Is the Infection of the SARS-CoV-2 Delta Variant Associated With the Outcomes of COVID-19 Patients?
- 1Pediatric Surgery Division, Department of Surgery/Genetics Working Group, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
- 2Department of Microbiology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
- 3Disease Investigation Center, Ministry of Agriculture Indonesia, Yogyakarta, Indonesia
- 4National Institute of Health Research and Development, Ministry of Health, Jakarta, Indonesia
- 5Pulmonology Division, Department of Internal Medicine, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital, Yogyakarta, Indonesia
- 6Centre of Tropical Medicine, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
- 7Department of Clinical Pathology and Laboratory Medicine, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital, Yogyakarta, Indonesia
- 8Department of Child Health, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
- 9Department of Computer Science and Electronics Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Yogyakarta, Indonesia
- 10Department of Physiology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/UGM Academic Hospital, Yogyakarta, Indonesia
- 11Balai Besar Teknik Kesehatan Lingkungan dan Pengendalian Penyakit, Yogyakarta, Indonesia
- 12Department of Anatomical Pathology/Genetics Working Group, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
- 13Department of Pharmacology and Therapy/Genetics Working Group, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
- 14RSUD Dr. Loekmono Hadi, Kudus, Indonesia
Background: Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) Delta variant (B.1.617.2) has been responsible for the current increase in Coronavirus disease 2019 (COVID-19) infectivity rate worldwide. We compared the impact of the Delta variant and non-Delta variant on the COVID-19 outcomes in patients from Yogyakarta and Central Java provinces, Indonesia.
Methods: In this cross-sectional study, we ascertained 161 patients, 69 with the Delta variant and 92 with the non-Delta variant. The Illumina MiSeq next-generation sequencer was used to perform the whole-genome sequences of SARS-CoV-2.
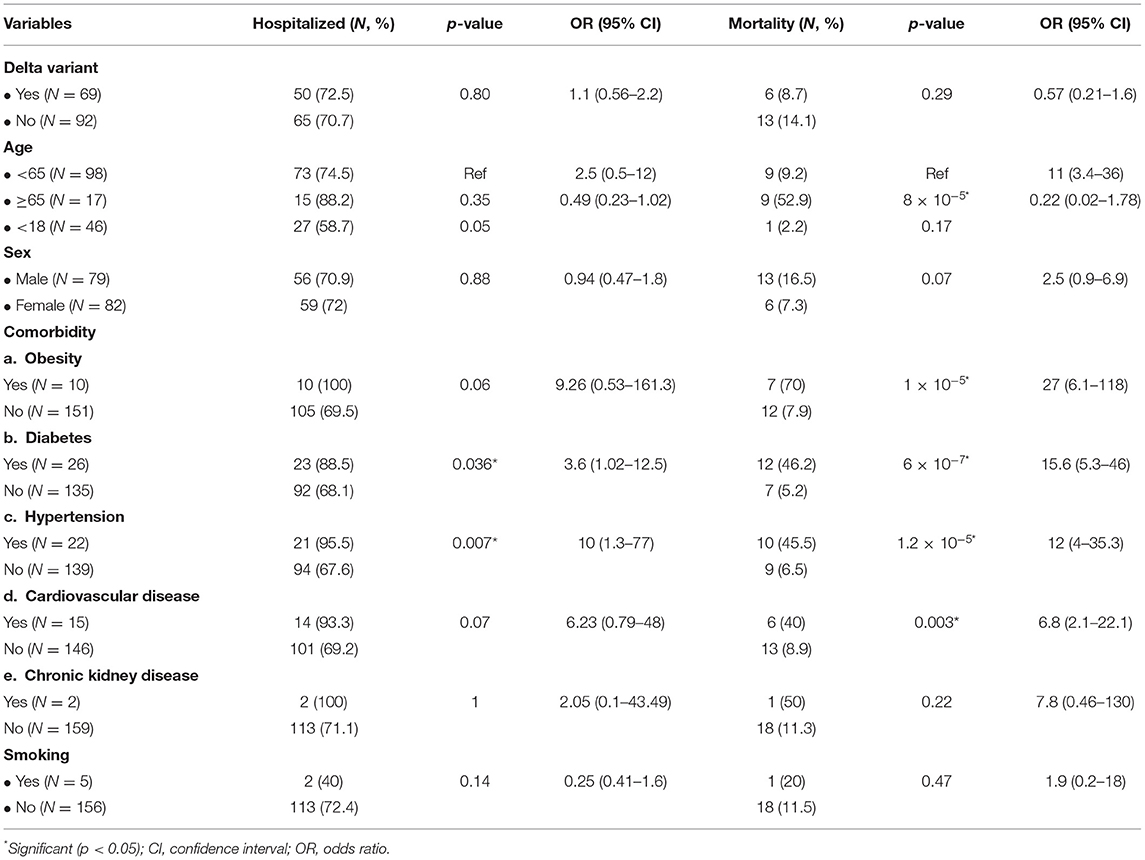
Results: The mean age of patients with the Delta variant and the non-Delta variant was 27.3 ± 20.0 and 43.0 ± 20.9 (p = 3 × 10−6). The patients with Delta variant consisted of 23 males and 46 females, while the patients with the non-Delta variant involved 56 males and 36 females (p = 0.001). The Ct value of the Delta variant (18.4 ± 2.9) was significantly lower than that of the non-Delta variant (19.5 ± 3.8) (p = 0.043). There was no significant difference in the hospitalization and mortality of patients with Delta and non-Delta variants (p = 0.80 and 0.29, respectively). None of the prognostic factors were associated with the hospitalization, except diabetes with an OR of 3.6 (95% CI = 1.02–12.5; p = 0.036). Moreover, the patients with the following factors have been associated with higher mortality rate than the patients without the factors: age ≥65 years, obesity, diabetes, hypertension, and cardiovascular disease with the OR of 11 (95% CI = 3.4–36; p = 8 × 10−5), 27 (95% CI = 6.1–118; p = 1 × 10−5), 15.6 (95% CI = 5.3–46; p = 6 × 10−7), 12 (95% CI = 4–35.3; p = 1.2 × 10−5), and 6.8 (95% CI = 2.1–22.1; p = 0.003), respectively. Multivariate analysis showed that age ≥65 years, obesity, diabetes, and hypertension were the strong prognostic factors for the mortality of COVID-19 patients with the OR of 3.6 (95% CI = 0.58–21.9; p = 0.028), 16.6 (95% CI = 2.5–107.1; p = 0.003), 5.5 (95% CI = 1.3–23.7; p = 0.021), and 5.8 (95% CI = 1.02–32.8; p = 0.047), respectively.
Conclusions: We show that the patients infected by the SARS-CoV-2 Delta variant have a lower Ct value than the patients infected by the non-Delta variant, implying that the Delta variant has a higher viral load, which might cause a more transmissible virus among humans. However, the Delta variant does not affect the COVID-19 outcomes in our patients. Our study also confirms that older age and comorbidity increase the mortality rate of patients with COVID-19.
Introduction
The recent pandemic of Coronavirus disease 2019 (COVID-19) continuously causes a tremendous impact on both global health and the economy, with millions of people losing their lives. The causative agent, severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2), is believed to emerge from bats as the natural reservoir of various strains of coronaviruses (CoV), including SARS-CoV-2 and the former SARS-CoV (1). Many SARS-CoV-2 infections in this ongoing pandemic have provided space and opportunity for continuous mutation and evolution, giving rise to novel variants with increased fitness and, thus, a significant impact on public health (2).
The SARS-CoV-2 variants of concern (VOC), including Alpha, Beta, Gamma, and Delta, have attracted public health authorities due to its capability on higher transmission; the possibility of affecting COVID-19 severity; and the impact of the effectiveness of public health measures, diagnosis, treatment, and vaccines (3–5). Among those VOC, the SARS-CoV-2 Delta variant (B.1.617.2) has been responsible for the current increase in COVID-19 infectivity rate worldwide, including Indonesia (6–10). The Delta variant is approximately two times more transmissible than the previous variants (11). The higher fitness and transmission capacity of the Delta variant is partly attributed to the notable mutations in the spike (S) region, including P681R and L452R, leading to a higher affinity of angiotensin-converting enzyme 2 (ACE-2) attachment (12, 13).
Currently, the Delta variant has been the most frequent variant circulating globally (14). Indeed, national genomic surveillance of SARS-CoV-2 variants has identified the Delta variant as the most dominant circulating variant in Indonesia (97.8%), followed by Alpha (1.7%) and Beta variants (0.5%) (10). Noteworthy, the Delta variant has been associated with a higher risk of hospitalization, more severe outcomes, admission of ICU, and mortality than other variants (15–17).
Several prognostic factors have been associated with COVID-19 illness (18–20). However, our knowledge about the role of the Delta variant on the COVID-19 outcomes is still very limited (15, 21). In this study, we compared the impact of the Delta variant and non-Delta variant infections on the COVID-19 outcomes, i.e., hospitalization and mortality, in patients from Yogyakarta and Central Java provinces, Indonesia.
Materials and Methods
Patients
This cross-sectional study ascertained 161 patients with COVID-19 (79 men and 82 women) from Yogyakarta and Central Java provinces. The patients were ascertained in this study if the PCR's Ct value was ≤25 according to our previous studies (22–24). The diagnostic criteria of COVID-19 were determined using PCR. The PCR was performed for patients with clinical manifestations of COVID-19 or close contact with the confirmed COVID-19 case.
Moreover, some patients (45/69, 65.2%) infected by the Delta variant were confirmed in clusters, while others (24/69, 34.8%) were not. The outcomes of patients with COVID-19 were hospitalization and mortality.
The sample size was determined using the cross-sectional design formula: type I error rate (α) of 0.05, power of the study (1–β) of 0.63, the odds ratio of Delta patients for hospitalization of 1.85, and proportion of hospitalization for non-Delta patients of 0.19 (15). The calculated total sample size was 160.
Prognostic Factors
According to previous studies, we associated the following prognostic factors with the hospitalization and mortality of patients with COVID-19: sex; age; comorbidities, including obesity, diabetes, hypertension, cardiovascular disease, and chronic kidney disease; and smoking (18–20).
Sample Collection
All samples were collected from either outpatient or hospitalized patients with COVID-19 from May 2020 to June 2021 from Yogyakarta and Central Java provinces. We diagnosed the first patient infected with a non-Delta and Delta variant on May 16, 2020, and May 25, 2021, respectively. Samples were collected from nasopharyngeal swabs by using viral transport media. Subsequently, the samples were sent to our institution for PCR.
Severe Acute Respiratory Syndrome Coronavirus 2 Whole-Genome Sequencing
According to our previous studies, SARS-CoV-2 WGS was performed for all samples with PCR's Ct value of ≤25 (22–24). First, single-stranded cDNA was synthesized from RNA extracted from the viral transport medium of patients with COVID-19 using SuperScript™ III First-Strand Synthesis System (Thermo Fisher Scientific, MA, United States). Then, the second strand was synthesized using COVID-19 ARTIC v3 primer pool design by SARS-CoV-2 ARTIC Network using Phusion™ High-Fidelity DNA Polymerase (Thermo Fisher Scientific, MA, United States). The library preparations were performed using the Illumina DNA Prep (Illumina, California, United States). The Illumina MiSeq next-generation sequencer was used to perform the whole-genome sequences of SARS-CoV-2. The assembly of our sample genomes was mapped into the reference genome from Wuhan, China (hCoV-19/Wuhan/Hu-1/2019, GenBank accession number: NC_045512.2) using Burrow–Wheeler Aligner (BWA) algorithm embedded in UGENE v. 1.30 (25).
Phylogenetic Study
We used a dataset of 250 available SARS-CoV-2 genomes extracted from GISAID from our region and others (Acknowledgment Table is provided in Supplementary Table 1) to reconstruct the phylogenetic tree. Multiple nucleotide sequence alignment was performed using the MAFFT program (https://mafft.cbrc.jp/alignment/server/). We used the neighbor-joining statistical method with 1,000 bootstrap replications (26, 27) to construct a phylogenetic tree from 29.420 nucleotide length of the open reading frame (ORF) of SARS-CoV-2, followed by computation of the evolutionary distances, and model the rate variation among sites by the Kimura 2-parameter method and the gamma distribution with estimated shape parameter (α) for the dataset, respectively (28). We used the DAMBE version 7 (29) to calculate the estimation of the gamma distribution, MEGA version 10 (MEGA X) (30), for phylogenetic reconstruction, and FigTree to visualize the Newick tree output from MEGA X.
Statistical Analysis
We presented data as mean ± SD and frequency (percentage). The normality of the continuous variables was evaluated using the Kolmogorov–Smirnov test. Missing or incomplete data were excluded from the final analysis. Chi-square or Fisher's exact tests with 95% confidence interval (CI) were used to find any significant association between independent variables and COVID-19 outcomes. Next, multivariate analysis was performed directly using a logistic regression test. We included all variables in multivariate analysis because those prognostic factors have been associated with the outcomes of patients with COVID-19 (18–20). The p-value of <0.05 was considered significant. All statistical analyses were performed using the IBM Statistical Package for the Social Sciences (SPSS) version 21 (Chicago, United States).
Ethical Approval
The Ethics Committee of the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital approved our study (KE/FK/0563/EC/2020).
Results
Phylogenetic Analysis
Phylogenetic analysis showed that about 69 samples (43%) of SARS-CoV-2 collected from Central Java and Yogyakarta provinces belonged to B.1.617.2 lineage (Delta variant), while 92 samples (57%) clustered in 14 different lineages based on the Pango nomenclature. They were B (1), B.1 (7), B.1.1 (2), B.1.459 (26), B.1.456 (1), B.1.462 (6), B.1.466.2 (21), B.1.468 (3), B.1.470 (11), B.1.570 (1), B.1.1.236 (1), B.1.36.19 (1), B.1.1.398 (3), and B.6 (1), and seven virus samples were not belonging to any of Pango lineages (“none”) (Figure 1). Except for the Delta variant, none of the virus samples collected from our study belonged to any VOC or variant of interest according to WHO labels for naming SARS-CoV-2 variants, including Alpha variant (B.1.7.7 + Q.x), which was first detected in Indonesia in January 2021. Delta variant (B.1.617.2 + AY.x) seemed to be the major VOC circulating in Indonesia, including in the Central Java and Yogyakarta provinces, from May 2021 up to now. Interestingly, we found that about 13% of virus samples from Central Java and Yogyakarta provinces were clustered into B.1.466.2 lineage (Figure 1) that is currently designated by WHO as a variant of alert for further monitoring.
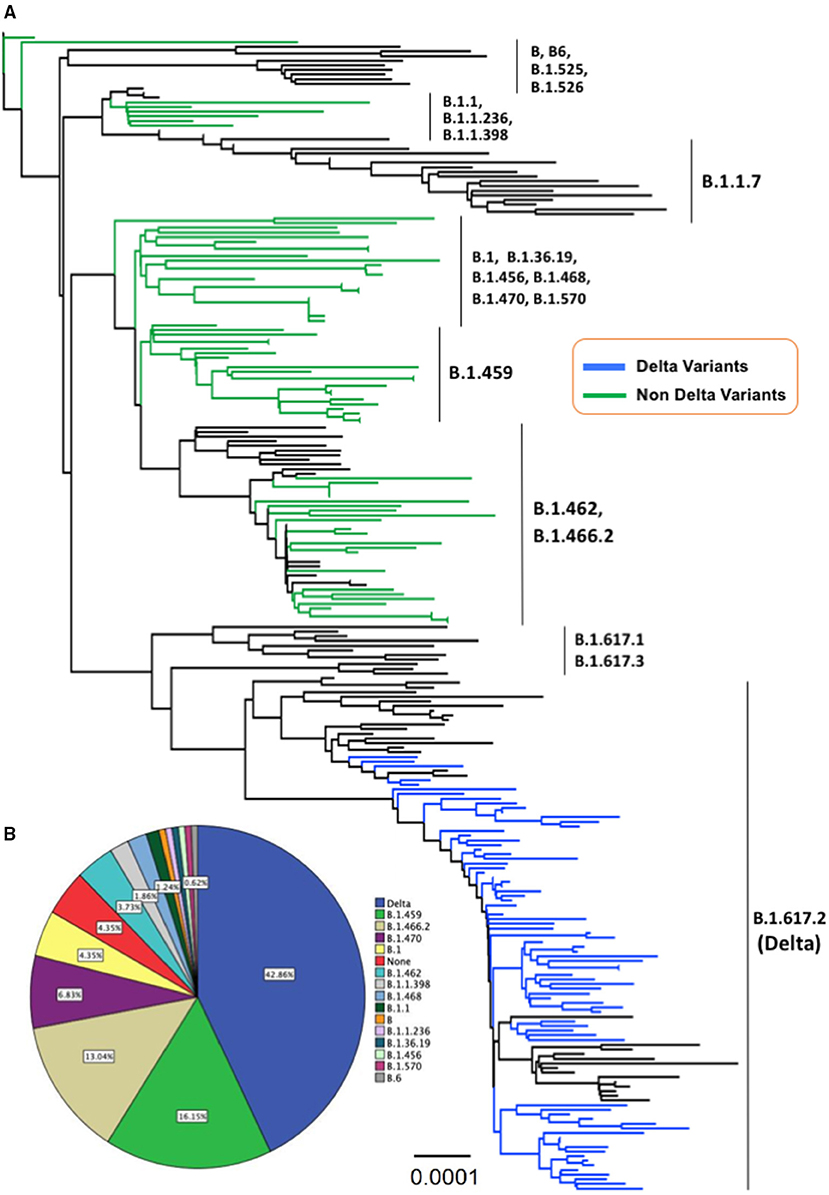
Figure 1. (A) The evolutionary history was inferred using the neighbor-joining method conducted in MEGA-X. The evolutionary distances were computed using the Kimura 2-parameter method with 1,000 bootstrap replication and are in the units of the number of base substitutions per site (0.0001) shown in the bottom tree. This analysis involved 250 nucleotide sequences with a total of 29,420 positions in the final dataset, and all ambiguous positions were removed for each sequence pair (pairwise deletion option). The Delta variant taxa are indicated in the blue line, whereas the non-Delta variant taxa appeared in the green line. (B) A pie chart illustrates the proportion of Delta variant and non-Delta samples detected in the present study.
Characteristics of Patients With COVID-19
Most Delta variants (65.2%) detected in our study were cluster cases. The mean age of patients with Delta and the non-Delta variant was 27.3 ± 20.0 and 43.0 ± 20.9 (p = 3x10−6), respectively. The patients with the Delta variant consisted of 23 men and 46 women, while the patients with the non-Delta variant involved 56 men and 36 women (p = 0.001). The Ct value of the Delta variant (18.4 ± 2.9) was significantly lower than that of the non-Delta variant (19.5 ± 3.8) (p = 0.043) (Table 1).
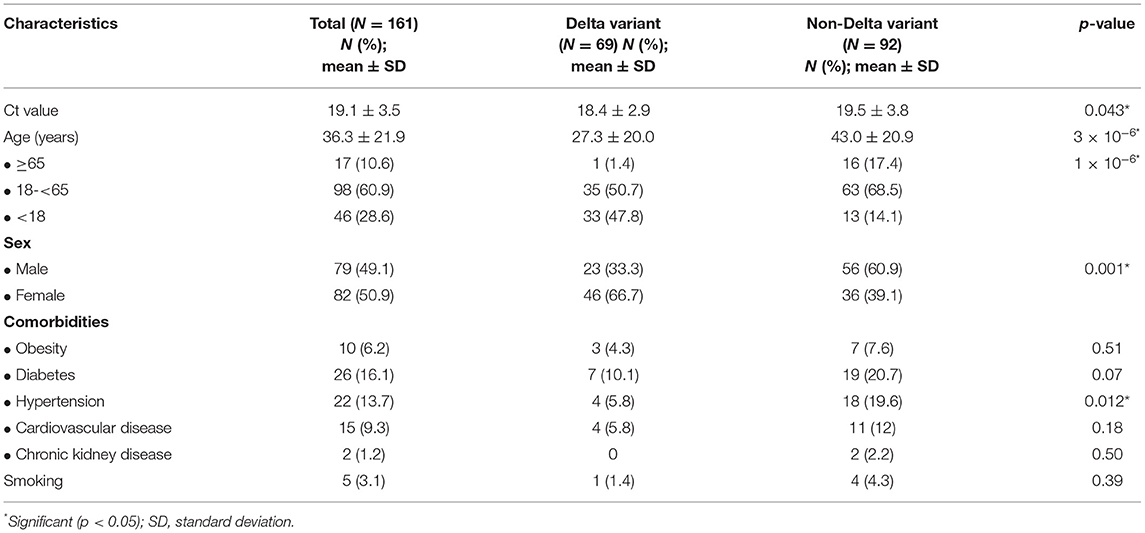
Table 1. Characteristics of patients with COVID-19 from Yogyakarta and Central Java provinces, Indonesia.
Association Between Independent Variables and COVID-19 Patients' Outcomes
Next, we associated the independent variables with the COVID-19 outcomes, hospitalization, and mortality. There were no significant differences in the hospitalization and mortality of patients with Delta and non-Delta variants (p = 0.80 and 0.29, respectively) (Table 2). None of the prognostic factors were associated with the hospitalization, except comorbidity of diabetes with the OR of 3.6 (95% CI = 1.02–12.5; p = 0.036) (Table 2). Moreover, the patients with the following factors have been associated with higher mortality rate than the patients without these factors: age ≥65 years, obesity, diabetes, hypertension, and cardiovascular disease with OR of 11 (95% CI = 3.4–36; p = 8 × 10−5), 27 (95% CI = 6.1–118; p = 1 × 10−5), 15.6 (95% CI = 5.3–46; p = 6 × 10−7), 12 (95% CI = 4–35.3; p = 1.2 × 10−5), and 6.8 (95% CI = 2.1–22.1; p = 0.003), respectively (Table 2).
Multivariate Analysis
Subsequently, we performed a multivariate analysis to find an independent factor affecting the COVID-19 outcomes. Multivariate analysis showed that age ≥65 years, obesity, diabetes, and hypertension were strong prognostic factors for the mortality of COVID-19 patients with the OR of 3.6 (95% CI = 0.58–21.9; p = 0.028), 16.6 (95% CI = 2.5–107.1; p = 0.003), 5.5 (95% CI=1.3–23.7; p = 0.021), and 5.8 (95% CI = 1.02–32.8; p = 0.047), respectively. In addition, no prognostic factors were associated with the hospitalization of patients with COVID-19 (Table 3).
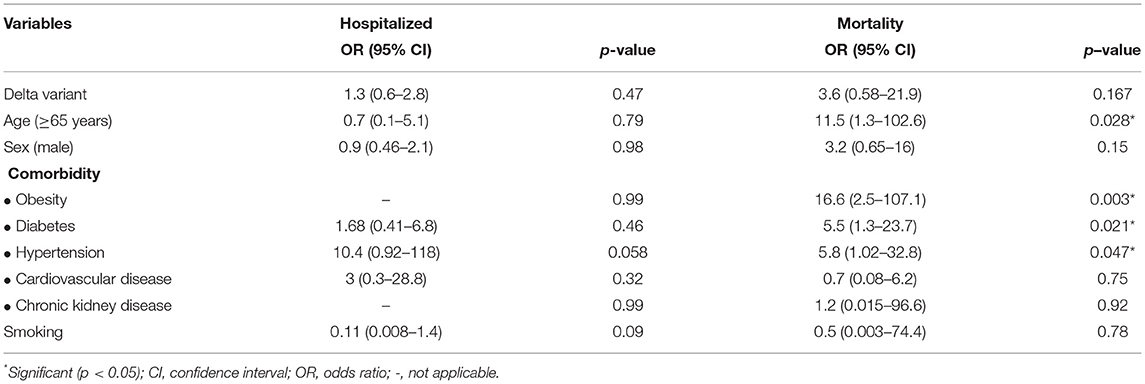
Table 3. Multivariate analysis of the association between independent variables and COVID patients' outcomes.
Discussion
We are able to show that the patients infected by the SARS-CoV-2 Delta variant have a significantly lower Ct value than the patients infected by the non-Delta variant. Our finding is compatible with previous studies (17, 31, 32). Our finding supports that the higher viral load of the Delta variant results in its characteristic of being more transmissible among humans (31, 33). It is associated with a higher reproductive number (R0) of the Delta variant (R0 = 7) than the parental SARS-CoV-2 and the Alpha variant (34). In addition, the Delta variant also has a significantly longer duration (18 days) of Ct value ≤30 than the original strain (13 days) (17). Again, this evidence implies higher transmissibility of the Delta variant than that of other strains.
In addition, it seems that the replication of SARS-CoV-2 is increased in a time-dependent manner during the early stage of infection. However, our study did not consider the interval days between the dates of the first infection and sample collection for the SARS-CoV-2 WGS. The SARS-CoV-2 WGS in our study was performed only based on the PCR's Ct value of ≤25 (22–24). These facts should be considered during the interpretation of our findings.
We also show that the Delta variant is not associated with the mortality and hospitalization of patients with COVID-19. Our findings are different from previous reports (15–17). Sheikh et al. (15) showed that the risk of hospitalization is two times higher in patients with Delta variant than in patients with the Alpha variant. Fisman et al. (16) revealed that the risk of hospitalization, admission of ICU, and mortality are significantly higher in the Delta variant than in N501Y-positive variants. They suggested that the Delta variant is more virulent than previous VOCs (16). Ong et al. (17) showed that the patients with the Delta variant were more severely affected with COVID-19 than the original variant. These differences might be due to differences in the host's genetic background (35). They identified a novel susceptibility locus for severe COVID-19 at the 3p21.31 gene cluster, consisting of SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6, and XCR1, and their functions are related to COVID-19 (35). Further study is necessary to identify the genetic susceptibility locus for severe COVID-19 in our patients. Another difference between our study and previous reports is that we compared the outcomes of the Delta variant and non-VOC, while other reports compared the Delta variant and other VOCs [9; 10->15, 16]. Moreover, we focused on the impact of the Delta variant on COVID-19 outcomes, regardless of whether the Delta variants were originated from independent cases or clusters.
Interestingly, ~50% of Delta variant infected children, higher than non-Delta variant (~15%). Similar findings were reported by a previous study showing that the frequency of the Delta variant is higher in children aged 5–9 years than that of the non-Delta variant (15). While the Delta variant infected more women than men, the non-Delta variant infected more men than women. However, multivariate analysis did not show an association between sex and COVID-19 outcomes. It is similar to a study by Ong et al. (17). It should be noted that the impact of sex on the COVID-19 outcomes is still controversial (17, 18, 36, 37). While some studies showed that male has a higher risk for severe COVID-19 (18, 36), other reports do not support the association (17, 37).
Our findings reveal that older age and comorbidities, including obesity, diabetes, and hypertension, are independent prognostic factors for the mortality of patients with COVID-19. Our findings were similar to previous studies (18, 30, 36). Several mechanisms have been proposed for the increased risk of COVID-19 in patients with diabetes, including an elevated level of ACE-2 receptors and furin, and dysregulated immune response, while the following factors contribute for the obesity to be associated with the worse prognosis of COVID-19: the compromised ventilation at the base of the lung and immune response (38). The use of the antihypertension drug, particularly ACE-2 inhibitors and angiotensin receptor blockers, is associated with the upregulated expression of the ACE-2 receptor, resulting in a higher possibility of respiratory failure (38). Unfortunately, we do not have complete data on the use of ACE-2 inhibitors and angiotensin receptor blockers in our patients. Therefore, it is challenging to conclude that the increased mortality of COVID-19 in patients with hypertension is due to antihypertension.
Several studies have shown that the vaccinated individuals might have significantly less severe outcomes of COVID-19 if infected with the Delta variant (15, 39, 40). Unfortunately, we did not have complete data on the vaccination status of patients with COVID-19. In addition, some studies showed that different prevention and control measures might have a different impact on the outcomes of patients with COVID-19 (41–43). The government of Indonesia has also applied public health measures, i.e., mitigation intervention, including compulsory mask-wearing, personal protective equipment, social distancing measures, travel and mass gathering restrictions, quarantine of travelers arriving from overseas, isolation of confirmed cases and close contacts, contact tracing and testing, and school closures. However, our study did not consider the role of those interventions on the outcomes of patients with COVID-19.
Our cross-sectional design that srestricts the ability of causal inference, the wide variation of the 95% CI of ORs, and the lack of demographic information (including occupation, income, education level, low power of the study, and small sample size) were the weaknesses of our study. Moreover, we only determined the impact of the Delta variant and some comorbidities on the COVID-19 outcomes by overall means without considering other variables affecting the data, including vaccination history and public health measures.
Conclusion
We show that the patients infected by the SARS-CoV-2 Delta variant have a lower Ct value than the patients infected by the non-Delta variant, implying that the Delta variant has a higher viral load, which might cause a more transmissible virus among humans. However, the Delta variant does not affect the COVID-19 outcomes in our patients. Our study also confirms that older age and comorbidity increase the mortality rate of patients with COVID-19.
Data Availability Statement
The datasets of SARS-CoV-2 genomes presented in this study can be found in GISAID (https://www.gisaid.org/). The accession number(s) can be found in the Supplementary Table 1.
Ethics Statement
The studies involving human participants were reviewed and approved by Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
G, KI, and NA conceived the study. G drafted the manuscript. MH, HW, and TW critically revised the manuscript for important intellectual content. M, DP, ITa, KV, MA, GG, LE, BN, EG, AD, AK, L, and SI performed the library preparation and NGS. G, MH, M, VS, Sl, ITr, ES, RK, KI, A, Si, I, NA, ED, DN, YP, SK, IL, NA, EA, TN, and TW collected the data. G, MH, HW, and M analyzed the data. All authors have read and approved the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was funded by the Ministry of Education, Culture, Research and Technology, Indonesia. The funders had no role in study design, data collection, and analysis, decision to publish, or manuscript preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Collaborator Members of the Yogyakarta-Central Java COVID-19 study group: Eko Budiono, Heni Retnowulan, Sumardi, Bambang Sigit Riyanto, Munawar Gani, Satria Maulana, Ira Puspitawati, Osman Sianipar (Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada [FK-KMK UGM]/RSUP Dr. Sardjito), Bagoes Poermadjaja (Balai Besar Besar Veteriner Wates, Yogyakarta), Indaryati and Havid Setyawan (Balai Besar Teknik Kesehatan Lingkungan dan Pengendalian Penyakit, Yogyakarta), Ludhang Pradipta Rizki and Sri Fatmawati (FK-KMK UGM), and Safitriani and Muhammad Taufiq Soekarno (PT. Pandu Biosains). We gratefully acknowledge the authors and the originating and submitting laboratories for their sequence and metadata shared through GISAID. We also thank Besar Besar Veteriner Wates (Disease Investigation Center Wates) where the virus samples were sequenced using the NGS Illumina MiSeq instrument. All submitters of data may be contacted directly via www.gisaid.org. The Acknowledgments Table for GISAID is reported as Supplementary Table 1.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.780611/full#supplementary-material
References
1. Hakim MS. SARS-CoV-2, Covid-19, and the debunking of conspiracy theories. Rev Med Virol. (2021) 14:e2222. doi: 10.1002/rmv.2222
2. Moustafa AM, Planet PJ. Jumping a moving train: SARS-CoV-2 evolution in real time. J Pediatric Infect Dis Soc. (2021) piab051. doi: 10.1093/jpids/piab051. [Epub ahead of print].
3. World Health Organization. Tracking SARS-CoV-2 Variants. Available online at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed August 16, 2021).
4. World Health Organization. Weekly Epidemiological Update on COVID-19 (2021). Available online at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-$-$10-august-2021 (accessed August 16, 2021).
5. Gushchin VA, Dolzhikova IV, Shchetinin AM, Odintsova AS, Siniavin AE, Nikiforova MA, et al. Neutralizing activity of sera from Sputnik V-vaccinated people against variants of concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow endemic SARS-CoV-2 variants. Vaccines. (2021) 9:779. doi: 10.3390/vaccines9070779
6. Herlihy R, Bamberg W, Burakoff A, Alden N, Severson R, Bush E, et al. Rapid increase in circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant - Mesa County, Colorado, April-June 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1084–7. doi: 10.15585/mmwr.mm7032e2
7. Kadri SS, Simpson SQ. Potential implications of SARS-CoV-2 delta variant surges for rural areas and hospitals. JAMA. (2021) 326:1003–4. doi: 10.1001/jama.2021.13941
8. Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, Konings F, Batra N, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. (2021) 26:2100509. doi: 10.2807/1560-7917.ES.2021.26.24.2100509
9. Musser JM, Christensen PA, Olsen RJ, Long SW, Subedi S, Davis JJ, et al. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. medRxiv [Preprint]. (2021). doi: 10.1101/2021.07.19.21260808
10. Badan Litbangkes Kementerian Kesehatan RI. Peta Sebaran Sekuens dan Varian Covid-19 di Indonesia. Available online at: https://www.litbang.kemkes.go.id/peta-sebaran-sekuens-dan-varian-covid-19 (accessed October 14, 2021).
11. Centers for Disease Control and Prevention. Delta Variant (2021). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html (accessed November 7, 2021).
12. Liu Y, Liu J, Johnson BA, Xia H, Ku Z, Schindewolf C, et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. bioRxiv [Preprint]. (2021). doi: 10.1101/2021.08.12.456173
13. Motozono C, Toyoda M, Zahradnik J, Saito A, Nasser H, Tan TS, et al. SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. (2021) 29:1124–36.e11. doi: 10.1016/j.chom.2021.06.006
14. Shiehzadegan S, Alaghemand N, Fox M, Venketaraman V. Analysis of the delta variant B.1.617.2 COVID-19. Clin Pract. (2021) 11:778–84. doi: 10.3390/clinpract11040093
15. Sheikh A, McMenamin J, Taylor B, Robertson C, Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. (2021) 397:2461–2. doi: 10.1016/S0140-6736(21)01358-1
16. Fisman DN, Tuite AR. Progressive increase in virulence of novel SARS-CoV-2 variants in Ontario, Canada. medRxiv [Preprint]. (2021). doi: 10.1101/2021.07.05.21260050
17. Ong SW, Chiew CJ, Ang LW, Mak TM, Cui L, Toh MP, et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). SSRN J. (2021). doi: 10.2139/ssrn.3861566. [Epub ahead of print].
18. Beeching NJ, Fletcher TE, Fowler R. BMJ Best Practice. Coronavirus Disease 2019 (COVID-19). Available online at: https://bestpractice.bmj.com/topics/en-us/3000168/prognosis (accessed August 16, 2021).
19. Cho Y, Cho Y, Choi HJ, Lee H, Lim TH, Kang H, et al. The effect of BMI on COVID-19 outcomes among older patients in South Korea: a nationwide retrospective cohort study. Ann Med. (2021) 53:1292–301. doi: 10.1080/07853890.2021.1946587
20. Zhou B, Kojima S, Kawamoto A, Fukushima M. COVID-19 pathogenesis, prognostic factors, and treatment strategy: urgent recommendations. J Med Virol. (2021) 93:2694–704. doi: 10.1002/jmv.26754
21. Shah SA, Moore E, Robertson C, McMenamin J, Katikireddi SV, Simpson CR, et al. Predicted COVID-19 positive cases, hospitalizations, and deaths associated with the Delta variant of concern, June-July, 2021. Lancet Digit Health. (2021). doi: 10.1016/S2589-7500(21)00175-8
22. Gunadi HW, Marcellus MS, Edwin Widyanto Daniwijaya LP, Endah Supriyati DA, Afiahayati S, Kristy Iskandar NA, et al. Full-length genome characterization and phylogenetic analysis of SARS-CoV-2 virus strains from Yogyakarta and Central Java, Indonesia. PeerJ. (2020) 8:e10575. doi: 10.7717/peerj.10575
23. Wibawa H, Hakim MS, Trisnawati I, El Khair R, Triasih R, Iskandar K, et al. Molecular epidemiology of SARS-CoV-2 isolated from COVID-19 family clusters. BMC Med Genomics. (2021) 14:144. doi: 10.1186/s12920-021-00990-3
24. Hakim MS, Wibawa H, Trisnawati I, Supriyati E, Khair RE, Iskandar K, et al. Association between prognostic factors and the outcomes of patients infected with SARS-CoV-2 harboring multiple spike protein mutations. Sci Rep. (2021) 11:21352. doi: 10.1038/s41598-021-00459-4
25. About UGENE. Unipro UGENE Online User Manual v. 1.30 - WIKI. Ugene.net. (2020). Available online at: https://ugene.net/wiki/display/UUOUM30/About$+$UGENE (accessed December 22, 2020).
26. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. (1897) 4:406–25.
27. Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. (1985) 39:783–91. doi: 10.1111/j.1558-5646.1985.tb00420.x
28. Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. (1980) 16:111–20. doi: 10.1007/BF01731581
29. Xia X. DAMBE7: new and improved tools for data analysis in molecular biology and evolution. Mol Biol Evol. (2018) 35:1550–2. doi: 10.1093/molbev/msy073
30. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. (2018) 35:1547–9 doi: 10.1093/molbev/msy096
31. Li B, Deng A, Li K, Hu Y, Li Z, Xiong Q, et al. Viral infection and transmission in a large well-traced outbreak caused by the Delta SARS-CoV-2 variant. medRxiv [Preprint]. (2021). doi: 10.1101/2021.07.07.21260122
32. Mlcochova P, Kemp S, Dhar MS, Papa G, Meng B, Mishra S, et al. SARS-CoV-2 B.1.617.2 delta variant emergence and vaccine breakthrough. Res Square Platform LLC. (2021). doi: 10.21203/rs.3.rs-637724/v1
33. Callaway E. Delta coronavirus variant: scientists brace for impact. Nature. (2021) 595:17–8. doi: 10.1038/d41586-021-01696-3
34. Burki TK. Lifting of COVID-19 restrictions in the UK and the delta variant. Lancet Respir Med. (2021) 9:e85. doi: 10.1016/S2213-2600(21)00328-3
35. Severe Covid-19 GWAS Group, Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. (2020) 383:1522–34. doi: 10.1056/NEJMoa2020283
36. Osibogun A, Balogun M, Abayomi A, Idris J, Kuyinu Y, Odukoya O, et al. Outcomes of COVID-19 patients with comorbidities in southwest Nigeria. PLoS ONE. (2021) 16:e0248281. doi: 10.1371/journal.pone.0248281
37. Chia PY, Ong SW, Chiew CJ, Ang LW, Chavatte JM, Mak TM, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. medRxiv [Preprint]. (2021). doi: 10.1101/2021.07.28.21261295
38. Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. (2020) 13:1833–9. doi: 10.1016/j.jiph.2020.07.014
39. Harder T, Külper-Schiek W, Reda S, Treskova-Schwarzbach M, Koch J, Vygen-Bonnet S, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Euro Surveill. (2021) 26. doi: 10.2807/1560-7917.ES.2021.26.41.2100920
40. Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta KD, et al. Effect of delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. (2021). doi: 10.1038/s41591-021-01548-7
41. Ansah JP, Matchar DB, Shao Wei SL, Low JG, Pourghaderi AR, Siddiqui FJ, et al. The effectiveness of public health interventions against COVID-19: lessons from the Singapore experience. PLoS ONE. (2021) 16:e0248742. doi: 10.1371/journal.pone.0248742
42. Ayouni I, Maatoug J, Dhouib W, Zammit N, Fredj SB, Ghammam R, et al. Effective public health measures to mitigate the spread of COVID-19: a systematic review. BMC Public Health. (2021) 21:1015. doi: 10.1186/s12889-021-11111-1
43. Chaudhry R, Dranitsaris G, Mubashir T, Bartoszko J, Riazi S. A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. EClinicalMedicine. (2020) 25:100464. doi: 10.1016/j.eclinm.2020.100464
Keywords: comorbidity, Ct value, delta variant, hospitalization, mortality, SARS-CoV-2, viral load, whole genome sequencing
Citation: Gunadi, Hakim MS, Wibawa H, Marcellus, Setiawaty V, Slamet, Trisnawati I, Supriyati E, El Khair R, Iskandar K, Afiahayati, Siswanto, Irene, Anggorowati N, Daniwijaya EW, Nugrahaningsih DAA, Puspadewi Y, Puspitarani DA, Tania I, Vujira KA, Ardlyamustaqim MB, Gabriela GC, Eryvinka LS, Nirmala BC, Geometri ET, Darutama AA, Kuswandani AA, Lestari, Irianingsih SH, Khoiriyah S, Lestari I, Ananda NR, Arguni E, Nuryastuti T and Wibawa T (2021) Is the Infection of the SARS-CoV-2 Delta Variant Associated With the Outcomes of COVID-19 Patients? Front. Med. 8:780611. doi: 10.3389/fmed.2021.780611
Received: 21 September 2021; Accepted: 09 November 2021;
Published: 09 December 2021.
Edited by:
Bin Luo, Lanzhou University, ChinaReviewed by:
Nur Izzah Ismail, The Chinese University of Hong Kong, ChinaZengliang Ruan, Southeast University, China
Copyright © 2021 Gunadi, Hakim, Wibawa, Marcellus, Setiawaty, Slamet, Trisnawati, Supriyati, El Khair, Iskandar, Afiahayati, Siswanto, Irene, Anggorowati, Daniwijaya, Nugrahaningsih, Puspadewi, Puspitarani, Tania, Vujira, Ardlyamustaqim, Gabriela, Eryvinka, Nirmala, Geometri, Darutama, Kuswandani, Lestari, Irianingsih, Khoiriyah, Lestari, Ananda, Arguni, Nuryastuti and Wibawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gunadi, drgunadi@ugm.ac.id
 Gunadi
Gunadi Mohamad Saifudin Hakim
Mohamad Saifudin Hakim Hendra Wibawa
Hendra Wibawa Marcellus
Marcellus Vivi Setiawaty4
Vivi Setiawaty4  Kristy Iskandar
Kristy Iskandar Khanza Adzkia Vujira
Khanza Adzkia Vujira Muhammad Buston Ardlyamustaqim
Muhammad Buston Ardlyamustaqim Gita Christy Gabriela
Gita Christy Gabriela Laudria Stella Eryvinka
Laudria Stella Eryvinka Abirafdi Amajida Darutama
Abirafdi Amajida Darutama Titik Nuryastuti
Titik Nuryastuti