Effects of Forestry Waste Neolamarckia cadamba Leaf Meal as an Additive on Fermentation Quality, Antioxidant Activity, and Bacterial Community of High-Moisture Stylo Silage
- 1State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, College of Forestry and Landscape Architecture, Guangdong Province Research Center of Woody Forage Engineering Technology, Guangdong Research and Development Center of Modern Agriculture (Woody Forage) Industrial Technology, South China Agricultural University, Guangzhou, China
- 2Inner Mongolia Key Laboratory of Microbial Ecology of Silage, Inner Mongolia Engineering Research Center of Development and Utilization of Microbial Resources in Silage, Inner Mongolia Academy of Agriculture and Animal Husbandry Science, Hohhot, China
- 3Dairy Science Department, National Research Centre, Cairo, Egypt
N. cadamba leaves, a byproduct of wood production, are always discarded in the field. N. cadamba leaves have strong antibacterial property, which might be recycled to inhibit undesirable bacteria and enhance the fermentation quality of silage. Ensiling, a traditional conservation method for animal feed, is commonly used all over the world. It is known that high-moisture forages, especially legumes, are difficult to ensile directly as much ammonia-N and butyric acid produced by undesirable bacteria will reduce the feeding value. To investigate the effects of N. cadamba leaf meal on the fermentation quality of stylo silage, 5% and 10% N. cadamba leaf meal were mixed with stylo for 30 days of ensiling in two independent experiments. Results showed that the silage pH and butyric acid content of stylo silage were decreased (p < 0.01) by 10% N. cadamba leaf meal. In experiment 2, contents of nonprotein-N and ammonia-N were significantly decreased (p < 0.05), while the true protein content was significantly increased (p < 0.05). The same results on the changing tendency were also obtained in experiment 1. In addition, N. cadamba leaf meal addition also decreased the bacterial diversity. The relative abundance of Clostridium and Lelliottia decreased, whereas that of Lactobacillus increased when N. cadamba leaf meal was added. It is worth noting that the addition of N. cadamba leaf meal also improved the antioxidant activity of stylo silage. The aforementioned results suggested that mixing N. cadamba leaf meal to high moisture forages could be an effective strategy to enhance silage fermentation quality, and it is also a feasible way to recycle N. cadamba leaves.
Introduction
N. cadamba is an evergreen, fast-growing, and semi-deciduous tree, and it is widely planted throughout the tropical or subtropical regions. It is traditionally used as an herbal medicine to cure various ailments in India. In recent years, it has been largely planted in countries like China, Malaysia, and Egypt for plywood and paper production (Zhao et al., 2017). The leaves with vast amount of biomass are discarded or burnt in the field, which produces greenhouse gases and air pollutants. Actually, it is a good resource for ruminants due to its high crude protein content (>22% DM, Wang et al., 2017) and digestibility (He et al., 2018). Better growth and slaughter performance and meat characteristics of goats were observed when N. cadamba leaves were added in their diet (Wang et al., 2017). Moreover, it contains many active metabolites, such as flavonoids and phenolic acids, which result in its strong antibacterial property. It is reported that the extract of N. cadamba leaves showed a strong inhibitory effect on undesirable bacteria like Bacillus, Escherichia, and Staphylococcus (Khandelwal et al., 2016; Pandey and Negi, 2016), which are also abundant in silage (Bai et al., 2020; Ni et al., 2020). Adding N. cadamba leaf meal to silage may not only decrease the moisture content but also inhibit the activities of undesirable microorganisms.
Ensiling is a traditional conservation method for fresh forages to avoid nutritional losses caused by weather. It is widely and commonly used due to the simple process, low-cost, and better preservation of nutrients (McDonald et al., 1991). But the activities of undesirable microorganisms often lead to protein degradation during ensiling. Moreover, most forages, especially legumes, are difficult to obtain a high quality of silage, owing to their high moisture and low soluble carbohydrate contents. As a result, the silage pH cannot decline rapidly, and undesirable microorganisms like Clostridia and Enterobacter can dominate, which lead to butyric acid production and proteolysis during ensiling (Wang et al., 2019a; Ni et al., 2020). Both butyric acid and proteolysis are undesirable in silage production. Butyric acid not only promotes the growth of spoilage microorganisms, resulting in the loss of dry matter, but also reduces animal intake directly (Wang et al., 2019a). Depending on the research of Tabacco et al. (2006), the degradation of proteins may lead to the inefficient rumen microbial nitrogen synthesis. More urinary nitrogen emission also has a negative impact on the environment (Adegbeye et al., 2019). As reported by Ni et al. (2020) and Wang et al. (2019a), the ammonia-N content could be higher than 20% total nitrogen, and the butyric acid content could be higher than 0.5% dry matter in legume silages like alfalfa and stylo. Thus, it is essential to find a suitable method to improve the conservation of high-moisture legume silages to minimize the economic loss and potential environmental pollution.
Therefore, stylo (Stylosanthes guianensis), an important legume in the tropical or subtropical regions, was used in the present study. In two independent experiments, 5% and 10% N. cadamba leaf meal were added to fresh stylo to investigate their effects on the fermentation quality, nitrogen fractions, antioxidant activities, and bacterial communities. In addition, to obviate the influence of the moisture content, 5% and 10% stylo meal were added separately to the fresh stylo.
Materials and Methods
Silage Preparation
Stylo (CIAT 184) and N. cadamba were grown in an experimental field at the South China Agricultural University (Guangdong, China). No herbicides and fertilizers were used during the growth stage. Stylo (at bloom stage) from two plots and leaves of N. cadamba (with a height of 1.5–2 m) from one plot (40 trees in total) were collected on 4 December 2018 (experiment 1) and 12 October 2019 (experiment 2), respectively. All the collected materials were chopped into 1–2 cm lengths. Prior to ensiling, stylo and N. cadamba leaves were dried at 65°C for 2 days and then ground immediately to obtain stylo meal (S) and N. cadamba leaf meal (N). In both experiments, fresh stylo was added with 0% (the control), 5% S, 10% S, 5% N, and 10% N (on a fresh matter basis). About 110 g of evenly mixed silage material was sealed in polyethylene plastic bags with a vacuum sealer. Three bags for each treatment were made, and these bags were kept in the shade (around 25°C). After 30 days of fermentation, silages were sampled to analyze their fermentation parameters, protein fractions, antioxidant activities, and bacterial communities.
Fermentation Characteristics, Chemical Components, and Antioxidant Capacity Analyses
The methods used in this part were similar to our previously study (He et al., 2020) unless otherwise specified. The silage sample (20 g) was fully soaked in sterilized physiological saline (180 ml), and then, the leaching solution was diluted in gradient. Then, the plate counting method was used to determine the number of lactic acid bacteria (LAB), coliform bacteria, yeasts, and molds. Another 20 g silage sample was mixed with 180 ml distilled water and stored at 4°C for 18 h. Then, one aliquot of the filtrate was used to measure the pH value with a pH meter. Organic acids including lactic acid, acetic acid, propionic acid, and butyric acid were determined using an HPLC method, in accordance with the condition and procedure of He et al. (2020).
About 50 g silage were oven-dried for calculating the dry matter (DM) and ground for chemical analysis. The determination methods of chemical compositions, including protein, water-soluble carbohydrates (WSC), neutral detergent fiber (NDF), acid detergent fiber (ADF), and tannin, were the same as that of Wang et al. (2019b). The antioxidant activity including radical 2, 2-diphenyl-1-picrylhy-drazyl (DPPH) and radical 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid), ammonium salt (ABTS)–scavenging activity, and ferric-reducing antioxidant power (FRAP) were measured with the method of He et al. (2020). The scavenging activity and reducing power were expressed as milligrams of trolox equivalents per gram of the dry matter (mg trolox g−1 DM).
Bacterial Community Sequencing Analysis
The stylo silage in the two experiments was sampled, and the total bacterial DNA was extracted with a DNA Kit (Omega Bio-Tek, Norcross, GA, United States), following the attached instructions. The specific steps were similar to Bai et al. (2020). The V3-V4 regions of 16S rDNA were amplified using the primers (341F: CCTACGGGNGGCWGCAG; 806R: GGACTACHVGGGTATCTAAT). Polymerase chain reactions (PCRs) were conducted in a 50 μl mixture (5 μl of 10 × KOD Buffer, 1.5 μl of each primer (5 μM), 1 μl of KOD polymerase, 5 μl of 2.5 mM dNTPs, and 100 ng of template DNA), and the same reaction procedures were reported by He et al. (2019b). After being purified and quantified, the purified PCR products were sequenced on an Illumina HiSeq 2500 Sequencing System (Illumina, Inc., San Diego, CA, United States), and the raw sequences were analyzed as according to the procedures of Wang et al. (2018). The bioinformatic data were analyzed using the free online platform (http://www.omicshare.com/tools). The α-diversity was calculated in the QIIME bioinformatic pipeline (https://qiime.org). The β-diversity was analyzed with principal component analysis (PCA). The relative abundances of different bacterial communities at the phylum and genus levels were also analyzed.
Statistical Analysis
The effects of mixing stylo meal and N. cadamba leaf meal on the stylo fermentation quality and chemical composition were analyzed with IBM SPSS 20.0 for Windows statistical software. The results were assessed using the one-way analysis of variance (ANOVA), with Duncan’s multiple range test. The statistical significance was determined at the p < 0.05 level. All the figures were made by Adobe Illustrator CS 6.0 software.
Results
Characteristics of Fresh Stylo and N. cadamba Leaves Prior to Ensiling
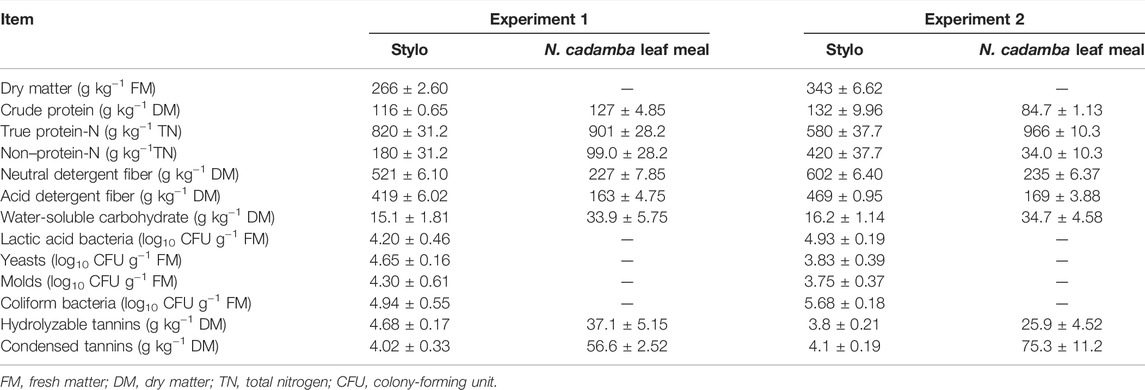
The chemical composition and microbial population of fresh stylo and N. cadamba leaf meal before ensiling in experiments 1 and 2 are listed in Table 1. The DM contents of stylo in the two experiments were 266 and 343 g kg−1, respectively. The protein contents of stylo in experiment 2 were slightly higher than those in experiment 1 (132 vs. 116 g kg−1 DM). But the NDF and ADF contents of stylo in experiment 1 were lower than those in experiment 2 (521 and 419 g kg−1 vs. 602 and 469 g kg−1 DM). The WSC contents of stylo in experiment 1 and 2 were very poor (15.1 and 16.2 g kg−1 DM, respectively). The LAB numbers of fresh stylo in our two experiments were insufficient (4.20 and 4.93 log10 CFU g−1 FM, respectively). More unfortunately, the counts of undesirable microorganisms, fungi, and coliform bacteria (4.65 and 3.83, 4.30 and 3.75, and 4.94 and 5.68 log10 CFU g−1 FM for yeasts, molds, and coliform bacteria, respectively) were relatively high. Tannin contents of N. cadamba leaves in experiments 1 and 2 (37.1 and 25.9 g kg−1, 56.5 and 75.3 g kg−1 DM for hydrolyzable tannins and condensed tannins, respectively) were abundant and far higher than those of stylo. In addition, N. cadamba leaf meal contains relatively higher WSC (34 g kg−1 DM), which were supposed to be helpful for fermentation.
Fermentation Quality and Microbial Population of Stylo Silage
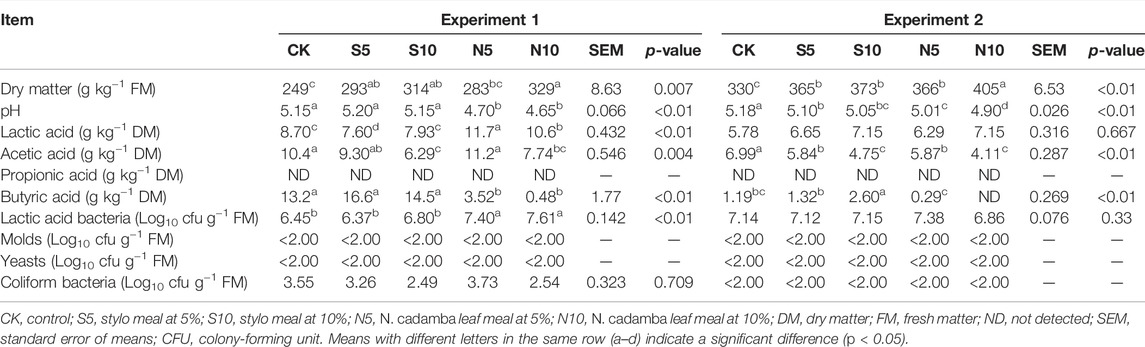
As shown in Table 2, the DM content of stylo ensiled alone (249 and 330 g kg−1 fresh matter) was relatively low, and the relatively high pH value (5.15 and 5.18, respectively) indicated the poor fermentation quality. The DM content and pH value were decreased significantly (p < 0.01) by N. cadamba leaf meal. Lactic acid, the dominant fermentation product in this study, increased by 10% N. cadamba leaf meal treatment in experiment 1 (8.70–10.6 g kg−1 DM) and experiment 2 (5.78–7.15 g kg−1 DM). The contents of acetic acid and butyric acid were significantly decreased (p < 0.01) when 10% N. cadamba leaf meal was added. For butyric acid, it decreased from 13.2 to 0.476 g kg−1 DM and from 1.19 to not detected in experiments 1 and 2, respectively.
Nitrogen Fractions and Antioxidant Activities of Stylo Silage
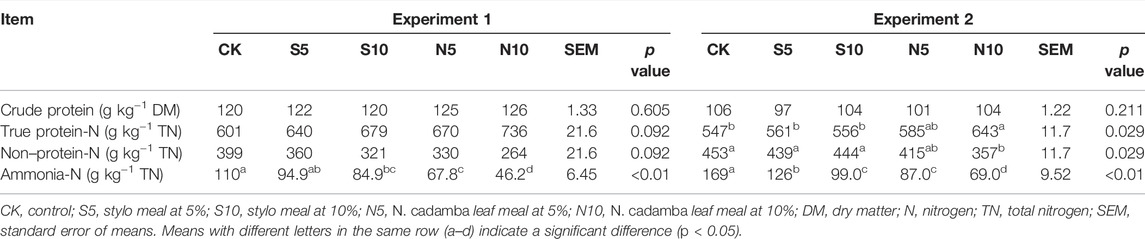
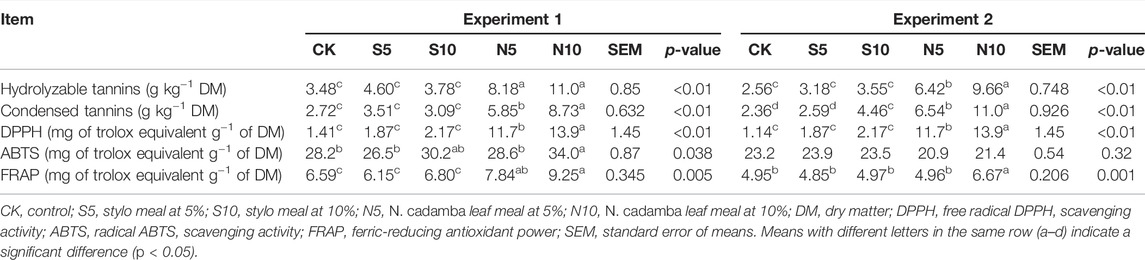
As shown in Table 3, in experiment 2, the 5% and 10% N. cadamba leaf meal increased the true protein content and decreased the nonprotein-N (p < 0.05) and ammonia-N (p < 0.01) contents, although the crude protein content showed no change. The same results on the changing tendency were also obtained in experiment 1. The ammonia-N content decreased from 110 to 46.2 g kg−1 total nitrogen and from 169 to 69.0 g kg−1 total nitrogen by 10% N. cadamba leaf meal in experiments 1 and 2. On the other hand, as shown in Table 4, the contents of hydrolyzable tannins and condensed tannin content increased in N. cadamba leaf meal-treated silage. Also, the DPPH scavenging activity and FRAP of N. cadamba leaf meal-treated stylo silage were significantly increased (p < 0.01). However, the addition of N. cadamba leaf meal had no significant effects on the ABTS scavenging activity of stylo silage.
Bacterial Diversity of Stylo Silage
Bacterial diversity of stylo silage mixed with stylo meal and N. cadamba leaf meal is shown in Table 5. The good coverage values for all the treatments in the two experiments were all above 0.99. The change in different indexes in the two experiments showed a similar trend. The Sobs, Shannon, Simpson, and Ace indices in experiments 1 and 2 decreased in 10% N. cadamba leaf meal treatment. The results of unweighted principal coordinate analysis (PCoA) could reflect the distinction of the bacterial community among the treatments. As shown in Figure 1, PCoA 1 and PCoA 2 were 66.1% and 12.1% of the total variance in experiment 1 and 38.0% and 15.3% of the total variance in experiment 2, respectively. The relative abundances of bacterial communities at the phylum and genus levels in the two experiments are presented in Figure 2 and Figure 3 Overall, Proteobacteria and Firmicutes were the main phyla in stylo silage. The bacterial composition of the two experiments is a little different, and the bacterial community was dramatically altered by N. cadamba leaf meal. In experiment 1, Clostridium (27.9%) was the most dominant genera in the control. The relative abundance of Clostridium increased to 45.3% and 41.7% after 5% and 10% stylo meals were added. In contrast, Clostridium decreased to 10.4% and 0.91% in N. cadamba leaf meal–treated silage (p < 0.01, Figure 4). In experiment 2, the relative abundance of Clostridium increased from 2.14% to 19.5% in 10% stylo meal–treated silage. The relative abundance of Clostridium decreased from 2.14% to 0.42% in N. cadamba leaf meal–treated silage. Lactobacillus was the most dominant species in experiment 1 (42.6% and 34.3%) when N. cadamba leaf meal was added. In experiment 2, the relative abundance of Lactobacillus increased from 1.88% to 5.81% after the addition of N. cadamba leaf meal. Moreover, Lelliottia was detected in stylo silage in experiment 2, and the relative abundance was relatively high (6.87%–12.0%), although Enterobacter was not detected in the present study. The good news is that the relative abundance of Lelliottia decreased by the addition of 10% N. cadamba leaf meal (p < 0.05, Figure 4).
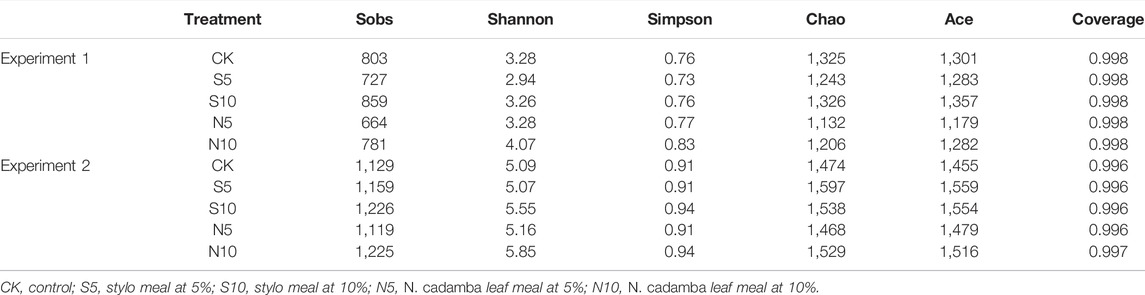
TABLE 5. Alpha diversity of the bacterial community of stylo silage mixed with stylo meal and N. cadamba leaf meal (n = 3).
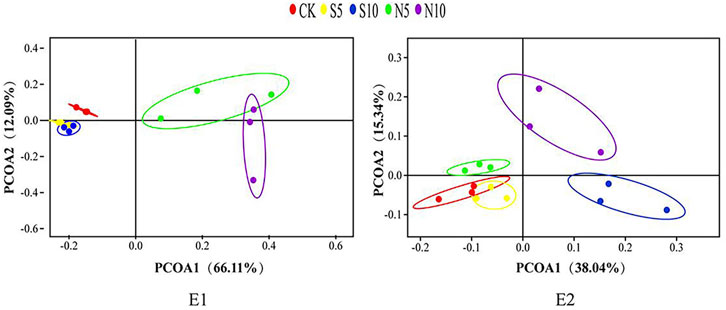
FIGURE 1. Principal component analysis (PCA) of bacterial communities of stylo silage mixed with stylo meal and N. cadamba leaf meal (E1 and E2, experiments 1 and 2; CK, control; S5 and S10, mixed with 5% and 10% stylo meal; N5 and N10, mixed with 5% and 10% N. cadamba leaf meal).
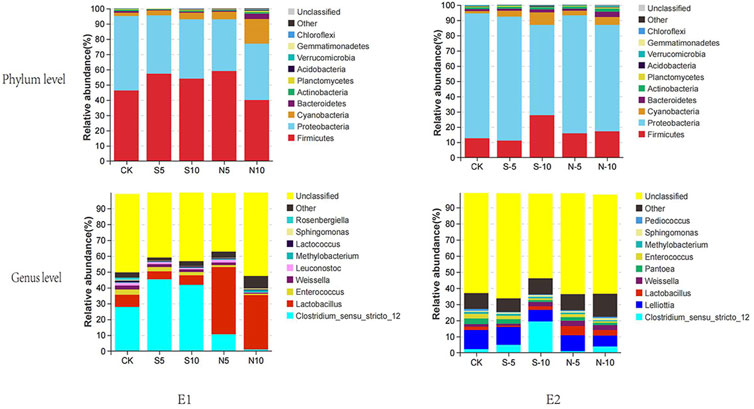
FIGURE 2. Relative abundance of bacterial communities at phylum and genus levels of stylo silage mixed with stylo meal and N. cadamba leaf meal (E1 and E2, experiments 1 and 2; CK, control; S5 and S10, mixed with 5% and 10% stylo meal; N5 and N10, mixed with 5% and 10% N. cadamba leaf meal).
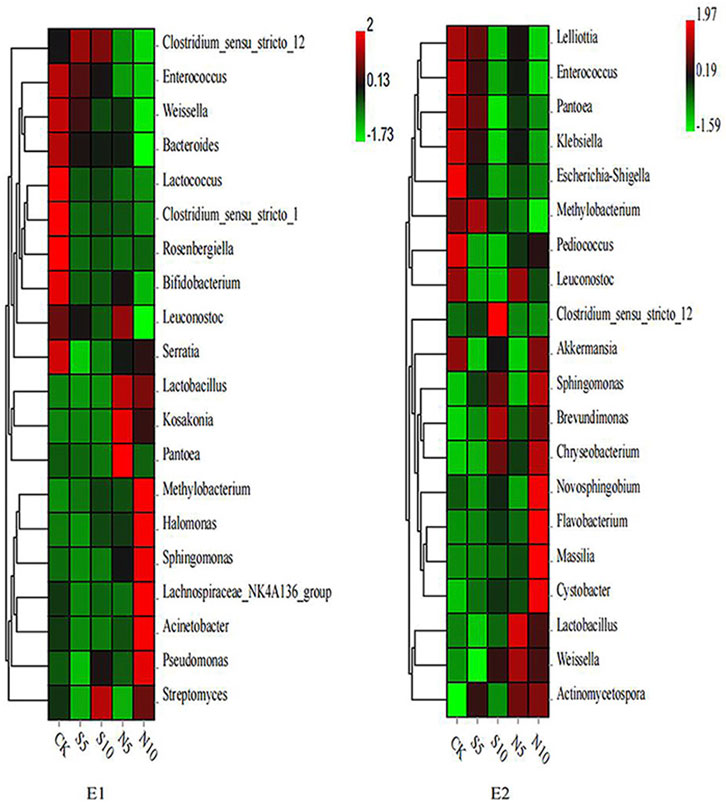
FIGURE 3. Heatmap of bacterial communities at the genus level of stylo silage mixed with stylo meal and N. cadamba leaf meal (E1 and E2, experiments 1 and 2; CK, control; S5 and S10, mixed with 5% and 10% stylo meal; N5 and N10, mixed with 5% and 10% N. cadamba leaf meal).
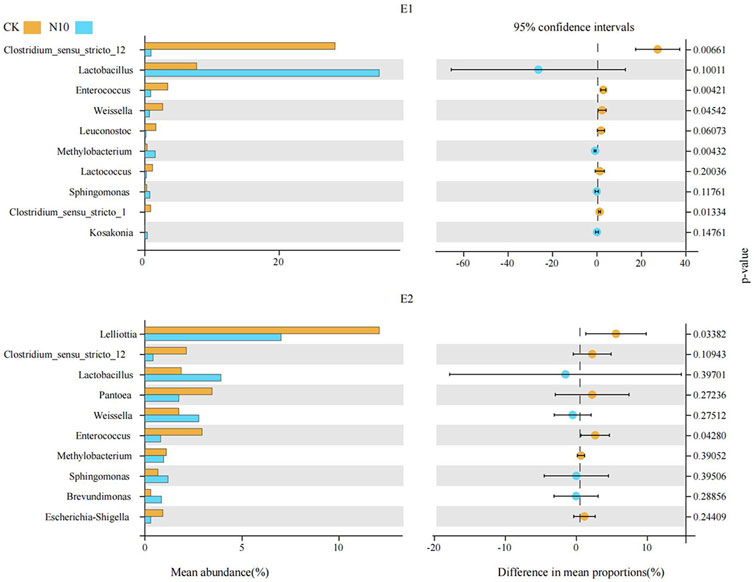
FIGURE 4. Difference in the bacterial community between the control and 10% N. cadamba leaf meal treatment based on Welch’s test (E1 and E2, experiments 1 and 2; CK, control; N10, mixed with 10% N. cadamba leaf meal).
Discussion
Characteristics of Fresh Stylo and N. cadamba Leaves Prior to Ensiling
The DM, NDF, and ADF contents of stylo in this study were comparable with the value reported by Wang et al. (2019a) but were lower than those showed by Liu et al. (2011). The crude protein content of N. cadamba leaves in experiment 1 was similar to that in our previous report (He et al., 2019a) and was much higher than those in experiment 2. Such variations might be due to the different factors like climate and season of harvest, which have influence on the chemical composition of the forage (Zhang et al., 2016). Trees like N. cadamba are widely planted for timber wood, and its leaf is the main byproduct. For a cleaner production of wood, using the residue as animal feed might be a good choice.
As the fermentation substrate, the WSC content of forage is an important factor for good silage fermentation quality. In our study, the WSC contents of stylo in experiment 1 and 2 were very poor and far lower than 60–70 g kg−1 DM, the theoretical requirement for obtaining well-preserved silage (Smith, 1962). The epiphytic LAB of fresh material is also a crucial factor, and at least five log10 CFU g−1 epiphytic LAB during ensiling are necessary to produce well-preserved silage (Cai et al., 1998). But the LAB numbers of stylo in our two experiments were below the value. Furthermore, the counts of undesirable microorganisms, like fungi and coliform bacteria, were relatively high. It indicates that measures should be taken to inhibit these undesirable microorganisms during ensiling. Jayanegara et al. (2018) reported that tannins had the ability to limit extensive proteolysis, which indicated N. cadamba leaf meal could be potentially used to enhance nitrogen conservation during the ensiling of legumes. Tannins (both hydrolyzable and condensed tannins) or tannin-containing forages are proved to be effective in reduction of greenhouse gases by ruminants (Kiggundu et al., 2019; Ugbogu et al., 2019). Therefore, mixing N. cadamba leaf meal might be helpful to reduce protein degradation, improve the fermentation quality of silage, and decrease the greenhouse gas emission from ruminants.
Fermentation Quality and Microbial Population of Stylo Silage
In this study, addition of N. cadamba leaf meal can significantly increase the DM contents of stylo silage. Excess moisture in silage could influence the silage pH and microbiota and increase the nitrogen level that is associated with the emission of nitrogen oxide. Higher DM contents could reduce the losses caused by effluent production and silo gas production during ensiling (Araújo et al., 2020). Silage mainly using lower pH inhibits the activities of undesirable microorganisms so as to preserve nutrients (McDonald et al., 1991). Therefore, the accumulation of organic acids in silage is crucial. Lactic acid, the dominant fermentation product in this study, increased in 10% N. cadamba leaf meal treatment in both experiment 1 and experiment 2. The production of butyric acid is unfavorable during ensiling. However, the content of butyric acid was not reduced by the addition of 10% stylo meal. It indicates that N. cadamba leaf meal enhances the fermentation quality, which is not merely because of the improvement of DM content. It might be because some chemicals in N. cadamba leaf meal changed the bacterial community and fermentation process during ensiling.
Nitrogen Fractions and Antioxidant Activities of Stylo Silage
As we all know, the ultimate goal of silage is to preserve nutrients. From Table 3, N. cadamba leaf meal increased the true protein content and decreased the nonprotein-N and ammonia-N contents. It indicates that N. cadamba leaf meal has a positive effect on the conservation of protein during ensiling. The ammonia-N content in silage is a crucial indicator of protein breakdown (Pahlow et al., 2003). The decrease of the ammonia-N content and nonprotein-N content by N. cadamba leaf meal addition indicates the benefit of N. cadamba leaves further. Tannins can protect proteins by forming insoluble complexes with forage proteins or inhibiting aminopeptidase, carboxypeptidase, and acid proteinase during the ensiling process (Li et al., 2018). Ding et al. (2013) reported that the addition of tannin acid could reduce ammonia-N and nonprotein-N contents in alfalfa silage. In our study, the contents of hydrolyzable tannins and condensed tannins increased in N. cadamba leaf meal–treated silage as expected. The higher content of tannins in N. cadamba leaf meal treatments might also explain the reduction of proteolysis. The DPPH scavenging activity of stylo silage was significantly increased in N. cadamba leaf meal treatment, which might also be because of higher tannin contents. A similar result has been reported by He et al. (2020), who also found that the antioxidant capacity increased by adding tannin acid in mulberry leaf and stylo silage.
Bacterial Diversity of Stylo Silage
Next-generation sequencing has been widely applied to monitor the dynamic changes of bacterial communities in the diverse ensiling period (He et al., 2019b; Wang et al., 2019b). The good coverage values for all treatments in the two experiments were all above 0.99 in the present study, which indicates that the data were representative. Moreover, OTUs in experiment 2 (1,450–1,580) were higher than those in experiment 1 (1,100–1,360). It might be because that the bacterial community of silage is influenced by many factors, such as forage varieties, collected period, silage treatments, and storage conditions (Yang et al., 2016; He et al., 2019b).
As shown in Figure 1, the bacterial community of stylo ensiled alone showed a clear separation from the samples treated with N. cadamba leaf meal. It suggests that N. cadamba leaf meal had a significant influence on the bacterial community of stylo silage. Ensiling is a process in which microorganisms influence and compete with each other. Thus, the better fermentation quality of stylo silage treated with N. cadamba leaf meal might be due to the change in the microbial community.
Clostridium, a kind of obligate anaerobic bacteria, is undesirable during ensiling (Driehuis et al., 2018; He et al., 2019b). It is reported that Clostridium can degrade protein, promote the growth of spoilage microorganisms, and prevent a rapid fall of silage pH (He et al., 2019a; Zheng et al., 2017). Furthermore, Clostridium in silage might be harmful to animal health because some species of it could colonize in the gastro and intestinal tract and then produce pathogenic toxins (Dunière et al., 2013), but Clostridium has low tolerance to a low moisture content. Reducing moisture by wilting is commonly and widely used in silage production to inhibit the activities of Clostridium (Pahlow et al., 2003). In this study, the relative abundance of Clostridium did not decrease as expected, although stylo meal addition decreased the moisture content. On the contrary, it increased in stylo meal–treated silage (Figures 2, 3). It indicates that well-fermented stylo silage is difficult to obtain by reducing the moisture content alone. The relative abundance of Clostridium was decreased by N. cadamba leaf meal, which was consistent with the decrease in ammonia-N and butyric acid contents. It suggests mixing N. cadamba leaf meal might be an effective method to inhibit Clostridia and improve the silage quality. Daglia (2012) considered that tannins had an extensive range of antimicrobial activity. It may partially explain the inhibiting effects of N. cadamba leaf meal on Clostridia in stylo silage.
Generally, Lactobacillus, Weissella, Enterococcus, and Lactococcus are common lactate-producing bacteria in silage (Pahlow et al., 2003; Yang et al., 2016; Ni et al., 2018). Weissella is considered an initial colonizer microorganism, and then, it is inhibited ultimately by the low pH value (<4.2) caused by organic acid accumulation (Graf et al., 2016). It might be because of the relatively high pH value (>4.5 in all samples) of stylo silage; Weissella was detected in the two experiments. Enterococcus and Lactococcus are always used as LAB inoculants during the silage production. It could reduce the ammonia-N content of silage and affect ruminal fermentation by improving ruminal microbial biomass production (Weinberg et al., 2003). In the present study, Enterococcus was decreased by N. cadamba leaf meal addition (Figure 4). It might be because of the increase in other genera like Lactobacillus.
Lelliottia, a gram-negative and non–spore-forming bacterium belonging to the family Enterobacteriaceae, was first separated from the genus Enterobacter in 2013, and it is reclassified subsequently as a novel genus, according to genotypic and phenotypic characteristics (Yuk et al., 2018). It is reported that Lelliottia has been isolated from drinking water (Kampfer et al., 2018), long-standing arsenic contaminated environment (Tian et al., 2019), and even fecal samples from hospitalized patients (Kmpfer et al., 2014). It is seldom reported in silage. However, Lelliottia was detected in stylo silage in experiment 2, and the relative abundance was relatively high although Enterobacter was not detected in the present study. Based on the similarity with Enterobacter, it might also play the same function in silage. The relative abundance of Lelliottia was decreased by 10% N. cadamba leaf meal addition. It could explain the reduction of ammonia-N in 10% N. cadamba leaf meal treatment. Ogunade et al. (2018) supposed that Pantoea had the ability to reduce the ammonia-N content and pH value in alfalfa silage. Confusingly, the relative abundance of Pantoea increased in 5% N. cadamba leaf meal treatment in experiment 1 but decreased in experiment 2. The relative abundance of Pantoea in both experiments was very low. So the role of Pantoea in stylo silage needs further study.
Conclusion
Compared with discarding in the field, using N. cadamba leaf meal as a silage additive might be a great choice to recycle this resource. Moreover, the present study revealed that mixing N. cadamba leaf meal is an effective way to improve the fermentation quality of legume silage. In N. cadamba leaves meal treated silage, silage pH, butyric acid, nonprotein-N and ammonia-N content decreases, and antioxidant activities, lactic acid, and true protein content increases. The relative abundance of Clostridium and Lelliottia decreased, while the relative abundance of Lactobacillus increased in stylo silage when N. cadamba leaf meal was added. It might be because of the abundant active compounds like tannins in N. cadamba leaves. The aforementioned results suggested that mixing N. cadamba leaf meal to high-moisture silage could be an effective strategy to improve the silage conservation and a feasible way to use this kind of forestry waste.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: BioProject, accession number PRJNA846587.
Author Contributions
SW: investigation and writing the original draft. LG: investigation and data curation. DC: investigation and software. YX:interpretation. AK: analysis. WZ: project administration and resources. XC: supervision and validation. QZ: methodology and project administration.
Funding
This work was financially supported by the Guangzhou Forestry Science and Technology Innovation Commission (Grant Nos. 2018KJCX001 and 2019KJCX001), the Guangdong Natural Science Foundation (2020A1515011253), and the National Natural Science Foundation of China Project (Grant No. 31771345).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adegbeye, M. J., Elghandour, M. M. M. Y., Monroy, J. C., Abegunde, T. O., Salem, A. Z. M., Barbabosa-Pliego, A., et al. (2019). Potential Influence of Yucca Extract as Feed Additive on Greenhouse Gases Emission for a Cleaner Livestock and Aquaculture Farming - A Review. J. Clean. Prod. 239, 118074. doi:10.1016/j.jclepro.2019.118074
Araújo, J. A. S., Almeida, J. C. C., Reis, R. A., Carvalho, C. A. B., and Barbero, R. P. (2020). Harvest Period and Baking Industry Residue Inclusion on Production Efficiency and Chemical Composition of Tropical Grass Silage. J. Clean. Prod. 266, 121953. doi:10.1016/j.jclepro.2020.121953
Bai, J., Xu, D., Xie, D., Wang, M., Li, Z., and Guo, X. (2020). Effects of Antibacterial Peptide-Producing Bacillus Subtilis and Lactobacillus Buchneri on Fermentation, Aerobic Stability, and Microbial Community of Alfalfa Silage. Bioresour. Technol. 315, 123881. doi:10.1016/j.biortech.2020.123881
Cai, Y., Benno, Y., Ogawa, M., Ohmomo, S., Kumai, S., and Nakase, T. (1998). Influence of Lactobacillus spp. from an Inoculant and of Weissella and Leuconostoc spp. from Forage Crops on Silage Fermentation. Appl. Environ. Microbiol. 64, 2982–2987. doi:10.1128/aem.64.8.2982-2987.1998
Daglia, M. (2012). Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 23, 174–181. doi:10.1016/j.copbio.2011.08.007
Ding, W., Guo, X., and Ataku, K. (2013). Characterization of Peptides in Ensiled Alfalfa Treated with Different Chemical Additives. Animal Sci. J. 84, 774–781. doi:10.1111/asj.12065
Driehuis, F., Wilkinson, J. M., Jiang, Y., Ogunade, I., and Adesogan, A. T. (2018). Silage Review: Animal and Human Health Risks from Silage. J. Dairy Sci. 101, 4093–4110. doi:10.3168/jds.2017-13836
Dunière, L., Sindou, J., Chaucheyras-Durand, F., Chevallier, I., and Thévenot-Sergentet, D. (2013). Silage Processing and Strategies to Prevent Persistence of Undesirable Microorganisms. Animal Feed Sci. Technol. 182, 1–15. doi:10.1016/j.anifeedsci.2013.04.006
Graf, K., Ulrich, A., Idler, C., and Klocke, M. (2016). Bacterial Community Dynamics During Ensiling of Perennial Ryegrass at Two Compaction Levels Monitored by Terminal Restriction Fragment Length Polymorphism. J. Appl. Microbiol. 120, 1479–1491. doi:10.1111/jam.13114
He, L., Zhou, W., Wang, Y., Wang, C., Chen, X., and Zhang, Q. (2018). Effect of Applying Lactic Acid Bacteria and Cellulase on the Fermentation Quality, Nutritive Value, Tannins Profile and In Vitro Digestibility of Neolamarckia cadamba Leaves Silage. J. Anim. Physiol. Anim. Nutr. 102, 1429–1436. doi:10.1111/jpn.12965
He, L., Wang, C., Xing, Y., Zhou, W., Pian, R., Yang, F., et al. (2019a). Dynamics of Proteolysis, Protease Activity and Bacterial Community of Neolamarckia Cadamba Leaves Silage and the Effects of Formic Acid and Lactobacillus Farciminis. Bioresour. Technol. 294, 122127. doi:10.1016/j.biortech.2019.122127
He, L., Chen, N., Lv, H., Wang, C., Zhou, W., Chen, X., et al. (2019b). Gallic Acid Influencing Fermentation Quality, Nitrogen Distribution and Bacterial Community of High-Moisture Mulberry Leaves and Stylo Silage. Bioresour. Technol. 295, 122255. doi:10.1016/j.biortech.2019.122255
He, L., Lv, H., Chen, N., Wang, C., Zhou, W., Chen, X., et al. (2020). Improving Fermentation, Protein Preservation and Antioxidant Activity of Moringa Oleifera Leaves Silage with Gallic Acid and Tannin Acid. Bioresour. Technol. 297, 122390. doi:10.1016/j.biortech.2019.122390
Jayanegara, A., Sujarnoko, T. U. P., Ridla, M., Kondo, M., and Kreuzer, M. (2018). Silage Quality as Influenced by Concentration and Type Of Tannins Present in the Material Ensiled: A Meta-Analysis. J. Anim. Physiol. Anim. Nutr. Berl. 103, 456–465. doi:10.1111/jpn.13050
Kampfer, P., Glaeser, S. P., Packroff, G., Behringer, K., Exner, M., Chakraborty, T., et al. (2018). Lelliottia aquatilis sp. nov., Isolated from Drinking Water. Int. J. Syst. Evol. Micr. 68, 2454–2461. doi:10.1099/ijsem.0.002854
Khandelwal, V., Bhatia, A. K., and Goel, A. (2016). Antimicrobial and Antioxidant Efficacy of Aqueous Extract of Anthocephalus cadamba Leaves. J. Pure Appl. Microbio. 10, 209–216. Available at: https://microbiologyjournal.org/antimicrobial-and-antioxidant-efficacy-of-aqueous-extract-of-anthocephalus-cadamba-leaves/
Kiggundu, N., Ddungu, S. P., Wanyama, J., Cherotich, S., Mpairwe, D., Zziwa, E., et al. (2019). Greenhouse Gas Emissions from Uganda's Cattle Corridor Farming Systems. Agric. Syst. 176, 102649. doi:10.1016/j.agsy.2019.102649
Kmpfer, P., Glaeser, S. P., Raza, M. W., Abbasi, S. A., and Perry, J. D. (2014). Pseudocitrobacter gen. nov. a Novel Genus of the Enterobacteriaceae with Two New Species Pseudocitrobacter faecalis sp. nov. and pseudocitrobacter anthropi sp. nov, Isolated from Fecal Samples from Hospitalized Patients in Pakistan. Syst. Appl. Microbiol. 37, 17–22. doi:10.1016/j.syapm.2013.08.003
Li, X., Tian, J., Zhang, Q., Jiang, Y., Hou, J., Wu, Z., et al. (2018). Effects of Applying Lactobacillus plantarum and Chinese Gallnut Tannin on the Dynamics Of Protein Degradation and Proteases Activity in Alfalfa Silage. Grass Forage Sci. 73, 648–659. doi:10.1111/gfs.12364
Liu, Q., Zhang, J., Shi, S., and Sun, Q. (2011). The Effects of Wilting and Storage Temperatures on the Fermentation Quality and Aerobic Stability of Stylo Silage. Anim. Sci. J. 82, 549–553. doi:10.1111/j.1740-0929.2011.00873.x
McDonald, P., Henderson, A. R., and Heron, S. (1991). The Biochemistry of Silage. Abersytwyth, U.K: Chalcombe Publications.
Ni, K., Zhao, J., Zhu, B., Su, R., Pan, Y., Ma, J., et al. (2018). Assessing the Fermentation Quality and Microbial Community of the Mixed Silage of Forage Soybean With Crop Corn or Sorghum. Bioresour. Technol. 265, 563–567. doi:10.1016/j.biortech.2018.05.097
Ni, K., Wang, X., Lu, Y., Guo, L., Li, X., and Yang, F. (2020). Exploring the Silage Quality of Alfalfa Ensiled with the Residues of Astragalus and Hawthorn. Bioresour. Technol. 297, 122249. doi:10.1016/j.biortech.2019.122249
Ogunade, I. M., Jiang, Y., Pech Cervantes, A. A., Kim, D. H., Oliveira, A. S., Vyas, D., et al. (2018). Bacterial Diversity and Composition of Alfalfa Silage as Analyzed by Illumina Miseq Sequencing: Effects of Escherichia coli O157: H7 and Silage Additives. J. Dairy Sci. 101, 2048–2059. doi:10.3168/jds.2017-12876
Pahlow, G., Muck, R. E., Driehuis, F., Elferink, S. J. W. H., and Spoelstra, S. F. (2003). “Microbiology of Ensiling,” in Silage Science and Technology. Editors D. R. Buxton, R. E. Muck, and J. H. Harrison (Madison, WI: American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc. Publications), 31–93.
Pandey, A., and Negi, P. S. (2016). Traditional Uses, Phytochemistry and Pharmacological Properties of Neolamarckia cadamba: a Review. J. Ethnopharmacol. 181, 118–135. doi:10.1016/j.jep.2016.01.036
Smith, L. H. (1962). Theoretical Carbohydrate Requirement for Alfalfa Silage Production. Agron. J. 54, 291–293. doi:10.2134/agronj1962.00021962005400040003x
Tabacco, E., Borreani, G., Crovetto, G. M., Galassi, G., Colombo, D., and Cavallarin, L. (2006). Effect of Chestnut Tannin on Fermentation Quality, Proteolysis, And Protein Rumen Degradability of Alfalfa Silage. J. Dairy Sci. 89, 4736–4746. doi:10.3168/jds.s0022-0302(06)72523-1
Tian, H., Wang, J., Li, J., Wang, Y., and He, W. (2019). Six New Families of Aerobic Arsenate Reducing Bacteria: leclercia, raoultella, kosakonia, lelliottia, yokenella, and kluyvera. Geomicrobiol. J. 36, 1–9. doi:10.1080/01490451.2018.1554726
Ugbogu, E. A., Elghandour, M. M. M. Y., Ikpeazu, V. O., Buendía, G. R., Molina, O. M., Arunsi, U. O., et al. (2019). The Potential Impacts of Dietary Plant Natural Products on The Sustainable Mitigation of Methane Emission From Livestock Farming. J. Clean. Prod. 213, 915–925. doi:10.1016/j.jclepro.2018.12.233
Wang, S., Liu, G., Li, Y., Cui, Z., Zhou, D., Liu, D., et al. (2017). Effect of Different Proportion Silage Anthocephalus Chinensis Substitute Silage Whole Plant Corn on Growth Performance, Slaughter Performance and Meat Quality of Lezhi Black Goat in Fattening Period. Feed Ind. 21, 37–44. doi:10.13302/j.cnki.fi.2017.2
Wang, Y., Wang, C., Zhou, W., Yang, F.-y., Chen, X.-y., and Zhang, Q. (2018). Effects of Wilting and Lactobacillus plantarum Addition on the Fermentation Quality and Microbial Community of Moringa oleifera Leaf Silage. Front. Microbiol. 9, 1817. doi:10.3389/fmicb.2018.01817
Wang, C., He, L., Xing, Y., Zhou, W., Yang, F., Chen, X., et al. (2019a). Fermentation Quality and Microbial Community of Alfalfa and Stylo Silage Mixed with Moringa Oleifera Leaves. Bioresour. Technol. 284, 240–247. doi:10.1016/j.biortech.2019.03.129
Wang, C., He, L., Xing, Y., Zhou, W., Yang, F., Chen, X., et al. (2019b). Effects of Mixing Neolamarckia cadamba Leaves on Fermentation Quality, Microbial Community of High Moisture Alfalfa and Stylo Silage. Microb. Biotechnol. 12, 869–878. doi:10.1111/1751-7915.13429
Weinberg, Z. G., Muck, R. E., and Weimer, P. J. (2003). The Survival of Silage Inoculant Lactic Acid Bacteria in Rumen Fluid. J. Appl. Microbiol. 94, 1066–1071. doi:10.1046/j.1365-2672.2003.01942.x
Yang, J., Tan, H., and Cai, Y. (2016). Characteristics of Lactic Acid Bacteria Isolates and their Effect on Silage Fermentation of Fruit Residues. J. Dairy Sci. 99, 5325–5334. doi:10.3168/jds.2016-10952
Yuk, K.-J., Kim, Y.-T., Huh, C.-S., and Lee, J.-H. (2018). Lelliottia jeotgali sp. nov., Isolated From a Traditional Korean Fermented Clam. Int. J. Syst. Evol. Microbiol. 68, 1725–1731. doi:10.1099/ijsem.0.002737
Zhang, Q., Yu, Z., Yang, H., and Na, R. S. (2016). The Effects of Stage of Growth and Additives With or Without Cellulase on Fermentation and In Vitro Degradation Characteristics of Leymus chinensis Silage. Grass Forage Sci. 71, 595–606. doi:10.1111/gfs.12210
Zhao, X., Tong, T., Li, H., Lu, H., Ren, J., Zhang, A., et al. (2017). Characterization of Hemicelluloses from Neolamarckia Cadamba (Rubiaceae) During Xylogenesis. Carbohydr. Polym. 156, 333–339. doi:10.1016/j.carbpol.2016.09.041
Keywords: recycle, mixed ensiling, microorganism, legume silage, tannin
Citation: Wu S, Gao L, Chen D, Xue Y, Kholif AE, Zhou W, Chen X and Zhang Q (2022) Effects of Forestry Waste Neolamarckia cadamba Leaf Meal as an Additive on Fermentation Quality, Antioxidant Activity, and Bacterial Community of High-Moisture Stylo Silage. Front. Environ. Sci. 10:925400. doi: 10.3389/fenvs.2022.925400
Received: 21 April 2022; Accepted: 17 June 2022;
Published: 22 July 2022.
Edited by:
Luciano Beneduce, University of Foggia, ItalyReviewed by:
Douglas Nkosi, Animal Production Institute, South AfricaJin Zhong, Institute of Microbiology (CAS), China
Yimin Cai, Japan International Research Center for Agricultural Sciences (JIRCAS), Japan
Copyright © 2022 Wu, Gao, Chen, Xue, Kholif, Zhou, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhou, wzhou@scau.edu.cn; Xiaoyang Chen, xychen@scau.edu.cn; Qing Zhang, zqing1988@126.com
 Shuo Wu
Shuo Wu Lin Gao1
Lin Gao1  Wei Zhou
Wei Zhou Qing Zhang
Qing Zhang