Relationship between Helicobacter pylori infection and gastrointestinal microecology
- 1Department of Gastroenterology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Pathology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
The prevalence of Helicobacter pylori (H. pylori) infection has exceeded 50% worldwide, and it is considered a high-risk factor for chronic gastritis, peptic ulcer, gastric adenocarcinoma, gastroesophageal reflux disease and functional dyspepsia. H. pylori drug resistance is a common problem worldwide. In recent years, the relationship between H. pylori infection and gastrointestinal microecology has received much attention. H. pylori infection changes the structure and composition of gastrointestinal microflora by regulating the gastrointestinal microecological environment, local pH value, cytokines and antimicrobial peptides, and immune response and then plays a crucial role in the occurrence and development of digestive system tumors, liver metabolism and extragastrointestinal diseases. The quadruple strategy of H. pylori eradication can also aggravate gastrointestinal microflora disorder. However, probiotics can reduce intestinal flora changes and imbalances through different mechanisms, thus enhancing the efficacy of H. pylori eradication therapy and reducing adverse reactions caused by eradication therapy. Therefore, this paper reviews the relationship between H. pylori infection and gastrointestinal microecology and its clinical application, providing a basis for clinical treatment.
1 Introduction
H. pylori is a microaerophilic gram-negative bacillus that colonizes the surface of the human gastric epithelium. It has a spiral-curved shape and flagella, which are available for motility (Warren and Marshall, 1983). It is considered a high-risk factor for chronic gastritis, peptic ulcer, gastric adenocarcinoma, gastric mucosa-associated lymphoid tissue lymphoma, gastroesophageal reflux disease and functional dyspepsia (Manabe et al., 2022; Zavros and Merchant, 2022). An epidemiological survey shows that the H. pylori infection rate has exceeded 50% worldwide, the infection rate in developed countries is approximately 30%, and it can be as high as 80% in developing countries (Jiang et al., 2021). The average H. pylori infection rate in China was 40% in the period 2015-2019 (Ren et al., 2022). The infection rate of H. pylori is related to economic situation, living conditions, sanitary conditions and living habits, occupation and quality of drinking water. It has a higher infection rate in situations of poor economic conditions, crowded living, poor sanitary conditions and bad living habits. In different populations, H. pylori is observed throughout life in the absence of appropriate treatment, and spontaneous clearance of the infection is very rare (Saito et al., 2021).
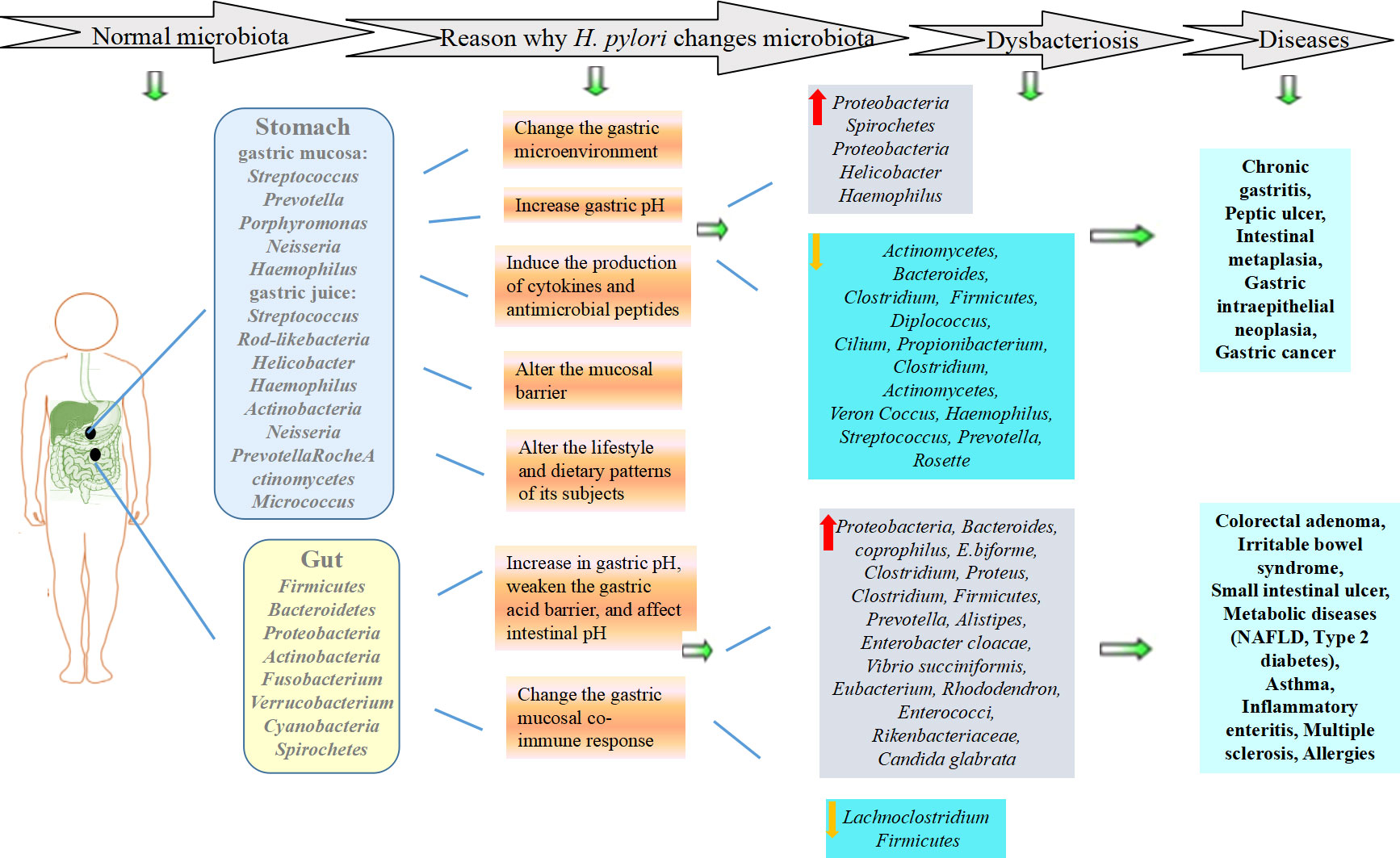
The stomach is now thought to contain more than 100 species of bacteria, and Streptococcus, Prevotella, Porphyromonas, Neisseria and Haemophilus are the dominant bacterial genera in the gastric mucosa, accounting for 70.5% of the total amount (Chen et al., 2021). Slightly different from the bacterial flora of gastric mucosa, the main bacteria in gastric juice of healthy people are ranked from high to low in abundance: Firmicutes, Proteobacteria, Bacteroides and Actinobacteria, among which Streptococcus and Rod-like bacteria of firmicutes; Helicobacter, Haemophilus and Neisseria of Proteobacteria; Prevotella of Bacteroidetes; and Roche, Actinomycetes and Micrococcus of Actinomycetes are the most widely distributed. The composition of the bacteria in the stomach is different from that in the mouth and throat, which suggested that the bacteria in the stomach were not migratory from upper regions. The gastric flora is affected by many factors. Compared with the intestinal flora, the gastric flora is characterized by low density, low specificity, poor stability and high fluctuation. According to previous research results, great differences were observed in the weight and number of bacteria in the gastric microecosystem of healthy adults, which might be due to the different influencing factors, such as regional span, cultural difference, dietary habit difference, etc. (Stewart et al., 2020). These differences may also be related to the age and sex of the population being studied, the method of testing the samples, the use of antibiotics, and whether other diseases were present.
The number of gut microbes is extremely large and includes bacteria, archaea, fungi, viruses, etc. These microorganisms colonize the gut after a series of complex processes, form relatively stable communities, and interact with the host to form a unity with a mutually beneficial symbiotic relationship. The gut microbial genome contains approximately 3.3×106 nonrepetitive genes, which is 150 times the number of genes in the human genome and considered the second genome of the human body. Harmful bacteria, opportunistic pathogens and probiotics are the three main flora in the gut. More than 99% of intestinal microorganisms are anaerobic bacteria, mainly including Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacterium, Verrucobacterium, Cyanobacteria and Spirochetes, the most important of which are Firmicutes (50% to 70%) and Bacteroidetes (10% to 30%) (Lensu and Pekkala, 2021). Its function is closely related to human health and not only affects the nutrient supply and energy balance but also participates in immune defense, endocrine regulation, barrier function maintenance, inhibition of pathogen colonization and many other physiological processes (Ruch and Engel, 2017). To identify the core composition pattern of human gut microbiota in a healthy state, Arumugam et al. proposed the concept of “enterotype” and believed that the composition of human gut microbiota could be divided into three types: “Enterotype 1” was mostly Bacteroides, “Enterotype 2” was mostly Prevotella, and “Enterotype 3” is mostly Ruminococcus (Arumugam et al., 2011). The three types represent three different symbiotic relationships between gut flora and the host and commonly do not change based on differences in race, sex, age, body weight, and health status. Once any kind of bacteria in the intestinal tract exceeds the normal range and reaches a certain pathogenic dose or changes in species and locations, etc., it will lead to dysbiosis. In recent years, studies have found that dysbiosis of intestinal flora may cause systemic lupus erythematosus, rheumatoid arthritis, diabetes, obesity, autism, multiple sclerosis and other extraintestinal system diseases as well as intestinal system diseases, such as inflammatory bowel disease and colon cancer (Jackson and Theiss, 2020; Scheithauer et al., 2020; Tomofuji et al., 2022).
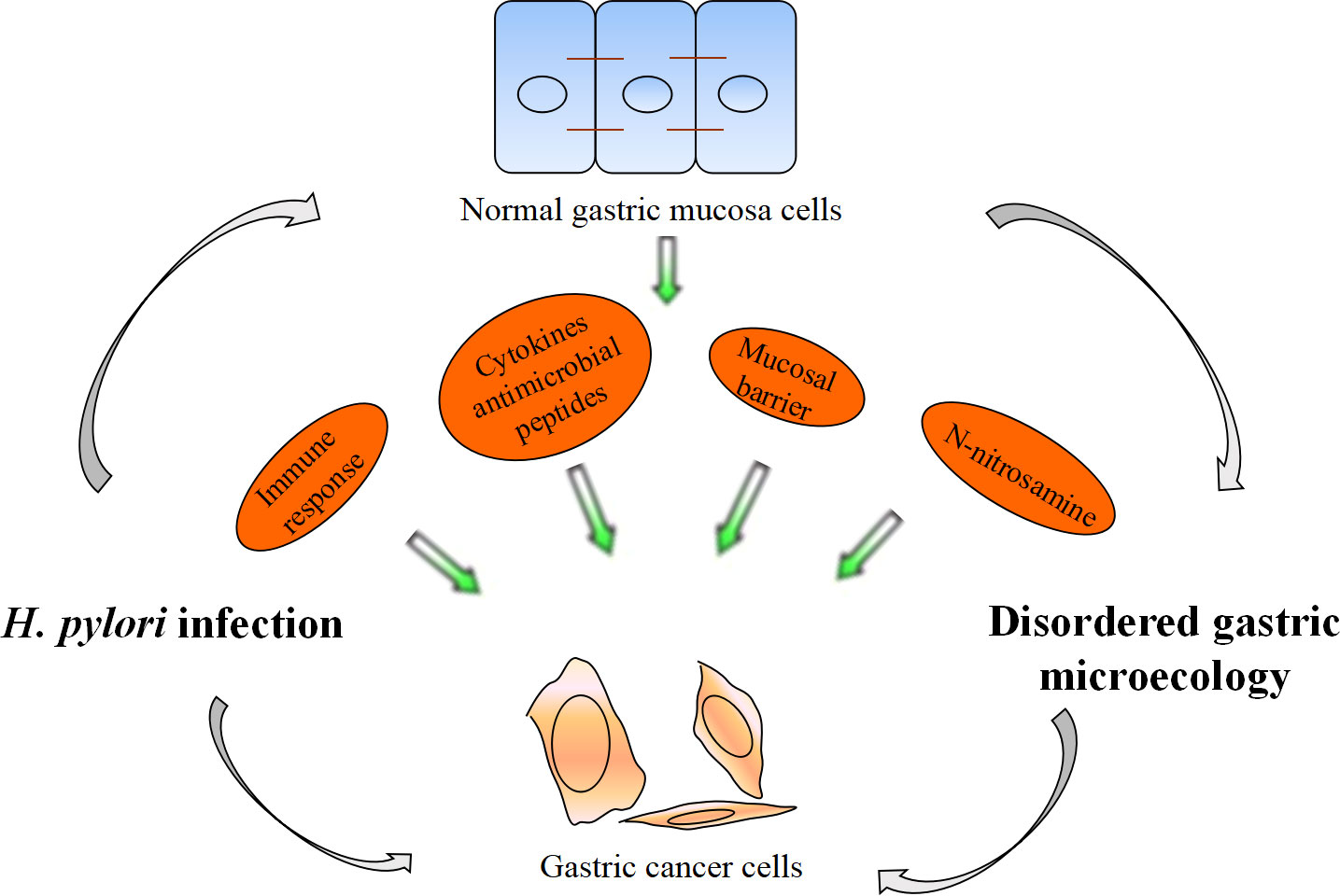
H. pylori infection can affect the gastrointestinal microecological environment, leading to disruption of biological barriers and bacterial translocation, which plays a common role in the occurrence and development of diseases (Lapidot et al., 2021) (Figure 1). In addition, H. pylori drug resistance is a common problem worldwide (Kuo et al., 2021). Probiotics can be divided into single strains and mixed strains, and probiotics represents the general term for beneficial bacteria living in the intestinal tract. They play an important role in the eradication of H. pylori, which also provides new ideas for the treatment of H. pylori infection. Therefore, this paper reviews the relationship between H. pylori infection and gastrointestinal microecology and the effect of their interaction on diseases to provide a basis for clinical treatment.
2 Effects of H. pylori infection on gastrointestinal microecology and diseases
2.1 H. pylori infection and gastric microecology
H. pylori dominates gastric mucosa-associated flora, and excessive proliferation of H. pylori may interfere with the gastric microecological environment, resulting in microecological disorders and inflammatory reactions, resulting in disordered gastric microflora and the occurrence of diseases. Studies have shown that H. pylori significantly reduces the diversity of the microbiota in the stomach. The changes in the proportion and genus level of each phylum are mainly manifested in increases in the relative abundance of Proteobacteria and Spirochetes but decreases in the abundance of Actinomycetes, Bacteroides, Clostridium and Firmicutes in the gastric mucosa. The relative abundance of Proteobacteria in the gastric juice increases, while the relative abundance of Diplococcus, Cilium, Propionibacterium, Clostridium, Actinomycetes, Veron Coccus, Haemophilus, Streptococcus and Prevotella decrease. (Bakhti and Latifi-Navid, 2021). At the genus level, Helicobacter and Haemophilus increase while Rosette decreases (Duan et al., 2022). This phenomenon further shows that H. pylori has advantages over other flora. It is not clear why H. pylori infection changes the gastric microecology, although possible reasons have been postulated (Cheok et al., 2021): H. pylori changes the gastric microenvironment, and the ammonia and bicarbonate produced by decomposing urea can also serve as substrates for other microbial communities; H. pylori infection induces the regulation of the H+/K+-ATPase D subunit promoter by the Nuclear Factor- Kappa B (NF-kB) P50 homologous dimer, which downregulates the expression of H+/K+-ATPase mRNA, and inhibits gastric acid secretion, thereby increasing gastric pH and creating favorable conditions for the colonization of other microorganisms; H. pylori can induce the production of cytokines and antimicrobial peptides, thereby leading to chronic gastritis and inhibiting other local microorganisms; H. pylori may directly alter the mucosal barrier by changing the expression of gastric mucin; H. pylori can alter the lifestyle and dietary patterns of its subjects; H. pylori can develop T regulatory-mediated tolerance to other bacterium, and suppress the effector T-cell responses (Sung et al., 2020; Marginean et al., 2021).
Recent research and practice have shown that H. pylori infection is the main cause of the occurrence and development of chronic gastritis, peptic ulcer, gastric cancer and other digestive diseases. The specific mechanism is still not completely clear, but changes in gastric microecology may be a main reason. H. pylori infection affects Th1-related IgG2 immune response, Interleukin (IL)-1β, IL-17 and Reg III γ transcription levels in the stomach and reactive oxygen species (ROS) levels by gastric flora, promoting the occurrence of superficial gastritis, atrophic gastritis, intestinal metaplasia and gastric intraepithelial neoplasia (Doulberis et al., 2021; Araujo et al., 2022). H. pylori infection promotes the occurrence of gastric cancer by regulating gastric flora (Figure 2). The next generation gene profile analysis of gastric microbiome composition showed that compared with the gastric microbiome of patients with chronic gastritis, gastric cancer patients had a microbiome imbalance characterized by lower diversity, lower H. pylori abundance and higher genotoxicity (Ferreira et al., 2018). Chronic H. pylori infection results in decreased gastric acid secretion, and non-H. pylori microbiota overgrowth promotes gastric mucosa malignancy by promoting inflammation, stimulating cell proliferation, changing stem cell dynamics and transforming nitrate into n-nitrosamine. In gastric cancer, the microbiota promotes nitrite reduction, and acidic nitrite can kill other bacteria. Nitrate can be used as an energy source and can change the gastric microbiota, causing ecological disorder. N-nitroso compounds formed during nitrite metabolism are important carcinogens. In addition, they release DNA-damaging reactive oxygen species and nitrogen (they are effective mutagens), continuously aggravating the gastric mucosal inflammatory response, accelerating the progression of gastric precancerous lesions, and promoting the occurrence of gastric adenocarcinoma (Sheweita and Alsamghan, 2020). H. pylori infection increased Escherichia coli, Lactobacillus, nitrate reductase bacteria, L. coleohominis and Spirobacter nitroso in gastric cancer and played a role in nitrite metabolism. Other bacteria, such as Clostridium, Hemophilus, Staphylococcus and Neisseria, may also be involved in the formation of these compounds (Engstrand and Graham, 2020; Liu et al., 2021). In addition, genomic DNA of Pseudomonas can be transferred into human somatic cells through some mechanism, thus upregulating proto-oncogene expression and promoting the occurrence of gastric adenocarcinoma (Rodrigues et al., 2019). Lactobacillus can promote immune tolerance and provide a platform for the colonization of other carcinogenic bacteria (Chen et al., 2021).
2.2 H. pylori infection and intestinal microecology
Studies have shown that H. pylori infection can not only affect the gastric microecology but also change the intestinal flora. The relationship between H. pylori infection and intestinal microecology is still not clear, and the conclusions in the literature are controversial (Fujimori, 2021). A study on children showed that the abundance and diversity of gastrointestinal flora were higher in children without H. pylori infection, among which Proteobacteria, Bacteroides, Clostridium, etc., were more abundant (Llorca et al., 2017). However, another study found that compared with uninfected children, H. pylori-positive children had an increased number of intestinal flora, such as Proteus, Clostridium, Firmicutes, and Prevotella (Kakiuchi et al., 2021). Lu found that the diversity of intestinal flora in H. pylori-infected patients was higher, in which the abundance of Alistipes and Enterobacter cloacae increased and the abundance of Lachnoclostridium decreased. H. pylori infection changes the balance of intestinal microecology (He et al., 2019). Researchers analyzed human feces and found that the diversity and complexity of the intestinal flora of H. pylori-infected patients were higher, in which Vibrio succiniformis, Rhododendron spp., Enterococci spp., and Rikenbacteriaceae spp. increased and the abundance of Candida glabrata and unclassified fungi improved (Dash et al., 2019). The underlying reason may be that H. pylori infection leads to the initiation of the mucosal coimmunity response as well as changes in gastric acid and gastrin secretion, resulting in an increase in gastric pH, weakening the gastric acid barrier, and simultaneously affecting intestinal pH, which in turn promotes changes in intestinal flora (Iino and Shimoyama, 2021). H. pylori infection may also change the distal intestinal flora through mucosal co-immune response by cytotoxin-associated gene A (CagA), Vacuolating cytotoxin A (VacA), which impairs cell polarity and affects host signaling pathways, thereby altering immune phenotypes and promoting inflammation (He et al., 2022).
An imbalance in H. pylori-related intestinal flora can lead to the occurrence of nonalcoholic fatty liver disease (NAFLD), colorectal adenoma, irritable bowel syndrome and small intestinal ulcer, and the mechanism may be that H. pylori invades the intestinal mucosa to increase intestinal permeability and leads to an imbalance of intestinal flora, which facilitates the entry of bacterial endotoxins into the liver through the portal vein, thereby promoting intestinal inflammation (Santos et al., 2020; Fujimori, 2021). It also affects the absorption of iron and vitamin B12 in the intestinal tract and the metabolic process of carbohydrates and amino acids in the host (Santos et al., 2020). H. pylori infection can increase Eubacterium, Bacteroides coprophilus, E.biforme and decrease Firmicutes, which are related to high density lipoprotein (HDL)/low density lipoprotein (LDL) ratio (Martin-Nunez et al., 2020). Meanwhile, H. pylori infection can affect the balance of gastrointestinal hormones such as leptin, ghrelin and gastrin, and alter the fermentation of non-digestible carbohydrates, the generation of short-chain fatty acids (SCFAs) and glucagon-like peptide-1 (GLP-1) through intestinal flora and may be involved in the occurrence of metabolic diseases such as insulin resistance, and type 2 diabetes (Martin-Nunez et al., 2021; Hu et al., 2022). In addition, H. pylori infection affects the intestinal microecosystem and can also lead to diseases of the extraintestinal system. H. pylori-induced changes in intestinal microflora were often accompanied by changes in immune-related gene expression in multiple organs. Changes in gut microecology and microenvironment caused by H. pylori infection (e.g., bacterial diversity and changes in gastric pH) affect the expression of some immune-related genes in the host, and changes in lung-related gene expression may be caused by H. pylori infection which promoting cumulative changes in immune and/or inflammatory responses. These changes can also be observed at distant sites in host organisms, and other members of the genus Helicobacter exhibit similar effects. For example, the natural colonization of liver Helicobacter pylori in the digestive tract of mice can lead to the transformation of intestinal microbiota, resulting in subclinical inflammation and severe immune damage (Li et al., 2020). Other studies found that intestinal flora disorders caused by H. pylori colonization can also play a protective role in some diseases through immune regulation, such as asthma, inflammatory enteritis, and multiple sclerosis (Borbet et al., 2019). Early H. pylori infection-induced changes in the microenvironment affect the expression of some immune-related genes in the host and then affect the local and even systemic immune system. The effect of H. pylori on the immune system is closely related to changes in gastric physiology and microflora and the occurrence and development of extragastric diseases. H. pylori affects the adaptive immune response by interfering with antigen presentation and T-cell response regulation through microecological imbalance, which plays an important role in the prevention of extragastric immune and inflammatory diseases (such as childhood asthma and allergies) and metabolic disorders (Zuo et al., 2021). H. pylori can regulate the levels of Treg cells and cytokines such as IL-10 and transforming growth factor β (TGF-β) in the lung through intestinal flora, prevent or regulate the Th2 response to allergens, and induce the allergen-specific immune response to immune tolerance transition. This prevents the development of asthma and other allergic diseases (Borbet et al., 2019). In addition, Tricorniaceae can reduce the colonization of Clostridium difficile and reduce the occurrence of intestinal inflammation (Castro-Fernandez et al., 2019). From the influence of H. pylori on disease, we can see that H. pylori has a complex effect on the host.
2.3 H. pylori eradication gastrointestinal microecology
Presently, a consensus report on the management of H. pylori infection recommends the use of quadruple therapy consisting of proton pump inhibitor (PPI), bismuth and two antibacterial drugs for 10 or 14 days (Liou et al., 2020). However, H. pylori eradication quadruple therapy has a certain impact on gastrointestinal flora (Guo et al., 2020). The powerful acid-suppressing effect of PPI can not only reduce the removal effect of exogenous bacteria in food by gastric acid and weaken the body’s own natural barrier but also weaken the body’s digestive function (Li et al., 2017). However, macromolecular nutrients that are not fully digested may promote the growth of pathogenic bacteria after entering the intestinal tract, which has a significant impact on the composition of the flora. In addition, PPI can also reduce the viscosity of gastric mucus, prolong gastric emptying time, and then induce the destruction of gastric microecological balance, resulting in a significant decrease in the abundance and diversity of commensal bacteria and increasing the growth of harmful bacteria such as Motococcaceae, Oxidobacteriaceae and Sphingobacteriaceae, Streptococcaceae, Veronococcaceae, Ruminococcaceae, Microococcaceae, Flavobacteriaceae and Enterococcaceae, thus resulting in osteoporosis, electrolyte imbalance, small intestinal bacterial overgrowth and Clostridium difficile infection (Le Bastard et al., 2021). Individuals with small intestinal bacterial overgrowth have no obvious symptoms in a short period of time, but bacterial overgrowth can lead to excessive fermentation of carbohydrates in the intestinal lumen and reduced absorption of iron, vitamin B12 and fat, which can lead to sequelae, such as abdominal distension in the later stage (Weitsman et al., 2022).
In addition, the long-term use of antibiotics is another important cause of gastrointestinal flora imbalance, damaging the gastrointestinal flora biological barrier, chemical barrier and immune barrier. The breakdown of the biological barrier manifests as a reduction in the number of antibiotic-sensitive bacteria and an increase in the number of drug-resistant bacteria. Then, the latter becomes the dominant flora. Therefore, it destroys the colonization ability of normal symbiotic flora, and exogenous pathogenic bacteria are more likely to invade the body (Zhou et al., 2021). Bacteroidetes, Bifidobacterium, Clostridium, Enterobacteriaceae and Lactobacillus are the most susceptible flora, and opportunistic pathogens such as Escherichia coli, Proteus, Morganella, Serratia, Streptococcus, Shigella, Klebsiella pneumoniae, etc., increase and replace the above normal flora to become the dominant flora. SCFA producing bacteria such as Lachnospiraceae, Ruminococcaceae, Eubacteriacea, Bacteroides, Faecalibacterium, Roseburia, Phascolarctobacterium also decrease with the short-term of the antibiotic treatment (Guillemard et al., 2021). The destruction of the chemical barrier can cause the intestinal microbiota to produce the enzyme cytochromatin (P450), which affects the oxidative metabolism of food and oral drugs (Jonaitis et al., 2020). Disruption of the immune barrier can affect gastrointestinal antigen presentation and innate immunity. The degree of influence of antibiotics on gastrointestinal microecology is related to various factors, such as types, doses, courses of treatment, routes of administration, and microbial resistance. The extensive and unreasonable application of antibiotics is the main reason for the increase in H. pylori drug resistance and the failure of eradication therapy (Megraud et al., 2021). Antimicrobial-associated gastrointestinal flora imbalance can cause a variety of clinical manifestations. The most common adverse reactions include diarrhea, nausea, vomiting, abdominal distension, and abdominal pain, which can increase the risk of the interruption and failure of treatment and occurrence of drug-resistant strains. Antimicrobial-associated gastrointestinal dysbiosis may also affect the long-term prognosis of the disease. The recovery time of gastrointestinal flora after antibiotics was also related to the type of antibiotics, and multiflora could recover to the level before treatment within 4 weeks; however, in some patients, the microbiota was in an “irritable” state for a long time after treatment, and it could take up to 4 years to fully recover to pretreatment levels (Jakobsson et al., 2010; Suzuki et al., 2021). At present, there is still a lack of sufficient clinical evidence for the effects of different H. pylori eradication regimens on gastrointestinal microecology. A good experimental design and standardized operation are the keys to obtain reliable research data.
3 Effect of intestinal probiotics on H. pylori eradication
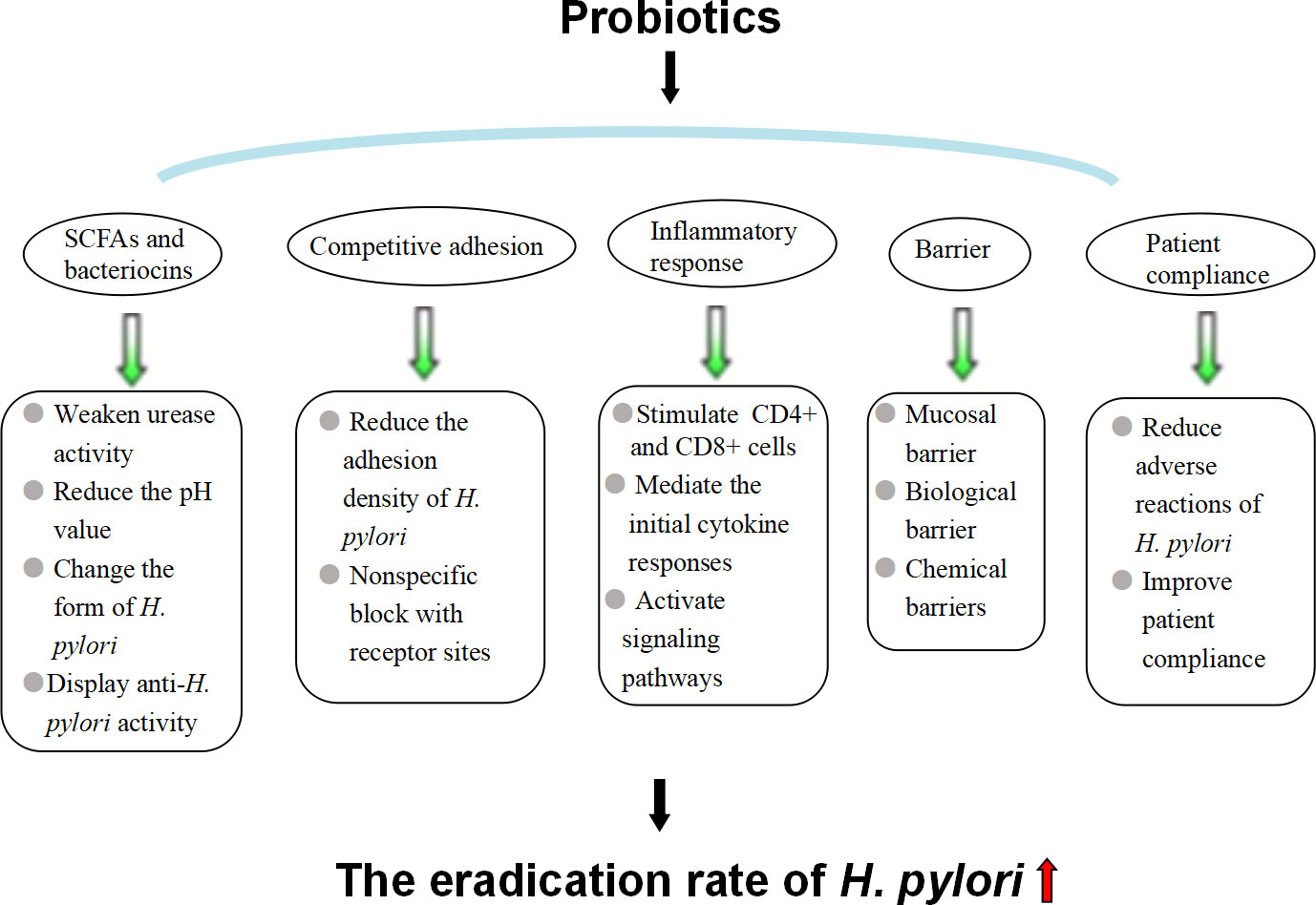
Intestinal probiotics are the general term for the original beneficial bacteria in the gut. Common probiotics include (1) Lactobacillus: L. helveticus, L. acidophilus, L. brevis, L. casei, L. johnsonii, L. rhamnosus, etc.; (2) Bifidobacterium; (3) Streptococcus: Streptococcus thermophilus, Streptococcus faecalis, Lactococcus, Streptococcus intermedia, etc.; and (4) yeast bacteria. Studies have shown that probiotic preparations combined with antibiotics can effectively relieve the clinical symptoms of patients with H. pylori infection, improve the effect of H. pylori eradication and reduce the incidence of adverse drug reactions (Baral et al., 2021) (Figure 3). Commonly used probiotic preparations in the clinic include L. acidophilus, Bacillus licheniformis, Bifidobacterium triple viable bacteria, Bacillus subtilis dual viable bacteria and Saccharomyces boulardii (Lu et al., 2022).
3.1 Probiotics improve the H. pylori radical cure effect
The compound L. acidophilus tablet is a compound tablet composed of four kinds of bacterial powder, including the Chinese strain L. acidophilus, Japanese strain L. acidophilus, Streptococcus faecalis and B. subtilis, and it represents a kind of intestinal flora adjustment drug (Ji et al., 2018). The main mechanism of its influence on H. pylori is mainly as follows: it secretes bacteriocin, lactic acid, hydrogen peroxide and other substances with antibacterial effects, inhibits the proliferation and growth process of H. pylori, and plays a role in eliminating H. pylori; it secretes some active substances against bacterial adhesion, leading to a decrease in the adhesion index of H. pylori to gastric epithelial adenocarcinoma cells; and it upregulates the expression level of small intestinal mucosal binding proteins, indirectly promoting the recovery of gastric mucosal permeability; and it inhibits the release of inflammatory factors, thereby inhibiting its pathogenicity (Takagi et al., 2016; Ji et al., 2018). Compound L. acidophilus combined with quadruple therapy improves the eradication rate and reduces the incidence of adverse reactions compared with quadruple therapy alone and can serve as a rescue regimen in cases of treatment failure (Karbalaei and Keikha, 2021).
B. licheniformis can treat bacteria by prompting the body to produce antibacterial active substances and kill pathogenic bacteria. After entering the digestive tract, B. licheniformis has an antagonistic effect on the pathogenic bacteria in the gastrointestinal tract while promoting the growth of beneficial flora to regulate the flora of the digestive tract. In addition, B. licheniformis can also cause a hypoxic environment in the digestive tract, which is conducive to the growth of anaerobic bacteria (Asaka et al., 2014). These two effects can not only regulate the function of the gastrointestinal tract but also improve adverse reactions during the treatment process, thereby preventing and treating gastrointestinal diseases (Feng et al., 2022). Therefore, the use of B. licheniformis to intervene in H. pylori eradication has played a positive role in the treatment.
Bifidobacterium triple viable bacteria is a live bacterial preparation made of three probiotics in an appropriate proportion. It is mainly composed of Bifidobacterium. The three together form a combined flora that can grow under different conditions. After entering the gastrointestinal tract, Bifidobacterium triple viable bacteria can reduce the pH value of the lesions, inhibit the growth of harmful bacteria, promote the reproduction of beneficial bacteria, and adjust the balance of the body flora (Di Pierro et al., 2020). Bifidobacterium triple viable bacterial capsules reduce the incidence of adverse reactions in the treatment of H. pylori-related gastritis and peptic gastric ulcer, and the clinical efficacy and eradication rates of gastritis and ulcers are significantly better than those of traditional therapies (Koretz, 2018).
B. subtilis dual live bacteria are composed of two probiotics, mainly B. subtilis, which can stimulate the release of active bacteria, such as Candidatus Soleaferrea, increase the activity of the body’s own beneficial bacteria, improve the immune function of the intestinal tract, and inhibit the reproduction of pathogens in the intestinal tract, such as Lachnospiraceae, Ruminiclostridium, Lachnospiraceae and Oxalobacter, thereby regulating intestinal flora (Zou et al., 2022). B. subtilis dual viable bacteria combined with quadruple therapy can improve the eradication rate of H. pylori and reduce adverse reactions.
Saccharomyces boulardii is a nonpathogenic yeast with natural resistance to antibacterial drugs, gastric acid and pepsin. It can have antibacterial and anti-inflammatory activities by inhibiting NF-κB from entering the nucleus and can stimulate the mucosal epithelium to secrete secretory IgA (sIgA), thus enhancing the body’s innate immune defense (Zhang et al., 2020).
3.2 Mechanism by which probiotics improve the eradication rate of H. pylori
3.2.1 SCFAs and bacteriocins
Studies have shown that probiotics and their metabolites can inhibit or kill H. pylori (Ge and Zheng, 2012). The substances that probiotics inhibit the growth of H. pylori mainly include SCFAs and bacteriocins (Xu et al., 2021). SCFAs, such as formic acid, acetic acid, propionic acid, butyric acid, and lactic acid, are produced by probiotics metabolizing carbohydrates. They inhibit the colonization and growth of H. pylori in the gastric mucosa by weakening urease activity, reducing the pH value in the stomach, and changing the form of H. pylori (Keikha and Karbalaei, 2021). Bacteriocin is a bactericidal or bacteriostatic substance produced by bacteria that exhibits a narrow activity inhibition spectrum for species of closely related strains. Studies have found that L. formus, B. subtilis, L. roche, Enterococcus faecalis, B. subtilis and Bifidobacterium can all release bacteriocin with anti-H. pylori activity, such as nisin from Streptococcus lactis, Amicoumacin A, Reuterin, etc., and their anti-H. pylori effects depend on the type and number of bacterial strains, which may be related to the diversity of clinical trials (Gotteland et al., 2006). The specific mechanism of bacteriocin: the bacteriocin nisin can kill H. pylori under the coordination of citric acid, similar to most bacteriocins. Nisin molecules are positively charged, containing three lysines at locus 12, 22 and 34 and one histidine at locus 31, which is advantageous for electrostatic and hydrophobic interactions with the cell membrane, inserting into the membrane formation, and can lead to cell autolysis and death (Liang et al., 2022). In addition, probiotics can produce substances with a broad spectrum of antibacterial effects, such as hydrogen peroxide, which can resist H. priori infection.
3.2.2 Competitive adhesion
Researchers studied the effect of Lactobacillus on H. pylori adhesion to gastric adenocarcinoma cells, detected the number of H. pylori adhering to gastric adenocarcinoma cells –AGS and MKN45 cells by urease test, and found that both live and dead Lactobacillus could adhere to AGS cells and MKN45 cells in large quantities, significantly reducing the adhesion density of H. pylori, although the inhibitory ability of dead bacteria was lower than that of live bacteria (Rokka et al., 2008; Takeda et al., 2017). Lactobacillus can bind to a variety of pathogenic bacterial receptors and inhibit the adhesion of pathogenic bacteria to gastric mucosa epithelial cells (Song et al., 2019; Lee et al., 2020). It is speculated that the nonspecific blocking of receptor sites by Lactobacillus may be the mechanism of its inhibition of H. pylori. Gastric epithelial cells have H. pylori-specific adhesion receptors, such as glycolipid receptor gangliotetracylsphingol and sulfatide. These studies showed that L. reuteri and H. pylori have common glycolipid specificity, which can secret adhesion factors and competitively inhibit the adhesion of H. pylori by binding to the binding site of gastric mucosal epithelial cells.
3.2.3 Inhibiting the inflammatory response to H. pylori infection
Probiotics can produce a cellular immune response in gastrointestinal mucosa, especially stimulating the activation and proliferation of CD4+ and CD8+ cells in the mucosa propria, increasing the production of secretory immunoglobulin A, reducing intestinal permeability, promoting the proliferation of epithelial cells, accelerating the regeneration of mucosal repair, and strengthening the role of the mucosal barrier. H. pylori stimulates gastric epithelial cells to secrete IL-8, mediating the initial cytokine responses and resulting in neutrophil and monocyte migration to the mucous membrane. Some probiotics have been shown to inhibit CagA protein expression and reduce the H. pylori-induced secretion of IL-8 by gastric mucosa epithelial cells, thus inhibiting the inflammatory responses (He et al., 2022). In addition to IL-8, H. pylori infection can also stimulate epithelial cells to produce a number of other inflammatory mediators, such as tumor necrosis factor α, inducible nitric oxide synthase, and cyclooxygenase-2, while probiotics block nuclear transport of the NF-κB signaling pathway by increasing the expression of cytokine signal transduction inhibitors, activating transcriptional activators and inactivating janus kinase (JAK) to upregulate the expression of suppressor of cytokine signal transduction 2 (SOCS2) or SOCS3 and activate signal transducerand activator of transcription 1 (STAT-1) and STAT-3 inactivation protein tyrosine kinase 2 (JAK2), thereby inhibiting the inflammatory response caused by H. pylori infection and inhibiting inflammation (Lee et al., 2010; Song et al., 2019). More research is needed because controversy remains about the underlying mechanism as it may be independent of STAT signaling (Sarajlic et al., 2020).
3.2.4 Strengthening mucosal, biological and chemical barriers to prevent H. pylori colonization
Probiotics can indirectly promote the recovery of gastric mucosal permeability, maintain the integrity of the mucosa, prevent the invasion of pathogenic bacteria, including H. pylori, and strengthen the mucosal barrier. Probiotics interact closely with mucosal epithelial cells to occupy the mucosal surface and improve the defense ability of epithelial cells, thus forming a biological barrier (Chakravarty and Gaur, 2019). Active substances, such as metabolites of probiotics, including small molecule acids, hydrogen peroxide and bacteritin, can kill pathogenic bacteria, including H. pylori, and prevent the colonization and invasion of pathogenic bacteria and opportunistic pathogens, forming a chemical barrier.
3.2.5 Reducing adverse reactions and improving patient compliance
Probiotic preparations can alleviate adverse reactions, such as taste disturbance, diarrhea, nausea, abdominal pain and constipation caused by antibiotics and significantly improving the compliance of patients with H. pylori eradication therapy, thereby increasing the H. pylori eradication rate (Zagari et al., 2018). Treatment compliance is an important factor in the success of H. pylori eradication (Nyssen et al., 2021). However, previous studies showed that probiotic preparations had the risk of systemic infection, as long-term use of B. subtilis carried the risk of cholangitis. Additionally, excessive immune stimulation in susceptible individuals may be induced, as the use of Saccharomyces cerevisiae and Saccharomyces boulardii improved the risk of fungalemia in patients with prolonged antibiotics or venous catheterization deleterious metabolic activities (Doron and Snydman, 2015). Therefore, careful consideration should be given to the clinical application of probiotics to patients with severe underlying diseases, low immunity, long-term antibiotic use, and central venous catheterization.
4 Discussion
In conclusion, H. pylori infection can change the structure and composition of gastrointestinal flora by regulating the local microenvironment, local pH value, cytokines and antimicrobial peptides, and immune response and then play a role in the occurrence and development of digestive system tumors, liver metabolism and extragastrointestinal diseases. The quadruple regimen of H. pylori eradication can aggravate gastrointestinal microflora disorder. Probiotics can reduce the change and imbalance of intestinal flora and improve the eradication rate of H. pylori. However there are still many challenges in this field. (1) The influence of H. pylori infection on gastrointestinal microecology is still controversial, and opposite conclusions have been obtained, which may be related to the infection time, host health status, age of research subjects, number of research samples, methods of sampling and detection, analysis from different phyla levels of gastrointestinal flora and other factors. (2) H. pylori eradication can restore the gastric acid barrier to a certain extent and then restore the balance of intestinal flora. At the same time, oral administration of large doses of antibiotics and PPIs can easily disrupt the normal symbiosis between the intestinal flora and the host, thereby weakening the protective effect of the biological barrier. Secondary changes in the intestinal flora after eradication may be related to the adverse reactions associated with eradication therapy and the recurrence of infection after eradication. However, it has not been determined whether H. pylori eradication causes long-term changes in intestinal flora. (3) The mechanism of H. pylori and gastrointestinal microecology in specific diseases and the causal relationship between H. pylori and diseases remain unclear. Different subtypes of gastric cancer are identified according to different types of ecological disorders, and personalized probiotic adjuvant therapy is developed. Future research should focus on analyzing bacterial imbalance in the oral cavity and stool as a noninvasive diagnostic marker and preventing and treating gastritis cancer by intervening with bacterial flora and studying its specific mechanism in nutrition, immunity, metabolism, signaling pathway and other aspects and the mechanism of interaction between H. pylori and other gastrointestinal microorganisms in gastric diseases. (4) Many studies have shown that probiotic adjuvant therapy can reduce changes and imbalances in the intestinal flora caused by eradication therapy, including antibiotics and PPIs, thus enhancing the efficacy of eradication therapy and reducing its adverse reactions. During H. pylori eradication therapy, probiotics as adjuvants can be used as follows: Try not to choose probiotics containing Enterococcus are more likely to develop resistance and resistance genes can be easily passed to H. pylori and other pathogenic bacteria by plasmids, which affects H. pylori eradication therapy; 2-4 weeks may be the optimal time to use probiotics; the time interval of probiotic and standard antibiotic treatments must be selected carefully so that probiotics can better colonize and play a role. However, homogeneity is difficult to control in research on the relationship between probiotics and effect of H. pylori eradication treatment because many factors need to be considered, such as the types of probiotics, dosage forms, types of eradication drugs, courses of treatment, and regional differences. It is necessary to carry out large-scale multicenter clinical studies to effectively improve the eradication rate of H. pylori and reduce the incidence of adverse reactions.
Author contributions
WX and LX wrote the manuscript; CX revised the review. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by The National Natural Science Foundation of China (No. 82100600), Zhejiang Provincial Natural Science Foundation of China under Grant (No. LQ20H030009), and Health Science and Technology Plan Project of Zhejiang Province (No. 2018KY367).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Araujo, G. R. L., Marques, H. S., Santos, M. L. C., da Silva, F. A. F., da Brito, B. B., Correa Santos, G. L., et al. (2022). Helicobacter pylori infection: How does age influence the inflammatory pattern? World J. Gastroenterol. 28, 402–411. doi: 10.3748/wjg.v28.i4.402
Arumugam, M., Raes, J., Pelletier, E., Le Paslier, D., Yamada, T., Mende, D. R., et al. (2011). Enterotypes of the human gut microbiome. Nature 473, 174–180. doi: 10.1038/nature09944
Asaka, M., Kato, M., Sakamoto, N. (2014). Roadmap to eliminate gastric cancer with helicobacter pylori eradication and consecutive surveillance in Japan. J. Gastroenterol. 49, 1–8. doi: 10.1007/s00535-013-0897-8
Bakhti, S. Z., Latifi-Navid, S. (2021). Interplay and cooperation of helicobacter pylori and gut microbiota in gastric carcinogenesis. BMC Microbiol. 21, 258. doi: 10.1186/s12866-021-02315-x
Baral, K. C., Bajracharya, R., Lee, S. H., Han, H. K. (2021). Advancements in the pharmaceutical applications of probiotics: Dosage forms and formulation technology. Int. J. Nanomed 16, 7535–7556. doi: 10.2147/IJN.S337427
Borbet, T. C., Zhang, X., Muller, A., Blaser, M. J. (2019). The role of the changing human microbiome in the asthma pandemic. J. Allergy Clin. Immunol. 144, 1457–1466. doi: 10.1016/j.jaci.2019.10.022
Castro-Fernandez, M., Marques-Ruiz, A., Camara-Baena, S., Grande-Santamaria, L. (2019). Clostridium difficile infection associated with bismuth-based quadruple therapy (Pylera((R))) for helicobacter pylori eradication. Gastroenterol Y Hepatol 42, 459–460. doi: 10.1016/j.gastre.2019.01.021
Chakravarty, K., Gaur, S. (2019). Role of probiotics in prophylaxis of helicobacter pylori infection. Curr. Pharm. Biotechnol. 20, 137–145. doi: 10.2174/1389201020666190227203107
Chen, M. J., Chen, C. C., Huang, Y. C., Tseng, C. C., Hsu, J. T., Lin, Y. F., et al. (2021). The efficacy of lactobacillus acidophilus and rhamnosus in the reduction of bacterial load of helicobacter pylori and modification of gut microbiota-a double-blind, placebo-controlled, randomized trial. Helicobacter 26, e12857. doi: 10.1111/hel.12857
Chen, C. C., Liou, J. M., Lee, Y. C., Hong, T. C., El-Omar, E. M., Wu, M. S. (2021). The interplay between helicobacter pylori and gastrointestinal microbiota. Gut Microbes 13, 1–22. doi: 10.1080/19490976.2021.1909459
Cheok, Y. Y., Lee, C. Y. Q., Cheong, H. C., Vadivelu, J., Looi, C. Y., Abdullah, S., et al. (2021). An overview of helicobacter pylori survival tactics in the hostile human stomach environment. Microorganisms 9, 2502. doi: 10.3390/microorganisms9122502
Dash, N. R., Khoder, G., Nada, A. M., Al Bataineh, M. T. (2019). Exploring the impact of helicobacter pylori on gut microbiome composition. PloS One 14, e0218274. doi: 10.1371/journal.pone.0218274
Di Pierro, F., Bertuccioli, A., Saponara, M., Ivaldi, L. (2020). Impact of a two-bacterial-strain formula, containing bifidobacterium animalis lactis BB-12 and enterococcus faecium L3, administered before and after therapy for helicobacter pylori eradication. Minerva Gastroenterol Dietol 66, 117–123. doi: 10.23736/S1121-421X.19.02651-5
Doron, S., Snydman, D. R. (2015). Risk and safety of probiotics. Clin. Infect. Dis. 60 Suppl 2, S129–S134. doi: 10.1093/cid/civ085
Doulberis, M., Pierre, N. T., Manzini, G., Papaefthymiou, A., Kountouras, J., Klukowska-Rotzler, J., et al. (2021). Helicobacter pylori-related metabolic parameters and premalignant gastric mucosa histological lesions in Swiss bariatric patients. Microorganisms 9, 1361. doi: 10.3390/microorganisms9071361
Duan, X., Chen, P., Xu, X., Han, M., Li, J. (2022). Role of gastric microorganisms other than helicobacter pylori in the development and treatment of gastric diseases. BioMed. Res. Int. 2022, 6263423. doi: 10.1155/2022/6263423
Engstrand, L., Graham, D. Y. (2020). Microbiome and gastric cancer. Digest Dis. Sci. 65, 865–873. doi: 10.1007/s10620-020-06101-z
Feng, S., Meng, C., Hao, Z., Liu, H. (2022). Bacillus licheniformis reshapes the gut microbiota to alleviate the subhealth. Nutrients 14, 1642. doi: 10.3390/nu14081642
Ferreira, R. M., Pereira-Marques, J., Pinto-Ribeiro, I., Costa, J. L., Carneiro, F., Machado, J. C., et al. (2018). Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 67, 226–236. doi: 10.1136/gutjnl-2017-314205
Fujimori, S. (2021). Progress in elucidating the relationship between helicobacter pylori infection and intestinal diseases. World J. Gastroenterol. 27, 8040–8046. doi: 10.3748/wjg.v27.i47.8040
Ge, R. T., Zheng, Q. (2012). Advances in Study of Probiotics in Treatment of Helicobacter pylori Infection. Chinese journal of gastroenterology 17, 686–688.
Gotteland, M., Brunser, O., Cruchet, S. (2006). Systematic review: are probiotics useful in controlling gastric colonization by helicobacter pylori? Alimentary Pharmacol. Ther. 23, 1077–1086. doi: 10.1111/j.1365-2036.2006.02868.x
Guillemard, E., Poirel, M., Schafer, F., Quinquis, L., Rossoni, C., Keicher, C., et al. (2021). A randomised, controlled trial: Effect of a multi-strain fermented milk on the gut microbiota recovery after helicobacter pylori therapy. Nutrients 13, 3171. doi: 10.3390/nu13093171
Guo, Y., Zhang, Y., Gerhard, M., Gao, J. J., Mejias-Luque, R., Zhang, L., et al. (2020). Effect of helicobacter pylori on gastrointestinal microbiota: a population-based study in linqu, a high-risk area of gastric cancer. Gut 69, 1598–1607. doi: 10.1136/gutjnl-2019-319696
He, C., Kong, F., Chai, X., Zou, C., Zhu, X., Zhao, D. (2022). Effect of probiotic-assisted eradication of cagA+/vacA s1m1 helicobacter pylori on intestinal flora. BioMed. Res. Int. 2022, 8607671. doi: 10.1155/2022/8607671
He, C., Peng, C., Wang, H., Ouyang, Y., Zhu, Z., Shu, X., et al. (2019). The eradication of helicobacter pylori restores rather than disturbs the gastrointestinal microbiota in asymptomatic young adults. Helicobacter 24, e12590. doi: 10.1111/hel.12590
Hu, Y., Xu, X., Ouyang, Y. B., He, C., Li, N. S., Xie, C., et al. (2022). Altered gut microbiota and short-chain fatty acids after vonoprazan-amoxicillin dual therapy for helicobacter pylori eradication. Front. Cell. Infect Microbiol. 12, 881968. doi: 10.3389/fcimb.2022.881968
Iino, C., Shimoyama, T. (2021). Impact of helicobacter pylori infection on gut microbiota. World J. Gastroenterol. 27, 6224–6230. doi: 10.3748/wjg.v27.i37.6224
Jackson, D. N., Theiss, A. L. (2020). Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes 11, 285–304. doi: 10.1080/19490976.2019.1592421
Jakobsson, H. E., Jernberg, C., Andersson, A. F., Sjolund-Karlsson, M., Jansson, J. K., Engstrand, L. (2010). Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PloS One 5, e9836. doi: 10.1371/journal.pone.0009836
Jiang, X., Xu, Z., Zhang, T., Li, Y., Li, W., Tan, H. (2021). Whole-Genome-Based helicobacter pylori geographic surveillance: A visualized and expandable webtool. Front. Microbiol. 12, 687259. doi: 10.3389/fmicb.2021.687259
Ji, W., Chen, W. Q., Tian, X. (2018). Efficacy of compound lactobacillus acidophilus tablets combined with quadruple therapy for helicobacter pylori eradication and its correlation with pH value in the stomach: a study protocol of a randomised, assessor-blinded, single-centre study. BMJ Open 8, e023131. doi: 10.1136/bmjopen-2018-023131
Jonaitis, P., Jonaitis, L., Kupcinskas, J. (2020). Role of genetic polymorphisms of cytochrome P450 2C19 in pantoprazole metabolism and pantoprazole-based helicobacter pylori eradication regimens. Curr. Drug Metab. 21, 830–837. doi: 10.2174/1389200221666200514081442
Kakiuchi, T., Tanaka, Y., Ohno, H., Matsuo, M., Fujimoto, K. (2021). Helicobacter pylori infection-induced changes in the intestinal microbiota of 14-year-old or 15-year-old Japanese adolescents: a cross-sectional study. BMJ Open 11, e047941. doi: 10.1136/bmjopen-2020-047941
Karbalaei, M., Keikha, M. (2021). Rescue effects of lactobacillus-containing bismuth regimens after helicobacter pylori treatment failure. New Microbes New Infect 42, 100904. doi: 10.1016/j.nmni.2021.100904
Keikha, M., Karbalaei, M. (2021). Probiotics as the live microscopic fighters against helicobacter pylori gastric infections. BMC Gastroenterol. 21, 388. doi: 10.1186/s12876-021-01977-1
Koretz, R. L. (2018). Probiotics in gastroenterology: How pro is the evidence in adults? Am. J. Gastroenterol. 113, 1125–1136. doi: 10.1038/s41395-018-0138-0
Kuo, C. J., Lee, C. H., Chang, M. L., Lin, C. Y., Lin, W. R., Su, M. Y., et al. (2021). Multidrug resistance: The clinical dilemma of refractory helicobacter pylori infection. J. Microbiol Immunol Infect 54, 1184–1187. doi: 10.1016/j.jmii.2021.03.006
Lapidot, Y., Reshef, L., Cohen, D., Muhsen, K. (2021). Helicobacter pylori and the intestinal microbiome among healthy school-age children. Helicobacter 26, e12854. doi: 10.1111/hel.12854
Le Bastard, Q., Berthelot, L., Soulillou, J. P., Montassier, E. (2021). Impact of non-antibiotic drugs on the human intestinal microbiome. Expert Rev. Mol. Diagn 21, 911–924. doi: 10.1080/14737159.2021.1952075
Lee, H. A., Kim, J. Y., Kim, J., Nam, B., Kim, O. (2020). Anti-helicobacter pylori activity of acomplex mixture of lactobacillus paracasei HP7 including the extract of perilla frutescens var. acuta and glycyrrhiza glabra. Lab. Anim. Res. 36, 40. doi: 10.1186/s42826-020-00073-x
Lee, J. S., Paek, N. S., Kwon, O. S., Hahm, K. B. (2010). Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling (SOCS) expression and signaling in helicobacter pylori infection: a novel mechanism. J. Gastroenterol. Hepatol. 25, 194–202. doi: 10.1111/j.1440-1746.2009.06127.x
Lensu, S., Pekkala, S. (2021). Gut microbiota, microbial metabolites and human physical performance. Metabolites 11, 716. doi: 10.3390/metabo11110716
Liang, Y., Yan, J., Chen, Z., Gu, Q., Li, P. (2022). Antibacterial effects of bacteriocin PLNC8 against helicobacter pylori and its potential mechanism of action. Foods 11, 1235. doi: 10.3390/foods11091235
Liou, J. M., Malfertheiner, P., Lee, Y. C., Sheu, B. S., Sugano, K., Cheng, H. C., et al. (2020). Screening and eradication of helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut 69, 2093–2112. doi: 10.1136/gutjnl-2020-322368
Liu, D., Chen, S., Gou, Y., Yu, W., Zhou, H., Zhang, R., et al. (2021). Gastrointestinal microbiota changes in patients with gastric precancerous lesions. Front. Cell. Infect Microbiol. 11, 749207. doi: 10.3389/fcimb.2021.749207
Li, J., Liu, L., Chen, Y. (2017). Advances in study on effects of Helicobacter pylori eradication therapy on intestinal microecology. Chinese journal of gastroenterology 22, 241–244.
Li, S., Wu, D., Cao, M., Yu, Z., Wu, M., Liu, Y., et al. (2020). Effects of choline supplementation on liver biology, gut microbiota, and inflammation in helicobacter pylori-infected mice. Life Sci. 259, 118200. doi: 10.1016/j.lfs.2020.118200
Llorca, L., Perez-Perez, G., Urruzuno, P., Martinez, M. J., Iizumi, T., Gao, Z., et al. (2017). Characterization of the gastric microbiota in a pediatric population according to helicobacter pylori status. Pediatr. Infect. Dis. J. 36, 173–178. doi: 10.1097/INF.0000000000001383
Lu, C. A., Zhang, M. B., Wang, J., Yang, G. R. (2022). Application progress of common probiotics in Helicobacter pylori infection. J Chronic Pathematology 23, 85–87.
Manabe, N., Matsueda, K., Haruma, K. (2022). Epidemiological review of gastroesophageal junction adenocarcinoma in Asian countries. Digestion 103, 29–36. doi: 10.1159/000519602
Marginean, C. O., Melit, L. E., Sasaran, M. O. (2021). Gastric microenvironment-a partnership between innate immunity and gastric microbiota tricks helicobacter pylori. J. Clin. Med. 10, 3258. doi: 10.3390/jcm10153258
Martin-Nunez, G. M., Cornejo-Pareja, I., Clemente-Postigo, M., Tinahones, F. J., Moreno-Indias, I. (2021). Helicobacter pylori eradication therapy affect the gut microbiota and ghrelin levels. Front. Med. 8, 712908. doi: 10.3389/fmed.2021.712908
Martin-Nunez, G. M., Cornejo-Pareja, I., Roca-Rodriguez, M. D. M., Clemente-Postigo, M., Cardona, F., et al. (2020). H. pylori eradication treatment causes alterations in the gut microbiota and blood lipid levels. Front. Med. 7, 417. doi: 10.3389/fmed.2020.00417
Megraud, F., Bruyndonckx, R., Coenen, S., Wittkop, L., Huang, T. D., Hoebeke, M., et al. (2021). Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 70, 1815–1822. doi: 10.1136/gutjnl-2021-324032
Nyssen, O. P., Perez-Aisa, A., Tepes, B., Castro-Fernandez, M., Kupcinskas, J., Jonaitis, L., et al. (2021). Adverse event profile during the treatment of helicobacter pylori: A real-world experience of 22,000 patients from the European registry on h. pylori management (Hp-EuReg). Am. J. Gastroenterol. 116, 1220–1229. doi: 10.14309/ajg.0000000000001246
Ren, S., Cai, P., Liu, Y., Wang, T., Zhang, Y., Li, Q., et al. (2022). Prevalence of helicobacter pylori infection in China: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 37, 464–470. doi: 10.1111/jgh.15751
Rodrigues, G., Silva, G. G. O., Buccini, D. F., Duque, H. M., Dias, S. C., Franco, O. L. (2019). Bacterial proteinaceous compounds with multiple activities toward cancers and microbial infection. Front. Microbiol. 10(1690). doi: 10.3389/fmicb.2019.01690
Rokka, S., Myllykangas, S., Joutsjoki, V. (2008). Effect of specific colostral antibodies and selected lactobacilli on the adhesion of helicobacter pylori on AGS cells and the helicobacter-induced IL-8 production. Scandinavian J. Immunol. 68, 280–286. doi: 10.1111/j.1365-3083.2008.02138.x
Ruch, T. R., Engel, J. N. (2017). Targeting the mucosal barrier: How pathogens modulate the cellular polarity network. Cold Spring Harbor Perspect. Biol. 9, a027953. doi: 10.1101/cshperspect.a027953
Saito, H., Nishikawa, Y., Masuzawa, Y., Tsubokura, M., Mizuno, Y. (2021). Helicobacter pylori infection mass screening for children and adolescents: a systematic review of observational studies. J. Gastrointestinal Cancer 52, 489–497. doi: 10.1007/s12029-021-00630-0
Santos, M. L. C., de Brito, B. B., da Silva, F. A. F., Sampaio, M. M., Marques, H. S., Oliveira, E. N., et al. (2020). Helicobacter pylori infection: Beyond gastric manifestations. World J. Gastroenterol. 26, 4076–4093. doi: 10.3748/wjg.v26.i28.4076
Sarajlic, M., Neuper, T., Vetter, J., Schaller, S., Klicznik, M. M., Gratz, I. K., et al. (2020). H. pylori modulates DC functions via T4SS/TNFalpha/p38-dependent SOCS3 expression. Cell Commun Signaling 18, 160.
Scheithauer, T. P. M., Rampanelli, E., Nieuwdorp, M., Vallance, B. A., Verchere, C. B., Van Raalte, D. H., et al. (2020). Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 11, 571731. doi: 10.3389/fimmu.2020.571731
Sheweita, S. A., Alsamghan, A. S. (2020). Molecular mechanisms contributing bacterial infections to the incidence of various types of cancer. Mediators Inflammation 2020, 4070419. doi: 10.1155/2020/4070419
Song, H., Zhou, L., Liu, D., Ge, L., Li, Y. (2019). Probiotic effect on helicobacter pylori attachment and inhibition of inflammation in human gastric epithelial cells. Exp. Ther. Med. 18, 1551–1562. doi: 10.3892/etm.2019.7742
Stewart, O. A., Wu, F., Chen, Y. (2020). The role of gastric microbiota in gastric cancer. Gut Microbes 11, 1220–1230. doi: 10.1080/19490976.2020.1762520
Sung, J. J. Y., Coker, O. O., Chu, E., Szeto, C. H., Luk, S. T. Y., Lau, H. C. H., et al. (2020). Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after helicobacter pylori eradication. Gut 69, 1572–1580. doi: 10.1136/gutjnl-2019-319826
Suzuki, S., Gotoda, T., Takano, C., Horii, T., Sugita, T., Ogura, K., et al. (2021). Long term impact of vonoprazan-based helicobacter pylori treatment on gut microbiota and its relation to post-treatment body weight changes. Helicobacter 26, e12851. doi: 10.1111/hel.12851
Takagi, A., Yanagi, H., Ozawa, H., Uemura, N., Nakajima, S., Inoue, K., et al. (2016). Effects of lactobacillus gasseri OLL2716 on helicobacter pylori-associated dyspepsia: A multicenter randomized double-blind controlled trial. Gastroenterol. Res. Pract. 2016, 7490452. doi: 10.1155/2016/7490452
Takeda, S., Igoshi, K., Tsend-Ayush, C., Oyunsuren, T., Sakata, R., Koga, Y., et al. (2017). Lactobacillus paracasei strain 06TCa19 suppresses inflammatory chemokine induced by helicobacter pylori in human gastric epithelial cells. Hum. Cell 30, 258–266. doi: 10.1007/s13577-017-0172-z
Tomofuji, Y., Kishikawa, T., Maeda, Y., Ogawa, K., Nii, T., Okuno, T., et al. (2022). Whole gut virome analysis of 476 Japanese revealed a link between phage and autoimmune disease. Ann. Rheumatic Dis. 81, 278–288. doi: 10.1136/annrheumdis-2021-221267
Warren, J. R., Marshall, B. (1983). Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1, 1273–1275. doi: 10.1016/S0140-6736(83)92719-8
Weitsman, S., Celly, S., Leite, G., Mathur, R., Sedighi, R., Barlow, G. M., et al. (2022). Effects of proton pump inhibitors on the small bowel and stool microbiomes. Digest Dis. Sci. 67, 224–232. doi: 10.1007/s10620-021-06857-y
Xu, Y., Yu, Y., Shen, Y., Li, Q., Lan, J., Wu, Y., et al. (2021). Effects of bacillus subtilis and bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poultry Sci. 100, 101358. doi: 10.1016/j.psj.2021.101358
Zagari, R. M., Romiti, A., Ierardi, E., Gravina, A. G., Panarese, A., Grande, G., et al. (2018). The “three-in-one” formulation of bismuth quadruple therapy for helicobacter pylori eradication with or without probiotics supplementation: Efficacy and safety in daily clinical practice. Helicobacter 23, e12502. doi: 10.1111/hel.12502
Zavros, Y., Merchant, J. L. (2022). The immune microenvironment in gastric adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 19, 451–467. doi: 10.1038/s41575-022-00591-0
Zhang, K., Zhang, X., Lv, A., Fan, S., Zhang, J. (2020). Saccharomyces boulardii modulates necrotizing enterocolitis in neonatal mice by regulating the sirtuin 1/NFkappaB pathway and the intestinal microbiota. Mol. Med. Rep. 22, 671–680. doi: 10.3892/mmr.2020.11138
Zhou, Y., Ye, Z., Wang, Y., Huang, Z., Zheng, C., Shi, J., et al. (2021). Long-term changes in the gut microbiota after triple therapy, sequential therapy, bismuth quadruple therapy and concomitant therapy for helicobacter pylori eradication in Chinese children. Helicobacter 26, e12809. doi: 10.1111/hel.12809
Zou, X. Y., Zhang, M., Tu, W. J., Zhang, Q., Jin, M. L., Fang, R. D., et al. (2022). Bacillus subtilis inhibits intestinal inflammation and oxidative stress by regulating gut flora and related metabolites in laying hens. Int. J. Anim. Biosci 16, 100474. doi: 10.1016/j.animal.2022.100474
Keywords: Helicobacter pylori, gastrointestinal microecology, probiotics, infection, eradication
Citation: Xu W, Xu L and Xu C (2022) Relationship between Helicobacter pylori infection and gastrointestinal microecology. Front. Cell. Infect. Microbiol. 12:938608. doi: 10.3389/fcimb.2022.938608
Received: 07 May 2022; Accepted: 04 August 2022;
Published: 18 August 2022.
Edited by:
Yi Hu, The First Affiliated Hospital of Nanchang University, ChinaReviewed by:
Peng Yu, Nanchang University, ChinaGuoling Zhou, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2022 Xu, Xu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengfu Xu, xiaofu@zju.edu.cn
 Wenting Xu
Wenting Xu Liming Xu2
Liming Xu2  Chengfu Xu
Chengfu Xu