Acute Kidney Injury Due to COVID-19 in Intensive Care Unit: An Analysis From a Latin-American Center
- 1Nephrology and Dialysis Center, Oswaldo Cruz German Hospital, São Paulo, Brazil
- 2Nephrology Division, University of São Paulo School of Medicine, São Paulo, Brazil
- 3Intensive Care Unit, Oswaldo Cruz German Hospital, São Paulo, Brazil
Introduction: The kidney may be affected by coronavirus (COVID-19) in the setting of acute kidney injury (AKI). Data about AKI in intensive care unit (ICU) patients in Latin America are scarce. We aimed to evaluate the risk of AKI, dialysis (HD), and death in ICU COVID-19 patients in a Brazilian center.
Methods: Analysis from medical records of COVID-19 patients in a Brazilian center.
Results: A total of 95 patients were analyzed. There was male predominance (64.2%), median age: 64.9 years, and previous history of hypertension and diabetes in 51.6 and 27.4%, respectively. AKI was diagnosed in 54 (56.8%) patients, and 32 (59.2%) of them required HD. Mortality rate was 17.9%. AKI patients when compared with no-AKI were more frequently hypertensive/diabetic and more often needed organ support therapies. Workups depicted more anemia, lymphopenia, and higher levels of inflammatory markers and higher mortality. Comparing patients who had undergone death to survivors, they were older, more frequently diabetic, and had worse SAPS3 and SOFA scores and need for organ support therapies, AKI, and HD. Multinomial logistic regression revealed that hypertension (p = 0.018) and mechanical ventilation (p = 0.002) were associated with AKI; hypertension (p = 0.002), mechanical ventilation (p = 0.008), and use of vasopressor (p = 0.027) to HD patients; and age >65 years (p = 0.03) and AKI (p = 0.04) were risk factors for death.
Conclusions: AKI was a common complication of ICU COVID-19 patients, and it was more frequent in patients with hypertension and need of organ support therapies. As well as age >65 years, AKI was an independent risk factor for death.
Introduction
The first cases of atypical pneumonia of unidentified etiology were reported on December 30, 2019, in Wuhan, China. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified by January 7, 2020, and the disease has since been named COVID-19. It was declared a pandemic in March 2020, affecting nearly every country. The initial clinical case series from China largely comprises hospitalized patients with severe pneumonia. Further data suggests that ~80% of the patients have a mild form of the disease, 20% require hospital admission, and ~5% require intensive care admission (1, 2).
The death rate from COVID-19 across different populations (3, 4) has its highest rate reported among older patients and those who are immunocompromised or have other comorbidities or lymphocytopenia (5, 6). Brazil is the most populated country in Latin America and was the first place to confirm a case on the continent on February 25, 2020, in the city of São Paulo (7). Since then, the number of people affected by the disease is growing, and Brazil has become, at the moment, the third country in the world in the number of total cases (8).
Acute kidney injury (AKI) is a syndrome. It is an important complication in patients admitted to hospitals (10–15% of all hospitalizations) (9) and in patients in the intensive care unit (ICU), where its prevalence can sometimes exceed 50% (10). The incidence of AKI in patients with COVID-19 depends on the profile of the analyzed patients, meaning their severity level and if they are in a ward, outpatient, or ICU environment. AKI during a hospital stay is reported with an average incidence of 11% (8–17%) overall with the highest ranges in the critically ill [23% (14–35%)] (11).
No data on AKI in Latin American countries has been published since the COVID-19 pandemic started. Thus, the purpose of this study is to assess the impact of AKI on the mortality and renal prognosis of patients with SARS-CoV-2 infection.
Methodology
Patient Selection
This is a retrospective, single-center study with the purpose of assessing the impact of AKI in patients with COVID-19. Adult patients hospitalized from February 28, 2020, to May 4, 2020, in the ICU of the Oswaldo Cruz German Hospital, Brazil, were chosen for the case study. All patients were diagnosed with COVID-19 infection based on reverse transcription polymerase chain reaction (RT-PCR) for the virus. Patients receiving palliative care or transferred to external services with no follow-up possibility were excluded from the case study.
Sample Characterization
The patients were characterized for gender, age, and the presence of comorbidities (hypertension, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, presence of active neoplasm, and immunosuppression use). The following complementary tests were analyzed: maximum D-dimer, C-reactive protein (CRP), lactate dehydrogenase (LDH), and blood count. We assessed the tests made on the day the nephrologist requested them for patients who had AKI, and we assessed the admission tests for those who did not have AKI. The organic dysfunctions assessed were mechanical ventilation required, use of extracorporeal membrane oxygenation (ECMO), vasopressor use, and renal replacement therapy required. AKI was defined as per the KDIGO (12) criteria based on the creatinine values and diuretic rhythm. AKI KDIGO 1 was defined as an increase of creatinine ≥0.3 mg/dl or 1.5–1.9 times baseline and/or urine output <0.5 ml/kg/h for 6–12 h. AKI KDIGO 2 was defined as an increase of creatinine of 2.0–2.9 times baseline and/or urine output <0.5 ml/kg/h for 12 h. AKI KDIGO 3 was defined as an increase of creatinine of 3.0 times baseline or increase in serum creatinine to ≥4.0 mg/dl or initiation of renal replacement therapy and/or urine output <0.3 ml/kg/h for ≥24 h or anuria for ≥12 h. The instituted treatments were also analyzed (use of antibiotics, anti-IL6, ivermectin, and nitazoxanide). The reference values for the lab tests are as follows: D-dimer: <500 ng/mL FEU, CRP: <1 mg/dL, LDH: 240 to 480 U/L. The following variables were analyzed: length of ICU stay, mechanical ventilation time, and total hospitalization period. Because many patients received a tracheotomy and the continuous use of CPAP/BIPAP was required, we considered the moment in which the patient no longer needed continuous CPAP/BIPAP support as the end of mechanical ventilation time.
Hemodialysis Protocol
The hemodialysis initiation timing and modality were defined by the nephrology team. We placed a hemodialysis catheter of 15.5 Fr in all patients, and the puncture site was chosen according to each patient's clinical setting. Our protocol indicates continuous venous–venous hemodiafiltration (CVVHDF) as the method of choice for those patients who required vasoactive drug infusion and/or presented evidence of hypervolemia. CVVHDF was performed using the Prismaflex system (Baxter, IL, USA) and AN69 ST150 (Baxter, IL, USA) as well as Oxiris® (Baxter, IL, USA) dialysis filters. The prescribed dialysis dose was 25–30 mL/kg/h of effluent and with regional citrate anticoagulation. Slow low-efficiency dialysis and classic hemodialysis were performed using a 4008S hemodialysis system (Fresenius, Germany) for patients with no need of vasoactive drugs.
Endpoint Analysis
The primary endpoint assessed was death attributed to AKI and dialysis need; the secondary endpoints were risk factors for AKI development, dialysis required, and death during the hospital stay.
Statistical Analysis
The distribution of variables was assessed with the Shapiro–Wilk test. Qualitative variables were expressed as proportions and compared against each other via the chi-squared test or Fisher's exact test. Variables following a parametric distribution were expressed as mean values ± standard error and compared against each other with Student's t-test. Variables with non-parametric distributions were expressed as median values (p25 and p75) and compared against each other with the Mann–Whitney U-test. Logistic regression was used in multivariate analysis. Statistical significance was attributed to p < 0.05. We used an ROC curve to analyze the age difference between patients who died and survived.
Ethical Aspects
The current study is a subanalysis of the Diagnóstico e prática clínica em síndromes gripais agudas durante a pandemia por SARS-COV2 em pacientes internados em unidades de baixa e alta complexidade hospitalar (Diagnosis and clinical practice in acute flu syndromes during the SARS-COV2 pandemic in patients hospitalized in low and high hospital complexity units) project, approved by the Brazilian Ethics Committee under number 30264920.6.1001.0070.
Results
From an initial sample of 102 patients, seven were excluded after receiving palliative care or being transferred to other centers throughout the hospital stay. Thus, 95 patients remained for the final data analysis. The baseline characteristics of the patients are outlined in Table 1. Most of them were older adult patients (64.9 ± 15.1 years of age) and men (64.2%), and diabetes and hypertension were the frequent comorbidities (51.6 and 27.4% of the patients, respectively). In addition, 11 (11.6%) patients had immunosuppression status with most of them (54%) being on chemotherapy treatment for an active cancer. Chronic kidney disease was present in 16.8% of the patients, three patients had CKD stage 2 (18.8%), seven had CKD stage 3 (43.8%), and six had CKD stage IV (37.4%). Regarding organic support therapies, the same proportion of patients was receiving mechanical ventilation and using vasopressors (54/56.8%), and three (3.7%) were submitted to ECMO therapy. AKI was diagnosed in 54 (56.8%) of the cases with 12 (22.2%) being KDIGO 1, four (7.4%) KDIGO 2, and 38 (70.3%) KDIGO 3. From the patients with AKI KDIGO 3, 32 (84.2%) required dialysis therapy, and the others continued with conservative treatment due to not presenting oliguria or hydroelectrolytic disorder nor acid-base indicating renal function replacement therapy. The average follow-up of the patients was 24 ± 9.3 days, and when completed, 17 (17.9%) patients died, 62 (65.3%) were discharged from the hospital, and 16 (16.8%) remained hospitalized. Analysis of urine spot was available in 40 patients, of which 21 (52%) had hematuria and 20 (50%) had proteinuria >300 mg/g. Hematuria was more frequent in patients who developed AKI (80.9 vs. 19%, p = 0.02), but similar results were not observed for proteinuria (56 vs. 25%, p = 0.11).
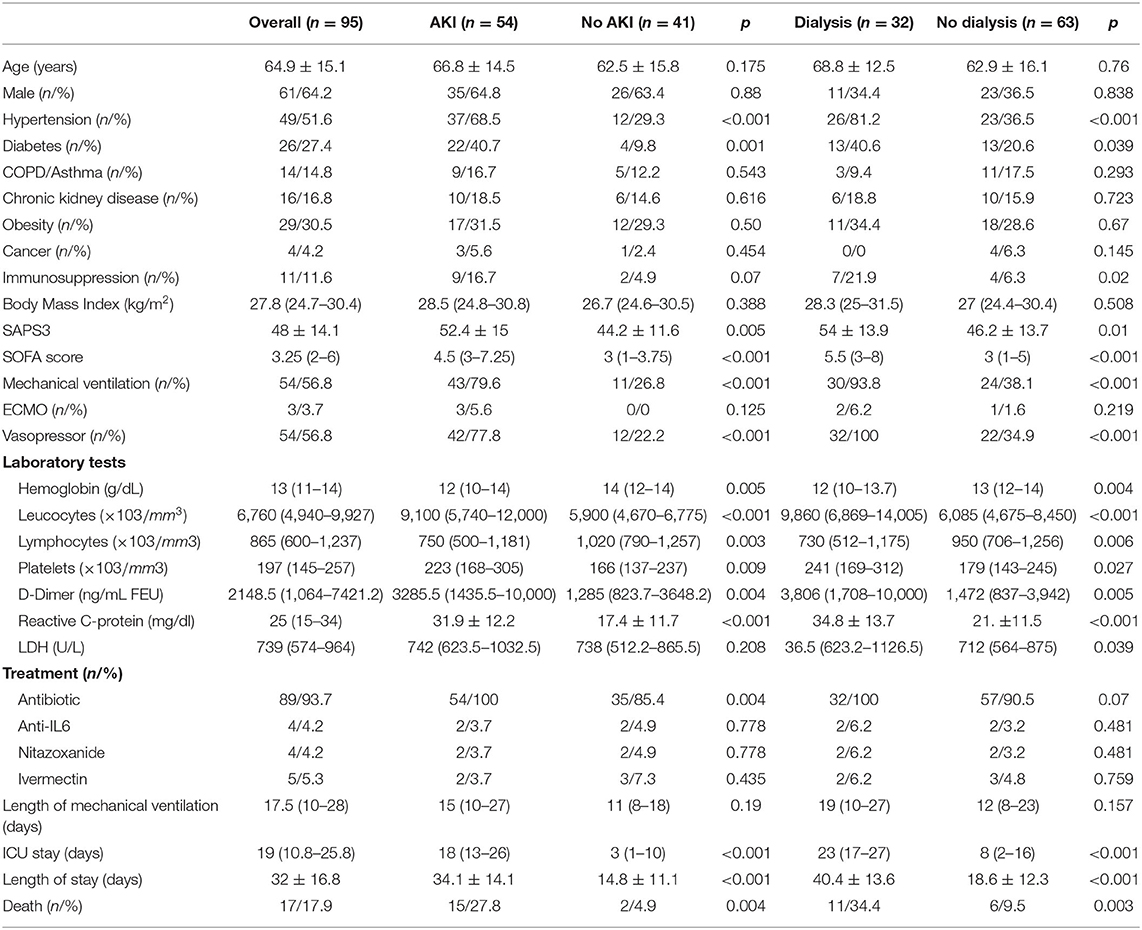
Table 1. Comparative clinical data, laboratory tests, and treatment in patients who presented with acute kidney injury and need of dialysis.
Patients who developed AKI were more frequently hypertensive (68.5 vs. 29.3%; p < 0.001) and diabetic (40.7 vs. 9.8%; p = 0.001) and received mechanical ventilation (79.6 vs. 26.8%; p < 0.001) and vasoactive drugs (77.8 vs. 22.2%; p < 0.001) more frequently when compared with those that did not (Table 1), therefore reflecting a higher severity that was confirmed through the worst SAPS3 (52.4 vs. 44.2; p = 0.005) and SOFA (4.5 vs. 3; p < 0.001) scores. Regarding their lab tests, they had the lowest hemoglobin results (12 vs. 14 g/dL; p = 0.005), the highest lymphopenia results (750 vs. 1,020/mm3; p = 0.003), and the highest D-dimer (3285.5 vs. 1,285 ng/mL FEU; p = 0.004) and CRP (31.9 vs. 17.4 mg/dL; p < 0.001) values. The adopted therapies included receiving antibiotics (100 vs. 85.4%; p = 0.004) more frequently. Being diagnosed with AKI increased the ICU stay (18 vs. 3 days; p < 0.001), hospital stay (34.1 vs. 14.8 days; p < 0.001), and increased the mortality rates (27.8 vs. 4.9%; p = 0.004).
Patients requiring hemodialysis were more frequently hypertensive (81.2 vs. 36.5%; p < 0.001), diabetic (40.6 vs. 20.6%; p = 0.001), and immunosuppressed (21.9 vs. 6.3%; p = 0.02) when compared with those who were not submitted to the procedure (Table 1). There was a higher indication rate of mechanical ventilation in patients under dialytic treatment (93.8 vs. 38.1%; p < 0.001), using vasoactive drugs (100 vs. 34.9%; p < 0.001), and with the worst SAPS3 (54 vs. 46.2; p = 0.01) and SOFA (5.5 vs. 3; p < 0.001) scores. They also had the lowest hemoglobin results (12 vs. 13 g/dL; p = 0.004), the highest lymphopenia results (730 vs. 950/mm3; p = 0.006), and the highest D-dimer (3,806 vs. 1,472 ng/mL FEU; p = 0.005) and CRP (34.8 vs. 21 mg/dL; p < 0.001) values. When hemodialysis was required, it increased the ICU stay (23 vs. 8 days; p < 0.001) and hospital stay (40.4 vs. 18.6 days; p < 0.001) and also increased the mortality rates (34.4 vs. 9.5%; p = 0.003).
From the 32 patients submitted to dialytic therapy, 26 (81.3%) underwent continuous renal replacement therapy and six (18.7 %) received intermittent hemodialysis with the average dialysis time being 11 ± 7 days. Furthermore, 11 (34.4%) of the patients receiving dialysis died, and 13 (40.62%) from the remaining 21 (65.6%) experienced renal function recovery and maintained an estimate glomerular filtration rate using CKD-EPI = 23 (18–50 ml/min/1.73 m2) until hospital discharge, and another 8 (25.6%) remained dialysis-dependent.
When we analyzed patients who died and compared them with those who were still alive (Table 2), those who died were older (78.6 vs. 61.9 years of age; p = 0.04), more frequently diabetic (47.1 vs. 23.1%; p = 0.04), immunosuppressed (29.4 vs. 7.7%; p = 0.01), and received mechanical ventilation (88.8 vs. 72.2%; p < 0.004), and vasoactive drugs (94.1 vs. 48.7%; p = 0.001) more frequently; they also developed AKI (88.2 vs. 50%; p = 0.004), and hemodialysis was required (54.7 vs. 26.9%; p = 0.003) more frequently; therefore reflecting the worst SAPS3 (62.8 vs. 45.8; p < 0.001) and SOFA (8 vs. 3; p < 0.001) scores, respectively. They had the lowest hemoglobin results (11.8 vs. 13 g/dL; p = 0.01), the highest lymphopenia results (650 vs. 940/mm3; p = 0.01), and the highest CRP (31.9 vs. 24 mg/dL; p < 0.02) values. There were no differences regarding the adopted therapies.
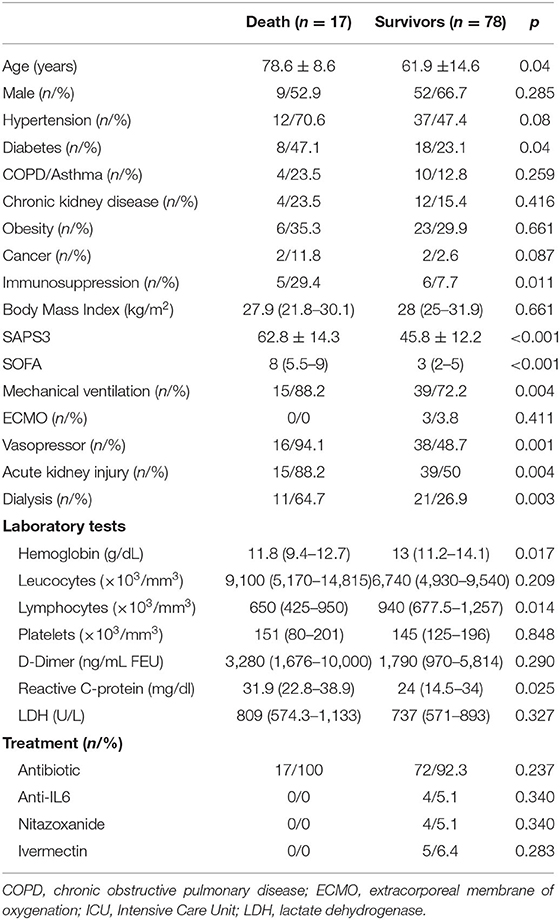
Table 2. Comparative clinical data, laboratory tests, and treatment in patients who had undergone death and survivors.
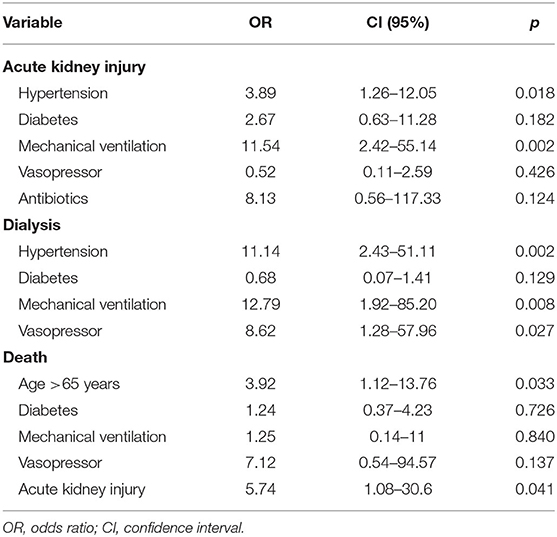
In the multivariate multinomial logistic regression analysis (Table 3), it was predicted that patients who developed AKI more commonly had a previous diagnosis of hypertension (OR: 3.89; CI: 1.26–12.05; p = 0.018) and need of mechanical ventilation (OR: 11.54; CI: 2.42–55.14; p = 0.002); in addition, hypertension (OR: 11.14; CI: 2.43–51.11; p = 0.002), mechanical ventilation (OR: 12.79; CI: 1.92–85.20; p = 0.008), and the use of vasopressor (OR: 8.62; CI: 1.28–57.96; p = 0.027) were more frequent in patients who required hemodialysis therapy. Age >65 years (OR: 3.92; CI: 1.12–13.76; p = 0.03) and AKI (OR: 5.74; CI: 1.08–30.6; p = 0.04) were independent risk factors for death.
Discussion
Several kidney problems associated with COVID have been previously described. The main problem is in the acute tubular injury spectrum (11, 12). Moreover, collapsing glomerulopathy (13–19) and thrombotic microangiopathy (20) cases have been described in the glomerulopathy spectrum. Other issues that have already been described are renal infarction (21, 22), acute pyelonephritis, rhabdomyolysis, and lymphocytic infiltrates (23).
It is claimed that renal injury mechanisms associated with COVID-19 involve inflammatory phenomena, crosstalk with other organs concurrently affected, and complications associated with organic dysfunction treatments. A phenomenon called a “cytokine storm” is characterized as a massive inflammatory cytokine release, mainly IL-6, therefore resulting in systemic vasodilation with increased vascular and endothelial permeability, which may ultimately lead to AKI and shock. Endothelial injury can also lead to dysfunctions in different organs, such as the heart and lungs, muscle injury, and rhabdomyolysis, in turn resulting in renal injury through an organic crosstalking mechanism. Furthermore, hypervolemia, loss of fluid to the third space, and endotoxin release are the last systemic complications described that contribute to AKI associated with viral conditions (24, 25).
The mechanisms of renal injury during COVID-19 are difficult to study due to the interference of several coexisting factors, such as polypharmacy, hypoxia, and cytokine storm. Just as in severe acute respiratory syndrome coronavirus, SARS-CoV-2 virus entry into target cells is facilitated by the presence of angiotensin-converting enzyme 2 (ACE2) expressed in respiratory cells as well as renal tubular cells and podocytes, making the hypothesis of a direct renal infection particularly intriguing (26).
Interestingly, viral nephropathies with direct tubular infection and viral replication within epithelial cells (i.e., BK virus nephropathy) are usually associated with substantial viruria due to the direct release of viral particles in the tubular lumen following cytopathic cell injury. Thus far, there is no solid evidence of a correlation between urinary levels of SARS-CoV-2 viral copies and the degree of kidney injury in patients with COVID-19 (27). Moreover, the presence of viral particles by pure morphologic evaluation (EM) does not by itself demonstrate a direct cytopathic effect nor a replicative potential because it only supports viral entry. More specific morphologic correlates of viral cytopathy (nuclear atypia/dysmorphology and syncytia) have not yet been shown.
A study analyzing the transcriptomics of renal cells in patients with COVID-19 (28) also demonstrates that the cytopathic virus effect may occur directly in proximal tubular cells and podocytes, therefore contributing to the onset of the disease through hyperexpression and recruitment of angiotensin-converting enzyme 2 (ACE2) and cellular transmembrane serine proteases (TMPRSSs), which are crucial for the virus internalization in affected cells.
In our study, the impact of AKI in the prognosis of critically ill patients with COVID is expressive. This finding has already been reported in a Chinese case study published by Cheng et al. (29) and in data from a retrospective study by 13 hospitals in the United States (30). The required renal replacement therapy results in poorer outcomes for these patients. Dialysis caused an almost ten-fold mortality increase when compared with patients without AKI in both studies. In our study, the presence of comorbidities (such as systemic hypertension and diabetes mellitus) was correlated with renal injury, therefore depicting the poor, preexisting conditions of these patients. In addition, the required mechanical ventilation and the use of vasopressor support in our data were important for the development of renal injury, thus expressing the serious conditions of the patients.
Another analysis of 3,099 ICU patients diagnosed with COVID-19 from 67 hospitals in the United States (31) revealed that 637 (20.6%) patients required dialysis. The mortality rate of those who needed renal replacement therapy was 63.3 and 33.8% were discharged from hospital still requiring dialysis. Moreover, in a follow-up 60 days after ICU admission, 18.1% of discharged patients were still maintained on regular dialysis.
In an English multicentric series (32) of 1,032 inpatients, of which 165 were in the ICU, the incidence of AKI in critical patients was 44.2% with a higher mortality in patients with AKI compared with non-AKI patients (52.1 vs. 22.8, p < 0.001) with similar results observed in French patients (33). In a Brazilian single-center study (34) with 201 ICU patients, 101 (50.2%) developed AKI and 32 (17%) required dialysis. The overall mortality was 14.4%, being higher in the AKI group of patients.
Risk factors for AKI in critical patients with COVID-19 vary according to the population. Older age, male gender, black race, obesity, diabetes, hypertension, heart failure, chronic kidney disease, leukocytosis, anemia, lymphopenia, increase in levels of serum inflammatory markers (D-dimer, PCR, and IL-6), and need for mechanical ventilation and vasoactive drugs as well as the use of angiotensin-converting enzyme inhibitors were associated with a higher risk of AKI development in COVID-19 critical patients (30–40). Herein, chronic kidney disease was more frequent in patients who developed AKI (18.5 vs. 14.6%), however with no statistical significance. This finding may be explained by the small number of patients with previous CKD diagnosis (16 patients) in addition to the fact that the diagnosis of CKD may reflect changes in renal function and/or evidence of structural changes with preserved renal function (41). Another interesting fact from our cohort is that some patients who developed AKI (15.3%), even reaching KDIGO 3 by the creatinine increase criteria, did not require renal replacement therapy because they did not present oliguria/anuria and some even polyuria. Due to the preserved diuresis, such patients did not develop uremia, hypervolemia, or other hydroelectrolytic disorders that required dialysis treatment. Regarding risk factors for mortality in COVID-19 patients, another case series (29–31, 34) found a correlation between obesity and mortality. This finding was not evidenced in our cohort; however, only 30.5% of the patients had an obesity diagnosis with most of them having a BMI in the range from 24.7 to 30.4 kg/m2 (p25; p75), differently from those from other series in which the obesity percentage and the BMI values were both higher.
Our study has some limitations, including being performed in a single center, and our data was collected from an electronic chart analysis from fewer than 100 critical patients; however, such factors resulted in standardized actions being taken and information registered in charts. Another limitation is the fact that it was performed in a private hospital; however, private hospitals were the first to receive patients with COVID-19 in Brazil.
Our study brings AKI data for critically ill patients with COVID-19 in a Brazilian center. AKI is a frequent finding, occurring in more than half of the patients, and dialysis is required in one third of the total case study. Previous comorbidities required organic systemic therapy use, lymphopenia, and elevated D-dimer, and CRP levels were associated with increased AKI development risk. Greater mortality rates were observed in older adult patients, and AKI was an independent risk factor for death. Therefore, we have exposed AKI data in critically ill patients with COVID-19 in a case study in Latin America, for which there is scarce data provided until now.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Brazilian Ethics Committee, number 30264920.6.1001.0070. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
PN, VS, and PC: conception or design, analysis and interpretation of data, drafting the article, providing intellectual content, and final approval of the version. SM, ÉO, LP, AB, LN, JF, DM, EB, and AS-J: conception or design, providing intellectual content, and final approval of the version. BF: drafting the article, providing intellectual content, and final approval of the version. AC-N: conception or design, drafting the article, analysis and interpretation of data, providing intellectual content, and final approval of the version. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Weekly. (2020) 2:113–22. doi: 10.46234/ccdcw2020.032
2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
3. Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. (2020) 323:1335. doi: 10.1001/jama.2020.4344
4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
5. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. (2020) 579:270–3. doi: 10.1038/s41586-020-2012-7
6. Cevik M, Bamford CGG, Ho A. COVID-19 pandemic-a focused review for clinicians. Clin Microbiol Infect. (2020) 26:842–7. doi: 10.1016/j.cmi.2020.04.023
7. Rodriguez-Morales AJ, Gallego V, Escalera-Antezana JP, Méndez CA, Zambrano LI, Franco-Paredes C, et al. COVID-19 in Latin America: the implications of the first confirmed case in Brazil. Travel Med Infect Dis. (2020) 35:101613. doi: 10.1016/j.tmaid.2020.101613
8. World Health Organization. Coronavirus Disease (COVID-19) Pandemic (2021). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed April 11, 2021).
9. Al-Jaghbeer M, Dealmeida D, Bilderback A, Ambrosino R, Kellum JA. Clinical decision support for in-hospital AKI. J Am Soc Nephrol. (2018) 29:654–60. doi: 10.1681/ASN.2017070765
10. Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41:1411–23. doi: 10.1007/s00134-015-3934-7
11. Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. (2020) 46:1339–48. doi: 10.1007/s00134-020-06153-9
12. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter Suppl. (2012) 2:1–138. doi: 10.1038/kisup.2011.31
13. Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. (2020) 31:1683–7. doi: 10.1681/ASN.2020040432
14. Rossi GM, Delsante M, Pilato FP, Gnetti L, Gabrielli L, Rossini G, et al. Kidney biopsy findings in a critically Ill COVID-19 patient with dialysis-dependent acute kidney injury: a case against “SARS-CoV-2 nephropathy”. Kidney Int Rep. (2020) 5:1100–5. doi: 10.1016/j.ekir.2020.05.005
15. Gaillard F, Ismael S, Sannier A, Tarhini H, Volpe T, Greze C, et al. Tubuloreticular inclusions in COVID-19–related collapsing glomerulopathy. Kidney Int. (2020) 98:241. doi: 10.1016/j.kint.2020.04.022
16. Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, et al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. (2020) 98:228–31. doi: 10.1016/j.kint.2020.04.006
17. Nasr SH, Kopp JB. COVID-19–associated collapsing glomerulopathy: an emerging entity. Kidney Int Reports. (2020) 5:759–61. doi: 10.1016/j.ekir.2020.04.030
18. Peleg Y, Kudose S, D'Agati V, Siddall E, Ahmad S, Nickolas T, et al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Reports. (2020) 5:940–5. doi: 10.1016/j.ekir.2020.04.017
19. Larsen CP, Bourne TD, Wilson JD, Sagga O, Sharshir MA. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19). Kidney Int Rep. (2020) 5:935–9. doi: 10.1016/j.ekir.2020.04.002
20. Jhaveri KD, Meir LR, Flores Chang BS, Parikh R, Wanchoo R, Barilla-LaBarca ML, et al. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. (2020) 98:509–12. doi: 10.1016/j.kint.2020.05.025
21. Tascón GC, Chiriboga DES, Ramos RL, Díaz DD, Ruiz CR, Procaccini FL, et al. Renal infarction in a patient with active COVID−19 infection. Nefrologia. (2021) 41:84–7. doi: 10.1016/j.nefroe.2020.04.005
22. Post A, den Deurwaarder ESG, Bakker SJL, de Haas RJ, van Meurs M, Gansevoort RT, et al. Kidney infarction in patients with COVID-19. Am J Kidney Dis. (2020) 76:431–5. doi: 10.1053/j.ajkd.2020.05.004
23. Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. (2020) 98:219–27. doi: 10.1016/j.kint.2020.04.003
24. Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. (2020) 16:308–10. doi: 10.1038/s41581-020-0284-7
25. Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. (2020) 8:738–42. doi: 10.1016/S2213-2600(20)30229-0
26. Delsante M, Rossi GM, Gandolfini I, Bagnasco SM, Rosenberg AZ. Kidney involvement in COVID-19: need for better definitions. JASN. (2020) 31:1380–3. doi: 10.1681/ASN.2020050630
27. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. (2020) 323:1843–34. doi: 10.1001/jama.2020.3786
28. Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. (2020) 46:1114–6. doi: 10.1007/s00134-020-06026-1
29. Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. (2020) 97:829–38. doi: 10.1016/j.kint.2020.03.005
30. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. (2020) 98:209–18. doi: 10.1016/j.kint.2020.05.006
31. Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, et al. AKI treated with renal replacement therapy in critically Ill patients with COVID-19. J Am Soc Nephrol. (2021) 32:161–76. doi: 10.1681/ASN.2020060897
32. Hamilton P, Hanumapura P, Castelino L, Henney R, Parker K, Kumar M, et al. Characteristics and outcomes of hospitalized patients with acute kidney injury and COVID-19. PLoS ONE. (2020) 15:e0241544. doi: 10.1371/journal.pone.0241544
33. Rubin S, Orieux A, Prevel R, Garric A, Bats ML, Dabernat S, et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J. (2020) 13:354–61. doi: 10.1093/ckj/sfaa099
34. Doher MP, Torres de Carvalho FR, Scherer PF, Matsui TN, Ammirati AL, Caldin da Silva B, et al. Acute kidney injury and renal replacement therapy in critically Ill COVID-19 patients: risk factors and outcomes: a single-center experience in Brazil. Blood Purif. (2020) 1–11. doi: 10.1159/000513425. [Epub ahead of print].
35. Kant S, Menez SP, Hanouneh M, Fine DM, Crews DC, Brennan DC, et al. The COVID-19 nephrology compendium: AKI, CKD, ESKD and transplantation. BMC Nephrol. (2020) 21:449. doi: 10.1186/s12882-020-02112-0
36. Lin L, Wang X, Ren J, Sun Y, Yu R, Li K, et al. Risk factors and prognosis for COVID-19-induced acute kidney injury: a meta-analysis. BMJ Open. (2020) 10:e042573. doi: 10.1136/bmjopen-2020-042573
37. Kellum JA, van Till JWO, Mulligan G. Targeting acute kidney injury in COVID-19. Nephrol Dial Transplant. (2020) 35:1652–62. doi: 10.1093/ndt/gfaa231
38. Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, et al. COVID-19-associated acute kidney injury: consensus report of the 25th acute disease quality initiative (ADQI) workgroup. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. (2020) 16:747–64. doi: 10.1038/s41581-020-00372-5
39. Cheng Y, Luo R, Wang X, Wang K, Zhang N, Zhang M, et al. The incidence, risk factors, and prognosis of acute kidney injury in adult patients with coronavirus disease 2019. Clin J Am Soc Nephrol. (2020) 15:1394–402. doi: 10.2215/CJN.04650420
40. Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. (2020) 16:14–25. doi: 10.2215/CJN.09610620
Keywords: acute kidney injury, COVID-19, intensive care unit, SARS-CoV-2, dialysis, continuous renal replacement therapy
Citation: Neves PDMdM, Sato VAH, Mohrbacher S, Ferreira BMC, Oliveira ÉS, Pereira LVB, Bales AM, Nardotto LL, Ferreira JN, Machado DJ, Bassi E, Silva-Júnior A, Chocair PR and Cuvello-Neto AL (2021) Acute Kidney Injury Due to COVID-19 in Intensive Care Unit: An Analysis From a Latin-American Center. Front. Med. 8:620050. doi: 10.3389/fmed.2021.620050
Received: 21 October 2020; Accepted: 16 April 2021;
Published: 04 June 2021.
Edited by:
Alain Le Moine, Université Libre de Bruxelles, BelgiumReviewed by:
Joelle L. Nortier, Université Libre de Bruxelles, BelgiumRichard Amerling, St. George's University, Grenada
Copyright © 2021 Neves, Sato, Mohrbacher, Ferreira, Oliveira, Pereira, Bales, Nardotto, Ferreira, Machado, Bassi, Silva-Júnior, Chocair and Cuvello-Neto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Precil Diego Miranda de Menezes Neves, precilmed61@yahoo.com.br
†These authors have contributed equally to this work
 Precil Diego Miranda de Menezes Neves
Precil Diego Miranda de Menezes Neves Victor Augusto Hamamoto Sato1†
Victor Augusto Hamamoto Sato1†  David José Machado
David José Machado