- 1Department of Earth and Environmental Sciences, Ludwig-Maximilians-Universität (LMU) München, Munich, Germany
- 2Volcano Science Center, United States Geological Survey, Menlo Park, CA, Unites States
- 3Department of Earth Sciences, Institute of Hazard, Risk and Resilience, Durham University, Durham, United Kingdom
- 4Institute of Public Health, University of Cambridge, Cambridge, United Kingdom
- 5Division of Clinical Pharmacology, Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Munich, Germany
Volcanic ash is a heterogeneous mineral dust that is typically composed of a mixture of amorphous (glass) and crystalline (mineral) fragments. It commonly contains an abundance of the crystalline silica (SiO2) polymorph cristobalite. Inhalation of crystalline silica can induce inflammation by stimulating the NLRP3 inflammasome, a cytosolic receptor complex that plays a critical role in driving inflammatory immune responses. Ingested material results in the assembly of NLRP3, ASC, and caspase-1 with subsequent secretion of the interleukin-1 family cytokine IL-1β. Previous toxicology work suggests that cristobalite-bearing volcanic ash is minimally reactive, calling into question the reactivity of volcanically derived crystalline silica, in general. In this study, we target the NLRP3 inflammasome as a crystalline silica responsive element to clarify volcanic cristobalite reactivity. We expose immortalized bone marrow-derived macrophages of genetically engineered mice and primary human peripheral blood mononuclear cells (PBMCs) to ash from the Soufrière Hills volcano as well as representative, pure-phase samples of its primary componentry (volcanic glass, feldspar, cristobalite) and measure NLRP3 inflammasome activation. We demonstrate that respirable Soufrière Hills volcanic ash induces the activation of caspase-1 with subsequent release of mature IL-1β in a NLRP3 inflammasome-dependent manner. Macrophages deficient in NLRP3 inflammasome components are incapable of secreting IL-1β in response to volcanic ash ingestion. Cellular uptake induces lysosomal destabilization involving cysteine proteases. Furthermore, the response involves activation of mitochondrial stress pathways leading to the generation of reactive oxygen species. Considering ash componentry, cristobalite is the most reactive pure-phase with other components inducing only low-level IL-1β secretion. Inflammasome activation mediated by inhaled ash and its potential relevance in chronic pulmonary disease was further evidenced in PBMC using the NLRP3 small-molecule inhibitor CP-456,773 (CRID3, MCC950). Our data indicate the functional activation of the NLRP3 inflammasome by volcanic ash in murine and human macrophages in vitro. Cristobalite is identified as the apparent driver, thereby contesting previous assertions that chemical and structural imperfections may be sufficient to abrogate the reactivity of volcanically derived cristobalite. This is a novel mechanism for the stimulation of a pro-inflammatory response by volcanic particulate and provides new insight regarding chronic exposure to environmentally occurring particles.
Introduction
Explosive volcanic eruptions generate vast plumes of ash. Their fall-out can affect extensive populated areas beyond the immediate vicinity of a volcano. Ash is defined as the portion of the erupted ejecta less than 2 mm in diameter, and it is a heterogeneous mixture of glassy fragments, containing variable amounts of crystals and older rock from the volcanic edifice (lithics). Since the 1980 eruption of Mount St. Helens, USA, when ash impacted more than a million inhabitants in the Pacific Northwest, it has been known that a substantial fraction of the ejecta is of micron, or even sub-micron, size and, therefore, potentially capable of being a human respiratory health hazard (1).
An exacerbation of airway problems, such as asthma and chronic bronchitis, due to the heightened levels of fine particles in the ambient air suspended from the ash deposits, was an expected finding at Mount St. Helens (2). The presence of a significant amount of respirable crystalline silica, mainly as cristobalite, however, was wholly unforeseen and has led to repeated toxicological testing of the ash to help establish the implications of ash exposure for human health [see review in reference (3)]. In particular, there was concern regarding the risks of developing silicosis in the general population and outdoor workers due to the established consequences of crystalline silica exposure, mainly as quartz, in industrial settings (4). The eruptions of the Soufrière Hills volcano on Montserrat, West Indies, starting in 1995 and lasting over 15 years, led to similar intensive study of the volcanic cristobalite hazard (3, 5). Ongoing work has constrained the presence of cristobalite in ash to eruptions that involve lava domes or incorporate pre-existing, altered flow-units (5, 6). This is because cristobalite forms by secondary mineralization or hydrothermal alteration in these environments and, therefore, is not present in primary magmatic ejecta. These discoveries have defined the environmental side of the hazard; however, the capacity of volcanic cristobalite to incite disease remains enigmatic (7, 8).
Previously, we have observed no systematic difference in reactivity when comparing ash containing crystalline silica, predominantly as cristobalite, and ash containing negligible amounts of crystalline silica (9, 10). Experimentally, cristobalite-bearing volcanic ash has incited granuloma formation in vivo (1, 11), but it is consistently less inflammatory and fibrogenic than would be expected for a crystalline silica-bearing dust (3, 12). However, we have recently reported on the propensity of volcanic ash to initiate an inflammatory immune response in vitro in macrophages (10). Crystalline silica in other mixed-mineral dusts is known to be variably reactive (13), whereby its pathogenicity may be altered by inherent structural and chemical defects along with effects imparted by other constituents in a mixed-phase dust; indeed, structural and chemical defects of volcanic cristobalite (which contains up to 3 wt.% aluminum) together with its presence in a heterogeneous dust have all been previously implicated in its reduced potency (8, 14, 15). The conflicting results to date have hindered efforts to provide health risk assessments (16), which consider all available evidence from in vitro and in vivo toxicological tests. A mechanistic understanding of the hazard posed by volcanic ash is needed to resolve the existing conundrum and provide appropriate public health advice during future volcanic eruptions.
Inflammation plays a pivotal role in crystal-driven disease progression and can be observed in patients with particle-induced lung diseases (17). Although the exact mechanisms of how pathogenic particles drive inflammation is not completely understood, it has been shown that danger-associated molecular patterns, such as endogenous uric acid (gout) or cholesterol (atherosclerosis) as well as exogenous particles like asbestos (asbestosis) or crystalline silica (silicosis), share features of crystalline particles that activate the NLRP3 inflammasome (also known as CIAS1, NALP3, or cryopyrin) (18–20). The NLRP3 inflammasome belongs to the Nod-like receptor pyrin-containing family of cytosolic receptors and, together with the adapter molecule apoptosis-associated speck-like protein containing a CARD domain (ASC), it forms a multi-protein platform that recruits and activates caspase-1. Caspase-1 belongs to the inflammatory caspases and leads to the processing and secretion of the pro-inflammatory cytokines interleukin-1 beta (IL-1β) and IL-18 into their active forms (21). While the exact upstream mechanism of NLRP3 activation remains unclear and is part of ongoing studies, the current understanding of NLRP3 inflammasome assembly mainly consists of a two-hit mechanism. NLRP3, as well as the pro-form of IL-1β, is not constitutively expressed and needs transcriptional priming. The first step in activation can be achieved by the germ line-encoded TLRs that usually sense microbial cell wall components or viral DNA and RNA molecules. The NLRP3 inflammasome senses crystalline danger signals that can occur during autoinflammatory diseases, such as gout or atherosclerosis, and environmental diseases, such as silicosis or asbestosis (18, 20, 22). IL-1 cytokines are potent mediators of innate immunity in response to crystalline silica exposure (23, 24) and have been implicated in the pathophysiology of human and experimental diseases (25, 26).
Here, we report on the propensity of volcanic cristobalite to activate the NLRP3 inflammasome, in the wake of a series of in-conclusive toxicological investigations of ash from recent major eruptions. The NLRP3 inflammasome has emerged as a central mechanism in mediating cellular responses to various endo- and exogenous signals and particles related to environmental and life-style diseases. Given the established hazard posed by respirable crystalline silica in occupational settings, the capacity of volcanic ash to stimulate IL-1β release by macrophages in vitro (10), and the observation that instigation of chronic disease by crystalline silica is NLRP3-dependent (27, 28), we have chosen to test the ability of cristobalite-bearing volcanic ash to promote inflammation by activating the inflammasome pathway.
Materials and Methods
Volcanic Ash Sample and Major Component Control Particles
Ash sample MRA5/6/99 is a respirable sample isolated from fresh ash that fell at Soufrière Hills volcano, Montserrat, on 5 June 1999. The ash was generated during a dome-collapse event, a particular style of eruption known to produce fine-grained, cristobalite-rich ash (5, 29). The respirable fraction was isolated using the Minisplit classification system at 16,000 rpm (British Rema, Sheffield, UK), which segregates particles within a vortex. The bulk tephra (sieved to 1 mm) was first separated to give a sub-10 μm fraction, and this fraction was further separated to give the sub-4 μm fraction. Sample MRA5/6/99 has been used extensively in ash characterization and toxicity studies; the ash is characterized in detail by Horwell et al. (29) and the crystallographic properties of the cristobalite it contains by Damby et al. (14). The sample comprises ~15 wt.% crystalline silica as cristobalite, with the other major constituents being volcanic glass (amorphous silica) and plagioclase feldspar; additional minor phases identified were hornblende, orthopyroxene, titanomagnetite, and oxides (10, 29).
Pure-phase mineral samples of the primary components were analyzed alongside MRA5/6/99 to constrain their reactivity in the NLRP3 inflammasome model. Cristobalite was synthesized by heating ultra-high purity quartz glass (Heraeus HOMOSIL® 101, Hanau, Germany) for 12 h in a platinum crucible at 1,600°C in air. As a representative feldspar, we sourced labradorite (feldspar), an intermediate member of the plagioclase series, from the Bavarian State Collection for Mineralogy (Munich, Germany). Anhydrous andesite glass was produced from high temperature (1,450°C) melting of a sub-sample of Soufrière Hills pumice (described below) in a Nabertherm HI 04/17 furnace (Lilienthal, Germany) in air for 12 h. The sample was then stirred under similar conditions in a second furnace to ensure a homogenous melt and rapidly quenched to produce glass. A cristobalite-free pumice sample from the 12 July 2003 eruption of Soufrière Hills volcano was included as the mineralogy is similar to MRA5/6/99, and it thus serves as a natural material control for the minor phases identified above [see reference (30)]. All componentry samples were ground dry in a mortar and pestle prior to use.
Sample Characterization
The particle size distributions of the samples were measured using a Coulter LS 230 Analyzer (Beckman Coulter Inc., CA, USA). Data were collected using the following refractive indices: 1.63 for Soufrière Hills ash, as optimized in Horwell (31), 1.49 for crystalline silica, 1.56 for feldspar, and 1.53 for synthetic andesitic glass (32). Data are the average of three 60-s runs and are analyzed according to the Mie scattering theory. The surface area of sample MRA5/6/99 is 3.6 m2/g as measured by the BET method of nitrogen adsorption (33).
Imaging of the volcanic ash sample was carried out on a Hitachi SU-70 FE-SEM (Hitachi, Ltd., Tokyo, Japan) in the GJ Russell Microscopy Facility, Department of Physics, Durham University. Images were collected at an operating voltage of 6.0 kV and a working distance of 14 mm. Sample mineralogy was confirmed by powder X-ray diffraction on a Bruker AXS D8 ADVANCE (Bruker Corp., MA, USA) with DAVINCI design in 2θ reflection mode using Cu radiation and a Ni filter in the Department of Chemistry, Durham University.
Cell Lines and Reagents
Wild-type and knock-out bone marrow-derived immortalized mouse macrophage cell lines (iMΦ) were generated with a recombinant retrovirus, carrying v-myc and v-raf(mil) oncogenes, as previously described by Hornung et al. (22). Cells were cultured in DMEM supplemented with l-glutamine, 10% FCS (all Gibco, Darmstadt, Germany) and ciprofloxacin (Sigma, Taufkirchen, Germany). Freshly isolated human peripheral blood mononuclear cells (PBMCs) from randomly selected donors were obtained using density gradient centrifugation with subsequent red blood cell lysis. Cells were kept and stimulated in RPMI supplemented with l-glutamine, 10% FCS (all Gibco, Darmstadt, Germany) and ciprofloxacin (Sigma, Taufkirchen, Germany). iMΦ were seeded at a density of 1 × 105 cells/96 well and PBMC at a density of 1 × 106 cells/96 well and primed with 200 ng/ml (iMΦ) or 100 pg/ml (PBMC) lipopolysaccharide (LPS, InvivoGen, Toulouse, France) for 2 h. For inhibitor studies, latrunculin A (Lat A), CA-074-ME, (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylic acid (APDC), and CP-456,773 (CRID3, MCC950) were used at 20 µM and applied 1 h prior to stimulation. Cells were then stimulated with ash and componentry samples as indicated or with 5 mM adenosine triphosphate (ATP), 200 ng/ml polydeoxyadenylic acid • polythymidylic acid (dAdT), 10 µM nigericin (all Sigma Aldrich, Taufkirchen, Germany), or 1 mM H-Leu-Leu-OMe Hydrochloride (Santa Cruz, Heidelberg, Germany). After 6 h, IL-1α, IL-1β, IL-6, and TNFα cytokine levels in supernatants were measured by ELISA (all BD Biosciences, Heidelberg, Germany) or for supernatants and cell lysates with western blot analysis.
Western Blot
Immortalized macrophages and human PBMC were seeded and stimulated, as described above, but in serum-free media. Supernatants were removed and processed for protein precipitation. Briefly, equivalent amounts of methanol and 20 vol.% chloroform were added to supernatants, vortexed, and centrifuged at 12k rcf for 5 min. The top aqueous layer was discarded and methanol was added to remove remaining chloroform from precipitated whole-protein pellets. Samples were centrifuged at 12k rcf for 5 min, supernatants were carefully removed and the protein pellets were air-dried. Precipitates were resolubilized in Lämmli buffer and heated at 95°C for 5 min. Samples were separated using SDS-PAGE and blotted for protein detection. Membranes were blocked with 3% BSA and incubated with goat anti-mouse caspase-1 p20 (Santa Cruz, Heidelberg, Germany), goat anti-mouse IL-1β, or goat anti-human IL-1β (all R&D Systems, Wiesbaden-Nordenstadt, Germany) pAb overnight. HRP-coupled donkey anti-goat IgG secondary antibodies were incubated for 2 h. HRP-coupled β-actin IgG mAb served as loading control.
Light Microscopy
iMΦ were treated with 500 µg/ml volcanic ash for 6 h. Particle-treated cells were imaged by light microscopy using a Zeiss Axiovert 200 M microscope (Zeiss, Oberkochen, Germany). Cell images were acquired using a 20× objective.
Confocal Laser Reflection and Immune Fluorescence Imaging
iMΦ were seeded in glass-bottom dishes (Thermo Scientific, Darmstadt, Germany) at a density of 1 × 105 cells/ml in complete DMEM and allowed to adhere. Cells were incubated with the quenching dye conjugate DQ-Ovalbumin (DQ-OVA) in the presence or absence of MRA5/6/99 for 4 h. Cells were washed and counterstained with the membrane dye Alexa Fluor® 647-conjugated cholera toxin B subunit (Ctx B) and the nucleic acid stain Hoechst 33342 (Invitrogen, Karlsruhe, Germany). Combined reflection and immune fluorescence data were acquired using a Leica TCS SP5 AOBS confocal laser scanning microscope with 63× magnification (Wetzlar, Germany).
Statistical Analysis
All results are expressed as mean ± SD. Comparisons and significance between two groups was assessed by Student’s t-test. The level of significance is assigned to p ≤ 0.05.
Results
Processing of Volcanic Ash by Macrophages
Macrophages are a first line of defense against inhaled particles and are responsible for coordinating an inflammatory immune response. Therefore, they are key targets for in vitro assessment of the hazard posed by atmospheric particles. Diverse crystalline material has been reported to activate the NLRP3 inflammasome via phagosomal destabilization with lysosomal content leaking into the cytosol. Consequently, this leads to activation of a plethora of endoproteases and oxidative stress molecules by a not yet completely understood mechanism (22, 34). However, particle size is known to be important for entering the endosomal compartment, as reported for silica crystals with an average size of 1–2 µm (22). Volcanic ash comprises a wide range of particle sizes depending on the fragmentation efficiency of the eruption (35), but can contain a substantial respirable component (31). To represent pulmonary exposures, we used an isolated respirable ash sample, obtained through fractioning and sieving methods previously described (29), that derived from a dome-collapse event at Soufrière Hills volcano (MRA5/6/99) and synthetic particles of its corresponding componentry (Figure 1A). The ash sample is particularly fine-grained: scanning electron microscopy and particle sizing data of the isolated sample show that the particles are <5 µm, with a mode of 2 µm (Figure 1B). Backscatter SEM imaging reveals a heterogeneous distribution of volcanic glass, feldspar, and cristobalite as the predominant phases (Figure 1C). The ash sample has been previously reported to be successfully internalized by differentiated THP-1 cells, the human monocytic cell line (10).
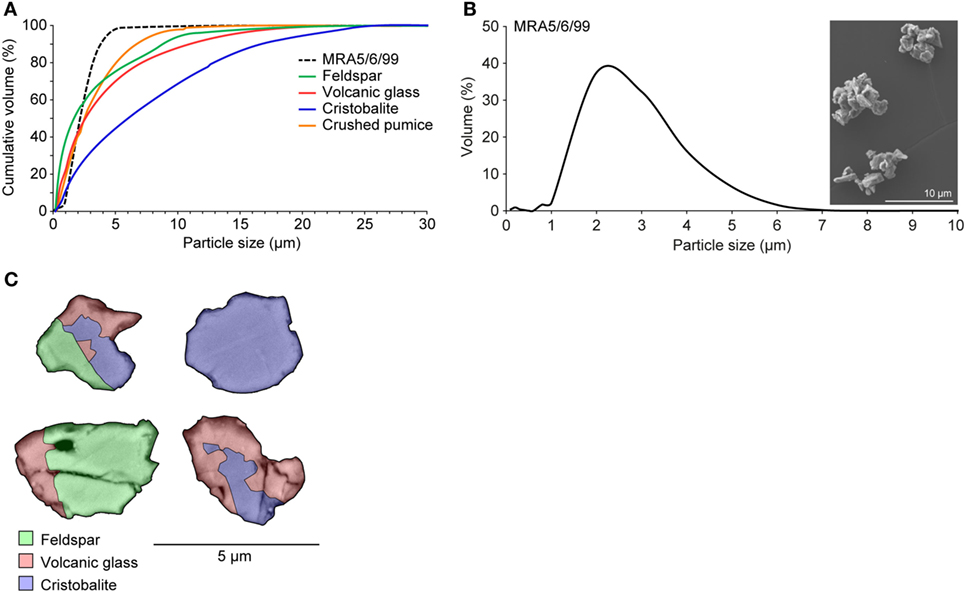
Figure 1. Characteristics of isolated volcanic ash and pure-phase componentry. (A) Cumulative particle size distributions of volcanic ash sample MRA5/6/99 and componentry samples. (B) Particle size distribution and SEM image of volcanic ash sample MRA5/6/99 (×4.00k magnification). All particle size data are the average of three runs. (C) False-color backscatter SEM image of ash in cross section (×10k magnification) evidencing the heterogeneous distribution of predominant phases: cristobalite, feldspar, and volcanic glass. Electron dispersive X-ray spectroscopy was employed for mineral identification. Images were collected at 8.0 kV and a 14.5 mm working distance.
Volcanic Ash Induces Inflammation and Leads to IL-1β Secretion
The inhalation and deposition of particulate pollutants, such as diesel exhaust, asbestos, or silica, in the small airways is known to induce inflammatory responses leading to sustained inflammation, lung fibrosis, and cancer (22, 25). The abundance of cristobalite in ash from Soufrière Hills volcano, as well as in ash from a large number of other dome-forming volcanoes [see (7) and references therein], and the potential for ash to induce the secretion of inflammatory cytokines prompted us to test the inflammatory response to ash particles further. Accordingly, we treated mouse macrophages with ash sample MRA5/6/99 and determined levels of TNFα, IL-6 and IL-1β in culture supernatants (Figures 2 and 3A). As expected, cells responded normally to the microbial TLR4 agonist LPS with high levels of TNFα and IL-6; however, no relevant cytokine secretion was detectable after exposure to volcanic ash without prior LPS stimulation (Figures 2 and 3A). Compared to other pro-inflammatory cytokines, pro-IL-1β lacks a signaling peptide sequence to exit the cell via golgi translocation. IL-1β is not constitutively expressed and requires transcriptional upregulation in response to a canonical NF-κB pathway or microbial stimuli, such as LPS, sensed by TLR receptors. As IL-1β itself is predominantly activated in a NLRP3/caspase-1-dependent manner, we investigated the involvement of the NLRP3 inflammasome in the response to volcanic ash.
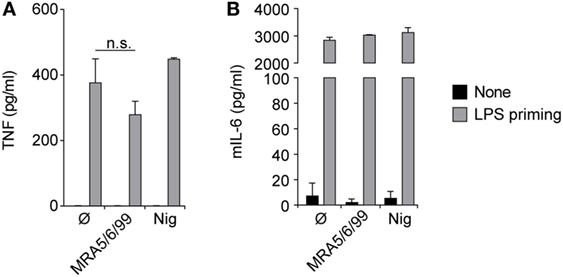
Figure 2. Volcanic ash does not induce a primary inflammatory response. Lipopolysaccharide (LPS)-primed and unprimed wild-type murine macrophages were stimulated with volcanic ash sample MRA5/6/99 or nigericin and (A) TNFα and (B) IL-6 from supernatants were examined by ELISA. Representative data from two independent experiments are shown.
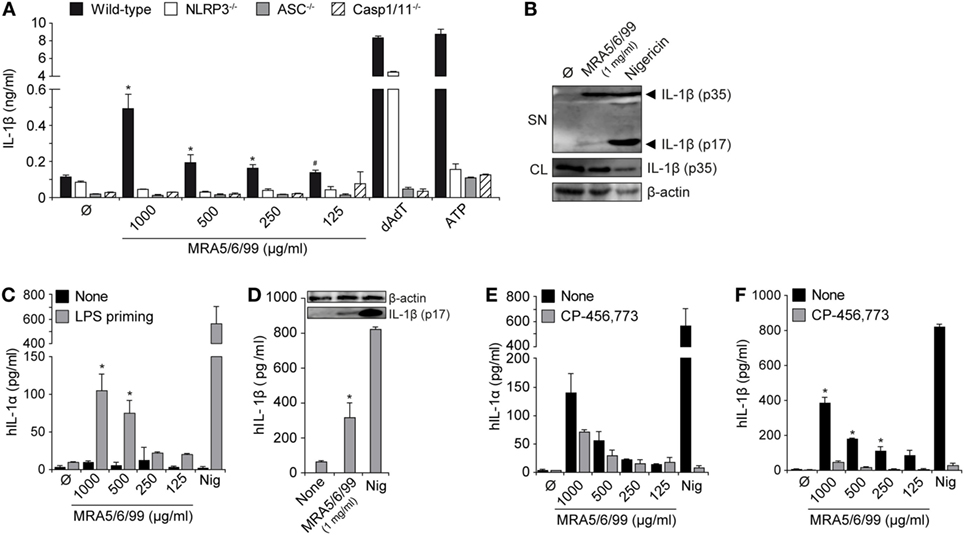
Figure 3. Volcanic ash-induced IL-1β production is NLRP3 inflammasome dependent. Lipopolysaccharide (LPS)-primed macrophages from wild-type or knock-out mice (NLRP3−/−, ASC−/−, and Casp-1/11−/−) were stimulated with MRA5/6/99 at indicated concentrations. (A) IL-1β from supernatants (SN) was measured with ELISA. (B) Mature IL-1β (p17) inSN and pro-IL-1β (p35) from cell lysates (CL) of cells stimulated with 1 mg/ml MRA5/6/99 was assessed by western blot. β-actin IgG mAb served as loading control. (C,D) Primary human peripheral blood mononuclear cells (PBMC) were either LPS-primed or left untreated and subsequently stimulated with MRA5/6/99. (C) IL-1α and (D) IL-1β levels in SN were determined by ELISA. Insertion (D) shows the western blot of bioactive IL-1β (p17) detected from SN. (E,F) IL-1α and IL-1β in SN of ash-treated human PBMC in the presence or absence of the NLRP3 inhibitor CP-456,773, assessed by ELISA. Positive controls are poly(dAdT), as a NLRP3-independent inducer, and adenosine triphosphate (ATP) or nigericin, as NLRP3-dependent inducers of IL-1β. Representative data from two independent experiments performed as triplicates are shown. (A) *p ≤ 0.05, compared to NLRP3−/−, ASC−/−, Caspase-1−/−; #p ≤ 0.05, compared to NLRP3−/− and ASC−/−; (C,D) *p ≤ 0.05, compared to none (Ø); (E,F) *p ≤ 0.05, compared to inhibitor (CP-456,773).
Volcanic Ash-Induced IL-1β Secretion Involves the NLRP3 Inflammasome and Caspase-1 Activation
Activation of the NLRP3 inflammasome results in assembly with the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and subsequent activation of caspase-1, with the release of active IL-1β. We stimulated immortalized wild-type macrophages or cells with deficiencies in the inflammasome signaling components NLRP3, ASC, or Caspase-1/11 with LPS (TLR4 ligand) and subsequently incubated the cells with volcanic ash sample MRA5/6/99 (Figure 3A). Only LPS-primed macrophages responded with high amounts of IL-1β, pointing toward the involvement of the NLRP3 inflammasome. Analysis of supernatants revealed a strong and dose-dependent secretion of IL-1β from wild-type macrophages whereas cells with defects in NLRP3 signaling (NLRP3−/−, ASC−/−, and Caspase-1/11−/−) components failed to secrete IL-1β in response to volcanic ash exposure (Figure 3A). As control served the double-stranded DNA mimic dAdT that signals NLRP3 independently via the DNA sensor absent in melanoma 2 (AIM2), still requiring the adapter molecule ASC as well as caspase-1 for the processing of IL-1β (36). ATP signaling via the ligand-gated cation channel P2×7 served as the NLRP3-dependent positive control. To further confirm proteolytic cleavage of IL-1β into its active form (p17 subunit), we performed a western blot analysis of whole cell lysates and the corresponding supernatants. As expected, volcanic ash was able to induce activation of mature IL-1β, however, in a moderate way compared to the positive control nigericin, a highly potent pore-forming toxin (Figure 3B).
We further analyzed the ash-induced release of IL-1 family cytokines (IL-1α and IL-1β) from freshly isolated primary human PBMCs (Figures 3C–E). The closely related homolog IL-1α is, in contrast to IL-1β, biologically active, although little is known about its activation and secretion. IL-1α represents a key alarmin from dying cells and plays a pivotal role in acute particle-induced lung inflammation (37). Here, we show that volcanic ash samples alone are not capable of inducing IL-1 secretion in the absence of LPS priming. In contrast, LPS-activated PBMCs secreted high amounts of IL-1α and IL-1β in a dose-dependent manner (Figure 3C). Secretion of bioactive IL-1β again was assessed by western blot showing the cleaved p17 fragment in supernatants of ash- or nigericin-treated samples (Figure 3D). Finally, the contribution of the NLRP3 inflammasome was confirmed using the specific NLRP3 inflammasome inhibitor CP-456,773 (CRID3, MCC950) that has been described earlier (38). The data show that CP-456,773 potently inhibited the release of IL-1β and, to a lesser extent, IL-1α (Figures 3E,F). Despite the well-described functions of IL-1β, IL-1α exists in two different forms, surface-bound pro-IL-1α and a mature secreted form. The fact that IL-1α can, on one hand, signal independently of inflammasome activation but, on the other hand, requires caspase-1 and mature IL-1β for secretion suggests the involvement of other inflammatory pathways, which are yet to be exactly investigated (39).
Cristobalite Is the Apparent Driver of IL-1β Secretion by Volcanic Ash
Volcanic ash is a heterogeneous dust, the componentry of which can vary substantially amongst eruptions and even within the same eruption. Therefore, identifying the phase(s) responsible for the observed reactivity is critical for hazard assessment in the event of an eruption, where ash componentry analysis is a first priority in rapid-response efforts (9, 40). Despite the fact that all of the mineral phases present in volcanic ash are not bio-soluble on the timescale of our experiments, or on the expected timescale of phagocytosis in vivo, physiologically relevant cations (K+, Na+, Ca2+) could be leached from the glass component via intra-cellular processing of particles in a matter of hours to days (41) by lysosomal fluid, a buffered, acidic (pH 5.5) solution largely comprising citric acid (42). This may augment the intra- and extra-cellular cation budget, an established condition implicated in inflammasome assembly and activation (43, 44). As neither the compositionally equivalent glass sample nor pumice, which is predominantly composed of glass, were comparably reactive, we discount particle alteration and glass leaching as primary controls on NLRP3 and subsequent caspase-1 activation by volcanic ash. This conclusion is substantiated by minimal (and equivalent) extraction of K+ and Na+ in leaching experiments conducted in dilute citric acid, the primary organic ligand in lysosomal fluid, and at a relevant pH (41). Therefore, we do not expect any significant alteration to the bulk phase assemblage throughout the experimental exposures, and contend that single-phase exposures are appropriate to probe the reactivity of the mixed-phase volcanic ash.
Of the componentry samples, the silica compound cristobalite triggered the strongest dose-dependent release of IL-1β (Figure 4A), suggesting it is the predominant driver behind production of IL-1β by volcanic ash. This aligns with the established propensity of cristobalite to induce production of IL-1β through activation of the NLRP3 inflammasome (45). We note, however, that intermediate levels of IL-1β were detected as well as the active caspase-1 subunit p20 in response to treatment of LPS-primed macrophages by all componentry samples (Figures 4B,C), thereby strengthening the evidence of NLRP3 involvement.
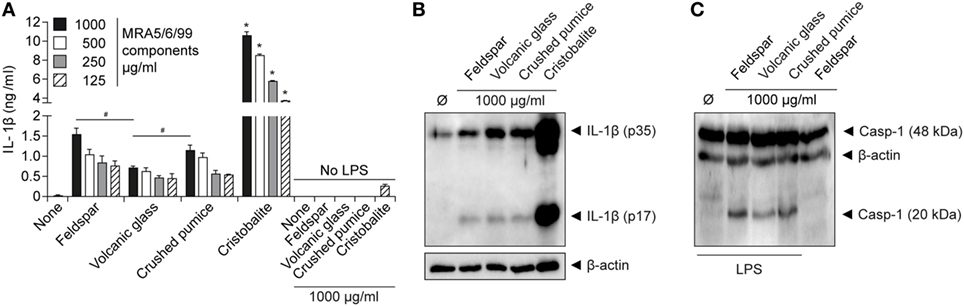
Figure 4. Cristobalite is the main component for volcanic ash-induced IL-1β release. Lipopolysaccharide (LPS)-primed and unprimed wild-type murine macrophages were stimulated with the major ash components (feldspar, volcanic glass, and cristobalite) and natural crushed pumice sample at indicated concentrations. (A) IL-1β from supernatants (SN) was examined by ELISA and (B) assessment of mature IL-1β (p17) from SN of LPS-primed macrophages by western blot. (C) Activated caspase-1 (p20 subunit) in supernatant detected by western blot analysis. Representative data from two independent experiments performed as triplicates are shown. *p ≤ 0.05, compared to other components; #p ≤ 0.05.
Volcanic Ash Acts through Translocation Into the Cytosol via Lysosomal Disruption and Reactive Oxygen Species (ROS) Production
To demonstrate that volcanic ash was internalized by macrophages, we incubated immortalized macrophages with the isolated ash sample for 4 h and imaged untreated and particle-treated cells by light microscopy (Figure 5A). The material was taken up by the cells, resulting in massive clustering of particles within the cell, and the intensity of optical refraction (darker cells) illustrates the capacity of the macrophages in particle clearance. This aligns with other recent observations of successful phagocytosis of volcanic ash by macrophages with little associated decrease in cell viability (10, 46). We next combined confocal laser reflection and fluorescence imaging to further consider cellular uptake of volcanic ash. We co-incubated the cells with the quenching dye DQ-OVA that, upon proteolytic processing, allowed us to monitor the endo-lysosomal compartment of living cells. As expected, DQ-OVA showed a distinct vesicular distribution in the absence of ash. In contrast, ash-treated cells showed enlarged vesicles with enhanced particle uptake (Figure 5B). Furthermore, ash and DQ-OVA translocated to the cytosol and showed partial co-localization. Faint fluorescence across the entire cytosol is evident of spreading and advanced dilution of the DQ quenching dye.
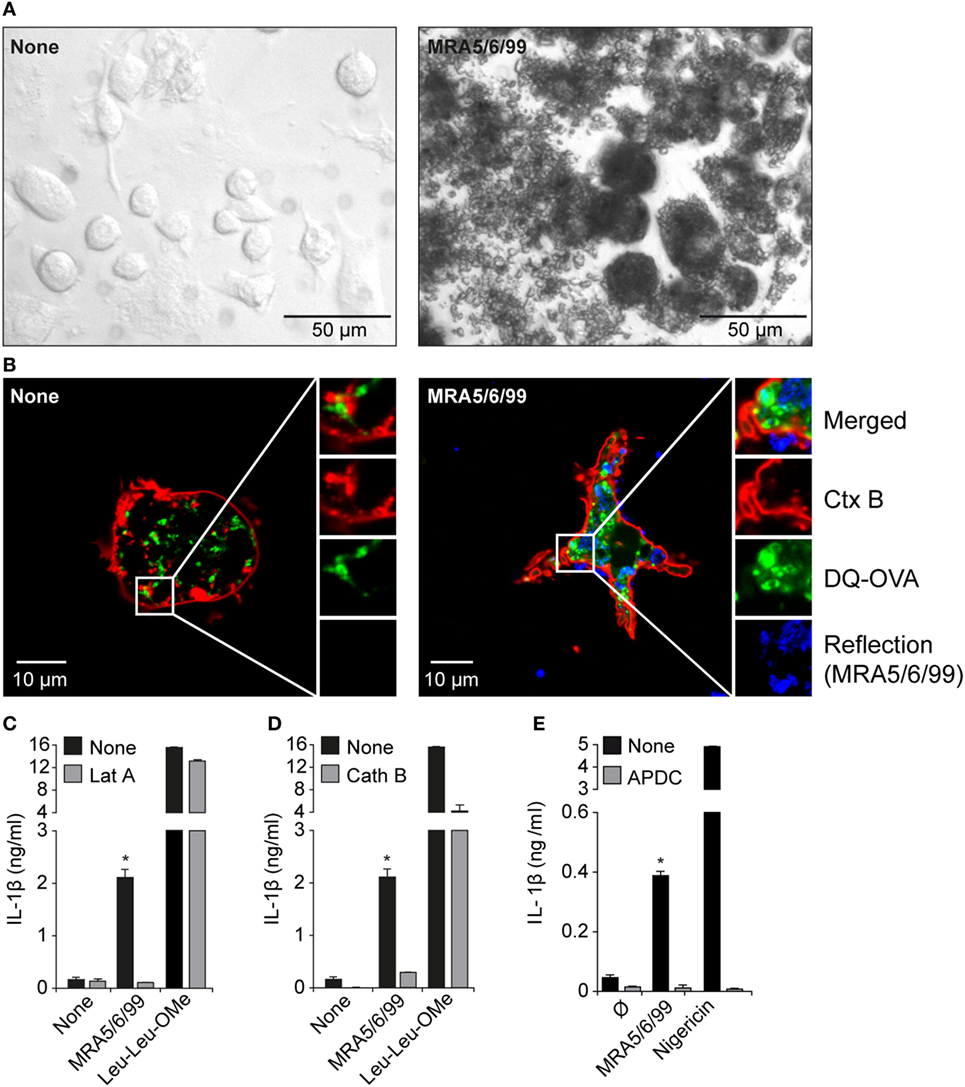
Figure 5. Internalization of volcanic ash by macrophages induces lysosomal damage and reactive oxygen species (ROS). (A) Light microscopy (20× magnification) and (B) laser scanning microscopy images (63× magnification) of untreated wild-type murine macrophages and macrophages treated with volcanic ash sample MRA5/6/99 for 4 h and stained with Ctx B (red) and DQ-Ovalbumin (DQ-OVA) (green). (C–E) LPS-primed immortalized macrophages were stimulated with 1 mg/ml MRA5/6/99 in the presence or absence of the endocytosis inhibitor latrunculin A (Lat A) (C), the cathepsin B inhibitor CA-074-Me (D), or the ROS scavenger (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylic acid (APDC) (E). Secretion of IL-1β in supernatants was measured by ELISA. Leu-Leu-OMe and nigericin served as lysosomotropic positive control and NLRP3-dependent inflammasome activator, respectively. Representative data from two independent experiments are shown. *p ≤ 0.05, compared to inhibitors (Lat A, Cath B, APDC).
To test if endosomal uptake was necessary for ash-induced cytokine production, we incubated cells with the endocytosis inhibitor Lat A prior to ash exposure of the cells and measured IL-1β secretion (Figure 5C). Lat A completely inhibited ash-induced IL-1β release, whereas it had no influence on cytokine secretion mediated by the pore-forming toxin nigericin, strengthening the role of lysosomal uptake in response to volcanic ash. The data further reveal that lysosomal uptake seems to be the main uptake mechanism leading to lysosomal rupture with subsequent IL-1β release. In terms of lysosomal destabilization, enzymes become proteolytically active and are released into cytosol for inflammasome activation. Cathepsins are protein-degrading enzymes that become cleaved upon lysosomal maturation and are released as active forms into the cytosol. The cathepsin family comprises multiple proteins involved in IL-1β processing with cathepsin B being a well-described player involved in NLRP3 activation (22, 47). To test the contribution of cathepsins in volcanic ash-induced NLRP3 activation, we pre-incubated wild-type macrophages with the cathepsin B inhibitor CA-074-Me prior to ash exposure and measured IL-1β secretion (Figure 5D). Inhibition of cathepsin B completely inhibited IL-1β secretion, compared to partial effects observed with the lysosomotropic agent l-leucyl-l-leucine methyl ester (Leu-Leu-OMe). With respect to particulate and non-particulate structures, the data further show that, in terms of NLRP3-dependent IL-1β processing, redundant roles of cathepsin family members might play a role, as previously described (48).
Particulate substances or fibers are likely candidates for the initiation of ROS (19). While ROS are an important feature of maintaining immune homeostasis, excessive production results in inflammation and carcinogenesis as postulated for inhaled asbestos fibers, with mechanisms such as “frustrated” phagocytosis being discussed (19, 49, 50). It has been shown that, in most scenarios, ROS are associate stimulators of particle-induced NLRP3 inflammasome activation. We show here that ROS production is involved in ash-induced IL-1β release, using the small-molecule ROS scavenger (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylic acid (APDC), which completely abrogated IL-1β secretion (Figure 5E). Previous work has shown that volcanic ash has the capacity to generate large amounts of ROS, largely due to the presence of non-crystalline-silica minerals (33, 51); however, the primary non-crystalline-silica components of volcanic ash have no history of inducing an inflammatory response (11, 52). In the present study, we observed low but dose-dependent production of IL-1β by the major constituents but, critically, the crushed pumice sample, which contains a similar assemblage of “non-crystalline-silica minerals,” was minimally reactive. Therefore, while auxiliary mineral-based ROS generation may be partially implicated in the observed volcanic ash reactivity, volcanic cristobalite is the likely candidate for NLRP3-mediated IL-1β production by volcanic ash.
Discussion
There is mounting evidence that, while not overtly toxic, volcanic ash exposure can result in general insult with the potential for chronic toxicity by inciting a low, but significant, pro-inflammatory response and resulting in delayed inflammation in vivo (10, 11). In this study, we identify a prominent role for ash-induced inflammation, and provide the first mechanism-specific response of macrophages to volcanic ash, whereby phagosomal uptake results in significant secretion of mature IL-1β. This secretion is caspase-1 and ASC dependent, identifying the involvement of an inflammasome-mediated pathway. Critically, silencing of the murine NLRP3 gene as well as inhibition of human NLRP3 completely abolished ash-induced IL-1β secretion. We show that endocytosis and cathepsins are involved in the induction of IL-1β secretion, which indicates that lysosomal maturation, with subsequent rupture and loss of lysosomal contents into the cytosol, is one major mechanism of volcanic ash-induced inflammation in vitro. The NLRP3 inflammasome as an ash-responsive element appears to be predominantly mediated by the crystalline silica phase (cristobalite), although other phases induced a low-level response. The componentry of volcanic ash is variable and intrinsically linked to eruption history, and, by isolating the reactivity of cristobalite, these data provide a new avenue for considering the propensity for volcanic ash to incite inflammation in order to better constrain the respiratory hazard posed during future eruptions.
The exact mechanism of how crystalline structures act on inflammasome activation remains elusive. Among others, including ROS involvement, discussed are scavenger receptors such as the macrophage receptor with collagenous structure (MARCO), CD36, or CD204, with MARCO identified as the dominant contributor in the C57BL/6 mouse model (53). However, the contribution of these receptors upon silica crystal uptake with subsequent IL-1β maturation remains controversial, since alveolar macrophages derived from MARCO-null mice show increased IL-1β cytokine release (54). Although scavenger receptor binding or membrane binding of crystalline particles without internalization may also be sufficient for silica-induced IL-1β production (55), lysosomal crystal uptake and procession is required for a potent inflammatory response. It is well accepted that the activation of the NLRP3 inflammasome pathway requires two steps: an initial, for example, NF-κB-mediated, priming step and a second stimulus for NLRP3 assembly with subsequent caspase-1 cleavage. Although we could clearly show that volcanic ash is capable of inducing NLRP3 activation in vitro, a link to the initial priming step is still missing. One explanation is provided by the fact that ROS seem to act upstream of NLRP3 activation, involving the NF-κB pathway upon phagocytosis (56, 57), although ROS are capable of performing both activation and inactivation of NF-κB pathways. However, volcanic ash exposure rarely occurs in isolation, for example, there will be concomitant exposure with anthropogenic pollution in populated regions (58), and other phases may initiate signaling. Another priming mechanism was described by Monick et al. (46), involving the ability of ash particles to promote bacterial infection by altered pathogen killing. This, in turn, would lead to an increased microbial burden with enhanced NF-κB signaling (Step 1) via pattern recognition receptors and result in a vicious circle.
Chronic inflammation resulting from particle-induced inflammasome activation, for example, in silicosis or asbestosis, is thought to derive from the inability of cells to destroy the ingested material, leading to successive rounds of apoptosis and re-ingestion of the crystalline material (4). Volcanic ash (including MRA5/6/99), however, has proven to be minimally apoptotic and necrotic to macrophages (10, 46), which suggests successful clearance of ash from the lungs. The combination of effective clearance and inflammasome activation may explain previous results from instillation experiments, such as those discussed above by Lee and Richards (11), who observed granuloma in the lymph nodes followed by considerably delayed lung inflammation, but no fibrosis. Prompt transport of ash to the lymphatic system would preclude an immediate and robust response in situ, yet inflammasome-initiated signaling may result in the delayed inflammation observed relative to pure crystalline silica. Despite this, in the most comprehensive clinical study to date, no hilar node enlargement was observed in chest X-rays of 37 children on Montserrat who had been exposed to volcanic ash from the Soufrière Hills volcano for 10 years (59). Clinical manifestations of exposure, therefore, are presently unknown.
The disparity in response between pure-phase cristobalite and volcanic ash is attributable, in part, to the heterogeneous nature of both the bulk ash (which is ~15 wt.% cristobalite) and the ash particles themselves (with individual particles often being comprised of various mineral components as seen in Figure 1C). However, the extent of this disparity, as also observed in previous work (10, 12), suggests that the reactivity of volcanic cristobalite itself is suppressed. Crystalline silica reactivity may be contingent on fracturing the surfaces of crystals in equilibrium (60). The high-energy nature of ash generation, through both explosive eruptions and dome-collapse events, ensures that volcanic cristobalite surfaces will be readily and commonly fractured. Nano-scale chemical and structural investigations of surface-exposed cristobalite crystals reveal no evidence for occlusion (8), and the presence of a reactive cristobalite surface has been implicated in the binding of similar proteins by cristobalite-rich volcanic ash and a crystalline silica standard (61). Therefore, intrinsic modifications may act to suppress volcanic cristobalite reactivity beyond the aforementioned diminished surface area-dose, as previously postulated (8, 14). In particular, all volcanic cristobalite is chemically impure, containing up to 3 wt.% aluminum (7, 8); incorporation of structural aluminum at an equivalent dopant concentration has been shown to suppress cristobalite reactivity (15), and may affect the presence of certain surface moieties (i.e., silanols) deemed critical for crystalline silica reactivity (62). Critically, however, volcanic cristobalite appears to be sufficiently reactive to have initiated a response here.
Significant advances in our understanding of the hazard posed by volcanic ash have been made in recent years; however, a serious concern regarding the crystalline silica polymorph cristobalite, the only toxic mineral phase appreciably present in volcanic ash, has persisted. The observations presented herein confirm adherence to the “variable entity” description of crystalline silica, as defined for quartz by Donaldson and Borm (13) and considered for volcanic cristobalite by Horwell et al. (8), whereby the reactive-silica burden for volcanic cristobalite is insufficient to initiate the more immediate and robust response observed with pure crystalline silica. As discussed, previously reported chemical and structural modifications have been hypothesized to alter the pathogenicity of volcanic cristobalite relative to a pure-phase standard (8). We show here, for the first time, that these modifications are insufficient to abrogate reactivity completely. With the potential for volcanic eruptions to impact millions of people, and with so few epidemiological studies having been conducted, mechanistic insight into the potential for ash to cause disease is of immediate public health value. Identification of an established pathway involved in other particle-induced diseases and through which volcanic ash can induce a chronic inflammatory response offers a foundation on which to provide health risk assessments during future volcanic crises.
Ethics Statement
This study was carried out in accordance with the Ethics Committee of the Ludwig-Maximilians-University of Munich (24.02.2006GP/cp).
Author Contributions
DED designed the study, performed the particle synthesis and characterization, and drafted the manuscript. PD designed the study, carried out the immunoassays, and drafted the manuscript. CH, PB, UK, MS, and DBD helped conceive the study. All authors read and approved the final manuscript and are accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare no conflict of interest. The funding bodies had no input into the design of the study, collection, analysis and interpretation of data, or writing of the manuscript.
Acknowledgments
The immortalized macrophage cell lines were a kind gift from Prof. Eicke Latz, Institute of Innate Immunity, University of Bonn, Germany. Thanks to Kai Uwe-Hess, Department of Earth and Environmental Sciences, Ludwig-Maximilians-Universität (LMU) München, Munich, Germany, for providing the sample of synthetic cristobalite. We are indebted to MC Leewis for providing an internal USGS review. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Funding
This work has been funded by the ERC Advanced Grant—Explosive Volcanism in the Earth System: Experimental Insights (EVOKES, no. 247076) to DBD, as well as by the DFG as part of the CRC 1123 (B7) to PD and MS; the International Doctoral Program i-Target: Immunotargeting of Cancer funded by the Elite Network of Bavaria to MS. UK acknowledges support by the Marie Curie Initial Training Network “VERTIGO,” funded through the European Seventh Framework Programme (FP7 2007–2013) under Grant Agreement number 607905.
References
1. Green FHY, Vallyathan V, Mentnech MS, Tucker JH, Merchant JA, Kiessling P, et al. Is volcanic ash a pneumoconiosis risk? Nature (1981) 293:216–7. doi:10.1038/293216a0
2. Bernstein RS, Baxter PJ, Falk H, Ing R, Foster L, Frost F. Immediate public health concerns and actions in volcanic eruptions: lessons from the Mount St. Helens eruptions, May 18–October 18, 1980. Am J Public Health (1986) 76:25–37. doi:10.2105/AJPH.76.Suppl.25
3. Horwell CJ, Baxter PJ. The respiratory health hazards of volcanic ash: a review for volcanic risk mitigation. Bull Volcanol (2006) 69:1–24. doi:10.1007/s00445-006-0052-y
4. Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med (1998) 157:1666–80. doi:10.1164/ajrccm.157.5.9707141
5. Baxter PJ, Bonadonna C, Dupree R, Hards VL, Kohn SC, Murphy MD, et al. Cristobalite in volcanic ash of the Soufriere Hills Volcano, Montserrat, British West Indies. Science (1999) 283:1142–5. doi:10.1126/science.283.5405.1142
6. Horwell CJ, Williamson BJ, Llewellin EW, Damby DE, Le Blond JS. The nature and formation of cristobalite at the Soufrière Hills volcano, Montserrat: implications for the petrology and stability of silicic lava domes. Bull Volcanol (2013) 75:696. doi:10.1007/s00445-013-0696-3
7. Damby DE. From Dome to Disease: The Respiratory Toxicity of Volcanic Cristobalite [Durham theses]. Durham, UK: Durham University e-theses (2012). p. 1–258.
8. Horwell CJ, Williamson BJ, Donaldson K, Le Blond JS, Damby DE, Bowen L. The structure of volcanic cristobalite in relation to its toxicity; relevance for the variable crystalline silica hazard. Part Fibre Toxicol (2012) 9:44. doi:10.1186/1743-8977-9-44
9. Horwell CJ, Baxter PJ, Hillman SE, Calkins JA, Damby DE, Delmelle P, et al. Physicochemical and toxicological profiling of ash from the 2010 and 2011 eruptions of Eyjafjallajökull and Grímsvötn volcanoes, Iceland using a rapid respiratory hazard assessment protocol. Environ Res (2013) 127:63–73. doi:10.1016/j.envres.2013.08.011
10. Damby DE, Murphy FA, Horwell CJ, Raftis J, Donaldson K. The in vitro respiratory toxicity of cristobalite-bearing volcanic ash. Environ Res (2016) 145:74–84. doi:10.1016/j.envres.2015.11.020
11. Lee SH, Richards RJ. Montserrat volcanic ash induces lymph node granuloma and delayed lung inflammation. Toxicology (2004) 195(2–3):155–65. doi:10.1016/j.tox.2003.09.013
12. Wilson MR, Stone V, Cullen RT, Searl A, Maynard RL, Donaldson K. In vitro toxicology of respirable Montserrat volcanic ash. Occup Environ Med (2000) 57(11):727–33. doi:10.1136/oem.57.11.727
13. Donaldson K, Borm PJA. The quartz hazard: a variable entity. Ann Occup Hyg (1998) 42(5):287–94. doi:10.1016/S0003-4878(98)00044-1
14. Damby DE, Llewellin EW, Horwell CJ, Williamson BJ, Najorka J, Cressey G, et al. The alpha-beta phase transition in volcanic cristobalite. J Appl Crystallogr (2014) 47(Pt 4):1205–15. doi:10.1107/S160057671401070X
15. Nattrass C, Horwell CJ, Damby DE, Brown D, Stone V. The effect of aluminium and sodium impurities on the in vitro toxicity and pro-inflammatory potential of cristobalite. Environ Res (2017) 159:164–75. doi:10.1016/j.envres.2017.07.054
16. Hincks TK, Aspinall WP, Baxter A, Sparks RSJ, Woo G. Long term exposure to respirable volcanic ash on Montserrat: a time series simulation. Bull Volcanol (2006) 68:266–84. doi:10.1007/s00445-005-0006-9
17. Kamp DW, Weitzman SA. The molecular basis of asbestos induced lung injury. Thorax (1999) 54:638–52. doi:10.1136/thx.54.7.638
18. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature (2006) 440(7081):237–41. doi:10.1038/nature04516
19. Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science (2008) 320:674–7. doi:10.1126/science.1156995
20. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature (2010) 464(7293):1357–61. doi:10.1038/nature08938
21. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell (2002) 10:417–26. doi:10.1016/S1097-2765(02)00599-3
22. Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol (2008) 9:847–56. doi:10.1038/ni.1631
23. Driscoll KE, Maurer JK. Cytokine and growth factor release by alveolar macrophages: potential biomarkers of pulmonary toxicity. Toxicol Pathol (1991) 19:398–405. doi:10.1177/0192623391019004-108
24. Davis GS, Pfeiffer LM, Hemenway DR. Persistent overexpression of interleukin-1beta and tumor necrosis factor-alpha in murine silicosis. J Environ Pathol Toxicol Oncol (1998) 17:99–114.
25. Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Curr Opin Pulm Med (2005) 11:169–73. doi:10.1097/01.mcp.0000152998.11335.24
26. Huaux F. New developments in the understanding of immunology in silicosis. Curr Opin Allergy Clin Immunol (2007) 7:168–73. doi:10.1097/ACI.0b013e32802bf8a5
27. Mossman BT, Glenn RE. Bioreactivity of the crystalline silica polymorphs, quartz and cristobalite, and implications for occupational exposure limits (OELs). Crit Rev Toxicol (2013) 43(8):632–60. doi:10.3109/10408444.2013.818617
28. Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A (2008) 105(26):9035–40. doi:10.1073/pnas.0803933105
29. Horwell CJ, Sparks RSJ, Brewer TS, Llewellin EW, Williamson BJ. The characterisation of respirable volcanic ash from the Soufriere Hills Volcano, Montserrat, with implications for health hazard. Bull Volcanol (2003) 65:346–62. doi:10.1007/s00445-002-0266-6
30. Horwell CJ, Hillman SE, Cole PD, Loughlin SC, Llewellin EW, Damby DE, et al. Controls on variations in cristobalite abundance in ash generated by the Soufrière hills volcano, Montserrat in the period 1997–2010. Geol Soc Lond Mem (2014) 39:399–406. doi:10.1144/M39.21
31. Horwell CJ. Grain size analysis of volcanic ash for the rapid assessment of respiratory health hazard. J Environ Monit (2007) 9(10):1107–15. doi:10.1039/b710583p
33. Horwell CJ, Fenoglio I, Ragnarsdottir KV, Sparks RSJ, Fubini B. Surface reactivity of volcanic ash from the eruption of Soufrière Hills volcano, Montserrat, West Indies with implications for health hazards. Environ Res (2003) 93(2):202–15. doi:10.1016/S0013-9351(03)00044-6
34. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol (2010) 11:136–40. doi:10.1038/ni.1831
35. Kueppers U, Scheu B, Spieler O, Dingwell DB. Fragmentation efficiency of explosive volcanic eruptions: a study of experimentally generated pyroclasts. J Volcanol Geotherm Res (2006) 153(1–2):125–35. doi:10.1016/j.jvolgeores.2005.08.006
36. Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature (2009) 458:514–8. doi:10.1038/nature07725
37. Rabolli V, Badissi AA, Devosse R, Uwambayinema F, Yakoub Y, Palmai-Pallag M, et al. The alarmin IL-1α is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Part Fibre Toxicol (2014) 11:69. doi:10.1186/s12989-014-0069-x
38. Primiano MJ, Lefker BA, Bowman MR, Bree AG, Hubeau C, Bonin PD, et al. Efficacy and pharmacology of the NLRP3 inflammasome inhibitor CP-456,773 (CRID3) in murine models of dermal and pulmonary inflammation. J Immunol (2016) 197(6):2421–33. doi:10.4049/jimmunol.1600035
39. Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity (2012) 36(3):388–400. doi:10.1016/j.immuni.2012.01.018
40. Damby DE, Horwell CJ, Baxter PJ, Delmelle P, Donaldson K, Dunster C, et al. The respiratory health hazard of tephra from the 2010 Centennial eruption of Merapi with implications for occupational mining of deposits. J Volcanol Geotherm Res (2013) 261:376–87. doi:10.1016/j.jvolgeores.2012.09.001
41. Fiantis D, Nelson M, Shamshuddin J, Goh TB, Van Ranst E. Leaching experiments in recent tephra deposits from Talang volcano (West Sumatra), Indonesia. Geoderma (2010) 156(3–4):161–72. doi:10.1016/j.geoderma.2010.02.013
42. Stebounova LV, Guio E, Grassian VH. Silver nanoparticles in simulated biological media: a study of aggregation, sedimentation, and dissolution. J Nanopart Res (2010) 13:233–44. doi:10.1007/s11051-010-0022-3
43. Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity (2013) 38(6):1142–53. doi:10.1016/j.immuni.2013.05.016
44. Schorn C, Frey B, Lauber K, Janko C, Strysio M, Keppeler H, et al. Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem (2011) 286:35–41. doi:10.1074/jbc.M110.139048
45. Sayan M, Mossman BT. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part Fibre Toxicol (2016) 13(51):1–15. doi:10.1186/s12989-016-0162-4
46. Monick MM, Baltrusaitis J, Powers LS, Borcherding JA, Caraballo JC, Mudunkotuwa I, et al. Effects of Eyjafjallajökull volcanic ash on innate immune system responses and bacterial growth in vitro. Environ Health Perspect (2013) 121(6):691–8. doi:10.1289/ehp.1206004
47. Hoegen T, Tremel N, Klein M, Angele B, Wagner H, Kirschning C, et al. The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release. J Immunol (2011) 187(10):5440–51. doi:10.4049/jimmunol.1100790
48. Orlowski GM, Colbert JD, Sharma S, Bogyo M, Robertson SA, Rock KL. Multiple cathepsins promote pro-IL-1β synthesis and NLRP3-mediated IL-1β activation. J Immunol (2015) 195(4):1685–97. doi:10.4049/jimmunol.1500509
49. Hansen K, Mossman BT. Generation of superoxide from alveolar macrophages exposed to asbestiform and nonfibrous particles. Cancer Res (1987) 47(6):1681–6.
50. Shukla A, Gulumian M, Hei TK, Kamp D, Rahman Q, Mossman BT. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic Biol Med (2003) 34(9):1117–29. doi:10.1016/S0891-5849(03)00060-1
51. Horwell CJ, Fenoglio I, Fubini B. Iron-induced hydroxyl radical generation from basalitic volcanic ash. Earth Planet Sci Lett (2007) 261:662–9. doi:10.1016/j.epsl.2007.07.032
52. Housley DG, Bérubé KA, Jones TP, Anderson S, Pooley FD, Richards RJ. Pulmonary epithelial response in the rat lung to instilled Montserrat respirable dusts and their major mineral components. Occup Environ Med (2002) 59:466–72. doi:10.1136/oem.59.7.466
53. Hamilton RF Jr, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med (2008) 44(7):1246–58. doi:10.1016/j.freeradbiomed.2007.12.027
54. Biswas R, Hamilton RF Jr, Holian A. Role of lysosomes in silica-induced inflammasome activation and inflammation in absence of MARCO. J Immunol Res (2014) 2014:304180. doi:10.1155/2014/304180
55. Hari A, Zhang Y, Tu Z, Detampel P, Stenner M, Ganguly A, et al. Activation of NLRP3 inflammasome by crystalline structures via cell surface contact. Sci Rep (2014) 4(7281):1–8. doi:10.1038/srep07281
56. Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol (2009) 183(2):787–91. doi:10.4049/jimmunol.0901363
57. Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol (2006) 72(11):1493–505. doi:10.1016/j.bcp.2006.04.011
58. Tomašek I, Horwell CJ, Damby DE, Barošová H, Geers C, Petri-Fink A, et al. Combined exposure of diesel exhaust particles and respirable Soufrière Hills volcanic ash causes a (pro-)inflammatory response in an in vitro multicellular epithelial tissue barrier model. Part Fibre Toxicol (2016) 13(1):67. doi:10.1186/s12989-016-0178-9
59. Baxter PJ, Searl AS, Cowie HA, Jarvis D, Horwell CJ. Evaluating the respiratory health risks of volcanic ash at the eruption of the Soufrière Hills Volcano, Montserrat, 1995 to 2010. Geol Soc Lond Mem (2014) 39:407–25. doi:10.1144/M39.22
60. Turci F, Pavan C, Leinardi R, Tomatis M, Pastero L, Garry D, et al. Revisiting the paradigm of silica pathogenicity with synthetic quartz crystals: the role of crystallinity and surface disorder. Part Fibre Toxicol (2015) 13(32):1–12. doi:10.1186/s12989-016-0136-6
61. Jones T, BéruBé K. The bioreactivity of the sub-10 µm component of volcanic ash: Soufrière Hills volcano, Montserrat. J Hazard Mater (2011) 194:128–34. doi:10.1016/j.jhazmat.2011.07.092
Keywords: inflammasome, NLRP3, reactive oxygen species, lysosomal damage, volcanic ash, cristobalite, silica, mineral dust
Citation: Damby DE, Horwell CJ, Baxter PJ, Kueppers U, Schnurr M, Dingwell DB and Duewell P (2018) Volcanic Ash Activates the NLRP3 Inflammasome in Murine and Human Macrophages. Front. Immunol. 8:2000. doi: 10.3389/fimmu.2017.02000
Received: 17 October 2017; Accepted: 22 December 2017;
Published: 22 January 2018
Edited by:
Rostyslav Bilyy, Danylo Halytsky Lviv National Medical University, UkraineReviewed by:
Luis Enrique Munoz, University of Erlangen-Nuremberg, GermanySeth Lucian Masters, Walter and Eliza Hall Institute of Medical Research, Australia
Copyright: © 2018 Damby, Horwell, Baxter, Kueppers, Schnurr, Dingwell and Duewell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David E. Damby, ddamby@usgs.gov;
Peter Duewell, peter.duewell@med.uni-muenchen.de
 David E. Damby
David E. Damby Claire J. Horwell3
Claire J. Horwell3 Max Schnurr
Max Schnurr Donald B. Dingwell
Donald B. Dingwell Peter Duewell
Peter Duewell