Abstract
Objective: Despite being the second most common neuropathy in the upper limb, surgical treatment of ulnar syndrome remains controversial, in part because the published studies are heterogeneous in terms of surgical technique and variables studied. This study aims to assess the poor satisfaction prognostic factors in several patients treated by the same surgeon and the same surgical technique.
Material and methods: We reviewed retrospectively all patients who underwent a submuscular anterior transposition of the ulnar nerve between 2011 to 2017. The duration of symptoms, grade of neuropathy (McGowan score and electromyographic scale study) pain, paresthesia and motor alterations were recorded before surgery and at present. Satisfaction was evaluated according to the modified Bishop score.
Results: A total of 43 cases in 42 patients were reviewed. with an average follow-up of 34.9 months. There was an improvement in pain in 75% of patients, paresthesia’s in 64% and motor alterations in 67%. According to the modified Bishop score, 82% of patients reported good or excellent results. Low satisfaction was associated with residuals postoperative symptoms (p<0.001), low postoperative McGowan grade (p<0.001), reinterventions cases (p=0.03) and with the duration of symptoms before surgery (p=0.003). No relationship between satisfaction and age, preoperative symptoms, preoperative grade of neuropathy or preoperative electromyographic alteration was found.
Conclusion: Submuscular transposition is a safe and effective technique for ulnar neuropathy. Overall, good and excellent satisfaction were archived in 82% of patients. The duration of preoperative symptoms are the main prognostic factors, so surgical treatment should be advised as soon as possible. On the other hand, periprosthetic fractures represent an independent risk factor for non-union.
Keywords
Ulnar neuropathy, Submuscular transposition, Outcomes, Prognostic
Introduction
Ulnar neuropathy is the second most common peripheral neuropathy in the upper limb after carpal tunnel syndrome, with an incidence of 30 cases per 100,000 persons/year [1,2]. It is characterized by the appearance of sensory symptoms such as pain, dysesthesia, and paresthesia, initially intermittent and then continuous, in the ulnar territory of the forearm and hand. In more advanced cases, there are motor symptoms as weakness and atrophy of the intrinsic muscles of the hand, especially in the first dorsal commissure.
The anatomical situation of the ulnar nerve, passing behind the medial humeral epicondyle, determines that the elbow is the main point of conflict [3], although there are other locations where the nerve may be affected. From a pathophysiological point of view, the nerve can undergo compressive phenomena, but also tensile phenomena inherent with elbow flexion and ischemic phenomena due to the collapse of intraneural microcirculation [4]. Conservative treatment seems to be effective with mild and moderate neuropathy, but currently, there is no consensus on which is the most successful surgical treatment when conservative treatment fails. The more employees’ surgical treatments are in situ decompression, epicondylectomy and anterior transpositions, either subcutaneous or submuscular. In the absence of quality randomized studies comparing the different surgical treatments, the choice of it is subject to the surgeon’s experience and preferences.
Although the anterior submuscular transposition is the technique that theoretically better addresses the underlying pathophysiological problems (eliminates all potential compression points, decrease the ulnar nerve tension with elbow flexion and house the nerve in a vascularized and protected bed), it has classically been reserved as a rescue treatment due to its technical difficulty and greater association with complications.
The purpose of this study is to analyze the prognostic factors of satisfaction after a standardized anterior submuscular transposition carried out by the same surgeon.
Material and Methods
A retrospective study was carried out with institutional review board approval. All patients older than 18 years treated surgically from 2011 to 2017 for ulnar nerve compression at the elbow by the same orthopedic surgeon (JM) were offered enrollment in the study. All of them were treated with the same anterior submuscular transposition technique.
The diagnosis was made based on clinical findings (paresthesia’s or hypoesthesia’s in the ulnar territory and loss of the intrinsic hand muscle strength) with subsequent confirmation using electromyographic study (EMG). We performed a standard neurophysiological protocol, following the American Association of Electrodiagnostic Medicine (AAEM) criteria [5]. Exclusion criteria were less than 12 months post-operatively follow-up and the inability to conduct a personal interview at the time of the study.
Between 2011 and 2017, 51 patients operated of 52 ulnar neuropathies (one case of bilateral neuropathy) were registered. Due to loss of follow-up or inability to conduct the personal interview, the final sample analyzed was 43 ulnar neuropathies (42 patients) with an average followup of 34.9 months (range, 12-72 months).
By reviewing the medical history, preoperative data were recognized, including demographic information, clinical symptoms, physical examination findings, and electromyographic results. The pain was assessed using the visual analog scale (VAS). We also recorded the presence and degree of paresthesia (1: absence; 2: intermittent; 3: persistent), the presence and degree of sensitivity alterations (1: absence of sensitivity; 2: hyposensitivity; 3: intact sensitivity) and strength alterations. The compression degree was stratified according to the McGowan scale in three grades [6]: grade I, paraesthesia and sensory symptoms detected; grade II, muscle weakness; Grade III, paresia and muscular atrophy. In five cases ulnar neuropathy was secondary to previous elbow fracture, none of which had an angular deformity. Five cases were a recurrence of previous in situ neurolysis (average time from neurolysis to transposition of 31 months). There was a case of simultaneous intervention of ulnar compression at the elbow and median nerve compression at the carpal tunnel. 17 cases were manual workers, 16 performed administrative or office jobs and nine were retired or unemployed for other reasons.
In all patients except in one case (post-traumatic neuropathy), conservative treatment was previously performed for at least six months with activity modifications, nonsteroidal anti-inflammatory drugs, and night splints.
For this study, a personal interview was conducted with all patients to know the current situation. The average time from surgery to personal interview was 34.90 (± 18.73) months. In this interview, we recorded the pain at present (VAS scale), the presence of paresthesia (1: absence; 2: intermittent; 3: persistent) and sensitivity alterations (1: absence of sensitivity; 2: hyposensitivity; 3: intact sensitivity). The interosseous strength was measured quantitatively by approximation of the third-fourth finger and the first-fifth finger clamp, both in the intervened hand as in the contralateral, using an approved Jamar dynamometer (Jamar Hydraulic Hand Dynamometer®, Preston, Jackson, Missouri, USA). The presence of positive Tinnel sign and positive maneuver with elbow flexion were also assessed.
The final satisfaction received was measured with the modified Bishop’s rating system [7-9], which, among other things, includes the patient’s ability to return to his job and classifies the perceived result as excellent, good, acceptable or poor (Table 1). All patients were asked if they would undergo surgery again.
| Modifted Bishop score | |
| Severity of residual symptoms (pain, paresthesia, dysesthesia, weakness, clumsiness) | |
| Asymptomatic | 3 |
| Mild (occasional) | 2 |
| Moderate | 1 |
| Severe | 0 |
| Improvement | |
| Better | 2 |
| Unchanged | 1 |
| Worse | 0 |
| Work status | |
| Able to return to previous work | 1 |
| Unable to return to previous work | 0 |
| Strength | |
| Intrinsic muscle strength normal (M5) | 2 |
| Intrinsic muscle strength reduced to M4 | 1 |
| Intrinsic muscle strength less than equal to M3 | 0 |
| Sensitivity (static two points discrimination) | |
| Normal (≤ 6mm) | 1 |
| Abnormal (> 6mm) | 0 |
| Total: 8-9, excellent; 5-7, good; 3-4, fair; 0-2, poor. | |
Table 1: Modified Bishop score.
Surgical technique
All these surgeries were performed by a senior doctor (JM) using uniform procedure techniques at a single institution. Surgical technique was a modification of the classic Learmonth procedure [10,11].
Under brachial plexus block anesthesia, a 12 cm curvilinear skin incision is made over the medial epicondyle, preserving the branches of the medial brachial cutaneous nerve in the subcutaneous tissue. After splitting the fascia, the ulnar nerve is identified in the posterior compartment, along the medial intermuscular septum, immediately proximal to its entry into the cubital tunnel. The first step is to secure a proximal release at armpit level, which is achieved with scissors and digital dissection. Then we investigate the existence of a thickening in the aponeurosis, known as Strtuher´s ligament and if it exists, it is divided. Then the medial intermuscular septum is identified and divided, releasing the triceps fibers that sometimes cover the nerve. Subsequently, the arcuate ligament, the most frequent point of compression, is carefully divided to leave the nerve fully released at epitrochlear level. Then the Osborne´s arcade is release, between the two heads of flexor carpi ulnaris, and we check distally for any other places of nerve compression. Once fully released of the ulnar nerve, the flexor-pronator mass is incised deeply for 2 cm at its epicondylar attachment, and the musculofascial flap was elevated and slid distally to provide a new groove for the nerve, taking special care that it runs freely through that tunnel and respecting its vascularization, as well as the muscular branches. The central two-thirds of the flexor-pronator mass was directly repaired using interrupted fascial stitches A compressive bandage is performed that allows early active mobilization in the postoperative period.
Results
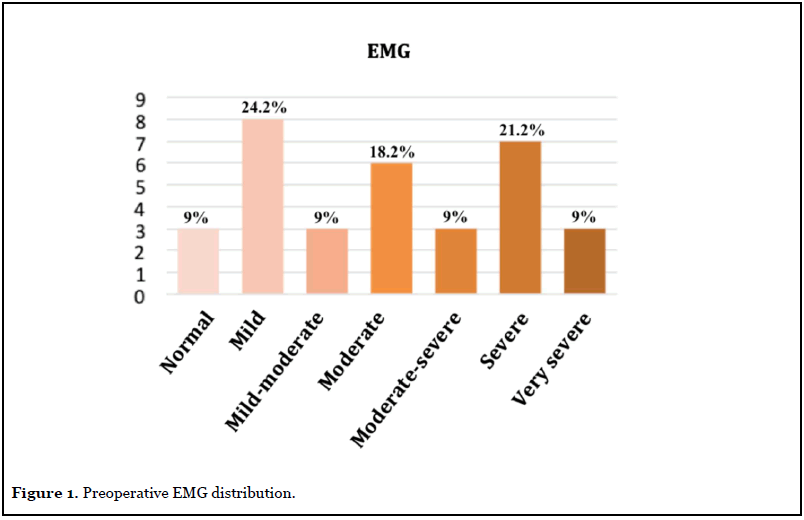
Demographic data are summarized in Table 2. Before surgery, 35 cases (81.4%) presented pain (VAS 5.39 ± 3.11), 42 cases (97.7%) had paresthesia’s, 39 cases (90.7%) reported hyposensitivity and 28 cases (65.1%) reported motor symptoms such as weakness, atrophy or positive Froment and Wartenberg signs. The McGowan scale degree was: nine grade I (20.9%), 25 grade II (58.1%) and nine grade III (20.9%). Preoperative EMG was available in 33 cases (Figure 1).
| Mean age (years) | 52.18 (± 14.23) |
| Gender, n (%) | |
|
20 (47.6) 22 (52.4) |
| Affected hand, n (%) | |
| - Right | 17 (40.5) |
| - Left | 24 (57.1) |
| - Bilateral | 1 (2.3) |
| Dominant hand, n (%) | |
| - Yes | 22 (51.2) |
| - No | 21 (48.8) |
| Handwork | |
| - Yes | 17 (40.5) |
| - No | 25 (59.5) |
| Mean follow-up (months) | 34.90 (± 18.73) |
| Mean duration of symptoms (months) | 19.60 (± 12.78) |
Table 2: Demographic data.
After surgery, there was a subjective pain improvement in 33 cases (76.7%), in an average of 4.3 points on the VAS scale (paired t-test, p <0.001) (Table 3). Of the eight cases who had no preoperative pain, seven remained painless and one had pain (VAS=5). Two cases with preoperative pain worsened after the intervention (from VAS=6 to VAS=7 in both).
| Preoperative | Postoperative | p | |
|---|---|---|---|
| Pain (VAS) | 5.39 ± 3.11 | 2.23 ± 2.62 | <0.001 |
| Worse | 3 (7.14%) | ||
| Unchanged | 7 (16.6%) | ||
| Better | 33 (76.1%) |
Table 3: Preoperative and postoperative Visual Analog Scale (VAS) pain score.
Of the 42 cases with paresthesia, 27 cases (64%) reported improvement (Wilkoxon test, p <0.001) (Table 4). Of the 39 cases with preoperative hyposensitivity, 36 (92.3%) reported improvement (Wilkoxon test, p<0.001). Three cases reported persistent hyposensitivity and there were no cases of worsening. 19 cases (44%) had a positive Tinel sign and three cases (6.9%) had a positive elbow flexion maneuver after surgery.
| Preoperative | Postoperative | |
|---|---|---|
| Absent | 1 | 24 |
| Intermittent | 26 | 14 |
| Persistent | 16 | 5 |
Table 4:Preoperative and postoperative paraesthesias degree distribution.
Of the 28 patients with strength alterations, 19 (67%) reported subjective improvement, although the objective study with dynamometer showed statistically significant differences (paired t-test, p <0.001) between the intervened hand and the contralateral, both in the approximation between third-fourth finger (7 ± 4.7 kg in the intervened limb; 8.9 ± 5 kg in the contralateral) and in the first-fifth finger clamp (4.5 ± 2.7 kg in the intervened limb; 6.8 ± 4 in the contralateral). In two cases the strength measurement was not assessable (one with bilateral surgery and one with previous fifth finger amputation).
There was a statistically significant postoperative McGowan scale improvement (Wilkoxon test, p=0.043) (Table 5). In 21 cases (48.8%) there was complete recovery of symptoms and a normal physical examination and they were classified as grade 0.
| Preoperative | Postoperative | |
|---|---|---|
| grade 0 | 0 | 21 |
| grade I | 9 | 16 |
| grade II | 25 | 5 |
| grade III | 9 | 1 |
Table 5: Preoperative and postoperative McGowan grade distribution.
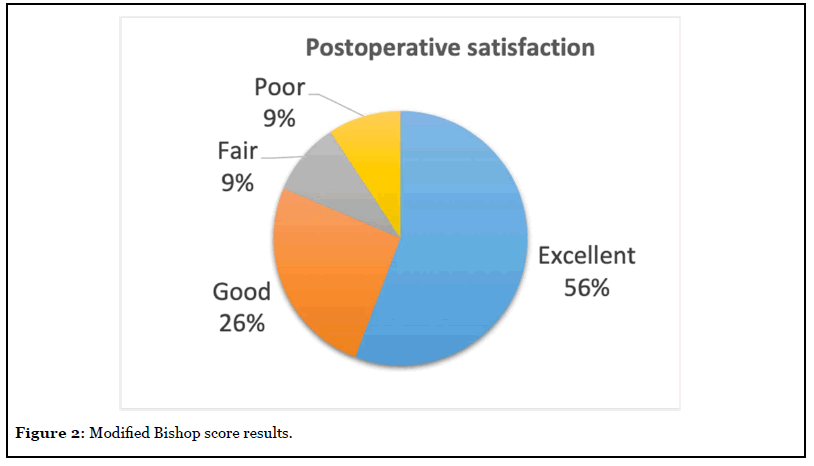
According to the modified Bishop score, we obtained 24 (55.8%) excellent, eleven (25.6%) good, four (9.3%) fair and four (9.3%) poor results (Figure 2). All the dissatisfied patients were manual workers. One presented relapse after a remission period of 4 months; one was later diagnosed about cervical pathology; one with previous fifth finger amputation continued with paresthesia’s; one has been unable to return to the previous job for an accidental section of the second finger flexor tendons during ulnar transposition recovery. 39 cases (90,7%) responded that they would be operated again.
A statistically significant relationship was found between final satisfaction and postoperative pain (Pearson, p<0.001), postoperative paresthesia’s (Wilkoxon test, p<0.001), alterations in postoperative sensitivity (Wilkoxon test, p<0.001), postoperative McGowan grade (Wilkoxon test, p< 0.001), duration of preoperative symptoms (Pearson, p=0.003) and reinterventions cases (Wilkoxon test, p=0.03). No statistically significant relationship was found with age, any of the preoperative symptoms, the preoperative McGowan grade or the preoperative EMG.
Postoperatively, one patient had a hematoma and accepted debridement. Subsequently, he received a good Modified Bishop score and had improved McGowan grade from II to 0. No infections, nerve injury, medial elbow pain, or elbow contractures were encountered.
Discussion
In 1958 Feindel and Stratford [12] minted the term “cubital tunnel syndrome” to refer to ulnar nerve compression at the elbow, by analogy with carpal tunnel syndrome. Even if they are both peripheral neuropathies, the cubital tunnel syndrome has greater pathophysiological complexity. Many compression points have been [13,14] and, in addition to the compressive phenomena, is important the elongation that suffers the nerve with the elbow flexion and the intraneural vascular compromise that this implies. Gelberman and Patel [4,15] showed that intraneural pressure increased more than extra neural pressure with elbow flexion above 90°, so not all the pressure suffered by the nerve could be explained by the tunnel pressure. During flexion, the traction suffered by the nerve causes a 30-40% reduction in its cross-sectional area and alters the intraneural microcirculation [16]. In this sense, only the transposition could eliminate all pathophysiological phenomena of ulnar neuropathy [17], and the submuscular would be the most appropriate when housing the nerve in a well-vascularized bed, protected from trauma and less susceptible to perineural scar tissue [18].
In the present study, the clinical results of submuscular transposition were satisfactory, with 82% of good or excellent results and only one case of post-surgical complication. Good results were recorded even in those subgroups in which worst results were expected as cases of severe compression or manual workers. Zimmerman [11] obtained similar results with 89% of good or excellent results in 142 patients and a minimum follow-up of 6 years. Similarly, Davis [19] describes an 82.5% clinical improvement in 45 submuscular transpositions and Lancigu [20] present a report of 82 patients in which 86% of patients were considered cured and 6% recurrence with a follow-up of 11.5 years. Fitzgerald [21] presents a series of 20 youth active patients, 19 of which obtained excellent results according to Bishop’s scale.
In our study, we found a relationship between the final satisfaction and residual symptoms and the postoperative McGowan grade. This result is expected since the Bishop score is based, among others, on residual symptoms. We also found the preoperative duration of symptoms and cases of reintervention as predictors of poor satisfaction. Studies are also contradictory in establishing satisfaction predictors [22], but several studies have found a relationship between satisfaction and duration of symptoms [23,24]. Yamamoto [25] conducted a retrospective study of 107 patients and concluded that age, symptoms duration, preoperative severity and nerve conduction velocity immediately after surgery were prognostic factors, while Kang [26] found the elderly, preoperative grip strength and preoperative twopoint discrimination as prognostic factors. In 2017, Suzuki [27] found the disease severity as the unique independent prognostic factor. In these studies, however, patients undergoing different surgical techniques.
Despite the good results mentioned and the theoretical advantages of submuscular transposition, comparative studies have not demonstrated superiority over in situ decompression or subcutaneous anterior transposition (an exception are the cases of ulnar subluxation, where transposition is better) [28]. Often, retrospective comparative studies and non-randomized prospective studies are not valid, since transposition is used in more severe neuropathies than in situ decompression or when consider transpositions group without distinction between subcutaneous or submuscular [29,30]. Randomized prospective studies found increased morbidity in the transposition group and no significant differences in clinical or electrophysiological results with submuscular transposition and in situ decompression [31,32]. Although Zarezadeh [33] found less residual pain in the submuscular transposition group concerning the subcutaneous transposition group, these results were not supported by a subsequent meta-analysis [34] which found no differences between the two techniques. However, it was based on only three studies with low and moderate quality. A previous meta-analysis of 906 cases [35] comparing in situ decompression, submuscular and subcutaneous transposition found a trend towards a better clinical outcome with transposition, although without statistical significance.
One reason for the inconclusive results is that different pre and postoperative evaluation systems have been used, such as the McGowan scale [6], the Messina criteria [36] the Dellon scoring system [37] or the Bishop score [9] among others. The severity of the disease may change according to the methods used. The severity of the disease may change according to the methods used. The lack of a standardized evaluation system makes comparison and reproducibility difficult. We use the McGowan classification because it is simple and easy to use.
Even assuming an equivalent neurological recovery, the revision rate is higher in cases of in situ decompression. While the overall recurrence rate, regardless of the surgical technique, has been estimated at 1.4% in a series of 25,977 cases [38] it described an incidence of up to 19% in cases of in situ decompression [39]. In these cases, the anterior transposition is the rescue treatment. However, the referred results of secondary transposition are worse than those of primary transposition [40, 41, 42]. This data is consistent with our results, so the benefits of a less invasive technique should be weighed against the higher revision rate associated with in situ decompression.
In our sample, there are three cases with normal preoperative EMG which improved after surgery and they all related excellent satisfaction. Ulnar nerve electrodiagnostic at the elbow is not as simple as the median nerve in the wrist and the correlation between clinical outcomes and postoperative electrodiagnostic has been questioned [19, 43, 44]. Although nerve conduction studies provide useful information in the diagnosis and monitoring of nerve compression syndromes, the diagnosis of ulnar neuropathy is eminently clinical.
The strength of this study is the homogeneity of the surgical technique in a broad spectrum of patients and, to our knowledge, the largest series published exclusively operated by the same surgeon. Considering this aspect, the sample size is relatively large. However, there are several limitations to our study. The retrospective design and patients lost to follow up are likely to be biased. A limited number of factors were analyzed and related to the satisfaction perceived by the patient, not with objective parameters. Although presurgical force is not objectively measured, we used the contralateral hand strength as a reference, based on studies that did not find statistically significant differences between dominant and non-dominant hand [45]. The inclusion of other factors can provide more valuable information. In the future, prospective and comparative studies are still needed to confirm our results.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
There is no financial support to declare.
References
2. Staples JR, Calfee R. Cubital tunnel syndrome: current concepts. Journal of the American Academy of Orthopaedic Surgeons. 2017 Oct 1;25(10): e215-24.
3. Karatas A, Apaydin N, Uz A, Tubbs SR, Loukas M, Gezen F. Regional anatomic structures of the elbow that may potentially compress the ulnar nerve. Journal of Shoulder and Elbow Surgery. 2009 Jul 1;18(4):627-31.
4. Gelberman RH, Yamaguchi KE, Hollstien SB, Winn SS, Heidenreich Jr FP, Bindra RR, et al. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. An experimental study in human cadavera. Journal of Bone and Joint Surgery. 1998 Apr 1;80(4):492-501.
5. AAEM Quality Assurance Committee. Practice Parameter for electrodiagnostic studies in ulnar neuropathy at the elbow. guidelines in electrodiagnostic medicine (2015). update from: AAEM, practice parameter for electrodiagnostic studies in ulnarneuropathy at the elbow, summary statement. Muscle Nerve. (1999) 22:408–11.
6. McGowan AJ. The results of transposition of the ulnar nerve for traumatic ulnar neuritis. The Journal of bone and joint surgery. British Volume. 1950 Aug;32(3):293-301.
7. Plancher KD, Bishai SK. Anterior intramuscular transposition of the ulnar nerve. Techniques in Orthopaedics. 2006 Dec 1;21(4):311-7.
8. Nouhan R, Kleinert JM. Ulnar nerve decompression by transposing the nerve and Z-lengthening the flexorpronator mass: clinical outcome. The Journal of Hand Surgery. 1997 Jan 1;22(1):127-31.
9. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. The Journal of Hand Surgery. 1989 Nov 1;14(6):972-9.
10. Learmonth JR. A technique for transplanting the ulnar nerve. Surgery, Gynecology and Obstetrics. 1942; 75:792-3.
11. JZimmerman RM, Jupiter JB, del Pino JG. Minimum 6-year follow-up after ulnar nerve decompression and submuscular transposition for primary entrapment. The Journal of Hand Surgery. 2013 Dec 1;38(12):2398-404.
12. Feindel W. The role of the cubital tunnel in tardy ulnar palsy. Canadian Journal of Surgery. 1958; 1:287-300.
13. Elhassan B, Steinmann SP. Entrapment neuropathy of the ulnar nerve. Journal of the American Academy of Orthopaedic Surgeons. 2007 Nov 1;15(11):672-81.
14. Felder III JM, Mackinnon SE, Patterson MM. The 7 Structures Distal to the Elbow That Are Critical to Successful Anterior Transposition of the Ulnar Nerve. Hand. 2019 Nov;14(6):776-81.
15. Patel VV, Heidenreich Jr FP, Bindra RR, Yamaguchi K, Gelberman RH. Morphologic changes in the ulnar nerve at the elbow with flexion and extension: a magnetic resonance imaging study with 3-dimensional reconstruction. Journal of Shoulder and Elbow Surgery. 1998 Jul 1;7(4):368-74.
16. Lundborg GO. Structure and function of the intraneural microvessels as related to trauma, edema formation, and nerve function. Journal of Bone and Joint Surgery. 1975 Oct 1;57(7):938-48.
17. Mitchell J, Dunn JC, Kusnezov N, Bader J, Ipsen DF, Forthman CL, Dykstra A. The effect of operative technique on ulnar nerve strain following surgery for cubital tunnel syndrome. Hand. 2015 Dec;10(4):707-11.
18. Lee SK, Sharma S, Silver BA, Kleinman G, Hausman MR. Submuscular versus subcutaneous anterior ulnar nerve transposition: a rat histologic study. The Journal of Hand Surgery. 2009 Dec 1;34(10):1811-4.
19. Davis GA, Bulluss KJ. Submuscular transposition of the ulnar nerve: review of safety, efficacy and correlation with neurophysiological outcome. Journal of Clinical Neuroscience. 2005 Jun 1;12(5):524-8.
20. Lancigu R, Saint Cast Y, Raimbeau G, Rabarin F. Dellon’s anterior submuscular transposition of the ulnar nerve: Retrospective study of 82 operated patients with 11.5 years’ follow-up. Chirurgie de la main. 2015 Oct 1;34(5):234-9.
21. Fitzgerald BT, Dao KD, Shin AY. Functional outcomes in young, active duty, military personnel after submuscular ulnar nerve transposition. The Journal of Hand Surgery. 2004 Jul 1;29(4):619-24.
22. Shi Q, MacDermid JC, Santaguida PL, Kyu HH. Predictors of surgical outcomes following anterior transposition of ulnar nerve for cubital tunnel syndrome: a systematic review. The Journal of Hand Surgery. 2011 Dec 1;36(12):1996-2001.
23. Lima S, Correia JF, Martins RM, Alves JM, Palheiras J, de Sousa C. subcuTaneous anTerior TransPosiTion for TreaTmenT of cubiTal Tunnel syndrome: is This meThod safe and effecTiVe? Revista Brasileira de Ortopedia (English Edition). 2012 Jan 1;47(6):748-53.
24. Lee SK, Lee GS, Choy WS. VY lengthening technique of the flexor-pronator mass for anterior submuscular transposition of the ulnar nerve in severe cubital tunnel syndrome: a long-term follow-up study. Annals of Plastic Surgery. 2018 May 1;80(5):533-8.
25. Shishido T, Yamamoto K, Masaoka T, Katori Y, Tanaka S. Postoperative clinical results in cubital tunnel syndrome. Orthopedics. 2006 Apr 1;29(4):347-53.
26. Kang HJ, Oh WT, Koh IH, Kim S, Choi YR. Factors influencing outcomes after ulnar nerve stability-based surgery for cubital tunnel syndrome: a prospective cohort study. Yonsei Medical Journal. 2016 Mar 1;57(2):455-60.
27. Suzuki T, Iwamoto T, Shizu K, Suzuki K, Yamada H, Sato K. Predictors of postoperative outcomes of cubital tunnel syndrome treatments using multiple logistic regression analysis. Journal of Orthopaedic Science. 2017 May 1;22(3):453-6.
28. Bimmler D, Meyer VE. Surgical treatment of the ulnar nerve entrapment neuropathy: submuscular anterior transposition or simple decompression of the ulnar nerve?: Long-term results in 79 cases. InAnnales de chirurgie de la main et du membre supérieur 1996 Jan 1 (Vol. 15, No. 3, pp. 148-157). Elsevier Masson.
29. Staples R, London DA, Dardas AZ, Goldfarb CA, Calfee RP. Comparative morbidity of cubital tunnel surgeries: a prospective cohort study. The Journal of Hand Surgery. 2018 Mar 1;43(3):207-13.
30. Zhang D, Earp BE, Blazar P. Rates of complications and secondary surgeries after in situ cubital tunnel release compared with ulnar nerve transposition: a retrospective review. The Journal of Hand Surgery. 2017 Apr 1;42(4):294-e1.
31. Gervasio O, Gambardella G, Zaccone C, Branca D. Simple decompression versus anterior submuscular transposition of the ulnar nerve in severe cubital tunnel syndrome: a prospective randomized study. Neurosurgery. 2005 Jan 1;56(1):108-17.
32. Biggs M, Curtis JA. Randomized, prospective study comparing ulnar neurolysis in situ with submuscular transposition. Neurosurgery. 2006 Feb 1;58(2):296-304.
33. Zarezadeh, A., Shemshaki, H., Nourbakhsh, M., Etemadifar, M.R., Moeini, M. and Mazoochian, F., 2012. Comparison of anterior subcutaneous and submuscular transposition of ulnar nerve in treatment of cubital tunnel syndrome: a prospective randomized trial. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences, 17(8), p.745.
34. Liu CH, Wu SQ, Ke XB, Wang HL, Chen CX, Lai ZL, et al. Subcutaneous versus submuscular anterior transposition of the ulnar nerve for cubital tunnel syndrome: a systematic review and meta-analysis of randomized controlled trials and observational studies. Medicine. 2015 Jul;94(29).
35. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. The Journal of Hand Surgery. 2008 Oct 1;33(8):1314-e1.
36. Messina A, Messina JC. Transposition of the ulnar nerve and its vascular bundle for the entrapment syndrome at the elbow. Journal of Hand Surgery. 1995 Oct;20(5):638-48.
37. Dellon AL, Coert JH. Results of the musculofascial lengthening technique for submuscular transposition of the ulnar nerve at the elbow. Journal of Bone and Joint Surgery. 2003 Jul 1;85(7):1314-20.
38. Camp CL, Ryan CB, Degen RM, Dines JS, Altchek DW, Werner BC. Risk factors for revision surgery following isolated ulnar nerve release at the cubital tunnel: a study of 25,977 cases. Journal of Shoulder and Elbow Surgery. 2017 Apr 1;26(4):710-5.
39. Krogue JD, Aleem AW, Osei DA, Goldfarb CA, Calfee RP. Predictors of surgical revision after in situ decompression of the ulnar nerve. Journal of Shoulder and Elbow Surgery. 2015 Apr 1;24(4):634-9.
40. Aleem AW, Krogue JD, Calfee RP. Outcomes of revision surgery for cubital tunnel syndrome. The Journal of Hand Surgery. 2014 Nov 1;39(11):2141-9.
41. Gabel GT, Amadio PC. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. Journal Bone and Joint Surgery. 1990 Feb 1;72(2):213-9.
42. Grandizio LC, Maschke S, Evans PJ. The management of persistent and recurrent cubital tunnel syndrome. The Journal of Hand Surgery. 2018 Oct 1;43(10):933-40.
43. Tapadia M, Mozaffar T, Gupta R. Compressive neuropathies of the upper extremity: update on pathophysiology, classification, and electrodiagnostic findings. The Journal of Hand Surgery. 2010 Apr 1;35(4):668-77.
44. Landau ME, Campbell WW. Clinical features and electrodiagnosis of ulnar neuropathies. Physical Medicine and Rehabilitation Clinics. 2013 Feb 1;24(1):49-66.
45. Torres-Coscoyuela M, González del Pino J, Yánez Calvo J, Bartolome del Valle E. Dynamometric study of the hand and thumb. Revista Española de Cirugía Ortopédica y Traumatología. 1999. 43(5):321-6.
