- 1Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital, Tianjin, China
- 2Department of Neurology, Tianjin Medical University General Hospital, Tianjin, China
- 3Laboratory of Epidemiology, Tianjin Neurological Institute, Tianjin, China
- 4Tianjin Neurological Institute, Key Laboratory of Post-Neuroinjury Neuro-repair and Regeneration in Central Nervous System, Ministry of Education and Tianjin City, Tianjin, China
- 5 Department of Endocrinology and Metabolism, The Second Hospital of Tianjin Medical University, Tianjin, China
- 6Department of Geriatrics, Tianjin Medical University General Hospital, Tianjin, China
Aims: Abnormal glucose regulation, which can present as diabetes and prediabetes, has become one of the most common chronic conditions. However, sex differences in the prevalence of and factors associated with abnormal glucose regulation remain unclear. Thus, we aimed to explore sex differences in the prevalence of and factors associated with abnormal glucose regulation in low-income adults in China aged ≥50 years with normal fasting plasma glucose levels.
Materials and Methods: A total of 2,175 individuals aged ≥50 years with normal fasting plasma glucose levels were recruited into this study. After an overnight fast of at least 10 h, individuals underwent an oral glucose tolerance test. Fasting and 2-h plasma glucose levels were measured to determine the state of glucose regulation.
Results: Women were more likely than men to have isolated-impaired glucose tolerance (i-IGT) overall (24.7% vs 20.8%; P= 0.034), among individuals aged <65 years (21.7% vs 15.9%; P= 0.012). Among men, independent risk factors for i-IGT were an age of ≥65 years, hypertension, and high serum uric acid (SUA) and triglyceride levels; independent risk factors for diabetes mellitus (DM) were an age of ≥75 years and alcohol consumption. Among women, independent risk factors for i-IGT were central obesity and high levels of high-sensitivity C-reactive protein and SUA; independent risk factors for DM were low education and an elevated white blood cell count.
Conclusions: Our findings suggest that conventional cardiovascular disease risk factors (i.e., age, hypertension, and dyslipidemia) associated with high risk of developing DM in men, but poor life style (i.e., obesity) and low education attainment in women. It is necessary for delay or stopping the development of DM among low-income adults in China to implement the personalized scheme of prevention DM between men and women, especially highlight control the risk factors in young and middle aged women.
Introduction
It is well established that outcomes of abnormal glucose regulation include prediabetes and diabetes. Over the past few decades, diabetes has become one of the most common noncommunicable chronic diseases (1, 2). The number of adults with diabetes worldwide increased to 422 million in 2014, almost four times that in 1980 (2). The prevalence of diabetes and prediabetes in China has reached 10.9% and 35.7% in 2013, respectively (3), and the prevalence of prediabetes is higher among rural residents than among urban residents (3). In addition to the vascular diseases resulting from diabetes (4), diabetes is also associated with the risk of death from nonvascular conditions, such as cancers, infectious diseases, and degenerative disorders (5). Furthermore, mortality associated with diabetes has been shown to be higher in rural areas than in urban areas, even though there is a greater number of patients in urban districts (6).
Numerous studies have reported the prevalence of and risk factors for diabetes in adults (1, 6). Some previous studies have shown that men are more likely than women to develop diabetes, although there is some discrepancy in the factors reported to be associated with diabetes, including age, body mass index (BMI), dyslipidemia, low educational attainment, and hypertension (7–12). In contrast, several epidemiological studies have demonstrated that the prevalence of diabetes was higher in women than in men and that risk factors for women developing diabetes were age, waist circumference, BMI, hypertension, low educational attainment, large family size, living environment, while risk factors for men were age, BMI, and hypertension (13, 14). Previous studies have shown that the prevalence of isolated impaired glucose tolerance (i-IGT) was estimated to be between 6.2% and 9.3% (15–17). The Chinese nationally representative survey conducted in 2010 demonstrated that the prevalence of i-IGT was 11.0% in men and 10.9% in women and that type 2 diabetes is mainly manifested by elevated blood glucose after meals (18). More than half of the number of individuals with diabetes has isolated elevated 2-h plasma glucose (2-h PG) levels (19), which rise sharply with age (20). However, few studies have reported sex differences in the prevalence of and factors associated with abnormal glucose regulation in people with normal fasting plasma glucose (FPG) levels, especially among low-income adults with low educational attainment.
The routine examination in China focuses on FPG but rarely measures 2-h PG, leading to missed diagnoses of diabetes. The rural population is large in China, and sanitation is lacking, thus people with diabetes in rural areas are more likely to develop diabetic complications. It is necessary to recognize risk factors for abnormal glucose regulation among men and women with normal FPG levels to perform the oral glucose tolerance test (OGTT) in time and to develop personalized diabetic prevention plans for those with different risk factors. Thus, this study aimed to explore the sex differences in the prevalence of and factors associated with abnormal glucose regulation in adults from a low-income population in northern China aged ≥50 years with normal FPG levels.
Materials and Methods
Participants
This population-based, cross-sectional study was conducted between April 2019 and May 2019. The participants were adults aged ≥50 years from 18 administrative villages in Yangjinzhuang, a township at Ji County in Tianjin, China. Generally, this population has relatively low income and educational levels, and 95% are farmers, with a 2014 per capita disposable income of <1,600 USD (21). The exclusion criteria for the study were as follows: (1) a definite diabetes history identified by a positive answer from participants to the question “Has a doctor told you before that you have diabetes?” and/or (2) an FPG level ≥6.1 mmol/L and <7 mmol/L, obtained from medical records or participant report.
This study was approved by the medical research ethics committee of Tianjin Medical University General Hospital; written informed consent was obtained from recruited individuals before study participation.
Data Collection
Trained researchers administered a standard questionnaire to participants that included information on education, occupation, income, medical history, and lifestyle characteristics. The interviewers were at local clinics in the participants’ residential areas and were trained according to a uniform standard. Demographic information, such as name, sex, and date of birth was obtained from existing records.
Lifestyle characteristics included cigarette smoking, alcohol consumption, physical exercise, and sleep duration. Cigarette smoking was defined as smoking >1 cigarette/day for at least 1 year; participants were categorized as non-smokers, former smokers (defined as those who had stopped smoking for at least 6 months), and current smokers. Alcohol consumption was defined as drinking more than 500 g alcohol/week >1 year; participants were categorized as non-drinkers, former drinkers (defined by temperance for at least 6 months), and current drinkers. Physical exercise was defined as being involved in moderate or vigorous activity ≥30 min/day for at least 3 days per week. Sleep duration was based on self-report. Participants were categorized into four groups according to sleep duration: <5 h, 5–6 h, 7–9 h, and >9 h.
Measurements
Physical examinations, including assessment of height, weight, waist circumference, and blood pressure (BP), were performed by trained physicians at the village clinics. Anthropometric measurements were conducted with participants wearing light clothing without a hat or shoes. Height and weight were measured while the participant was in a fully vertical position using a calibrated instrument, zeroed before each measurement. Waist circumference was measured with a non-stretching measuring tape placed on the participant’s horizontal plane at the midpoint between the top of the iliac crest and the bottom of the costal margin in the midaxillary line. BP, including systolic BP (SBP) and diastolic BP (DBP), was obtained using an electronic sphygmomanometer; participants were asked to take off their coats and roll up their sleeves to the shoulders after 5 min of quiet rest. Two measurements were made 5 min apart, and the second measurement was the reported value.
Before undergoing the oral glucose tolerance test (OGTT), participants were asked to maintain their daily physical activity and diet for at least 3 days. After participants had fasted overnight for at least 10 h, they had venous blood samples taken for measurement of FBP, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), high-sensitivity C-reactive protein (hs-CRP), and serum uric acid (SUA) levels. Participants then drank a standard 75-g glucose solution, and venous blood samples were drawn 120 min later for the measurement of 2-h PG. All blood samples were immediately delivered to Guangzhou KingMed Diagnostics Group Co., Ltd., an Independent Clinical Laboratories industry in China, for all measurements.
Carotid ultrasonography was performed using a Mindary M7 portable ultrasound system. A professional radiologist used a scanner to scan and store images of both common carotid arteries, both carotid sinuses, and both proximal internal carotid arteries. Later, carotid intima-media thickness (CIMT) was measured by two experienced imaging physicians based on blood vessel images.
Definitions
BMI was calculated as the participant’s weight (in kg) divided by the square of the participant’s height (m2). According to the BMI, participants were categorized as underweight (BMI ≤ 18.5 kg/m2), normal weight (18.5 kg/m2 <BMI< 24.0 kg/m2), overweight (24.0 kg/m2 ≤ BMI< 28.0 kg/m2), or obese (BMI ≥28 kg/m2) (22). Central obesity was defined as a waist circumference >90 cm for men and >85 cm for women (23). Hypertension was defined as an SBP ≥140 mmHg, DBP ≥90 mmHg, or for participants taking antihypertension medications. Diabetes was defined as an FPG level ≥7.0 mmol/L, a 2-h PG level ≥11.1 mmol/L, a previous history of diagnosed diabetes, or by the use of hypoglycemic drugs (24). Individuals with normal FPG levels were categorized as having normal glucose tolerance (NGT, 2-h PG level <7.8 mmol/L), isolated-impaired glucose tolerance (i-IGT, 2-h PG level 7.8-11.0 mmol/L), or diabetes mellitus (DM, 2-h PG level ≥11.1 mmol/L).
Statistical Analyses
Continuous variables, including age, number of years of formal education, BMI, SBP, DBP, FPG level, 2-h PG level, white blood cell (WBC) count, hs-CRP level, SUA level, TC level, TG level, HDL-C level, LDL-C level, and CIMT, are presented as means with standard deviations and were compared between NGT and i-IGT/DM groups using Student’s t-tests. Categorical variables, such as age group, education group, income group, smoking status, alcohol consumption status, the number of days per week that a participant engaged in physical activity, and hypertension status, are presented as numbers with frequencies and were compared between NGT and i-IGT/DM groups using chi-squared tests. Binary logistic regression analyses were used to evaluate the associations between DM and i-IGT prevalence with those factors found to be statistically significant in the univariate analysis. The associations are presented as odds ratios (ORs) and 95% confidence intervals (CIs). P-values <0.05 in the two-tailed tests were considered statistically significant. SPSS for Windows (version 22.0; IBM Corp., Armonk, NY, USA) was used for analyses.
Results
A total of 2,647 persons were recruited to the study, and 472 were excluded for having an elevated FPG level (FPG ≥6.1 mmol/L); thus, 2,175 participants were included in the final analyses.
Demographic Characteristics
In this study, 53.9% (n = 1,172) were women, and 54.6% (n = 1,187) were individuals aged 50–65 years. The mean number of years of formal education was only 5.53 years overall, and 97.2% of participants had an annual income <6,000 yuan. Among participants, 74.6% had hypertension, and 64.6% were overweight or obese. The mean SBP and DBP were 149.03 mmHg and 84.66 mmHg, respectively, and the FPG and 2-h PG levels were 5.26 mmol/L and 6.75 mmol/L, respectively (Table 1).
Sex Differences in the Prevalence of i-IGT and DM
Among all participants, women were more likely than men to have i-IGT (24.7% vs. 20.8%; P = 0.034), and this sex difference was also true among individuals aged <65 years (P<0.05). There were no significant sex differences in DM prevalence among all participants or by education or income subgroup (P>0.05; Table 2).
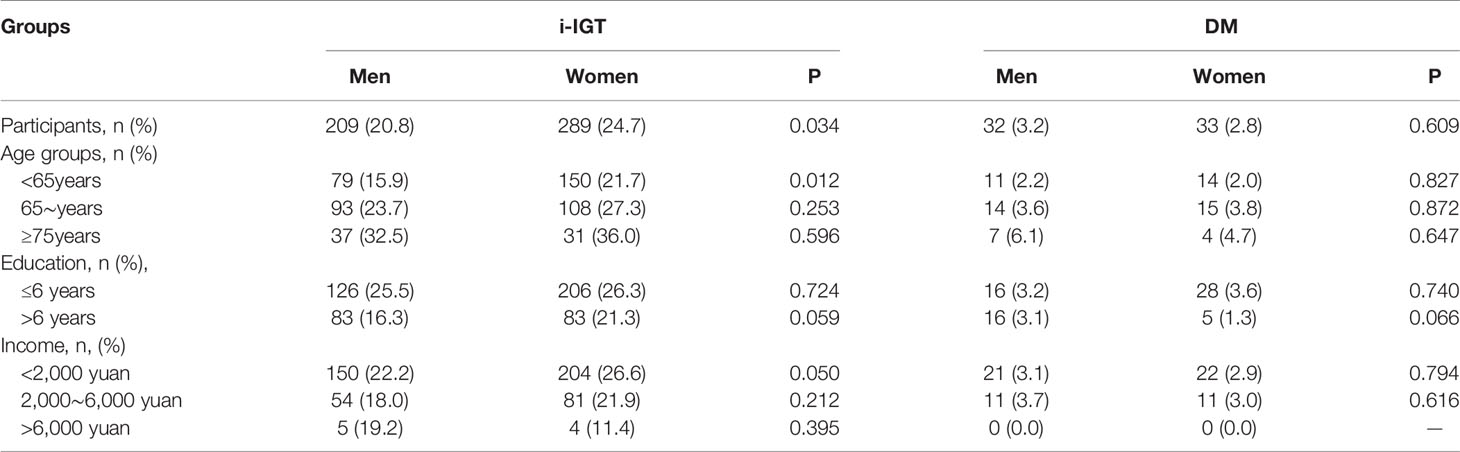
Table 2 Sex differences in the prevalence of abnormal glucose regulation by demographic characteristics.
Risk Factors for Abnormal Glucose Regulation in Men and Women
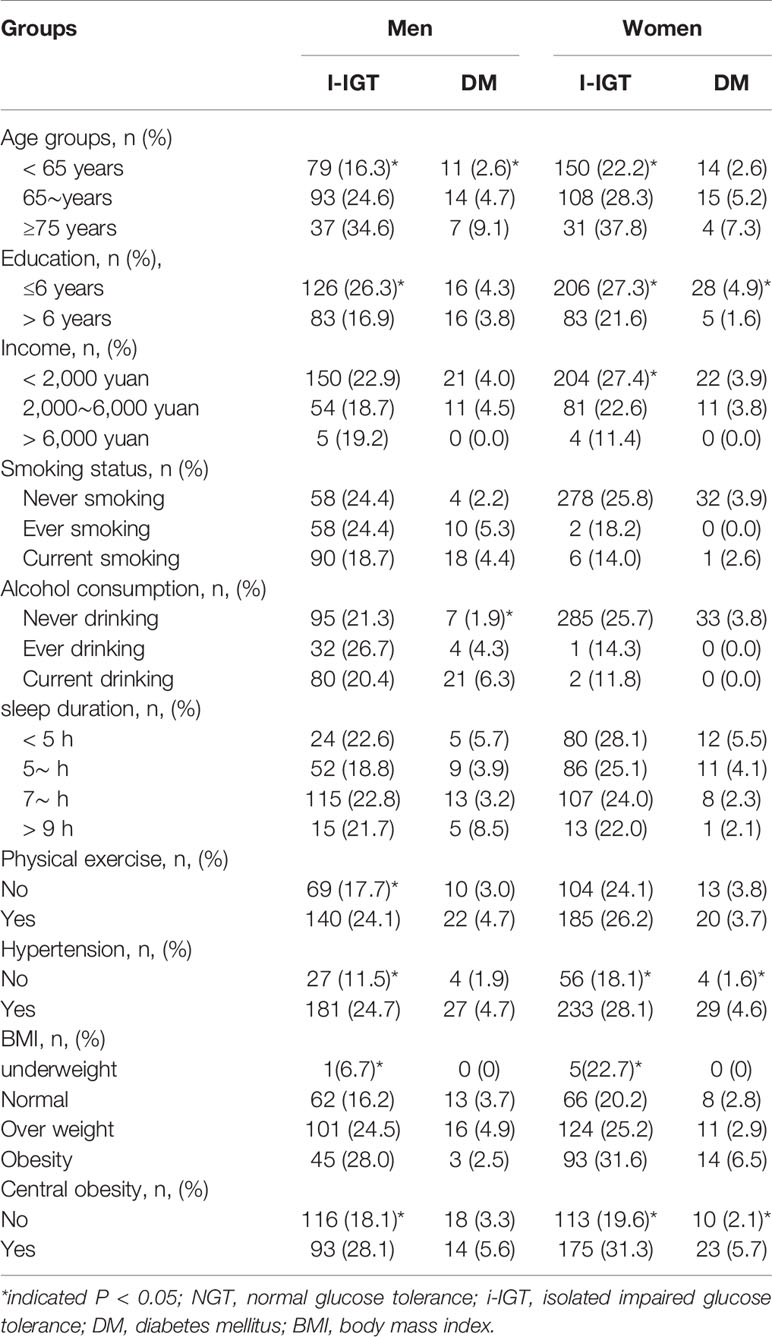
Table 3 shows that, among men, the prevalence of i-IGT increased with increasing age, BP, and BMI (P<0.05), and the prevalence of i-IGT was significantly higher among those whose formal education was ≤6 years and who had central obesity (P<0.001). Older men and those who consumed alcohol had a greater risk of DM than did those <65 years of age and those who had never consumed alcohol (P<0.05). Among women, the prevalence of i-IGT was greater in those with hypertension and obesity (P<0.001). Women who had less formal education and less income were at an increased risk of i-IGT (P= 0.035). Furthermore, women with ≤6 years of education had a higher risk of DM (P= 0.016), and the prevalence of DM was higher among women with hypertension and central obesity (P<0.05).
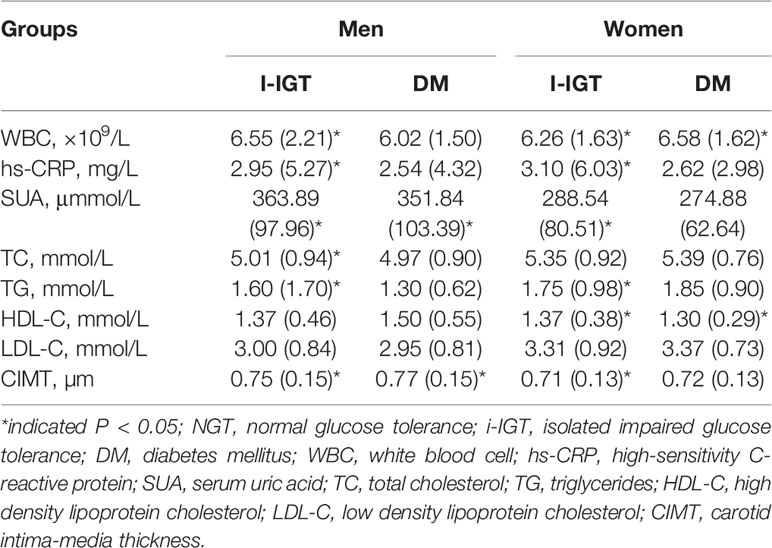
As shown in Table 4, men with elevated WBC counts, hs-CRP levels, SUA levels, TC levels, TG levels, and increased CIMT had an increased risk of i-IGT (P<0.05). Additionally, men with high levels of SUA and increased CIMT had an increased risk of DM (P<0.05). Women with i-IGT had lower HDL-C levels, higher WBC counts, hs-CRP, SUA, TG, and CIMT than those with NGT did (P<0.05). The pattern of association described above for HDL-C and WBC was also consistent for women with DM (P<0.05).
Risk Factors Identified in the Multivariate Analysis for Abnormal Glucose Regulation in Men
In the multivariate analysis, an age of ≥65 years, the presence of hypertension, and high SUA and TG levels were independent risk factors for i-IGT among men. When compared with that among men <65 years of age, the prevalence of i-IGT was 63.7% higher among men who were in the 65–74-year age group (OR, 1.637; 95% CI, 1.065–2.517; P= 0.025) and 111.8% higher among men in the ≥75-year age group (OR, 2.118; 95% CI, 1.177–3.813; P= 0.012). Men with hypertension had a 109.5% increase in the prevalence of i-IGT compared with men with normal BP (OR, 2.095; 95% CI, 1.238–3.544; P= 0.006). Furthermore, the prevalence of i-IGT among men increased by 4% with each 1-µmol/L increase in SUA level (OR, 1.004; 95% CI, 1.002-1.006; P<0.001) and by 22.2% with each 1-mmol/L increase in TG level (OR, 1.222; 95% CI, 1.027–1.455; P= 0.024; Figure 1).
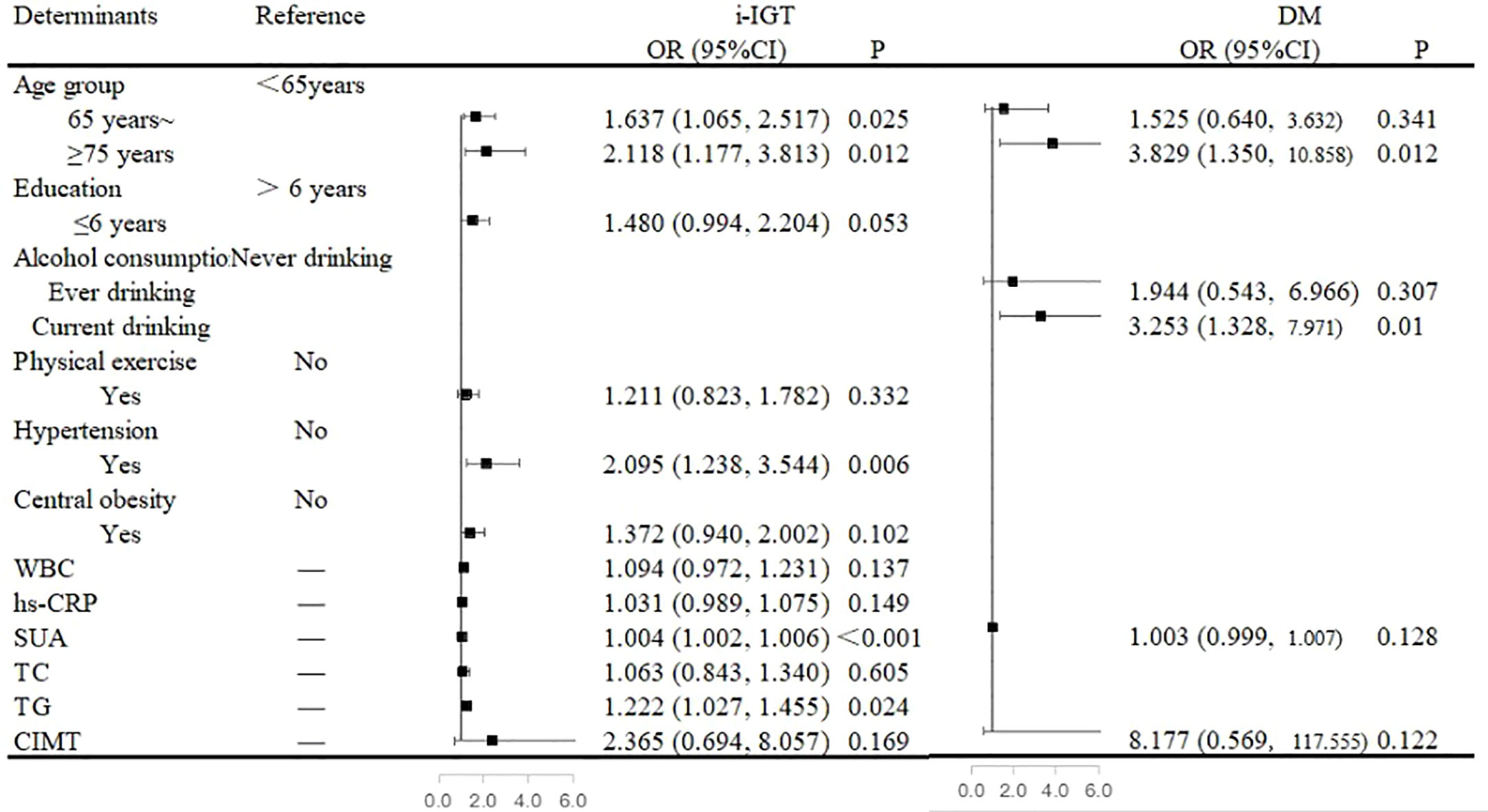
Figure 1 Associated factors of abnormal glucose regulation in the multivariate analyses in men. Figure 1 showed that an age of ≥65 years, the presence of hypertension, and high serum uric acid (SUA) and triglycerides (TG) levels were independent risk factors for isolated-impaired glucose tolerance (i-IGT) among men. An age of ≥75 years and alcohol consumption were independent risk factors for diabetes mellitus (DM) among men.
An age of ≥75 years and alcohol consumption were independent risk factors for DM among men. When compared with that among men <65 years of age, the prevalence of DM was almost three-fold higher among men aged ≥75 years (OR, 3.829; 95% CI, 1.350–10.858; P= 0.012). Moreover, alcohol consumption was associated with a 225.3% increased risk of DM among men (OR, 3.253; 95% CI, 1.328-7.971; P= 0.010; Figure 1).
Risk Factors Identified in the Multivariate Analysis for Abnormal Glucose Regulation in Women
Among women, central obesity and high levels of hs-CRP and SUA were independent risk factors for i-IGT. The prevalence of i-IGT was 40.2% higher for women who had central obesity than for women who did not (OR, 1.402; 95% CI, 1.025–1.917; P= 0.034). Each 1-µmol/L increase in SUA level increased the prevalence of i-IGT by 2% among women (OR, 1.002; 95% CI, 1.000-1.004; P= 0.028). Similarly, women with a high level of hs-CRP were at greater risk of having i-IGT (OR, 1.047; 95% CI, 1.008–1.088; P= 0.018; Figure 2).
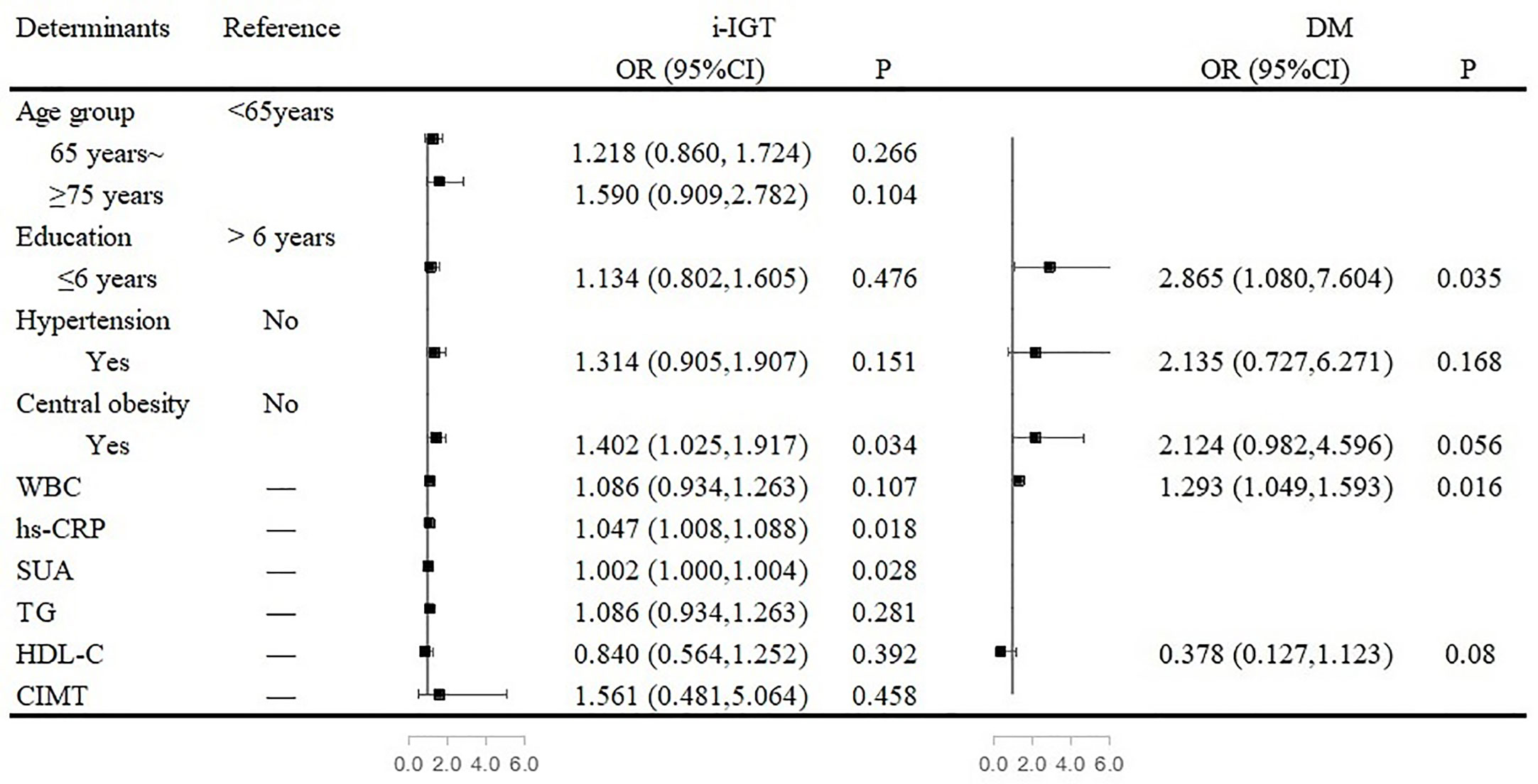
Figure 2 Associated factors of abnormal glucose regulation in the multivariate analyses in women. Figure 2 showed that central obesity and high levels of high-sensitivity C-reactive protein (hs-CRP) and serum uric acid (SUA) were independent risk factors for isolated-impaired glucose tolerance (i-IGT). Low education and elevated white blood cell (WBC) count were independent risk factors for diabetes mellitus (DM) among women.
Low education and elevated WBC count were independent risk factors for DM among women. Women who received a formal education of <6 years had a 186.5% higher risk of DM than did women with longer formal educations (OR, 2.865; 95% CI, 1.080–7.604; P<0.001). Furthermore, for every 1 × 109/L increase in WBC count, the risk of DM increased by 29.3% (OR, 1.293; 95% CI, 1.049–1.593; P= 0.016; Figure 2).
Discussion
In this cross-sectional study, we explored sex differences in the prevalence of and risk factors associated with abnormal glucose regulation in low-income adults aged 50 years or older in northern China with normal FPG levels. Women were more likely than men overall to develop i-IGT, and this was also true for individuals aged <65 years; there was no significant sex-based difference in DM prevalence. Among men, an age of ≥65 years, hypertension, and high SUA and TG levels were independent risk factors for i-IGT; an age of ≥75 years and alcohol consumption were independent risk factors for DM. Among women, central obesity and high levels of hs-CRP and SUA were independent risk factors for i-IGT; low educational attainment and an elevated WBC count were independent risk factors for DM.
The prevalence of diabetes in China has increased sharply in the past few decades (25), from 2.5% in 1994 (26) to 10.9% in 2013 (3). Some studies reporting sex differences in the prevalence of diabetes reported that women were more susceptible to diabetes than were men (14). A meta-analysis associated with inland residents in China showed that the prevalence of diabetes was higher among women than among men (11.6% vs 9.3%) (25). However, other studies suggested that men were more likely to have diabetes (7–9, 27). Similarly, a cross-sectional study of 46,239 individuals in China showed that male sex was an independent risk factor for diabetes (OR, 1.26; 95% CI, 1.12–1.43) (18). The National Health and Nutrition Examination Surveys in America demonstrated that the prevalence of prediabetes was higher in women than in men among adults aged ≥50 years (28). In the present study, we observed that women were more likely overall to have i-IGT and that this was also true among individuals aged <65 years. In contrast, we did not observe a significant sex-based difference in DM prevalence. Different study designs and populations may explain these discrepant conclusions. It is well-known that women usually live longer than men; therefore, it may be reasonable to devote more attention to understanding women with prediabetes and diabetes in order to promote their health, especially those with a low income and aged <65 years.
Many studies have explored the association between advanced age and diabetes (25, 29). A 12-year follow-up study that enrolled individuals aged 40–69 years reported that age was an independent risk factor for progression to diabetes (HR, 1.02; 95% CI, 1.02–1.03) (30). Similarly, the China National Diabetes and Metabolic Disorders Study, conducted from June 2007 to May 2008, demonstrated that older age was significantly associated with an increased risk of diabetes (18). In accordance with the results of previous studies, we concluded that advanced age was an independent risk factor for i-IGT (≥65 years) and diabetes (≥75 years), but among men alone in this low-income population. The decrease in insulin secretion, especially in people with impaired glucose tolerance, may partly explain the effect of advanced age on diabetes and prediabetes (31).
Previous reports on the association between education and diabetes are inconsistent. The China Kadoorie Biobank study, which included 0.5 million adults recruited from 10 diverse areas in China, concluded that, compared with having no formal education, having a college education or higher was associated with a 21% increase in the prevalence of diabetes (OR, 1.21; 95% CI, 1.09–1.35) and a 27% increase in the incidence of diabetes (HR, 1.27; 95% CI, 1.07–1.51) among men, whereas having a college education or higher was associated with a 31% decrease in the prevalence of diabetes (OR, 0.69; 95% CI, 0.63–0.76) and a 20% decrease in the incidence of diabetes (HR, 0.80; 95% CI, 0.67–0.95) among women (32). However, there are also some studies hold the opposite view (27, 33). A prospective study in low-, middle-, and high-income countries, including China, revealed that a low education level was an independent risk factor for diabetes (OR, 1.10; 95% CI, 1.02-1.19) (33). In the current study, low education was an independent risk factor for diabetes among women alone. Similar to socioeconomic status, level of education can affect health through complex mechanisms, such as health care, environmental exposure, and health behavior (34).
The association between alcohol consumption and the risk of developing DM remains controversial. People currently consuming 8–14 drinks/week have been reported to have a lower risk of diabetes than did those consuming ≤1 drink/week for both men (HR, 0.84; 95% CI, 0.70–1.00) and women (HR, 0.71; 95% CI, 0.56–0.91) (35). However, reductions in the risk of developing diabetes among moderate drinkers appears to apply to women and non-Asian populations alone (36). Studies in Asia, including China and Japan, suggest that regular drinkers and heavy drinkers have an increased risk of developing diabetes than do social drinkers (37, 38). Even for moderate drinkers, a higher alcohol intake increased the risk of developing diabetes (39). In accordance with results of the studies mentioned above, current drinking was found to be an independent risk factor for i-IGT among men in this cross-sectional study.
Obesity and central obesity are accepted risk factors for diabetes (7–9, 40). Central obesity has been shown to significantly increase the risk of prediabetes (OR, 1.22; 95% CI, 1.06–1.40) and diabetes (OR, 1.39; 95% CI, 1.18–1.63) (18). In this study, women with central obesity were at a higher risk of having i-IGT. In humans with obesity, reduced G protein pathway suppressor 2 (GPS2) expression in macrophages causes elevated systemic and adipose tissue inflammation, insulin resistance, and diabetes (41, 42). These changes may contribute to the greater risk of IGT in those who are obese, but the cause of the sex disparity remains unclear.
The positive association between hypertension and diabetes has been established in previous studies (18, 29). A 10-year, community-based, prospective study in Korea demonstrated that individuals with prehypertension, stage 1 hypertension, and stage 2 hypertension had a 23%, 26%, and 60% increased risk of diabetes, respectively, compared with individuals with normal blood pressure (<120/80 mmHg) (43). In addition, usual blood pressure (95 mmHg <SBP ≤195 mmHg; 65 mmHg <DBP ≤115 mmHg) also increased the risk of new-onset diabetes; each 20-mmHg elevation in SBP and 10-mmHg elevation in DBP increased the risk of new-onset diabetes by 58% and 52%, respectively, when compared with BP within the normal range (44). Among men in this low-income population, hypertension was an independent risk factor for i-IGT. The links between obesity, diabetes, and BP may be attributed to adipokine dysregulation in perivascular adipose tissue (45).
Previous studies have also reported a positive association between TG level and diabetes (46–48).In the present study, we found that TG level was an independent risk factor for i-IGT among men, resulting in a 23.4% increase in the prevalence of i-IGT with each 1-mmol/L increase in TG level. The association between TG and diabetes may be partly mediated by insulin resistance and increased insulin secretion (49, 50). As a result, lipid management is essential, especially for those with chronic diseases.
Diabetes is an inflammatory disease (51). In a cross-sectional study of a Chinese population, there was a U-shaped association between WBC count and the incidence of diabetes (52). However, other studies have indicated that WBC count increased the risk of incident diabetes, even when it was within the normal range (53). A substudy of working-class individuals in Hong Kong indicated that WBC count was an independent predictor for hyperglycemia and increased insulin resistance among Chinese men (54). In the present study, WBC count was an independent risk factor for diabetes in women. Decreased insulin sensitivity and incidence of diabetes mediated by elevated WBC count may partly explain this association (55).
hs-CRP is a major acute-phase protein marking systematic inflammation in chronic disease. Some studies have demonstrated a positive association between hs-CRP level and diabetes (56–58). Similarly, we concluded that women with high levels of hs-CRP were at an increased risk of i-IGT in this low-income population. hs-CRP level has been shown to predict changes in insulin sensitivity and future insulin resistance even in non-diabetic adults; thus, hs-CRP level may indicate the imminent development of type 2 diabetes (59, 60).
Higher levels of uric acid were associated with an increased risk of diabetes, both in quantitative and qualitative analyses (61–64). Consistent with the results of these studies, uric acid level was an independent risk factor for i-IGT in both sexes in this low-income population. This association may be explained by higher levels of uric acid inducing oxidative stress, which favors the development of diabetes (65, 66).
There are several limitations of this cross-sectional study. First, we did not measure insulin levels, so we could not evaluate insulin resistance or function of islet beta-cells. Second, we explored the prevalence of abnormal glucose regulation and associated risk factors using people living in Tianjin rural areas; therefore, our findings cannot be generalized to other populations. Third, because this was a cross-sectional study, we could not identify causal associations. Fourth, nutritional factors, as important risk factors for diabetes, were not measured in this low-income population. Finally, OGTT was not performed in people with impaired fasting glucose and known diabetes. The conclusions of the study may be weak without the data of impaired fasting glucose and known diabetes. Therefore, we plan to perform OGTT under careful management among all participants in future research.
Conclusion
In this population-based, cross-sectional study, women were more likely than men to develop i-IGT overall, and this was also true for individuals aged <65 years. Our findings suggest that conventional cardiovascular disease risk factors (i.e., age, hypertension, and dyslipidemia) are associated with a high risk of developing DM in men, while poor life style (i.e., obesity) and low education attainment are risk factors in women. It is necessary to implement the personalized scheme between men and women for delay or stopping the development of DM and to perform OGTT for early diagnosis of diabetes in men and women with multiple risk factors, especially women with a low income and aged less than 65 years.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding authors.
Ethics Statement
This study was approved by the medical research ethics committee of Tianjin Medical University General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JC, JW, and XN were involved in conception, design, and data collection. XZ and JL were involved in manuscript drafting for this article. XZ, JL, SS, YY, DQ, CW, QL, YL, JT, JW, XN, and JC were involved in data collection and case diagnosis for this article. JW and XN were involved in data analysis, and data interpretation. JC, JW, and XN were involved critical review for this article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XY declared a shared affiliation with the authors, to the handling editor, at time of review.
Acknowledgments
We thank all participants of the Tianjin Brain Study and all local medical care professionals for their valuable contribution.
References
1. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA (2013) 310(9):948–59. doi: 10.1001/jama.2013.168118
2. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet (2016) 387(10027):1513–30. doi: 10.1016/S0140-6736(16)00618-8
3. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA (2017) 317(24):2515–23. doi: 10.1001/jama.2017.7596
4. Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet (2010) 375(9733):2215–22. doi: 10.1016/S0140-6736(10)60484-9
5. Seshasa SRK, Kaptoge S, Thompson A, Angelantonio DE, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med (2011) 364(9):829–41. doi: 10.1056/NEJMoa1008862
6. Bragg F, Holmes MV, Iona A, Guo Y, Du H, Chen Y, et al. Association Between Diabetes and Cause-Specific Mortality in Rural and Urban Areas of China. JAMA (2017) 317(3):280–9. doi: 10.1001/jama.2016.19720
7. Mota M, Popa SG, Mota E, Mitrea A, Catrinoiu D, Cheta DM, et al. Prevalence of diabetes mellitus and prediabetes in the adult Romanian population: PREDATORR study. J Diabetes (2016) 8(3):336–44. doi: 10.1111/1753-0407.12297
8. Adekanmbi VT, Uthman OA, Erqou S, Echouffo-Tcheugui JB, Harhay MN, Harhay MO. Epidemiology of prediabetes and diabetes in Namibia, Africa: A multilevel analysis. J Diabetes (2019) 11(2):161–72. doi: 10.1111/1753-0407.12829
9. Pham NM, Eggleston K. Prevalence and determinants of diabetes and prediabetes among Vietnamese adults. Diabetes Res Clin Pract (2016) 113:116–24. doi: 10.1016/j.diabres.2015.12.009
10. Supiyev A, Kossumov A, Kassenova A, Nurgozhin T, Zhumadilov Z, Peasey A, et al. Diabetes prevalence, awareness and treatment and their correlates in older persons in urban and rural population in the Astana region, Kazakhstan. Diabetes Res Clin Pract (2016) 112:6–12. doi: 10.1016/j.diabres.2015.11.011
11. Kaiser A, Vollenweider P, Waeber G, Marques-Vidal P. Prevalence, awareness and treatment of type 2 diabetes mellitus in Switzerland: the CoLaus study. Diabetes Med (2012) 29(2):190–7. doi: 10.1111/j.1464-5491.2011.03422.x
12. Aguayo A, Urrutia I, González-Frutos T, Martínez R, Martínez-Indart L, Castaño L, et al. Prevalence of diabetes mellitus and impaired glucose metabolism in the adult population of the Basque Country, Spain. Diabetes Med (2017) 34(5):662–6. doi: 10.1111/dme.13181
13. Satman I, Omer B, Tutuncu Y, Kalaca S, Gedik S, Dinccag N, et al. Twelve-year trends in the prevalence and risk factors of diabetes and prediabetes in Turkish adults. Eur J Epidemiol (2013) 28(2):169–80. doi: 10.1007/s10654-013-9771-5
14. Duboz P, Chapuis-Lucciani N, Boëtsch G, Gueye L. Prevalence of diabetes and associated risk factors in a Senegalese urban (Dakar) population. Diabetes Metab (2012) 38(4):332–6. doi: 10.1016/j.diabet.2012.02.011
15. Soriguer F, Goday A, Bosch-Comas A, Bordiú E, Calle-Pascual A, Carmena R, et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@bet.es Study. Diabetologia (2012) 55(1):88–93. doi: 10.1007/s00125-011-2336-9
16. Wilmot EG, Edwardson CL, Biddle SJ, Gorely T, Henson J, Khunti K, et al. Prevalence of diabetes and impaired glucose metabolism in younger ‘at risk’ UK adults: insights from the STAND programme of research. Diabetes Med (2013) 30(6):671–5. doi: 10.1111/dme.12173
17. Meisinger C, Strassburger K, Heier M, Thorand B, Baumeister SE, Giani G, et al. Prevalence of undiagnosed diabetes and impaired glucose regulation in 35-59-year-old individuals in Southern Germany: the KORA F4 Study. Diabetes Med (2010) 27(3):360–2. doi: 10.1111/j.1464-5491.2009.02905.x
18. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med (2010) 362(12):1090–101. doi: 10.1056/NEJMoa0908292
19. Qiao Q, Nakagami T, Tuomilehto J, Borch-Johnsen K, Balkau B, Iwamoto Y, et al. Comparison of the fasting and the 2-h glucose criteria for diabetes in different Asian cohorts. Diabetologia (2000) 43(12):1470–5. doi: 10.1007/s001250051557
20. Metter EJ, Windham BG, Maggio M, Simonsick EM, Ling SM, Egan JM, et al. Glucose and insulin measurements from the oral glucose tolerance test and mortality prediction. Diabetes Care (2008) 31(5):1026–30. doi: 10.2337/dc07-2102
21. National Bureau of Statistics of China. China Statistical Yearbook. Beijing: China Statistics Press (2015).
22. Zhou BF. Effect of body mass index on all-cause mortality and incidence of cardiovascular diseases–report for meta-analysis of prospective studies open optimal cut-off points of body mass index in Chinese adults. BioMed Environ Sci (2002) 15(3):245–52. doi: CNKI:SUN:SWYX.0.2002-03-007
23. Tian Y, Jiang C, Wang M, Cai R, Zhang Y, He Z, et al. BMI, leisure-time physical activity, and physical fitness in adults in China: results from a series of national surveys, 2000-14. Lancet Diabetes Endocrinol (2016) 4(6):487–97. doi: 10.1016/S2213-8587(16)00081-4
24. Diabetes branch of the Chinese Medical Association. Guidelines for the prevention and treatment of type 2 diabetes in China (2017 edition). Chin J Diabetes Mellitus (2018) 10(1):4–67. doi: 10.3760/cma.j.issn.1674-5809.2018.01.003
25. Yang L, Shao J, Bian Y, Wu H, Shi L, Zeng L, et al. Prevalence of type 2 diabetes mellitus among inland residents in China (2000-2014): A meta-analysis. J Diabetes Investig (2016) 7(6):845–52. doi: 10.1111/jdi.12514
26. Pan XR, Yang WY, Li GW, Liu J. Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative Group. Diabetes Care (1997) 20(11):1664–9. doi: 10.2337/diacare.20.11.1664
27. Xu G, Liu B, Sun Y, Du Y, Snetselaar LG, Hu FB, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ (2018) 362:k1497. doi: 10.1136/bmj.k1497
28. Caspersen CJ, Thomas GD, Beckles GL, Bullard KM. Secular changes in prediabetes indicators among older-adult Americans, 1999-2010. Am J Prev Med (2015) 48(3):253–63. doi: 10.1016/j.amepre.2014.10.004
29. Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol (2017) 5(8):585–96. doi: 10.1016/S2213-8587(17)30174-2
30. Han SJ, Kim HJ, Kim DJ, Lee KW, Cho NH. Incidence and predictors of type 2 diabetes among Koreans: A 12-year follow up of the Korean Genome and Epidemiology Study. Diabetes Res Clin Pract (2017) 123:173–80. doi: 10.1016/j.diabres.2016.10.004
31. Szoke E, Shrayyef MZ, Messing S, Woerle HJ, van Haeften TW, Meyer C, et al. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care (2008) 31(3):539–43. doi: 10.2337/dc07-1443
32. Wu H, Bragg F, Yang L, Du H, Guo Y, Jackson CA, et al. Sex differences in the association between socioeconomic status and diabetes prevalence and incidence in China: cross-sectional and prospective studies of 0.5 million adults. Diabetologia (2019) 62(8):1420–9. doi: 10.1007/s00125-019-4896-z
33. Dagenais GR, Gerstein HC, Zhang X, McQueen M, Lear S, Lopez-Jaramillo P, et al. Variations in Diabetes Prevalence in Low-, Middle-, and High-Income Countries: Results From the Prospective Urban and Rural Epidemiological Study. Diabetes Care (2016) 39(5):780–7. doi: 10.2337/dc15-2338
34. Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood) (2002) 21(2):60–76. doi: 10.1377/hlthaff.21.2.60
35. He X, Rebholz CM, Daya N, Lazo M, Selvin E. Alcohol consumption and incident diabetes: The Atherosclerosis Risk in Communities (ARIC) study. Diabetologia (2019) 62(5):770–8. doi: 10.1007/s00125-019-4833-1
36. Knott C, Bell S, Britton A. Alcohol Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of More Than 1.9 Million Individuals From 38 Observational Studies. Diabetes Care (2015) 38(9):1804–12. doi: 10.2337/dc15-0710
37. Lai YJ, Hu HY, Lee YL, Ko MC, Ku PW, Yen YF, et al. Frequency of alcohol consumption and risk of type 2 diabetes mellitus: A nationwide cohort study. Clin Nutr (2019) 38(3):1368–72. doi: 10.1016/j.clnu.2018.06.930
38. Heianza Y, Arase Y, Saito K, Tsuji H, Fujihara K, Hsieh SD, et al. Role of alcohol drinking pattern in type 2 diabetes in Japanese men: the Toranomon Hospital Health Management Center Study 11 (TOPICS 11). Am J Clin Nutr (2013) 97(3):561–8. doi: 10.3945/ajcn.112.043364
39. Peng M, Zhang J, Zeng T, Hu X, Min J, Tian S, et al. Alcohol consumption and diabetes risk in a Chinese population: a Mendelian randomization analysis. Addiction (2019) 114(3):436–49. doi: 10.1111/add.14475
40. Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of Prediabetes Among Adolescents and Young Adults in the United States, 2005-2016. JAMA Pediatr (2019) 2:e194498. doi: 10.1001/jamapediatrics.2019.4498
41. Fan R, Toubal A, Goñi S, Drareni K, Huang Z, Alzaid F, et al. Loss of the co-repressor GPS2 sensitizes macrophage activation upon metabolic stress induced by obesity and type 2 diabetes. Nat Med (2016) 22(7):780–91. doi: 10.1038/nm.4114
42. Wang T, Lu J, Shi L, Chen G, Xu M, Xu Y, et al. Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol (2020) 8(2):115–24. doi: 10.1016/S2213-8587(19)30425-5
43. Cho NH, Kim KM, Choi SH, Park KS, Jang HC, Kim SS, et al. High Blood Pressure and Its Association With Incident Diabetes Over 10 Years in the Korean Genome and Epidemiology Study (KoGES). Diabetes Care (2015) 38(7):1333–8. doi: 10.2337/dc14-1931
44. Knowles JW, Reaven G. Usual Blood Pressure and New-Onset Diabetes Risk: Evidence From 4.1 Million Adults and a Meta-Analysis. J Am Coll Cardiol (2016) 67(13):1656–7. doi: 10.1016/j.jacc.2015.12.065
45. Saxton SN, Clark BJ, Withers SB, Eringa EC, Heagerty AM. Mechanistic Links Between Obesity, Diabetes, and Blood Pressure: Role of Perivascular Adipose Tissue. Physiol Rev (2019) 99(4):1701–63. doi: 10.1152/physrev.00034.2018
46. Cui J, Sun J, Wang W, Yasmeen N, Ke M, Xin H, et al. Triglycerides and total cholesterol concentrations in association with IFG/IGT in Chinese adults in Qingdao, China. BMC Public Health (2018) 18(1):444. doi: 10.1186/s12889-018-5286-z
47. Lin D, Qi Y, Huang C, Wu M, Wang C, Li F, et al. Associations of lipid parameters with insulin resistance and diabetes: A population-based study. Clin Nutr (2018) 37(4):1423–9. doi: 10.1016/j.clnu.2017.06.018
48. Malmström H, Walldius G, Carlsson S, Grill V, Jungner I, Gudbjörnsdottir S, et al. Elevations of metabolic risk factors 20 years or more before diagnosis of type 2 diabetes: Experience from the AMORIS study. Diabetes Obes Metab (2018) 20(6):1419–26. doi: 10.1111/dom.13241
49. Simental-Mendía LE, Rodríguez-Morán M, Simental-Saucedo L, Guerrero-Romero F. Insulin secretion is increased in non-diabetic subjects with fasting hypertriglyceridaemia. Diabetes Metab Res Rev (2013) 29(3):214–9. doi: 10.1002/dmrr.2379
50. Caporaso NE, Jones RR, Stolzenberg-Solomon RZ, Medgyesi DN, Kahle LL, Graubard BI. Insulin Resistance in Healthy U.S. Adults: Findings from the National Health and Nutrition Examination Survey (NHANES). Cancer Epidemiol Biomarkers Prev (2020) 29(1):157–68. doi: 10.1158/1055-9965.EPI-19-0206
51. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol (2011) 11(2):98–107. doi: 10.1038/nri2925
52. Du X, Zhu B, Hu G, Mao W, Wang S, Zhang H, et al. U-shape association between white blood cell count and the risk of diabetes in young Chinese adults. Diabetes Med (2009) 26(10):955–60. doi: 10.1111/j.1464-5491.2009.02801.x
53. Twig G, Afek A, Shamiss A, Derazne E, Tzur D, Gordon B, et al. White blood cells count and incidence of type 2 diabetes in young men. Diabetes Care (2013) 36(2):276–82. doi: 10.2337/dc11-2298
54. So WY, Tong PC, Ko GT, Ma RC, Ozaki R, Kong AP, et al. Low plasma adiponectin level, white blood cell count and Helicobacter pylori titre independently predict abnormal pancreatic beta-cell function. Diabetes Res Clin Pract (2009) 86(2):89–95. doi: 10.1016/j.diabres.2009.08.010
55. Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes (2002) 51(2):455–61. doi: 10.2337/diabetes.51.2.455
56. Effoe VS, Correa A, Chen H, Lacy ME, Bertoni AG. High-Sensitivity C-Reactive Protein Is Associated With Incident Type 2 Diabetes Among African Americans: The Jackson Heart Study. Diabetes Care (2015) 38(9):1694–700. doi: 10.2337/dc15-0221
57. Mahajan A, Tabassum R, Chavali S, Dwivedi OP, Bharadwaj M, Tandon N, et al. High-sensitivity C-reactive protein levels and type 2 diabetes in urban North Indians. J Clin Endocrinol Metab (2009) 94(6):2123–7. doi: 10.1210/jc.2008-2754
58. Lainampetch J, Panprathip P, Phosat C, Chumpathat N, Prangthip P, Soonthornworasiri N, et al. Association of Tumor Necrosis Factor Alpha, Interleukin 6, and C-Reactive Protein with the Risk of Developing Type 2 Diabetes: A Retrospective Cohort Study of Rural Thais. J Diabetes Res (2019) 2019:9051929. doi: 10.1155/2019/9051929
59. Yan Y, Li S, Liu Y, Bazzano L, He J, Mi J, et al. Temporal relationship between inflammation and insulin resistance and their joint effect on hyperglycemia: the Bogalusa Heart Study. Cardiovasc Diabetol (2019) 18(1):109. doi: 10.1186/s12933-019-0913-2
60. Fizelova M, Jauhiainen R, Kangas AJ, Soininen P, Ala-Korpela M, Kuusisto J, et al. Differential Associations of Inflammatory Markers With Insulin Sensitivity and Secretion: The Prospective METSIM Study. J Clin Endocrinol Metab (2017) 102(9):3600–9. doi: 10.1210/jc.2017-01057
61. Viazzi F, Leoncini G, Vercelli M, Deferrari G, Pontremoli R. Serum uric acid levels predict new-onset type 2 diabetes in hospitalized patients with primary hypertension: the MAGIC study. Diabetes Care (2011) 34(1):126–8. doi: 10.2337/dc10-0918
62. Kodama S, Saito K, Yachi Y, Asumi M, Sugawara A, Totsuka K, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care (2009) 32(9):1737–42. doi: 10.2337/dc09-0288
63. Shani M, Vinker S, Dinour D, Leiba M, Twig G, Holtzman EJ, et al. High Normal Uric Acid Levels Are Associated with an Increased Risk of Diabetes in Lean, Normoglycemic Healthy Women. J Clin Endocrinol Metab (2016) 101(10):3772–8. doi: 10.1210/jc.2016-2107
64. Liu J, Tao L, Zhao Z, Mu Y, Zou D, Zhang J, et al. Two-Year Changes in Hyperuricemia and Risk of Diabetes: A Five-Year Prospective Cohort Study. J Diabetes Res (2018) 30:6905720. doi: 10.1155/2018/6905720
65. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol (2007) 293(2):C584–96. doi: 10.1152/ajpcell.00600.2006
Keywords: sex differences, abnormal glucose regulation, prevalence, risk factors, epidemiology
Citation: Zhang X, Liu J, Shao S, Yang Y, Qi D, Wang C, Lin Q, Liu Y, Tu J, Wang J, Ning X and Cui J (2021) Sex Differences in the Prevalence of and Risk Factors for Abnormal Glucose Regulation in Adults Aged 50 Years or Older With Normal Fasting Plasma Glucose Levels. Front. Endocrinol. 11:531796. doi: 10.3389/fendo.2020.531796
Received: 01 February 2020; Accepted: 29 December 2020;
Published: 19 February 2021.
Edited by:
Marc R. Blackman, Washington DC VA Medical Center, United StatesReviewed by:
Antonello Lorenzini, University of Bologna, ItalyXilin Yang, Tianjin Medical University, China
Copyright © 2021 Zhang, Liu, Shao, Yang, Qi, Wang, Lin, Liu, Tu, Wang, Ning and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingqiu Cui, cuijingqiu2020@163.com; Xianjia Ning, xjn0906@gmail.com; Jinghua Wang, jhw8799@yahoo.com
†These authors have contributed equally to this work
 Xinxin Zhang1†
Xinxin Zhang1† Jie Liu
Jie Liu Yuan Yang
Yuan Yang Conglin Wang
Conglin Wang Qiuxing Lin
Qiuxing Lin Jinghua Wang
Jinghua Wang Xianjia Ning
Xianjia Ning Jingqiu Cui
Jingqiu Cui