Serum Complement C1q Activity Is Associated With Obstructive Coronary Artery Disease
- 1Department of Clinical Laboratory, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 2Department of Clinical Laboratory, Henan Provincial People's Hospital, People's Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 3Department of Clinical Laboratory, ShenQiu People's Hospital, ShenQiu, Henan, China
Background: Complement C1q plays a dual role in the atherosclerosis. Previous studies showed inconsistent results about the association of serum C1q levels and coronary artery disease (CAD). Here, we explored the associations of serum C1q activity with CAD, coronary stenosis severity, cardiovascular biomarkers, and 1-year restenosis after coronary artery revascularization.
Methods: We enrolled 956 CAD patients and 677 controls to evaluate the associations of serum complement C1q activity to the presence and severity of obstructive CAD and non-obstructive CAD. Serum C1q activity and the concentrations of laboratory markers were measured in all subjects. All the data were analyzed using SPSS22.0 software.
Results: Serum C1q activity in Obstructive CAD and Non-Obstructive CAD groups was significantly higher than the control group (195.52 ± 48.31 kU/L and 195.42 ± 51.25 kU/L vs. 183.44 ± 31.75 kU/L, P < 0.05). Greater C1q activity was significantly correlated with higher total cholesterol (TC) and triglyceride (TG) levels. C1q activity was associated with an increased Odds Ratio (OR) of CAD (OR = 1.322, 95% CI 1.168–1.496, P < 0.05) and 1-year restenosis after revascularization (the highest OR = 3.544, 95% CI 1.089–12.702, P < 0.05). Complement C1q activity was not correlated with Gensini score in the Obstructive CAD group after adjustment for confounders. C1q activity has low value in predicting the incidence of CAD.
Conclusion: Serum complement C1q activity is associated with obstructive CAD.
Introduction
Coronary artery disease (CAD) is projected as one of the leading causes of disease burden in the world in 2030 (1). Atherosclerosis (AS) is a pathological change throughout the whole pathogenesis of CAD. The formation of atherosclerotic plaque is related to endothelial dysfunction, large amount of lipid deposition in the arterial wall, aggravation of immune response, proliferation of vascular smooth muscle cells, and accumulation of extracellular matrix. Studies demonstrated that the complement system (CS) triggered inflammatory response and participates in the occurrence and development of AS (2). Atherosclerotic lesions contained several triggers such as C-reactive protein for the activation of the complement cascade. Previous studies indicated that the classical pathway rather than the alternative pathway was activated in plaques (3).
Complement C1q is a complex glycoprotein composed of 18 polypeptide chains. The C-terminal globular head region mediates the recognition of different molecular structures, and the N-terminal collagen like tail mediates the immune effector mechanism. As one of the starting components of classical complement pathway, C1q plays positive and negative effects on AS (4). Many experimental studies have shown that C1q played a beneficial role in the early stage of AS. C1q binds apoptotic cells and cell debris from atherosclerotic plaques, and plays an important role in their disposal (5–9). C1q played a beneficial role in early atherosclerosis by regulating macrophage molecular signal through complement independent pathway and modulating the uptake of atherogenic lipoprotein. On the contrary, C1q might be involved in accelerating the formation of atherosclerosis due to its proinflammatory effect (4). C1q could have a role in regulating collagen-induced platelet activation, production of reactive oxygen species (ROS), and associated leukocyte recruitment during vessel wall injury (10). Previous studies showed inconsistent results on the association of serum complement C1q level and the severity of different types of CAD with variant study groups and small sample size (11–13). In this study, we enrolled a total of 956 CAD patients and 677 healthy controls to assess the associations of serum C1q activity with the stenosis degree of coronary artery, cardiometabolic phenotypes, and the incidence of CAD and 1-year restenosis.
Subjects and Methods
Subjects
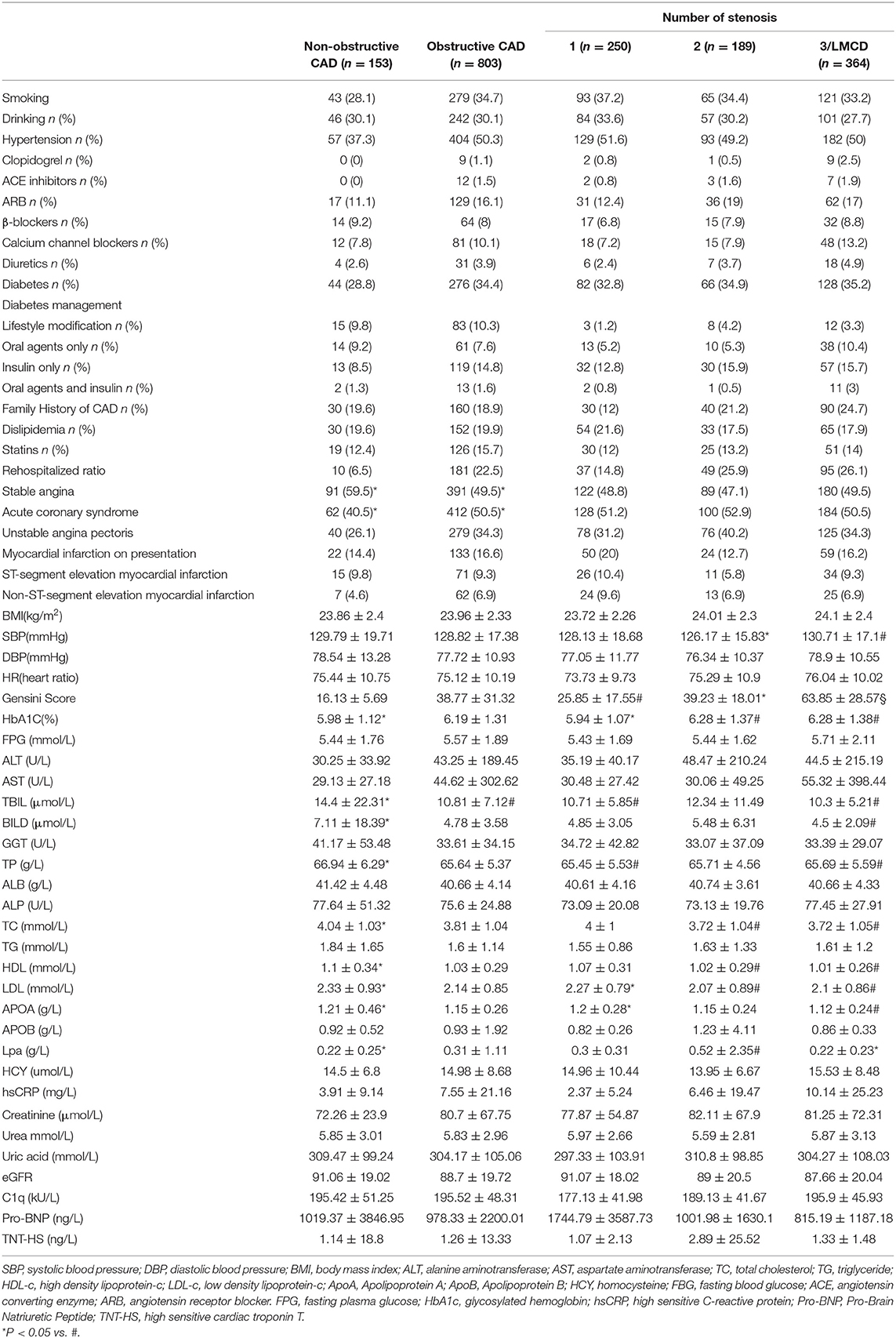
We recruited 1,336 patients who received coronary angiography (CAG) from June 2019 to June 2020 in The First Affiliated Hospital of Zhengzhou University at Henan Province. According to arteriography, patients were divided into normal or near-normal coronary arteries (NNCAs, n = 380), non-obstructive CAD group (n = 153), and obstructive CAD group (n = 803). NNCAs were defined as having all coronary artery stenosis ≤20%. According to previous studies, non-obstructive CAD was defined as having at least one coronary artery stenosis between 21 and 49% (14–16). Obstructive CAD was defined as angiographically demonstrated stenosis of >50% in major coronary vessel, with or without clinical symptoms of angina, negative changes of cardiac biomarkers, and typical electrocardiographic patterns (17). The severity of coronary artery stenosis was quantified by the number of diseased vessels with ≥50% stenosis and modified Gensini scores. We first categorized each patient by the number of diseased vessels with ≥50% stenosis in a single, double, or triple-vessel distribution. We defined vessel distribution by the left anterior descending artery and its branches, the left circumflex artery and its branches, and the right coronary artery and its branches. Patients with ≥50% stenosis of left main coronary artery were classified into the three-vessel obstructive CAD group (18). So, we finally created four categories of CAD extent: non-obstructive CAD, 1-, 2-, 3-vessel/left main obstructive CAD.
The control group consisted of 677 healthy persons without cardiovascular disease via physical examination including cardiac echocardiography, chest CT, and electrocardiogram every 2 years. The controls were frequency-matched to the cases on age and sex. Subjects were excluded if they (1) underwent percutaneous coronary intervention, coronary bypass surgery, or angioplasty; (2) had cardiac diseases like cardiomyopathy, valvular or congenital heart disease, heart failure, coronary spasm, or myocardial bridge; (3) patients who had malignant tumors, acute or chronic infection, autoimmune disease; (4) patients whose blood pressure ≥180/110 mmHg after taking standard antihypertensive drugs, patients whose ALT or AST was three times higher than normal, patients whose estimated glomerular filtration rate (eGFR) <15 mL/(min·1.73 m2). The study protocol followed the declaration of Helsinki, and The Zhengzhou University Committee approved this study (approval number 2020-KY-172).
During the study period, 485 of 956 patients underwent percutaneous coronary intervention (PCI) due to the presence of indications for revascularization. Re-stenosis was defined as having a >50% decrease in the luminal diameter as determined by quantitative coronary angiography. These 485 patients were followed up for 12 months after revascularization to track restenosis ratios. The follow-up was ascertained through telephone interviews with participants and verification by checking medical records at 30 days and 6 months after revascularization.
Collection and Definition of Clinical Variables
Demographic factors including age, sex, smoking status (ever and never), alcohol drinking status (ever and never), type of diabetes management (lifestyle modification, oral agents, insulin, and oral agents plus insulin), and medicines were documented by checking participants' medical records and questionnaires. Weight and height were measured on the day of enrollments. Dyslipidemia and hypertension were defined based on the previously published guidelines (19, 20). Venous blood samples were collected from upper limbs before CAG. Serum complement C1q activity was tested by immunity transmission turbidimetry on a Cobas 8000 Analyzer (Roche Diagnostics, Germany). Fasting plasma glucose (FPG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), triglycerides (TG), high sensitive C-reactive protein (hs-CRP), liver function, and renal function were measured on a Cobas 8000 Analyzer (Roche Diagnostics, Germany) using standard methods. Hemoglobin A1c (HbA1c) was assayed by high-performance liquid chromatography on a Premer-Premier Hemoglobin Testing System.
Selective CAG was performed in all patients using the standard Judkins technique. The localization of coronary artery disease and the rate of lumen stenosis were determined by CAG. Gensini score of coronary artery equals the sum of all segment scores. Each segment score equals segment weighting factor multiplied by a severity score. Segment weighting factor assigned to specific coronary segment are five for left main coronary artery, 2.5 for proximal left anterior descending coronary artery (LAD) and proximal left circumflex branch, 1.5 for mid-segment of LAD, 0.5 for second diagonal branch and posterolateral branch, and one for other branches. Severity score allocated to the definite percentage luminal diameter reduction are 1 for 0–25%, 2 for 26–50%, 4 for 51–75%, 8 for 76–90%, 16 for 91–99%, and 32 for 100% stenosis (21). We used the Gensini score as a dependent variable to describe and judge the severity of coronary stenosis.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (normally distributed data), median and quartile spacing [M (P25-P75)] (non-normally distributed data). Categorical variables are expressed as the frequency and its percentage. Continuous variables were analyzed using Student's t-test in normally distributed data, and Mann-Whitney test in non-normally distributed data. Categorical variables were analyzed using the Chi-square test. All the statistical analyses were executed using Statistical Package for Social Science (SPSS, version 22.0). The association of C1q with obstructive CAD and 1-year restenosis was analyzed by logistic regression models with three progressive degrees of adjustment. Model 1 was a crude model without any confounders; model 2 was adjusted for age, sex, and cardiovascular risk factors including smoking habit, alcohol drinking habit, overweight, hypertension, and dyslipidemia; model 3 was additionally adjusted for laboratory tests including HbA1c, FPG, alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea nitrogen (UREA), uric acid (UA), estimated glomerular filtration rate (eGFR), homocysteine (HCY), and lipid profiles. For each model, C1q was first analyzed as a continuous variable, and then as an ordinal variable based on its quartile distribution. A two-sided value of P < 0.05 was considered statistically significant.
Results
Clinical Characteristics
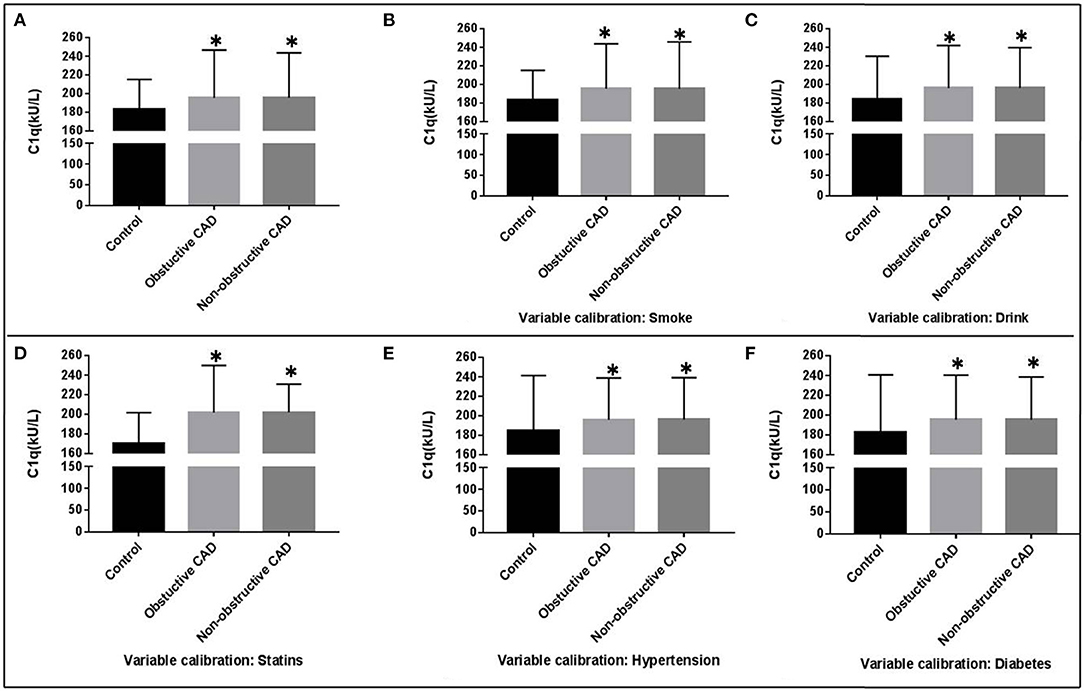
The general characteristics of the study groups were detailed in Table 1 and Supplementary Table 1. There was no statistically significant difference between CAD group and controls for age and gender. Overall, CAD patients were older, more likely to be smokers, obese, hypertensive, dyslipidemia, and hyperglycemia. Comparing with the controls, individuals in the CAD group showed significantly lower plasma concentrations of Total proteins (TP), Albumin (ALB), TC, HDL-C, and ApoA. Diastolic blood pressure (DBP), systolic blood pressure (SBP), body mass index (BMI), and the plasma concentrations of HbA1c, FBP, TG, C1q, liver function, and renal function were significantly higher in the CAD group compared to the control (P < 0.05). Complement C1q activity in obstructive CAD and non-Obstructive CAD groups identified as 195.52 ± 48.31 kU/L and 195.42 ± 51.25 kU/L, were significantly higher than the control group (183.44 ± 31.75 kU/L, P < 0.05) (Table 1, Figure 1). Even after adjusting for after calibrating smoke, drink, hypertension, dyslipidemia, diabetes, and the history of taking statins, serum complement C1q activity in the CAD groups were significantly higher than in the control group (P < 0.05; Figure 1). However, there was no significant difference in the four categories of CAD extent for C1q, Pro-Brain Natriuretic Peptide (Pro-BNP), and high sensitive cardiac troponin T (TNT-HS) (Table 1). There was a significant higher proportion of documented stable angina in the non-obstructive CAD than that in the obstructive CAD group (59.5 vs. 49.5%).
C1q and Cardiometabolic Phenotypes
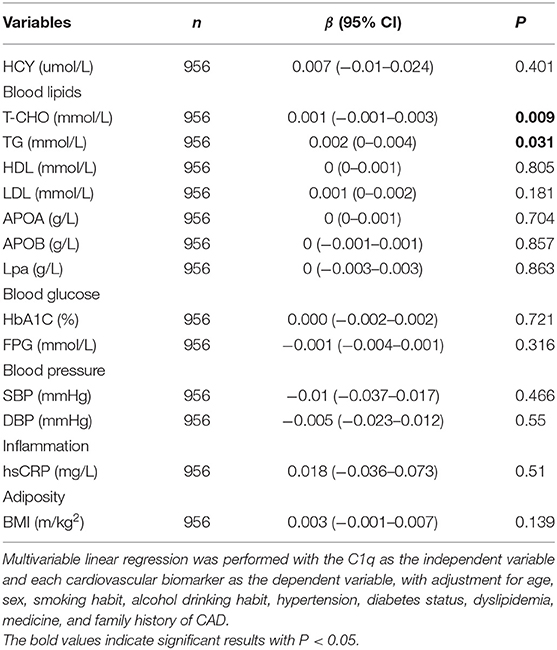
We tested 14 biomarkers, i.e., blood lipids (LDL-C, HDL-C, TC, and TG), blood glucose (FPG, HbA1c), blood pressure (systolic and diastolic), proinflammatory measures (hs-CRP), and adiposity measure (BMI). Overall, greater C1q activity was significantly correlated with higher TC levels (β = 0.001, P = 0.009) and TG (β = 0.002, P = 0.031), but not with 12 other biomarkers after adjustment for confounders (Table 2).
C1q Activity and Coronary Stenosis Severity in Obstructive CAD as Determined by Gensini Scores
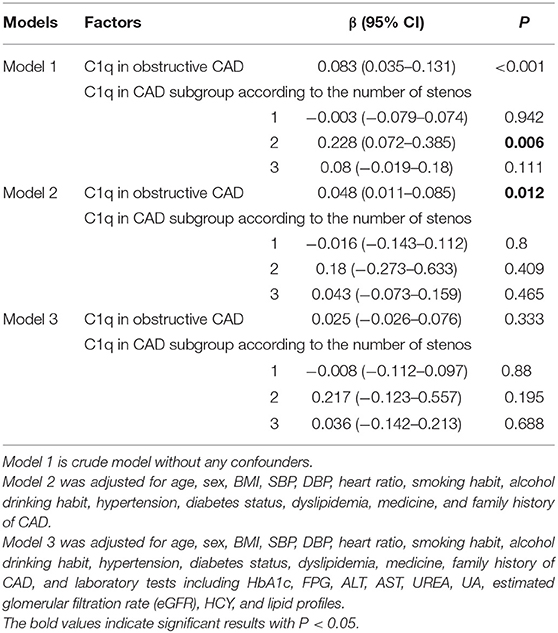
We analyzed the correlation between the serum complement C1q activity and Gensini score. Spearman analysis showed no significant direct correlation (P > 0.05) in the non-obstructive CAD. The regression model of complement C1q was established in the obstructive CAD samples (Table 3).
Complement C1q activity exhibited a positive correlation with Gensini score in the obstructive CAD group without adjustment for the cofounders (β = 0.083, 95% CI 0.035–0.131, P < 0.001; Table 3). After calibrating age, sex, BMI, SBP, DBP, HR, smoking habit, alcohol drinking habit, hypertension, diabetes status, dyslipidemia, medicine, and family history of CAD (model 2), the serum complement C1q activity remained a positive correlation with Gensini score in the obstructive CAD group (β = 0.048, 95% CI 0.011–0.085, P = 0.012; Table 3). Complement C1q activity showed no place in the regression model of Gensini score after additional adjustment for laboratory tests (P > 0.05; Table 3).
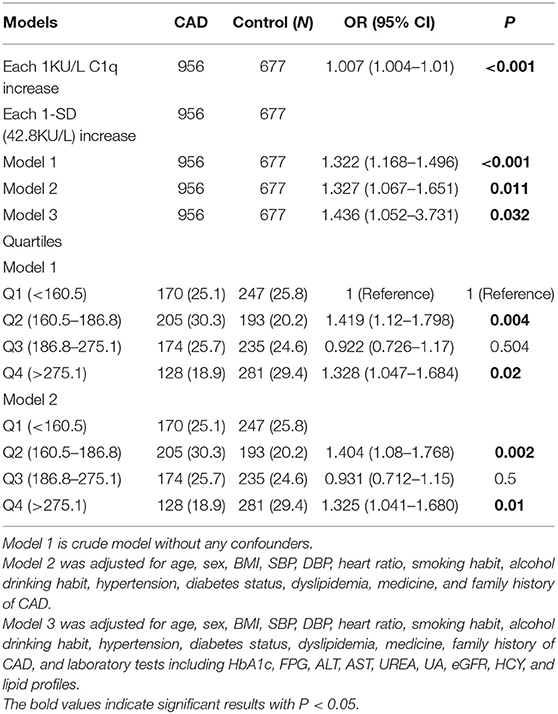
Logistic Regression Analysis of the Risk of C1q Activity for CAD and 1-Year Restenosis
In the whole cohort of 956 patients, each 1-SD increase (42.8 KU/L) in C1q activity was associated with a 1.322-fold (95 confidence interval [95% CI] (1.168–1.496), Table 4) increased Odds Ratio (OR) of CAD in the crude model. When further adjusted for age, sex, BMI, SBP, DBP, HR, smoking habit, alcohol drinking habit, hypertension, diabetes status, dyslipidemia, medicine, and family history of CAD (model 2), the OR for CAD was strengthened to 1.327 (95% CI 1.067–1.651) per 1-SD increase in C1q activity. Ordinal logistic regression showed that each 1-SD increase in C1q activity associated with 43.6% higher odds of having CAD after adjustment for laboratory tests in model 3. When analyzed with the bottom quartile of C1q as the reference, the OR for CAD was 1.419 in the second quartile, 0.922 in the third quartile, and 1.328 in the top quartile (Table 4).
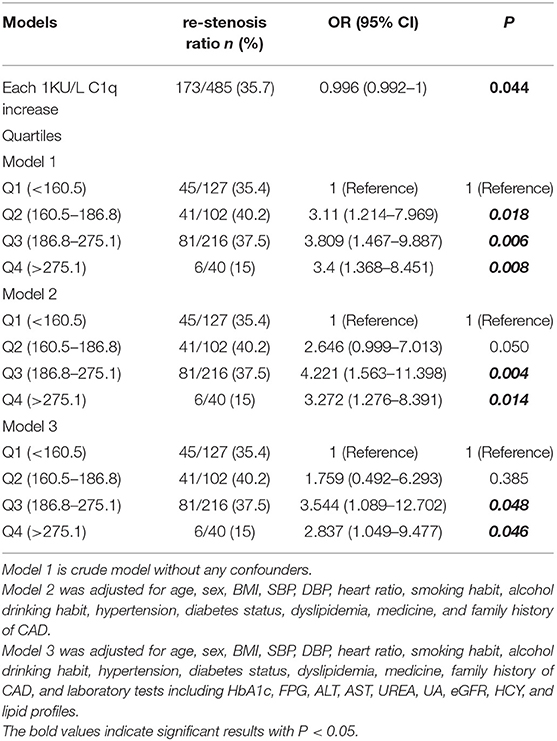
In the cohort 485 patients underwent PCI treatment and were followed by 12 months to track the restenosis ratio. The restenosis ratio was 35.7% (173/485). In the crude model, the three ordinal levels of C1q were estimated as risk factors for 1-year restenosis after PCI (OR = 3.11, 95% CI 1.214–7.969, OR = 3.809, 95% CI 1.467–9.887, and OR = 3.4, 95% CI 1.368–8.451 P < 0.05, Table 5). After adjustment for age, sex, BMI, SBP, DBP, HR, smoking habit, alcohol drinking habit, hypertension, diabetes status, dyslipidemia, medicine, and family history of CAD (model 2), the ORs were attenuated to 3.272 the top quartile levels of C1q, and increased to 3.809 in the third quartile levels of C1q (P < 0.05, Table 5). After additional adjustment for laboratory tests (model 3), the association remained statistic significant (OR = 3.544, 95% CI 1.089–12.702, and OR = 2.837, 95% CI 1.049–9.477. P < 0.05, Table 5).
ROC Curve
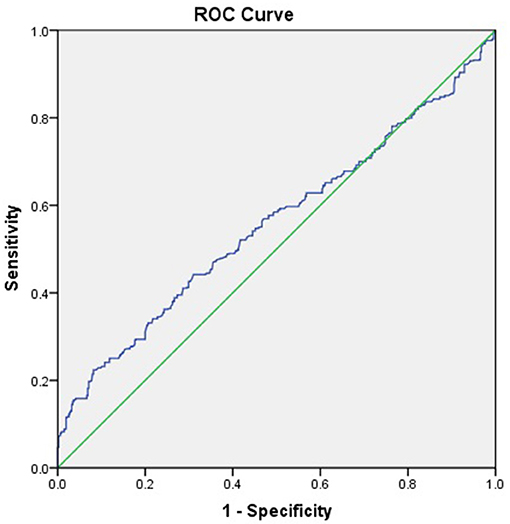
The ROC curve of complement C1q activity as a predictor of CAD suggested that the C1q had low value in predicting the incidence of CAD (C1q = 197.5 KU/L, AUC = 0.558, 95%CI = 0.525–0.592, P = 0.001, sensitivity 44.2 %, Specificity 69.0%; Figure 2).
Discussion
Ischemic heart disease (IHD) represented a large burden on individuals and health care resources worldwide (22). Traditionally, it was equated to coronary artery disease (CAD). However, clinical, angiographic, and autoptic found just some cases were caused by severe or complicated atherosclerotic plaques (23). A large percentage of patients with IHD had minimal or no epicardial coronary vascular disease. In our study, we also found that there was a higher ratio of stable angina in the CAD group without severe or complicated atherosclerotic plaques. IHD hides a multifaceted and complex pathophysiological paradigm including micro and macrovascular dysfunction, atherosclerotic plaque rupture, inflammation, endothelium dependent and independent dysfunction, ion channels (24), and nervous system impairment (25, 26).
Complement C1q was an initiator of the classic pathway (2). However, C1q plays an important role independent of complement activation in many systems. C1q plays a dual role in the chronic inflammatory disease of atherosclerosis. On the one hand, C1q helps maintain the size and complexity of early atherosclerotic lesions by docking on phagocytes and clearing apoptotic cells (27). Apoptosis is associated with the development of human atherosclerosis lesion necrotic core as well as instability of complex plaques. On the other, complement activation via C1q exacerbates pathology in the atherosclerotic lesion in later stages of the disease (28, 29). Upregulating inflammatory signaling in endothelial cells and leucocytes contributes to the development and rupture of vulnerable plaque as well as consequent acute coronary syndrome (30). C1 inhibitor limited neointimal plaque formation and inflammation. This might involve blockade of complement activation, inhibition of leukocyte recruitment, and reduced triglyceride levels, thus providing a multimodal approach to treat arterial disease (31–33).
In this work, we found complement C1q activity in obstructive CAD and non-obstructive CAD groups was significantly higher than the control group. This result was inconsistent with part of previous reports. Xiao-ning Ni reported that level of complement C1q in AMI group was lower significantly than control group and unstable angina group (13). Cavusoglu found that the reduced baseline plasma levels of complement C1q were strong predictors of all-cause mortality in a population of either known or suspected CAD and emerged as a strong and independent predictor of all-cause mortality at 10 years (34). There are several reasons for the inconsistency. First, previous studies only enrolled high-risk participants who were referred for coronary angiography, which may cause selection bias that influences the stability of results. Second, the CAD groups were categorized according to different standards. Xiao-ning Ni used the patients with coronary artery stenosis <50% showed by CAG as control group. Third, the previous studies measured the C1q quantity, and in our study we tested the C1q activity. In addition, the sample size was relatively small in these two previous studies.
Consisted with the previous study (13), we confirmed that complement C1q activity was not correlated with Gensini score in the obstructive CAD group after adjustment for confounders. Complement C1q activity showed no place in the regression model of Gensini score after additional adjustment for laboratory tests. Greater C1q activity was significantly correlated with higher TC levels (β = 0.001, P = 0.009) and TG levels (β = 0.002, P = 0.031). This correlation may explain part of the disappeared association between C1q activity and Gensini score in the model 3 with adjustment for laboratory tests including lipid profiles.
In order to further explore the association of C1q activity and coronary stenosis in patients with obstructive CAD, this study constructed a logistic regression model and found that C1q activity was associated with an increased OR of obstructive CAD and 1-year restenosis after revasculation. Complement deposition and activation might in fact be the first steps in lesion initiation in the arterial wall, even preceding monocyte infiltration (35, 36). C1q has been confirmed existed in atherosclerotic lesions. C1q is expressed by dendritic cells, macrophages, foam cells, and endothelium cells in atherosclerotic arterial wall (35, 36). The production and deposition of C1q activated the complement system, promoting the formation of membrane attack complex C5b-9, inducing the activation and proliferation of smooth muscle cells and endothelial cells, and promoting the continuous formation of plaques through positive feedback and aggravating vascular stenosis (37). Complement C1q-induced arterial remodeling and arteriosclerosis in patients with hypertension by activating β-catenin signaling (38). The pro-atherosclerotic effect of C1q might explain that the higher activity of C1q was related to the onset of obstructive CAD.
C1q activity in non-obstructive CAD groups was also significantly higher than the control group. Non-obstructive CAD groups represented patients with coronary microvascular disease (CMD). CMD refers to pathologic changes within the small vessels of the coronary circulation, in the absence of obstructive lesions in the major vessels. Contemporary evidence strongly supported the coexistence of CMD with atherosclerosis in some patients (39–41). The impaired endothelial function is a hallmark of IHD (42). Endothelial dysfunction is present in CMD, and this can lead to complement activation. Blood contact with a damaged endothelium would lead to a certain degree of complement system activation (43). Platelets might have an ability to interact with both the classical and alternative pathways of complement activation (44). Higher level of terminal complement complex (TCC) C5b-9 was found in plasma of CMD group compared to those with angiographically proven coronary atherosclerosis (45). However, no parallel activation of the classical or the alternative complement pathway was observed in the Horvath study. Low levels of several lectin pathway products might reflect upstream consumption and consequent, downstream terminal complement complex activation. Complement activation might contribute to the increased cardiovascular risk of non-obstructive CAD by promoting endothelial and microvascular dysfunction.
Stenting is one of the most common procedures used to treat stenosis. Compared with conservative treatment, invasive treatment reduced mortality after acute myocardial infarction (46). However, the full hemocompatibility of the different kinds of stents was not yet reached (47, 48). The constant insult of vessel wall injury due to the metal stent resulted in the non-specific inflammatory response, inducing the migration of smooth muscle cells and myofibroblasts to tunica intima (49–51). PCI may induce a local inflammatory response that contributes to restenosis. The damaged intima integrity, causing exposure of sub-cellular components to the extracellular milieu, attracts C1q, leading to activation of the downstream effectors of complement C1q (52), which has been shown to play a role in enhancing the phagocytosis of immune complexes and apoptotic cells (53, 54). In this regard, PCI might enhance the pro-atherosclerotic effect of C1q activity by inducing non-specific inflammatory response. The association between baseline C1q activity and 1-year restenosis remained statistic significant after adjustment for age, sex, BMI, SBP, DBP, HR, smoking habit, alcohol drinking habit, hypertension, diabetes status, dyslipidemia, medicine, and family history of CAD and laboratory tests (Regression analysis model 2 and 3). Although our data could not elucidate the causality between the C1q activity and pathogenesis of 1-year restenosis, the statistic significant association suggested that higher C1q activity at baseline might predict an increased OR of 1-year restenosis after revasculation.
On the other hand, the increase of C1q activity may be a kind of immune regulation. The increased C1q activity inhibited local inflammatory response in peripheral blood by combining with adiponectin. Therefore, it is an active defense against acute cardiovascular events and helps maintain autoimmune tolerance (55). Regrettably, ROC curve suggested that the accuracy in CAD by serum complement C1q activity was low.
In conclusion, complement C1q activity in obstructive CAD and non-obstructive CAD groups was significantly higher than the control group. Greater C1q activity was significantly correlated with higher TC and TG levels. Complement C1q activity exhibited a positive correlation with Gensini score in the obstructive CAD group, especially in the obstructive CAD with two stenosis vessels. C1q activity was associated with an increased OR of CAD and 1-year restenosis after revasculation. However, complement C1q activity was not correlated with Gensini score in the obstructive CAD group after adjustment for confounders. C1q activity has low value in predicting the incidence of CAD.
Our study has limitations. First, although we used a large sample size, we could not establish a causal relationship between C1q activity and obstructive CAD owing to our observational design. Second, the observation of the significant association between C1q and higher TC and TG levels should be interpreted cautiously, because all cardiovascular biomarkers were measured at a single time point, which might bias the results. Third, 1 year of follow-up was not long enough to assess the association of C1q activity with long-term outcomes after coronary revascularization. Finally, the mechanism of the interaction between complement C1q activity and atherosclerosis in patients with coronary heart disease is not clear.
Data Availability Statement
The original contributions generated for this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by The Zhengzhou University Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SG performed the experiments, analyzed the data, prepared figures and tables, authored or reviewed drafts of the paper. XM performed the data collection and analysis. XL, HO, and YG performed the laboratory tests and clinical data collection. LM prepared figures and tables, reviewed drafts of the paper, and approved the final draft. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.618173/full#supplementary-material
References
1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
2. Yasojima K, Schwab C, Mcgeer EG, Mcgeer PL. Complement components, but not complement inhibitors, are upregulated in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. (2001) 21:1214–9. doi: 10.1161/hq0701.092160
3. Fumagalli S, Perego C, Zangari R, De Blasio D, Oggioni M, De Nigris F, et al. Lectin pathway of complement activation is associated with vulnerability of atherosclerotic plaques. Front Immunol. (2017) 8:288. doi: 10.3389/fimmu.2017.00288
4. Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? J Thromb Haemost. (2011) 9:428–40. doi: 10.1111/j.1538-7836.2010.04172.x
5. Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. (1997) 158:4525–8.
6. Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. (2000) 192:359–66. doi: 10.1084/jem.192.3.359
7. Navratil JS, Watkins SC, Wisnieski JJ, Ahearn JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. (2001) 166:3231–9. doi: 10.4049/jimmunol.166.5.3231
8. Nauta AJ, Trouw LA, Daha MR, Tijsma O, Nieuwland R, Schwaeble WJ, et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. (2002) 32:1726–36. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R
9. Martin M, Blom AM. Complement in removal of the dead - balancing inflammation. Immunol Rev. (2016) 274:218–32. doi: 10.1111/imr.12462
10. Skoglund C, Wetterö J, Bengtsson T. C1q regulates collagen-dependent production of reactive oxygen species, aggregation and levels of soluble P-selectin in whole blood. Immunol Lett. (2012) 142:28–33. doi: 10.1016/j.imlet.2011.11.003
11. Seifert PS, Kazatchkine MD. The complement system in atherosclerosis. Atherosclerosis. (1988) 73:91–104. doi: 10.1016/0021-9150(88)90030-5
12. Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, et al. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. (2007) 170:416–26. doi: 10.2353/ajpath.2007.060406
13. Ni XN, Yan SB, Zhang K, Sai WW, Zhang QY, Ti Y, et al. Serum complement C1q level is associated with acute coronary syndrome. Mol Immunol. (2020) 120:130–5. doi: 10.1016/j.molimm.2020.02.012
14. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. (2010) 362:886–95. doi: 10.1056/NEJMoa0907272
15. Douglas PS, Patel MR, Bailey SR, Dai D, Kaltenbach L, Brindis RG, et al. Hospital variability in the rate of finding obstructive coronary artery disease at elective, diagnostic coronary angiography. J Am Coll Cardiol. (2011) 58:801–9. doi: 10.1016/j.jacc.2011.05.019
16. Ouellette ML, Loffler AI, Beller GA, Workman VK, Holland E, Bourque JM. Clinical characteristics, sex differences, and outcomes in patients with normal or near-normal coronary arteries, non-obstructive or obstructive coronary artery disease. J Am Heart Assoc. (2018) 7:e007965. doi: 10.1161/JAHA.117.007965
17. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'agostino RB Sr., Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2014) 63:2935–59. doi: 10.1016/j.jacc.2013.11.005
18. Lee KY, Hwang BH, Kim TH, Kim CJ, Kim JJ, Choo EH, et al. Computed tomography angiography images of coronary artery stenosis provide a better prediction of risk than traditional risk factors in asymptomatic individuals with type 2 diabetes: a long-term study of clinical outcomes. Diabetes Care. (2017) 40:1241–8. doi: 10.2337/dc16-1844
19. National Cholesterol Education Program Expert Panel on Detection, E and Treatment of High Blood Cholesterol In, A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. (2002) 106:3143–421. doi: 10.1161/circ.106.25.3143
20. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. (2014) 311:507–20. doi: 10.1001/jama.2013.284427
21. Montorsi P, Ravagnani PM, Galli S, Rotatori F, Veglia F, Briganti A, et al. Association between erectile dysfunction and coronary artery disease. Role of coronary clinical presentation and extent of coronary vessels involvement: the COBRA trial. Eur Heart J. (2006) 27:2632–9. doi: 10.1093/eurheartj/ehl142
22. Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. (2014) 129:1483–92. doi: 10.1161/CIRCULATIONAHA.113.004042
23. Crea F, Battipaglia I, Andreotti F. Sex differences in mechanisms, presentation and management of ischaemic heart disease. Atherosclerosis. (2015) 241:157–68. doi: 10.1016/j.atherosclerosis.2015.04.802
24. Severino P, D'amato A, Pucci M, Infusino F, Birtolo LI, Mariani MV, et al. Ischemic heart disease and heart failure: role of coronary ion channels. Int J Mol Sci. (2020) 21:3167. doi: 10.3390/ijms21093167
25. Fox KA, Metra M, Morais J, Atar D. The myth of 'stable' coronary artery disease. Nat Rev Cardiol. (2020) 17:9–21. doi: 10.1038/s41569-019-0233-y
26. Severino P, D'amato A, Pucci M, Infusino F, Adamo F, Birtolo LI, et al. Ischemic heart disease pathophysiology paradigms overview: from plaque activation to microvascular dysfunction. Int J Mol Sci. (2020) 21:8118. doi: 10.3390/ijms21218118
27. Ogden CA, Decathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. (2001) 194:781–95. doi: 10.1084/jem.194.6.781
28. Haskard DO, Boyle JJ, Mason JC. The role of complement in atherosclerosis. Curr Opin Lipidol. (2008) 19:478–82. doi: 10.1097/MOL.0b013e32830f4a06
29. Vlaicu SI, Tatomir A, Rus V, Mekala AP, Mircea PA, Niculescu F, et al. The role of complement activation in atherogenesis: the first 40 years. Immunol Res. (2016) 64:1–13. doi: 10.1007/s12026-015-8669-6
30. Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. (2014) 276:618–32. doi: 10.1111/joim.12296
31. Thielmann M, Marggraf G, Neuhauser M, Forkel J, Herold U, Kamler M, et al. Administration of C1-esterase inhibitor during emergency coronary artery bypass surgery in acute ST-elevation myocardial infarction. Eur J Cardiothorac Surg. (2006) 30:285–93. doi: 10.1016/j.ejcts.2006.04.022
32. Fattouch K, Bianco G, Speziale G, Sampognaro R, Lavalle C, Guccione F, et al. Beneficial effects of C1 esterase inhibitor in ST-elevation myocardial infarction in patients who underwent surgical reperfusion: a randomised double-blind study. Eur J Cardiothorac Surg. (2007) 32:326–32. doi: 10.1016/j.ejcts.2007.04.038
33. Shagdarsuren E, Bidzhekov K, Djalali-Talab Y, Liehn EA, Hristov M, Matthijsen RA, et al. C1-esterase inhibitor protects against neointima formation after arterial injury in atherosclerosis-prone mice. Circulation. (2008) 117:70–8. doi: 10.1161/CIRCULATIONAHA.107.715649
34. Cavusoglu E, Kassotis JT, Anwar A, Marmur JD, Hussain SW, Yanamadala S, et al. Usefulness of complement C1q to predict 10-year mortality in men with diabetes mellitus referred for coronary angiography. Am J Cardiol. (2018) 122:33–8. doi: 10.1016/j.amjcard.2018.03.008
35. Yasojima K, Schwab C, Mcgeer EG, Mcgeer PL. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. (2001) 158:1039–51. doi: 10.1016/S0002-9440(10)64051-5
36. Cao W, Bobryshev YV, Lord RS, Oakley RE, Lee SH, Lu J. Dendritic cells in the arterial wall express C1q: potential significance in atherogenesis. Cardiovasc Res. (2003) 60:175–86. doi: 10.1016/S0008-6363(03)00345-6
37. Cubedo J, Padro T, Badimon L. Coordinated proteomic signature changes in immune response and complement proteins in acute myocardial infarction: the implication of serum amyloid P-component. Int J Cardiol. (2013) 168:5196–204. doi: 10.1016/j.ijcard.2013.07.181
38. Sumida T, Naito AT, Nomura S, Nakagawa A, Higo T, Hashimoto A, et al. Complement C1q-induced activation of beta-catenin signalling causes hypertensive arterial remodelling. Nat Commun. (2015) 6:6241. doi: 10.1038/ncomms7241
39. Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). J Interv Cardiol. (2010) 23:511–9. doi: 10.1111/j.1540-8183.2010.00598.x
40. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. (2014) 129:2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507
41. Indorkar R, Kwong RY, Romano S, White BE, Chia RC, Trybula M, et al. Global coronary flow reserve measured during stress cardiac magnetic resonance imaging is an independent predictor of adverse cardiovascular events. JACC Cardiovasc Imaging. (2019) 12:1686–95. doi: 10.1016/j.jcmg.2018.08.018
42. Padro T, Manfrini O, Bugiardini R, Canty J, Cenko E, De Luca G, et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on 'coronary microvascular dysfunction in cardiovascular disease'. Cardiovasc Res. (2020) 116:741–55. doi: 10.1093/cvr/cvaa003
43. Torzewski M, Bhakdi S. Complement and atherosclerosis-united to the point of no return? Clin Biochem. (2013) 46:20–5. doi: 10.1016/j.clinbiochem.2012.09.012
44. Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol Immunol. (2010) 47:2170–5. doi: 10.1016/j.molimm.2010.05.009
45. Horvath Z, Csuka D, Vargova K, Kovacs A, Molnar AA, Gulacsi-Bardos P, et al. Elevated C1rC1sC1inh levels independently predict atherosclerotic coronary heart disease. Mol Immunol. (2013) 54:8–13. doi: 10.1016/j.molimm.2012.10.033
46. Lee J, Seo KW, Park JS, Yang HM, Lim HS, Choi BJ, et al. Managing nonagenarians with acute myocardial infarction: invasive versus conservative treatment. Cardiol Res Pract. (2020) 2020:8885518. doi: 10.1155/2020/8885518
47. Basoli A, Cametti C, Faraglia V, Gili T, Rizzo L, Taurino M. Hemocompatibility of carotid artery stents: alterations of the electrical parameters of erythrocyte cell membrane-a word of caution. Vasc Endovascular Surg. (2010) 44:190–7. doi: 10.1177/1538574409359336
48. Basoli A, Cametti C, Satriani FG, Mariani P, Severino P. Hemocompatibility of stent materials: alterations in electrical parameters of erythrocyte membranes. Vasc Health Risk Manag. (2012) 8:197–204. doi: 10.2147/VHRM.S28979
49. Celik T, Iyisoy A, Barindik N, Isik E. Glucocorticoids in the prevention of in-stent restenosis: the role of inflammation. Int J Cardiol. (2009) 135:403–5. doi: 10.1016/j.ijcard.2008.01.057
50. Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J. (2015) 36:3320–31. doi: 10.1093/eurheartj/ehv511
51. Megaly M, Alani F, Cheng CI, Ragina N. Risk factors for the development of carotid artery in-stent restenosis: multivariable analysis. Cardiovasc Revasc Med. (2021) 24:65–9. doi: 10.1016/j.carrev.2020.09.005
52. Rossen RD, Michael LH, Kagiyama A, Savage HE, Hanson G, Reisberg MA, et al. Mechanism of complement activation after coronary artery occlusion: evidence that myocardial ischemia in dogs causes release of constituents of myocardial subcellular origin that complex with human C1q in vivo. Circ Res. (1988) 62:572–84. doi: 10.1161/01.RES.62.3.572
53. Bohlson SS, O'conner SD, Hulsebus HJ, Ho MM, Fraser DA. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front Immunol. (2014) 5:402. doi: 10.3389/fimmu.2014.00402
54. Hulsebus HJ, O'conner SD, Smith EM, Jie C, Bohlson SS. Complement Component C1q programs a pro-efferocytic phenotype while limiting TNFalpha production in primary mouse and human macrophages. Front Immunol. (2016) 7:230. doi: 10.3389/fimmu.2016.00230
Keywords: serum complement C1q, obstructive CAD, Gensini score, restenosis, non-obstructive CAD
Citation: Guo S, Mao X, Li X, Ouyang H, Gao Y and Ming L (2021) Serum Complement C1q Activity Is Associated With Obstructive Coronary Artery Disease. Front. Cardiovasc. Med. 8:618173. doi: 10.3389/fcvm.2021.618173
Received: 26 October 2020; Accepted: 08 March 2021;
Published: 29 April 2021.
Edited by:
Yun Fang, University of Chicago, United StatesReviewed by:
Paolo Severino, Sapienza University of Rome, ItalyMarie Guerraty, University of Pennsylvania, United States
Copyright © 2021 Guo, Mao, Li, Ouyang, Gao and Ming. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Ming, 149429901@qq.com
 Shuren Guo
Shuren Guo Xiaohuan Mao2
Xiaohuan Mao2  Yuhua Gao
Yuhua Gao Liang Ming
Liang Ming