- Department of Critical Care Medicine, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
Sepsis represents a global health concern, and patients with severe sepsis are at risk of experiencing MODS (multiple organ dysfunction syndrome), which is associated with elevated mortality rates and a poorer prognosis. The development of sepsis involves hyperactive inflammation, immune disorder, and disrupted microcirculation. It is crucial to identify targets within these processes to develop therapeutic interventions. One such potential target is Panx1 (pannexin-1), a widely expressed transmembrane protein that facilitates the passage of molecules smaller than 1 KDa, such as ATP. Accumulating evidence has implicated the involvement of Panx1 in sepsis-associated MODS. It attracts immune cells via the purinergic signaling pathway, mediates immune responses via the Panx1-IL-33 axis, promotes immune cell apoptosis, regulates blood flow by modulating VSMCs’ and vascular endothelial cells’ tension, and disrupts microcirculation by elevating endothelial permeability and promoting microthrombosis. At the level of organs, Panx1 contributes to inflammatory injury in multiple organs. Panx1 primarily exacerbates injury and hinders recovery, making it a potential target for sepsis-induced MODS. While no drugs have been developed explicitly against Panx1, some compounds that inhibit Panx1 hemichannels have been used extensively in experiments. However, given that Panx1’s role may vary during different phases of sepsis, more investigations are required before interventions against Panx1 can be applied in clinical. Overall, Panx1 may be a promising target for sepsis-induced MODS. Nevertheless, further research is needed to understand its complex role in different stages of sepsis fully and to develop suitable pharmaceutical interventions for clinical use.
1 Introduction
Sepsis, a life-threatening condition triggered by a dysregulated immune response, arises from severe infections, trauma, burns, shock, and major surgeries. This global health concern accounts for approximately 50 million cases and 11 million deaths yearly (1). Among patients with severe sepsis, the development of MODS leads to a poor prognosis (2). Those diagnosed with sepsis and MODS exhibit significantly higher mortality and rehospitalization rates than those without MODS (3). To improve clinical management and devise novel therapeutics, it’s crucial to comprehend the mechanisms underlying MODS in sepsis. However, despite ongoing research, viable therapeutic targets for septic MODS have yet to be identified.
The Panx (pannexin) protein family is found in vertebrates, first identified in 2000 (4). Panx and hemichannel Cx (connexin) function as unselective channels in vertebrates. Initially considered a transmembrane channel due to extracellular glycosylation modification (5), Panx1 was later found to form intercellular cell-to-cell channels, indicating its role as a hemichannel (6). The Panx family comprises three members: Panx1, widely expressed in various tissues such as eyes, kidneys, liver, CNS (central nervous system), vascular endothelium, alveolar epithelium, and immune cells; Panx2, predominantly found in CNS with subcellular localization on intracellular membranes; and Panx3, exclusively detected in skin and osteoblasts (7, 8). Panx1 acts as a non-selective hemichannel, allowing the passage of molecules smaller than 1 KDa, including ATP (adenosine-triphosphate) and dye molecules (9). Its involvement spans various physiological and pathological processes across different cell types. Hypoxia and mechanical stress can activate Panx1 hemichannels on erythrocytes, leading to ATP release, which, in turn, dilates vascular endothelium through the purinergic signaling pathway (10, 11). In neurons under hypoxic and glucose-deficient conditions, NMDAR (N-methyl-d-aspartate receptor) signaling or K+ current activates SFK (Src kinase), which phosphorylates Panx1, resulting in agonistic toxicity and impaired neuronal recovery in inflammatory conditions (12, 13). Additionally, Panx1 involves tumor migration and metastasis, as mechanical forces on tumor cells induce the opening of Panx1 hemichannels on vascular endothelium, leading to ATP release that promotes metastasis (14). Elevated Panx1 levels are associated with a poor prognosis in certain cancers (15).
Studies have demonstrated Panx1’s role in inflammation (16, 17). At the organ level, Panx1 has been implicated in the inflammatory response in various organs, including the heart, brain, lungs, kidneys, and liver (18–22). Furthermore, Panx1’s involvement in developing systemic inflammatory disorders, such as sepsis, has been investigated (23, 24). However, it’s not clear whether Panx1 is potential as a therapeutic target for sepsis-induced MODS. This article aims to provide an overview of Panx1 in inflammation, immunosuppression, and microcirculation, as well as its role in the inflammatory damage of multiple organs. This article aims to shed light on Panx1’s potential therapeutic value against sepsis-induced MODS.
2 Panx1’s contribution to hyperinflammation
According to Sepsis-3 criteria, sepsis is characterized as a dysregulated host response to infection, wherein excessive inflammation and immunosuppression coincide; in cases where the body fails to effectively eliminate a high pathogen load, an exaggerated inflammatory response ensues, coupled with paradoxical anti-inflammatory regulation. The early phase of sepsis exhibits hyperinflammation involving multiple cell types and complex networks. While Panx1 is not directly engaged in the initial process, it plays a significant role by releasing signaling molecules like ATP, which can contribute to the proinflammatory response. Specifically, Panx1 promotes inflammasome activation (16) and facilitates the migration of immune cells (25) (Figure 1). It is also involved in releasing neutrophil extracellular traps via the purinergic signaling pathway (26, 27).
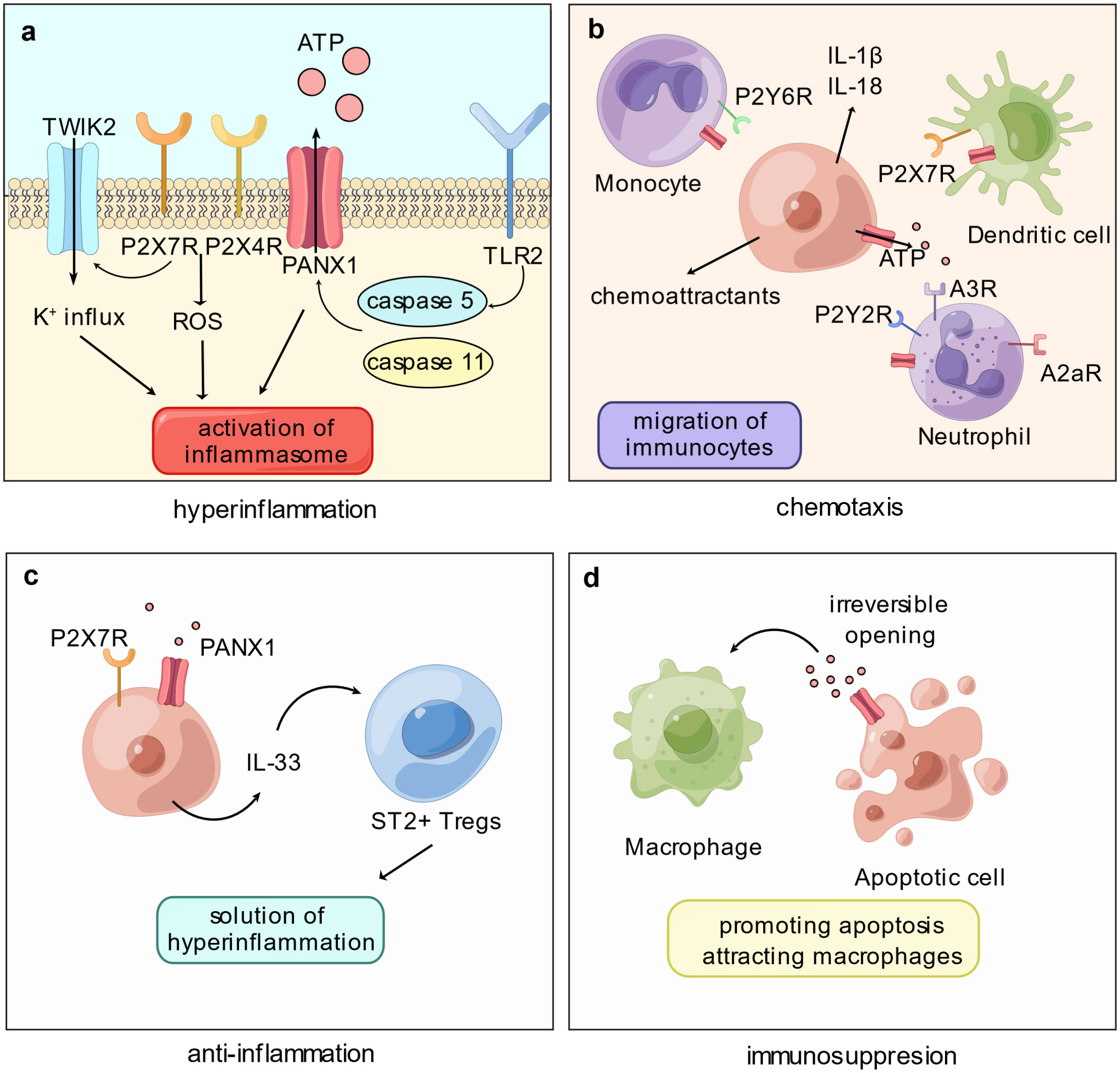
Figure 1 Panx1 participates in immune disorder by proinflammation and immunosuppression. (A) The proinflammatory role of Panx1 is associated with activation of the inflammasome. Cleavage of Panx1 by caspase proteins, such as caspase-5 or -11, opens Panx1 and leads to downstream signaling pathways that activate inflammasome: Purinergic stimulation on P2X7R and P2X4R promotes the production of cytoplasmic ROS; P2X7R mediates the K+ influx via TWIK2 channel. (B) Opening of Panx1 recruits immune cells mainly via the purinergic signaling pathway. ATP released via Panx1 attracts monocytes, neutrophils, and dendritic cells, meanwhile Panx1 on immune cells mediates migration. Release of proinflammatory molecules or chemoattractants promoted by Panx1 hemichannel activation, such as IL-1β and IL-18, certainly elevates immunocyte migration. (C) Panx1 contributes to immunosuppression by mediating the production of IL-33. As an immunoregulatory molecule, IL-33 binds ST2+ Tregs and assists resolution of hyperinflammation. (D) Activation of Panx1 hemichannel elevates apoptosis level of immune cells, promoting immune paralysis. Further, ATP released by Panx1 acts as “find-me” signals and attracts macrophages.
2.1 Panx1’s role in inflammasome activation
In sepsis, the delicate balance between protective and detrimental inflammation becomes crucial, as excessive inflammation can damage tissue. Inflammasome activation plays a vital role in hyperinflammation during sepsis, as it triggers the production and release of proinflammatory cytokines, such as IL-1β (Interleukin 1β) and IL-18 (Interleukin 18), and induces inflammatory cellular death, including pyroptosis and necroptosis. The inflammasome is a protein complex that responds to infection or damage. Cytoplasmic receptors like NLRP3 (NOD-like receptor thermal protein domain associated protein 3) recruit ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) to activate caspase-1 (28). In contrast, LPS (lipopolysaccharides) can activate caspase-11 in murine or caspase-4/5 in humans in a non-canonical manner, leading to the activation of NLRP3 and caspase-1 (29). Mature caspase-1 cleavages IL-1β and IL-18 into maturity, both of which play critical roles in the early phase of sepsis, with elevated levels exacerbating the inflammatory response (30). Moreover, downstream inflammatory cellular death can lead to tissue injury and further amplify inflammation in sepsis (31).
Panx1 has been found to promote inflammasome activation via the purinergic signaling pathway (16). However, the mechanism linking Panx1 to downstream NLRP3 activation has yet to establish fully. The prevailing hypothesis suggests that Panx1 activates inflammasome by releasing ATP and activating P2X7 receptors, which are ATP-gated ion channels. Although initially believed to be a K+ efflux channel (32), recent studies have shown that P2X7 receptors only allow the influx of Ca2+ and Na+ upon activation, and the K+ current via TWIK2 (two-pore domain weakly inwardly rectifying K+ channel 2), promoted by P2X7 receptors, activates NLRP3 (33). Moreover, inflammasome activation is associated with producing cytoplasmic ROS (reactive oxygen species), which is enhanced by P2X7 and P2X4 receptors upon ATP signaling from Panx1 (34, 35). Since P2X7 and P2X4 receptors form heterodimeric trimers, they are structurally and functionally interdependent (36, 37), suggesting that the inflammasome activation by Panx1 and P2X7 receptors may also involve P2X4 receptors.
In addition to NLRP3, Panx1 may also be involved in the activation of the non-canonical inflammasome, such as NLRP1, which is associated with the upregulation of M1 macrophages (the proinflammatory phenotype of macrophages) (38), as well as neuronal aging (39). Additionally, Panx1 has been found to promote AIM2 (absent in melanoma 2) activation, contributing to neuronal damage in conditions like subarachnoid hemorrhage (40), myocardial death in heart failure (41), pyroptosis of retinal cells due to ocular hypertension (42), and inflammatory response of Kupffer cells following hepatic ischemia-reperfusion injury (43). Furthermore, Panx1’s involvement in the non-classical pathway is evident, where murine caspase-11 cleaves the C-terminus of Panx1, leading to NLRP3 activation (21). Moreover, TLR2 interactions with human-derived caspase-5 can also lead to Panx1 cleavage and promote an inflammatory response (44, 45).
The role of Panx1 in inflammasome activation and pyroptosis has been a topic of debate among researchers. Some studies suggest that Panx1 likely plays a role in inflammasome activation, supported by experiments using Panx1 hemichannel inhibitors like probenecid, which have demonstrated inhibitory effects on inflammation responses (40, 46). However, this method may be limited, as probenecid inhibits P2X7 receptors (47). On the other hand, some experiments have demonstrated that inflammasome can still be activated in macrophages even in the absence of Panx1, suggesting that under certain conditions, Panx1 may not be a mandatory component for inflammasome activation (48, 49). To gain a comprehensive understanding, more extensive and detailed studies are required to fully elucidate the involvement of Panx1 in inflammasome activation and pyroptosis.
2.2 Panx1’s function in mediating immune cell chemotaxis
Chemokines, such as C5a and leukotrienes, play a pivotal role in orchestrating immune response by driving immune cells, especially neutrophils, from bone marrow or blood to sites of inflammation or organs during uncontrolled inflammatory responses (50). The degree of immune cell infiltration in organs strongly correlates with organ damage and mortality in sepsis. Consequently, interventions targeting chemotaxis hold promise as potential therapeutic strategies to mitigate organ injury and enhance survival rates (51–53). Immune cells, like neutrophils, employ an amoeboid mode of migration characterized by minimal adherence to the extracellular matrix, largely dependent on cytoskeletal contractility and mechanosensitive channels (54). Panx1 emerges as a crucial player in purinergic and Ca2+ signaling and cytoskeleton control within this complex regulatory network. This section provides a concise overview of Panx1’s involvement in chemotaxis, with more comprehensive insights available in Harcha’s review (17).
In the context of neutrophil migration, Panx1 localizes at the leading edge of the cellular membrane alongside F-actin (55). By releasing ATP, Panx1 indirectly influences neutrophil movement bidirectionally. The purinergic pathway, mediated by Panx1, exerts a dual role in regulating neutrophil behavior: ATP activates polarly distributed P2Y2 receptors, while adenosine, derived from ATP, stimulates A3 receptors, creating an autocrine regulatory network that enhances gradient sensitivity and sustains cell polarity (55, 56). At the rear of neutrophils, adenosine interacts with A2a receptors, counteracting agonistic signaling and triggering the cAMP/PKA pathway (57).
In DCs (dendritic cells), Panx1 collaborates with P2X7 receptors to form an autocrine loop. In the presence of elevated extracellular ATP levels, P2X7 receptors activate and induce Ca2+ influx, leading to Panx1 hemichannel opening and consequent ATP release. This process triggers the activation of CaMKII (calmodulin-dependent protein kinase II), ultimately altering cytoskeletal structure, and enabling rapid DC migration (25). Panx1 connects the purinergic pathway with Ca2+ signaling in DCs via CaMKII, which can directly open Panx1 hemichannels (58).
While monocytes and macrophages share a common origin and exhibit similar markers, the exact involvement of Panx1 in monocyte migration remains somewhat enigmatic. Monocytes in the bloodstream can differentiate into either DCs or macrophages. In an ATP-mediated autocrine loop, they release ATP to activate P2Y6 receptors in response to CCL2-induced (C-C motif ligand 2) chemotaxis (59). The specific role of Panx1 in monocyte migration has yet to be entirely elucidated, even though both Cx and Panx1 are expressed in these cells (60). Panx1 on apoptotic cells serves as a “find-me” signal by releasing ATP to attract macrophages. Nevertheless, it remains unclear whether Panx1 on macrophages also actively mediates chemotaxis. Prior investigations have suggested inhibiting Panx1 hemichannels can impede microglial migration (macrophages within the nervous system) and reduce their numbers at the injury site (61). However, this effect may be associated with reduced levels of pro-inflammatory cytokines (62). Further investigations are warranted to fully comprehend the intricate role of Panx1 in mediating immune cell chemotaxis.
3 Panx1’s involvement in immunosuppression
Sepsis presents a complex immune landscape characterized by both hyperactive inflammation and immunosuppression. During the late phase of sepsis, the body’s ability to eliminate pathogens is hindered by abnormal immune system suppression, resulting in inflammation-related immunosuppression. This factor significantly contributes to secondary infections and the development of MODS, which remains a primary cause of poor prognosis in septic patients (63). While Panx1 has been discussed for its role in fueling inflammatory responses, it also exhibits a dual function in the immune response, as it is involved in mediating Tregs (regulatory T cells) and promoting immune cell death. This suggests that Panx1 actively suppresses immune response (Figure 1).
3.1 Panx1’s anti-inflammatory effects through Treg regulation
In sepsis, an immunosuppressive state ensues, characterized by the release of anti-inflammatory cytokines, immune cell death, T-cell exhaustion, and elevated levels of immunomodulatory cells, notably Tregs (64). Recent research by Medina et al. has shed light on Panx1’s role in mediating Tregs and its capacity to curtail inflammation through the purinergic signaling pathway (65). While extracellular ATP acts as a danger signal to initiate the inflammatory response by activating the inflammasome and recruiting immune cells, its degradation into adenosine by ectonucleotidases CD39 and CD73 can help limit inflammation and regulate immune responses. Adenosines facilitate intercellular communication between Tregs and Teffs (effector T cells), inhibiting Teff cell proliferation within the airway. This study underscores the significance of Panx1-dependent crosstalk between Treg and Teff cells in mitigating inflammation.
The Panx1-IL-33 (interleukin-33) axis is crucial in resolving excessive inflammation in the liver (66). Upon LPS stimulation, hepatic cells release ATP, activating P2X7 receptors and producing IL-33, a member of the IL-1 protein family that is not activated by caspase-1-dependent cleavage. This cytokine fosters the recruitment of liver-infiltrating Tregs expressing its receptor, ST2, thereby resolving hyperinflammation in sepsis (66). Notably, in liver transplantation models infected with MRSA (methicillin-resistant Staphylococcus aureus), the Panx1-IL-33 axis is distinct in enhancing bacterial elimination. Panx1-mediated purinergic signals activate P2X2 receptors, leading to IL-33 production in hepatocytes. Consequently, this axis recruits macrophages and neutrophils, effectively reducing MRSA infection (67). These studies highlight the diverse mechanisms by which the Panx1-IL-33 axis regulates immune responses in the liver, with low levels of Panx1 potentially limiting its immunoregulatory effects.
Moreover, studies have suggested that Panx1 may also influence the population of infiltrating phagocytes during peritonitis development, further emphasizing its involvement in immunomodulation (68). As previously mentioned, Panx1 acts as an upstream modulator, influencing pro-inflammatory and anti-inflammatory responses via the purinergic pathway. However, given the intricate nature of this regulatory network, the mechanisms underlying Panx1’s anti-inflammatory effects are not yet fully elucidated, underscoring the need for further research in this area.
3.2 Panx1’s contribution to immunosuppression through immunocyte death
In sepsis, the impairment of effector immune cells through various forms of programmed cell death exacerbates the immunosuppressive state. Pyroptosis, apoptosis, autophagy, and ferroptosis have been observed in immune cells during sepsis, collectively contributing to compromised immune function and hindering the body’s ability to combat infections (64). While the relationship between Panx1 and autophagy has yet to be definitively established (69), a study by Su et al. has reported a potential role of Panx1 in promoting ferroptosis of human renal cells during ischemia-reperfusion injury of the kidney (70). However, further investigations are needed to elucidate these connections fully.
Pyroptosis, a lytic and pro-inflammatory type of cell death, is characterized by cell swelling, pore formation, and rapid disruption of membrane integrity. It is activated by the inflammasome (71), and as discussed earlier, Panx1 may promote pyroptosis by activating the inflammasome (35, 72). Yang et al. reported that Panx1 promotes pyroptosis of macrophages upon LPS challenge, where cytosolic LPS induces caspase-11-dependent cleavage of Panx1, activating downstream P2X7 receptors (31). High levels of pyroptosis in immune cells impair the host’s ability to eliminate infections and worsen the overall condition (73). Notably, ablation of PANX1 has been shown to reduce the level of pyroptosis and enhance resistance to sepsis.
In contrast, apoptosis is a non-inflammatory type of programmed cellular death frequently observed during sepsis. During apoptosis, the C-terminus of Panx1 undergoes irreversible cleavage, a critical functional structure required for Panx1 activity (74). The effector proteins caspase-3/7 shear the C-terminus of Panx1, particularly the DVVD region at the C-terminus, as reported by Chekeni et al. (75), resulting in a sustained increase in Panx1 hemichannel activity, ultimately promoting apoptosis (49, 75–77). Furthermore, during apoptosis, Panx1 releases ATP as “find-me” signals, which attract macrophages to efficiently clear apoptotic cells (75, 78). Studies have indicated that Panx1 participates in Fas-induced apoptosis of leukocytes via the caspase-8/Panx1/P2X7R signaling cascade, wherein caspase-8 induces the cleavage of Panx1 and promotes cellular death in Jurkat cells (79). In neurons, elevated levels of Panx1 may exacerbate apoptosis by promoting the TLR2/TLR4/NF-κB pathway (80), mediating Ca2+ current, and upregulating the levels of caspase-3 and Bax, a member of the pro-apoptotic BCL-2 (B cell lymphoma-2) protein family, further contributing apoptosis (81). Nevertheless, whether Panx1 expression levels influence the apoptosis level of immune cells requires further investigation.
4 Panx1’s regulation of blood flow and implications for microcirculation disorder
Under normal physiological conditions, the body carefully regulates microcirculatory blood flow to ensure adequate oxygen supply to tissues. However, in the context of sepsis, the release of a multitude of pro-inflammatory molecules into the bloodstream disrupts microcirculation, encompassing vessels with a diameter of approximately 100 μm, such as arterioles, capillaries, venules, and micro-lymphatics. This disruption affects various crucial elements involved in microcirculation, including endothelial cells, immune cells, red blood cells, white blood cells, and platelets. Consequently, a significant number of capillaries become dysfunctional, and microcirculatory blood flow undergoes abnormal redistribution, contributing to the establishment of organ dysfunction (82).
Notably, Panx1is expressed in various cells of the microcirculation, such as vascular smooth muscle cells (83, 84) and vascular endothelial cells (85). The widespread distribution of the Panx1 protein suggests its potential impact on microcirculation in several ways, such as regulation of blood flow, promotion of microcirculatory thrombosis, and modulation of endothelial permeability (Figure 2).
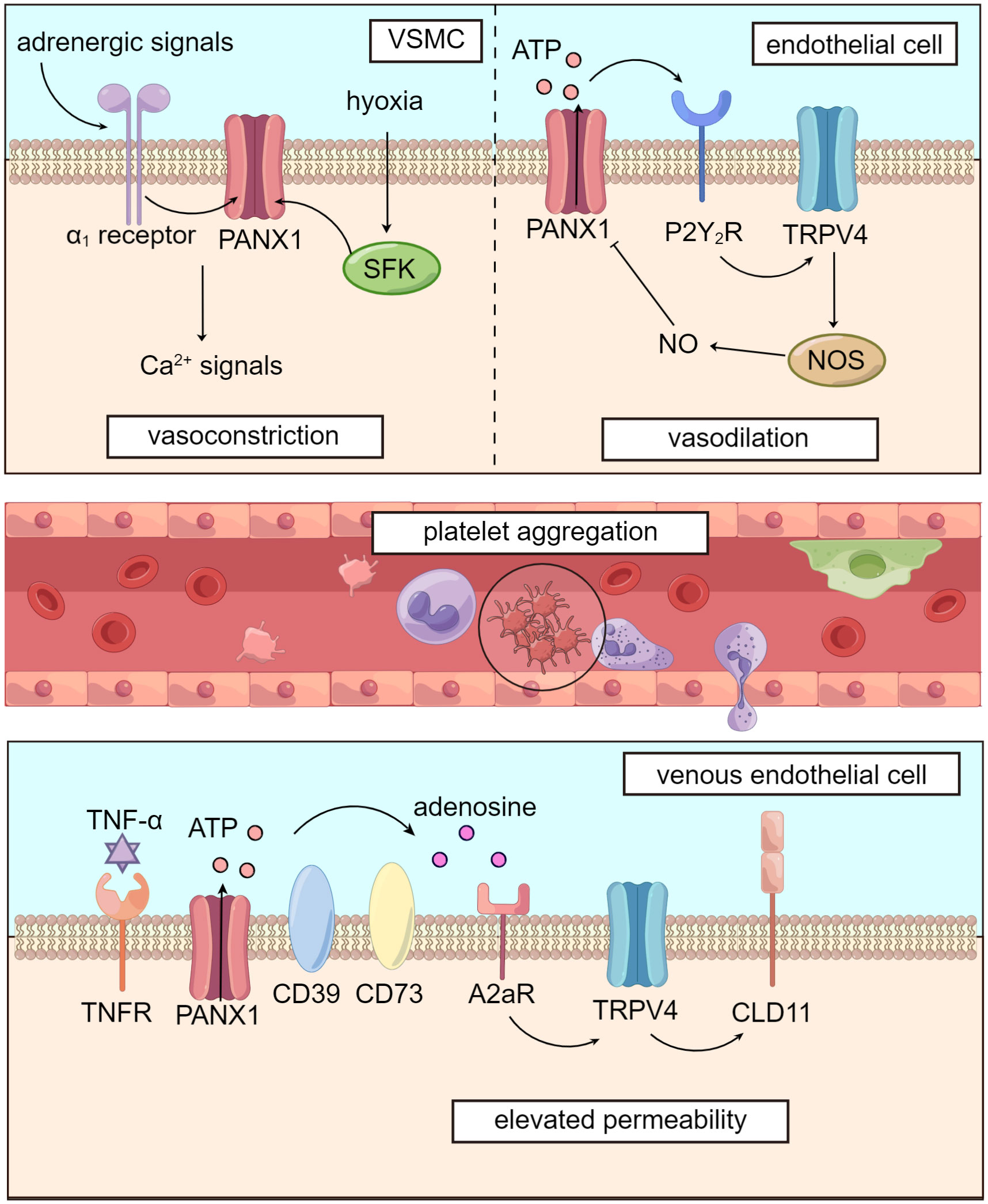
Figure 2 Panx1 mediates blood flow and dampens microcirculation. Panx1 regulates vascular tone in several ways. In VSMCs, adrenergic signals or phosphorylation by SFK opens Panx1 and leads to downstream Ca2+ signals, causing vasoconstriction. In vascular endothelial cells, Panx1 promotes activation of TRPV4 channel via purinergic signaling and enhances activity of NOS, leading to NO-dependent vasodilation. Activation of Panx1 hemichannels, which is widely distributed in the circulatory system, dampens microcirculation by promoting platelet aggregation and elevating venous permeability.
4.1 Panx1’s role in regulating blood flow
Panx1 plays a significant role in regulating blood flow through its influence on vascular tone. In VSMCs (vascular smooth muscle cells), Panx1 is coupled to α1-adrenergic receptors and located on the caveolae of the cell membrane. Panx1 promotes VSMC contraction, following sympathetic adrenergic signals stimulation, resulting in vasoconstriction and increased blood pressure (83, 84). The inhibition of Panx1 hemichannels using specific inhibitors like mefloquine and probenecid has been shown to reduce blood pressure by attenuating adrenergic-mediated vasoconstriction (83).
Panx1 is also involved in blood flow regulation under hypoxic conditions in the pulmonary circulation. Hypoxia induces the phosphorylation of the Tyr198 site of Panx1 in PASMCs (pulmonary artery smooth muscle cells) by SFK. This activation leads to the opening of Panx1 hemichannels and subsequent Ca2+ influx, facilitating pulmonary vasoconstriction and blood transfer from under-ventilated to well-ventilated areas during mechanical ventilation (86). Previous studies have found that TRPV4 (transient receptor potential cation channel subfamily V member 4), a channel that mediates Ca2+ influx, is involved in PASMCs’ response to hypoxia and subsequent vasoconstriction (87). However, Panx1 conducts Ca2+ influx through a TRPV4-independent pathway (86). Further research is needed to verify the TRPV4-independent mechanism by which Panx1 regulates vascular tone in VSMCs, as interactions between Panx1 and TRPV4 appear to participate in regulating vascular tension, as we will explore in subsequent sections.
Panx1 has been implicated in various signaling pathways in regulating vascular endothelial tension, with a particular focus on the NO (nitric oxide) pathway (88, 89). Studies have shown that Panx1 is closely linked with TRPV4, contributing to lowering pulmonary artery pressure (85). In murine PASMCs, Panx1 hemichannels, P2Y2 receptors, and TRPV4 are co-localized on caveolae, forming the Panx1-ATP-P2Y2R-PLC (phospholipase C)-PKCα (protein kinase C α)-TRPV4 pathway (84, 90). This signaling cascade leads to NO-mediated pulmonary artery relaxation, as TRPV4 facilitates Ca2+ influx, which activates NO synthase in vascular endothelial cells (89). Furthermore, this pathway exhibits a negative feedback mechanism: NO-induced S-nitrosylation of specific sites on the Panx1 protein, namely Cys-40, and Cys-346, inhibits Panx1 opening, downregulating the activity of the signaling pathway (91).
Panx1 may also impact NO production for NO-dependent regulation of vascular tone through inhibitory actions on eNOS (endothelial nitric oxide synthase). By modulating phosphorylation levels at Ser-1177 of eNOS, Panx1 can ultimately lead to reduced NO synthesis (92, 93). Additionally, Panx1 may promote vascular relaxation through NO-independent pathways, such as EDH (endothelium-derived hyperpolarization) regulation, as reported by Gaynullina et al. (94).
Beyond its involvement in vascular smooth muscle cells and endothelial cells, Panx1’s widespread distribution extends to erythrocytes, which also influences vasodilation regulation. Studies suggest that in arterioles, ATP released from erythrocytes reaches higher concentrations in the cell-free layer, activating purinergic signaling pathways in vascular endothelial cells (95). This leads to Ca2+ wave propagation and relaxation of upstream VSMCs, ultimately resulting in vessel dilation (10). This supports the notion that hypoxia-induced opening of Panx1 hemichannels dilates blood vessels and modulates tissue perfusion (96). Moreover, erythrocytes may release substantial amounts of ATP in capillaries under the influence of hematocrit. This leads to endothelial cell hyperpolarization, propagating along the capillary network to upstream vessels, thereby regulating oxygen supply (95, 97). This mechanism is reminiscent of the EDH-like regulation described above.
4.2 Panx1 in promoting microthrombosis
During sepsis, the immune response triggered by invading pathogens also activates pathways involved in hemostasis. While coagulation activation can promote innate immunity (98), extensive coagulation during sepsis may lead to microcirculation disruption and even disseminated intravascular coagulation (DIC). Panx1, expressed on human platelets and interacting with P2X1 receptors, plays a crucial role in platelet aggregation by promoting Ca2+ influx (99, 100).
When endothelial cells are damaged, collagen is exposed, activating GPVI (glycoprotein VI) on platelets, which in turn promotes SFK phosphorylation. The interaction between SFK and Panx1 leads to the opening of Panx1 and the subsequent release of ATP. ATP then activates P2X1 receptors on platelets, causing a further influx of Ca2+ and platelet aggregation (99). Notably, the genetic variation of PANX1 400A>C (rs1138800) encodes a gain-of-function with increased platelet reactivity to collagen in healthy individuals, providing additional evidence for the involvement of Panx1 in inducing platelet aggregation (101).
Furthermore, Panx1 on platelets responds to mechanical stress by releasing ATP, inducing inward Ca2+ flow, and promoting platelet aggregation, thereby contributing to arterial thrombosis (99). Deletion of PANX1 prevents the aggregation of platelets (102), underscoring the potential of Panx1 as an inhibitory target for microthrombosis.
Given its role in promoting microthrombosis, Panx1 represents a critical factor in the delicate balance between beneficial coagulation and harmful microcirculation disruption during sepsis. Targeting Panx1 may offer a promising therapeutic approach to mitigate the adverse effects of excessive coagulation and microthrombosis in septic patients. However, further research is needed to fully understand the complex interplay between Panx1, platelet function, and microcirculation in the context of sepsis, ultimately leading to the development of targeted therapies.
4.3 Panx1’s impact on endothelial permeability and leakage
During infections, the endothelial barrier is dynamically regulated to allow leukocyte access to tissues through diapedesis, facilitating pathogen clearance. However, sepsis severely compromises the integrity of the endothelial barrier, leading to persistent permeability elevation, leakage, edema, hemodynamic disorder, and potentially respiratory failure. Panx1 has been identified as a key player in increasing vascular permeability, and intriguingly, its effect appears to be contingent on the type of blood vessel involved.
Studies by the same research group revealed that venous endothelial cells express higher levels of Panx1 and are more susceptible to Panx1-induced permeability elevation than arterial endothelial cells (103, 104). In response to stimulation signals like TNF-α (tumor necrosis factor-α), Panx1 hemichannel is activated through SFK-mediated phosphorylation at the Tyr198 site. This activation opens Panx1, leading to the release of ATP. Extracellular ATP is then broken down into adenosine by ectonucleotidases CD39 and CD73. Adenosine binds to A2 receptors, initiating downstream activation of TRPV4 through the cAMP/PKA pathway. Consequently, the arrangement of CLD11 (claudin 11), a major cadherin in endothelial cells, is disrupted, resulting in increased venous permeability. However, it’s important to note that these experiments were conducted on human umbilical vein endothelial cells. A2 receptors may vary across different endothelial cells, such as those in the alveolar microcirculation (105). Therefore, further research is required to clear the role of this Panx1-involved pathway in the microcirculation of specific organs.
In addition to its impact on venous permeability, Panx1 has been implicated in the inflammatory response of microcirculation-associated cells (20), such as pyroptosis, apoptosis, and immune cell chemotaxis, which can contribute to increases vascular permeability. Several potential mechanisms have been proposed for how Panx1 influences vascular permeability: Panx1 can upregulate VCAM1 (vascular cell adhesion molecule 1) on venous endothelium, promoting immune cell chemotaxis and adhesion (104); Panx1-mediated Ca2+ influx can trigger the NK-κB cascade, leading to inflammation (106); and Panx1 hemichannels on erythrocytes can be activated by fluid shear, promoting gap formation between endothelial cells, further increasing vascular permeability (107).
While PANX1 is expressed in lymphatic vessels, limited studies suggest a possible association between Panx1 in lymphatic vessels and lipid metabolism and atheromatous plaque development (108). However, there is currently no evidence to indicate that Panx1 is directly involved in the clearance of edematous exudate. The understanding of Panx1’s role in endothelial permeability and leakage is an area of ongoing research, and further investigations are needed to comprehensively unravel its involvement in the complex processes underlying sepsis-induced microcirculation disruption.
5 Panx1 in organ dysfunction during sepsis
Sepsis-3 defines sepsis as a dysregulated host response to infection, often resulting in organ failure (109). The organs commonly affected during sepsis are the lungs, kidneys, liver, heart, and brain. The severity and extent of organ failure can vary from patient to patient, with some experiencing mild dysfunction in one or two organ systems. In contrast, others may face multiple organ failures, posing life-threatening situations. Identifying the specific target organs affected during sepsis is crucial for effective management. In this section, we will focus on Panx1’s involvement in various organs and its role in promoting inflammatory injury recognizing that its functions can differ significantly across different organs (Figure 3).
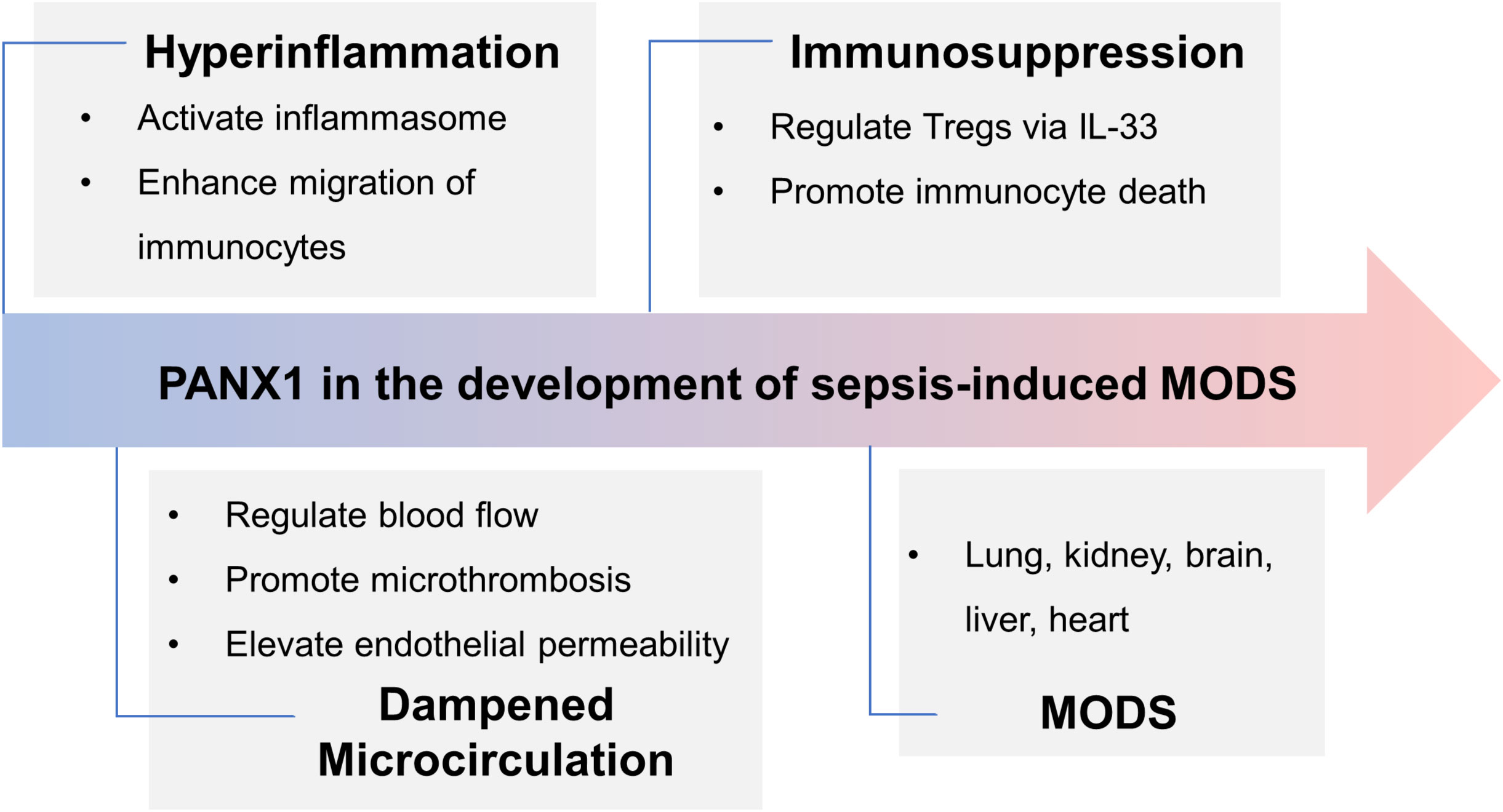
Figure 3 Panx1’s role in the development of sepsis-induced MODS. In the early phase of sepsis, Panx1 contributes to hyperinflammation by activating inflammasome and attracting immunocytes, while it promotes immunosuppression via IL-33-mediated Tregs and immunocyte apoptosis in the late phase. Activation of Panx1 hemichannels dampens microcirculation, aggravating hemodynamic disorder and organ dysfunction. Further, expression of Panx1 in different organs deteriorates injury. Overall, during sepsis, Panx1 contributes to MODS.
5.1 Lungs
Panx1 is expressed on the pulmonary vascular endothelial and epithelial cells (20, 110). Its precise function in lung inflammation remains largely unexplored. Studies have focused on understanding Panx1’s physiological mechanisms in the lungs. For instance, elevated hydrostatic pressure was found to activate Panx1 hemichannels in pulmonary epithelial cells, resulting in the efflux of K+ through the purinergic pathway, indicating a potential role for Panx1 in pressure transduction of non-agitated cells (111). Mechanical stress has been implicated in Panx1 hemichannel’s activation, with piezo 1 channel activation leading to the opening of Panx1 hemichannels on alveolar epithelial type I cells and influencing surfactant secretion by alveolar epithelial type II cells (110).
Given this information, it is reasonable to consider Panx1’s potential involvement in the development of ALI (acute lung injury). The integrity of the alveolar-capillary barrier is crucial for normal blood-air exchange, and any damage to this barrier can result in ALI or ARDS (acute respiratory distress syndrome). ALI can be triggered by inhaled pathogens, mechanical stress, and pro-inflammatory molecules (112). Pulmonary circulation plays a vital role, with arterioles, capillaries, and veins collectively contributing to pulmonary vascular resistance. When insulted by factors such as pulmonary endothelial dysfunction, microthrombosis, altered vascular permeability, vasoactive mediator imbalance, hypoxic pulmonary vasoconstriction, and vascular remodeling (113–115), pulmonary vascular pressure may increase, leading to pulmonary vascular dysfunction and contributing to severe diseases like ARDS and sepsis (116, 117).
During inflammation, pro-inflammatory molecules like TNF-α may activate Panx1 hemichannels in the lung, triggering the activation and recruitment of immune cells in the alveolar microcirculation. Simultaneously, mechanical stress arising from improper mechanical ventilation or autonomous respiratory attempts may activate Panx1 hemichannels in the alveolar microcirculation, leading to pyroptosis and further damaging the alveolar-capillary barrier, exacerbating pulmonary edema and promoting an inflammatory response. Although there is limited research on Panx1’s specific role in ALI, a recent study has suggested its potential involvement in pulmonary ischemia-reperfusion injury, pointing toward its potential therapeutic significance in treating ALI (20).
5.2 Kidney
Immunodetection studies have provided insights into the distribution of Panx1 within the renal system, revealing its presence in various segments of renal tubules, including proximal tubules, thin descending limbs, and collecting ducts. Additionally, Panx1 expression is observed explicitly in VSMCs of afferent and efferent arterioles within the renal vasculature (118). This diverse expression pattern suggests a potential role for Panx1 in renal functions.
One intriguing aspect of Panx1’s involvement in renal physiology is its implication in ATP-independent control of renin secretion from juxtaglomerular cells, which modulates intracellular Ca2+ concentration (119). Moreover, Panx1’s mechanosensitivity has been associated with the development of renal diseases (120).
Under hazardous conditions, such as ischemia-reperfusion injury, Panx1 has been shown to cause damage to renal tubular epithelial cells through the purinergic pathway, leading to the subsequent activation of inflammasome (121). Yin et al. demonstrated that in hypoxia/reoxygenation conditions simulating renal ischemia-reperfusion injury, upregulated caspase-11 cleaves Panx1, triggering inflammasome activation and resulting in cell injury and death. This suggests the potential involvement of the caspase-11/Panx1/NLRP3 pathway in renal I/R (ischemia/reperfusion) injury (21). Panx1’s role in kidney inflammation is also evident in vascular endothelial cells (122). Additionally, Panx1 on macrophages enhances the inflammatory response, with necrotic tubular epithelial cells generating danger signals that activate the inflammasome via the TLR2/caspase-5/Panx1 pathway, leading to the transformation of macrophages into a pro-inflammatory phenotype (44).
Furthermore, it has been demonstrated that Panx1 is involved in ferroptosis following kidney I/R injury (70). Inhibitors of Panx1 hemichannels, such as probenecid, have shown the potential to reduce renal I/R damage, highlighting the significance of Panx1 in the context of AKI (46, 121).
5.3 Brain
Panx1 is found in various regions of the CNS, including the cortex, hippocampus, striatum, thalamus, and cerebellum. It is predominantly found in principal excitatory neurons, GABAergic interneurons, and residing immune cells (123). Interestingly, Panx1 expression is not constant throughout life; it is most prominent during early development and gradually diminishes with age, suggesting a role for Panx1 in CNS development (124).
Within the CNS, Panx1 hemichannels play a pro-inflammatory role and can be activated by various factors. For instance, after ischemia, activation of SFK leads to the phosphorylation of Panx1 hemichannel at Tyr-308, resulting in hypoxia-induced depolarization, mitochondrial dysfunction, Ca2+ dysregulation, and neuronal death (13, 125). Furthermore, Panx1 hemichannels can be activated by nitrosylation in their intracellular region by NO, leading to neuronal inflammatory damage (91, 126).
In addition to directly causing neuronal injury, Panx1 can influence blood flow regulation and contribute to ischemic injury in the nervous system. Interestingly, vascular endothelial cell-specific PANX1 knockdown, but not VSMCs, has reduced cerebral infarct volume after I/R injury during middle cerebral artery occlusion (127).
During the early phase of inflammation, activation of Panx1 hemichannels can lead to inflammatory cellular death through the purinergic pathway, amplifying the inflammatory response and causing a large-scale release of pro-inflammatory molecules, such as IL-1β and HMGB1 (high mobility group box 1). Panx1 contributes to brain dysfunction in sepsis-induced encephalopathy by promoting pyroptosis (72). The inhibition of Panx1 hemichannels, such as with probenecid, has shown promise in reducing levels of inflammatory factors (e.g., IL-1β, IL-6, and TNF-α) in the CNS, as well as improving memory retention and ameliorating behavioral deficits (128).
These findings collectively highlight the significant role of Panx1 in CNS physiology and pathology, indicating its potential as a therapeutic target for managing neuroinflammatory conditions, specifically in sepsis. Further research is essential to fully comprehend the precise mechanisms and therapeutic implications of Panx1 modulation in the CNS.
5.4 Liver
Despite the growing evidence suggesting that Panx1 increases the inflammatory response, its specific role in the liver hasn’t been extensively discussed. However, recent studies have shed light on the significant involvement of Panx1 in liver physiology and pathology.
Panx1 is highly expressed in both murine and human livers, under both healthy and diseased situations (129), and primarily engages in inflammation and immunomodulation within the liver. In non-alcoholic hepatitis, Panx1 has been implicated in mediating the production of IL-1β, contributing to the development of hepatic inflammation (130). Additionally, Panx1 appears to play a role in liver fibrosis through an ATP-dependent mechanism (131, 132).
In models of infection following liver transplantation, Panx1 exhibits immunomodulatory effects through the Panx1-P2X2R-IL-33 signaling axis. This leads to increased infiltration of Treg cells, thereby reducing bacterial infection-induced liver injury (66, 67).
Intriguingly, the role of Panx1 in the progression of acute liver failure appears to be sequential. Specifically, PANX1 knockdown effectively reduces liver injury within the first 24 hours, as evidenced by relatively low levels of serum AST (aspartate transaminase) and ALT (alanine aminotransferase). However, beyond the initial 24 hours, PANX1 deletion shows no significant protective effect, and by 48 hours, PANX1 knockdown fails to reduce the necrotic area (133). This suggests that Panx1 may have a time-sensitive impact on inflammatory injury in the liver, and its role in liver pathophysiology is more complex than that of a simple pro-inflammatory regulator.
5.5 Heart
Panx1 is widely expressed in the cardiovascular system and is crucial in cardiac rhythm regulation (134). The Cl- permeability of Panx1 determines its contribution to cardiac rhythm. When Ca2+ efflux from the sarcoplasmic reticulum activates Panx1 hemichannels on cardiomyocytes, it leads to high conductance. However, in the absence of Ca2+ current, episodic opening of Panx1 causes action potentials, potentially resulting in arrhythmias (135).
Panx1 serves distinct roles during acute and chronic heart inflammation, operating through separate signaling pathways. In cases of I/R injury, Panx1 and P2X7 receptors are involved in the release of cardioprotectants. Pre- or postconditioning with P2X7R agonists, such as ATP, has been shown to reduce the damaged area, indicating that the cardioprotective effects associated with Panx1 and P2X7 receptors are likely mediated through the release of ATP. However, the exact mechanism remains unknown (136, 137).
However, in chronic inflammation, such as myocardial fibrosis, ATP via Panx1, combined with P2X6 receptors, activates the heterotrimeric G12 family G protein. The activation promotes the expression of fibrogenic genes, leading to myocardial fibrosis (138). The role of Panx1 in the heart during sepsis and its potential therapeutic efficacy remains to be discovered due to a lack of studies in this specific context.
6 Pharmacological inhibition of Panx1 hemichannels
There are currently no drugs available that specifically target and inhibit Panx1. Still, some compounds have been reported to exhibit inhibitory effects on Panx1 activity and have been extensively studied in experimental settings. Among these compounds, probenecid, carbenoxolone, and 10panx1 have shown promise and potential for future therapeutic approaches (139, 140). Detailed information can be referred to a Review by Koval et al. (141).
Probenecid, a medication commonly used for gout, is clinically used to increase effective concentrations of antibiotics, chemotherapeutics, and other drugs (142). Probenecid has been found to inhibit Panx1 currents in a concentration-response manner (143) and has been widely used to inhibit the transport activity of Panx1 hemichannels. It can suppress inflammasome activity (144), inhibit α-adrenergic receptor-mediated vasoconstriction (145), and enhance microtubule stability (146). Besides Panx1, probenecid broadly inhibits other transport channels and suppresses receptors, such as P2X7 receptors, which can limit its application in experiments (47). Nevertheless, probenecid significantly alleviates sepsis-associated damage in experimental settings. Studies have revealed that probenecid can mitigate cerebral I/R injury (147), skeletal muscle cellular energy crisis, and histopathological damage in sepsis (148), as well as promote recovery from renal I/R injury (121). Interestingly, published reports demonstrated that probenecid efficiently inhibits virus replication in cells and murine models (149, 150). Along with its contribution to anti-inflammation, probenecid may be a competent agent in treating SARS-CoV-2-associated sepsis.
Initially developed as an anti-ulcer medication, Carbenoxolone inhibits Panx1 activity by binding to its first extracellular loop of Panx1 (151). It has been demonstrated to inhibit Panx1 hemichannels in various cell types, including neurons, astrocytes, and macrophages (152–154). Although widely used to investigate Panx1’s role in various physiological and pathological processes, its non-specificity and off-target effects may limit its experimental application (155, 156). Carbenoxolone exerts protective impacts in septic conditions (46). The underlying mechanisms may involve its inhibition of Cx hemichannels, which also allows the passage of ATP, and blockage of HMGB1 release (154, 157), in addition to its interference with the Panx1 protein. Carbenoxolone can potentially treat sepsis by alleviating vascular leakage and renal injury in septic conditions (46, 158).
10panx1, a 10-amino acid peptide derived from the extracellular loop of the Panx1 protein, provides selective inhibition of Panx1 without affecting ATP-evoked currents, distinguishing it from probenecid and carbenoxolone (159). Studies have shown that 10panx1 effectively inhibits Panx1-mediated ATP release and inflammasome activation, reducing inflammatory responses in various experimental models (160–162). However, it’s essential to note that the long-term safety and efficacy of 10panx1 as a therapeutic agent for sepsis are still being investigated, as research by Chen et al. has suggested that 10panx1 may exacerbate sepsis-induced animal lethality (23).
Though inhibitors of Panx1 show promising outcomes in animal models of sepsis, there is still a long way before clinical application in treating sepsis, as no clinical trials have been reported yet. Moreover, as discussed above, Panx1 involves both hyperinflammation and immunosuppression. As a result, solely targeting it may not be sufficient to address the complexity of sepsis and sepsis-induced MODS. It could worsen immune disorders and organ damage in patients. Therefore, further evidence is required to understand the pattern of Panx1 hemichannel activation in sepsis before exploring its clinical therapeutic potential. More comprehensive research is needed to elucidate Panx1’s role and potential as a therapeutic target in sepsis and sepsis-induced MODS.
7 Conclusion
This comprehensive review delves into the potential roles of Panx1 in the pathogenesis of sepsis, examining its contribution to the excessive inflammatory response in the early phase, immunosuppression in the late phase, and impairment of microcirculation. Considering Panx1’s expression in multiple organs and its involvement in organ inflammatory injury, it is reasonable to suggest that Panx1 may play a role in the development of MODS. Inhibiting Panx1 hemichannels emerges as a promising therapeutic strategy against sepsis and sepsis-associated MODS; however, further research is essential before its clinical translation can be realized. As the scientific investigation on Panx1 hemichannels in sepsis is limited, it becomes crucial to explore its distinct functions at different phases of sepsis and understand how it contributes to organ injuries. This makes Panx1 an intriguing potential therapeutic target for sepsis and sepsis-induced MODS, offering a fresh approach to therapeutic interventions against these challenging disorders. Given its significant involvement in various critical processes, Panx1 represents a promising therapeutic target for sepsis and MODS.
Author contributions
XC wrote the paper, SY proposed the theme of article, LM assisted in writing, and YL and HH provided guidance. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Fundamental Research Funds for the Central Universities (3332022011), CAMS Innovation Fund for Medical Sciences (CIFMS) from Chinese Academy of Medical Sciences (2021-I2M-1-062) and National High-Level Hospital Clinical Research Funding (2022-PUMCH-B-115, 2022-PUMCH-D-005).
Acknowledgments
Figure 1 and 2 of this review are orchestrated by Figdraw.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet (2020) 395(10219):200–11. doi: 10.1016/S0140-6736(19)32989-7
2. Vincent JL, Nelson DR, Williams MD. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit Care Med (2011) 39(5):1050–5. doi: 10.1097/CCM.0b013e31820eda29
3. Farrah K, McIntyre L, Doig CJ, Talarico R, Taljaard M, Krahn M, et al. Sepsis-associated mortality, resource use, and healthcare costs: A propensity-matched cohort study. Crit Care Med (2021) 49(2):215–27. doi: 10.1097/CCM.0000000000004777
4. Kelmanson IV, Shagin DA, Usman N, Matz MV, Lukyanov SA, Panchin YV. Altering electrical connections in the nervous system of the pteropod mollusc Clione limacina by neuronal injections of gap junction mRNA. Eur J Neurosci (2002) 16(12):2475–6. doi: 10.1046/j.1460-9568.2002.02423.x
5. Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem (2007) 282(43):31733–43. doi: 10.1074/jbc.M702422200
6. Palacios-Prado N, Soto PA, López X, Choi EJ, Marquez-Miranda V, Rojas M, et al. Endogenous pannexin1 channels form functional intercellular cell-cell channels with characteristic voltage-dependent properties. Proc Natl Acad Sci U.S.A. (2022) 119(18):e2202104119. doi: 10.1073/pnas.2202104119
7. Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, et al. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci (2007) 120(Pt 21):3772–83. doi: 10.1242/jcs.009514
8. Iwamoto T, Nakamura T, Ishikawa M, Yoshizaki K, Sugimoto A, Ida-Yonemochi H, et al. Pannexin 3 regulates proliferation and differentiation of odontoblasts via its hemichannel activities. PloS One (2017) 12(5):e0177557. doi: 10.1371/journal.pone.0177557
9. Wang J, Ambrosi C, Qiu F, Jackson DG, Sosinsky G, Dahl G. The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci Signal (2014) 7(335):ra69. doi: 10.1126/scisignal.2005431
10. Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U.S.A. (2006) 103(20):7655–9. doi: 10.1073/pnas.0601037103
11. Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, et al. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol (2010) 299(4):H1146–52. doi: 10.1152/ajpheart.00301.2010
12. Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science (2006) 312(5775):924–7. doi: 10.1126/science.1126241
13. Weilinger NL, Lohman AW, Rakai BD, Ma EM, Bialecki J, Maslieieva V, et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci (2016) 19(3):432–42. doi: 10.1038/nn.4236
14. Furlow PW, Zhang S, Soong TD, Halberg N, Goodarzi H, Mangrum C, et al. Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat Cell Biol (2015) 17(7):943–52. doi: 10.1038/ncb3194
15. Jalaleddine N, El-Hajjar L, Dakik H, Shaito A, Saliba J, Safi R, et al. Pannexin1 is associated with enhanced epithelial-to-mesenchymal transition in human patient breast cancer tissues and in breast cancer cell lines. Cancers (Basel) (2019) 11(12). doi: 10.3390/cancers11121967
16. Gombault A, Baron L, Couillin I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol (2012) 3:414. doi: 10.3389/fimmu.2012.00414
17. Harcha PA, López-López T, Palacios AG, Sáez PJ. Pannexin channel regulation of cell migration: focus on immune cells. Front Immunol (2021) 12:750480. doi: 10.3389/fimmu.2021.750480
18. Good ME, Young AP, Wolpe AG, Ma M, Hall PJ, Duffy CK, et al. Endothelial pannexin 1 regulates cardiac response to myocardial infarction. Circ Res (2021) 128(8):1211–3. doi: 10.1161/CIRCRESAHA.120.317272
19. Chen SP, Qin T, Seidel JL, Zheng Y, Eikermann M, Ferrari MD, et al. Inhibition of the P2X7-PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain (2017) 140(6):1643–56. doi: 10.1093/brain/awx085
20. Sharma AK, Charles EJ, Zhao Y, Narahari AK, Baderdinni PK, Good ME, et al. Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol (2018) 315(2):L301–l312. doi: 10.1152/ajplung.00004.2018
21. Yin F, Zheng PQ, Zhao LQ, Wang YZ, Miao NJ, Zhou ZL, et al. Caspase-11 promotes NLRP3 inflammasome activation via the cleavage of pannexin1 in acute kidney disease. Acta Pharmacol Sin (2022) 43(1):86–95. doi: 10.1038/s41401-021-00619-2
22. Xiao F, Waldrop SL, Khimji AK, Kilic G. Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells. Am J Physiol Cell Physiol (2012) 303(10):C1034–44. doi: 10.1152/ajpcell.00175.2012
23. Chen W, Zhu S, Wang Y, Li J, Qiang X, Zhao X, et al. Enhanced macrophage pannexin 1 expression and hemichannel activation exacerbates lethal experimental sepsis. Sci Rep (2019) 9(1):160. doi: 10.1038/s41598-018-37232-z
24. Li X, Kondo Y, Bao Y, Staudenmaier L, Lee A, Zhang J, et al. Systemic adenosine triphosphate impairs neutrophil chemotaxis and host defense in sepsis. Crit Care Med (2017) 45(1):e97–e104. doi: 10.1097/CCM.0000000000002052
25. Sáez PJ, Vargas P, Shoji KF, Harcha PA, Lennon-Duménil AM, Sáez JC. ATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2X(7) receptors. Sci Signal (2017) 10(506). doi: 10.1126/scisignal.aah7107
26. Alarcon P, Manosalva C, Quiroga J, Belmar I, Alvarez K, Diaz G, et al. Oleic and linoleic acids induce the release of neutrophil extracellular traps via pannexin 1-dependent ATP release and P2X1 receptor activation. Front Vet Sci (2020) 7:260. doi: 10.3389/fvets.2020.00260
27. Quiroga J, Alarcon P, Manosalva C, Taubert A, Hermosilla C, Hidalgo MA, et al. Mitochondria-derived ATP participates in the formation of neutrophil extracellular traps induced by platelet-activating factor through purinergic signaling in cows. Dev Comp Immunol (2020) 113:103768. doi: 10.1016/j.dci.2020.103768
28. Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell (2014) 156(6):1193–206. doi: 10.1016/j.cell.2014.02.008
29. Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature (2014) 514(7521):187–92. doi: 10.1038/nature13683
30. Ge Y, Huang M, Yao YM. Recent advances in the biology of IL-1 family cytokines and their potential roles in development of sepsis. Cytokine Growth Factor Rev (2019) 45:24–34. doi: 10.1016/j.cytogfr.2018.12.004
31. Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity (2015) 43(5):923–32. doi: 10.1016/j.immuni.2015.10.009
32. Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem (2007) 282(26):18810–8. doi: 10.1074/jbc.M610762200
33. Di A, Xiong S, Ye Z, Malireddi RKS, Kometani S, Zhong M, et al. The TWIK2 potassium efflux channel in macrophages mediates NLRP3 inflammasome-induced inflammation. Immunity (2018) 49(1):56–65 e4. doi: 10.1016/j.immuni.2018.04.032
34. Hung SC, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, et al. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PloS One (2013) 8(7):e70210. doi: 10.1371/journal.pone.0070210
35. Draganov D, Gopalakrishna-Pillai S, Chen YR, Zuckerman N, Moeller S, Wang C, et al. Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci Rep (2015) 5:16222. doi: 10.1038/srep16222
36. Schneider M, Prudic K, Pippel A, Klapperstuck M, Braam U, Muller CE, et al. Interaction of purinergic P2X4 and P2X7 receptor subunits. Front Pharmacol (2017) 8:860. doi: 10.3389/fphar.2017.00860
37. Adamiak M, Bujko K, Thapa A, Pensato V, Brzezniakiewicz-Janus K, Ratajczak J, et al. The P2X4 purinergic receptor has emerged as a potent regulator of hematopoietic stem/progenitor cell mobilization and homing-a novel view of P2X4 and P2X7 receptor interaction in orchestrating stem cell trafficking. Leukemia (2022) 36(1):248–56. doi: 10.1038/s41375-021-01352-9
38. Qi Q, Wang XX, Li JL, Chen YQ, Chang JR, Xi J, et al. Neuroprotective effects of the pannexin-1 channel inhibitor: probenecid on spinal cord injury in rats. Front Mol Neurosci (2022) 15:848185. doi: 10.3389/fnmol.2022.848185
39. Mawhinney LJ, de Rivero Vaccari JP, Dale GA, Keane RW, Bramlett HM. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci (2011) 12:123. doi: 10.1186/1471-2202-12-123
40. Zheng Y, Tang W, Zeng H, Peng Y, Yu X, Yan F, et al. Probenecid-blocked pannexin-1 channel protects against early brain injury via inhibiting neuronal AIM2 inflammasome activation after subarachnoid hemorrhage. Front Neurol (2022) 13:854671. doi: 10.3389/fneur.2022.854671
41. Onódi Z, Ruppert M, Kucsera D, Sayour AA, Tóth VE, Koncsos G, et al. AIM2-driven inflammasome activation in heart failure. Cardiovasc Res (2021) 117(13):2639–51. doi: 10.1093/cvr/cvab202
42. Pronin A, Pham D, An W, Dvoriantchikova G, Reshetnikova G, Qiao J, et al. Inflammasome activation induces pyroptosis in the retina exposed to ocular hypertension injury. Front Mol Neurosci (2019) 12:36. doi: 10.3389/fnmol.2019.00036
43. Kim HY, Kim SJ, Lee SM. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS J (2015) 282(2):259–70. doi: 10.1111/febs.13123
44. Liu C, Shen Y, Huang L, Wang J. TLR2/caspase-5/Panx1 pathway mediates necrosis-induced NLRP3 inflammasome activation in macrophages during acute kidney injury. Cell Death Discovery (2022) 8(1):232. doi: 10.1038/s41420-022-00814-y
45. Parzych K, Zetterqvist AV, Wright WR, Kirkby NS, Mitchell JA, Paul-Clark MJ. Differential role of pannexin-1/ATP/P2X(7) axis in IL-1β release by human monocytes. FASEB J (2017) 31(6):2439–45. doi: 10.1096/fj.201600256
46. Huang G, Bao J, Shao X, Zhou W, Wu B, Ni Z, et al. Inhibiting pannexin-1 alleviates sepsis-induced acute kidney injury via decreasing NLRP3 inflammasome activation and cell apoptosis. Life Sci (2020) 254:117791. doi: 10.1016/j.lfs.2020.117791
47. Bhaskaracharya A, Dao-Ung P, Jalilian I, Spildrejorde M, Skarratt KK, Fuller SJ, et al. Probenecid blocks human P2X7 receptor-induced dye uptake via a pannexin-1 independent mechanism. PloS One (2014) 9(3):e93058. doi: 10.1371/journal.pone.0093058
48. Chen KW, Demarco B, Broz P. Pannexin-1 promotes NLRP3 activation during apoptosis but is dispensable for canonical or noncanonical inflammasome activation. Eur J Immunol (2020) 50:170–7. doi: 10.1002/eji.201948254
49. Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, et al. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol (2011) 186(11):6553–61. doi: 10.4049/jimmunol.1100478
50. Lerman YV, Kim M. Neutrophil migration under normal and sepsis conditions. Cardiovasc Hematol Disord Drug Targets (2015) 15(1):19–28. doi: 10.2174/1871529X15666150108113236
51. Souto FO, Alves-Filho JC, Turato WM, Auxiliadora-Martins M, Basile-Filho A, Cunha FQ. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am J Respir Crit Care Med (2011) 183(2):234–42. doi: 10.1164/rccm.201003-0416OC
52. Martin EL, Souza DG, Fagundes CT, Amaral FA, Assenzio B, Puntorieri V, et al. Phosphoinositide-3 kinase gamma activity contributes to sepsis and organ damage by altering neutrophil recruitment. Am J Respir Crit Care Med (2010) 182(6):762–73. doi: 10.1164/rccm.201001-0088OC
53. Sharma A, Matsuo S, Yang WL, Wang Z, Wang P. Receptor-interacting protein kinase 3 deficiency inhibits immune cell infiltration and attenuates organ injury in sepsis. Crit Care (2014) 18(4):R142. doi: 10.1186/cc13970
54. Yamada KM, Sixt M. Mechanisms of 3D cell migration. Nat Rev Mol Cell Biol (2019) 20(12):738–52. doi: 10.1038/s41580-019-0172-9
55. Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, et al. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal (2010) 3(125):ra45. doi: 10.1126/scisignal.2000549
56. Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science (2006) 314(5806):1792–5. doi: 10.1126/science.1132559
57. Bao Y, Chen Y, Ledderose C, Li L, Junger WG. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J Biol Chem (2013) 288(31):22650–7. doi: 10.1074/jbc.M113.476283
58. Lopez X, Palacios-Prado N, Guiza J, Escamilla R, Fernandez P, Vega JL, et al. A physiologic rise in cytoplasmic calcium ion signal increases pannexin1 channel activity via a C-terminus phosphorylation by CaMKII. Proc Natl Acad Sci U.S.A. (2021) 118(32). doi: 10.1073/pnas.2108967118
59. Campwala H, Sexton DW, Crossman DC, Fountain SJ. P2Y(6) receptor inhibition perturbs CCL2-evoked signalling in human monocytic and peripheral blood mononuclear cells. J Cell Sci (2014) 127(Pt 22):4964–73. doi: 10.1242/jcs.159012
60. Saez PJ, Shoji KF, Aguirre A, Saez JC, et al. Regulation of hemichannels and gap junction channels by cytokines in antigen-presenting cells. Mediators Inflammation (2014) 2014:742734. doi: 10.1155/2014/742734
61. Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, et al. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PloS One (2011) 6(1):e15973. doi: 10.1371/journal.pone.0015973
62. Garg C, Seo JH, Ramachandran J, Loh JM, Calderon F, Contreras JE. Trovafloxacin attenuates neuroinflammation and improves outcome after traumatic brain injury in mice. J Neuroinflamm (2018) 15(1):42. doi: 10.1186/s12974-018-1069-9
63. Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA (2011) 306(23):2594–605. doi: 10.1001/jama.2011.1829
64. Liu D, Huang SY, Sun JH, Zhang HC, Cai QL, Gao C, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res (2022) 9(1):56. doi: 10.1186/s40779-022-00422-y
65. Medina CB, Chiu YH, Stremska ME, Lucas CD, Poon I, Tung KS, et al. Pannexin 1 channels facilitate communication between T cells to restrict the severity of airway inflammation. Immunity (2021) 54(8):1715–1727 e7. doi: 10.1016/j.immuni.2021.06.014
66. Wang P, Shi B, Wang C, Wang Y, Que W, Jiang Z, et al. Hepatic pannexin-1 mediates ST2(+) regulatory T cells promoting resolution of inflammation in lipopolysaccharide-induced endotoxemia. Clin Transl Med (2022) 12(5):e849. doi: 10.1002/ctm2.849
67. Li H, Yu X, Shi B, Zhang K, Yuan L, Liu X, et al. Reduced pannexin 1-IL-33 axis function in donor livers increases risk of MRSA infection in liver transplant recipients. Sci Transl Med (2021) 13(606). doi: 10.1126/scitranslmed.aaz6169
68. Wang H, Xing Y, Mao L, Luo Y, Kang L, Meng G. Pannexin-1 influences peritoneal cavity cell population but is not involved in NLRP3 inflammasome activation. Protein Cell (2013) 4(4):259–65. doi: 10.1007/s13238-013-2114-1
69. Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ (2014) 21(1):79–91. doi: 10.1038/cdd.2013.75
70. Su L, Jiang X, Yang C, Zhang J, Chen B, Li Y, et al. Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. J Biol Chem (2019) 294(50):19395–404. doi: 10.1074/jbc.RA119.010949
71. Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev (2011) 243(1):206–14. doi: 10.1111/j.1600-065X.2011.01044.x
72. Lei Y, Zhou R, Sun X, Tang F, Gao H, Chen L, et al. The pannexin-1 channel regulates pyroptosis through autophagy in a mouse model of sepsis-associated encephalopathy. Ann Transl Med (2021) 9(24):1802. doi: 10.21037/atm-21-6579
73. Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol (2013) 13(12):862–74. doi: 10.1038/nri3552
74. Purohit R, Bera AK. Mutational effects of Pannexin 1 R217H depend on the carboxyl-terminus. Biochem Biophys Res Commun (2021) 548:143–7. doi: 10.1016/j.bbrc.2021.02.060
75. Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature (2010) 467(7317):863–7. doi: 10.1038/nature09413
76. Dourado M, Wong E, Hackos DH. Pannexin-1 is blocked by its C-terminus through a delocalized non-specific interaction surface. PloS One (2014) 9(6):e99596. doi: 10.1371/journal.pone.0099596
77. Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, et al. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J Biol Chem (2012) 287(14):11303–11. doi: 10.1074/jbc.M111.323378
78. Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature (2009) 461(7261):282–6. doi: 10.1038/nature08296
79. Aguirre A, Shoji KF, Saez JC, Henriquez M, Quest AF. FasL-triggered death of Jurkat cells requires caspase 8-induced, ATP-dependent cross-talk between Fas and the purinergic receptor P2X(7). J Cell Physiol (2013) 228(2):485–93. doi: 10.1002/jcp.24159
80. Wu LY, Ye ZN, Zhou CH, Wang CX, Xie GB, Zhang XS, et al. Roles of pannexin-1 channels in inflammatory response through the TLRs/NF-kappa B signaling pathway following experimental subarachnoid hemorrhage in rats. Front Mol Neurosci (2017) 10:175. doi: 10.3389/fnmol.2017.00175
81. Huang Y, Lin J, Chen X, Lin J. Pannexin-1 contributes to the apoptosis of spinal neurocytes in spinal cord injury. Front Physiol (2021) 12:656647. doi: 10.3389/fphys.2021.656647
82. Miranda M, Balarini M, Caixeta D, Bouskela E. Microcirculatory dysfunction in sepsis: pathophysiology, clinical monitoring, and potential therapies. Am J Physiol Heart Circ Physiol (2016) 311(1):H24–35. doi: 10.1152/ajpheart.00034.2016
83. Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, et al. Pannexin1 regulates alpha1-adrenergic receptor- mediated vasoconstriction. Circ Res (2011) 109(1):80–5. doi: 10.1161/CIRCRESAHA.110.237594
84. DeLalio LJ, Keller AS, Chen J, Boyce AKJ, Artamonov MV, Askew-Page HR, et al. Interaction between pannexin 1 and caveolin-1 in smooth muscle can regulate blood pressure. Arterioscler Thromb Vasc Biol (2018) 38(9):2065–78. doi: 10.1161/ATVBAHA.118.311290
85. Daneva Z, Ottolini M, Chen YL, Klimentova E, Kuppusamy M, Shah SA, et al. Endothelial pannexin 1-TRPV4 channel signaling lowers pulmonary arterial pressure in mice. Elife (2021) 10. doi: 10.7554/eLife.67777
86. Grimmer B, Krauszman A, Hu X, Kabir G, Connelly KA, Li M, et al. Pannexin 1: a novel regulator of acute hypoxic pulmonary vasoconstriction. Cardiovasc Res (2022) 118(11):2535–47. doi: 10.1093/cvr/cvab326
87. Goldenberg NM, Wang L, Ranke H, Liedtke W, Tabuchi A, Kuebler WM. TRPV4 is required for hypoxic pulmonary vasoconstriction. Anesthesiology (2015) 122(6):1338–48. doi: 10.1097/ALN.0000000000000647
88. Burboa PC, Puebla M, Gaete PS, Duran WN, Lillo MA. Connexin and pannexin large-pore channels in microcirculation and neurovascular coupling function. Int J Mol Sci (2022) 23(13). doi: 10.3390/ijms23137303
89. Marziano C, Hong K, Cope EL, Kotlikoff MI, Isakson BE, Sonkusare SK. Nitric oxide-dependent feedback loop regulates transient receptor potential vanilloid 4 (TRPV4) channel cooperativity and endothelial function in small pulmonary arteries. J Am Heart Assoc (2017) 6(12). doi: 10.1161/JAHA.117.007157
90. Daneva Z, Marziano C, Ottolini M, Chen YL, Baker TM, Kuppusamy M, et al. Caveolar peroxynitrite formation impairs endothelial TRPV4 channels and elevates pulmonary arterial pressure in pulmonary hypertension. Proc Natl Acad Sci U.S.A. (2021) 118(17). doi: 10.1073/pnas.2023130118
91. Lohman AW, Weaver JL, Billaud M, Sandilos JK, Griffiths R, Straub AC, et al. S-nitrosylation inhibits pannexin 1 channel function. J Biol Chem (2012) 287(47):39602–12. doi: 10.1074/jbc.M112.397976
92. Gaete PS, Lillo MA, Puebla M, Poblete I, Figueroa XF. CGRP signalling inhibits NO production through pannexin-1 channel activation in endothelial cells. Sci Rep (2019) 9(1):7932. doi: 10.1038/s41598-019-44333-w
93. Lillo MA, Gaete PS, Puebla M, Burboa PC, Poblete I, Figueroa XF. Novel pannexin-1-coupled signaling cascade involved in the control of endothelial cell function and NO-dependent relaxation. Oxid Med Cell Longev (2021) 2021:2678134. doi: 10.1155/2021/2678134
94. Gaynullina D, Shestopalov VI, Panchin Y, Tarasova OS. Pannexin 1 facilitates arterial relaxation via an endothelium-derived hyperpolarization mechanism. FEBS Lett (2015) 589(10):1164–70. doi: 10.1016/j.febslet.2015.03.018
95. Gou Z, Zhang H, Abbasi M, Misbah C. Red blood cells under flow show maximal ATP release for specific hematocrit. Biophys J (2021) 120(21):4819–31. doi: 10.1016/j.bpj.2021.09.025
96. Kirby BS, Sparks MA, Lazarowski ER, Lopez Domowicz DA, Zhu H, McMahon TJ. Pannexin 1 channels control the hemodynamic response to hypoxia by regulating O(2)-sensitive extracellular ATP in blood. Am J Physiol Heart Circ Physiol (2021) 320(3):H1055–h1065. doi: 10.1152/ajpheart.00651.2020
97. Ellis CG, Milkovich S, Goldman D. What is the efficiency of ATP signaling from erythrocytes to regulate distribution of O(2) supply within the microvasculature? Microcirculation (2012) 19(5):440–50. doi: 10.1111/j.1549-8719.2012.00196.x
98. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol (2013) 13(1):34–45. doi: 10.1038/nri3345
99. Taylor KA, Wright JR, Vial C, Evans RJ, Mahaut-Smith MP. Amplification of human platelet activation by surface pannexin-1 channels. J Thromb Haemost (2014) 12(6):987–98. doi: 10.1111/jth.12566
100. Wright JR, Amisten S, Goodall AH, Mahaut-Smith MP. Transcriptomic analysis of the ion channelome of human platelets and megakaryocytic cell lines. Thromb Haemost (2016) 116(2):272–84. doi: 10.1160/TH15-11-0891
101. Molica F, Morel S, Meens MJ, Denis JF, Bradfield PF, Penuela S, et al. Functional role of a polymorphism in the Pannexin1 gene in collagen-induced platelet aggregation. Thromb Haemost (2015) 114(2):325–36. doi: 10.1160/TH14-11-0981
102. Molica F, Meens MJ, Pelli G, Hautefort A, Emre Y, Imhof BA, et al. Selective inhibition of Panx1 channels decreases hemostasis and thrombosis in vivo. Thromb Res (2019) 183:56–62. doi: 10.1016/j.thromres.2019.09.028
103. Maier-Begandt D, Comstra HS, Molina SA, Kruger N, Ruddiman CA, Chen YL, et al. A venous-specific purinergic signaling cascade initiated by Pannexin 1 regulates TNFalpha-induced increases in endothelial permeability. Sci Signal (2021) 14(672). doi: 10.1126/scisignal.aba2940
104. Lohman AW, Leskov IL, Butcher JT, Johnstone SR, Stokes TA, Begandt D, et al. Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat Commun (2015) 6:7965. doi: 10.1038/ncomms8965
105. Batori R, Kumar S, Bordan Z, Cherian-Shaw M, Kovacs-Kasa A, MacDonald JA, et al. Differential mechanisms of adenosine- and ATPgammaS-induced microvascular endothelial barrier strengthening. J Cell Physiol (2019) 234(5):5863–79. doi: 10.1002/jcp.26419
106. Yang Y, Delalio LJ, Best AK, Macal E, Milstein J, Donnelly I, et al. Endothelial pannexin 1 channels control inflammation by regulating intracellular calcium. J Immunol (2020) 204(11):2995–3007. doi: 10.4049/jimmunol.1901089
107. Xu S, Li X, LaPenna KB, Yokota SD, Huke S, He P. New insights into shear stress-induced endothelial signalling and barrier function: cell-free fluid versus blood flow. Cardiovasc Res (2017) 113(5):508–18. doi: 10.1093/cvr/cvx021
108. Molica F, Meens MJ, Dubrot J, Ehrlich A, Roth CL, Morel S, et al. Pannexin1 links lymphatic function to lipid metabolism and atherosclerosis. Sci Rep (2017) 7(1):13706. doi: 10.1038/s41598-017-14130-4
109. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
110. Diem K, Fauler M, Fois G, Hellmann A, Winokurow N, Schumacher S, et al. Mechanical stretch activates piezo1 in caveolae of alveolar type I cells to trigger ATP release and paracrine stimulation of surfactant secretion from alveolar type II cells. FASEB J (2020) 34(9):12785–804. doi: 10.1096/fj.202000613RRR
111. Richter K, Kiefer KP, Grzesik BA, Clauss WG, Fronius M. Hydrostatic pressure activates ATP-sensitive K+ channels in lung epithelium by ATP release through pannexin and connexin hemichannels. FASEB J (2014) 28(1):45–55. doi: 10.1096/fj.13-229252
112. Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet (2022) 400(10358):1145–56. doi: 10.1016/S0140-6736(22)01485-4
113. Price LC, McAuley DF, Marino PS, Finney SJ, Griffiths MJ, Wort SJ. Pathophysiology of pulmonary hypertension in acute lung injury. Am J Physiol Lung Cell Mol Physiol (2012) 302(9):L803–15. doi: 10.1152/ajplung.00355.2011
114. Pandolfi R, Barreira B, Moreno E, Lara-Acedo V, Morales-Cano D, Martinez-Ramas A, et al. Role of acid sphingomyelinase and IL-6 as mediators of endotoxin-induced pulmonary vascular dysfunction. Thorax (2017) 72(5):460–71. doi: 10.1136/thoraxjnl-2015-208067
115. Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care (2010) 14(5):R169. doi: 10.1186/cc9264
116. Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med (1977) 296(9):476–80. doi: 10.1056/NEJM197703032960903
117. Sibbald WJ, Paterson NA, Holliday RL, Anderson RA, Lobb TR, Duff JH. Pulmonary hypertension in sepsis: measurement by the pulmonary arterial diastolic-pulmonary wedge pressure gradient and the influence of passive and active factors. Chest (1978) 73(5):583–91. doi: 10.1378/chest.73.5.583
118. Jelicic I, Vukojevic K, Racetin A, Caric D, Glavina Durdov M, Saraga-Babic M, et al. Expression of pannexin 1 in the human kidney during embryonal, early fetal and postnatal development and its prognostic significance in diabetic nephropathy. Biomedicines (2022) 10(5). doi: 10.3390/biomedicines10050944
119. Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol (2012) 303(10):F1454–9. doi: 10.1152/ajprenal.00206.2011
120. Verschuren EHJ, Rigalli JP, Castenmiller C, Rohrbach MU, Bindels RJM, Peters DJM, et al. Pannexin-1 mediates fluid shear stress-sensitive purinergic signaling and cyst growth in polycystic kidney disease. FASEB J (2020) 34(5):6382–98. doi: 10.1096/fj.201902901R
121. El-Maadawy WH, Hassan M, Badawy MH, AbuSeada A, Hafiz E. Probenecid induces the recovery of renal ischemia/reperfusion injury via the blockade of Pannexin 1/P2X7 receptor axis. Life Sci (2022) 308:120933. doi: 10.1016/j.lfs.2022.120933
122. Jankowski J, Perry HM, Medina CB, Huang L, Yao J, Bajwa A, et al. Epithelial and endothelial pannexin1 channels mediate AKI. J Am Soc Nephrol (2018) 29(7):1887–99. doi: 10.1681/ASN.2017121306
123. Yeung AK, Patil CS, Jackson MF. Pannexin-1 in the CNS: Emerging concepts in health and disease. J Neurochem (2020) 154(5):468–85. doi: 10.1111/jnc.15004
124. Sanchez-Arias JC, Liu M, Choi CSW, Ebert SN, Brown CE, Swayne LA. Pannexin 1 regulates network ensembles and dendritic spine development in cortical neurons. eNeuro (2019) 6(3). doi: 10.1523/ENEURO.0503-18.2019
125. Weilinger NL, Tang PL, Thompson RJ. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J Neurosci (2012) 32(36):12579–88. doi: 10.1523/JNEUROSCI.1267-12.2012
126. Zhang L, Deng T, Sun Y, Liu K, Yang Y, Zheng X. Role for nitric oxide in permeability of hippocampal neuronal hemichannels during oxygen glucose deprivation. J Neurosci Res (2008) 86(10):2281–91. doi: 10.1002/jnr.21675
127. Good ME, Eucker SA, Li J, Bacon HM, Lang SM, Butcher JT, et al. Endothelial cell Pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight (2018) 3(6). doi: 10.1172/jci.insight.96272
128. Zhang Z, Lei Y, Yan C, Mei X, Jiang T, Ma Z, et al. Probenecid relieves cerebral dysfunction of sepsis by inhibiting pannexin 1-dependent ATP release. Inflammation (2019) 42(3):1082–92. doi: 10.1007/s10753-019-00969-4
129. Leroy K, Vilas-Boas V, Gijbels E, Vanderborght B, Devisscher L, Cogliati B, et al. Expression of connexins and pannexins in diseased human liver. EXCLI J (2022) 21:1111–29. doi: 10.17179/excli2022-5163
130. Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology (2011) 54(1):133–44. doi: 10.1002/hep.24341
131. Feig JL, Mediero A, Corciulo C, Liu H, Zhang J, Perez-Aso M, et al. The antiviral drug tenofovir, an inhibitor of Pannexin-1-mediated ATP release, prevents liver and skin fibrosis by downregulating adenosine levels in the liver and skin. PloS One (2017) 12(11):e0188135. doi: 10.1371/journal.pone.0188135
132. Crespo Yanguas S, da Silva TC, Pereira IVA, Maes M, Willebrords J, Shestopalov VI, et al. Genetic ablation of pannexin1 counteracts liver fibrosis in a chemical, but not in a surgical mouse model. Arch Toxicol (2018) 92(8):2607–27. doi: 10.1007/s00204-018-2255-3
133. Willebrords J, Maes M, Pereira IVA, da Silva TC, Govoni VM, Lopes VV, et al. Protective effect of genetic deletion of pannexin1 in experimental mouse models of acute and chronic liver disease. Biochim Biophys Acta Mol Basis Dis (2018) 1864(3):819–30. doi: 10.1016/j.bbadis.2017.12.013
134. Meens MJ, Kwak BR, Duffy HS. Role of connexins and pannexins in cardiovascular physiology. Cell Mol Life Sci (2015) 72(15):2779–92. doi: 10.1007/s00018-015-1959-2
135. Kienitz MC, Bender K, Dermietzel R, Pott L, Zoidl G. Pannexin 1 constitutes the large conductance cation channel of cardiac myocytes. J Biol Chem (2011) 286(1):290–8. doi: 10.1074/jbc.M110.163477
136. Vessey DA, Li L, Kelley M. P2X7 receptor agonists pre- and postcondition the heart against ischemia-reperfusion injury by opening pannexin-1/P2X(7) channels. Am J Physiol Heart Circ Physiol (2011) 301(3):H881–7. doi: 10.1152/ajpheart.00305.2011
137. Vessey DA, Li L, Kelley M. Ischemic preconditioning requires opening of pannexin-1/P2X(7) channels not only during preconditioning but again after index ischemia at full reperfusion. Mol Cell Biochem (2011) 351(1-2):77–84. doi: 10.1007/s11010-011-0713-9
138. Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, et al. P2Y6 receptor-Galpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J (2008) 27(23):3104–15. doi: 10.1038/emboj.2008.237
139. Willebrords J, Maes M, Crespo Yanguas S, Vinken M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol Ther (2017) 180:144–60. doi: 10.1016/j.pharmthera.2017.07.001
140. Dahl G, Qiu F, Wang J. The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology (2013) 75:583–93. doi: 10.1016/j.neuropharm.2013.02.019
141. Koval, Schug, Isakson. Pharmacology of pannexin channels. Curr Opin Pharmacol (2023) 69:102359. doi: 10.1016/j.coph.2023.102359
142. García-Rodríguez C, Mujica P, Illanes-González J, López A, Vargas C, Sáez JC, et al. Probenecid, an old drug with potential new uses for central nervous system disorders and neuroinflammation. Biomedicines (2023) 11(6). doi: 10.3390/biomedicines11061516
143. Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol (2008) 295(3):C761–7. doi: 10.1152/ajpcell.00227.2008
144. Jian Z, Ding S, Deng H, Wang J, Yi W, Wang L, et al. Probenecid protects against oxygen-glucose deprivation injury in primary astrocytes by regulating inflammasome activity. Brain Res (2016) 1643:123–9. doi: 10.1016/j.brainres.2016.05.002
145. Nyberg M, Piil P, Kiehn OT, Maagaard C, Jorgensen TS, Egelund J, et al. Probenecid inhibits alpha-adrenergic receptor-mediated vasoconstriction in the human leg vasculature. Hypertension (2018) 71(1):151–9. doi: 10.1161/HYPERTENSIONAHA.117.10251
146. Xu X, Wicki-Stordeur LE, Sanchez-Arias JC, Liu M, Weaver MS, Choi CSW, et al. Probenecid disrupts a novel pannexin 1-collapsin response mediator protein 2 interaction and increases microtubule stability. Front Cell Neurosci (2018) 12:124. doi: 10.3389/fncel.2018.00124
147. Wei R, Wang J, Xu Y, Yin B, He F, Du Y, et al. Probenecid protects against cerebral ischemia/reperfusion injury by inhibiting lysosomal and inflammatory damage in rats. Neuroscience (2015) 301:168–77. doi: 10.1016/j.neuroscience.2015.05.070
148. He H, Liu D, Long Y, Wang X, Yao B. The pannexin-1 channel inhibitor probenecid attenuates skeletal muscle cellular energy crisis and histopathological injury in a rabbit endotoxemia model. Inflammation (2018) 41(6):2030–40. doi: 10.1007/s10753-018-0846-z
149. Perwitasari O, Yan X, Johnson S, White C, Brooks P, Tompkins SM, et al. Targeting organic anion transporter 3 with probenecid as a novel anti-influenza a virus strategy. Antimicrob Agents Chemother (2013) 57(1):475–83. doi: 10.1128/AAC.01532-12
150. Murray J, Hogan RJ, Martin DE, Blahunka K, Sancilio FD, Balyan R, et al. Probenecid inhibits SARS-CoV-2 replication in vivo and in vitro. Sci Rep (2021) 11(1):18085. doi: 10.1038/s41598-021-97658-w
151. Michalski K, Kawate T. Carbenoxolone inhibits Pannexin1 channels through interactions in the first extracellular loop. J Gen Physiol (2016) 147(2):165–74. doi: 10.1085/jgp.201511505
152. Dong S, Zhang K, Shi Y. Carbenoxolone has the potential to ameliorate acute incision pain in rats. Mol Med Rep (2021) 24(1). doi: 10.3892/mmr.2021.12159
153. Bravo D, Ibarra P, Retamal J, Pelissier T, Laurido C, Hernandez A, et al. Pannexin 1: a novel participant in neuropathic pain signaling in the rat spinal cord. Pain (2014) 155(10):2108–15. doi: 10.1016/j.pain.2014.07.024
154. Li W, Li J, Sama AE, Wang H. Carbenoxolone blocks endotoxin-induced protein kinase R (PKR) activation and high mobility group box 1 (HMGB1) release. Mol Med (2013) 19(1):203–11. doi: 10.2119/molmed.2013.00064
155. Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem (2005) 92(5):1033–43. doi: 10.1111/j.1471-4159.2004.02947.x
156. de Groot JR, Veenstra T, Verkerk AO, Wilders R, Smits JP, Wilms-Schopman FJ, et al. Conduction slowing by the gap junctional uncoupler carbenoxolone. Cardiovasc Res (2003) 60(2):288–97. doi: 10.1016/j.cardiores.2003.07.004
157. Cea LA, Balboa E, Vargas AA, Puebla C, Brañes MC, Escamilla R, et al. De novo expression of functional connexins 43 and 45 hemichannels increases sarcolemmal permeability of skeletal myofibers during endotoxemia. Biochim Biophys Acta Mol Basis Dis (2019) 1865(10):2765–73. doi: 10.1016/j.bbadis.2019.06.014
158. Zhang J, Yang GM, Zhu Y, Peng XY, Li T, Liu LM. Role of connexin 43 in vascular hyperpermeability and relationship to Rock1-MLC20 pathway in septic rats. Am J Physiol Lung Cell Mol Physiol (2015) 309(11):L1323–32. doi: 10.1152/ajplung.00016.2015
159. Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J (2006) 25(21):5071–82. doi: 10.1038/sj.emboj.7601378
160. Loureiro AV, Moura-Neto LI, Martins CS, Silva PIM, Lopes MBS, Leitão RFC, et al. Role of Pannexin-1-P2X7R signaling on cell death and pro-inflammatory mediator expression induced by Clostridioides difficile toxins in enteric glia. Front Immunol (2022) 13:956340. doi: 10.3389/fimmu.2022.956340
161. Maes M, McGill MR, da Silva TC, Abels C, Lebofsky M, Weemhoff JL, et al. Inhibition of pannexin1 channels alleviates acetaminophen-induced hepatotoxicity. Arch Toxicol (2017) 91(5):2245–61. doi: 10.1007/s00204-016-1885-6
Keywords: pannexin 1 (Panx1), sepsis, MODS, inflammasome, microcirculation
Citation: Chen X, Yuan S, Mi L, Long Y and He H (2023) Pannexin1: insight into inflammatory conditions and its potential involvement in multiple organ dysfunction syndrome. Front. Immunol. 14:1217366. doi: 10.3389/fimmu.2023.1217366
Received: 09 May 2023; Accepted: 10 August 2023;
Published: 30 August 2023.
Edited by:
Ronald Sluyter, University of Wollongong, AustraliaReviewed by:
Juan Saez, Universidad de Valparaiso, ChileZhang Meng, Chongqing General Hospital, China
Nan Wang, Fourth Affiliated Hospital of Anhui Medical University, China
Copyright © 2023 Chen, Yuan, Mi, Long and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Long, ly_icu@aliyun.com; Huaiwu He, tjmuhhw@163.com
 Xiangyu Chen
Xiangyu Chen Siyi Yuan
Siyi Yuan Liangyu Mi
Liangyu Mi Yun Long
Yun Long Huaiwu He
Huaiwu He