- 1Experimental Diabetes Unit, Division of Immunology, Transplantation and Infectious Diseases, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 2Immunology and Allergy Unit, Department of Medicine, Solna, Karolinska Institute and University Hospital, Stockholm, Sweden
The intestinal barrier provides the host with a strong defense line against the external environment playing also a pivotal role in the crosstalk between the gut microbiota and the immune system. Notably, increasing lines of evidence concerning autoimmune disorders such as Multiple Sclerosis (MS) report an imbalance in both intestinal microbiota composition and mucosal immunity activation, along with an alteration of gut barrier permeability, suggesting this complex network plays a crucial role in modulating the course of autoimmune responses occurring in tissues outside the gut such as the central nervous system (CNS). Here, we review current knowledge on how gut inflammation and breakage of gut barrier integrity modulates the interplay between the commensal gut microbiota and the immune system and its role in shaping brain immunity.
Introduction
The human gastrointestinal tract is equipped with physical and biological barriers whose function is not only to isolate the internal host's milieu from the external environment but also to regulate the immune system, absorption of nutrients, and to limit the access of microorganisms, both commensal, and pathogens. Hence, the intestinal mucosa operates in a dynamic manner in order to maintain intestinal integrity and immune homeostasis. Alterations of gut barrier integrity have been associated not only to inflammatory bowel diseases (IBD) but also to autoimmune disorders occurring outside the gut, such as Multiple Sclerosis (MS) both in experimental models and humans. These alterations are associated with dysbiosis, i.e., modification of microbiota composition, along with a persistent activation of the immune system within the gut mucosa (1). The mechanisms through which gut dysbiosis, breakage of gut barriers, and brain autoimmunity are linked are still unknown, but it has been proposed that a condition of dysbiosis can promote inflammation and morphological and functional changes of intestinal mucosa thus favoring uncontrolled passage of macromolecules, microorganisms or their derivates from the intestine to the systemic circulation where they activate myelin-reactive T cells. Here we will review the current knowledge on how the commensal microbiota and the gut barriers modulate the immune system within the gut mucosa and systemically and how this could influence the pathogenesis of MS (2, 3).
The Crosstalk Between the Commensal Microbiota and the Immune System
More and more revalued and increasingly considered by the recent scientific bibliography is the concept of intestinal ecosystem, by which functions and interactions among mucosal barrier, local immune system, and intestinal microflora are defined. Inside the gut microbial community, the esteemed number of species changes, but it is generally accepted that the human microbial compartment includes at least 1014 microbial cells, overall 10 times more than the total human somatic cells (4). The advances of molecular biology techniques, including ribosomal RNA 16S sequencing and metagenomics, have been instrumental in identifying the large biodiversity of the mammalian intestinal microbiota. In fact, microbiota organization is extremely diverse and influenced by many environmental and time factors. In the mammalian microbiota are five predominant phyla present (Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria e Verrucomicrobia). Firmicutes include several genera such as Clostridium, Lactobacillus and Ruminococcus, as well as Eubacterium, Fecalibacterium, and Roseburia; Bacteroidetes include Bacteroides, Prevotella and Xylanibacter genera; Proteobacteria involve Escherichia and Desulfovibrio, while Akkermansia genus belong to Verrucomicrobia phylum (5). Although every mammalian intestine harbors a unique microbiota profile, there is a balance in its composition conferring benefits toward the host, whose alteration negatively affects the overall health status (6). Indeed, studies of germ-free (GF) or antibiotic-treated mice demonstrated that functions of commensal bacteria are important in several mechanisms, including, but not restricted to, the maturation of the immune system, and other physiological processes like resistance to pathogenic infections, nutrient absorption and maintenance of intestinal barrier functions (7, 8). All these functions are closely dependent on the heterogeneous and dynamic features of the surrounding environment and they are also fundamental in maintaining the homeostasis in the intestinal environment.
Over the past few years, broad evidence of a pivotal role of the microbiota in shaping the immune responses has been collected. It has long been acknowledged that, when compared to wild-type, germ free mice exhibit imbalanced immune development characterized by immature gut-associated lymphoid tissues (GALT), decreased numbers of intestinal lymphocytes and decreased levels of both antimicrobial peptides and immunoglobulin A, two components that are essential to contrast enteric infections (9). On the other hand, the intestinal immune system is crucial to discriminate between invasive or opportunistic organisms and harmless ones, i.e., the commensal microbiota, adopting an immunological tolerance toward the latter. Hence, intestinal microbiota regulates the development and function of the immune system which in turn, shape the microbial community and regulates immune responses against the bacterial species on mucosal surfaces.
Specific resident bacteria play an important role in shaping the immunological features of different immune cell subsets. Hence, knowledge of mechanisms through which the gut microbiota regulate T cells differentiation is highly relevant in the field of immunology and autoimmune disease.
For example, it has been observed that GF mice show reduced number of Th1 and Th17 cells, as well as a reduction of IL-17 and IL-22 cytokines and overall, a decrease of Lamina Propria (LP) associated -CD4+ lymphocytes (10–12). Th17 cells, in particular, normally play a key role in host defense from pathogens, and several studies also describe their involvement in the pathogenesis of autoimmune diseases (13). A decrease in this cell subset has been correlated to the absence or reduction of segmented filamentous bacteria (SFB) in the human microbiota, suggesting their potent capacity in triggering Th17 cells differentiation and thereby, IL-17 and IL-22 induction (14, 15). Resident intestinal bacteria are also essential for the induction and the development of Foxp3+ cells in the gut. In the absence of commensals, the amount of Foxp3+ Helios− Treg (intestinal Treg) is significantly reduced at colonic lamina propria level (16). However, by re-balancing the intestinal microbiota with certain bacterial species, intestinal Treg subset can be restored (16–18). Indeed, colonization of GF mice with Clostridium strains triggered the transforming growth factor β (TGF-β) expression by intestinal epithelial cells, and thereby, promoting the differentiation of Foxp3+ T regulatory lymphocytes in the colonic lamina propria (18). Furthermore, in a mouse model of experimental colitis, through a polysaccharide-A (PSA)- mediated mechanism, a molecule univocally expressed on its surface, the commensal Bacteroides fragilis has been shown to be able to stimulate the development of T regulatory cells and to increase their suppressive capacity by inducing IL-10 anti-inflammatory cytokine (19). With similar mechanisms, the gut bacteria also have an important role in CD8+ T cell-mediated immune responses in the intestine. In fact, a reduced number of intestinal CD8+ T cells have been observed in GF mice, suggesting that signals from commensals are necessary in order to maintain the pool as well as the function of intestinal CD8+ T lymphocytes (20). Furthermore, gut microbiota also affects the development of intestinal-resident B lymphocytes residing at lamina propria level, given that GF mice have a reduced number of B cell in the intestinal LP (21). Such cell population is also responsible for the IgA production, which in turn, are strong regulators of microbiota composition. Hence, this has importance not only in order to reach a broad diversification for IgA at mucosal sites, but also to establish immune tolerance toward commensal microorganisms (22). Collectively, these studies demonstrate that our intestinal microbial community has a broad and long-lasting effect on the development and induction of T and B cell responses in the gastrointestinal tract.
Recent studies showed that is not the presence of a single or a consortium of bacterial strains that modify gut mucosal immunity but rather the functional metabolic profile that different bacterial species induce within the intestinal milieu. In fact, the gut microorganisms produce a broad range of metabolites through the anaerobic fermentation of exogenous and undigested dietary components as well as endogenous ones that are synthetized by both gut bacteria and the host. The interaction between microbial metabolic products and the host immune cells within the gut mucosa is fundamental to regulate differentiation of T cells with regulatory (Treg cells) or effector (Teff cells) properties. Among these metabolites there are, for instance, short-chain fatty acids (SCFAs), mainly acetate, propionate, and butyrate. Resulting all from dietary fiber fermentation, acetate and propionate are mainly produced by Bacteroidetes, while butyrate is produced by Firmicutes (23). SCFAs have been studied for a long time for their capacity to increase the number and function of regulatory T lymphocytes in the gut (24). In addition, it has been reported that these metabolites are involved in promoting gut barrier function and inducing anti-inflammatory effects through the inhibition of the transcription factor NF-κB, as well as in influencing gene transcription by counteracting histone-deacetylase (HDAC) activity (25–27). Another example of microbial metabolites that affect the intestinal immune system activity is provided by tryptophan derivatives metabolites that bind the aryl hydrocarbon receptor (AhR). Initially recognized for its involvement in xenobiotics metabolism, AhR was further investigated for its role in regulating mucosal immune responses (28). In such overview, tryptophan catabolism and production of indole-3-aldehyde by the commensal Lactobacillus reuteri has been shown to be able to inhibit the overgrowth of Candida albicans in mice gut by binding AhR and triggering the IL-22 pathway, thus protecting the mucosa from infection (29). In addition to tryptophan, intestinal bacteria are involved in the production of arginine derivatives such as polyamines, diamine, spermidine, and spermine, capable of exerting immune-modulatory effects, for instance, by enhancing the homeostasis of both intestinal mucosa and resident immune cells (30, 31). Hence, microbiota regulate immune responses in the gastrointestinal tract both directly and indirectly, by coordinating immune cell subsets to different conditions and regulating inflammatory responses. For these reasons, to elucidate molecular and metabolic pathways, regulatory metabolites as well as microorganisms involved in their production, is crucial to understand the pathogenesis of immune-mediated disorders.
The Role of Physical and Biological Gut Barriers in Regulating the Crosstalk Between the Commensal Microbiota and the Immune System
The complex and dynamic interaction between the commensal microorganisms and the gut immune system is regulated at the gut barriers level through several mechanisms. Intestinal microorganisms and/or microbial metabolites may affect both immune system and epithelial barrier and, at the same time, intestinal epithelial cells (IECs) and the mucus layer are able to strongly influence immune responses and to shape the microbial composition. This complex network is fundamental to maintain immune homeostasis at the intestinal level.
Although gut commensals play a key role in orchestrating intestinal immune responses, an abnormal stimulation of mucosal immunity as well as a microbial systemic spreading, may lead to harmful intestinal inflammation and excessive immune activation. To avoid such an occurrence, intestinal microbes are compartmentalized in the lumen and remain isolated from intestinal immune system by physical and functional barriers working in a coordinated manner, consisting of the mucus layer, the intestinal epithelial barrier (IEB) and the gut vascular barrier (GVB) (8). In the colon, the mucus layer covering the epithelial one is organized in two zones: an inner dense layer, usually sterile and inhabited by immune system cells, and an outer layer colonized by commensal bacteria (32). The intestinal mucus is composed mainly by mucins which represent complex agglomerates of glycoproteins characterized by specific O-linked glycans produced by globet cells (33). The main function of the mucus layer is to limit the interaction between the gut mucosa and harmful molecules or pathogens present in the intestinal lumen and thus, to regulate the passage of food and microbial products into the gut tissue and systemic circulation. However, it is now clear that the mucus layer is also fundamental to both shaping the composition of gut microbial community and regulate the interaction between commensals and the immune system. The ability to adhere to host epithelial cells and mucus has long been considered a key property underlying colonization by both pathogenic and beneficial bacteria (34–36). Hence, under certain conditions, a host may favor the colonization and competitiveness of beneficial microbial strains, as well as can promote the secretion of specific factors able to reduce adhesion and thus, limiting a strain's colonization (34). For instance, the colonic mucus layer allows microbial adhesion and growth by exposing mucins glycans which serve as both a nutritional source and attachment sites for bacterial adhesins (37, 38). Since mucin glycosylation is distinctive for each species, this phenomenon likely favors the microbial colonization in a selective manner (39, 40). In this regard, several studies showed that altered glycosylation profiles of mucins are correlated with increased inflammation in both mouse models and humans, underlying the role of mucin glycosylation in maintenance of intestinal homeostasis (41–43). Furthermore, mucin proteins provide a nutrient harvest for intestinal microbiota. Many intestinal bacteria are known to be mucin-degrading microorganisms, involving, for instance, Akkermansia muciniphila (44), Bacteroides thetaiotaomicron (45), and Bacteroides fragilis (46). Released saccharides can then be used by the same bacteria as carbohydrate sources or by other resident commensals which are biologically unable to degrade polysaccharides constituting mucins (33, 47). Importantly, the mucin glycans can control microbial translocation by regulating bacterial mucin degradation.
The dense layer of transmembrane mucins that covers the entire intestinal mucosa (the mucin barrier) does not simply act as a diffusion barrier but exerts important dynamic functions that are able also to improve the intestinal immune properties, thereby regulating the interaction between commensals and the gut immune system. For instance, MUC2 is important for the cooperation between goblet cells and CD103+ dendritic cells that leads to Treg cell differentiation and immune tolerance (48). In addition, a recent study showed that glycans associated with MUC2 induced the assembly of galectin-3-Dectin-1-FcγRIIB receptor complex and the activation of β-catenin which in turn, by inhibiting the nuclear factor-κB gene transcription, suppressed inflammatory but not tolerogenic intestinal DCs (49). Besides MUC2, MUC1 also have a strong anti-inflammatory function acting as a decoy molecule on the apical cell surface of enterocytes to limit bacterial adherence, translocation and inflammation.
Another important intestinal barrier is represented by the IEB. This anatomical structure is mainly composed of a tight junction (TJ), a adherent junction (AJ) and desmosomes. This organization is fundamental in order to maintain its integrity as well as to regulate paracellular trafficking of solutes and fluids. While AJs are necessary for assembly of TJs, the latter are made up of several kind of proteins which include transmembrane proteins (claudin, occludin), peripheral membrane proteins (zonula occludens) and regulatory molecules (2). Overall, these proteins allow the stabilization of TJs and contribute to the regulation of molecular signaling across IECs and TJs, thereby regulating gut permeability (2, 50, 51). The passage of components from the lumen through the mucosal layer is achieved by two different mechanisms, which involve paracellular and transcellular routes. The first pathway allows the passage of water, solutes and ions, and is mainly due to TJs. The regulation of paracellular permeability involve cytoskeletal contraction, endocytosis of TJ proteins, transcriptional regulation of TJ genes and epithelial cell apoptosis (52). In contrast, macromolecules such as proteins, bacterial products or microorganisms use the transcellular route which is mainly ascribed to M-cells overlying isolated lymphoid follicles or Peyer's patches (PP) (52–56).
An impairment of intestinal epithelial barrier has been recognized as a crucial mechanism for the onset of several inflammatory and immune-mediate disorders. Interestingly, increased permeability of the intestinal barrier has been ranked among those factors that may be responsible for bacterial translocation (57). Indeed, as result of gut barrier imbalances, some microorganisms, bacterial products, or toxins may translocate across the epithelium in an uncontrolled manner, leading to the onset of inflammatory disorders which may arise both in the gut and in distal organs, as described by the recent literature (50, 58–62). Current evidences have identified two main routes through which bacterial translocation may occur: transcellular or paracellular routes. While the first pathway is under the control of membrane pumps and channel, the second is due to an impairment of TJs (63). Although only a few evidences have been reported, transcellular migration has been observed in rats, where E.coli and P.mirabilis have been detected within intact enterocytes (64). Since appear to be related to an impairment of TJs, paracellular translocation has been instead associated to direct damage to enterocytes and their support structures, along with significant changes in intestinal TJs gene expression, and downregulation of both zonulin 1 and occludin (65, 66). Hence, functions of intestinal mucosa depend, at least in part, on many structural and dynamic factors, including IECs, the integrity of cellular plasma membranes, TJs and their protein components which contribute to maintain a homeostatic intestinal environment that impacts the overall health of the host.
Interestingly, the crosstalk between IECs and the mucosal immune system plays a key role to ensure the correct functioning of the immunological network operating at the intestinal level. Indeed, by detecting antigens or secreting anti-microbial molecules in response to commensal bacteria, IECs provide immune signals to these cells, thereby controlling the mucosal immune response (67).For instance, IECs sensing of gut microbial components through toll-like receptors (TRLs) protects from epithelial damage following administration of dextran sulfate sodium (DSS) (68). At the same time, intestinal immune cells exert a control on IEC-derived cytokines, such as thymic stromal lymphopoietin (TSLP), by secreting IL-12 and thus, ensuring the host ability to mount appropriate immune response toward pathogens (69). Commensal microorganisms also contribute to maintain the IEB integrity, for instance, by regulating tight junction expression and IECs proliferation (70, 71). Importantly, IECs form a real network with components of the mucosal immune system such as intraepithelial lymphocytes and immune cells residing in the intestinal lamina propria, contributing also in shaping the regulatory features of the latter in order to ensure the homeostasis. In this context, by using a culture of human monocyte-derived DCs with epithelial cells, a recent study highlights the capacity of IECs to induce anti-inflammatory DCs (72). Investigating about this phenomenon, researchers showed how this process was mediated by a combination of TSLP and other factors expressed by epithelial cells. TSLP induced-DCs did not release interleukin 12 as well as they did not drive Th1 responses toward bacteria, demonstrating that in steady state conditions the interplay between DCs and IECs is instrumental in order to maintain an anti-inflammatory environment (72).
In the intestinal barriers, gut bacteria induce also the production of secretory IgA and anti-microbial peptides (AMPs), such as RegIIIγ and alpha-defensins, which have been shown to have a crucial role in maintaining spatial compartmentalization of commensal bacteria outside the host epithelium as well as in exerting modulatory functions on both chemotaxis and TRLs signaling (73, 74). IgA also contribute to the compartmentalization of commensals by binding them and preventing their interaction with epithelial cells and their subsequent internalization. Interestingly, it has been observed that in a steady state, non- invasive bacteria residing in mice gut are coated with IgA and thus, favoring microbes to reach PP in order to induce a broader and more diversified amount of species-specific IgA in mice (22). Thus, immunoglobulin A also play a pivotal role in maintaining intestinal homeostasis.
At mucosal surfaces level, IECs-associated inflammasomes also have been demonstrated as one of those mechanisms of regulation, necessary to provide tissue homeostasis and host defense to infection (75). Several studies have described that inflammasomes play a key role in shaping the intestinal bacteria composition, such as that inflammasome-deficient mice show an aberrant microbiota, with an overgrowth of the bacterial phyla Bacteroidetes, particularly Prevotellaceae and TM7, and a decrease in the representation of Lactobacillaceae (76, 77). The subsequent dysbiosis enhances inflammatory responses in the intestine, thereby predisposing the host to develop inflammatory bowel disease (76). Moreover, mice lacking inflammasomes exhibit an imbalance in intestinal barrier functions that affect tissue homeostasis at distal organs (77, 78). Interestingly, studies in both humans and mice have identified the expression of NLRP6 inflammasome to be involved in colonic homeostasis, where it acts at the host-microbiome interface (76, 79). Specifically, it has been observed that Nlrp6−/− mice present alterations in globet cell mucus secretion, suggesting its putative role in the maintenance of mucus barrier (80). In addition, by triggering the production of epithelial IL-18 in response to commensals, it also promotes the release of AMPs (79). Therefore, dysfunctions in this regulatory system may result in imbalances of AMPs production, thereby leading to dysbiosis and intestinal auto-immune phenomena. This has been clearly demonstrated in NLRP6 deficient mice, in which colonic epithelial cells showed reduced levels of IL-18 and altered microbiota composition (76). Interestingly, a recent study demonstrated that microbiota-associated metabolites such as taurine, histamine and spermine can modulate NLRP6 inflammasome signaling, IL-18 secretion and release of AMPs, thereby promoting intestinal dysbiosis (81). Hence, by playing a pivotal role in both intestinal mucus secretion and induction of AMPs, NLRP6 inflammasome is indirectly involve in controlling bacterial adherence and colonization.
Recently, another important gut barrier has been identified, which also play a key role in modulating the interaction between the gut bacteria and the host: the GVB. The GVB is characterized by the presence of tight junctions and adherent junctions, in addition to several cell type such as endothelial cells but also pericytes and fibroblast which are involved in the maintenance of the endothelial wall integrity (82). GVB integrity is fundamental to prevent the entry of bacteria components into the gut mucosa and systemic circulation, where they can potentially trigger activation of immune cells including self-reactive T cells. The latter hypothesis is supported by the observation that damage of the GVB is present in patients affected by an extra-intestinal autoimmune disease ankylosing spondylitis (83).
In summary, it is now clear that the intestinal barriers play a central role in the biological network linking the intestinal microbiota and mucosal immune system, not only because it is physically and biologically positioned between them, but also because it participates actively in maintaining immune homeostasis in the gut. Hence, an imbalance of intestinal barrier or inflammation phenomena compromising its integrity, which might lead to the translocation of commensal or pathogenic bacteria, resulting in inflammation and activation of effector immune cells in the gut mucosa and, possibly, systemically and at sites distal from the intestine (Figure 1).
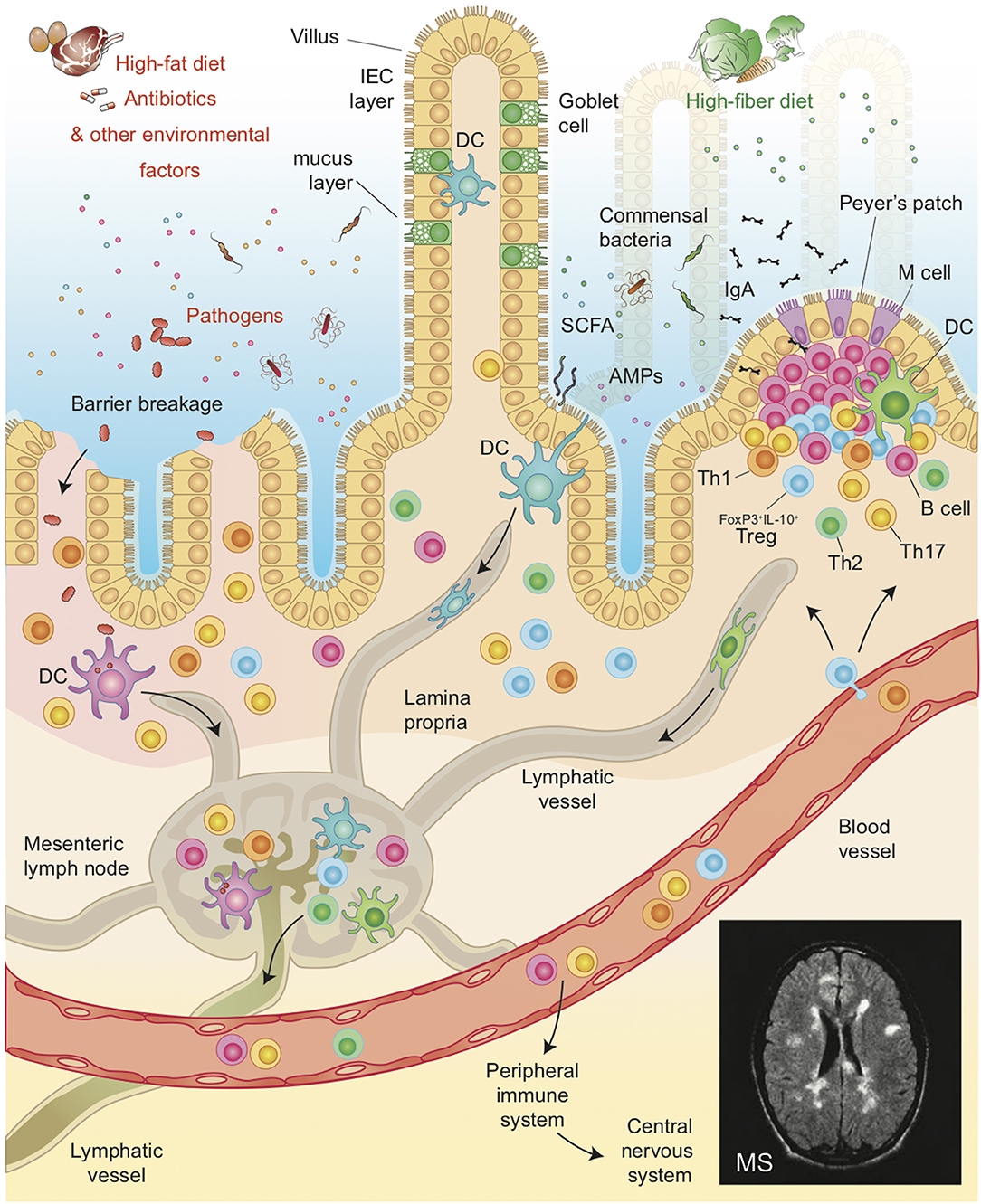
Figure 1. Illustration of the host intestinal barrier, including the complex interaction among IEB, and mucus layer and its role in modulating the cross-talk between commensal bacteria and the GALT. Environmental factors such as diet, pathogens or antibiotics affect the pathogenesis of MS by altering gut microbiota composition, intestinal permeability and by favoring the translocation of bacteria or microbial products, thereby shaping auto-immune responses both in the gut and in peripheral organs such as the Central Nervous System in Multiple Sclerosis.
How Does the Gut Environment Modulate Brain Autoimmunity?
The intestinal environment can regulate the pathogenesis of extra-intestinal autoimmune diseases such as MS through different mechanisms (84). A pro-inflammatory gut microbiota profile and alterations of the immune-regulatory function of the gut barriers could drive immune cells residing in the intestinal mucosa toward an effector phenotype. An increased differentiation of T effector cells and reduction of tolerogenic mechanisms, i.e., differentiation of Treg cells (FoxP3+ and IL-10-secreting) may affect the functional phenotype and enhance aggressiveness T cells that circulate in the gut mucosa possibly including autoimmune myelin-reactive T cells (84). Alternatively, a damage of the different physical and biological gut barriers could favor the uncontrolled translocation of bacterial components, e.g., bacterial antigens, metabolites, toxins, from the gut mucosa into the systemic circulation where they can activate myelin-reactive T cells either in the peripheral lymphoid organs (lymph nodes) or in the brain tissue (83).
The Role of the Microbiota
The influence of the gut microbiota in immune-mediated diseases extends beyond the intestine. Although the underlying mechanisms are yet to be characterized, several recent reports demonstrated that the gut microbiota is implied in the pathogenesis of different extra-intestinal autoimmune diseases such as Type 1 Diabetes (T1D), Rheumatoid Arthritis (RA) and MS. In support of a role of dysbiosis in the autoimmune pathogenesis of MS, there are epidemiological observations showing that many environmental factors involved in MS pathogenesis such as diet composition, hygienic conditions and the use of antibiotics, act by modifying the gut microbiota (85). In animal models of relapsing-remitting (RR) MS, it was shown that the TCR transgenic SJL/J mouse model that spontaneously develop experimental autoimmune encephalomyelitis (EAE) within 3–8 months of age, is protected from brain autoimmunity in the absence of microbiota when hosted under specific pathogen-free (SPF) conditions (84).
Importantly, alterations in the composition of the gut microbiota have also been found in individuals with RRMS compared to healthy controls. For instance, recent lines of evidence revealed overall decreased levels in both Bacteroidetes and Firmicutes phyla as well as in Prevotella strains in patients with RRMS compared to controls (86, 87). Interestingly, the alterations of gut microbiota composition, i.e., decreased relative abundance of Prevotella, are selectively found in RRMS patients with an active disease, meaning that those modifications could be linked with activation of brain autoimmunity and onset of MS relapse (1). Also, it is interesting to note that RRMS patients treated with Interferon-β and glatiramer acetate, show an increase in Prevotella genus, notoriously associated with high-fiber ingestion and beneficial role via butyrate synthesis (88). By evaluating individuals with MS, others observed a reduction in Firmicutes and Butyricimonas, which are butyrate-producing microorganisms (89). Likewise, another study detected an altered microbiota with decreased of bacteria with tolerogenic properties such as Clostridia XIVa and IV groups and Bacteroidetes members (87). In another study they have been found significant differences within the RR-MS patient cohort and particularly a trend toward reduced species richness in patients compared to controls (90, 91).
The mechanisms through which dysbiosis promotes MS pathogenesis are still unknown. The current hypotheses to link the gut dysbiosis with extra-intestinal autoimmune diseases such as MS hold that self-reactive T cells could be activated by mechanisms of molecular mimicry, bystander activation, and/or amplification of autoimmunity by the microbiota-induced pro-inflammatory milieu (58, 59, 92–96). For example, in animal models it has been clearly demonstrated that the gut microbiota modulate the Th17/Treg balance during the development of EAE. Those experiments allowed the characterization of some commensal bacterial species able to modulate brain autoimmunity. For instance, the colonization of GF mice with SFB, selectively promotes the differentiation of Th17 lymphocytes in both the lamina propria and CNS thus worsening disease activity in EAE (84). Conversely, Bacteroidetes fragilis was able to prevent intestinal inflammation and brain autoimmunity through a polysaccharide A- mediated mechanisms, which induced an increase of both Foxp3+ T regulatory cell and IL-10-secreting Type 1 regulatory T cells (Tr1), thus limiting Th17 cell expansion (97, 98). Similarly, oral administration of Lactobacillus spp. and Bifidobacterium bifidum strongly ameliorated the EAE clinical score by increasing Treg cell frequency in mice (99). Furthermore, the treatment with human gut-derived commensal Prevotella suppressed EAE, by decreasing pro-inflammatory Th1 and Th17 cells and enhancing Treg cells frequency (100). A recent study also confirmed that in humans the gut microbiota could modulate MS pathogenesis by promoting Th17 cell differentiation in the gut. Specifically, a reduced abundance of Prevotella strains and increased in Streptococcus oralis in patients with active RRMS correlated with increased frequency of intestinal Th17 cells (1). Collectively, these research findings demonstrate that altered profiles in the microbiota composition may be directly responsible for brain autoimmunity. However, as previously mentioned, the metabolic profile induced by microbiota rather than the single bacterial strain is fundamental in shaping gut immunity and plays a role in regulating extra-intestinal autoimmune disorders and EAE. In line with this idea, mice lacking the Aryl-hydrocarbon Receptor (AhR), an environmental sensor that detects dietary, microbial and metabolic cues such as tryptophan derivatives, show an increased susceptibility to EAE and are more prone to develop infection with Citrobacter rodentium and Listeria monocytogenes. The latter finding suggests that the metabolic environment and, specifically, tryptophan derivatives metabolites, play an important role in modulating brain autoimmunity (29, 101).
The Role of the Gut Barrier Integrity
As previously mentioned, components of the gut microbial strains could directly trigger activation of autoimmune T cells and/or their acquisition of an effector Th17 cell phenotype. However, an intestinal dysbiosis with decreased microbial function and diversity could promote extra-intestinal autoimmune diseases also by inducing inflammation and alterations of the gut barrier integrity (102, 103). In support to the latter hypothesis, studies in humans and animal models indicate that, along with intestinal inflammation and dysbiosis, an increased intestinal permeability may play a pathogenic role in brain autoimmunity (59, 95). An intestinal barrier dysfunction has been observed at the onset of EAE, in which increased permeability and altered expression of TJs have been associated to a disruption of the mucosal balance between Th1/Th17 and Treg cell subsets in intestinal lamina propria, Peyer's patches and mesenteric lymph nodes (MLN) (104). A recent study furthermore showed that the degree of intestinal permeability impairment is closely related to EAE severity (105).
Also, in humans altered gut barrier functions and composition have been observed in MS patients. The intestinal barrier permeability in patients can be inferred by using a test that directly measures the ability of lactulose and mannitol to permeate the intestinal mucosa, then revealing the degree of gut permeability and mucosal alterations of the small intestine (95). A recent study, by applying this test, detected a significant increase in intestinal permeability in RRSM patients compared to healthy controls. In fact, the concentration of mannitol recovered in urine samples was strongly decreased in MS patients than in controls, suggesting the presence of abnormalities in the mechanism of intestinal absorption (95). A previous report, by using the lactulose/mannitol test, reported an increased intestinal permeability in individuals affected by RRMS and showed that the bowel inflammatory scenario correlated with systemic immune alterations (expression of the CD45RO isoform of the leukocyte common antigen on peripheral blood CD20+ B cells) (106). Further confirmation of diminished intestinal barrier function in MS has been provided by the detection of elevated serum zonulin levels in both RRMS and Secondary Progressive Multiple Sclerosis patients (SPMS) (107). Interestingly, in this study RRMS patients in remission phase showed serum zonulin levels comparable to controls, suggesting that an impairment of gut barrier functions may be ranked not only among those factors involved in the onset of auto-immune response, but also among those implicated in the relapse phases (107). There is another finding showing that alterations of the gut barrier integrity may lead to MS pathogenesis, namely that elevated systemic lipopolysaccharide (LPS) levels, a biomarker of increased bacterial translocation, are detectable in the plasma samples of MS individuals (68). Interesting, in those patients the bacterial translocation was associated with the presence of increased in vitro Th17-like responses and higher neurological disabilities (68).
Concluding Remarks
Collectively, several lines of evidence indicate that functional and morphological alterations of the intestinal barrier are associated with dysbiosis and are present in humans and animal models of RRMS (95, 108). Persistent dysbiosis, responsible for the overgrowth of harmful bacterial species, and chronic exposures to molecules originating from microbial translocation, may shape the autoimmune T cell repertoire in MS patients toward an effector Th17 cell type interfering with Treg function (109). However, although some preliminary indications exist suggesting that gut barriers are altered in MS and EAE, the putative role of the different physical and biological gut barrier in the pathogenesis of brain autoimmunity requires yet to be fully investigated.
Author Contributions
MA reviewed the literature and wrote the manuscript. ML reviewed the literature and the final manuscript. CS reviewed the manuscript and prepared the figure. MF wrote the manuscript.
Funding
This work was supported by JDRF INO-2018-640-A-N.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. (2017) 3:e1700492. doi: 10.1126/sciadv.1700492
2. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. (2009) 9:799–809. doi: 10.1038/nri2653
3. Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. (2017) 11:821–834. doi: 10.1080/17474124.2017.1343143
4. Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol. (2013) 14:646–53. doi: 10.1038/ni.2604
5. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. (2016) 22:1079–89. doi: 10.1038/nm.4185
6. Charbonneau MR, Blanton LV, DiGiulio DB, Relman DA, Lebrilla CB, Mills DA, et al. A microbial perspective of human developmental biology. Nature. (2016) 535:48–55. doi: 10.1038/nature18845
7. Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. (2001) 291:881–4. doi: 10.1126/science.291.5505.881
8. Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. (2010) 28:623–67. doi: 10.1146/annurev-immunol-030409-101330
9. Kabat AM, Srinivasan N, MaloyKJ. Modulation of immune development and function by intestinal microbiota. Trends Immunol. (2014) 35:507–17. doi: 10.1016/j.it.2014.07.010
10. Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. (2005) 122:107–18. doi: 10.1016/j.cell.2005.05.007
11. Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. (2012) 149:1578–93. doi: 10.1016/j.cell.2012.04.037
12. Macpherson AJ, Martinic MM, Harris N. The functions of mucosal T cells in containing the indigenous commensal flora of the intestine. Cell Mol Life Sci. (2002) 59:2088–96. doi: 10.1007/s000180200009
13. Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. (2010) 140:845–58. doi: 10.1016/j.cell.2010.02.021
14. Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. (2009) 31:677–89. doi: 10.1016/j.immuni.2009.08.020
15. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. (2009) 139:485–98. doi: 10.1016/j.cell.2009.09.033
16. Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. (2014) 146:1477–88. doi: 10.1053/j.gastro.2014.01.060
17. Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. (2011) 34:794–806. doi: 10.1016/j.immuni.2011.03.021
18. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. (2011) 331:337–41. doi: 10.1126/science.1198469
19. Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. (2010) 107:12204–9. doi: 10.1073/pnas.0909122107
20. Helgeland L, Dissen E, Dai KZ, Midtvedt T, Brandtzaeg P, Vaage JT. Microbial colonization induces oligoclonal expansions of intraepithelial CD8 T cells in the gut. Eur J Immunol. (2004) 34:3389–400. doi: 10.1002/eji.200425122
21. Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. (2013) 501:112–5. doi: 10.1038/nature12496
22. Fransen F, Zagato E, Mazzini E, Fosso B, Manzari C, El Aidy S, et al. BALB/c and C57BL/6 mice differ in polyreactive IgA abundance, which impacts the generation of antigen-specific IgA and microbiota diversity. Immunity. (2015) 43:527–40. doi: 10.1016/j.immuni.2015.08.011
23. Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. (2003) 62:67–72. doi: 10.1079/PNS2002207
24. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. (2013) 341:569–73. doi: 10.1126/science.1241165
25. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. (2014) 40:833–42. doi: 10.1016/j.immuni.2014.05.014
26. Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. (2011) 12:5–9. doi: 10.1038/ni0111-5
27. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. (2013) 504:446–50. doi: 10.1038/nature12721
28. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. (2016) 16:341–52. doi: 10.1038/nri.2016.42
29. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. (2013) 39:372–85. doi: 10.1016/j.immuni.2013.08.003
30. Löser C, Eisel A, Harms D, Fölsch UR. Dietary polyamines are essential luminal growth factors for small intestinal and colonic mucosal growth and development. Gut. (1999) 44:12–6. doi: 10.1136/gut.44.1.12
31. Kibe R, Kurihara S, Sakai Y, Suzuki H, Ooga T, Sawaki E, et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep. (2014) 4:4548. doi: 10.1038/srep04548
32. Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. (2011) 108 (Suppl. 1):4659–65. doi: 10.1073/pnas.1006451107
33. Sicard JF, Le Bihan G, Vogeleer P, Jacques M, Harel J. Interactions of intestinal bacteria with components of the intestinal mucus. Front Cell Infect Microbiol. (2017) 7:387. doi: 10.3389/fcimb.2017.00387
34. McLoughlin K, Schluter J, Rakoff-Nahoum S, Smith AL, Foster KR. Host selection of microbiota via differential adhesion. Cell Host Microbe. (2016) 19:550–9. doi: 10.1016/j.chom.2016.02.021
35. Freter R. Mechanisms of association of bacteria with mucosal surfaces. Ciba Found Symp. (1981) 80:36–55. doi: 10.1002/9780470720639.ch4
36. Hartley CL, Neumann CS, Richmond MH. Adhesion of commensal bacteria to the large intestine wall in humans. Infect Immun. (1979) 23:128–32.
37. Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol. (2016) 16:639–49. doi: 10.1038/nri.2016.88
38. Juge N. Microbial adhesins to gastrointestinal mucus. Trends Microbiol. (2012) 20:30–9. doi: 10.1016/j.tim.2011.10.001
39. Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. (2008) 320:1647–51. doi: 10.1126/science.1155725
40. Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. (2006) 127:423–33. doi: 10.1016/j.cell.2006.08.043
41. An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. (2007) 204:1417–29. doi: 10.1084/jem.20061929
42. Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. (2011) 121:1657–66. doi: 10.1172/JCI45538
43. Larsson JM, Karlsson H, Crespo JG, Johansson ME, Eklund L, Sjövall H, et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm Bowel Dis. (2011) 17:2299–307. doi: 10.1002/ibd.21625
44. Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov, sp. nov, a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. (2004) 54(Pt 5):1469–76. doi: 10.1099/ijs.0.02873-0
45. Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, et al. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. (2003) 299:2074–6. doi: 10.1126/science.1080029
46. Macfarlane GT, Gibson GR. Formation of glycoprotein degrading enzymes by Bacteroides fragilis. FEMS Microbiol Lett. (1991) 61:289–93. doi: 10.1016/0378-1097(91)90567-T
47. Hoskins LC, Agustines M, McKee WB, Boulding ET, Kriaris M, Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. (1985) 75:944–53. doi: 10.1172/JCI111795
48. Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. (2002) 295:1726–9. doi: 10.1126/science.1069094
49. Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. (2013) 342:447–53. doi: 10.1126/science.1237910
50. Nagpal R, Yadav H. Bacterial translocation from the gut to the distant organs: an overview. Ann Nutr Metab. (2017) 71 (Suppl. 1):11–6. doi: 10.1159/000479918
51. Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. (2003) 18:479–97. doi: 10.1046/j.1440-1746.2003.03032.x
52. Barreau F, Hugot JP. Intestinal barrier dysfunction triggered by invasive bacteria. Curr Opin Microbiol. (2014) 17:91–8. doi: 10.1016/j.mib.2013.12.003
53. Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. (1997) 273:C1378–85. doi: 10.1152/ajpcell.1997.273.4.C1378
54. Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, et al. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. (2005) 16:5040–52. doi: 10.1091/mbc.e05-03-0193
55. Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, et al. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. (2000) 113 (Pt 11):2085–90. doi: 10.1016/S0016-5085(00)84547-3
56. Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. (2003) 171:6164–72. doi: 10.4049/jimmunol.171.11.6164
57. Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. (1995) 3:149–54. doi: 10.1016/S0966-842X(00)88906-4
58. Sorini C, Cosorich I, Lo Conte M, De Giorgi L, Facciotti F, Lucianò R, et al. Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes. Proc Natl Acad Sci USA. (2019). 116:15140–9. doi: 10.1073/pnas.1814558116
59. Buscarinu MC, Romano S, Mechelli R, Pizzolato Umeton R, Ferraldeschi M, Fornasiero A, et al. Intestinal permeability in relapsing-remitting multiple sclerosis. Neurotherapeutics. (2018) 15:68–74. doi: 10.1007/s13311-017-0582-3
60. DeMeo MT, Mutlu EA, Keshavarzian A, Tobin MC. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. (2002) 34:385–96. doi: 10.1097/00004836-200204000-00003
61. Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. (2007) 22:464–71. doi: 10.1111/j.1440-1746.2007.04933.x
62. Slyepchenko A, Maes M, Machado-Vieira R, Anderson G, Solmi M, Sanz Y, et al. Intestinal dysbiosis, gut hyperpermeability and bacterial translocation: missing links between depression, obesity and type 2 diabetes. Curr Pharm Des. (2016) 22:6087–106. doi: 10.2174/1381612822666160922165706
63. MacFie J. Current status of bacterial translocation as a cause of surgical sepsis. Br Med Bull. (2004) 71:1–11. doi: 10.1093/bmb/ldh029
64. Wells LC, Erlandsen SL. Bacterial translocation: intestinal epithelial permeability. In: Rombeau JL, Takala J, editors. Gut Dysfunction in Critical Illness (Update in Intensive Care and Emergency Medicine). Vol. 26. Berlin; Heidelberg: Springer (1996).
65. Wells CL, van de Westerlo EM, Jechorek RP, Erlandsen SL. Exposure of the lateral enterocyte membrane by dissociation of calcium-dependent junctional complex augments endocytosis of enteric bacteria. Shock. (1995) 4:204–10. doi: 10.1097/00024382-199509000-00009
66. Joly Condette C, Khorsi-Cauet H, Morlière P, Zabijak L, Reygner J, Bach V, et al. Increased gut permeability and bacterial translocation after chronic chlorpyrifos exposure in rats. PLoS ONE. (2014) 9:e102217. doi: 10.1371/journal.pone.0102217
67. Goto Y, Kiyono H. Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immunol Rev. (2012) 245:147–63. doi: 10.1111/j.1600-065X.2011.01078.x
68. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. (2004) 118:229–41. doi: 10.1016/j.cell.2004.07.002
69. Regoli M, Man A, Gicheva N, Dumont A, Ivory K, Pacini A, et al. Morphological and functional characterization of IL-12Rbeta2 chain on intestinal epithelial cells: implications for local and systemic immunoregulation. Front Immunol. (2018) 9:1177. doi: 10.3389/fimmu.2018.01177
70. Ivanov II, Littman DR. Modulation of immune homeostasis by commensal bacteria. Curr Opin Microbiol. (2011) 14:106–14. doi: 10.1016/j.mib.2010.12.003
71. Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. (2008) 295:G1025–34. doi: 10.1152/ajpgi.90227.2008
72. Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. (2005) 6:507–14. doi: 10.1038/ni1192
73. Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. (2011) 334:255–8. doi: 10.1126/science.1209791
74. Gallo RL, Hooper L. V. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. (2012) 12:503–16. doi: 10.1038/nri3228
75. Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. (2011) 32:171–9. doi: 10.1016/j.it.2011.02.002
76. Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. (2011) 145:745–57. doi: 10.1016/j.cell.2011.04.022
77. Elinav E, Thaiss CA, Flavell RA. Analysis of microbiota alterations in inflammasome-deficient mice. Methods Mol Biol. (2013) 1040:185–94. doi: 10.1007/978-1-62703-523-1_14
78. Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. (2012) 482:179–85. doi: 10.1038/nature10809
79. Levy M, Shapiro H, Thaiss CA, Elinav E. NLRP6: a multifaceted innate immune sensor. Trends Immunol. (2017) 38:248–260. doi: 10.1016/j.it.2017.01.001
80. Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. (2014) 156:1045–59. doi: 10.1016/j.cell.2014.01.026
81. Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. (2015) 163:1428–43. doi: 10.1016/j.cell.2015.10.048
82. Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. (2015) 350:830–4. doi: 10.1126/science.aad0135
83. Ciccia F, Guggino G, Rizzo A, Alessandro R, Luchetti MM, Milling S, et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. (2017) 76:1123–32. doi: 10.1136/annrheumdis-2016-210000
84. Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. (2011) 479:538–41. doi: 10.1038/nature10554
85. Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. (2015) 26:26191. doi: 10.3402/mehd.v26.26191
86. Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med. (2015) 63:729–34. doi: 10.1097/JIM.0000000000000192
87. Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS ONE. (2015) 10:e0137429. doi: 10.1371/journal.pone.0137429
88. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. (2011) 334:105–8. doi: 10.1126/science.1208344
89. Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. (2016) 7:12015. doi: 10.1038/ncomms12015
90. Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. (2016) 6:28484. doi: 10.1038/srep28484
91. Colpitts SL, Kasper LH. Influence of the gut microbiome on autoimmunity in the central nervous system. J Immunol. (2017) 198:596–604. doi: 10.4049/jimmunol.1601438
92. Lerner A, Aminov R, Matthias T. Dysbiosis may trigger autoimmune diseases via inappropriate post-translational modification of host proteins. Front Microbiol. (2016) 7:84. doi: 10.3389/fmicb.2016.00084
93. Horai R, Zárate-Bladés CR, Dillenburg-Pilla P, Chen J, Kielczewski JL, Silver PB, et al. Microbiota-dependent activation of an autoreactive T cell receptor provokes autoimmunity in an immunologically privileged site. Immunity. (2015) 43:343–53. doi: 10.1016/j.immuni.2015.07.014
94. Cole DK, Bulek AM, Dolton G, Schauenberg AJ, Szomolay B, Rittase W, et al. Hotspot autoimmune T cell receptor binding underlies pathogen and insulin peptide cross-reactivity. J Clin Invest. (2016) 126:2191–204. doi: 10.1172/JCI85679
95. Buscarinu MC, Cerasoli B, Annibali V, Policano C, Lionetto L, Capi M, et al. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult Scler. (2017) 23:442–6. doi: 10.1177/1352458516652498
96. Pearson JA, Kakabadse D, Davies J, Peng J, Warden-Smith J, Cuff S, et al. Altered gut microbiota activate and expand insulin B15-23-reactive CD8+ T cells. Diabetes. (2019) 68:1002–13. doi: 10.2337/db18-0487
97. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. (2008) 453:620–5. doi: 10.1038/nature07008
98. Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. (2010) 3:487–95. doi: 10.1038/mi.2010.29
99. Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE. (2010) 5:e9009. doi: 10.1371/journal.pone.0009009
100. Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. (2017) 20:1269–77. doi: 10.1016/j.celrep.2017.07.031
101. Shi LZ, Faith NG, Nakayama Y, Suresh M, Steinberg H, Czuprynski CJ. The aryl hydrocarbon receptor is required for optimal resistance to Listeria monocytogenes infection in mice. J Immunol. (2007) 179:6952–62. doi: 10.4049/jimmunol.179.10.6952
102. Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. (2012) 3:4–14. doi: 10.4161/gmic.19320
103. Rosser EC, Mauri C. A clinical update on the significance of the gut microbiota in systemic autoimmunity. J Autoimmun. (2016) 74:85–93. doi: 10.1016/j.jaut.2016.06.009
104. Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. (2017) 14:9–21. doi: 10.1038/nrgastro.2016.169
105. Secher T, Kassem S, Benamar M, Bernard I, Boury M, Barreau F, et al. Oral administration of the probiotic strain Escherichia coli nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front Immunol. (2017) 8:1096. doi: 10.3389/fimmu.2017.01096
106. Yacyshyn B, Meddings J, Sadowski D, Bowen-Yacyshyn MB. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig Dis Sci. (1996) 41:2493–8. doi: 10.1007/BF02100148
107. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. (2011) 91:151–75. doi: 10.1152/physrev.00003.2008
108. Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain. (2018) 141:1900–16. doi: 10.1093/brain/awy131
Keywords: autoimminity, microbiota, T cells, gut barrier, central nervous system
Citation: Antonini M, Lo Conte M, Sorini C and Falcone M (2019) How the Interplay Between the Commensal Microbiota, Gut Barrier Integrity, and Mucosal Immunity Regulates Brain Autoimmunity. Front. Immunol. 10:1937. doi: 10.3389/fimmu.2019.01937
Received: 23 May 2019; Accepted: 30 July 2019;
Published: 16 August 2019.
Edited by:
Mats Bemark, University of Gothenburg, SwedenReviewed by:
Claudio Nicoletti, University of Florence, ItalyBjörn Ragnar Weström, Lund University, Sweden
Copyright © 2019 Antonini, Lo Conte, Sorini and Falcone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marika Falcone, falcone.marika@hsr.it
 Martina Antonini
Martina Antonini Marta Lo Conte
Marta Lo Conte Chiara Sorini
Chiara Sorini Marika Falcone
Marika Falcone