The Role of Sphingolipid Metabolism in Bone Remodeling
- State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Engineering Research Center of Oral Translational Medicine, Ministry of Education, National Engineering Laboratory for Oral Regenerative Medicine, West China Hospital of Stomatology, West China School of Public Health, West China Fourth Hospital, Sichuan University, Chengdu, China
Emerging studies of bioactive lipids have made many exciting discoveries in recent years. Sphingolipids and their metabolites perform a wide variety of cellular functions beyond energy metabolism. Emerging evidence based on genetically manipulated mouse models and molecular biology allows us to obtain new insights into the role sphingolipid played on skeletal remodeling. This review summarizes studies or understandings of the crosstalk between sphingomyelin, ceramide, and sphingosine-1-phosphate (S1P) of sphingolipids family and the cells, especially osteoblasts and osteoclasts of the bone through which bone is remodeled during life constantly. This review also shows agonists and antagonists of S1P as possible therapeutic options and opportunities on bone diseases.
Background
Bone is an important tissue to provide biomechanical and structural supports to the body (Lee and Karsenty, 2008). Besides, it is a dynamic organ that undergoes bone formation and resorption. Bone is formed through two forms: endochondral and intramembranous ossification (Soltanoff et al., 2009), formation of which begins when mesenchymal cells adhere, and the osteogenesis can be made through the transformation of the pre-existing mesenchymal cells into bone tissue or sometimes by the replacement of the cartilage by bone (Zaidi, 2007). Then bone remodeling takes place after bone formation and development and continues during the whole lifetime (Kronenberg, 2003; Roberts et al., 2015; Serra-Vinardell et al., 2020). Development and lifelong remodeling of the bone involve some major bone-related cells, such as osteoblasts (Matsuo and Irie, 2008), osteoclast precursors, osteoclasts, osteocytes, bone lining cells, bone marrow stem cells (Lee et al., 2017), adipocytes, fibroblasts, immune cells (Arron and Choi, 2000; Pacifici, 2010, 2013; Rauner et al., 2013; Purdue et al., 2014; Pietschmann et al., 2016), and non-osteogenic cell populations by the blood supply. Their proliferation, differentiation, death, and dynamic balance determine the shape and function of bone. Besides, some other cellular systems, including cartilage, also play important roles. Among these cells, osteoblasts and osteoclasts play a critical role in bone remodeling. The maintenance of bone size, shape, integrity, and function of bone depends on the exquisite balance between osteoblasts and osteoclasts (Huang et al., 2007; Soltanoff et al., 2009; Matsuoka et al., 2014). Mainly osteoclasts are responsible for bone resorption, and osteoblasts are responsible for new bone formation. The imbalance can result in abnormal bone architecture or function; therefore, bone metabolism diseases will occur, such as osteoporosis and osteopetrosis (Zaidi, 2007). But osteoblasts and osteoclasts are closely connected through cytokine, cell-bone matrix contact, or direct cell-cell contact. Besides, the communication between osteoblasts and osteoclasts happens at various stages of bone remodeling (Matsuo and Irie, 2008). Lipid is an important nutrient of the body by offering energy, essential fatty acids (FAs), and other derivatives and influences many cell types, cell functions, and signaling pathways. Lipid could also be divided into eight categories, including fatty acyls, glycerolipid, glycerophospholipid, sphingolipid, sterol lipid, prenol lipid, saccharolipid, and polyketide. Each of them contains distinct classes and subclasses of molecules (Fahy et al., 2005; Liebisch et al., 2020). Lipid metabolism that is a complex process associated with biosynthesis and degradation controls the level of lipid. Lipid metabolism also involves the hydrolysis of lipid (Mu and Porsgaard, 2005) and then its hydrolysis product is absorbed, packaged, and transported to the rest of the cells or tissues.
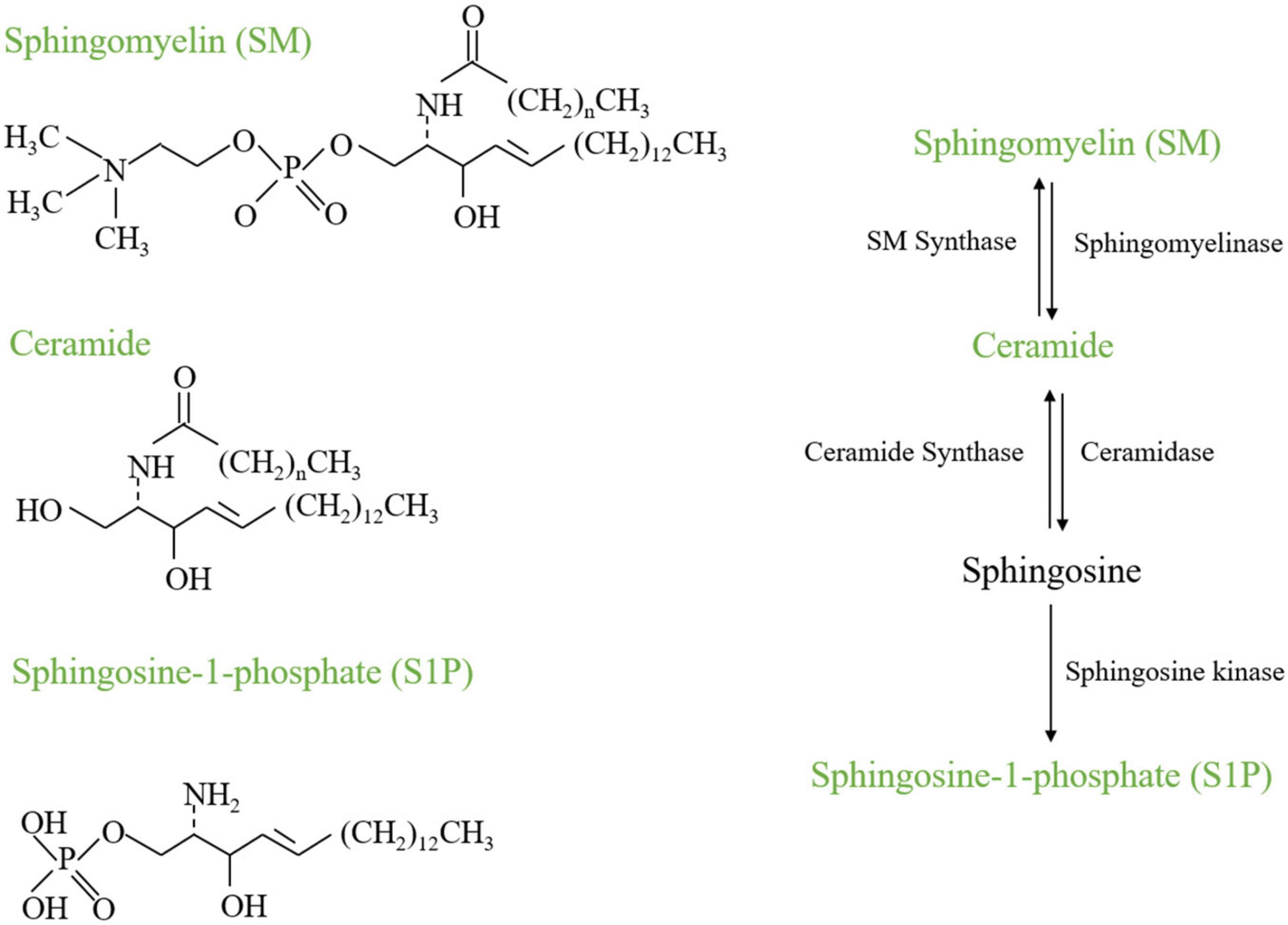
Lipids play an important role in bone remodeling and bone disease. The study about the relationship between lipid metabolism and biomineralization was early in 1963 (Irving, 1963). The presence of lipid within a porous compartment of cortical bone restricts radial permeability, possibly influencing the metabolic functions of osteoblasts and osteocytes (Wen et al., 2010). Recently, a study found the obstruction of vascular invasion during bone healing tends to chondrogenic rather than osteogenic differentiation of skeletal progenitor cells, due to a decreased availability of extracellular lipids. Thus, lipid availability has been found to determine the fate of skeletal progenitor cells (van Gastel et al., 2020). It highlights that lipid plays a crucial role in a signal pathway, cell types, and functions in bone biology, and suggests its significance in bone pathology. Indeed, emerging data suggest the sphingolipid metabolism plays a critical role in skeletogenesis. Recent studies indicate the multifaceted influences of the sphingolipid on osteoblasts, osteoclasts, and the pivotal interaction underlying bone homeostasis-osteoblast and osteoclast crosstalk and highlight the multifaced roles of sphingolipid metabolism on bone remodeling. Hence, this review will focus on sphingolipid metabolism that regulates bone development and remodeling, involving the representative Sphingomyelin, Ceramide, and sphingosine 1-phosphate (Figure 1), teasing out their roles in the crosstalk of osteoblasts and osteoclasts.
Sphingolipids Metabolism
Sphingolipids carry a long-chain sphingoid base with the 2-amino group amide-linked to a fatty acid, which forms ceramide, the core unit. Then different types of sphingolipids are formed by polar head groups added. The family of sphingolipids is defined by characters of the fatty acid, including carbon length, degree of unsaturation, and hydroxylation, along with other modifications of the LCBs and the polar head group (Hannun and Obeid, 2008; Teixeira and Costa, 2016). The sphingolipid metabolism shares a similar spatial organization which is highly conserved. It is governed by an integrated network of common synthetic and catabolic pathways that are modulated in response to different stimuli despite the diversity of sphingolipids (Hannun and Obeid, 2008; Teixeira and Costa, 2016).
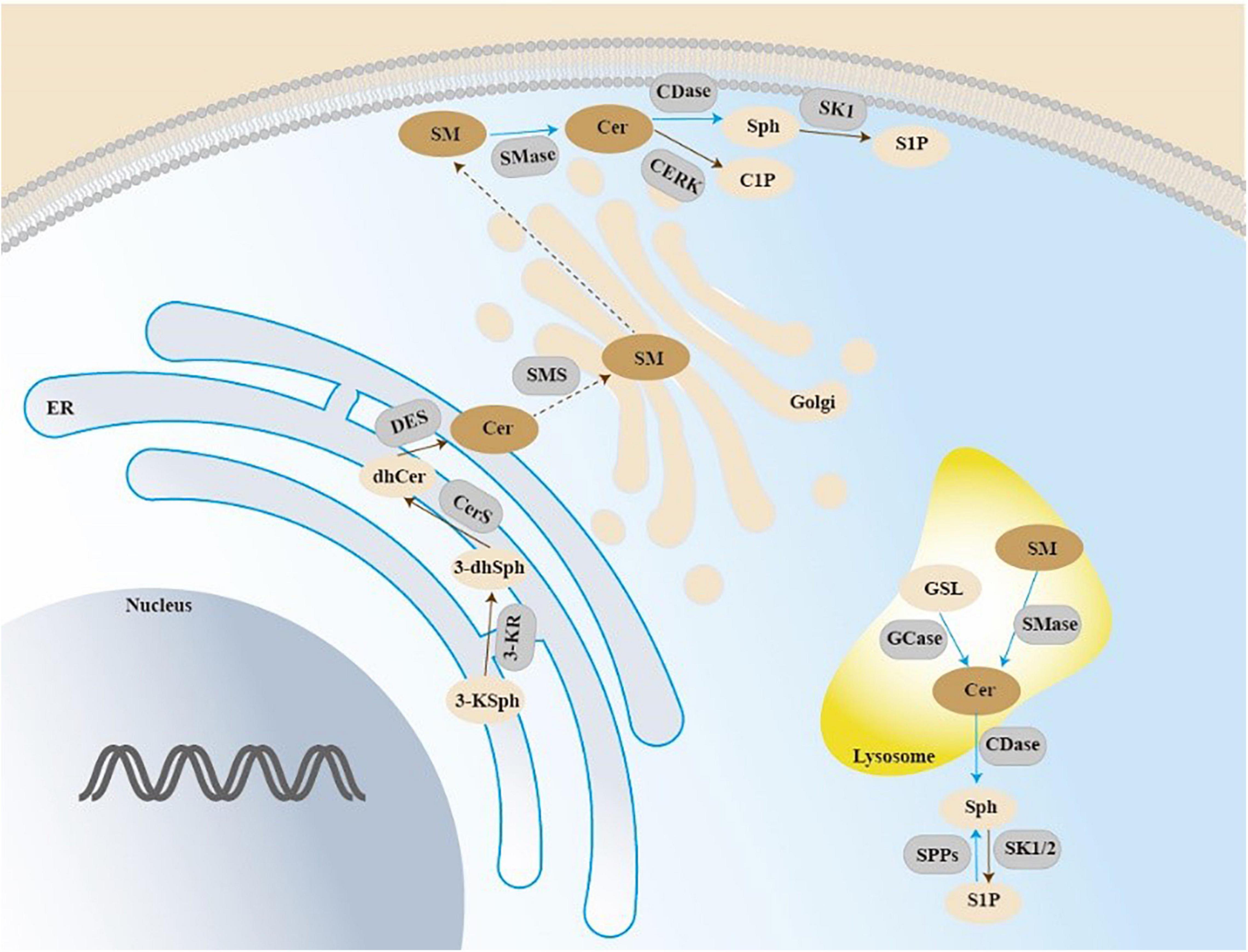
Sphingolipid biosynthesis de novo taking place in the endoplasmic reticulum (ER) involves a condensation reaction of serine and palmitoyl-CoA to produce 3-ketodihydrosphingosine catalyzed by serine palmitoyltransferase (SPT) (Merrill, 2002; Teixeira and Costa, 2016). 3-ketodihydrosphingosine generation is converted to the dihydroxyceramide (DHS) by ketodihydrosphingosine reductase (Kihara and Igarashi, 2004). DHS is acylated to dihydroceramides catalyzed by ceramide synthase (CERS1-6). Then dihydroceramide is catalyzed by dihydroceramide desaturases DES1 and DES2 to generate ceramide (Ternes et al., 2002). Ceramide serves as a substrate for enzymes that produce sphingolipids, sphingomyelin included. The ceramide is transported to the Golgi compartment by transfer protein CERT or vesicular, and then converted into sphingomyelin by sphingomyelin synthases (SMS). There are two isoforms of SMS, which is SMS1 and SMS2. SMS1 is only located in the Golgi apparatus while SMS2 is located in both the Golgi apparatus and the plasma membrane. These enzymes have been classified into three categories—acidic, alkaline, and neutral sphingomyelinases (Stoffel, 1999). The hydrolysis of ceramide generates sphingosine, which can generate sphingosine-1-phosphate (S1P) (Figure 2). The current review will discuss the role of some important sphingolipids in bone remodeling.
De novo sphingolipid biosynthesis begins at the endoplasmic reticulum (ER) with the condensation reaction of serine and palmitoyl-CoA forming 3-ketosphingosine. 3-ketodihydrosphingosine generation is converted to the dihydroxyceramide, and then acylated to dihydroceramides by ceramide synthase (CerS 1-6). Dihydroxyceramide is dehydrated between carbons 4 and 5 by dihydroceramide desaturase (DES) to form ceramide, and then be translocated to the Golgi. The action of SMS on ceramide results in the production of sphingomyelin (SM). Acid sphingomyelinase (SMase) is an enzyme converting sphingomyelin into ceramide. GSL can be transported intracellularly in the lysosome to generate ceramide. Ceramide can be generated through degradation of SM in the lysosome or at the plasma membrane by SMase. Then hydrolysis of ceramide by ceramidase generates sphingosine, which can be phosphorylated and generate sphingosine-1-phosphate (S1P).
Sphingomyelin and Bone Remodeling
Sphingomyelin exists in diverse species, from protozoa to mammals. It is the major component of the double membrane-bound sphingolipids, which is generated from ceramide and phosphatidylcholine by sphingomyelin synthase and is implicated in cell survival, proliferation, migration, and inflammation (Tafesse et al., 2006; Taniguchi and Okazaki, 2014). However, its bioactive function mainly relies on its hydrolysis and downstream lipids, including ceramide and S1P. SM is essential for bone formation and normal mineralization. The abnormity of SM could cause defective bone mineralization, including osteoporosis, severe short stature, neonatal fractures, osteogenesis imperfecta, spondylometaphyseal dysplasia, severe bone and tooth mineralization defects, and gross skeletal abnormalities.
(1) Sphingomyelin and bone formation
Sphingomyelin is essential for bone formation. The local SM catabolism is found to be essential for the mineralization process in healthy bones. Catalyzing SM hydrolysis forms phosphocholine and ceramide, which are highly expressed in bone and are required for normal mineralization (During et al., 2015). Mutations in the sphingomyelin synthase 2 gene (SGMS2) could cause defective bone mineralization (Pekkinen et al., 2019). Patients with skeletal phenotypes and osteoporosis were identified with mutations in the SGMS2 gene encoding for the SMS2, with some sharing the same nonsense variant to yield a catalytically inactive enzyme and presenting with childhood-onset osteoporosis. In addition, others had a missense variant to enhance the rate of sphingomyelin production by blocking the export of a functional enzyme from the endoplasmic reticulum, with severe short stature, neonatal fractures, and spondylometaphyseal dysplasia. A recent case reported a family with moderately severe bone fragility and multiple sclerotic skull lesions similar to the osteogenesis imperfecta mentioned above; however, no pathogenic variant was found in SGMS2 (Makitie et al., 2021). Knocking down the SMS2 suppressed TRAP-positive multinucleated cells’ formation through co-culture of bone marrow cells and osteoblasts, which indicated that knockdown of SMS2 inhibits osteoclastogenesis through decreasing RANKL expression in primary osteoblasts of mice (Yoshikawa et al., 2019). In addition, the recent study found that based on metabolomic analysis, giant cell tumor of bone (GCTB), SM was checked as the most dysregulated phospholipid in GCTB, with high expression of SMS1 and SMS2, and low expression of nSMase2 (Quiroz-Acosta et al., 2021).
(2) Sphingomyelin and bone resorption
The excessive accumulation or catabolism of SM seemed to be linked to bone resorption. SMPD3 encodes neutral sphingomyelinase 2, the expression of which is restricted to the cartilage, bone, and brain (Khavandgar et al., 2011). Currently, there are two established SMPD3-deficient mouse models fro/fro model and SMPD3-/- model (Aubin et al., 2005; Stoffel et al., 2005). Both the two SMPD3-deficient mouse models show severe bone and tooth mineralization defects, and gross skeletal abnormalities. The fro mutation completely abolishes the enzymatic activity without affecting the location of SMPD3, thus reduction of nSMase activity in skeletal tissues marked by abnormal bone mineralization defects with high expression of SMPD3 is found in fro/fro mice (Khavandgar et al., 2011). Fro/fro mice also showed delayed mantle dentin mineralization and a consequent delay in enamel formation, but these tooth abnormalities progressively improved with time (Khavandgar et al., 2013). So far, no abnormalities of bone in mice lacking SMPD1 or SMPD2 activity have been reported. All of these revealed the excessive accumulation of SM properly could be tightly associated with bone defects. On the other side, although there is an increase of SM in bone marrow while there is a significant reduction of SM in the mineralized tissue part of OVX rat femurs (During et al., 2020), which suggested that excessive catabolism of SM could be associated with bone resorption. But more investigations will be needed regarding the potential role of SM in bone and the underlying mechanism.
Ceramide and Bone Remodeling
Ceramide can arise from the endoplasmic reticulum by de novo synthesis (Hirschberg et al., 1993) and it can also be generated from the hydrolysis of SM by sphingomyelinases either at the plasma membrane or in endosomes or lysosomes. Ceramide is a bioactive lipid that serves as a second messenger in the regulation of cell death pathways and metabolism in response to stress, apoptotic triggers, and chemotherapy (Ryland et al., 2011; Kurek et al., 2013), involving extrinsic mechanism by mimicking the cytotoxicity of TNF (Hannun, 1994), and an intrinsic mechanism by modifying enzymes to regulate the level of ceramide (Morales et al., 2007), further leading to signal cascade and cell death by the downstream of Bcl2. However, S1P opposes the proapoptotic function of ceramide (Rutherford et al., 2013) and the ratio of S1P and ceramide is described as a rheostat of sphingolipid which is involved in the pathogenesis of certain cancers and this rheostat is one of the targets of anticancer drugs (Dyatlovitskaya et al., 2001). Ceramide plays an important role in bone metabolism. The abnormity of ceramide could cause osteoblast metabolic disorder and dysfunction, and thus influence the bone formation, and some special ceramides (C16:0, C18:0, C18:1, and C24:1) are correlated with bone resorption markers.
(1) Ceramide and bone formation
The alteration in the intracellular levels of ceramide could play a vital role in bone formation. C2-ceramide is reported to promote osteoblast viability, while high concentration (≥2 × 10–6M) reduces osteoblast viability. Increasing intracellular levels of ceramide also increase osteoblast apoptosis, determined by nuclear appearance and DNA fragmentation (Hill and Tumber, 2010). Endogenous cellular ceramide concentrations increase after TNF-α treatment, while the apoptosis of osteoblasts is triggered by TNF-α-generated ceramide by activating NF-κB signaling pathway. In addition, reducing the production of ceramide by dexamethasone inhibits TNF-α-induced activation of NF-κB and apoptosis in osteoblasts (Chae et al., 2000). Some external or internal stimuli impair the viability or physiological function of osteoblast through ceramide accumulation. Sodium nitroprusside enhances the release of intracellular ceramides C22 and C24 to decrease osteoblast viability (Olivier et al., 2005). Elevated palmitic acid intake significantly increases C16 ceramide accumulation and thus reduces osteoblast function in vitro and bone formation markers in vivo (Alsahli et al., 2016). In obese mice with palmitic acid or oleic acid-enriched high fat diet, ceramide accumulation in osteoblasts and suppresses bone formation (Alsahli et al., 2016). The influence of ceramide on osteoblast is in a dose- and time-dependent manner, and increasing levels of intracellular ceramide with either an inhibitor of ceramide metabolism or sphingomyelinase increased osteoblast apoptosis (Hill and Tumber, 2010).
(2) Ceramide and bone resorption
The role of ceramide in apoptosis is studied extensively, and recently ceramide is reported to be involved in bone cell survival, cell death, and bone resorption. However, at present, few experimental data directly link it with the mineralization of skeletal tissue. DES1 is one of the enzymes that form ceramide through the de novo pathway. The DES1 null mice show a normal skeletal structure, although they have multiple physiologic anomalies such as weight loss and growth impairment (Holland et al., 2007). The C24:1 ceramide in serum extracellular vesicles increases with age and could induce senescence in human bone marrow stromal cells (BMSCs) (Khayrullin et al., 2019). In patients 65 years or older with hip surgery, age was correlated with circulating levels of C16:0, C18:0, and C24:1 ceramide positively. Higher levels of C16:0, C18:0, C18:1, and C24:1 ceramide were positively related to bone resorption markers in both blood and bone marrow samples. C18:0 and C24:1 ceramide directly increased osteoclastogenesis in vitro (Kim et al., 2019). In support, the Postmenopausal Osteoporosis Mouse model found a significant reduction of three to five SM species and increased six metabolites, of which five were ceramide species (Zhao et al., 2018).
Sphingosine-1-Phosphate and Bone Remodeling
Sphingosine-1-phosphate (S1P) is a natural lipid molecule that is formed by the phosphorylation of sphingosine and is derived from cell membrane sphingolipid (Spiegel and Milstien, 2002, 2003) as the product of sphingosine kinase(SK)1 and/or 2-mediated phosphorylation of sphingosine. In addition, S1P can either be converted back to sphingosine by specific S1P phosphatases or degraded by S1P lyase to form hexadecenal and phosphoethanolamine (Pebay et al., 2007). S1P is a common first or second messenger and serves as a mediator in regulating cell migration, death (Olivera and Spiegel, 1993), proliferation (Zhang et al., 1991; Cuvillier et al., 1996), and apoptosis (Cuvillier et al., 1996). Furthermore, it is involved in cell adhesion, cell motility, smooth muscle contraction, and platelet aggregation (Takuwa, 2002). It has been acquired in extensive study in cardiovascular, nervous, and immune systems, and its role in promoting angiogenesis is well-established (Alvarez et al., 2007). S1P acts either directly on intracellular targets or combines its known surface G-protein-coupled receptors S1P1–5 as a common second messenger. The binding of S1P to these receptors induces differential signaling pathway, and sometimes overlapping. S1P and its S1P1–5 receptors are expressed in variable systems, including vascular, immune, nervous, and reproductive systems (Hla, 2004). In recent years, S1P is implicated in osteogenesis-related processes, such as cell recruitment, cell differentiation, osteoblast survival, and coupling with osteoclasts.
S1P is important for osteoblast survival and the migration of osteoblasts and osteoclasts. The abnormity of S1P could cause osteopenia, reduced bone formation, and rheumatoid arthritis.
Sphingosine-1-Phosphate and Bone Formation
Homing
Inhibiting S1P degradation or downregulate S1P1 receptors to dissipate the gradient between blood and bone marrow can reduce the number of circulating progenitor cells (Bendall and Basnett, 2013). The fractures in bone lead to increased S1P levels and hematopoietic stem cell migration (Golan et al., 2013). Various agents during mobilization are closely associated with the bone remodeling (Mendez-Ferrer et al., 2008), CXCR4 antagonist AMD3100 (Broxmeyer et al., 2005; Pusic and DiPersio, 2010) included. Using S1P lyase inhibitor to increase the bone marrow S1P concentration is shown to attenuate AMD3100-mediated progenitor cells mobilization in mice (Ratajczak et al., 2010; Golan et al., 2012; Juarez et al., 2012). Mice lacking SPK1 have impaired AMD3100-mediated progenitor cell mobilization, and besides, suppression of S1P1 receptor inhibits AMD3100-mediated progenitor cell mobilization (Golan et al., 2012; Juarez et al., 2012).
S1P stimulates mesenchymal (skeletal) cell chemotaxis by activating JAK/STATs and FAK/PI3K/AKT signaling pathways through S1P1 and S1P2 coordinately (Quint et al., 2013). S1P could be produced by osteoclast precursors during differentiation, and it enhances osteoblast survival in serum-deprived conditions (Ryu et al., 2006). In addition, it is a chemorepellent for pre-osteoblasts (Roelofsen et al., 2008), and increased osteoblast chemotaxis at the range of 0.01–1 μM.
Differentiation
As S1P could affect the migration of osteoblasts, additional efforts are made to know the role of S1P on the proliferation and differentiation of osteoblasts. S1P can act as an osteoanabolic molecule (Keller et al., 2014). The data from 4091 participants of the SHIP-Trend population-based study reveals a positive between serum levels of S1P, bone formation markers, and serum calcium, but not resorption markers. S1P participates in the proliferative process in human osteoblasts via MAP kinase activation (Carpio et al., 1999), and S1P-driven human osteoblast proliferation is predominantly linked to PKCα isoform (Lampasso et al., 2002).
For animals, in mice increasing S1P levels by conditionally deleting or inhibiting S1P lyase could increase bone formation, bone mass, and bone strength, and interestingly decreased white adipose tissue (Weske et al., 2018). It has been identified S1P receptors were in the key cells involved in bone remodeling, as S1P1–3 receptors are expressed in osteoblasts but S1P4–5 failed to be detected in primary osteoblasts (Grey et al., 2004), or much lower S1P4 receptor mRNA level and no detectable S1P5 receptor mRNA in osteoblasts (Keller et al., 2014). Besides, S1P1 and S1P3 receptors are increasing at the early stage of osteoblastogenesis. The Sgpl1–/– mice, which lack the S1P lyase (Vogel et al., 2009; Liu et al., 2012), display high extracellular S1P levels and it causes various organ abnormalities, one of which the trabecular bone mass is remarkably increased at the age of 6 weeks. A study found that the S1P1-deficient mice died in utero (Allende et al., 2003), but S1P3-deficient mice do not display obvious abnormalities (Ishii et al., 2001). The S1P3-deficient mice at 3 months of age have no difference compared with wild mice, while the 8-month-old S1P3-deficient mice displayed osteopenia, reduced bone formation, and unaffected bone resorption parameters (Keller et al., 2014). But the bone mass or bone remodeling parameters are not an alteration in mice lacking S1P1 receptor specifically in osteoblasts both at 3 and 8 months. S1P1 and S1P3 are the candidate receptors controlling bone formation in response to S1P.
In primary rat osteoblasts, S1P is a potent osteoblast mitogen and the proliferative action of S1P is involved Gi protein, intracellular calcium, and p42/p44 MAP kinases (Grey et al., 2004). In C2C12 myoblasts, S1P receptor-mediated signaling plays a vital role in osteoblast differentiation by MEK1/2-ERK1/2 signaling pathway enhanced BMP-2-Smad signaling (Sato et al., 2012). In SaOS-2 and MC3T3-E1, two osteoblast-like cell lines, S1P activates the PI3K/Akt signaling pathway to the promotion of nuclear translocation of β-catenin in osteoblast-like cells, and upregulates osteoprotegerin and osteoblast differentiation markers (Matsuzaki et al., 2013). SphK1 is expressed in human and mouse osteoblastic cells, which secrete a large amount of S1P, and the process is accompanied by decreased levels of S1P1 and S1P2, but increased levels of S1P3. The autocrine S1P/S1P3 signaling is a core signaling pathway during differentiation to mature osteoblasts by regulating runx2, which plays a key role in transcription factor associated with osteoblast differentiation and osteoblastic maturation (Brizuela et al., 2014). S1P significantly increases matrix mineralization in wild-type mice and a rapid phosphorylation Erk1/2, while both are not detected in S1P3 receptor-deficient mice during osteogenesis. In addition, during the process, S1P negatively regulated S1P3 receptor in wild-type cultures.
Sphingosine-1-Phosphate and Bone Resorption
Homing
Some causes of bone disease depend on the recruitment of osteoclasts into resorption sites or due to the migration of inflammatory cells by chemokine gradients. S1P promotes the entry of osteoclast precursors into the bone from blood and promotes osteoclast differentiation (Ishii et al., 2009, 2010). OP-positive chemotaxis is prominent in gradients with low maximal concentrations of S1P and with high maximal S1P concentrations, Cells with properties of osteoclast precursors express S1P1 receptors, and using S1P1 agonist SEW2871 can stimulate motility of osteoclast precursor-containing monocytoid populations. In addition, OC/monocyte (CD11b) lineage-specific conditional S1P1 receptor knockout mice increased osteoclasts attaching to the bone surface to make bone osteoporosis changes (Ishii et al., 2009), and Gi and Rac are involved in S1P1 receptor-mediated chemoattraction. While osteoclast precursors also express S1P2, which mediates negative chemotaxis of osteoclast precursors. The S1P2-mediated chemorepulsion overrides S1P1 upgradient motion. S1P2 inhibited the chemotaxis of BMMs by treatment with S1P2 siRNA. The combined results indicate that cell migration controlled by S1P relies on the gradient between tissues, the doses of S1P, or the balance between S1P and other chemokines.
Differentiation
Bone loss in many diseases, including osteoporosis, rheumatoid arthritis etc. (Redlich and Smolen, 2012), is featured with proinflammatory cytokines, and RANKL is the most important molecule among them to regulate OC differentiation. In addition, inflammatory conditions are always associated with high levels of S1P (Lee et al., 2012). It shows that postmenopausal women had higher S1P plasma levels, and positively correlated with low bone mineral density, compared to premenopausal women and men (Lee et al., 2012). The SK1 deficiency in mice alleviated periodontal alveolar bone loss, and S1P dose-dependently increased chemotaxis of murine bone marrow-derived monocytes. It is also shown a significantly higher level of S1P in synovial fluid of patients with rheumatoid arthritis (Lai et al., 2012). In addition, SK1 deficiency in mice decreased inflammation and joint erosions in murine arthritis (Baker et al., 2010).
S1P is a coupling factor between osteoclasts and osteoblasts. It impacts OC precursor differentiation by regulating RANKL or its downstream signaling pathway. Increased S1P production and secretion and upregulated SPHK1 expression are observed in a bone marrow-derived macrophage model system by RANKL stimulation. The osteoclastogenesis is greatly increased by adding S1P to BMM/osteoblast co-culture system, indicating that S1P affects the osteoclastogenesis (Ryu et al., 2006). One of the important mechanisms for bone resorption is that osteoclast-secreted S1P increases RANKL. However, deletion of cathepsin k in osteoclasts, which is secreted by osteoclasts to degrade collagen and other matrix proteins, increased the SPHK1 expression, and conditioned media from cathepsin k-deficient osteoclasts, in which the levels of S1P elevated, increased alkaline phosphatase and mineralized nodules in osteoblast culture (Lotinun et al., 2013). Sphingosylphosphorylcholine (SPC), a biological lipid that can be converted to S1P by autotaxin and share receptors with S1P, is reported to inhibit RANKL-induced osteoclast differentiation. But SPC-induced inhibitory effects are not altered by several antagonists of S1P receptors (Lee et al., 2021), suggesting the independence of S1P and SPC on surface receptors, and thus denying the speculation of receptor competition.
S1P1–2 receptors are detected in osteoclast precursor cells and mRNA for all S1P receptors except S1P5 in bone marrow-derived macrophages and differentiating osteoclasts (Ryu et al., 2006; Keller et al., 2014). S1P2 seems closely linked to osteoclasts. S1P2 played an important role in regulating proinflammatory cytokine release induced by the oral bacterial pathogen Aa. shRNA of S1P2 reduced IL-1β, IL-6, and TNF-α levels in BMMs induced by Aa. In addition, knockdown of S1P2 suppressed p-PI3K, p-ERK, p-JNK, p-p38 MAPK, and p-NF-κBp65 levels induced by Aa. Furthermore, knockdown of S1P2 significantly suppressed factors associated with osteoclast formation/activity, including the nuclear factor of activated T-cells cytoplasmic calcineurin-dependent 1(Nfatc 1), acid phosphatase 5 (Acp5), cathepsin K(Ctsk), osteoclast-associated receptor (Oscar) (Yu, 2016). S1P2-deficient mice exhibit moderate osteopetrosis because of a decrease in osteoclastic bone resorption. Using S1P2 antagonist JTE013 can change the migration of osteoclast precursors and relieved osteoporosis in a mice model by limiting op localization and reduced osteoclasts (Ishii et al., 2010). However, another study showed S1P2-deficient mice were osteopenic and obese. S1P signaling through S1P2 potently stimulated osteoblastogenesis by inversely regulating osterix and PPAR-γ at the expense of adipogenesis, and simultaneously the osteoclastogenesis is inhibited through p38-GSK3β-β-catenin and WNT5a-LRP5 pathway (Weske et al., 2018).
Treatment of Bone Disease by Targeting Sphingomyelin and Downstream Pathway
The appropriate level of sphingomyelin without excessive anabolism or catabolism plays an essential role in bone remodeling and prevents bone diseases. Adjusting the level of sphingomyelin in bone is the targeting treatment by activating the SMS or SMase. Sphingomyelin performed its function by its hydrolysis. While it lacks adequate studies of Ceramide, more research about S1P on bone disease is needed, thus introducing some possible treatments about S1P on bone diseases.
Agonists
FTY720 is a mimetic of natural sphingosine and therefore can be recognized by part of the cellular sphingosine enzymatic machinery (Zemann et al., 2006; Mechtcheriakova et al., 2007). It is suggested that FT720 is a prodrug and FTY720-P is its phosphorylation by SphKs, most efficiently by SphK2, can act as a mimetic of S1P as an S1P receptor agonist (Billich et al., 2003) and specifically binds to four out of five S1P receptors, except S1P2 (Brinkmann et al., 2002; Spiegel and Milstien, 2011). It is described that daily injection of the nonselective S1P receptor agonist FTY720 protects against ovariectomy-induced bone loss (Ishii et al., 2009). In addition, the trabecular bone volume is increased in wild-type mice treatment with FTY720 daily, whereas S1P3-deficient mice do not respond (Keller et al., 2014). Although FTY720-P is an agonist of S1P1/3/4/5, its effects are inhibitory on S1P receptor function in the longer term. The mechanism of its antagonism function is suggested to be associated with receptor internalization and in part is based on the ability to target the S1P1 receptor to the proteasomal degradation pathway through poly-ubiquitination (Oo et al., 2007). Treatment with FTY720 relieved ovariectomy-induced osteoporosis by facilitating recirculation of osteoclast precursor-containing cell populations and reducing the number of mature osteoclasts attached to the bone in mice (Ishii et al., 2009). KPR-203 has a structural similarity with FTY720. KPR203 and FTY720 both have a similar high affinity for the S1P1 receptor. To date, the effect of KRP-203 on bone remodeling has not yet been reported. SEW2871 is an S1P1 receptor-selective agonist, and not active for the S1P2–5 receptors unlike FTY720 of a nonselective S1P receptor agonist. SEW2871 can induce the recruitment of macrophages (Lien et al., 2006; Takabe et al., 2008), and the hydrogels incorporating mixed SEW2871 and PRP promoted bone regeneration to a great extent, which suggests macrophage recruitment contributed to PRP-induced bone regeneration (Kim et al., 2014). Cells with the properties of osteoclast precursors express S1P1 receptors and exhibit positive chemotaxis along an S1P gradient in vitro. In addition, intravital two-photon imaging of bone tissues showed that SEW2871 stimulated motility of osteoclast precursor-containing monocytoid populations in vivo. Osteoclast/monocyte lineage-specific conditional S1P1 knockout mice showed osteoporotic changes due to increased osteoclast attachment to the bone surface (Ishii et al., 2009).
Antagonist
VPC23019 is an unselective S1P1 and S1P3 antagonist. Targeted ablation of cathepsin K which is secreted by osteoclasts to degrade collagen and other matrix proteins during bone resorption, in hematopoietic cells, and specifically in osteoclasts and cells of monocyte-osteoclast lineage causes increased bone volume and bone formation rate. In contrast, the targeted deletion of cathepsin K in osteoblasts did not get those results. The deletion of cathepsin K in osteoclasts increases SK1 expression. Conditioned media from cathepsin K-deficient osteoclasts with elevated levels of S1P increased alkaline phosphatase and mineralized nodules in osteoblast culture with an increased RANKL/OPG ratio. However, VPC 23019 inhibited these process (Lotinun et al., 2013). JTE013, a specific and most used competitive S1P2 receptor antagonist, was synthesized at the Central Pharmaceutical Institute in Japan in 2001 (Takabe et al., 2008). It was shown to antagonize the binding of radiolabeled S1P in Chinese hamster ovary cells overexpressing S1P2 receptor (Pyne and Pyne, 2011). JTE013 suppresses PI3K, MAPKs, and NF-κB and inhibits the release of IL-1β,IL-6,TNF-α, and S1P in murine bone marrow cells. In addition, JTE013 suppressed osteoclastogenesis and bone resorption through changing monocyte migration behavior induced by RANKL in murine bone marrow cultures (Hsu et al., 2019).
Conclusion
Although the published studies have displayed the critical role of sphingomyelin metabolism in osteoblasts, osteoclasts, and bone remodeling, the studies on the mechanism are still few. The sphingomyelin is essential in bone formation, but the excessive accumulation or catabolism of SM properly could be tightly associated with bone resorption. At present, limited direct evidence is available on the roles of sphingomyelin in osteoblasts and osteoclasts, and further, the enzymes of sphingomyelin are little studied in the bone tissue. As for Ceramide, despite the pro-apoptosis function in osteoblasts, the links of the differentiation of osteoblasts to ceramide are still unknown. In addition, recently, osteoclast can be mediated by ceramide. The comparatively sufficient studies of S1P on bone remodeling allow us to further study the treatment of associated bone diseases, although the role of S1P is complicated and depending on the different receptors of S1P1–5.
Author Contributions
TW and LL conceived of the presented idea. TQ finished the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2017YFA0104800), China Postdoctoral Science Foundation (2019M653434), and the Nature Science Foundation of China (82071092). The Fundamental Research Funds for the Central Universities (2021SCU12140).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allende, M. L., Yamashita, T., and Proia, R. L. G. (2003). Protein-coupled receptor S1p1 acts within endothelial cells to regulate vascular maturation. Blood 102, 3665–3667. doi: 10.1182/blood-2003-02-0460
Alsahli, A., Kiefhaber, K., Gold, T., Muluke, M., Jiang, H., Cremers, S., et al. (2016). Palmitic acid reduces circulating bone formation markers in obese animals and impairs osteoblast activity Via C16-ceramide accumulation. Calcif. Tissue Int. 98, 511–519. doi: 10.1007/s00223-015-0097-z
Alvarez, S. E., Milstien, S., and Spiegel, S. (2007). Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 18, 300–307. doi: 10.1016/j.tem.2007.07.005
Arron, J. R., and Choi, Y. (2000). Bone versus immune system. Nature 408, 535–536. doi: 10.1038/35046196
Aubin, I., Adams, C. P., Opsahl, S., Septier, D., Bishop, C. E., Auge, N., et al. (2005). Deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat. Genet. 37, 803–805. doi: 10.1038/ng1603
Baker, D. A., Barth, J., Chang, R., Obeid, L. M., and Gilkeson, G. S. (2010). Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine tnf-alpha-induced arthritis. J. Immunol. 185, 2570–2579. doi: 10.4049/jimmunol.1000644
Bendall, L. J., and Basnett, J. (2013). Role of sphingosine 1-phosphate in trafficking and mobilization of hematopoietic stem cells. Curr. Opin. Hematol. 20, 281–288. doi: 10.1097/moh.0b013e3283606090
Billich, A., Bornancin, F., Devay, P., Mechtcheriakova, D., Urtz, N., and Baumruker, T. (2003). Phosphorylation of the immunomodulatory drug Fty720 by sphingosine kinases. J. Biol. Chem. 278, 47408–47415. doi: 10.1074/jbc.m307687200
Brinkmann, V., Davis, M. D., Heise, C. E., Albert, R., Cottens, S., Hof, R., et al. (2002). The immune modulator fty720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277, 21453–21457. doi: 10.1074/jbc.c200176200
Brizuela, L., Martin, C., Jeannot, P., Ader, I., Gstalder, C., Andrieu, G., et al. (2014). Osteoblast-derived sphingosine 1-phosphate to induce proliferation and confer resistance to therapeutics to bone metastasis-derived prostate cancer cells. Mol. Oncol. 8, 1181–1195. doi: 10.1016/j.molonc.2014.04.001
Broxmeyer, H. E., Orschell, C. M., Clapp, D. W., Hangoc, G., Cooper, S., Plett, P. A., et al. (2005). Rapid mobilization of murine and human hematopoietic stem and progenitor cells with amd3100, a cxcr4 antagonist. J. Exp. Med. 201, 1307–1318. doi: 10.1084/jem.20041385
Carpio, L. C., Stephan, E., Kamer, A., and Dziak, R. (1999). Sphingolipids stimulate cell growth via map kinase activation in osteoblastic cells. Prostaglandins Leukot. Essent. Fatty Acids 61, 267–273. doi: 10.1054/plef.1999.0100
Chae, H. J., Chae, S. W., Kang, J. S., Bang, B. G., Cho, S. B., Park, R. K., et al. (2000). Dexamethasone suppresses tumor necrosis factor-alpha-induced apoptosis in osteoblasts: possible role for ceramide. Endocrinology 141, 2904–2913. doi: 10.1210/endo.141.8.7604
Cuvillier, O., Pirianov, G., Kleuser, B., Vanek, P. G., Coso, O. A., Gutkind, S., et al. (1996). Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381, 800–803. doi: 10.1038/381800a0
During, A., Coutel, X., Bertheaume, N., Penel, G., and Olejnik, C. (2020). Long term ovariectomy-induced osteoporosis is associated with high stearoyl-coa desaturase indexes in rat femur. Calcif. Tissue Int. 106, 315–324. doi: 10.1007/s00223-019-00637-7
During, A., Penel, G., and Hardouin, P. (2015). Understanding the local actions of lipids in bone physiology. Prog. Lipid Res. 59, 126–146. doi: 10.1016/j.plipres.2015.06.002
Dyatlovitskaya, E. V., Kandyba, A. G., Kozlov, A. M., and Somova, O. G. (2001). Sphinganine in sphingomyelins of tumors and mouse regenerating liver. Biochem. Biokhim. 66, 502–504. doi: 10.1023/a:1010250600604
Fahy, E., Subramaniam, S., Brown, H. A., Glass, C. K., Merrill, A. H. Jr., Murphy, R. C., et al. (2005). Comprehensive classification system for lipids. J. Lipid Res. 46, 839–861.
Golan, K., Kollet, O., and Lapidot, T. (2013). Dynamic cross talk between s1p and cxcl12 regulates hematopoietic stem cells migration, development and bone remodeling. Pharmaceuticals 6, 1145–1169. doi: 10.3390/ph6091145
Golan, K., Vagima, Y., Ludin, A., Itkin, T., Cohen-Gur, S., Kalinkovich, A., et al. (2012). S1p promotes murine progenitor cell egress and mobilization via s1p1-mediated ros signaling and sdf-1 release. Blood 119, 2478–2488. doi: 10.1182/blood-2011-06-358614
Grey, A., Xu, X., Hill, B., Watson, M., Callon, K., Reid, I. R., et al. (2004). Osteoblastic cells express phospholipid receptors and phosphatases and proliferate in response to sphingosine-1-phosphate. Calcif. Tissue Int. 74, 542–550. doi: 10.1007/s00223-003-0155-9
Hannun, Y. A. (1994). The sphingomyelin cycle and the second messenger function of ceramide. J. Biol. Chem. 269, 3125–3128. doi: 10.1016/s0021-9258(17)41834-5
Hannun, Y. A., and Obeid, L. M. (2008). Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150. doi: 10.1038/nrm2329
Hill, P. A., and Tumber, A. (2010). Ceramide-induced cell death/survival in murine osteoblasts. J. Endocrinol. 206, 225–233. doi: 10.1677/JOE-10-0068
Hirschberg, K., Rodger, J., and Futerman, A. H. (1993). The long-chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem. J. 290(Pt 3), 751–757. doi: 10.1042/bj2900751
Hla, T. (2004). Physiological and pathological actions of sphingosine 1-phosphate. Semin. Cell Dev. Biol. 15, 513–520. doi: 10.1016/j.semcdb.2004.05.002
Holland, W. L., Brozinick, J. T., Wang, L. P., Hawkins, E. D., Sargent, K. M., Liu, Y., et al. (2007). Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated- fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179. doi: 10.1016/j.cmet.2007.01.002
Hsu, L. C., Reddy, S. V., Yilmaz, O., and Yu, H. (2019). Sphingosine-1-phosphate receptor 2 controls podosome components induced by rankl affecting osteoclastogenesis and bone resorption. Cells 8:17. doi: 10.3390/cells8010017
Huang, W., Yang, S., Shao, J., and Li, Y. P. (2007). Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 12:3068–3092. doi: 10.2741/2296
Irving, J. T. (1963). Calcification of the organic matrix of enamel. Arch. Oral Biol. 8, 773–774. doi: 10.1016/0003-9969(63)90010-4
Ishii, I., Friedman, B., Ye, X., Kawamura, S., McGiffert, C., Contos, J. J., et al. (2001). Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its g protein-coupled receptor, lp(b3)/edg-3. J. Biol. Chem. 276, 33697–33704. doi: 10.1074/jbc.M104441200
Ishii, M., Egen, J. G., Klauschen, F., Meier-Schellersheim, M., Saeki, Y., Vacher, J., et al. (2009). Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458, 524–528. doi: 10.1038/nature07713
Ishii, M., Kikuta, J., Shimazu, Y., Meier-Schellersheim, M., and Germain, R. N. (2010). Chemorepulsion by blood S1p regulates osteoclast precursor mobilization and bone remodeling in vivo. J. Exp. Med. 207, 2793–2798. doi: 10.1084/jem.20101474
Juarez, J. G., Harun, N., Thien, M., Welschinger, R., Baraz, R., Pena, A. D., et al. (2012). Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by cxcr4 antagonists in mice. Blood 119, 707–716. doi: 10.1182/blood-2011-04-348904
Keller, J., Catala-Lehnen, P., Huebner, A. K., Jeschke, A., Heckt, T., Lueth, A., et al. (2014). Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat. Commun. 5:5215. doi: 10.1038/ncomms6215
Khavandgar, Z., Alebrahim, S., Eimar, H., Tamimi, F., McKee, M. D., and Murshed, M. (2013). Local regulation of tooth mineralization by sphingomyelin phosphodiesterase 3. J. Dent. Res. 92, 358–364. doi: 10.1177/0022034513478429
Khavandgar, Z., Poirier, C., Clarke, C. J., Li, J., Wang, N., McKee, M. D., et al. (2011). Cell-autonomous requirement for neutral sphingomyelinase 2 in bone mineralization. J. Cell Biol. 194, 277–289. doi: 10.1083/jcb.201102051
Khayrullin, A., Krishnan, P., Martinez-Nater, L., Mendhe, B., Fulzele, S., Liu, Y., et al. (2019). Very long-chain c24:1 ceramide is increased in serum extracellular vesicles with aging and can induce senescence in bone-derived mesenchymal stem cells. Cells 8:37. doi: 10.3390/cells8010037
Kihara, A., and Igarashi, Y. (2004). Fvt-1 is a mammalian 3-ketodihydrosphingosine reductase with an active site that faces the cytosolic side of the endoplasmic reticulum membrane. J. Biol. Chem. 279, 49243–49250. doi: 10.1074/jbc.M405915200
Kim, B. J., Lee, J. Y., Park, S. J., Lee, S. H., Kim, S. J., Yoo, H. J., et al. (2019). Elevated ceramides 18:0 and 24:1 with aging are associated with hip fracture risk through increased bone resorption. Aging 11, 9388–9404. doi: 10.18632/aging.102389
Kim, Y. H., Furuya, H., and Tabata, Y. (2014). Enhancement of bone regeneration by dual release of a macrophage recruitment agent and platelet-rich plasma from gelatin hydrogels. Biomaterials 35, 214–224. doi: 10.1016/j.biomaterials.2013.09.103
Kronenberg, H. M. (2003). Developmental regulation of the growth plate. Nature 423, 332–336. doi: 10.1038/nature01657
Kurek, K., Lukaszuk, B., Piotrowska, D. M., Wiesiolek, P., Chabowska, A. M., and Zendzian-Piotrowska, M. (2013). Metabolism, physiological role, and clinical implications of sphingolipids in gastrointestinal tract. BioMed Res. Int. 2013:908907.
Lai, W. Q., Chia, F. L., and Leung, B. P. (2012). Sphingosine kinase and sphingosine-1-phosphate receptors: novel therapeutic targets of rheumatoid arthritis? Future Med. Chem. 4, 727–733. doi: 10.4155/fmc.12.28
Lampasso, J. D., Marzec, N., Margarone, J. III, and Dziak, R. (2002). Role of protein kinase C alpha in primary human osteoblast proliferation. J. Bone Mineral Res. 17, 1968–1976. doi: 10.1359/jbmr.2002.17.11.1968
Lee, H. Y., Cho, K. M., Kim, M. K., Lee, M., Kim, H., Choi, C. Y., et al. (2021). Sphingosylphosphorylcholine blocks ovariectomy-induced bone loss by suppressing Ca(2+) /calmodulin-mediated osteoclast differentiation. J. Cell. Mol. Med. 25, 473–483. doi: 10.1111/jcmm.16101
Lee, N. K., and Karsenty, G. (2008). Reciprocal regulation of bone and energy metabolism. Trends Endocrinol. Metab. 19, 161–166. doi: 10.1016/j.tem.2008.02.006
Lee, S. H., Lee, S. Y., Lee, Y. S., Kim, B. J., Lim, K. H., Cho, E. H., et al. (2012). Higher circulating sphingosine 1-phosphate levels are associated with lower bone mineral density and higher bone resorption marker in humans. J. Clin. Endocrinol. Metab. 97, E1421–E1428.
Lee, W. C., Guntur, A. R., Long, F., and Rosen, C. J. (2017). Energy metabolism of the osteoblast: implications for osteoporosis. Endocr. Rev. 38, 255–266. doi: 10.1210/er.2017-00064
Liebisch, G., Fahy, E., Aoki, J., Dennis, E. A., Durand, T., Ejsing, C. S., et al. (2020). Update on lipid maps classification, nomenclature, and shorthand notation for ms-derived lipid structures. J. Lipid Res. 61, 1539–1555. doi: 10.1194/jlr.S120001025
Lien, Y. H., Yong, K. C., Cho, C., Igarashi, S., and Lai, L. W. (2006). S1p(1)-selective agonist, sew2871, ameliorates ischemic acute renal failure. Kidney Int. 69, 1601–1608. doi: 10.1038/sj.ki.5000360
Liu, X., Zhang, Q. H., and Yi, G. H. (2012). Regulation of metabolism and transport of sphingosine-1-phosphate in mammalian cells. Mol. Cell. Biochem. 363, 21–33. doi: 10.1007/s11010-011-1154-1
Lotinun, S., Kiviranta, R., Matsubara, T., Alzate, J. A., Neff, L., Luth, A., et al. (2013). Osteoclast-specific cathepsin k deletion stimulates s1p-dependent bone formation. J. Clin. Investig. 123, 666–681. doi: 10.1172/JCI64840
Makitie, R. E., Pekkinen, M., Morisada, N., Kobayashi, D., Yonezawa, Y., Nishimura, G., et al. (2021). Novel ifitm5 variant associated with phenotype of osteoporosis with calvarial doughnut lesions: a case report. Calcif. Tissue Int. 109, 626–632. doi: 10.1007/s00223-021-00878-5
Matsuo, K., and Irie, N. (2008). Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 473, 201–209. doi: 10.1016/j.abb.2008.03.027
Matsuoka, K., Park, K. A., Ito, M., Ikeda, K., and Takeshita, S. (2014). Osteoclast-derived complement component 3a stimulates osteoblast differentiation. J. Bone Mineral Res. 29, 1522–1530. doi: 10.1002/jbmr.2187
Matsuzaki, E., Hiratsuka, S., Hamachi, T., Takahashi-Yanaga, F., Hashimoto, Y., Higashi, K., et al. (2013). Sphingosine-1-phosphate promotes the nuclear translocation of beta-catenin and thereby induces osteoprotegerin gene expression in osteoblast-like cell lines. Bone 55, 315–324. doi: 10.1016/j.bone.2013.04.008
Mechtcheriakova, D., Wlachos, A., Sobanov, J., Bornancin, F., Zlabinger, G., Baumruker, T., et al. (2007). Fty720-phosphate is dephosphorylated by lipid phosphate phosphatase 3. FEBS Lett. 581, 3063–3068. doi: 10.1016/j.febslet.2007.05.069
Mendez-Ferrer, S., Lucas, D., Battista, M., and Frenette, P. S. (2008). Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447. doi: 10.1038/nature06685
Merrill, A. H. Jr. (2002). De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 277, 25843–25846. doi: 10.1074/jbc.R200009200
Morales, A., Lee, H., Goni, F. M., Kolesnick, R., and Fernandez-Checa, J. C. (2007). Sphingolipids and cell death. Apoptosis 12, 923–939.
Mu, H., and Porsgaard, T. (2005). The metabolism of structured triacylglycerols. Prog. Lipid Res. 44, 430–448. doi: 10.1016/j.plipres.2005.09.002
Olivera, A., and Spiegel, S. (1993). Sphingosine-1-phosphate as second messenger in cell proliferation induced by pdgf and fcs mitogens. Nature 365, 557–560. doi: 10.1038/365557a0
Olivier, S., Fillet, M., Malaise, M., Piette, J., Bours, V., Merville, M. P., et al. (2005). Sodium nitroprusside-induced osteoblast apoptosis is mediated by long chain ceramide and is decreased by raloxifene. Biochem. Pharmacol. 69, 891–901. doi: 10.1016/j.bcp.2004.11.030
Oo, M. L., Thangada, S., Wu, M. T., Liu, C. H., Macdonald, T. L., Lynch, K. R., et al. (2007). Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 282, 9082–9089. doi: 10.1074/jbc.M610318200
Pacifici, R. (2013). Osteoimmunology and its implications for transplantation. Am. J. Transplant. 13, 2245–2254. doi: 10.1111/ajt.12380
Pacifici, R. T. (2010). Cells: critical bone regulators in health and disease. Bone 47, 461–471. doi: 10.1016/j.bone.2010.04.611
Pebay, A., Bonder, C. S., and Pitson, S. M. (2007). Stem cell regulation by lysophospholipids. Prostaglandins & Other Lipid Mediat. 84, 83–97. doi: 10.1016/j.prostaglandins.2007.08.004
Pekkinen, M., Terhal, P. A., Botto, L. D., Henning, P., Makitie, R. E., Roschger, P., et al. (2019). Osteoporosis and skeletal dysplasia caused by pathogenic variants in sgms2. JCI Insight 4:e126180. doi: 10.1172/jci.insight.126180
Pietschmann, P., Mechtcheriakova, D., Meshcheryakova, A., Foger-Samwald, U., and Ellinger, I. (2016). Immunology of osteoporosis: a mini-review. Gerontology 62, 128–137. doi: 10.1159/000431091
Purdue, P. E., Crotti, T. N., Shen, Z., Swantek, J., Li, J., Hill, J., et al. (2014). Comprehensive profiling analysis of actively resorbing osteoclasts identifies critical signaling pathways regulated by bone substrate. Sci. Rep. 4:7595. doi: 10.1038/srep07595
Pusic, I., and DiPersio, J. F. (2010). Update on clinical experience with amd3100, an sdf-1/cxcl12-cxcr4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr. Opin. Hematol. 17, 319–326. doi: 10.1097/MOH.0b013e328338b7d5
Pyne, N. J., and Pyne, S. (2011). Selectivity and specificity of sphingosine 1-phosphate receptor ligands: “off-targets” or complex pharmacology? Front. Pharmacol. 2:26. doi: 10.3389/fphar.2011.00026
Quint, P., Ruan, M., Pederson, L., Kassem, M., Westendorf, J. J., Khosla, S., et al. (2013). Sphingosine 1-phosphate (s1p) receptors 1 and 2 coordinately induce mesenchymal cell migration through s1p activation of complementary kinase pathways. J. Biol. Chem. 288, 5398–5406. doi: 10.1074/jbc.M112.413583
Quiroz-Acosta, T., Flores-Martinez, Y. M., Becerra-Martinez, E., Perez-Hernandez, E., Perez-Hernandez, N., and Banuelos-Hernandez, A. E. (2021). Aberrant sphingomyelin (31)p-nmr signatures in giant cell tumour of bone. Biochem. Cell Biol. Biochim. Biol. Cell. 5, 1–8. doi: 10.1139/bcb-2020-0599
Ratajczak, M. Z., Lee, H., Wysoczynski, M., Wan, W., Marlicz, W., Laughlin, M. J., et al. (2010). Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia 24, 976–985. doi: 10.1038/leu.2010.53
Rauner, M., Sipos, W., Thiele, S., and Pietschmann, P. (2013). Advances in osteoimmunology: pathophysiologic concepts and treatment opportunities. Int. Arch. Allergy Immunol. 160, 114–125. doi: 10.1159/000342426
Redlich, K., and Smolen, J. S. (2012). Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 11, 234–250.
Roberts, S. J., van Gastel, N., Carmeliet, G., and Luyten, F. P. (2015). Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone 70, 10–18. doi: 10.1016/j.bone.2014.08.007
Roelofsen, T., Akkers, R., Beumer, W., Apotheker, M., Steeghs, I., van de Ven, J., et al. (2008). Sphingosine-1-phosphate acts as a developmental stage specific inhibitor of platelet-derived growth factor-induced chemotaxis of osteoblasts. J. Cell. Biochem. 105, 1128–1138. doi: 10.1002/jcb.21915
Rutherford, C., Childs, S., Ohotski, J., McGlynn, L., Riddick, M., MacFarlane, S., et al. (2013). Regulation of cell survival by sphingosine-1-phosphate receptor s1p1 via reciprocal erk-dependent suppression of bim and pi-3-kinase/protein kinase c-mediated upregulation of mcl-1. Cell Death Dis. 4:e927. doi: 10.1038/cddis.2013.455
Ryland, L. K., Fox, T. E., Liu, X., Loughran, T. P., and Kester, M. (2011). Dysregulation of sphingolipid metabolism in cancer. Cancer Biol. Ther. 11, 138–149. doi: 10.4161/cbt.11.2.14624
Ryu, J., Kim, H. J., Chang, E. J., Huang, H., Banno, Y., and Kim, H. H. (2006). Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 25, 5840–5851. doi: 10.1038/sj.emboj.7601430
Sato, C., Iwasaki, T., Kitano, S., Tsunemi, S., and Sano, H. (2012). Sphingosine 1-phosphate receptor activation enhances bmp-2-induced osteoblast differentiation. Biochem. Biophys. Res. Commun. 423, 200–205. doi: 10.1016/j.bbrc.2012.05.130
Serra-Vinardell, J., Roca-Ayats, N., De-Ugarte, L., Vilageliu, L., Balcells, S., and Grinberg, D. (2020). Bone development and remodeling in metabolic disorders. J. Inherited Metab. Dis. 43, 133–144.
Soltanoff, C. S., Yang, S., Chen, W., and Li, Y. P. (2009). Signaling networks that control the lineage commitment and differentiation of bone cells. Crit. Rev. Eukaryot. Gene Expr. 19, 1–46. doi: 10.1615/critreveukargeneexpr.v19.i1.10
Spiegel, S., and Milstien, S. (2002). Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 277, 25851–25854. doi: 10.1074/jbc.r200007200
Spiegel, S., and Milstien, S. (2003). Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4, 397–407.
Spiegel, S., and Milstien, S. (2011). The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11, 403–415. doi: 10.1038/nri2974
Stoffel, W. (1999). Functional analysis of acid and neutral sphingomyelinases in vitro and in vivo. Chem. Phys. Lipids 102, 107–121. doi: 10.1016/s0009-3084(99)00079-1
Stoffel, W., Jenke, B., Block, B., Zumbansen, M., and Koebke, J. (2005). Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proc. Natl. Acad. Sci. U.S.A. 102, 4554–4559. doi: 10.1073/pnas.0406380102
Tafesse, F. G., Ternes, P., and Holthuis, J. C. (2006). The multigenic sphingomyelin synthase family. J. Biol. Chem. 281, 29421–29425. doi: 10.1074/jbc.R600021200
Takabe, K., Paugh, S. W., Milstien, S., and Spiegel, S. (2008). “Inside-out” Signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 60, 181–195. doi: 10.1124/pr.107.07113
Takuwa, Y. (2002). Subtype-specific differential regulation of rho family g proteins and cell migration by the edg family sphingosine-1-phosphate receptors. Biochim. Biophys. Acta 1582, 112–120.
Taniguchi, M., and Okazaki, T. (2014). The role of sphingomyelin and sphingomyelin synthases in cell death, proliferation and migration-from cell and animal models to human disorders. Biochim. Biophys. Acta 1841, 692–703. doi: 10.1016/j.bbalip.2013.12.003
Teixeira, V., and Costa, V. (2016). Unraveling the role of the target of rapamycin signaling in sphingolipid metabolism. Progr. Lipid Res. 61, 109–133. doi: 10.1016/j.plipres.2015.11.001
Ternes, P., Franke, S., Zahringer, U., Sperling, P., and Heinz, E. (2002). Identification and characterization of a sphingolipid delta 4-desaturase family. J. Biol. Chem. 277, 25512–25518. doi: 10.1074/jbc.M202947200
van Gastel, N., Stegen, S., Eelen, G., Schoors, S., Carlier, A., Daniels, V. W., et al. (2020). Lipid availability determines fate of skeletal progenitor cells via sox9. Nature 579, 111–117. doi: 10.1038/s41586-020-2050-1
Vogel, P., Donoviel, M. S., Read, R., Hansen, G. M., Hazlewood, J., Anderson, S. J., et al. (2009). Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS One 4:e4112. doi: 10.1371/journal.pone.0004112
Wen, D., Androjna, C., Vasanji, A., Belovich, J., and Midura, R. J. (2010). Lipids and collagen matrix restrict the hydraulic permeability within the porous compartment of adult cortical bone. Ann. Biomed. Eng. 38, 558–569. doi: 10.1007/s10439-009-9858-z
Weske, S., Vaidya, M., Reese, A., von Wnuck Lipinski, K., Keul, P., Bayer, J. K., et al. (2018). Targeting sphingosine-1-phosphate lyase as an anabolic therapy for bone loss. Nat. Med. 24, 667–678. doi: 10.1038/s41591-018-0005-y
Yoshikawa, Y., Yoshizawa, T., Domae, E., Hirai, Y., Kamada, A., Okazaki, T., et al. (2019). Knockdown of sphingomyelin synthase 2 inhibits osteoclastogenesis by decreasing rankl expression in mouse primary osteoblasts. Biomed. Res. 40, 189–196. doi: 10.2220/biomedres.40.189
Yu, H. (2016). Sphingosine-1-phosphate receptor 2 regulates proinflammatory cytokine production and osteoclastogenesis. PLoS One 11:e0156303. doi: 10.1371/journal.pone.0156303
Zaidi, M. (2007). Skeletal remodeling in health and disease. Nat. Med. 13, 791–801. doi: 10.1038/nm1593
Zemann, B., Kinzel, B., Muller, M., Reuschel, R., Mechtcheriakova, D., Urtz, N., et al. (2006). Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug fty720. Blood 107, 1454–1458. doi: 10.1182/blood-2005-07-2628
Zhang, H., Desai, N. N., Olivera, A., Seki, T., Brooker, G., and Spiegel, S. (1991). Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol. 114, 155–167. doi: 10.1083/jcb.114.1.155
Keywords: sphingolipid, bone remodeling, osteoblast, osteoclast, S1P
Citation: Qi T, Li L and Weidong T (2021) The Role of Sphingolipid Metabolism in Bone Remodeling. Front. Cell Dev. Biol. 9:752540. doi: 10.3389/fcell.2021.752540
Received: 03 August 2021; Accepted: 11 October 2021;
Published: 29 November 2021.
Edited by:
Chunyi Wen, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Jianquan Chen, Soochow University, ChinaHua Su, Huazhong University of Science and Technology, China
Copyright © 2021 Qi, Li and Weidong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liao Li, lliao@scu.edu.cn; Tian Weidong, drtwd@sina.com
 Tang Qi
Tang Qi  Liao Li
Liao Li Tian Weidong
Tian Weidong