A novel approach for fibrous dysplasia assessment using combined planar and quantitative SPECT/CT analysis of Tc-99m-diphosphonate bone scan in correlation with biological bone turnover markers of disease activity
- 1Department of Nuclear Medicine and Molecular Imaging, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
- 2Interdisciplinary Centre for Bone Diseases, Service of Rheumatology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
- 3Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
Purpose: To investigate the emerging role of Tc-99m-labeled diphosphonate (Tc-99m-DPD) uptake quantification by SPECT/CT in fibrous dysplasia (FD) bone lesions and its correlation with biological bone turnover markers (BTMs) of disease activity.
Materials and methods: Seven patients (49 ± 16 years) with a confirmed diagnosis of FD were included in this retrospective study. Bone scans with Tc-99m-DPD and quantitative SPECT/CT (xSPECT/CT) were performed. SUVmax (maximum standard unit value) and SUVmean (mean standard unit value) were measured in all FD bone lesions. The skeletal burden score (SBS) was assessed on planar scintigraphy and multiplied by mean SUVmax and SUVmean to generate two new parameters, SBS_SUVmax and SBS_SUVmean, respectively. Planar and xSPECT/CT quantitative measures were correlated with biological BTMs of disease activity, including fibroblast growth factor 23 (FGF-23), alkaline phosphatase (ALP), procollagen 1 intact N-terminal propeptide (P1NP) and C-terminal telopeptide (CTX), as well as scoliosis angle measured on radiographs. Statistical significance was evaluated with Spearman’s correlations.
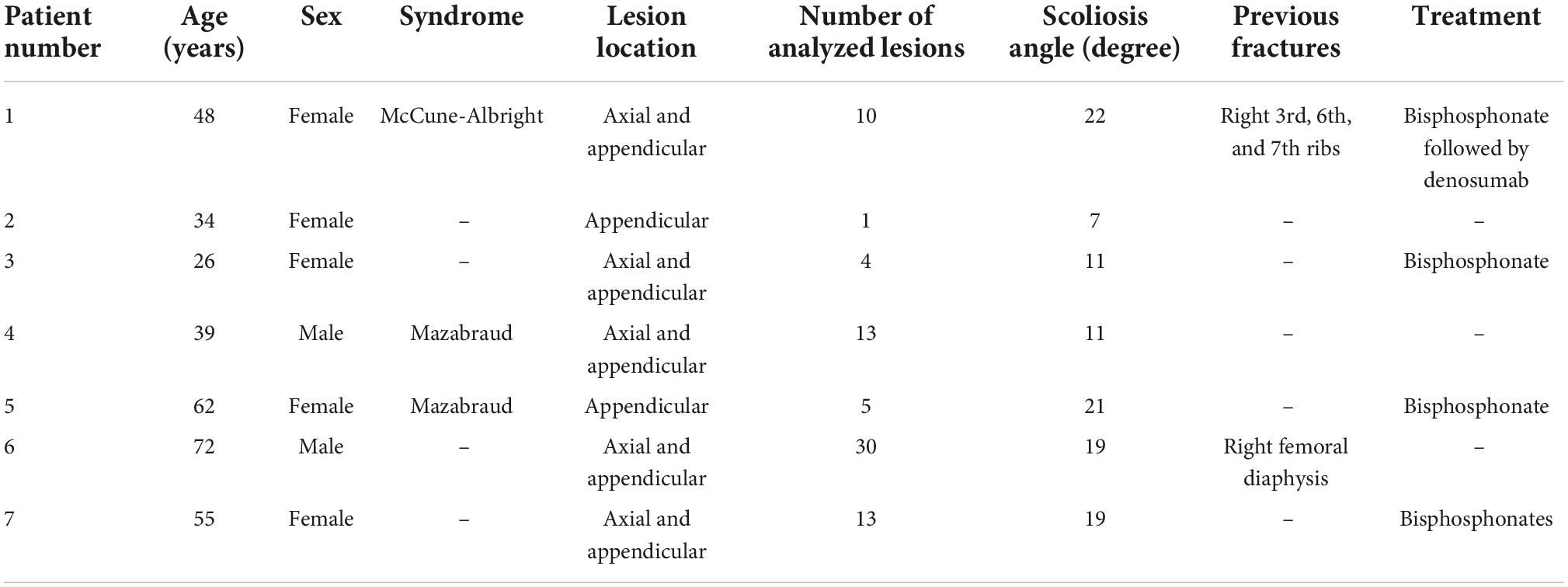
Results: A total of 76 FD bone lesions were analyzed, showing an average SUVmax and SUVmean (g/mL) of 13 ± 7.3 and 8 ± 4.5, respectively. SBS, SBS_SUVmax and SBS_SUVmean values were 30.8 ± 25.6, 358 ± 267 and 220.1 ± 164.5, respectively. Mean measured values of FGF-23 (pg/mL), ALP (U/L), P1NP (μg/L) and CTX (pg/mL) were 98.4 (22–175), 283.5 (46–735), 283.1 (31–1,161) and 494 (360–609), respectively. Mean scoliosis angle was 15.7 (7–22) degrees. We found a very strong positive correlation between planar-derived SBS and CTX (r = 0.96, p = 0.010), but no significant correlation between SUVmax or SUVmean and biological BTMs. SBS_SUVmax showed a strong to very strong positive correlation with CTX (ρ = 0.99, p = 0.002), FGF-23 (ρ = 0.91, p = 0.010), ALP (ρ = 0.82, p = 0.020), and P1NP (ρ = 0.78, p = 0.039), respectively.
Conclusion: This study showed that biological BTMs are significantly correlated with diphosphonate uptake on bone scan, quantified by a new parameter combining information from both planar and quantitative SPECT/CT. Further analysis of bone scan quantitative SPECT/CT data in a larger patient population might help better characterize the skeletal disease burden in FD, and guide treatment and follow-up.
Introduction
Fibrous dysplasia (FD) is a benign, non-hereditary congenital bone disorder caused by impaired osteogenesis secondary to an activating somatic mutation in the GNAS gene, leading to mutations in the alpha subunit of the Gs protein (1, 2). FD is characterized by intramedullary fibro-osseous proliferative lesions and may present in a monostotic (single bone) or polyostotic (multiple bones) form, or as a feature of two rare syndromes, namely the McCune-Albright syndrome or the Mazabraud syndrome (1, 3). Symptoms of FD include bone pain, fractures, bone deformities and neurological deficits (4). Bone turnover markers (BTMs), namely alkaline phosphatase (ALP), procollagen 1 intact N-terminal propeptide (P1NP) and C-terminal telopeptide (CTX), have been used as markers of disease activity, but serum levels may be influenced by age, comorbidities and treatments, including bisphosphonates and denosumab (2, 5, 6). FGF-23 is a phosphate-regulating hormone overproduced in FD lesions which levels correlate with the disease burden and can also be used as a biomarker of disease activity (7). The skeletal burden score (SBS) derived from planar 99Tc-methylene diphosphonate (99Tc-MDP) bone scan evaluation has been validated as a reliable instrument for measuring the global skeletal burden of FD, based on estimation of the percentage of affected skeleton area on whole-body images. However, SBS derived from 99Tc-MDP is limited as it is a semiquantitative method based on two-dimensional imaging (8). To overcome these limitations, Van der Bruggen et al. proposed to quantify the skeletal burden in FD using sodium fluoride PET/CT (9). Their study showed a strong correlation of Na18F-PET/CT FD burden measurements with biological BTMs and suggested a possible role of this technique in treatment follow-up. The major limitation of Na18F-PET/CT is its low availability and high cost, as opposed to bone scintigraphy. Recently, technological advances enabled to quantify 99mTc-DPD uptake in single-photon emission computed tomography (SPECT) coupled with computed tomography (CT) (xSPECT/CT, Symbia Intevo, Siemens Healthineers, Erlangen, Germany). The xSPECT showed an accurate activity recovery within 10% of the expected value for objects > 10 mL, which is similar to PET/CT (10).
The aim of our study was to investigate the correlation between Tc-99m-DPD xSPECT/CT uptake quantification of FD bone lesions and biological BTMs and scoliosis, as a complication of FD.
Materials and methods
Patient selection
Between 2016 and 2021, 17 patients with a confirmed diagnosis of FD were treated in our Interdisciplinary Centre for Bone Diseases at Lausanne University Hospital. Of these, 2 patients were excluded from this retrospective analysis because they did not have bone scintigraphy and 8 because they did not undergo quantitative SPECT/CT. Hence, our final study population consisted of 7 patients: 2 males and 5 females, with a median age of 59 years (range, 26–72 years), with confirmed diagnosis of FD based on histopathology or as part of a clinical syndrome (Mazabraud or McCune-Albright syndromes), available BTMs values and Tc-99m-DPD xSPECT/CT images. We retrospectively collected clinical, biological and radiological data from the patients’ hospital medical records. The local Ethics Research Committee of the State of Vaud approved the research protocol (CER-VD #2018-01513). All patients participating in this study had signed an institutional general consent for retrospective use of their data in clinical research.
Biological bone turnover markers measurements
Dosage of biological markers were performed on early morning fasting blood samples. Results of the most recent BTMs dosage in respect to bone imaging were used. ALP, P1NP and CTX were measured in the central clinical routine laboratory of the Lausanne University Hospital; intact FGF-23 was measured by ELISA (Kainos, Tokyo, Japan) at the Inselspital (Bern, Switzerland). For P1NP and CTX premenopausal women normal ranges are used as reference (P1NP: < 58.6 μg/L; CTX: < 573 ng/L). Normal values are 36–120 UI/L for ALP, and 10–50 pg/mL for FGF-23.
Tc-99m-labeled diphosphonate single-photon emission computed tomography/computed tomography acquisition and analysis
All patients underwent whole-body planar imaging with low-energy high-resolution collimators, and a scanning speed of 12 cm/min, followed by quantitative SPECT/CT (Symbia Intevo, Siemens Healthineers, Erlangen, Germany) on regions with high uptake on planar scintigraphy. The xSPECT was acquired in average at 3 h and 22 min ± 31 min after intravenous injection of 10 MBq/kg of 99mTc-DPD with a mean patient dose of 798 ± 58 MBq. Images were acquired with 3 degrees rotation/step and 12 s/projection with a 256 × 256 matrix. Reduced-dose CT was acquired using 120 kV and 25 reference mAs modulation (Siemens Care Dose, Symbia Intevo, Erlangen, Germany). Images were reconstructed to generate xSPECT data allowing SUVbw quantification on post-processed images and measurement of SUVmax and SUVmean (g/mL) using xSPECT reconstruction algorithm.
For each patient, the SUVmax and SUVmean of all FD bone lesions visible on xSPECT and CT were measured with a 42% thresholding and classified according to their location in the axial or appendicular skeleton (Figure 1). All FD bone lesions were visually assessed based on pathological high uptake, excluding uptake due to degenerative changes. The SBS was assessed on planar scintigraphy for all patients in consensus by two nuclear medicine specialists (MJ and MNL) as described by Collins et al., and SBS_SUVmax and SBS_SUVmean were generated by multiplying SBS by mean SUVmax and SUVmean of all lesions for each patient, respectively (8).
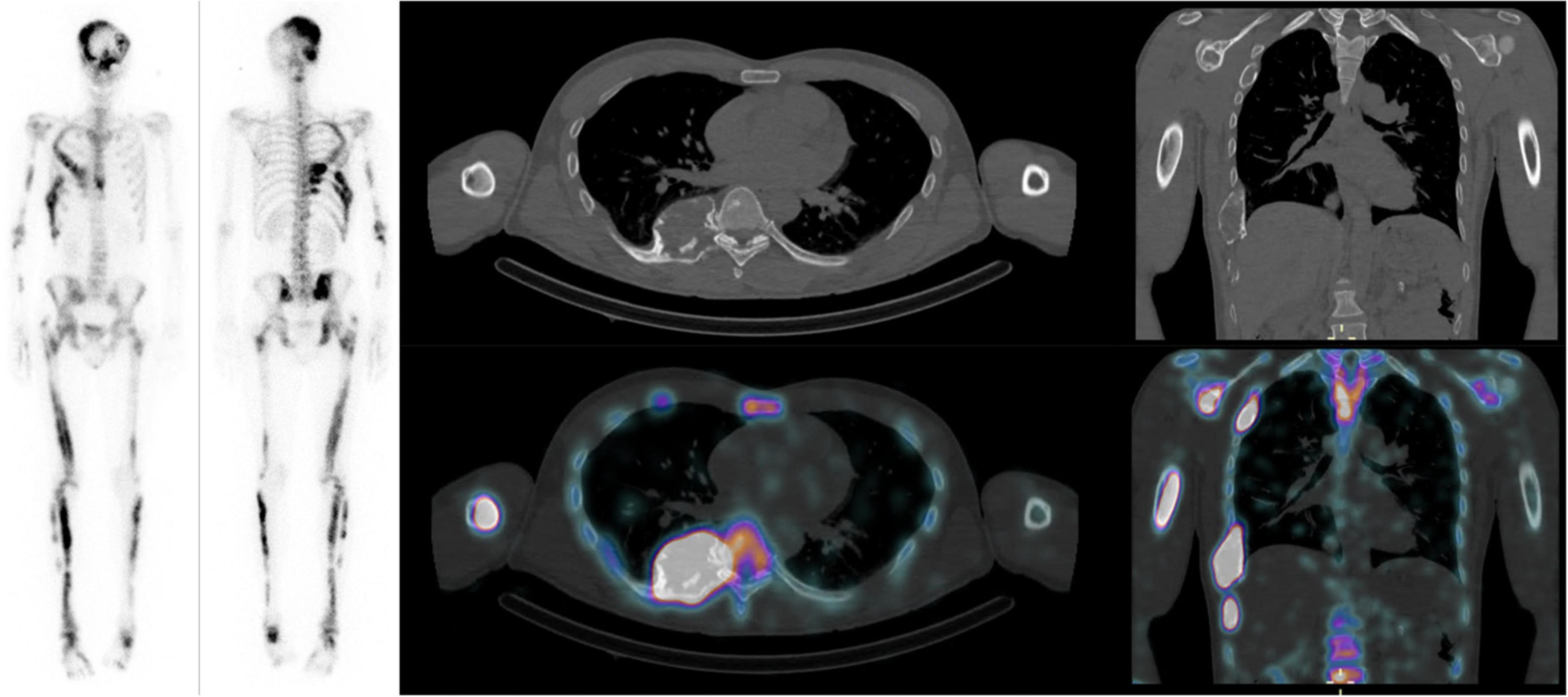
Figure 1. Forty-year-old male patient with Mazabraud syndrome: Mean SUVmax and SUVmean of 11 and 6.7 g/mL, respectively. SBS: 46.3, FGF-23: 175 (10–50 pg/mL), CTX: 531 pg/mL, P1NP: 383 (15–78 μg/L), ALP: 277 (36–108) U/L, scoliosis angle: 11 degrees.
Scoliosis Cobb angles were measured using the Carestream Vue PACS’s orthopedics workflow tool by a musculoskeletal radiologist.
Statistical analysis
Continuous variables are reported as mean ± standard deviation (SD) and range.
Categorical data were analyzed using Fisher’s exact test or chi-squared test, as appropriate. For comparison of two groups, the Student’s t-test was used when assumptions for parametric tests were met; otherwise, the Mann-Whitney U-test was used.
Planar SBS and all xSPECT/CT quantitative measures were correlated with biological BTMs of disease activity, including FGF-23, ALP, P1NP and CTX, as well as with scoliosis angle using Spearman’s correlation. Statistical analysis was performed using STATA (version 14.0; STATA Corp., College Station, Texas, USA). P-values less than 0.05 were considered as statistically significant.
Results
Study population
Seven patients with confirmed FD, quantitative SPECT/CT and BTMs dosage were retrospectively identified and included in this study. They had been referred for evaluation of FD confirmed on histopathological analysis (n = 6) and/or within the spectrum of a known syndrome (n = 3, 1 McCune-Albright syndrome and 2 Mazabraud syndromes). One patient with McCune-Albright syndrome did not have histopathological confirmation (Table 1). FD lesions were assessed on Tc-99m-DPD xSPECT/CT in all 7 patients. All seven included patients had a polyostotic form of FD. Four out of seven (57%) patients had previously been treated with bisphosphonates. A total number of 76 lesions for all patients were measured on xSPECT/CT with a mean number of lesions per patient of 10.9 ± 9.6. No difference was observed in terms of distribution of the 76 lesions between the axial (n = 50) and appendicular (n = 26) skeleton (p = 0.273).
Biological bone turnover markers
Mean measured values of biological BTMs were: FGF-23, 98.4 ± 56.3 pg/mL (22–175); ALP, 283.5 ± 243.6 U/L (46–735); P1NP, 283.1 ± 406.7μg/L (31–1,161); and CTX, 494 ± 90.5 pg/mL (360–609). Scoliosis was reported in all patients with a mean angle of 15.7 ± 5.9 (7–22) degrees.
Quantitative Tc-99m-labeled diphosphonate single-photon emission computed tomography/computed tomography
A total number of 76 FD bone lesions were analyzed, showing a mean SUVmax and SUVmean of 13 ± 7.3 and 8 ± 4.5 g/mL, respectively. Mean SBS score was 30.8 ± 25.6. Mean SBS_SUVmax and SBS_SUVmean were 358 ± 267 and 220 ± 165 g/mL, respectively. We found significantly higher values of SUVmax and SUVmean in axial skeleton lesions (14.5 ± 1.1 and 8.9 ± 0.6 g/mL, respectively) compared to appendicular skeleton lesions (10 ± 1.2 and 6.2 ± 0.8 g/mL, respectively) (p = 0.010 and p = 0.013, respectively).
Correlations between bone turnover markers and imaging quantification
Spearman correlations results are shown in Table 2. We found a very strong positive correlation between planar-derived SBS and CTX (ρ = 0.96, p = 0.01), a statistical trend for a positive correlation with FGF-23 (ρ = 0.81, p = 0.053) and ALP (ρ = 0.79, p = 0.060) but no correlation with P1NP (ρ = 0.69, p = 0.089). No significant correlation was found between SUVmax or SUVmean and biological BTMs. Combining both SBS and SUV increased the strength of the correlations: SBS_SUVmax and SBS_SUVmean showed a strong to very strong positive correlation with CTX (ρ = 0.99, p = 0.002 and ρ = 0.99, p = 0.001), FGF-23 (ρ = 0.91, p = 0.010 and ρ = 0.91, p = 0.013), ALP (ρ = 0.82, p = 0.020 and ρ = 0.88, p = 0.019), and P1NP (ρ = 0.78, p = 0.039 and ρ = 0.78, p = 0.028), as shown in Figure 2. There was no correlation between bone scan quantitative measures and scoliosis angle. No significant correlation was found between SUVmax or SUVmean of axial skeleton FD lesions with scoliosis angle (ρ = 0.33, p = 0.472 and ρ = 0.36, p = 0.432, respectively).
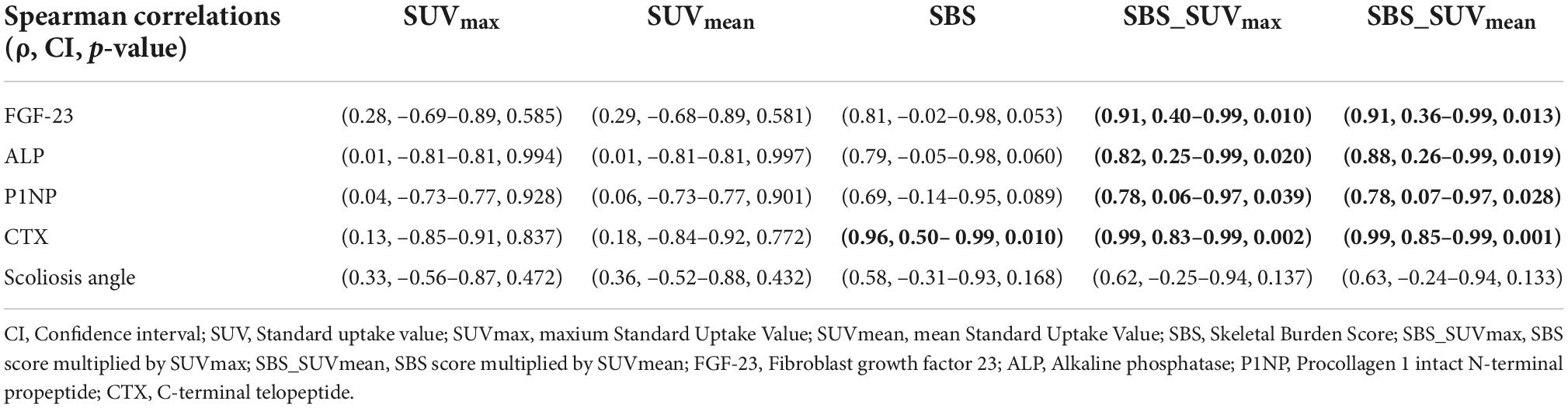
Table 2. Correlations between bone scintigraphy quantitative measures and biological bone turnover markers of disease activity and scoliosis angle.
Discussion
In this study, we propose a novel approach to quantify the FD disease burden on Tc-99m-DPD bone scan using a combination of planar and absolute SPECT/CT quantification by multiplying the well-known SBS score by the average SUVmax and SUVmean values of the patient’s FD lesions, leading to a disease-extension-corrected SUV value, which we termed SBS_SUVmax and SBS_SUVmean, respectively. We showed that SBS_SUVmax and SBS_SUVmean improved the correlation of bone imaging with disease activity as compared to SBS and SUV values alone: correlations between the results of this combined quantitative bone scan approach and biological activity as assessed by BTMs were strong to very strong and highly statistically significant.
To differentiate monostotic from polyostotic forms of FD and to evaluate the disease extent, patients with FD routinely undergo a Tc-99m-diphosphonate bone scan as recommended by current guidelines (11). Collins et al. have developed and validated the SBS, which is derived from a weighted score based on the estimation of the amount of FD lesions in anatomical segments on bone scintigraphy (8). The SBS correlated positively with BTMs, such as ALP, bone-specific ALP, osteocalcin, pyridinium cross-links, deoxypyridinoline cross-links, and N-telopeptide (8). However, this score has its limitations as it remains semiquantitative and is unable to quantify bone turnover activity within individual FD lesions, which may be of clinical relevance. 18F-NaF-PET/CT overcomes this limitation by allowing single lesion bone turnover quantification (12). Van der Bruggen et al. demonstrated that 18F-NaF-PET/CT SUV thresholds could discriminate healthy bone from FD lesions (9). In addition, their study showed a strong relationship between serum markers of bone formation and measurements of FD burden by 18F-NaF-PET/CT. Whereas SBS is not influenced by medical treatment, total lesion fluorination was higher in patients at baseline than in patients treated with biphosphonates, implying a potential role for Na18F-PET/CT in treatment response assessment (9). Papadakis et al. also investigated the role of 18F-NaF-PET/CT in FD skeletal burden assessment, and showed that BTMs, including ALP, N-telopeptides, and osteocalcin, were strongly correlated with total volume of all 18F-NaF-positive FD lesions. These authors also found a very strong correlation between SBS derived from 18F-NaF PET/CT and 99mTc-MDP scintigraphy (13). The drawback of 18F-NaF PET/CT is that it is not reimbursed in many countries and/or not readily available, constituting an obstacle to its use in routine clinical practice.
Recently, absolute quantification of bone scintigraphy has become available and has been the subject of several studies. For example, Yamane et al. showed in a pediatric population that SUV was higher at the epiphyseal plates of children under 15 years of age compared to older subjects, consistent with higher osteoblastic activity (14). Another study in an adult population showed a significant difference in quantitative 99mTc-DPD uptake on bone xSPECT/CT between prostate cancer bone metastases and spinal and pelvic osteoarthritic changes, with higher SUVmax and SUVmean in metastases (15). Therefore, the quantification of radiotracer uptake on bone scan might help differentiating between benign and malignant pathologies or other rheumatological disorders with high bone turnover, which needs to be confirmed by prospective studies.
To the best of our knowledge, our study is the first to report the absolute quantification of 99mTc-DPD uptake on xSPECT/CT in FD. We introduced new quantitative parameters, SBS_SUVmax and SBS_SUVmean, to evaluate the disease burden. The main motivation for this quantitative model was to optimize the information extracted from bone scintigraphy combining both the overall disease activity assessed by SBS on a whole-body basis and the degree of tracer uptake assessed by mean SUV measurements of lesions in the xSPECT/CT field of view, as xSPECT/CT was not acquired on the whole body.
In our study, SBS alone correlated only with CTX, with a statistical trend toward a positive association with FGF-23 and ALP, while SUVmax and SUVmean did not correlate with any BTMs. In contrast, SBS_SUVmax and SBS_SUVmean correlated with all four BTMs. Multiplying mean SUVmax and SUVmean of all lesions on xSPECT/CT by SBS could allow a more accurate characterization of bone turnover and disease activity in FD. The advantage of this quantification method is that bone scan is widely available and SPECT/CT quantification methods are increasingly implemented with new gamma cameras.
Finally, 18F-FDG PET/CT has also been studied in FD and has shown a significant role in detecting malignant transformation of FD and optimizing patient management strategies in a complementary manner to 99mTc-MDP SPECT/CT (16). Therefore, quantification of bone scintigraphy may increase the accuracy of SPECT/CT in predicting disease activity, allowing this modality to remain competitive in the era of novel multimodal imaging, with particular potential as a biomarker for assessing disease activity and for monitoring treatment response.
This is a preliminary retrospective study, and thus its main limitation is the small number of patients included. However, the relatively large number of FD lesions analyzed increased the accuracy of the bone scan scores calculated for each patient. Further analyses of larger patient populations are needed to confirm the observed correlation between bone scintigraphy quantification and biological BTMs. In this study, SUV thresholding was used with a standard level of 42%, limiting reproducibility issues. However, an optimized approach to lesion delineation should be evaluated in larger future studies and compared with a standard fixed-level thresholding approach. In our study, the values of the scintigraphic quantitative measures did not correlate with the scoliosis angle. Interpretation of this observation remains limited as the range of scoliosis angles measured in our population was low.
Conclusion
This preliminary study showed a significant correlation between biological BTMs of disease activity and diphosphonate uptake on bone scan, quantified by a new parameter combining information from both planar and quantitative SPECT/CT. This approach could become the routine technique for clinical assessment of skeletal burden in FD due to its widespread availability. Further analysis of bone scan quantitative SPECT/CT data might help better characterize the skeletal disease burden in FD, and its role in guiding treatment and follow-up.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Research Committee of the State of Vaud. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
MJ and MN designed the work, performed and interpreted the data analysis, and drafted and revised the work. NH participated to data collection. FB performed the radiologic measurements and revised the work. BA-R, EG, NS, and JP critically revised the work. All authors contributed to the article and approved the submitted version.
Funding
Open access funding was provided by the University of Lausanne.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Boyce AM, Collins MT. Fibrous dysplasia/mccune-albright syndrome: a rare, mosaic disease of gαs activation. Endocr Rev. (2019) 41:345–70. doi: 10.1210/endrev/bnz011
3. Munksgaard PS, Salkus G, Iyer VV, Fisker RV. Mazabraud’s syndrome: case report and literature review. Acta Radiol Short Rep. (2013) 2:2047981613492532. doi: 10.1177/2047981613492532
4. DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. (2005) 87:1848–64. doi: 10.2106/JBJS.D.02942
5. Majoor BC, Appelman-Dijkstra NM, Fiocco M, van de Sande MA, Dijkstra PS, Hamdy NA. Outcome of long-term bisphosphonate therapy in mccune-albright syndrome and polyostotic fibrous dysplasia. J Bone Miner Res. (2017) 32:264–76. doi: 10.1002/jbmr.2999
6. Florenzano P, Pan KS, Brown SM, Paul SM, Kushner H, Guthrie LC, et al. Age-related changes and effects of bisphosphonates on bone turnover and disease progression in fibrous dysplasia of bone. J Bone Miner Res. (2019) 34:653–60. doi: 10.1002/jbmr.3649
7. Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. (2003) 112:683–92. doi: 10.1172/JCI18399
8. Collins MT, Kushner H, Reynolds JC, Chebli C, Kelly MH, Gupta A, et al. An instrument to measure skeletal burden and predict functional outcome in fibrous dysplasia of bone. J Bone Miner Res. (2005) 20:219–26. doi: 10.1359/JBMR.041111
9. van der Bruggen W, Hagelstein-Rotman M, de Geus-Oei L-F, Smit F, Dijkstra PDS, Appelman-Dijkstra NM, et al. Quantifying skeletal burden in fibrous dysplasia using sodium fluoride PET/CT. Eur J Nucl Med Mol Imaging. (2020) 47:1527–37. doi: 10.1007/s00259-019-04657-1
10. Gnesin S, Leite Ferreira P, Malterre J, Laub P, Prior JO, Verdun FR. Phantom validation of Tc-99m absolute quantification in a SPECT/CT commercial device. Comput Math Methods Med. (2016) 2016:4360371. doi: 10.1155/2016/4360371
11. Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, Offiah A, et al. Best practice management guidelines for fibrous dysplasia/McCune-albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis. (2019) 14:139. doi: 10.1186/s13023-019-1102-9
12. Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med. (2010) 51:1826–9. doi: 10.2967/jnumed.110.077933
13. Papadakis GZ, Manikis GC, Karantanas AH, Florenzano P, Bagci U, Marias K, et al. 18F-NaF PET/CT imaging in fibrous dysplasia of bone. J Bone Miner Res. (2019) 34:1619–31. doi: 10.1002/jbmr.3738
14. Yamane T, Kuji I, Seto A, Matsunari I. Quantification of osteoblastic activity in epiphyseal growth plates by quantitative bone SPECT/CT. Skeletal Radiol. (2018) 47:805–10. doi: 10.1007/s00256-017-2861-9
15. Tabotta F, Jreige M, Schaefer N, Becce F, Prior JO, Nicod Lalonde M. Quantitative bone SPECT/CT: high specificity for identification of prostate cancer bone metastases. BMC Muscul Dis. (2019) 20:619. doi: 10.1186/s12891-019-3001-6
Keywords: fibrous dysplasia, SPECT/CT, scintigraphy, bone scan, quantitative imaging, bone turnover markers
Citation: Jreige M, Hall N, Becce F, Aubry-Rozier B, Gonzalez Rodriguez E, Schaefer N, Prior JO and Nicod Lalonde M (2022) A novel approach for fibrous dysplasia assessment using combined planar and quantitative SPECT/CT analysis of Tc-99m-diphosphonate bone scan in correlation with biological bone turnover markers of disease activity. Front. Med. 9:1050854. doi: 10.3389/fmed.2022.1050854
Received: 22 September 2022; Accepted: 07 November 2022;
Published: 25 November 2022.
Edited by:
Giorgio Treglia, Ente Ospedaliero Cantonale (EOC), SwitzerlandReviewed by:
Edel Noriega-Álvarez, Hospital General Universitario de Ciudad Real, SpainSharjeel Usmani, Kuwait Cancer Control Center, Kuwait
Copyright © 2022 Jreige, Hall, Becce, Aubry-Rozier, Gonzalez Rodriguez, Schaefer, Prior and Nicod Lalonde. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie Nicod Lalonde, marie.nicod-lalonde@chuv.ch
 Mario Jreige
Mario Jreige Nicolas Hall2
Nicolas Hall2  Fabio Becce
Fabio Becce Niklaus Schaefer
Niklaus Schaefer John O. Prior
John O. Prior Marie Nicod Lalonde
Marie Nicod Lalonde