Abstract
Direct oral anticoagulants (DOAC) are used in several indications for the prevention and treatment of thrombotic events. As highlighted by data from clinical trials and case studies, all DOAC carry the risk of bleeding despite careful selection and patient management. Previous publications have demonstrated the limited knowledge of many physicians concerning the indications for, and correct management of, these anticoagulants. Health institutions should develop risk minimisation strategies and educational materials to prevent major adverse events related to DOAC administration. Major bleeding events are reported in clinical practice and specific antidotes are emerging from Phase III trials. Some antidotes are licensed but their high cost might limit routine use. We therefore illustrate approaches and tools that can help physicians prescribe DOAC appropriately. We focus on screening for modifiable bleeding risk factors and adapting doses according to the individual benefit-risk profile. We also provide recommendations on managing a missed dose, switching, bridging, and resumption.
INTRODUCTION
Bleeding events have been reported in all the major clinical trials comparing direct oral anticoagulants (DOAC) with other anticoagulants despite regular monitoring of adverse events, strong medication adherence, and careful patient selection.1-4
Large randomised trials comparing bleeding risks of different DOAC are not available as DOAC have always been compared with warfarin, low molecular weight heparin (LMWH), and antiplatelet agents. When DOAC were prescribed at prophylactic doses in orthopaedic surgery, rates of bleeding were similar to LMWHs.5 When DOAC were prescribed for patients with non-valvular atrial fibrillation (NVAF) or venous thromboembolism (VTE), a recent meta-analysis showed a lower rate of fatal bleeding and case-fatality of major bleeding (MB) events for DOAC than with warfarin.6 Another meta-analysis showed that patients with a creatinine clearance (CrCl) between 50–80 mL/min who received DOAC had significantly fewer MB events than those receiving vitamin K antagonists (VKA). For patients with CrCl <50 mL/min, the difference in MB was not statistically significant, but using indirect comparisons, apixaban was associated with significantly fewer MB episodes than other DOAC.7
Post-marketing surveillance registries provide real-world data on the safety and efficacy of DOAC. A retrospective cohort study of Medicare beneficiaries with NVAF found a higher incidence of MB with dabigatran than with warfarin, regardless of anatomical site.8 A smaller Italian cohort study revealed a safe profile of either 110 mg or 150 mg dabigatran twice daily (bid), regarding fatal bleeding in patients at high risk of haemorrhage and thromboembolism.9 The Dresden New Oral Anticoagulants (NOAC) registry found that the routine safety profile of dabigatran etexilate (DE) was no worse than that reported in the RE-LY trial even if selection bias might exist.10 The Danish national registry found a higher bleeding rate in previous VKA users than VKA-naïve patients. This observation may reflect patient selection and ‘drug switching’ practices.11 Concerning rivaroxaban, a large observational study showed that the MB rate was generally consistent with registration trial results and that fatal bleeds were rare.12 The MB rate with rivaroxaban from the Dresden NOAC registry was lower than that for VKA.13 However, the ROCKET AF trial identified age, sex, diastolic blood pressure, prior gastrointestinal bleeding, prior aspirin use, and anaemia as clinically relevant factors associated with MB risk with rivaroxaban and VKA.14 This review aims to discuss strategies to prevent DOAC-related bleeding in normal clinical practice.
HOW TO PREVENT MAJOR BLEEDING
Various aspects of the management of patients treated with DOAC should be highlighted to reduce the incidence of MB.
Appropriate Use of Direct Oral Anticoagulants
Off-label use or misuse
The off-label use or misuse of DOAC means use outside of approved indication, at an inappropriate dose, or an inappropriate choice of DOAC according to patient characteristics. Inappropriate use is frequent (occurring in 8–49% of patients) and may cause infra or supra-therapeutic anticoagulation carrying risks of thromboembolism or severe, even fatal bleeding (Table 1).11,15-19 A recent publication indicated a significant risk for patients related to lack of physicians’ knowledge about DOAC and highlighted the need for additional education and training.20
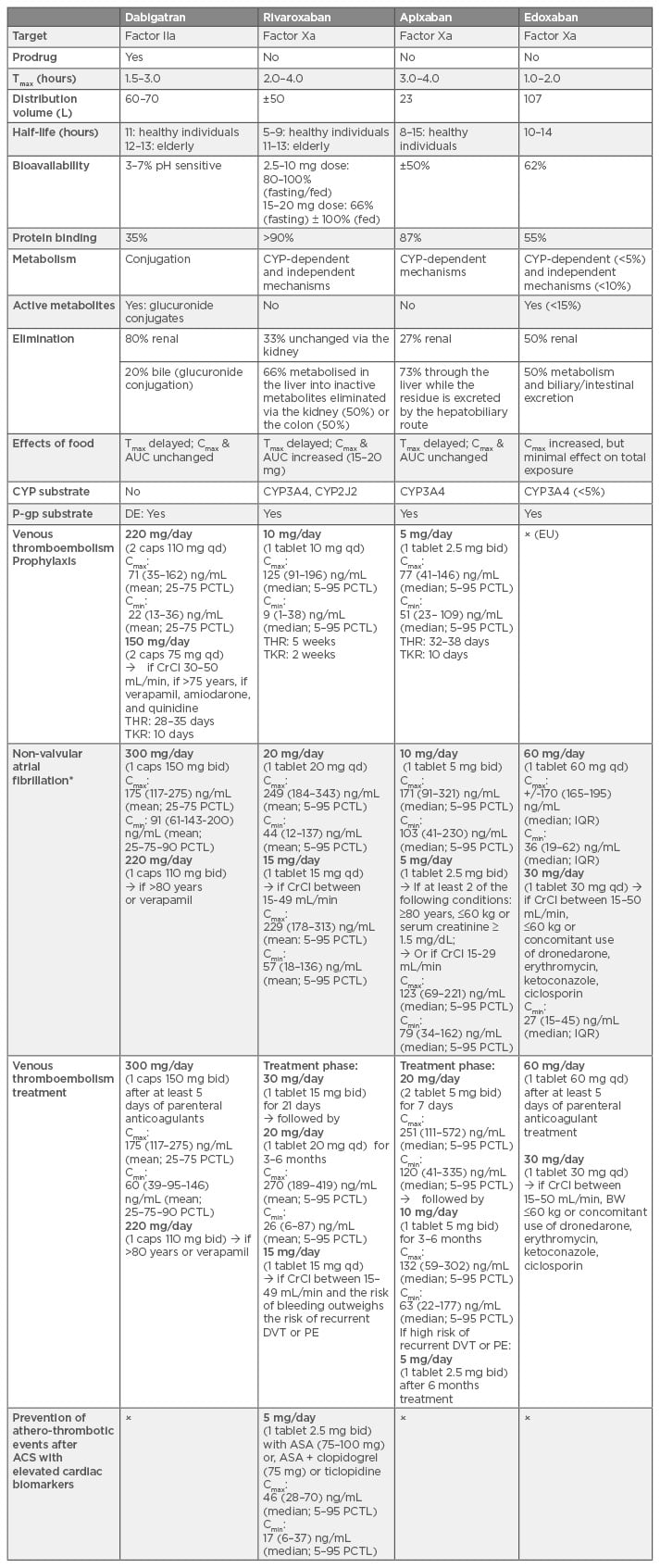
Table 1: Summary of pharmacokinetic properties, indication, and dose regimens of direct oral anticoagulants in the European Union.29,35,46,54,62-83
Tmax: time to reach peak concentration; Cmax: peak concentration; AUC: area under the curve; CYP3A4: cytochrome P450 isozyme 3A4; P-gp: P-glycoprotein; BRCP: breast cancer resistance protein; caps: capsule; qd: once daily; bid: twice daily; CrCl: creatinine clearance; DVT: deep-vein thrombosis; PE: pulmonary embolism; THR: total hip replacement; TKR: total knee replacement; ASA: acetylsalicylic acid; ACS: acute coronary syndrome; Cmax and Cmin: peak and trough concentrations providing from the clinical trials; PCTL: percentiles; IQR: interquartile range; BW: body weight; DE: dabigatran etexilate.
x = off-label; EU: European Union.
Renal function
Renal failure is the most common risk factor associated with bleeding in elderly patients, and should therefore be assessed before initiating DOAC and during treatment.21 The updated European Heart Rhythm Association (EHRA) practical guide on the use of DOAC suggests that renal function should be checked every 6 months in patients >75–80 years (especially those on DE or edoxaban), and in frail patients.22 The proposed recheck interval (in months) is the CrCl divided by 10, if the CrCl is <60 mL/min. More frequent checks are recommended in patients with conditions that might affect renal function. In clinical trials of DOAC for NVAF, drug eligibility and dosing were determined using the Cockcroft– Gault equation to estimate CrCl. The modification of diet in renal disease equation (used to estimate glomerular filtration rate) tends, at low estimate glomerular filtration rate values, to overestimate renal function compared with the Cockcroft–Gault equation.23,24 This overestimation may lead to incorrect decisions about eligibility or dose.
Bioavailability
If DE capsules are opened, the bioavailability increases to 75% and the bleeding risk is greatly enhanced.25 Therefore, DE should not be given via a gastrostomy or jejunostomy and capsules should not be opened or crushed before administration. Rivaroxaban and apixaban have similar bioavailability when administered in crushed form mixed with apple sauce or water through a nasogastric tube or gastrostomy.26,27 To the best of our knowledge, there are currently no data available for edoxaban.
Patient body weight
There is evidence that anticoagulant clearance rates increase with body weight, however optimal dosing strategies for DOAC in obese patients remain unknown.28 In the RE-LY study, dabigatran concentrations tended to increase with decreasing body weight.28 Close clinical surveillance is recommended by several authorities because of limited clinical experience in these populations.29,30
Kubitza et al.31 showed that rivaroxaban peak concentration (Cmax) was increased by 24% in subjects weighing ≤50 kg while the area under the curve (AUC) was unaffected (difference was <25%) by body weight resulting in a small (15%) increase in prolongation of prothrombin time, which was not considered to be clinically relevant. Similarly to dabigatran, no dose adjustment is currently proposed by the European or American agencies.32
Regarding apixaban, a 30% and 20% increase in Cmax and AUC, respectively, have been reported in patients weighing <50 kg.33 Since body weight seems to have a modest effect on apixaban exposure, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) recommend dose adjustment in patients weighing <60 kg (2.5 mg bid instead of 5 mg bid) in the presence of additional risk factors, namely age ≥80 years or serum creatinine >1.5 mg/dL.34,35
Dose adjustment in patients weighing <60 kg is also recommended for edoxaban (30 mg once daily since Cmax and AUC in patients with low body weight (median 55 kg) were increased by 40% and 13%, respectively, compared with patients with high body weight (median 84 kg) in a population pharmacokinetic analysis of the ENGAGE AF-TIMI 48 study in NVAF.36
Patients with impaired hepatic function
The manufacturer’s recommendations for DOAC in patients with impaired hepatic function are based on both Child–Pugh classification and liver-related exclusion criteria applied in clinical trials (elevated liver enzymes [aspartate transaminase/alanine transaminase] >2-times the upper limit of normal or total bilirubin ≥1.5-times the upper limit of normal).37 The EMA contraindicates the use of dabigatran in patients with hepatic impairment or liver disease expected to have any impact on survival.29
In contrast to dabigatran, liver metabolism is an important route of elimination for FXa inhibitors. Approximately two-thirds of the administered rivaroxaban dose is metabolised by the liver via CYP3A4, 2J2, and CYP-independent mechanisms into inactive metabolites.32 Apixaban undergoes liver metabolism mainly via CYP3A4/5, but other isoenzymes are also involved, while edoxaban is metabolised via carboxylesterase-1 hydrolysis, conjugation, or oxidation by CYP3A4/5 (<10%). Therefore, the EMA contraindicates their use in cases of hepatic disease associated with coagulopathy and clinically relevant bleeding risk. Apixaban and edoxaban are not recommended in patients with severe hepatic impairment, rivaroxaban is also contraindicated in cirrhotic patients with Child–Pugh B/C. Although one limited study showed a similar bleeding risk in Child–Pugh A/B cirrhosis patients treated with apixaban or rivaroxaban compared with traditional anticoagulants, further evaluations and larger studies are needed to better inform clinicians’ decision-making.38
Drug interactions
In addition to these specific populations, DOAC also interact with P-glycoprotein substrates and CYP3A4 inhibitors, which may greatly increase their plasma concentrations, and hence, bleeding risks. Table 2 summarises drug-drug interactions reported in the literature as well as recommendations for dose adaptation and contraindications for the EMA and FDA prescribing guidelines.22,39,40 As there is an increased risk of bleeding, DOAC should be used with caution if a concomitant use of antiplatelet agents is indicated29,32,35 and non-steroidal anti-inflammatory drugs (NSAID) should be avoided if possible.28,31,39 Concomitant use with any other anticoagulant is strictly contraindicated, except during switching procedures from DOAC to VKA or when unfractionated heparin (UFH) is given at doses necessary to maintain an open central venous or arterial catheter.29
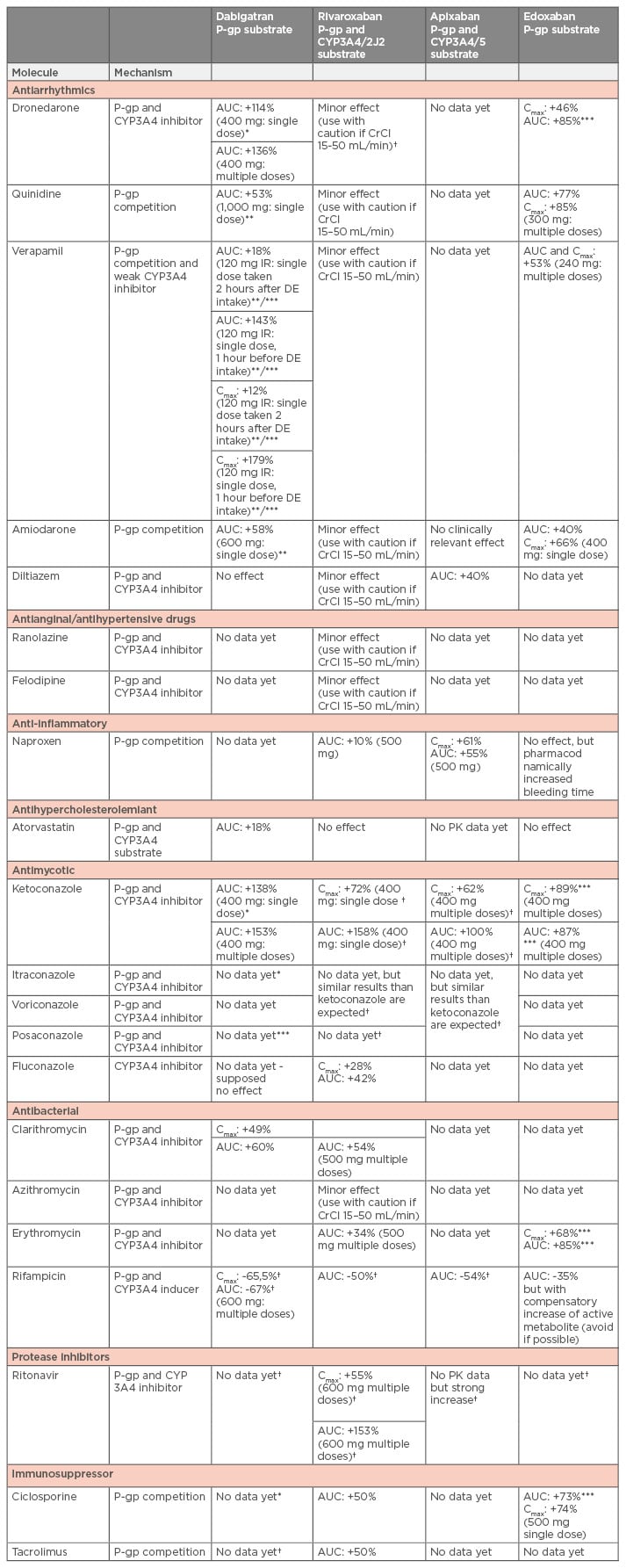
Table 2: Summary of drug-drug interactions with available recommendations for dose adaptation or contraindications by the competent authorities.22,39,40,83
*Contraindicated by the European Medicines Agency (EMA).
**The EMA recommends dose reduction for dabigatran: from 220 mg qd to 150 mg qd in prevention of VTE in joint replacement.
***The EMA recommends dose reduction for dabigatran: from 150 mg bid to 110 mg bid) in patients with NVAF or VTE; and for edoxaban a dose reduction of 50% (30 mg qd) in patients with NVAF or VTE.
†Concomitant treatment with these drugs are not recommended by the EMA.
NVAF: non-valvular arterial fibrillation; VTE: venous thromboembolism; P-gp: p-glycoprotein; CYP: cytochrome; AUC: area under the curve; CrCl: creatinine clearance; PK: pharmacokinetics; Cmax/Cmin: peak and trough concentrations providing from the clinical trials; qd: once daily; bid: twice daily; DE: dabigatran etexilate.
Screening for Bleeding Risk Factors
Non-modifiable and potentially reversible clinical features associated with higher bleeding risks must be carefully identified when initiating DOAC therapy and assessed regularly throughout treatment. Various risk stratification scores can help clinical decision-making.41,42 The bleeding risk score HAS-BLED has shown better performance than the HEMORR(2)HAGES and ATRIA risk scores in predicting MB in anticoagulated AF patients.41 A value ≥3 indicates a high risk for haemorrhage and suggests review and correction of modifiable risk factors (hypertension, renal/ hepatic impairment, alcohol excess, concomitant use of non-steroidal anti-inflammatory drugs, antiplatelet agents, or selective serotonin reuptake inhibitors). The simple five-element ORBIT AF bleeding risk score (older age [>75 years], reduced haemoglobin/haematocrit history of anaemia, bleeding history, insufficient kidney function, and treatment with antiplatelet agents) had the best ability to predict MB in patients with NVAF compared with HAS-BLED and ATRIA risk scores.43 Screening for injuries (e.g. recent intracranial haemorrhage) and conditions (e.g. colon cancer) that may lead to MB is essential before starting DOAC therapy.
Particular attention should be paid to renal protective strategies for patients on dabigatran and edoxaban. These patients should be informed about the impact of concurrent medications (e.g. NSAID) or comorbidities (e.g. dehydration) on their renal function, which could lead to an enhanced and prolonged anticoagulant effect.
Adapting Direct Oral Anticoagulant Dosage to Individual Benefit-Risk Ratios
Why and when?
Several criteria should be taken into account when considering drug monitoring: 1) intra and 2) inter-individual variability in plasma drug level, both justifying the identification of the optimal dose for the individual at the start of treatment, 3) variability and reproducibility of the testing method, 4) correlation between drug level and clinical outcome, and 5) the therapeutic value of drug monitoring.
DOAC demonstrate high intra and inter-individual variability depending on patients’ age, renal and hepatic function, drug-drug interactions, and body weight.44 While package inserts recommend dose adaptation according to the patient’s characteristics, they do not give guidance for patients with multiple interfering factors in whom a targeted plasma level cannot be reached. The importance of targeted plasma levels is supported by analysis of large trials (RE-LY and the ENGAGE-AF TIMI 48), which showed that plasma concentrations of dabigatran and edoxaban were correlated with the incidence of MB and thrombotic events.45-47 Although there are currently no published data for rivaroxaban and apixaban, data from the FDA Clinical Pharmacology and Biopharmaceutics Reviews (available at http://www.fda.gov) clearly suggest an association between drug exposure and safety outcomes. Thus, patients may benefit from individualised dose tailoring.
In addition, specific situations require an assessment of the intensity of anticoagulation to prevent bleeding and other complications. These include the peri-procedural management of urgent surgery or elective procedures, before thrombolysis or percutaneous coronary intervention, and bridging therapy. Patients on dual antiplatelet therapy added to DOAC could also benefit from a reduced posology to prevent bleeding.48
How to handle and perform such measurements?
Compared to VKA, the effect of DOAC on clotting tests varies greatly depending on the time between the last dose and blood sampling (Table 1).49 Therefore, the time of the last dose in relation to the blood sampling must be known in order to interpret the results of a particular test.
In ambulatory patients, regulatory documents only state thresholds associated with a risk of bleeding for dabigatran. The cut-offs proposed for apixaban, rivaroxaban, and edoxaban are based on the pharmacokinetic characteristics of these drugs where it is suggested that exceeding the highest percentile of a trough concentration carries a risk of bleeding. Regarding which test should be used, Table 3 summarises recommendations depending on the situation and the drug. While limitations have been mentioned for routine coagulation tests such as prothrombin time, activated partial thromboplastin time, and even thrombin time,50,51 the aim of this comprehensive approach is to provide reliable options, even for laboratories where more specific tests are not available, to give physicians sufficient information to support their clinical decisions.
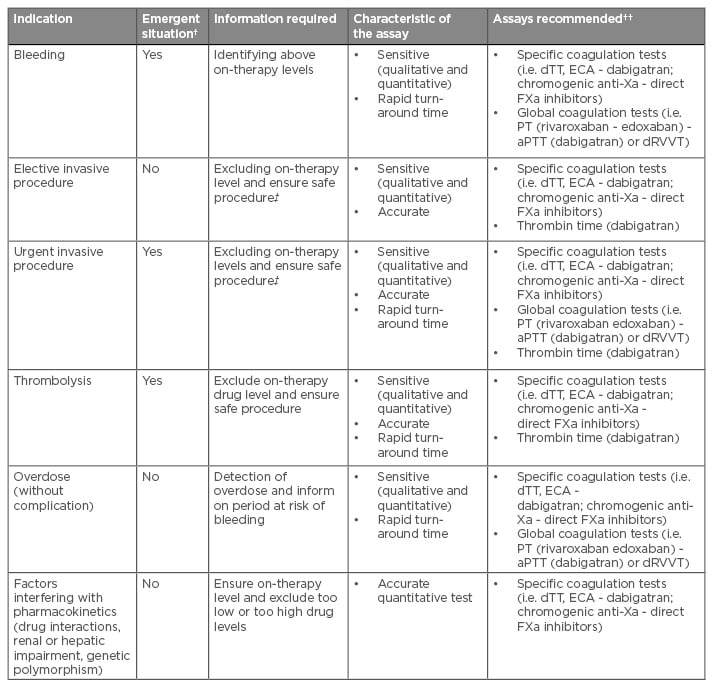
Table 3: Current indication, clinical need, and required assay characteristics for the measurement of direct oral anticoagulants.49-51
†Emergency situations are those in which anticoagulant effects should be measured within 30 minutes.
††Assays recommended are presented from the more to the less suitable among those able to respond the clinical need.
‡The assessment will depend on the type of procedure.
aPTT: activated partial thromboplastin time; dRVVT: dilute Russell’s Viper Venom Time; dTT: dilute thrombin time; ECA: ecarin chromogenic assays; PT: prothrombin time; FXa: factor Xa.
Dealing with Missed Doses
If a missed dose is noticed within 6 hours of the correct time for dabigatran/apixaban or within 12 hours for edoxaban/rivaroxaban, patients should take the forgotten dose immediately. Beyond these times, the dose should be skipped and the next scheduled dose should be taken.22 A double dose should never be taken to make up for a missed dose except during the first 21 days of rivaroxaban administration for VTE treatment, where two 15 mg tablets may be taken simultaneously if a dose is missed to ensure a total daily dose of 30 mg.32
Adherence to Switching Procedures
The correct timing of switching procedures is essential to avoid excessive anticoagulation due to additional effects and to reduce thromboembolic risk due to transitory low anticoagulation. This timing has been fixed for each DOAC taking into account the drug profile and trial protocols.52 The Dresden NOAC registry reported that 25% of patients did not have an international normalised ratio (INR) measurement before switching from VKA to DE or rivaroxaban.53
Practically, when VKA are switched to DOAC, VKA should be discontinued and DOAC should be started as soon as the INR is ≤2 for dabigatran and apixaban, ≤2.5 for edoxaban, or ≤3 for rivaroxaban. The EMA recommends that for patients with a deep vein thrombosis, rivaroxaban should be initiated once the INR is ≤2.5.22
When DOAC are switched to VKA, DOAC should be administered concomitantly until the INR reaches an appropriate anticoagulation level. As DOAC can have an impact on the INR measurements, this should be taken just before the next scheduled administration of the DOAC and be rechecked 24 hours later. Close monitoring of the INR is recommended within the first month of switching. For edoxaban, the administration of a half dose is recommended when VKA is started.22 For DE, the starting time of the VKA should be based on CrCl as follows: 3 days if CrCl is >50 mL/min, 2 days if CrCl is 30–50 mL/min, and 1 day if CrCl is 15–30 mL/min.29,30
When switching from DOAC to parenteral anticoagulants (LMWH or UFH), these should be started when the next DOAC dose is due. Inversely, DOAC should be started at the same time or up to 2 hours before the next parenteral anticoagulant dose. For intravenous UFH, DOAC should be started at the time of discontinuation of the infusion.22,29 The EMA recommends that edoxaban should be started 4 hours after stopping the UFH infusion.54 The renal function should be assessed before switching from heparin to DOAC.
Adherence to Bridging Procedures
The peri-procedural interruption of DOAC treatment depends on the bleeding risk of the procedure and the patient’s thromboembolic risk. Interruption may require bridging therapy with LMWH or UFH, especially for patients with high thromboembolic risks (CHADS2 score >4, recent stroke, VTE, or transient ischaemic attack within the past 3 months, hypercoagulable state) and in cases of severe renal impairment when interruption is >96 hours.55 Recent data showed increased peri-procedural MB events in patients bridged with heparins after DOAC were stopped, with no decrease in the incidence of cardiovascular or thrombotic events compared with patients who were not bridged.56-58 The BRIDGE-trial showed similar results concerning patients on warfarin.59 The first prospective perioperative study including patients receiving dabigatran showed a safe profile in terms of haemorrhagic and thrombotic events without the use of LMWH bridging.60 The use of LMWH bridging after antiplatelet therapy cessation in patients with coronary stents who underwent non-cardiac surgery, showed a worse ischaemic outcome at 30 days and a significant risk of bleeding compared with the group who did not stop antiplatelet therapy and had no bridging.61 Therefore, arguments for bridging therapy should be balanced, as it may be harmful for some patients, and further studies are needed to validate the safety of bridging therapy in specific populations, for example, thrombophilic patients or patients with high CHADS2 score (5–6).
Resuming DOAC after an invasive procedure should only be considered when haemostasis is achieved, usually 6–8 hours after procedures with a standard risk of bleeding or atraumatic spinal/epidural anaesthesia. For procedures with a high risk of bleeding, DOAC at full therapeutic dosage should be deferred until 48–72 hours after the invasive procedure. This interval is justified by the fact that specific antidotes are not available everywhere in case of postoperative bleeding or re-operation.22 Furthermore, the high cost of these antidotes may severely limit their use in clinical practice. For cases of prolonged fasting, immobilisation, or patients with high thrombotic risk profile, short-term administration of reduced venous thromboprophylactic or an intermediate dose of LMWH can be considered before resuming DOAC.22 Renal function should be assessed postoperatively when anticoagulants are resumed.
CONCLUSIONS
Despite the considerable advantages that DOAC bring to the field of anticoagulation, their inappropriate use can lead to a higher than expected risk of bleeding. Continuous education of healthcare practitioners and patients about dose adjustment and compliance guidance should be supported in all health institutions. Modifiable bleeding risk factors should be identified before initiation of DOAC and reviewed regularly. Individual benefit-risk may be improved in some clinical settings or in patients at risk of supra or infra-therapeutic plasma levels by altering the dose following coagulation monitoring. Protocols for switching, bridging, and resuming anticoagulants should be standardised and systems should be designed to optimise the management of patients treated with DOAC.