of interest
MM has served as a speaker, consultant, and advisory member for, or has organized courses with scholarships or funding from: AbbVie, Almirall, Casen Fleet, Chiesi, Falk Pharma, Faes Farma, Ferring, Janssen, MSD, Otsuka Pharmaceutical, Pfizer, Roche, Shire Pharmaceuticals, Takeda, Tillotts Pharma, and Vifor Pharma. MC was previously an employee of Vifor Pharma. ÁL has been an advisor to Vifor Pharma. CV has organized courses with scholarships or funding from: AbbVie, Bayer, Boston Scientific, Faes Farma, Ferrer, Ferring, Fujifilm, Gilead, Intercept, MSD, Mylan, Norgine, Salvat, Takeda, and Vifor Pharma. AJH has served as a speaker, consultant, and advisory board member for, or has received research funding from Vifor Pharma. JPG has served as a speaker, consultant, and advisory board member for, or has received research funding from: AbbVie, Allergan, Biogen, Casen Fleet, Celgene, Chiesi, Diasorin, Falk Pharma, Faes Farma, Ferring, Gebro Pharma, Gilead, Janssen, Kern Pharma, Mayoly, MSD, Mylan, Otsuka Pharmaceutical, Pfizer, Phathom, Roche, Sandoz, Shire Pharmaceuticals, Takeda, Tillotts Pharma, and Vifor Pharma. XC has served as a speaker, consultant, and advisory board member for, or has received research funding from: AbbVie, Allergan, Ferring, Janssen, Kern Pharma, MSD, Pfizer, Sandoz, Takeda, and Vifor Pharma. SGL has served as a speaker, consultant, and advisory member for, or has received research funding from: AbbVie, Dr Falk Pharma, Faes Farma, Ferring, Janssen, MSD, Pfizer, Shire Pharmaceuticals, Takeda, Tillotts Pharma, and Vifor Pharma. CMdA has served as a speaker, consultant, and advisory board member for, or has received research funding from: Allergan, BioGaia, Casen Recordati, Norgine, Vifor Pharma, and Zambon. MP has served as a speaker for Vifor Pharma. CJ has served as a speaker, consultant, and advisory member for, or has received support for attending meetings and/or travel, or organized courses with scholarships or funding from: Amgen, Bial, Sanofi, Vifor Pharma, and Zambon. JAGE has previously managed grants, given talks, participated in consultancies, moderated tables at congresses and conferences, or organized courses with scholarships or funding for/from: Alexion, Amgen, Braun, Celgene, CSL Behring, Ferrer, GSK, Inmucor, Jansen, Novartis, Novo Nordisk, Octapharma, Roche, Sandoz, Sanofi, Terumo, UCB Pharm, Uriach, Vifor, Wellspect HealthCare, and Zambon.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. (2019) 42–43:101610. doi: 10.1016/j.bpg.2019.04.003
3. Mearin F, Lanas A, Bujanda L, Canelles P, Cotter J, Hervas A, et al. Open questions and misconceptions in the diagnosis and management of anemia in patients with gastrointestinal bleeding. Gastroenterol Hepatol. (2018) 41:63–76. doi: 10.1016/j.gastrohep.2017.08.012
4. Miilunpohja S, Jyrkka J, Karkkainen JM, Kastarinen H, Heikkinen M, Paajanen H, et al. Long-term mortality and causes of death in endoscopically verified upper gastrointestinal bleeding: Comparison of bleeding patients and population controls. Scand J Gastroenterol. (2017) 52:1211–8. doi: 10.1080/00365521.2017.1347811
5. Oakland K, Chadwick G, East JE, Guy R, Humphries A, Jairath V, et al. Diagnosis and management of acute lower gastrointestinal bleeding: Guidelines from the British Society of Gastroenterology. Gut. (2019) 68:776–89. doi: 10.1136/gutjnl-2018-317807
6. Lanas-Gimeno A, Lanas A. Risk of gastrointestinal bleeding during anticoagulant treatment. Expert Opin Drug Saf. (2017) 16:673–85. doi: 10.1080/14740338.2017.1325870
7. Oakland K, Guy R, Uberoi R, Hogg R, Mortensen N, Murphy MF, et al. Acute lower GI bleeding in the UK: Patient characteristics, interventions and outcomes in the first nationwide audit. Gut. (2018) 67:654–62. doi: 10.1136/gutjnl-2016-313428
8. Ng KS, Nassar N, Soares D, Stewart P, Gladman MA. Acute lower gastrointestinal haemorrhage: Outcomes and risk factors for intervention in 949 emergency cases. Int J Colorectal Dis. (2017) 32:1327–35. doi: 10.1007/s00384-017-2844-2
9. Hearnshaw S, Travis S, Murphy M. The role of blood transfusion in the management of upper and lower intestinal tract bleeding. Best Pract Res Clin Gastroenterol. (2008) 22:355–71. doi: 10.1016/j.bpg.2007.10.012
10. Baradarian R, Ramdhaney S, Chapalamadugu R, Skoczylas L, Wang K, Rivilis S, et al. Early intensive resuscitation of patients with upper gastrointestinal bleeding decreases mortality. Am J Gastroenterol. (2004) 99:619–22. doi: 10.1111/j.1572-0241.2004.04073.x
11. Bosch MA, Contreras E, Madoz P, Ortiz P, Pereira A, Pujol MM. The epidemiology of blood component transfusion in Catalonia, Northeastern Spain. Transfusion. (2011) 51:105–16. doi: 10.1111/j.1537-2995.2010.02785.x
12. Tinegate H, Chattree S, Iqbal A, Plews D, Whitehead J, Wallis JP. Ten-year pattern of red blood cell use in the North of England. Transfusion. (2013) 53:483–9. doi: 10.1111/j.1537-2995.2012.03782.x
13. Wallis JP, Wells AW, Chapman CE. Changing indications for red cell transfusion from 2000 to 2004 in the North of England. Transfus Med. (2006) 16:411–7. doi: 10.1111/j.1365-3148.2006.00702.x
14. Ferraris VA, Davenport DL, Saha SP, Bernard A, Austin PC, Zwischenberger JB. Intraoperative transfusion of small amounts of blood heralds worse postoperative outcome in patients having noncardiac thoracic operations. Ann Thorac Surg. (2011) 91:1674–80. doi: 10.1016/j.athoracsur.2011.01.025
15. Goel R, Patel EU, Cushing MM, Frank SM, Ness PM, Takemoto CM, et al. Association of perioperative red blood cell transfusions with venous thromboembolism in a North American registry. JAMA Surg. (2018) 153:826–33. doi: 10.1001/jamasurg.2018.1565
16. Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, et al. Health care-associated infection after red blood cell transfusion: A systematic review and meta-analysis. JAMA. (2014) 311:1317–26. doi: 10.1001/jama.2014.2726
17. Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: A meta-analysis and systematic review. Am J Med. (2014) 127:124–31.e3. doi: 10.1016/j.amjmed.2013.09.017
18. Yang TO, Cairns BJ, Reeves GK, Green J, Beral V. Cancer risk among 21st century blood transfusion recipients. Ann Oncol. (2017) 28:393–9. doi: 10.1093/annonc/mdw555
19. Kherad O, Restellini S, Martel M, Sey M, Murphy MF, Oakland K, et al. Outcomes following restrictive or liberal red blood cell transfusion in patients with lower gastrointestinal bleeding. Aliment Pharmacol Ther. (2019) 49:919–25. doi: 10.1111/apt.15158
20. Kola G, Sureshkumar S, Mohsina S, Sreenath GS, Kate V. Restrictive versus liberal transfusion strategy in upper gastrointestinal bleeding: A randomized controlled trial. Saudi J Gastroenterol. (2021) 27:13–9. doi: 10.4103/sjg.SJG_152_20
21. Odutayo A, Desborough MJ, Trivella M, Stanley AJ, Dorée C, Collins GS, et al. Restrictive versus liberal blood transfusion for gastrointestinal bleeding: A systematic review and meta-analysis of randomised controlled trials. Lancet Gastroenterol Hepatol. (2017) 2:354–60. doi: 10.1016/s2468-1253(17)30054-7
22. Wang J, Bao YX, Bai M, Zhang YG, Xu WD, Qi XS. Restrictive vs liberal transfusion for upper gastrointestinal bleeding: A meta-analysis of randomized controlled trials. World J Gastroenterol. (2013) 19:6919–27. doi: 10.3748/wjg.v19.i40.6919
23. García-Erce JA, Gomollón F, Muñoz M. Blood transfusion for the treatment of acute anaemia in inflammatory bowel disease and other digestive diseases. World J Gastroenterol. (2009) 15:4686–94. doi: 10.3748/wjg.15.4686
24. Ripollés-Melchor J, Jericó-Alba C, Quintana-Díaz M, García-Erce JA. From blood saving programs to patient blood management and beyond. Med Clin (Barc). (2018) 151:368–73. doi: 10.1016/j.medcli.2018.02.027
25. Ducrocq G, Gonzalez-Juanatey JR, Puymirat E, Lemesle G, Cachanado M, Durand-Zaleski I, et al. Effect of a restrictive vs liberal blood transfusion strategy on major cardiovascular events among patients with acute myocardial infarction and anemia: The REALITY randomized clinical trial. JAMA. (2021) 325:552–60. doi: 10.1001/jama.2021.0135
26. Jairath V, Hearnshaw S, Brunskill SJ, Doree C, Hopewell S, Hyde C, et al. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database Syst Rev. (2010) 9:CD006613. doi: 10.1002/14651858.CD006613.pub3
27. Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Update 2021. Endoscopy. (2021) 53:300–32. doi: 10.1055/a-1369-5274
28. Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, James MW, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): A pragmatic, open-label, cluster randomised feasibility trial. Lancet. (2015) 386:137–44. doi: 10.1016/s0140-6736(14)61999-1
29. Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. (2013) 368:11–21. doi: 10.1056/NEJMoa1211801
30. Goodnough LT, Murphy MF. Do liberal blood transfusions cause more harm than good? BMJ. (2014) 349:g6897. doi: 10.1136/bmj.g6897
31. Goodnough LT, Panigrahi AK. Blood transfusion therapy. Med Clin North Am. (2017) 101:431–47. doi: 10.1016/j.mcna.2016.09.012
32. Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: A retrospective observational study in four major adult tertiary-care hospitals. Transfusion. (2017) 57:1347–58. doi: 10.1111/trf.14006
33. Ballester-Clau R, Torres Vicente G, Voltà-Pardo T, López-Barroso L, Cucala-Ramos M, Reñé-Espinet JM, et al. Clinical experience with ferric carboxymaltose in the management of anemia in acute gastrointestinal bleeding. Eur J Gastroenterol Hepatol. (2019) 31:116–22. doi: 10.1097/meg.0000000000001282
34. Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, Dyer C, et al. Restrictive vs liberal blood transfusion for acute upper gastrointestinal bleeding: Rationale and protocol for a cluster randomized feasibility trial. Transfus Med Rev. (2013) 27:146–53. doi: 10.1016/j.tmrv.2013.04.001
35. Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. (2016) 10:CD002042. doi: 10.1002/14651858.CD002042.pub4
36. Murphy MF, Palmer A. Patient blood management as the standard of care. Hematology Am Soc Hematol Educ Program. (2019) 2019:583–9. doi: 10.1182/hematology.2019000063
37. European Commission [EC]. Infographic on organs, blood, tissues and cells in the EU. (2021). Available online at: https://ec.europa.eu/health/publications/infographic-organs-blood-tissues-and-cells-eu_en (accessed February 1 2022).
38. International Foundation for Patient Blood Management [IFPBM]. Manifesto for European action on patient blood management (PBM). (2020). Available online at: https://www.ifpbm.org/images/EU%20PBM%20Manifesto%20February%202020%2024.pdf (accessed January 25, 2022).
39. European Commission [EC]. Commission staff working document: Evaluation of the Union legislation on blood, tissues and cells. (2019). Available online at: https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/swd_2019_376_en.pdf (accessed January 25, 2022).
40. European Commission [EC]. Building national programmes of patient blood management (PBM) in the EU – A guide for health authorities. (2017). Available oline at: https://op.europa.eu/en/publication-detail/-/publication/5ec54745-1a8c-11e7-808e-01aa75ed71a1/language-en (accessed January 25, 2022).
41. Joint United Kingdom (UK) Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee. Patient blood management – An evidence-based approach to patient care. (2014). Available online at: https://www.transfusionguidelines.org/document-library/documents/national-pbm-recommendationsfinaljune2014-pdf/ (accessed January 25, 2022).
42. Quintana-Díaz M, Andrés-Esteban EM, Sánchez-Serrano J, Martínez-Virto A, Juárez-Vela R, García-Erce JA. Transfusions in the emergency department: More than a blood transfusion. Rev Clin Esp. (2020) 220:393–9. doi: 10.1016/j.rce.2019.10.002
43. Simancas-Racines D, Montero-Oleas N, Vernooij RWM, Arevalo-Rodriguez I, Fuentes P, Gich I, et al. Quality of clinical practice guidelines about red blood cell transfusion. J Evid Based Med. (2019) 12:113–24. doi: 10.1111/jebm.12330
44. Gombotz H. Patient blood management: A patient-orientated approach to blood replacement with the goal of reducing anemia, blood loss and the need for blood transfusion in elective surgery. Transfus Med Hemother. (2012) 39:67–72. doi: 10.1159/000337183
45. Jericó C, Osorio J, García-Erce JA, Pera M. Patient blood management strategies for iron deficiency anemia management in gastric cancer. Eur J Gastroenterol Hepatol. (2019) 31:547–8. doi: 10.1097/meg.0000000000001383
46. Althoff FC, Neb H, Herrmann E, Trentino KM, Vernich L, Fullenbach C, et al. Multimodal patient blood management program based on a three-pillar strategy: A systematic review and meta-analysis. Ann Surg. (2019) 269:794–804. doi: 10.1097/SLA.0000000000003095
47. Klein HG, Hrouda JC, Epstein JS. Crisis in the sustainability of the U.S. blood system. N Engl J Med. (2018) 378:305–6. doi: 10.1056/NEJMc1714807
48. Fredrick J, Berger JJ, Menitove JE. Strategic issues currently facing the US blood system. Transfusion. (2020) 60:1093–6. doi: 10.1111/trf.15769
49. Roman MA, Abbasciano RG, Pathak S, Oo S, Yusoff S, Wozniak M, et al. Patient blood management interventions do not lead to important clinical benefits or cost-effectiveness for major surgery: A network meta-analysis. Br J Anaesth. (2021) 126:149–56. doi: 10.1016/j.bja.2020.04.087
50. Warner MA, Schulte PJ, Hanson AC, Madde NR, Burt JM, Higgins AA, et al. Implementation of a comprehensive patient blood management program for hospitalized patients at a large United States medical center. Mayo Clin Proc. (2021) 96:2980–90. doi: 10.1016/j.mayocp.2021.07.017
51. Hwang JH, Fisher DA, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, et al. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. (2012) 75:1132–8. doi: 10.1016/j.gie.2012.02.033
52. Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. (2015) 47:a1–46. doi: 10.1055/s-0034-1393172
53. Barkun AN, Almadi M, Kuipers EJ, Laine L, Sung J, Tse F, et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the International Consensus Group. Ann Intern Med. (2019) 171:805–22. doi: 10.7326/m19-1795
54. Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG Clinical Guideline: Upper gastrointestinal and ulcer bleeding. Am J Gastroenterol. (2021) 116:899–917. doi: 10.14309/ajg.0000000000001245
55. Snook J, Bhala N, Beales ILP, Cannings D, Kightley C, Logan RP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. (2021) 70:2030–51. doi: 10.1136/gutjnl-2021-325210
56. NHS Blood and Transplant. National comparative audit of lower gastrointestinal bleeding and the use of blood. Resulst from a National audit. (2016). Available online at: https://hospital.blood.co.uk/audits/national-comparative-audit/surgical-audits/lower-gastrointestinal-bleeding-audit/ (accessed February 9, 2022).
57. Oakland K, Jairath V, Murphy MF. Advances in transfusion medicine: Gastrointestinal bleeding. Transfus Med. (2018) 28:132–9. doi: 10.1111/tme.12446
58. Donovan K, Stanworth S, Jairath V. The optimal use of blood components in the management of gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. (2019) 4:101600. doi: 10.1016/j.bpg.2019.02.002
59. Joosten A, Alexander B, Delaporte A, Lilot M, Rinehart J, Cannesson M. Perioperative goal directed therapy using automated closed-loop fluid management: The future? Anaesthesiol Intensive Ther. (2015) 47:517–23. doi: 10.5603/AIT.a2015.0069
60. Kendrick JB, Kaye AD, Tong Y, Belani K, Urman RD, Hoffman C, et al. Goal-directed fluid therapy in the perioperative setting. J Anaesthesiol Clin Pharmacol. (2019) 35:S29–34. doi: 10.4103/joacp.JOACP_26_18
61. Tomescu DR, Scarlatescu E, Bubenek-Turconi SI. Can goal-directed fluid therapy decrease the use of blood and hemoderivates in surgical patients? Minerva Anestesiol. (2020) 86:1346–52. doi: 10.23736/s0375-9393.20.14154-3
62. García-Erce JA, Quintana Díaz M, Muñoz Gómez M. Transfusion and anticoagulation in the management of nonvariceal upper gastrointestinal bleeding in Spain [article in Spanish]. Gastroenterol Hepatol. (2013) 36:115–6. doi: 10.1016/j.gastrohep.2012.09.004
63. Lanas A, Calvet X, Feu F, Ponce J, Gisbert JP, Barkun A, et al. First Spanish consensus on peptic ulcer bleeding management. Consenso sobre Hemorragia Digestiva por Ulcera Peptica [article in Spanish]. Med Clin (Barc). (2010) 135:608–16. doi: 10.1016/j.medcli.2010.07.002
64. Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA. (2016) 316:2025–35. doi: 10.1001/jama.2016.9185
65. Jairath V, Desborough MJ. Modern-day management of upper gastrointestinal haemorrhage. Transfus Med. (2015) 25:351–7. doi: 10.1111/tme.12266
66. National Institute for Health and Care Excellence [NICE]. Blood transfusion [NICE guideline NG24]. (2015). Available online at: https://www.nice.org.uk/guidance/ng24 (accessed January 25, 2022).
67. Leal-Noval SR, Muñoz M, Asuero M, Contreras E, García-Erce JA, Llau JV, et al. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: The 2013 update of the “Seville Document”. Blood Transfus. (2013) 11:585–610. doi: 10.2450/2013.0029-13
68. Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: Patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. (2011) 60:1327–35. doi: 10.1136/gut.2010.228437
69. Oakland K, Guy R, Uberoi R, Seeney F, Collins G, Grant-Casey J, et al. Study protocol: First nationwide comparative audit of acute lower gastrointestinal bleeding in the UK. BMJ Open. (2016) 6:e011752. doi: 10.1136/bmjopen-2016-011752
70. Abraham I, Sun D. The cost of blood transfusion in Western Europe as estimated from six studies. Transfusion. (2012) 52:1983–8. doi: 10.1111/j.1537-2995.2011.03532.x
71. Lanas A, Perez-Aisa MA, Feu F, Ponce J, Saperas E, Santolaria S, et al. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. Am J Gastroenterol. (2005) 100:1685–93. doi: 10.1111/j.1572-0241.2005.41833.x
72. Muñoz Gómez M, Bisbe Vives E, Basora Macaya M, García Erce JA, Gómez Luque A, Leal-Noval SR, et al. Forum for debate: Safety of allogeneic blood transfusion alternatives in the surgical/critically ill patient. Med Intensiva. (2015) 39:552–62. doi: 10.1016/j.medin.2015.05.006
73. Jericó C, Beverina I, Quintana-Diaz M, Salvadori U, Melli C, Rondinelli MB, et al. Efficacy and safety of high-dose intravenous iron as the first-choice therapy in outpatients with severe iron deficiency anemia. Transfusion. (2020) 60:1443–9. doi: 10.1111/trf.15870
74. Gani F, Cerullo M, Ejaz A, Gupta PB, Demario VM, Johnston FM, et al. Implementation of a blood management program at a tertiary care hospital: Effect on transfusion practices and clinical outcomes among patients undergoing surgery. Ann Surg. (2019) 269:1073–9. doi: 10.1097/SLA.0000000000002585
75. Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. (2011) 365:2453–62. doi: 10.1056/NEJMoa1012452
76. Cooper HA, Rao SV, Greenberg MD, Rumsey MP, McKenzie M, Alcorn KW, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol. (2011) 108:1108–11. doi: 10.1016/j.amjcard.2011.06.014
77. Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. (2013) 165:964–71.e1. doi: 10.1016/j.ahj.2013.03.001
78. Kansagara D, Dyer E, Englander H, Fu R, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: A systematic review. Ann Intern Med. (2013) 159:746–57. doi: 10.7326/0003-4819-159-11-201312030-00007
79. Sherwood MW, Wang Y, Curtis JP, Peterson ED, Rao SV. Patterns and outcomes of red blood cell transfusion in patients undergoing percutaneous coronary intervention. JAMA. (2014) 311:836–43. doi: 10.1001/jama.2014.980
80. Docherty AB, O’Donnell R, Brunskill S, Trivella M, Doree C, Holst L, et al. Effect of restrictive versus liberal transfusion strategies on outcomes in patients with cardiovascular disease in a non-cardiac surgery setting: Systematic review and meta-analysis. BMJ. (2016) 352:i1351. doi: 10.1136/bmj.i1351
81. Carson JL, Stanworth SJ, Alexander JH, Roubinian N, Fergusson DA, Triulzi DJ, et al. Clinical trials evaluating red blood cell transfusion thresholds: An updated systematic review and with additional focus on patients with cardiovascular disease. Am Heart J. (2018) 200:96–101. doi: 10.1016/j.ahj.2018.04.007
82. Ortiz P, Mingo A, Lozano M, Vesga MA, Grifols JR, Castrillo A, et al. Guide for transfusion of blood components [article in Spanish]. Med Clin (Barc). (2005) 125:389–96. doi: 10.1157/13079172
83. Ghosh K. Non haematological effects of iron deficiency – a perspective. Indian J Med Sci. (2006) 60:30–7. doi: 10.4103/0019-5359.19676
84. Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. (2011) 434:365–81. doi: 10.1042/bj20101825
85. Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. (2010) 30:105–22. doi: 10.1146/annurev.nutr.012809.104804
86. Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. (2021) 397:233–48. doi: 10.1016/S0140-6736(20)32594-0
87. World Health Organization [WHO]. Iron deficiency anaemia – Assessment, prevention, and control. A guide for programme managers. (2001). Available online at: https://apps.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/index.html (accessed January 25, 2022).
89. Muñoz M, Gómez-Ramírez S, Besser M, Pavía J, Gomollón F, Liumbruno GM, et al. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus. (2017) 15:422–37. doi: 10.2450/2017.0113-17
90. Planella de Rubinat M, Teixido Amoros M, Ballester Clau R, Trujillano Cabello J, Ibarz Escuer M, Rene Espinet JM. Incidence and predictive factors of iron deficiency anemia after acute non-variceal upper gastrointestinal bleeding without portal hypertension [article in Spanish]. Gastroenterol Hepatol. (2015) 38:525–33. doi: 10.1016/j.gastrohep.2015.02.012
91. Salvadori U, Sandri M, Melli C, Polese F, Simeoni M, Capelli S, et al. Ferric carboxymaltose reduces the number of red blood cell units transfused and allows transfusion independence to be obtained in patients with iron deficiency anemia secondary to gastrointestinal chronic blood loss. Transfusion. (2016) 56:2720–6. doi: 10.1111/trf.13794
92. Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part II: Iron deficiency and iron overload. J Clin Pathol. (2011) 64:287–96. doi: 10.1136/jcp.2010.086991
93. Muñoz M, Villar I, García-Erce JA. An update on iron physiology. World J Gastroenterol. (2009) 15:4617–26. doi: 10.3748/wjg.15.4617
94. García Erce JA, Altés A, López Rubio M, Remacha AF. Management of iron deficiency in various clinical conditions and the role of intravenous iron: Recommendations of the Spanish Erythropathology Group of the Spanish Society of Haematology and Haemotherapy. Rev Clin Esp. (2020) 220:31–42. doi: 10.1016/j.rce.2019.09.004
95. Gargallo-Puyuelo CJ, Alfambra E, García-Erce JA, Gomollon F. Iron treatment may be difficult in inflammatory diseases:Inflammatory bowel disease as a paradigm. Nutrients. (2018) 10:1959. doi: 10.3390/nu10121959
96. Ko CW, Siddique SM, Patel A, Harris A, Sultan S, Altayar O, et al. AGA clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology. (2020) 159:1085–94. doi: 10.1053/j.gastro.2020.06.046
97. Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. (2016) 91:31–8. doi: 10.1002/ajh.24201
98. Rimon E, Kagansky N, Kagansky M, Mechnick L, Mashiah T, Namir M, et al. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. (2005) 118:1142–7. doi: 10.1016/j.amjmed.2005.01.065
99. Schrier SL. So you know how to treat iron deficiency anemia. Blood. (2015) 126:1971. doi: 10.1182/blood-2015-09-666511
100. Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLoS One. (2015) 10:e0117383. doi: 10.1371/journal.pone.0117383
101. Koduru P, Abraham BP. The role of ferric carboxymaltose in the treatment of iron deficiency anemia in patients with gastrointestinal disease. Therap Adv Gastroenterol. (2016) 9:76–85. doi: 10.1177/1756283X15616577
102. Ferrer-Barcelo L, Sanchis Artero L, Sempere Garcia-Arguelles J, Canelles Gamir P, Gisbert JP, Ferrer-Arranz LM, et al. Randomised clinical trial: Intravenous vs oral iron for the treatment of anaemia after acute gastrointestinal bleeding. Aliment Pharmacol Ther. (2019) 50:258–68. doi: 10.1111/apt.15327
103. Cotter J, Baldaia C, Ferreira M, Macedo G, Pedroto I. Diagnosis and treatment of iron-deficiency anemia in gastrointestinal bleeding: A systematic review. World J Gastroenterol. (2020) 26:7242–57. doi: 10.3748/wjg.v26.i45.7242
104. Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. (2015) 9:211–22. doi: 10.1093/ecco-jcc/jju009
105. Auerbach M, Pappadakis JA, Bahrain H, Auerbach SA, Ballard H, Dahl NV. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. Am J Hematol. (2011) 86:860–2. doi: 10.1002/ajh.22153
106. Auerbach M, Strauss W, Auerbach S, Rineer S, Bahrain H. Safety and efficacy of total dose infusion of 1,020 mg of ferumoxytol administered over 15 min. Am J Hematol. (2013) 88:944–7. doi: 10.1002/ajh.23534
107. Reinisch W, Staun M, Tandon RK, Altorjay I, Thillainayagam AV, Gratzer C, et al. A randomized, open-label, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol. (2013) 108:1877–88. doi: 10.1038/ajg.2013.335
108. Bhandari S, Pereira DIA, Chappell HF, Drakesmith H. Intravenous irons: From basic science to clinical practice. Pharmaceuticals (Basel). (2018) 11:82. doi: 10.3390/ph11030082
109. Lichtenstein GR, Onken JE. Improved hemoglobin response with ferric carboxymaltose in patients with gastrointestinal-telated iron-deficiency anemia versus oral iron. Dig Dis Sci. (2018) 63:3009–19. doi: 10.1007/s10620-018-5204-3
110. Martin-Malo A, Borchard G, Flühmann B, Mori C, Silverberg D, Jankowska EA. Differences between intravenous iron products: Focus on treatment of iron deficiency in chronic heart failure patients. ESC Heart Fail. (2019) 6:241–53. doi: 10.1002/ehf2.12400
111. Vifor Pharma UK Limited. Ferinject (ferric carboxymaltose) [summary of product characteristics]. (2020). Available online at: https://www.medicines.org.uk/emc/product/5910/smpc#gref (accessed January 25, 2022).
112. Vifor Pharma UK Limited. Venofer (iron sucrose) 20 mg iron/ml, solution for injection or concentrate for solution for infusion [summary of product characteristics]. (2020). Available online at: https://www.medicines.org.uk/emc/product/5911 (accessed January 25, 2022).
113. Evstatiev R, Marteau P, Iqbal T, Khalif IL, Stein J, Bokemeyer B, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. (2011) 141:846–53.e1–2. doi: 10.1053/j.gastro.2011.06.005
114. Ganzoni AM. [Intravenous iron-dextran: Therapeutic and experimental possibilities]. Schweiz Med Wochenschr. (1970) 100:301–3.
115. Bager P, Dahlerup JF. Randomised clinical trial: Oral vs. intravenous iron after upper gastrointestinal haemorrhage – a placebo-controlled study. Aliment Pharmacol Ther. (2014) 39:176–87. doi: 10.1111/apt.12556
116. Goodnough LT, Hollenhorst MA. Clinical decision support and improved blood use in patient blood management. Hematology Am Soc Hematol Educ Program. (2019) 2019:577–82. doi: 10.1182/hematology.2019000062
117. Di Bartolomeo E, Merolle L, Marraccini C, Canovi L, Berni P, Guberti M, et al. Patient blood management: Transfusion appropriateness in the post-operative period. Blood Transfus. (2019) 17:459–64. doi: 10.2450/2019.0035-19
118. Beal EW, Bagante F, Paredes A, Akgul O, Merath K, Cua S, et al. Perioperative use of blood products is associated with risk of morbidity and mortality after surgery. Am J Surg. (2019) 218:62–70. doi: 10.1016/j.amjsurg.2018.11.015
119. Gomollón F, Gisbert JP. Current management of iron deficiency anemia in inflammatory bowel diseases: A practical guide. Drugs. (2013) 73:1761–70. doi: 10.1007/s40265-013-0131-2
Keywords: anemia, ferric carboxymaltose (FCM), gastrointestinal bleeding, iron supplementation, patient blood management, transfusion
Citation: Montoro M, Cucala M, Lanas Á, Villanueva C, Hervás AJ, Alcedo J, Gisbert JP, Aisa Áp, Bujanda L, Calvet X, Mearin F, Murcia Ó, Canelles P, García López S, Martín de Argila C, Planella M, Quintana M, Jericó C and García Erce JA (2022) Indications and hemoglobin thresholds for red blood cell transfusion and iron replacement in adults with gastrointestinal bleeding: An algorithm proposed by gastroenterologists and patient blood management experts. Front. Med. 9:903739. doi: 10.3389/fmed.2022.903739
Received: 24 March 2022; Accepted: 11 August 2022;
Published: 15 September 2022.
Edited by:
Alicia Mohr, University of Florida, United StatesReviewed by:
Bálint Mihály Eross, University of Pécs, HungarySinem Civriz Bozdağ, Ankara University, Turkey
Copyright © 2022 Montoro, Cucala, Lanas, Villanueva, Hervás, Alcedo, Gisbert, Aisa, Bujanda, Calvet, Mearin, Murcia, Canelles, García López, Martín de Argila, Planella, Quintana, Jericó and García Erce. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miguel Montoro, maimontoro@gmail.com
†These authors have contributed equally to this work
 Miguel Montoro
Miguel Montoro Mercedes Cucala
Mercedes Cucala Ángel Lanas
Ángel Lanas Cándido Villanueva7,8
Cándido Villanueva7,8  Javier P. Gisbert
Javier P. Gisbert Luis Bujanda
Luis Bujanda Xavier Calvet
Xavier Calvet Fermín Mearin
Fermín Mearin Santiago García López
Santiago García López Montserrat Planella
Montserrat Planella Manuel Quintana
Manuel Quintana Carlos Jericó
Carlos Jericó José Antonio García Erce
José Antonio García Erce