Alcohol Abuse Associated With Increased Risk of Angiographic Vasospasm and Delayed Cerebral Ischemia in Patients With Aneurysmal Subarachnoid Hemorrhage Requiring Mechanical Ventilation
- 1Department of Neurosurgery, Tangdu Hospital, The Fourth Military Medical University, Xi'an, China
- 2Department of Public Health, School of Health Sciences and Practice, New York Medical College, Valhalla, NY, United States
Objective: Although alcohol abuse has been indicated to cause cerebral aneurysm development and rupture, there is limited data on the impact of alcohol abuse on outcomes after an aneurysmal subarachnoid hemorrhage (aSAH). This study aims to investigate whether alcohol abuse increases the risk of angiographic vasospasm and delayed cerebral ischemia (DCI) in critically ill patients with aSAH.
Methods: We conducted a secondary analysis based on a retrospective study in a French university hospital intensive care unit (ICU). Patients with aSAH requiring mechanical ventilation hospitalized between 2010 and 2015 were included. Patients were segregated according to alcohol abuse (yes or no). Multivariable logistic regression analysis was used to identify the independent risk factors associated with angiographic vasospasm and DCI.
Results: The patient proportion of alcohol abuse was dramatically greater in males than that in females (p < 0.001). The Simplified Acute Physiology Score II (SAPSII) score on admission did not show a statistical difference. Neither did the World Federation of Neurosurgical Societies (WFNS) and Fisher scores. Patients with alcohol abuse were more likely to develop angiographic vasospasm (OR 3.65, 95% CI 1.17–11.39; p = 0.0260) and DCI (OR 3.53, 95% CI 1.13–10.97; p = 0.0294) as evidenced by multivariable logistic regression analysis.
Conclusions: In this study, patients with alcohol abuse are at higher odds of angiographic vasospasm and DCI, which are related to poor prognosis following aSAH. These findings are important for the prevention and clinical management of aSAH.
Introduction
Alcohol abuse is associated with an increased risk of death and cardiovascular disease (CVD). Excessive alcohol intake is one of the top three leading causes of premature deaths in the US (the other two are smoking and obesity) (1, 2). Also, the prevalence of alcohol use is still rising (3). Alcohol abuse is a chronic disease characterized by uncontrolled drinking and preoccupation with alcohol. The patients generally exhibit an inability to control drinking due to both a physical and emotional dependence on alcohol. Notably, it has been indicated that alcohol abuse may cause cerebral aneurysm development and rupture, leading to aneurysmal subarachnoid hemorrhage (aSAH) (4–7). Also, reduced alcohol intake may substantially decrease subarachnoid hemorrhage (SAH) risk (4).
The clinical outcomes following aSAH are associated with multiple factors, including the patient's severity on admission, angiographic vasospasm, and delayed cerebral ischemia (DCI) (8–11). Angiographic vasospasm is the arterial narrowing of large cerebral vessels observed on a radiological test such as CT angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA) (12). Additionally, angiographic vasospasm is considered a critical factor leading to DCI, which causes poor outcomes or death in up to 30% of patients with SAH (11, 13). While alcohol abuse is a potential risk factor for aSAH, the impact of alcohol abuse on outcomes after aSAH has not been fully evaluated. Herein, we investigated the effect of alcohol abuse on angiographic vasospasm and DCI in a cohort of patients with aSAH requiring mechanical ventilation.
Methods
Data Source
We obtained data from the “DATADRYAD” database (https://datadryad.org/search). This website permitted users to freely download the raw dataset. According to Dryad Terms of Service, we cited the Dryad data package in this study [Dryad data package: Chalard et al. (14), Long-term outcome in patients with aSAH requiring mechanical ventilation, Dryad, Dataset, https://doi.org/10.5061/dryad.47d7wm3b4].
Study Cohort
Chalard et al. completed the entire dataset in the previous study (14). The details were described in the original article. Between January 2010 and December 2015, adult patients with aSAH hospitalized in the neuro-ICU were recruited. Only patients with mechanical ventilation were included in the current study. CTA was performed in all patients at admission to confirm SAH was caused by an aneurysm rupture.
Patients Characteristics and Clinical Outcomes Collection
The following variables were collected: age, sex, tobacco use, alcohol abuse, diabetes, CVD, Simplified Acute Physiology Score II (SAPSII), World Federation of Neurosurgical Societies (WFNS) score, Fisher score, aneurysm location, and presence of intracerebral hemorrhage (ICH). Type of aneurysm treatment procedure and presence of angiographic vasospasm and DCI were also recorded.
Statistical Analysis
Continuous variables were expressed as mean ± SD (normal distribution) and categorical variables were expressed in frequency (percentage). The Student's t-test (normal distribution) and chi-square test (categorical variables) were used to determine statistical differences between the means and proportions of the groups. Univariate logistic regression analysis was initially performed to identify factors of potential risk, then a multivariable logistic regression model was used to identify independently associated risk factors for the outcomes. The variables included in the multivariable logistic regression analysis were selected on the basis of their associations with the outcomes of interest or a change in effect estimate of more than 10%. All of the analyses were performed using the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A p-value < 0.05 (two-sided) were considered statistically significant.
Results
Patient Demographics and Outcomes
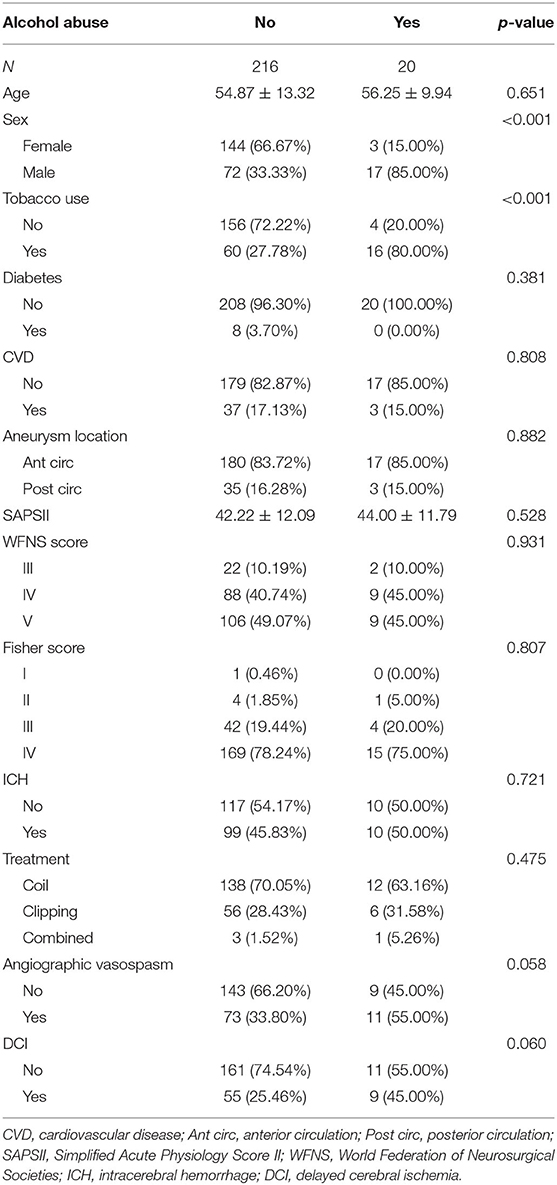
There were 236 patients in this cohort, including 20 patients with alcohol abuse and 216 patients without alcohol abuse. As shown in Table 1, the mean age of non-alcohol abuse patients was 54.87 (SD = 13.32). The mean age of patients with alcohol abuse was 56.25 (SD = 9.94). The comparison of age in the two groups of patients indicated no statistical significance (p = 0.651). There were 17 male patients included in the group of alcohol abuse, which was of statistical significance compared with the proportion of alcohol abuse in the female patients (p < 0.001). There were 16 patients with tobacco use in alcohol abuse patients with a percentage of 80.00%, which was markedly greater than that in non-alcohol abuse patients (p < 0.001). There were 8 patients with diabetes in the non-alcohol abuse group with a percentage of 3.7%. In patients with alcohol abuse, no patients had diabetes. There were 37 patients with CVD in the non-alcohol abuse group (17.13%). In the alcohol abuse group, 3 patients had CVD (15.00%). No statistical significance was observed as to diabetes and CVD (p = 0.381, 0.808, respectively) in these two groups.
As for the location of the aneurysm, there were 180 patients with anterior circulation aneurysms in the non-alcohol abuse group (83.72%). In the alcohol abuse group, 17 patients had anterior circulation aneurysms (85.00%). No statistical significance was detected between the two groups (p = 0.882). The mean SAPS II of non-alcohol abuse patients was 42.22 (SD = 12.09). The mean SAPS II of alcohol abuse patients was 44.00 (SD = 11.79). The differences between SAPS II, WFNS score, and Fisher score were of no statistical significance (p = 0.528, 0.931, 0.807, respectively). There were 99 patients with ICH in the non-alcohol abuse group (45.83%). In the alcohol abuse group, 10 patients exhibited ICH (50.00%). No statistical significance was detected in the comparison of ICH between the two groups (p = 0.721).
A total of 138 patients were treated with a coil and 56 patients were treated with clipping in the non-alcohol abuse group. No statistical significance was observed in the comparison of treatment between the two groups (p = 0.475). A total of 73 patients (33.80%) presented with angiographic vasospasm in the non-alcohol abuse group, and 11 patients (55.00%) presented with angiographic vasospasm in the alcohol abuse group, with the p-value approaching significance (p = 0.058). There were 55 patients (25.46%), who presented with DCI in the non-alcohol abuse group, vs. 9 patients (45.00%) with DCI in the non-alcohol abuse group, a difference that also nearly reached statistical significance (p = 0.060).
Univariate Analysis of Risk Factors for Angiographic Vasospasm and DCI
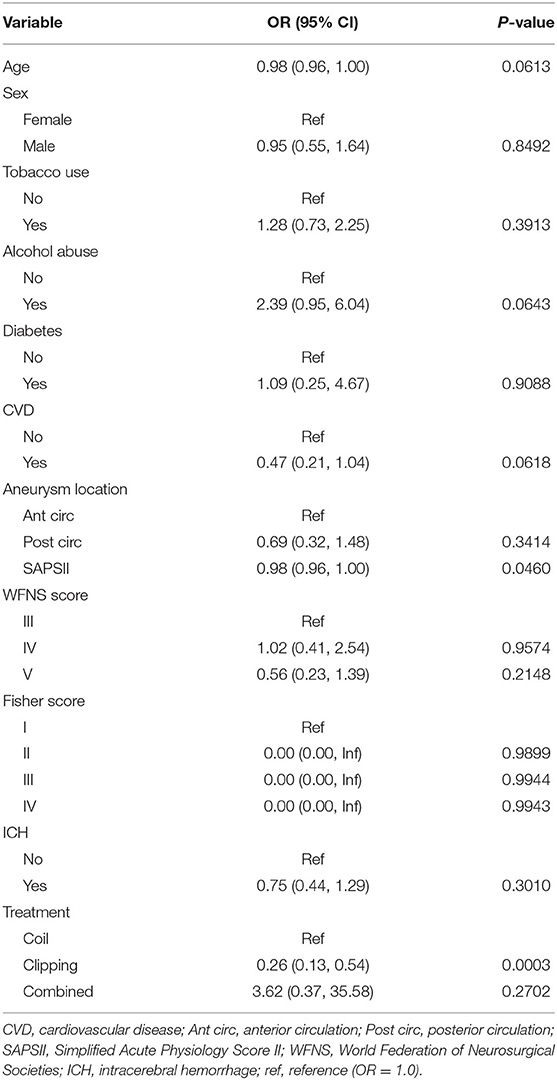
The risk factors for angiographic vasospasm and DCI were then identified using univariate logistic regression analysis. Alcohol abuse [odds ratio (OR) 2.39, 95% confidence interval (CI) 0.95–6.04; p = 0.0643] was likely associated with an increased risk of angiographic vasospasm, with the p-value approaching significance. Clipping treatment was associated with a reduced likelihood of angiographic vasospasm (OR 0.26, 95% CI 0.13–0.54; p = 0.0003). The results of the univariate analysis of risk factors for angiographic vasospasm are presented in Table 2.
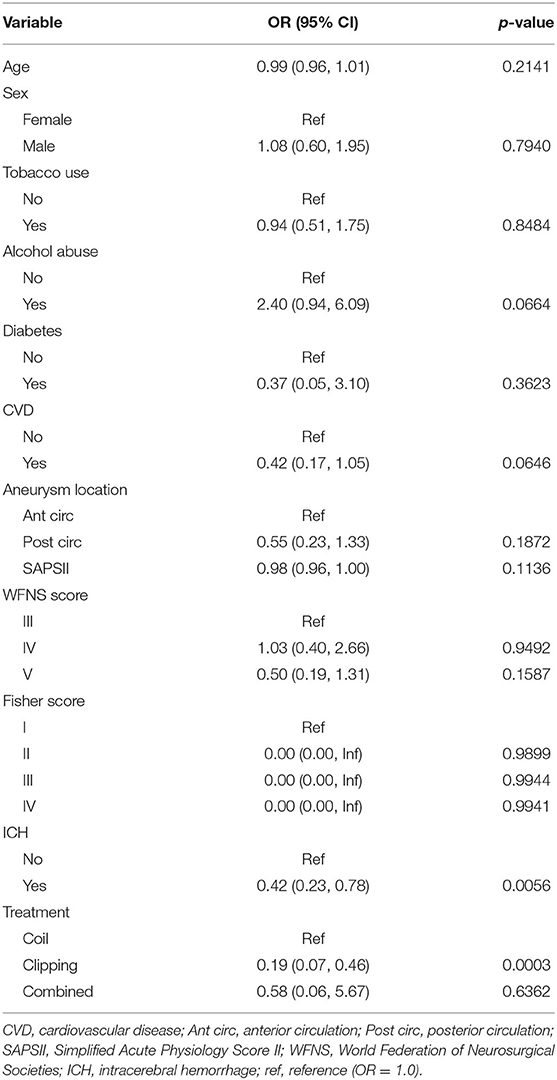
Alcohol abuse (OR 2.40, 95% CI 0.94–6.09; p = 0.0664) was likely associated with increased risk of DCI, with the p-value nearly reaching statistical significance. ICH (OR 0.42, 95% CI.23–0.78; p = 0.0056) and clipping treatment were associated with a reduced likelihood of DCI (OR 0.19, 95% CI 0.07–0.46; p = 0.0003). The results of the univariate analysis of risk factors for DCI are presented in Table 3.
Multivariable Analysis of Independent Risk Factors for Angiographic Vasospasm and DCI
The multivariable logistic regression analysis used non-adjusted (crude) and adjusted models to examine the independent risk factors. As shown in Table 4, in model I (adjusted for age and sex), the OR is 2.75, 95% CI 1.03–7.36 (p = 0.0440). In model II (adjusted for age, sex, Fisher score, and treatment), the OR is 3.61, 95% CI 1.17–11.17 (p = 0.0259). In model III (adjusted for age, sex, Fisher score, treatment, CVD, and SAPS II), the OR is 3.65, 95% CI 1.17–11.39 (p = 0.0260). The results of multivariable logistic regression analysis indicated that alcohol abuse was independently associated with increased odds of angiographic vasospasm after adjusting for age, sex, Fisher score, treatment, CVD, and SAPS II.
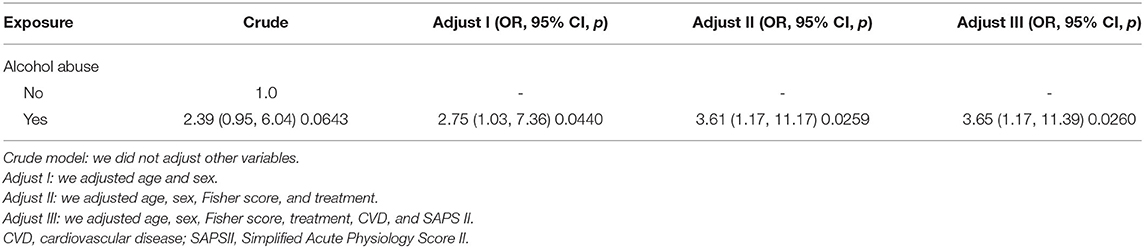
Table 4. Relationship between alcohol abuse and angiographic vasospasm in different models by multivariable logistic regression analysis.
Similarly, the ORs of alcohol abuse for DCI were calculated in different adjusted models (Table 5). The results suggested that alcohol abuse was independently associated with increased odds of DCI (OR 3.53, 95% CI 1.13–10.97; p = 0.0294, model III).
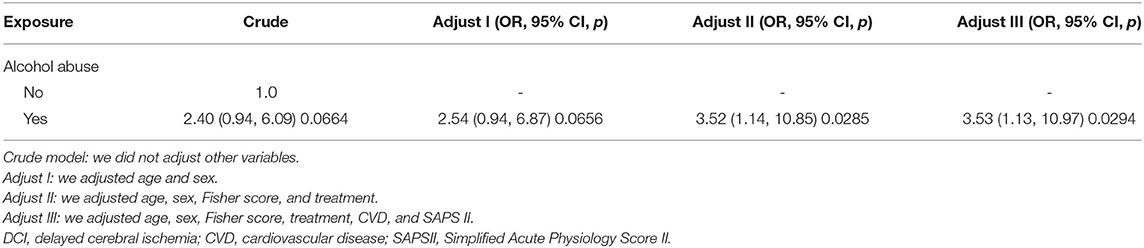
Table 5. Relationship between alcohol abuse and DCI in different models by multivariable logistic regression analysis.
Discussion
Angiographic vasospasm and DCI are strongly associated with the outcomes following aSAH (11, 13). This study aims to have a better understanding of the risk factors involved that may lead to angiographic vasospasm and DCI. Alcohol abuse has about 2.5-fold higher odds of angiographic vasospasm and DCI via multivariable logistic regression analysis when adjusting for other potential confounding variables. Our findings provide important evidence for the prevention and clinical management of aSAH.
Although previous studies have suggested that alcohol abuse increases the risk of aSAH (4), the role of alcohol abuse in the development of angiographic vasospasm and DCI has not been fully investigated yet. Because alcohol abuse is present in a large proportion of patients with aSAH, especially in men, identification of alcohol abuse as an independent risk factor of angiographic vasospasm and DCI is of great clinical significance in determining how closely the patients should be monitored for these two events.
Alcohol has been suggested to induce vessel constriction via various mechanisms. Alcohol can increase the activity of the sympathetic nervous system and the release of catecholamines, leading to the constriction of blood vessels (15, 16). Besides, alcohol has been indicated to decrease the levels of electrically charged (i.e., ionized) magnesium in plasma (17). A delicate balance between magnesium and calcium ions is needed to maintain vascular tone at a normal level. Magnesium triggers vessel relaxation and calcium, on the contrary, causes vessel constriction. When the level of magnesium ions is reduced by alcohol, the calcium ions will predominate, resulting in vessel constriction. These mechanisms help to explain alcohol abuse as an independent risk factor for angiographic vasospasm after aSAH in this study.
Apart from the effect on vascular tone, alcohol may lead to endothelial dysfunction. A high dose of alcohol consumption leads to an increased endothelin level, which is also involved in vessel constriction (18, 19). Alcohol abuse also leads to oxidative stress. For example, a high dose of alcohol leads to the overproduction of reactive oxygen species, leading to the peroxidation of lipids, proteins, and DNA and ultimately to necrosis and apoptosis (20). The increased level of endothelin induced by alcohol abuse is also associated with oxidative stress (18). The blockade of oxidative stress may prevent endothelial dysfunctions induced by alcohol. A study by Sacanella et al. indicated that alcohol abuse increases the expression of intercellular adhesion molecule (ICAM-1) and E-selectin, which participates in the adhesion and migration of inflammatory cells. As for inflammation, a high dose of alcohol consumption may cause an increase in tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) (21), contributing to the aggregation of inflammatory cells and vessel constriction or spasm.
Alcohol also acts on coagulation and fibrinolysis. It has been demonstrated that heavy alcohol intake is associated with lower fibrinolytic capacity and a more procoagulant state, with an elevation in the plasma levels of factor VII, fibrinogen, and viscosity (22). Fibrinolysis is mainly regulated by two proteins in the blood: tissue plasminogen activator (tPA) and plasminogen activator inhibitor 1 (PAI-1). TPA promotes fibrinolysis, whereas PAI-1 inhibits fibrinolytic activity. Heavy alcohol consumption has been indicated to stimulate PAI-1 production and thereby suppress fibrinolysis (23). Fibrinolysis suppression may lead to subsequent thrombosis and vessel spasms.
Angiographic vasospasm has been indicated to be present in up to 70% of patients following SAH (9), and it has been regarded as an important factor causing DCI for a long time. However, recent findings have suggested that angiographic vasospasm alone is not sufficient to trigger DCI (9). DCI is only found in about 30% of patients and does not always fall within the vascular distribution of the angiographic vasospasm (24). DCI, therefore, may occur in an area that does not involve angiographic vasospasm. In our study, DCI was found in 27% of patients, which is nearly 30%. For the non-alcohol abuse group, 73 patients presented with angiographic vasospasm and 55 with DCI. For the alcohol abuse group, 11 with angiographic vasospasm, and 9 with DCI. Notably, the patients with DCI all presented with the presence of angiographic vasospasm, which verified the critical role of angiographic vasospasm in the development of DCI following aSAH. However, further research is still needed to find out other causes of DCI.
Limitations
There are some limitations to our study. First, this is a single-center study based on a French patients cohort, and only patients with mechanical ventilation were analyzed. Therefore, the generalizability of the results to other geographical areas and the patient groups may be limited. Second, this is a retrospective study, so we can only conclude that alcohol abuse was associated with angiographic vasospasm and DCI, but causation cannot be proved. Therefore, only an OR could be calculated, not the relative risk. Third, due to raw data limitations, the dose of alcohol consumption was not recorded, which would otherwise provide more details regarding the effect of alcohol on angiographic vasospasm and DCI.
Conclusions
In this study, we have demonstrated that patients with alcohol abuse havehas about 2.5-fold higher odds of angiographic vasospasm and DCI compared to non-alcohol abuse patients after aSAH, independent of age, sex, Fisher score, treatment, CVD, and SAPS II. Our findings may be helpful in monitoring patients with known risk factors for the development of angiographic vasospasm and DCI following aSAH.
Data Availability Statement
The dataset is available on Dryad at DOI: 10.5061/dryad.47d7wm3b4.
Ethics Statement
The studies involving human participants were reviewed and approved by Comite de Protection des Personnes Sud-Mediterannee IV, Montpellier, France; ID: Q-2015-09-07. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
LZhao wrote the first draft of the manuscript. All authors contributed to the conception and design of the study, statistical analysis, manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very grateful to the data providers of the study.
References
1. O'Keefe EL, DiNicolantonio JJ, O'Keefe JH, Lavie CJ. Alcohol and CV health: Jekyll and Hyde J-curves. Prog Cardiovasc Dis. (2018) 61:68–75. doi: 10.1016/j.pcad.2018.02.001
2. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. (2004) 291:1238–45. doi: 10.1001/jama.291.10.1238
3. Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry. (2017) 74:911–23. doi: 10.1001/jamapsychiatry.2017.2161
4. Kissela BM, Sauerbeck L, Woo D, Khoury J, Carrozzella J, Pancioli A, et al. Subarachnoid hemorrhage: a preventable disease with a heritable component. Stroke. (2002) 33:1321–6. doi: 10.1161/01.STR.0000014773.57733.3E
5. Juvela S, Hillbom M, Numminen H, Koskinen P. Cigarette smoking and alcohol consumption as risk factors for aneurysmal subarachnoid hemorrhage. Stroke. (1993) 24:639–46. doi: 10.1161/01.STR.24.5.639
6. King JT Jr. Epidemiology of aneurysmal subarachnoid hemorrhage Neuroimaging. Clin N Am. (1997) 7:659–68.
7. Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. (2005) 36:2773–80. doi: 10.1161/01.STR.0000190838.02954.e8
8. Galea JP, Dulhanty L, Patel HC, UK and Ireland Subarachnoid Hemorrhage Database Collaborators. Predictors of outcome in aneurysmal subarachnoid hemorrhage patients: observations from a multicenter data set. Stroke. (2017) 48:2958–63. doi: 10.1161/STROKEAHA.117.017777
9. Crowley RW, Medel R, Dumont AS, Ilodigwe D, Kassell NF, Mayer SA, et al. Angiographic vasospasm is strongly correlated with cerebral infarction after subarachnoid hemorrhage. Stroke. (2011) 42:919–23. doi: 10.1161/STROKEAHA.110.597005
10. Rumalla K, Lin M, Ding L, Gaddis M, Giannotta SL, Attenello FJ, et al. Risk factors for cerebral vasospasm in aneurysmal subarachnoid hemorrhage: a population-based study of 8346 patients. World Neurosurg. (2021) 145:e233–e41. doi: 10.1016/j.wneu.2020.10.008
11. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. (2014) 10:44–58. doi: 10.1038/nrneurol.2013.246
12. Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. (2010) 41:2391–5. doi: 10.1161/STROKEAHA.110.589275
13. Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. (2006) 354:387–96. doi: 10.1056/NEJMra052732
14. Chalard K, Szabo V, Pavillard F, Djanikian F, Dargazanli C, Molinari N, et al. Long-term outcome in patients with aneurysmal subarachnoid hemorrhage requiring mechanical ventilation. PLoS ONE. (2021) 16:e0247942. doi: 10.1371/journal.pone.0247942
15. Russ R, Abdel-Rahman AR, Wooles WR. Role of the sympathetic nervous system in ethanol-induced hypertension in rats. Alcohol. (1991) 8:301–7. doi: 10.1016/0741-8329(91)90433-W
16. O'Connor AD, Rusyniak DE, Bruno A. Cerebrovascular and cardiovascular complications of alcohol and sympathomimetic drug abuse. Med Clin North Am. (2005) 89:1343–58. doi: 10.1016/j.mcna.2005.06.010
17. Altura BM, Altura BT. Role of magnesium and calcium in alcohol-induced hypertension and strokes as probed by in vivo television microscopy, digital image microscopy, optical spectroscopy, 31P-NMR, spectroscopy and a unique magnesium ion-selective electrode. Alcohol Clin Exp Res. (1994) 18:1057–68. doi: 10.1111/j.1530-0277.1994.tb00082.x
18. Soardo G, Donnini D, Varutti R, Moretti M, Milocco C, Basan L, et al. Alcohol-induced endothelial changes are associated with oxidative stress and are rapidly reversed after withdrawal. Alcohol Clin Exp Res. (2005) 29:1889–98. doi: 10.1097/01.alc.0000183004.28587.23
19. Zilkens RR, Burke V, Hodgson JM, Barden A, Beilin LJ, Puddey IB. Red wine and beer elevate blood pressure in normotensive men. Hypertension. (2005) 45:874–9. doi: 10.1161/01.HYP.0000164639.83623.76
20. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. (2007) 87:315–424. doi: 10.1152/physrev.00029.2006
21. Luedemann C, Bord E, Qin G, Zhu Y, Goukassian D, Losordo DW, et al. Ethanol modulation of TNF-alpha biosynthesis and signaling in endothelial cells: synergistic augmentation of TNF-alpha mediated endothelial cell dysfunctions by chronic ethanol. Alcohol Clin Exp Res. (2005) 29:930–8. doi: 10.1097/01.ALC.0000171037.90100.6B
22. Lee KW, Lip GY. Effects of lifestyle on hemostasis, fibrinolysis, and platelet reactivity: a systematic review. Arch Intern Med. (2003) 163:2368–92. doi: 10.1001/archinte.163.19.2368
23. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. (1997) 21:42–52.
Keywords: aneurysmal subarachnoid hemorrhage, alcohol abuse, retrospective study, clinical outcomes, angiographic vasospasm, delayed cerebral ischemia
Citation: Zhao L, Cheng C, Peng L, Zuo W, Xiong D, Zhang L, Mao Z, Zhang J, Wu X, Jiang X, Wang P and Li W (2022) Alcohol Abuse Associated With Increased Risk of Angiographic Vasospasm and Delayed Cerebral Ischemia in Patients With Aneurysmal Subarachnoid Hemorrhage Requiring Mechanical Ventilation. Front. Cardiovasc. Med. 9:825890. doi: 10.3389/fcvm.2022.825890
Received: 30 November 2021; Accepted: 21 March 2022;
Published: 10 May 2022.
Edited by:
Anne-Clémence Vion, INSERM U1087 Institut du Thorax, FranceReviewed by:
Nicolas K. Khattar, University of Louisville, United StatesJing Xu, Johns Hopkins University, United States
Copyright © 2022 Zhao, Cheng, Peng, Zuo, Xiong, Zhang, Mao, Zhang, Wu, Jiang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weixin Li, neuropub@163.com; Lei Zhao, conrad03037@163.com
 Lei Zhao
Lei Zhao Chao Cheng1
Chao Cheng1  Liwei Peng
Liwei Peng Weixin Li
Weixin Li