- Department of Dermatology and Allergy Biederstein, School of Medicine, Technische Universität München, Munich, Germany
For some years now the basophil activation test (BAT) using flow cytometry has emerged as a powerful tool and sensitive marker that can be used to detect clinically relevant allergies, provide information on the severity of an allergic reaction, and monitor therapies. Compared to other in vitro diagnostic tests, BAT seems to have a better informative value in terms of clinical relevance. In general, the BAT can be used for the diagnosis of the most common forms of IgE-mediated allergy such as hymenoptera venom allergy, inhalant allergy, food allergy, and drug allergy. Various basophil markers and parameters have been established which, depending on the trigger of the respective allergy, can provide information on the clinical relevance of sensitization, on the development of natural tolerance, on trigger thresholds, and on the severity of the allergic reaction. The BAT also serves as a suitable follow-up instrument for various therapeutic approaches such as specific immunotherapy, desensitization protocols, or use of anti-IgE-antibodies for the various diseases. Quality controls for routine use, standardization, and automatization are expected to expand the range of applications for the above-mentioned indications.
Introduction
Cellular in vitro tests can be used for the allergy diagnosis of type I allergies and serve for the detection of indirect sensitization on basophils (due to their easier availability compared to mast cells). In recent years the basophil activation test (BAT) which measures activation markers after incubation with allergens or other triggers by flowcytometry has emerged as the most widely used test for this purpose.
In most studies the activation marker CD63 was favored, occasionally also CD203c. CD63, a membrane component of the basophil granules, is not a basophil-specific marker and is also expressed on other blood cells. Therefore, further labeling is necessary for the identification of basophils. Possible markers include anti-CCR3, anti-IgE, anti-CRTH2, CD203c, or anti-CD123. CD203c, an ectoenzyme located both on the plasma membrane and in the cytoplasmic compartment of basophils, is a basophil-specific marker and is expressed constitutively. The test can be performed with full blood, washed basophils, or donor basophils. This and various protocols are the main differences between the BATs used in different laboratories. CD203c and CD63 markers are upregulated after IgE receptor aggregation but have partially different metabolic pathways and follow different kinetics. Interleukin-3 potentiates the allergen-induced CD63 expression without upregulating CD63 itself, whereas it increases CD203c expression even without allergen.
Results of the BATs are usually expressed as percentages of activated basophils (% CD63+ cells), sometimes also as MFI (mean fluorescent intensity). This basophil reactivity measures the number of basophils that respond to a given stimulus. Maximal basophils reactivity is the maximal activity induced by a given stimulus. Additionally, further parameters such as results of the determination of the half-maximal concentration (EC50, CD-sens, basophil sensitivity), the calculation of a ratio (CD63 ratio), of allergen-induced CD63 activation in comparison to an IgE-dependent positive control (anti-IgE of anti-FcεRI), or of the area under the curve (AUC) in dose-response curves turned out to be of value for the assessment of clinically relevant allergies and therapy outcomes (1–4). Details can be found in an EAACI position paper (1).
Elucidation of Clinically Relevant Allergy
Food Allergy
For food allergies, the sensitivity of the BAT varies between 62 and 90% and the specificity between 80 and 100% depending on the allergen. In general, cellular tests are useful to detect the trigger of an IgE-mediated reaction to food if conventional diagnostics is negative or not available and a provocation test is expected to be potentially life-threatening. In recent years, more and more studies have been published which see the basophil activation test as a diagnostic tool prior to oral provocation being only necessary in remaining unclear cases (1).
In 2014, Santos et al. could show that the BAT discriminates between allergy and tolerance in peanut-sensitized children. Receiver operator curves (ROC) showed that the BAT with a peanut extract was better than skin prick test (SPT) and sIgE to Ara h 2 and peanut for this purpose. The application of BAT as a second or third step in the diagnostic workup dramatically reduced the need for oral provocation tests. It was recommended to perform oral food challenges in cases with equivocal BAT as well as in BAT-negative patients (5). Other authors showed that a negative CD-sens to peanut of Ara h 2 excluded an allergy (6). Certain parameters of the BAT using a peanut extract correlated with the severity of the reaction (CD63 ratio) and with the amount of eliciting allergen (CD-sens) (2, 7). Interestingly, only the use of a peanut extract and not of Ara h 2 in the BAT was associated to the eliciting dose of peanut in allergic patients (8).
In milk allergic children BAT helped in deciding when to reintroduce cow's milk in their diet showing that CD63 ratio reflected the severity of reaction to oral challenge (9). This parameter was also significantly higher among patients with milk allergy who reacted to baked milk than among those who tolerated it (10). As a consequence, the BAT reduced the need for a food challenge in children suspected of IgE-mediated cow's milk allergy (11).
Baked egg-reactive children had significantly increased basophil activation in response to intermediated stimulation levels of egg white protein compared to tolerant children, but there was a great overlap in basophil activation between these groups, which made it difficult to use it in clinical practice (12).
CD63 and CD203c expression at several allergen concentrations differed between individuals allergic or sensitized to hazelnut, too. In this study, EC50 of allergen-induced CD203c expression displayed a better discrimination compared to CD63, but there was no significant difference between patients with oral allergy syndrome and systemic reactions (13).
Similarly, basophil activation with peach extract was higher in mugwort pollen-related peach allergic patients than in tolerant subjects, but the BAT results were comparable in patients with oral allergy syndrome and systemic reactions, limiting its utility in predicting severity. In contrast, the basophil activation with Pru p 3 correlated not only with clinical allergy but also with the severity of symptoms having the best diagnostic performance compared to determination of sIgE (14).
Also for rare food allergies, e.g., the alpha-gal syndrome, it could be shown that the BAT differentiates between patients with a clinically relevant allergy and asymptomatic alpha-gal sensitization. Especially the parameter CD63 ratio for low concentrations of alpha-gal turned out to be a reliable basophil parameter and was better than sIgE to alpha-gal (4).
In another study it was shown that the BAT using hydrolyzed wheat protein and ω-5 gliadin was highly useful for diagnosing the subtypes of hydrolyzed wheat protein WDEIA (wheat-dependent exercise-induced anaphylaxis) and conventional WDEIA indicating an IgE-response to different protein components (15). Despite a tendency to higher wheat CD-sens values, only the combination of CD-sens and sIgE to wheat or wheat components was useful in the prediction of wheat challenge outcome (16).
Due to good results of CD203c sesame-induced basophil expression joint utilization of BAT and skin prick test with a high protein concentration sesame extract, this approach may also obviate the need for oral food challenge in most patients with sesame food allergy (17).
Hymenoptera Venom Allergy
For hymenoptera venom allergies, the sensitivity for the BAT varies between 85 and 100% and the specificity between 83 to 100% (1). There is no correlation between basophil activation and the clinical severity of the sting reaction reported by patients (18).
Because diagnostic sting challenges for insect venom allergies are not performed routinely for ethical reasons, this cellular test can be used in diagnostics for the detection of an IgE mediated reaction, especially if skin tests and specific IgE antibodies to insect venom extracts are negative (19). Although the component resolved diagnosis has made significant progress in specific IgE determination for insect venom allergic patients, there are still individuals in which only the BAT showed positive results (20). The use of recombinantly produced CCD-free hymenoptera venom allergens also lead to an improvement of the BAT results compared to the total hymenoptera venom extracts, both in terms of the number of positive results and the level of activation (21).
The BAT turned out to be helpful also in cases of double sensitization to bee and vespid venom and a clinical reaction to only one insect species or in cases of insect stings that cannot be clearly assigned to a particular insect species from the clinical history. In about one third of the patients information about the clinically relevant insect could be obtained by the BAT incubating the cells with bee and wasp venom extracts and, if necessary, by calculating the half-maximum concentration of the dose-response curves and forming a ratio (22–24). The clinical relevance of such BAT results could be confirmed in patients with double sensitization (skin test and specific IgE antibodies) and exclusive monosensitization to vespid venom in the BAT: 92% of the patients tolerated a sting challenge test with the bee (BAT negative) without systemic reaction, and 7% suffered from a mild systemic reaction (25). Thus, unnecessary specific immunotherapy can be avoided.
Inhalant Allergy
The sensitivity of the BAT for house dust mites, pollen, latex, or cat hair is 91–100% for both extracts and recombinant major allergens, and the specificity is between 96 and 100% (1).
Due to the good sensitivity of conventional diagnostics, cellular tests are used less for diagnostic purposes in routine, but the usefulness of the BAT and component-resolved diagnosis in distinguishing between symptomatic allergic rhinitis patients and asymptomatic sensitization to house dust mite could be demonstrated. Symptomatic patients showed a lower threshold for in vitro basophil activation and a higher AUC. There was also a positive correlation between the number of recognized house dust mite allergens and the AUC of basophil activation (26).
BAT seems to be advantageous in the diagnosis of local allergic rhinitis (LAR) because it was able to diagnose at least 50% of these cases allergic to house dust mite extracts and was more sensitive than detection of nasal specific IgE and less time-consuming than nasal provocation tests (27, 28). Similar results were shown for LAR patients with olive tree pollen (29). Based on these studies BAT has been shown to have a sensitivity of 50.0–66.6% and a specificity of 90.0–91.7% in LAR. These results reinforce the usefulness of BAT, a rational step of a diagnostic approach in LAR before nasal provocation tests.
Drug Allergy
In general, sensitivity of the BAT for most drugs is significantly lower than the sensitivity of the allergens mentioned above. The sensitivity of the BAT for beta-lactam antibiotics is about 50% with a positive predictive value of about 90%. In order to obtain relevant information about the sensitization of a patient by this test, the BAT should be carried out within half a year after the clinical reaction, since the cells' reactivity to the antibiotics decreases thereafter. Sensitivity for quinolones is slightly better (about 64%) with a positive predictive value of about 90% (30).
The sensitivity of BAT in hypersensitivity reactions to NSAIDs being independent of IgE-/FcεRI cross-linking is very low (20–40%) with specificities of 40–100%; only BAT with pyrazolones showed better results (sensitivity about 54%, specificity about 95%) (30, 31).
For radio contrast media the sensitivity is about 60% with positive predictive values of about 97%. The sensitivity for muscle relaxants varies between 54 and 92% for BAT (specificity: 100%) with a positive predictive value of about 96% (30). Algorithms for allergy workup in perioperative hypersensitivity reactions include the BAT before considering drug provocation tests: Negative skin testing and BAT results might increase confidence in performing drug provocation tests (32–34).
The studies to date show that cellular tests with drugs should only be used as a supplement to existing diagnostics, and they are not a substitute for provocation tests (30).
Therapy Outcome
Over the last few years, it has become apparent that the BAT can serve as a suitable follow-up instrument for various therapeutic approaches such as specific immunotherapy, desensitization protocols, or use of anti-IgE-antibodies for various allergic diseases.
Immunotherapy in Food Allergy
During a 12-months sublingual immunotherapy (SLIT) for peanut allergy in children a significantly decreased basophil activity after stimulation with the two lowest concentrations of a crude peanut extract could be demonstrated (35). Others showed that 2-years responders of a SLIT had significantly lower percent CD63+ basophils than non-responders for the lower peanut stimulant levels, but there are also studies demonstrating that peanut-induced basophil response was most reduced in the immune tolerant group after 24 months of oral immunotherapy (OIT), although differences between immune tolerant and non-tolerant participants did not achieve statistical significance (36, 37). Using the CD63 ratio with a crude peanut extract, a significant decrease of this parameter at all concentrations after 3 to 5 years of peanut SLIT was observed (38).
In a pilot study the utility of BAT for monitoring the acquisition of clinical tolerance after oral desensitization to cow's milk over 12 months was shown (39). Furthermore, milk-induced %CD63 and %CD203c expression was significantly lower in patients >24 months of oral immunotherapy vs. in patients <24 months of treatment (40).
Also, a decrease in antigen-specific CD63 basophil expression (egg white, ovomucoid, ovalbumin) was associated with the development of tolerance to egg by specific oral tolerance induction after 15 days and 1 month, respectively, of the buildup phase (41, 42).
In contrast, a 6 month or 12 month SLIT with a peach extract lead to an increase in basophil activation following stimulation with rPru p3 (43, 44).
Immunotherapy With Hymenoptera Venoms
A basophil activation decrease using mostly submaximal concentrations of insect venoms was only observed in part of the studies up to 18 months after beginning of venom immunotherapy (VIT), but was found throughout all studies after 2 years of treatment, and maintained until the completion of a 3–5-years immunotherapy period (45–50). A significant difference was also shown for submaximal concentrations of bee venom in patients reacting to a sting challenge compared to patients not reacting at the end (mean 4.4. years) of VIT (51). The depression of allergen-specific basophil response also lasted 1 year after completing 4–6.5 years of immunotherapy (47). In a BAT inhibition assay incubating blood of donor patients with insect venom allergy with sera from patients undergoing VIT for at least 1 year, the basophil response was almost completely inhibited at submaximal allergen concentrations (52). It was shown that patients who reacted after discontinuation of immunotherapy in field re-stings had a persistence of high basophil activation at submaximal concentrations in contrast to protected patients (53).
Immunotherpy With Inhalant Allergens
First indications of the benefit of BAT for the monitoring of specific immunotherapy (SIT) with pollen were shown in patients with Japanese cedar pollinosis. Significant reductions in the allergen-induced CD203c response in basophils were observed in part of the subjects already 1 month after beginning of a rush immunotherapy (54). CD-sens dropped significantly after reaching the maintenance dose of SIT for birch or grass allergy compared to before (55). Similarly, a decrease in allergen-induced basophil activation at submaximal allergen concentrations was demonstrated at the end of a short-term preseasonal immunotherapy over 7 weeks and additionally at the peak of the grass pollen season after immunotherapy (56). CD63 expression decreased also 8 months after an immunotherapy with an olive pollen allergoid compared to baseline values (57). Basophil sensitivity was significantly lower after 1 month of treatment with subcutaneous immunotherapy (SCIT) to grass pollen when compared to SLIT-tablet treatment, and although the differences diminished towards the end of the study (15 months), they remained significant (58). Interestingly, a decrease in basophil sensitivity after 3 weeks of treatment predicted long-term improvement in seasonal combined symptom and medication scores during 3 years of treatment in grass pollen allergic patients (59). Grass pollen immunotherapy induced sustained suppression of the allergen-specific basophil response during initiation and after 1–2 years after completion of treatment (60). In contrast to these studies, a significant decrease in the basophil activation to various grass allergens was not found after 2 or 4 months of a SLIT with a five-grass-pollen tablet vs. placebo using a defined allergen challenge chamber (61).
For house dust mite (HDM) allergy a significant decrease in BAT results in the course of specific immunotherapy with HDM allergens in children was shown. CD-sens seemed to be a better monitoring parameter than the plain percentage of CD63-expressing basophils (62). Another study demonstrated that after the first and second year of HDM immunotherapy, CD63 expression was lower in atopic dermatitis active group than in the atopic dermatitis control group (63), but others did not find a significant change of basophil reactivity to HDM during 24 months of immunotherapy nor a significant association between the change in clinical symptoms and a change in basophil reactivity (64). A phase I study with timothy grass and dust mite dual-SLIT for pollen allergy showed that basophil activation for these two allergens decreased after 24 months of SLIT compared to baseline (65).
During a latex sublingual immunotherapy in children BAT determinations showed significant decreases in recombinant and natural latex allergens in the active group at 6 months, but not at 12 months (66).
Desensitization of Drugs
It was shown in single cases that desensitization protocols can be monitored by the decrease of basophil sensitivity to the eliciting drug. This was published for insulin, pertuzumab, adalimumab, and brentuximab (67–70). For other drugs, e.g., etanercept and platinum compounds, this could not be constantly demonstrated (71, 72).
Anti-IgE Treatment
In patients with chronic urticaria in whom omalizumab is licensed there was no significant difference in activation of donor basophils incubated with patients' serum before and after 3 months of treatment (73).
In contrast, in patients with peanut allergy, individually dosed omalizumab in vivo could be monitored by CD-sens based on peanut induced basophil activation in vitro and facilitated peanut oral immunotherapy (74, 75). In severe cow's milk allergy, CD-sens monitoring during omalizumab treatment helped in the decision for performing food challenge (76).
Timothy allergic patients who received omalizumab for 3 months had a decline in CD-sens during the treatment and stayed below the starting value for at least 3 months after the treatment (77). A decrease of CD-sens after a 4-months treatment with omalizumab was also seen in cat allergic patients (78). Furthermore, 12–14 months after closing of 6-years omalizumab treatment, a downregulation of basophil reactivity was still seen (79).
Conclusion and Perspectives
This overview showed that the flowcytometric measurement of allergen-induced basophil activation and the calculation of basophil parameters from the dose-response curves could help to gain better estimates of in vivo reactions in a number, but not all type-I allergic diseases in comparison to conventional diagnostics (Table 1). Especially the consideration of results in the submaximal allergen range proved to be particularly relevant and should be pursued further. A thorough characterization of the patients which were not completely transparent in all studies is a prerequisite. Furthermore, quality controls for routine use, standardization, and automatization are expected to expand the range of applications for the above-mentioned indications.
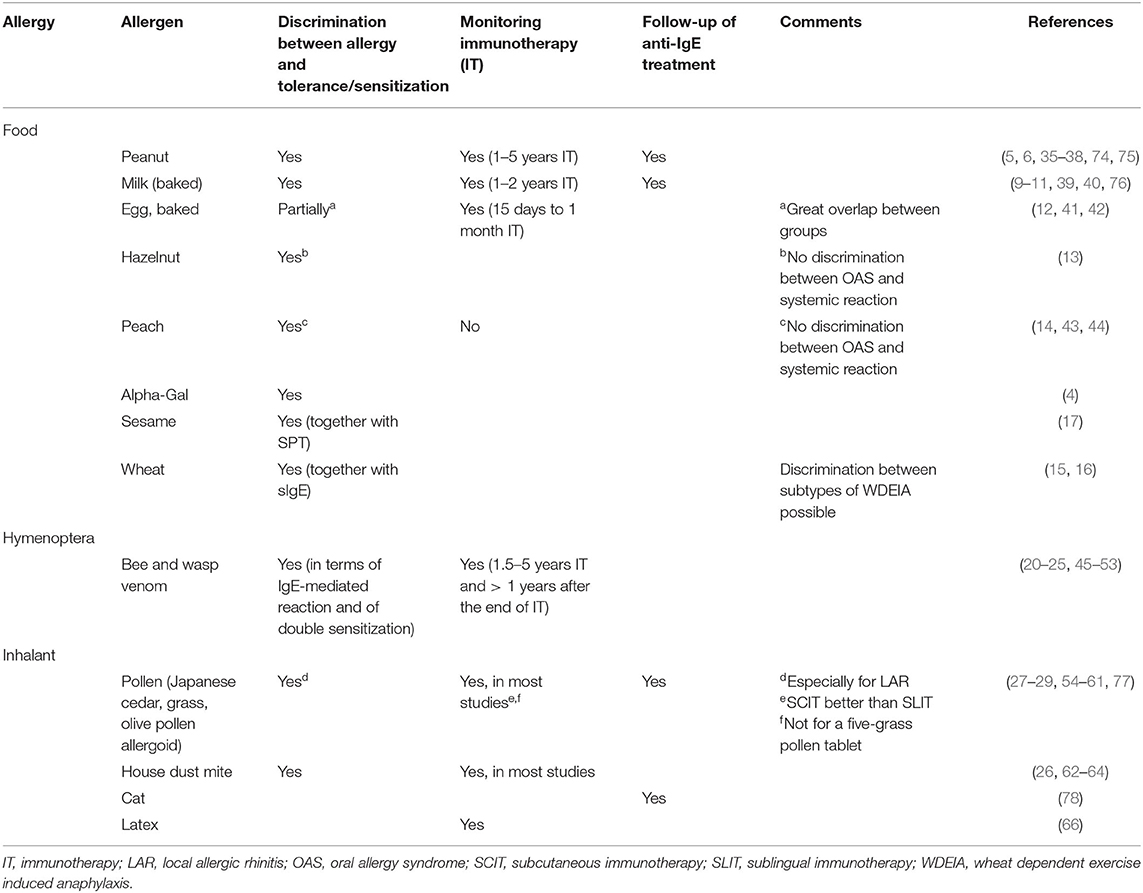
Table 1. Overview over possible current applications of BAT for discrimination between clinically relevant allergy and tolerance, monitoring immunotherapy, and follow-up of anti-IgE treatment for food, hymenoptera, and inhalant allergies according to the literature.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
BE received methodological and technical support from the company BÜHLMANN Laboratories (Schönenbuch, Switzerland).
References
1. Hoffmann HJ, Santos AF, Mayorga C, Nopp A, Eberlein B, Ferrer M, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergy disease. Allergy. (2015) 70:1393–405. doi: 10.1111/all.12698
2. Santos AF, Du Toit G, Douiri A, Radulovic S, Stephens A, Turcanu V, et al. Distinct parameters of the basophil activation test reflect the severity and threshold of allergic reactions to peanut. J Allergy Clin Immunol. (2015) 135:179–86. doi: 10.1016/j.jaci.2014.09.001
3. Hemmings O, Kwok M, McKendry R, Santos AF. The basophil activation test: old and new applications in allergy. Curr Allergy Asthma Rep. (2018) 18:77. doi: 10.1007/s11882-018-0831-5
4. Mehlich J, Fischer J, Hilger C, Swiontek K, Morisset M, Codreanu-Morel F, et al. The basophil activation test differentiates between patients with alpha-gal syndrome and asymptomatic alpha-gal sensitization. J Allergy Clin Immunol. (2019) 143:182–9. doi: 10.1016/j.jaci.2018.06.049
5. Santos AF, Douiri A, Bécares N, Wu SY, Stephens A, Radulovic S, et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol. (2014) 134:645–52. doi: 10.1016/j.jaci.2014.04.039
6. Glaumann S, Nopp A, Johansson SG, Rudengeren M, Borres MP, Nilsson C. Basophil allergen threshold sensitivity, CD-sens, IgE-sensitization and DBPCFC in peanut-sensitized children. Allergy. (2012) 67:242–7. doi: 10.1111/j.1398-9995.2011.02754.x
7. Chinthrajah RS, Purington N, Andorf S, Rosa JS, Mukai K, Hamilton, et al. Development of a tool predicting severity of allergic reaction during peanut challenge. Ann Allergy Asthma Immunol. (2018) 121:69–76.e2. doi: 10.1016/j.anai.2018.04.020
8. Chapuis A, Thevenot J, Coutant F, Messaoudi K, Michaud E, Pereira B, et al. Ara h 2 basophil activation test does not predict clinical reactivity to peanut. J Allergy Clin Immunol Pract. (2018) 6:1772–4. doi: 10.1016/j.jaip.2018.01.021
9. Rubio A, Vivinus-Nébot M, Bourrier T, Saggio B, Albertini M, Bernard A. Benefit of the basophil activation test in deciding when to reintroduce cow's milk in allergic children. Allergy. (2011) 66:92–100. doi: 10.1111/j.1398-9995.2010.02432.x
10. Ford LS, Bloom KA, Nowak-Wegrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow's milk tolerance. J Allergy Clin Immunol. (2013) 131:180–6.e1–3. doi: 10.1016/j.jaci.2012.06.003
11. Ruinemans-Koerts J, Schmidt-Hieltjes Y, Jansen AD, Savelkouel HFJ, Plaisier A, van Setten P. The basophil activation test reduces the need for a food challenge test in children suspected of IgE-mediated cow's milk allergy. Clin Exp Allergy. (2019) 49:350–56. doi: 10.1111/cea.13307
12. Berin MC, Grishin A, Masilamani M, Leung DYM, Sicherer SH, Jones M, et al. Egg-specific IgE and basophil activation but not egg-specific T-cell counts correlate with phenotypes of clinical egg allergy. J Allergy Clin Immunol. (2018) 142:149–58.e8. doi: 10.1016/j.jaci.2018.01.044
13. Lötzsch B, Dölle S, Vieths S, Worm M. Exploratory analysis of CD63 and CD203c expression in basophils from hazelnut sensitized and allergic individuals. Clin Transl Allergy. (2016) 13:6–45. doi: 10.1186/s13601-016-0134-7
14. Deng S, Yin J. Clinical utility of basophil activation test in diagnosis and predicting severity of mugwort pollen-related peach allergy. World Allergy Organ J. (2019) 12:100043. doi: 10.1016/j.waojou.2019.100043
15. Chinuki Y, Kaneko S, Dekio I, Takahashi H, Tokuda R, Nagao M, et al. CD203c expression–based basophil activation test for diagnosis of wheat-dependent exercise-induced anaphylaxis. J. Allergy Clin. Immunol. (2012) 129:5: 1404–6. doi: 10.1016/j.jaci.2012.02.049
16. Nilsson N, Nilsson C, Hedlin G, Johannsson SG, Borres MP, Nopp A. Combining analyses of basophil allergen threshold sensitivity, CD-sens, and IgE antibodies to hydrolyzed wheat, ω-5 gliadin and timothy grass enhances the prediction of wheat challenge outcome. Int Arch Allergy Immunol. (2013) 162:50–7. doi: 10.1159/000350923
17. Appel MY, Nachshon L, Elizur A, Levy MB, Katz Y, Goldberg MR. Evaluation of the basophil activation test and skin prick testing for the diagnosis of sesame food allergy. Clin Exp Allergy. (2018) 48:1025–34. doi: 10.1111/cea.13174
18. Eberlein-König B, Schmidt-Leidescher C, Rakoski J, Behrendt H, Ring J. In vitro basophil activation using CD63 expression in patients with bee and wasp venom allergy. J Investig Allergol Clin Immunol. (2006) 16:5−10.
19. Korosec P, Erzen P, Silar M, Bajrovic N, Kopac P, Kosnik M. Basophil responsiveness in patients with insect sting allergies and negative venom-specific immunoglobulin E and skin prick test results. Clin Exp Allergy. (2009) 39:1730–7. doi: 10.1111/j.1365-2222.2009.03347.x
20. Ebo DG, Faber M, Sabato V, Leysen J, Bridts CH, De Clerck LS. Component-resolved diagnosis of wasp (yellow jacket) venom allergy. Clin Exp Allergy. (2013) 43:255–61. doi: 10.1111/cea.12057
21. Schiener M, Eberlein B, Moreno-Aguilar C, Pietsch G, Serrano P, McIntyre M, et al. Application of recombinant antigen 5 allergens from seven allergy-relevant Hymenoptera species in diagnostics. Allergy. (2017) 72:98–108. doi: 10.1111/all.13000
22. Eberlein-König B, Rakoski J, Behrendt H, Ring J. Use of CD63 expression as marker of in vitro basophil activation in identifying the culprit in insect venom allergy. J Investig Allergol Clin Immunol. (2004) 14:10–6.
23. Sturm GJ, Jin C, Kranzelbinder B, Hemmer W, Sturm EM, Griesbacher A, et al. Inconsistent results of diagnostic tools hamper the differentiation between bee and vespid venom allergy. PLoS ONE. (2011) 6:e20842. doi: 10.1371/journal.pone.0020842
24. Eberlein B, Krischan L, Darsow U, Ollert M, Ring J. Double positivity to bee and wasp venom: improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J Allergy Clin Immunol. (2012) 130:155–61. doi: 10.1016/j.jaci.2012.02.008
25. Bokanovic D, Arzt-Gradwohl L, Schwarz I, Schrautzer C, Laipold K, Aberer W, et al. Possible utility of basophil activation test in dual honeybee and vespid sensitization. J Allergy Clin Immunol Pract. (2020) 8:392–394.e5. doi: 10.1016/j.jaip.2019.06.008
26. Zidarn M, Robič M, Krivec A, Šilar M, Resch-Marat Y, Vrtala S, et al. Clinical and immunological differences between asymptomatic HDM-sensitized and HDM-allergic rhinitis patients. Clin Exp Allergy. (2019) 49:808–18. doi: 10.1111/cea.13361
27. Gómez E, Campo P, Rondón C, Barrionuevo E, Blanca-Lopéz N, Torres J, et al. Role of the basophil activation test in the diagnosis of local allergic rhinitis. J Allergy Clin Immunol. (2013) 132:975–6. e1–5. doi: 10.1016/j.jaci.2013.07.016
28. Duarte Ferreira R, Ornelas C, Silva S, Morgado R, Pereira D, Escaleira D, et al. Contribution of in vivo and in vitro testing for the diagnosis of local allergic rhinitis. J Investig Allergol Clin Immunol. (2019) 29:46–8. doi: 10.18176/jiaci.0321
29. Campo P, Villalba M, Barrionuevo E, Rondón C, Salas M, Galindo L, et al. Immunologic responses to the major allergen of olea europaea in local and systemic allergic rhinitis subjects. Clin Exp Allergy. (2015) 45:1702–12. doi: 10.1111/cea.12600
30. Mayorga C, Doña I, Perez-Inestrosa E, Fernández TD, Torres MJ. The value of in vitro tests to diminish drug challenges. Int J Mol Sci. (2017) 7:18. doi: 10.3390/ijms18061222
31. Decuyper II, Mangodt EA, Van Gasse AL, Claesen K, Uyttebroek A, Faber M, et al. In vitro diagnosis of immediate drug hypersensitivity anno 2017: Potentials and limitations. Drugs R D. (2017) 17:265–78. doi: 10.1007/s40268-017-0176-x
32. Takazawa T, Sabato V, Ebo EG. In vitro diagnostic tests for perioperative hypersensitivity, a narrative review: potential, limitations, and perspectives. Br J Anaesth. (2019) 123:e117–25. doi: 10.1016/j.bja.2019.01.002
33. Garvey LH, Ebo DG, Mertes PM, Dewachter P, Garcez T, Kopac P. An EAACI position paper on the investigation of perioperative immediate hypersensitivity reactions. Allergy. (2019) 74:1872–84. doi: 10.1111/all.13820
34. Li J, Best OG, Rose MA, Green SL, Fulton RB, Fernando SL. Integrating basophil activation tests into evaluation of perioperative anaphylaxis to neuromuscular blocking agents. Br J Anaesth. (2019) 123:e135–e43. doi: 10.1016/j.bja.2019.02.024
35. Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. (2011) 127:640–6.e1. doi: 10.1016/j.jaci.2010.12.1083
36. Burks AW, Wood, Jones RA SM, Sicherer SH, Fleischer DM, Scurlock AM, et al. Sublingual immunotherapy for peanut allergy: long-term follow-up of a randomized multicenter trial. J Allergy Clin Immunol. (2015) 135:1240–8.e1-3. doi: 10.1016/j.jaci.2014.12.1917
37. Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol. (2014) 133:500–10. doi: 10.1016/j.jaci.2013.12.1037
38. Kim EH, Yang L, Ye P, Guo R, Li Q, Kulis D, et al. Long-term sublingual immunotherapy for peanut allergy in children: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. (2019) 144:1320–6.e1. doi: 10.1016/j.jaci.2019.07.030
39. Nucera E, Pecora V, Buonomo A, Rizzi A, Aruanno A, Pascolini L, et al. Utility of basophil activation test for monitoring the acquisition of clinical tolerance after oral desensitization to cow's milk: pilot study. United European Gastroenterol J. (2015) 3:272–6. doi: 10.1177/2050640615570694
40. Elizur A, Appel MY, Goldberg MR, Yichie T, Levy MB, Nachshon L, et al. Clinical and laboratory 2-year outcome of oral immunotherapy in patients with cow's milk allergy. Allergy. (2016) 71:275–8. doi: 10.1111/all.12794
41. Vila L, Moreno A, Gamboa PM, Martinez-Aranguren R, Sanz ML. Decrease in antigen-specific CD63 basophil expression is associated with the development of tolerance to egg by SOTI in children. Pediatr Allergy Immunol. (2013) 24:463–8. doi: 10.1111/pai.12070
42. Gamboa PM, Garcia-Lirio E, Gonzalez C, Martinez-Aranguren RM, Sanz ML. Is the quantification of antigen-specific basophil activation a useful tool for monitoring oral tolerance induction in children with egg allergy? J Investig Allergol Clin Immunol. (2016) 26:25–30. doi: 10.18176/jiaci.0004
43. Garrido-Fernández S, Garcia BE, Sanz ML, Echechipia S, Lizaso MT, Tabar AI. Are basophil activation and sulphidoleukotriene determination useful tests for monitoring patients with peach allergy receiving sublingual immunotherapy with a Pru p 3-enriched peach extract? J Investig Allergol Clin Immunol. (2014) 24:106–13.
44. Gomez F, Bogas G, Gonzalez M, Campo P, Salas M, Diaz-Perales A, et al. The clinical and immunological effects of Pru p 3 sublingual immunotherapy on peach and peanut allergy in patients with systemic reactions. Clin Exp Allergy. (2017) 47:339–50. doi: 10.1111/cea.12901
45. Erdmann SM, Sachs B, Kwiecien R, Moll-Slodowy S, Sauer I, Merk HF. The basophil activation test in wasp venom allergy: sensitivity, specificity and monitoring specific immunotherapy. Allergy. (2004) 59:1102–9. doi: 10.1111/j.1398-9995.2004.00624.x
46. Ebo DG, Hagendorens MM, Schuerwegh AJ, Beirens LM, Bridts CH, De Clerck S, et al. Flow-assisted quantification of in vitro activated basophils in the diagnosis of wasp venom allergy and follow-up of wasp venom immunotherapy. Cytometry B Clin Cytom. (2007) 72:196–203. doi: 10.1002/cyto.b.20142
47. Eržen R, Košnik M, Silar M, Korošec P. Basophil response and the induction of a tolerance in venom immunotherapy: a long-term sting challenge study. Allergy. (2012) 67:822–30. doi: 10.1111/j.1398-9995.2012.02817.x
48. Žitnik SE, Vesel T, Avčin T, Šilar M, Košnik M, Korošec P. Monitoring honeybee venom immunotherapy in children with the basophil activation test. Pediatr Allergy Immunol. (2012) 23:166–72. doi: 10.1111/j.1399-3038.2011.01233.x
49. Bidad K, Nawijn MC, van Oosterhout AJ, van der Heide S, Elberink JN. Basophil activation test in the diagnosis and monitoring of mastocytosis patients with wasp venom allergy on immunotherapy. Cytometry B Clin Cytom. (2014) 86:183–90. doi: 10.1002/cytob.21181
50. Rodriguez Trabado A, Cámarar Hijón C, Ramos Canrarino A, Romero-Chala S, Garcia-Trujillo JA, Fernandez Pereira LM. Short-, intermediate-, and long-term changes in basophil reactivity induced by venom immunotherapy. Allergy Asthma Immunol Res. (2016) 8:412–20. doi: 10.4168/aair.2016.8.5.412
51. Kucera P, Cvackova M, Hulikova K, Juzova O, Pachl J. Basophil activation can predict clinical sensitivity in patients after venom immunotherapy. J Investig Allergol Clin Immunol. (2010) 20:110–6.
52. Arzt L, Bokanovic D, Schrautzer C, Laipold K, Möbs C, Pfützner W. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. (2018) 73:1223–31. doi: 10.1111/all.13368
53. Peternelj A, Silar M, Erzen R, Kosnik M, Korosec P. Basophil sensitivity in patients not responding to venom immunotherapy. Int Arch Allergy Immunol. (2008) 146:248–54. doi: 10.1159/000116361
54. Nagao M, Hiraguchi Y, Hosoki K, Tokuda R, Usui T, Masuda S, et al. Allergen-induced basophil CD203c expression as a biomarker for rush immunotherapy in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. (2008) 146 (Suppl. 1):47–53. doi: 10.1159/000126061
55. Nopp A, Cardell LO, Johansson SG, Oman H. CD-sens: a biological measure of immunological changes stimulated by ASIT. Allergy. (2009) 64:811–4. doi: 10.1111/j.1398-9995.2008.01900.x
56. Özdemir K, Sin BA, Güloglu D, Ikinciogullari A, Gençtürk Z, Misirligil Z. Short-term preseasonal immunotherapy: is early clinical efficacy related to the basophil response? Int Arch Allergy Immunol. (2014) 164:237–45. doi: 10.1159/000365628
57. Gokmen NM, Ersoy R, Gulbahar O, Ardeniz O, Sin A, Unsel M, et al. Desensitization effect of preseasonal seven-injection allergoid immunotherapy with olive pollen on basophil activation: the efficacy of olive pollen-specific preseasonal allergoid immunotherapy on basophils. Int Arch Allergy Immunol. (2012) 159:75–82. doi: 10.1159/000335251
58. Aasbjerg K, Backer V, Lund G, Holm J, Nielsen, Holse NC, et al. Immunological comparison of allergen immunotherapy tablet treatment and subcutaneous immunotherapy against grass allergy. Clin Exp Allergy. (2014) 44:417–28. doi: 10.1111/cea.12241
59. Schmid JM, Würtzen PA, Siddhuray P, Jogdand P, Peters CG, Dahl R, et al. Basophil sensitivity reflects long-term clinical outcome of subcutaneous immunotherapy in grass pollen-allergic patients. Allergy. (2020) doi: 10.1111/all.14264. [Epub ahead of print].
60. Zidarn M, Košnik M, Šilar M, Bajrović N, Korošec P. Sustained effect of grass pollen subcutaneous immunotherapy on suppression of allergen-specific basophil response; a real-life, nonrandomized controlled study. Allergy. (2015) 70:547–55. doi: 10.1111/all.12581
61. Van Overtvelt L, Baron-Bodo V, Norio S, Moussu, Ricarte H C, Horak F, et al. Changes in basophil activation during grass-pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy. (2011) 66:1530–7. doi: 10.1111/j.1398-9995.2011.02696.x
62. Czarnobilska EM, Bulanda M, Spiewak R. The usefulness of the basophil activation test in monitoring specific immunotherapy with house dust mite allergens. Postepy Dermatol Alergol. (2018) 35:93–8. doi: 10.5114/ada.2018.73169
63. Sánchez J, Cardona R. Effect of immunotherapy on basophil activation induced by allergens in patients with atopic dermatitis. Rev Alerg Mex. (2014) 61:168–77.
64. Kim SH, Kim SH, Chung SJ, Kim JH, Lee SY, Kim BK. Changes in basophil activation during immunotherapy with house dust mite and mugwort in patients with allergic rhinitis. Asia Pac Allergy. (2018) 24:8–e6. doi: 10.5415/apallergy.2018.8.e6
65. Swamy RS, Reshamwala N, Hunter T, Vissamsetti S, Santos CB, Baroody M, et al. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol. (2012) 130:215–24.e7. doi: 10.1016/j.jaci.2012.04.021
66. Lasa Luaces EM, Tabar Purroy AI, García Figueroa BE, Anda Apiñaniz M, Sanz Laruga ML, Raulf-Heimsoth M, et al. Component-resolved immunologic modifications, efficacy, and tolerance of latex sublingual immunotherapy in children. Ann. Allergy Asthma Immunol. (2012) 108:367–72. doi: 10.1016/j.anai.2012.03.005
67. Luyasu S, Hougardy N, Hasdenteufel F, Jacquenet S, Weber E, Moneret-Vautrin A, et al. Anaphylactic shock due to recombinant human insulin: follow-up of a desensitization protocol by basophil activation test. Rev Med Internet. (2011) 32:39–42. doi: 10.1016/j.revmed.2010.10.350
68. González-de-Olano D, Morgado JM, Juárez-Guerrero R, Sánchez-Muñoz L, Letellez-Fernández J, Malón-Giménez D, et al. Positive basophil activation test following anaphylaxis to pertuzumab and successful treatment with rapid desensitization. J Allergy Clin Immunol Pract. (2016) 4:338–40. doi: 10.1016/j.jaip.2015.10.007
69. Thévenot J, Ferrier le Bouëdec MC, Buisson A, Bommelaer G, D'Incan M, Rouzaire P. Rapid desensitization to adalimumab is associated with decreased basophil sensitivity. J Investig Allergol Clin Immunol. (2019) 29:141–3. doi: 10.18176/jiaci.0350
70. de la Varga Martínez R, Gutiérrez Fernández D, Áñez GA, Foncubierta Fernández A, Andrés García JA, Medina Varo F. Use of the basophil activation test in monitoring clinical tolerance after desensitization to brentuximab vedotin. Ann Allergy Asthma Immunol. (2017) 118:745–7. doi: 10.1016/j.anai.2017.04.015
71. De la Varga Martínez R, Gutiérrez Fernández D, Foncubierta Fernández A, Andrés García JA, Medina Varo F. Rapid subcutaneous desensitization for treatment of hypersensitivity reactions to etanercept in two patients with positive basophil activation test. Allergol Int. (2017) 66:357–9. doi: 10.1016/j.alit.2016.09.002
72. Giavina-Bianchi P, Galvão VR, Picard M, Caiado J, Castells MC. Basophil activation test is a relevant biomarker of the outcome of rapid desensitization in platinum compounds-allergy. J Allergy Clin Immunol Pract. (2017) 5:728–36. doi: 10.1016/j.jaip.2016.11.006
73. Jörg L, Pecaric-Petkovic T, Reichenbach S, Coslovsky M, Stalder O, Pichler W, et al. Double-blind placebo-controlled trial of the effect of omalizumab on basophils in chronic urticaria patients. Clin Exp Allergy. (2018) 48:196–204. doi: 10.1111/cea.13066
74. Brandström J, Vetander M, Lilja G, Johansson SG, Sundqvist AC, Kalm F, et al. Individually dosed omalizumab: an effective treatment for severe peanut allergy. Clin Exp Allergy. (2017) 47:540–50. doi: 10.1111/cea.12862
75. Brandström J, Vetander M, Sundqvist AC, Lilja G, Johansson SGO, Melén E, et al. Individually dosed omalizumab facilitates peanut oral immunotherapy in peanut allergic adolescents. Clin Exp Allergy. (2014) 49:1328–41. doi: 10.1111/cea.13469
76. Nilsson C, Nordvall L, Johansson SG, Nopp A. Successful management of severe cow's milk allergy with omalizumab treatment and CD-sens monitoring. Asia Pac Allergy. (2014) 4:257–60. doi: 10.5415/apallergy.2014.4.4.257
77. Nopp A, Johansson SG, Ankerst J, Bylin G, Cardell LO, Grönneberg R, et al. Basophil allergen threshold sensitivity: a useful approach to anti-IgE treatment efficacy evaluation. Allergy. (2006) 61:298–302. doi: 10.1111/j.1398-9995.2006.00987.x
78. Johansson SG, Nopp A, Oman H, Ankerst J, Cardell LO, Grönneberg R, et al. The size of the disease relevant IgE antibody fraction in relation to ‘total-IgE' predicts the efficacy of anti-IgE (Xolair) treatment. Allergy. (2009) 64:1472–7. doi: 10.1111/j.1398-9995.2009.02051.x
Keywords: basophil activation test, basophil parameters, food allergy, hymenoptera venom allergy, inhalant allergy, drug allergy, immunotherapy, anti-IgE-treatment
Citation: Eberlein B (2020) Basophil Activation as Marker of Clinically Relevant Allergy and Therapy Outcome. Front. Immunol. 11:1815. doi: 10.3389/fimmu.2020.01815
Received: 16 May 2020; Accepted: 07 July 2020;
Published: 21 August 2020.
Edited by:
Simon Blank, Helmholtz Zentrum München, GermanyReviewed by:
Mitja Kosnik, University Clinic of Pulmonary and Allergic Diseases Golnik, SloveniaMaximilian Schiener, Roche Diagnostics GmbH, Germany
Copyright © 2020 Eberlein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernadette Eberlein, bernadette.eberlein@tum.de
 Bernadette Eberlein
Bernadette Eberlein