Introduction
Obesity has been one of the crucial problems worldwide, especially for younger generation [1]. It includes broader problems from medical, social, environmental and public health points of view [2]. For younger people, obesity may bring metabolic and cardiovascular impairement which would give grave influence for long life [3]. Consequently, several strategies will be required for the treatment for obesity, including lifestyle intervention, pharmacological treatment and other methods. As recent statistics, 38.2 million children <5 years old are obese or overweight [4]. Formerly, obesity problem was considered the matter of high-income countries, but recently it is found in also low-/middle-income countries. Approximately half children <5 years with obese or overweight have lived in Asian region. For the treatment of pediatric patients with obesity, pharmacotherapeutic agents had not approved by European Medicines Agency (EMA) until 2020. However, EMA has decided to authorize the therapy in April 2021, for the application of Liraglutide as a Glucagon-Like Peptide Receptor Analog (GLP-1RA). Clinical effect and safety were analyzed for 251 cases for a randomized, double-blind trial. As a result, 26.1% of cases showed >10%, and 43.3% cases showed >5% of weight reduction associated clinical effect [3]. As one of GLP-1RA, semaglutide has been also effective for weight reduction and decreased daily profile of blood glucose [5]. It can also show clinical efficacy for delaying stomach emptying and suppressing appetite leading to lose weight. As long-acting GLP-1RA, semaglutide can be applied for two ways, which are injection once-weekly and oral formulation once-daily. Thus, it is only agent of GLP-1RA that can be administered per os [6]. Oral semaglutide intake should be conducted for empty stomach when waking up. Furthermore, it has wide range of beneficial efficacy, including diabetic complication, cardiovascular outcomes, Chronic Kidney Disease (CKD) or Diabetic Kidney Disease (DKD), and non-alcoholic steatohepatitis [7]. Consequently, these clinical mechanisms of semaglutide would possibly extend to other beneficial function and indications. Obesity and Type 2 Diabetes (T2D) are closely correlated, and these combination will cause a variety of diabetic complications. They include impaired function of several organs, such as heart, kidney, nerve and macro-/ micro-angiopathy [8-10].
In
recent medical situation of COVID-19 worldwide, the combined situation will
become higher risk for patients with obesity and T2D [11,12]. As mentioned
above, GLP-1RA has beneficial efficacy on obesity and T2D, by controlling
appetite and stomach emptying [13,14]. Furthermore, it shows advantageous
effects for decreasing mortality and Major Cardiovascular Averse Events (MACE)
in comparison with other regimens [15]. Authors
and collaborators have developed diabetic clinical research especially for Low
Carbohydrate Diet (LCD) [16, 17]. Furthermore, various reports were presented
such as Continuous Glucose Monitoring (CGM), Meal Tolerance Test (MTT), Sodium-Glucose
Transport Protein 2 Inhibitors (SGLT-2i), GLP-1RA and others. Among our medical
practice, we have experienced an impressive young female patient with obesity,
T2D and fatty liver. By applying oral semaglutide, she showed remarkable
clinical efficacy. Her general clinical progress and some related discussion
would be described in this article [18-20].
Case Presentation
History
and physical: The
patient is a 24-year-old female patient with Type 2 diabetes (T2D). She was
diagnosed as T2D three years ago. After that she was provided Oral Hypoglycemic
Agents (OHAs) and her HbA1c was almost stable 6.5% to 7.2%. The important problem
has been obesity for years. When diagnosed as T2D, her body weight was 114kg.
After that, she was advised to continue Low Carbohydrate Diet (LCD) and weight
was decreased to 110kg. From Jan 2020, her weight has been stable about
107-109kg (Figure 1).

Figure 1: Clinical progress of the case with HbA1c, body weight and treatment.
As
to her physical examination on Jan 2022, her physique showed 165cm in height,
107 kg in weight with 39.3 kg/m2 of Body Mass Index (BMI). The
consciousness is alert, speech is normal, and neurological findings were
unremarkable. Her lung, heart, abdomen and orthopedic problems are negative.
Several
exams: Biochemical
laboratory tests were conducted in Feb 2022. The results were as follows: WBC
8300 /μL, RBC 6.02 x 106 /μL, Hb 13.0 g/dL, Ht 45.7%, Plt 36.6 x 104
/μL, AST 24 U/L, ALT 35 U/L, r-GT 34 U/L (-86), LDL 142 mg/dL, TG 148 mg/dL,
HDL 61 mg/dL, BUN 16 mg/dL, Cr 0.50 mg/dL, uric acid 5.3 mg/dL. Other
examinations were conducted during 2021-2022. Chest X-P and ECG
(Electrocardiogram) were unremarkable. She has continued to reveal abnormal
liver function tests, which were followed up every 4 months (Table 1). The data showed the
elevation of AST, ALT, g-GTP until June 2021, and they were normalized after
Oct 2021.
Medical
Problems and Medicine: From previous history and situation, medical
problems and related medication can be summarized in the following.
·
T2D: She has three
years of T2D, and has been provided Metformin 1000mg, Canagliflozin 100mg,
Teneligliptin Hydrobromide Hydrate 20mg and voglibose 0.2mg per day.
·
Gastro Esophageal
Reflux Disease (GERD):
She has slight upper Gastrointestinal (GI) symptoms for years, and then has
taken nizatidine 150mg per day.
·
Fatty liver: She revealed
continuous elevated liver function tests including AST, ALT, r-GTP. According
to abdominal echography, she had moderate fatty liver. Since she is young,
abdominal CT scan was not performed. She did not have special medicine for
fatty liver. By continuing LCD, taking Canagliflozin as SGLT2i and diabetic
treatment, her liver function was followed up. Her elevated biochemical data
was relieved in Oct 2021 (Table 1).
Obesity: Three years ago, her body weight was 114kg at the maximum point. At that time, BMI was 41.9 kg/m2. After that, BMI was decreased to 39.3 kg/m2 in Jan 2022.
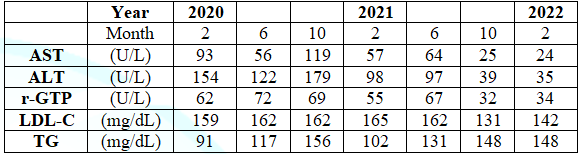
Table 1: Clinical progress of abnormal liver function test in every 4 months.
Clinical progress: Her body weight
and HbA1c were increased in Jan 2022, and then her OHAs were decided to change.
She had been provided Teneligliptin for DPP4i, and then changed to oral
semaglutide (Rybelsus) 3mg/day as GLP-1RA. After that, Rybelsus was increased
to 7mg and 14 mg for each 4 weeks (Figure
1). As a result, HbA1c was reduced from 6.3% to 5.6%, and weight was also
decreased from 107kg to 103kg for 4 months. Before increasing the dose of
Rybelsus, she was informed of the possibility of GIAEs, but she did not feel
any GIAEs during the period until 14mg intake per day.
Ethical
Considerations: Current
report has been basically conducted with principles of ethics. They include the
Declaration of Helsinki, and also some commentary from Ethical Guideline from
the Research for Human aspect. These contents were along with the Good Clinical
Practice (GCP). Authors and collaborators had established hospital ethical
committee for arguing several ethical matters. This committee has been present
in Kanaiso Hospital including several related professional members. They are
hospital director, surgeon, physician, pharmacist, nutritionist and legal
specialty. During the meeting of the committee, enough discussion and
perspectives were conducted. As a result, agreements were provided according to
the current investigation. The informed consent was given with written
agreement document from the patient.
Discussion
As
to applicable diagnosis and treatment of diabetes, American Diabetes
Association (ADA) has presented standard guideline for medical care in Jan 2022
[21]. Recent recommendation for diabetic pharmacological OHAs include SGLT2-i
and Glucagon-Like Peptide 1 receptor agonist (GLP-1RA) [22]. For the reason,
GLP-1RA shows various beneficial efficacy for metabolic, cardiovascular and
renal mechanism. Several types of GLP-1RAs have been prevalent in medical
practice [23]. They have some categories as follows [24]: i) subcutaneous
injection once a day (liraglutide and lixisenatide), ii) subcutaneous injection
two times a day (exenatide), iii) subcutaneous injection once a week
(exenatide, duraglutide and semaglutide), and iv) oral semaglutide formulation
that was from Peptide Innovation for Early diabetes treatment (PIONEER) trials.
As one of GLP-1Ras, semaglutide has been evaluated for various beneficial
clinical efficacy [25,26]. Semaglutide includes two kinds of injective
administration and oral formulation [27]. In particular, Rybelsus as an oral
semaglutide has been used for actual practice associated with pharmacological
beneficial mechanism [28]. In addition, it shows benefits for significant
improvement of glucose control and reduction of body weight [29]. Authors and
our diabetic team have reported various diabetic patients treated with
effective agents [30,31]. Among them, we have presented a recent report of a
case with remarkable efficacy of Rybelsus [20]. Rybelsus shows clinical effect of weight
reduction. Concerning anti-obesity agents, FDA of US approved some kinds of
medicines, such as semaglutide and liraglutide, as well as orlistat,
naltrexone/bupropion and phentermine/topiramate [31]. Once-weekly semaglutide
revealed efficacy for obese people, and some Gastrointestinal Adverse Events
(GIAEs) were found. Those data were from 1st to 3rd trials
of Semaglutide Treatment Effect in People with Obesity (STEP) associated with
simultaneous analysis of reduction degree [32]. In this case, remarkable
decrease of HbA1c and weight was found after increasing dose of Rybelsus from
7mg to 14 mg/day. Some probable factors may contribute this clinical progress.
She usually skips her breakfast for long as her lifestyle.
Rybelsus is
administered just after waking up with 50-120mL of water followed by more than
30 min fasting [32]. Her fasting time period was rather long about 120 min.
From previous analysis of fasting time, clinical efficacy of Rybelsus would
increase as the fasting time becomes long [33]. These data were obtained by the
investigation of PIONEER 2 and 3 [34,35]. Semaglutide concentration in the
blood was analyzed for time period from 4 hours to 24 hours, and fasting time
in the case of 15, 30, 60, 120 min. As a result, the level is stable during
4-24 hours. As the standard level is set 1.00 at 15 min, concentration ratio
for 4 hours would be elevated 1.67, 2.60 and 3.06 times in 30, 60 and 120 min,
respectively [36]. Thus, fasting time period after intake would become crucial
factor for clinical efficacy. Consequently, current case seemed to have enough
blood concentration because of long fasting time period. When Rybelsus is provided to diabetic
patients, possible problem would be Gastrointestinal Adverse Events (GIAEs) [37].
This case did not feel any GIAEs, and it may be due to usual intaking of
nizatidine 150mg/day for the treatment of GERD. From mentioned above, the case
has been tolerated Rybelsus well and had significant efficacy for the
improvement of weight, glucose control and fatty liver. Some limitation would
be present for this report. The case is young female with high BMI, T2D, fatty
liver and GERD. Rybelsus may contribute much for such combined medical
problems. Fasting time period will be possibly in discussion for the
personalized medical treatment for lifestyle, the severity of T2D, the degree
of obesity, fatty liver and other factors. In summary, Rybelsus showed
significant clinical efficacy for young female patient with obesity, T2D and
fatty liver. This report will become hopefully a useful reference for
personalized therapy in the future.
Conflict of
interest
The authors declare no conflict of interest.
References
1.
Lobstein T and Jewell J. What is a "high"
prevalence of obesity? Two rapid reviews and a proposed set of thresholds for
classifying prevalence levels (2022) Obes Rev 23: e13363. https://doi.org/10.1111/obr.13363
2. Howell NA and Booth GL. The weight of place: Built environment correlates of obesity and diabetes (2022) Endocr Rev 24: bnac005. https://doi.org/10.1210/endrev/bnac0055201344
3.
Nicolucci A and Maffeis C. The adolescent with
obesity: what perspectives for treatment? (2022) Ital J Pediatr 48: 9. https://doi.org/10.1186/s13052-022-01205-w
4.
World Health
Organization (WHO). Obesity and overweight.
5.
Liu Y and Luo X. New practice in semaglutide on type-2
diabetes and obesity: clinical evidence and expectation (2022) Front Med 16: 17-24. https://doi.org/10.1007/s11684-021-0873-2 .
6. Gallwitz B and Giorgino F. Clinical Perspectives on the Use of Subcutaneous and Oral Formulations of Semaglutide. (2021) Front Endocrinol (Lausanne) 12: 645507. https://doi.org/10.3389/fendo.2021.645507
7.
Gasbjerg LS, Bergmann NC, Stensen S, Christensen MB,
Rosenkilde MM, et al. Evaluation of the incretin effect in humans using GIP and
GLP-1 receptor antagonists. (2020) Peptides 125: 170183. https://doi.org/10.1016/j.peptides.2019.170183
8.
Shieh JC, Huang PT and Lin YF. Alzheimer's disease and
Diabetes: Insulin Signaling as the Bridge Linking Two Pathologies (2020) Mol
Neurobiol 57: 1966-1977. https://doi.org/10.1007/s12035-019-01858-5
9.
Tadic M, Grassi G and Cuspidi C. Cardiorespiratory
fitness in patients with type 2 diabetes: A missing piece of the puzzle (2021)
Heart Fail 26: 301-308.
https://doi.org/10.1007/s10741-020-10015-3
10.
Catrina SB and Zheng X. Hypoxia and hypoxia-inducible
factors in diabetes and its complications (2021) Diabetologia 64: 709-716. https://doi.org/10.1007/s00125-021-05380-z
11.
Drucker DJ. Diabetes, obesity, metabolism, and
SARS-CoV-2 infection: the end of the beginning (2021) Cell Metab 33: 479-498. https://doi.org/10.1016/j.cmet.2021.01.016
12. Cefalu WT and Rodgers GP. COVID-19 and metabolic diseases: a heightened awareness of health inequities and a renewed focus for research priorities (2021) Cell Metab 2021 Mar 33:473-478. https://doi.org/10.1016/j.cmet.2021.02.006
13.
Song Y, Koehler JA, Baggio LL, Powers AC, Sandoval DA,
et al. Gut-proglucagon-derived peptides are essential for regulating glucose
homeostasis in mice (2019) Cell Metab 30: 976-986.e3.
14.
Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, et
al. Semaglutide improves postprandial glucose and lipid metabolism, and delays
first-hour gastric emptying in subjects with obesity (2018) Diabetes Obes Metab
20: 610-619.
https://doi.org/10.1111/dom.13120
15. Orsini FM, Gentilella R, Corcos A, Torre E and
Genovese S. Changing the approach to type 2 diabetes treatment: A comparison of
glucagon-like peptide-1 receptor agonists and sulphonylureas across the
continuum of care (2021) Diabetes Metab Res 37: e3434. https://doi.org/10.1002/dmrr.3434 .
17.
Muneta T, Hayashi M, Nagai Y, Matsumoto M, Bando H, et
al. Ketone Bodies in the Fetus and Newborn During Gestational Diabetes and
Normal Delivery (2022) Int J Diab 3: 142-148.
18.
Ebe K, Bando H, Muneta T, Bando M and Yonei Y.
Investigation of C-peptide Index for Carbo70 by the severity of diabetic state
(2018) Clin Med Case Rep 22: 113.
19.
Takehisa Y and Bando H. Blood Glucose and Insulin
Values on Daily Profile, M Value and Meal Tolerance in Patients with Type 2
Diabetes Mellitus (T2DM) (2020) Diab Res Open Access 2: 85-94. https://doi.org/10.36502/2020/droa.6174
20.
Bando H, Yamashita H, Kato Y, Kato Y, Ogura K, et al.
Remarkable Efficacy of Blood Glucose and Weight by Oral Semaglutide (Rybelsus)
For Short Period (2022) SunText Rev Case Rep Image 23: 143. https://doi.org/10.51737/2766-4589.2022.043
21.
American Diabetes Association; Standards of Medical
Care in Diabetes-2022 Abridged for Primary Care Providers. (2022) Clin Diabetes
40: 10-38. https://doi.org/10.2337/cd22-as01
22.
ADA Professional Practice Committee, 9. Pharmacologic
Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022
(2022) Diabetes Care 45: S125-S143. https://doi.org/10.2337/dc22-S009
23.
Tak YJ and Lee SY. Anti-Obesity Drugs: Long-Term
Efficacy and Safety: An Updated Review (2021) World J Mens Health 39: 208-221. https://doi.org/10.5534/wjmh.200010
24.
Bando H. Effective oral formulation of semaglutide
(Rybelsus) for diabetes and obesity due to absorption enhancer development
(2022) Int J Endocrinol Diabetes 5: 130.
https://doi.org/10.36266/IJED/130
25.
Zhong P, Zeng H, Huang M, Fu W and Chen Z. Efficacy
and safety of once-weekly semaglutide in adults with overweight or obesity: a
meta-analysis. Endocrine (2022) Epub ahead of print. PMID: 34981419. https://doi.org/10.1007/s12020-021-02945-1
26.
Wadden TA, Bailey TS and Billings LK. Effect of
subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral
therapy on body weight in adults with overweight or obesity: The STEP 3
randomized clinical trial (2021) JAMA 325 :1403-1413.
https://doi.org/10.1001/jama.2021.1831
27.
Bando H. Useful Oral Administration of Glucagon-Like
Peptide 1 Receptor Agonist (GLP-1RA) as Semaglutide (Rybelsus) for Type 2
Diabetes Mellitus (T2DM) (2022) Asp Biomed Clin Case Rep 5: 38-41. https://doi.org/10.36502/2022/ASJBCCR.6260
28.
Takehisa Y and Bando H. Elderly diabetic patients with
effective add-on therapy of dulaglutide as a GLP-1 receptor analogue (GLP1 RA)
(2020) Edel J Biomed Res Rev 2: 31-35. https://doi.org/10.33805/2690-2613.113
29.
Ebe K, Bando H, Muneta T, Bando M and Yonei Y.
Remarkable improvement of glucose variability by Sodium–glucose cotransporter 2
(SGLT2) inhibitors using continuous glucose monitoring (CGM) (2019) Diabetes
Case Rep 4:1. https://doi.org/10.4172/2572-5629.1000139
30.
ADAProfessional Practice Committee. 8. Obesity and
weight management for the prevention and treatment of type 2 diabetes: Standards
of Medical Care in Diabetes-2022 (2022) Diabetes Care 45: S113-S124. https://doi.org/10.2337/dc22-S008
31.
Wharton S, Calanna S, Davies M, Dicker D, Goldman B, et
al. Gastrointestinal tolerability of once-weekly semaglutide 2.4 mg in adults
with overweight or obesity, and the relationship between gastrointestinal adverse
events and weight loss (2022) Diabetes Obes Metab 24: 94-105. https://doi.org/10.1111/dom.14551
32.
Rybelsus (Semaglutide)
[US Prescribing Information]. Available online:
33.
Bækdal TA, Breitschaft A, Donsmark M, Maarbjerg SJ,
Søndergaard FL, et al. Effect of Various Dosing Conditions on the
Pharmacokinetics of Oral Semaglutide, a Human Glucagon-Like Peptide-1 Analogue
in a Tablet Formulation (2021) Diabetes Ther 12: 1915-1927. https://doi.org/10.1007/s13300-021-01078-y
34.
Rosenstock J, Allison D, Birkenfeld AL, Blicher TM,
Deenadayalan S, et al.. Effect of Additional Oral Semaglutide vs Sitagliptin on
Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin
Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial (2019) JAMA
321: 1466-1480. https://doi.org/10.1001/jama.2019.2942
35.
Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C,
Gumprecht J, et al. Oral Semaglutide Versus Empagliflozin in Patients With Type
2 Diabetes Uncontrolled on Metformin: The PIONEER 2 (2019) Trial Diabetes Care
42: 2272-2281.
https://doi.org/10.2337/db19-54-OR
36.
Miki K, Bando H, Hayashi K, Dohi A and Kamoto A.
Longer Fasting After Rybelsus Administration Contributes Higher Efficacy (2022)
SunText Rev Med Clin Res 3: 150. https://doi.org/10.51737/2766-4813.2022.050
37. Ji L, Dong X, Li Y, Li Y, Lim S, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30-week, double-blind, phase 3a, randomized trial (2021) Diabetes Obes Metab 23: 404-414. https://doi.org/10.1111/dom.14232
Corresponding author
Hiroshi Bando, Tokushima University/Medical Research, Nakashowa 1-61, Tokushima 770-0943, Japan, Tel: +81-90-3187-2485, E-mail: pianomed@bronze.ocn.ne.jp Citation
Bando H, Yamashita H, Kato Y, Ogura K, Kato Y,
et al. Clinically both effects of weight and glucose variability by oral semaglutide
(Rybelsus) for younger female patient with Type 2 Diabetes (T2D) (2022) J
Obesity and Diabetes 5: 11-14.
Keywords
Type 2 diabetes, Obesity, Oral semaglutide, Weight.


 PDF
PDF