Function of the hand as a predictor of early diagnosis and progression of Alzheimer’s dementia: A systematic review
Abstract
BACKGROUND:
The dominant feature of Alzheimer’s dementia (AD) is gradual cognitive decline, which can be reflected by reduced finger dexterity.
OBJECTIVE:
This review analyzed reports on hand function in AD patients to determine the possibility of using it for an early diagnosis and for monitoring the disease progression of AD.
METHODS:
PubMed, Web of Science, EMBASE, and Cochrane library were searched systematically (search dates: 2000–2022), and relevant articles were cross-checked for related and relevant publications.
RESULTS:
Seventeen studies assessed the association of the handgrip strength or dexterity with cognitive performance. The hand dexterity was strongly correlated with the cognitive function in all studies. In the hand dexterity test using the pegboard, there was little difference in the degree of decline in hand function between the healthy elderly (HE) group and the mild cognitive impairment (MCI) group. On the other hand, there was a difference in the hand function between the HE group and the AD group. In addition, the decline in hand dexterity is likely to develop from moderate to severe dementia. In complex hand movements, movement speed variations were greater in the AD than in the HE group, and the automaticity, regularity, and rhythm were reduced.
CONCLUSIONS:
HE and AD can be identified by a simple hand motion test using a pegboard. The data can be used to predict dementia progression from moderate dementia to severe dementia. An evaluation of complex hand movements can help predict the transition from MCI to AD and the progression from moderate to severe dementia.
1.Introduction
AD accounts for over half of all dementia cases [1]. The dominant feature of AD is a gradual decline in cognition, particularly memory function and orientation. Although cognitive impairment can be observed, even in the preclinical phase of AD [2], and memory dysfunction can be observed in all older age group [3]. Therefore, other markers in addition to cognitive function are needed to detect people at high risk of developing AD. The loss of fine motor skills has been investigated to identify the behavioral markers of neurodegenerative diseases [4, 5]. In particular, the relationship with muscle weakness in the hand has been reported [6, 7]. Decreased finger dexterity may reflect cognitive decline, and an assessment of the hand motor function should be of interest for use as an early diagnosis of AD and a possible predictor of disease progression.
The hand function is an important part of the human movement repertoire and is essential in many activities that demand well-coordinated hand and arm movements [8]. Grip strength is sensitive to age-related and biological function changes in the hand functions. Decreased grip strength is a feature of age-related muscle strength loss and can indicate overall health in older adults [9]. Hand agility is the ability to make precisely coordinated movements of the fingers of one or both hands to grasp, manipulate, or assemble very small objects [10, 11, 12]. Hand agility is more complex than the grip strength, and dexterity can be an expression of creativity and precision in a range of activities [13]. Hand agility is a highly complex physical function that requires a combination of planning in the center that receives the senses, execution of the hand muscles, and attention [14]. Hand skill is developed continuously throughout one’s life and is learned through motivation, purpose, and repetition of similar tasks. The process requires a complex neural network [13].
It is important to determine if the cognitive decline is due to normal aging or a precursor to AD. If changes in grip strength and dexterity can be used as a standard for determining whether cognitive impairment is due to normal aging or a precursor symptom of dementia, it will be possible to cope with dementia more quickly. It is highly usable and accessible as grip strength and can be measured in the community without the need for expensive equipment or special facilities. Therefore, if dementia can be predicted based on hand dexterity and grip strength, it can greatly contribute to a healthy old age.
In this study, we analyzed research related to hand function in dementia patients and investigated the possibility of using it as an early diagnosis of Alzheimer’s disease and a predictor of disease progression.
2.Methods
2.1Search strategy
The literature search for this study was conducted in the same way by connecting the two words “dementia and dexterity” OR “dementia and hand function” OR “fine motor and Alzheimer’s” OR “fine motor and Alzheimer’s disease” AND “grip” in PubMed, Web of Science core collection, EMBASE, and Cochrane library. When searching with the above keywords, almost no studies before 2000 appeared. Therefore, we set the research period from 2000 to 2022. The research search and selection were conducted through a database search according to the PRISMA (preferred reporting items for systematic reviews and meta-analysis) flow chart [15].
Eight hundred and forty-three articles were initially retrieved by entering the search term. Of these, 328 overlapping papers were excluded, 449 papers that met the exclusion conditions among 515 papers, and papers without a full text were excluded. The final 17 papers were used for analysis. Two hundred and nine articles from Pub-Med, 159 articles from the Web of Science core collection, 473 articles from EMBASE, and two articles from the Cochrane library were found (Fig. 1).
Figure 1.
PRISMA flow diagram for trials included and excluded from the systematic review.
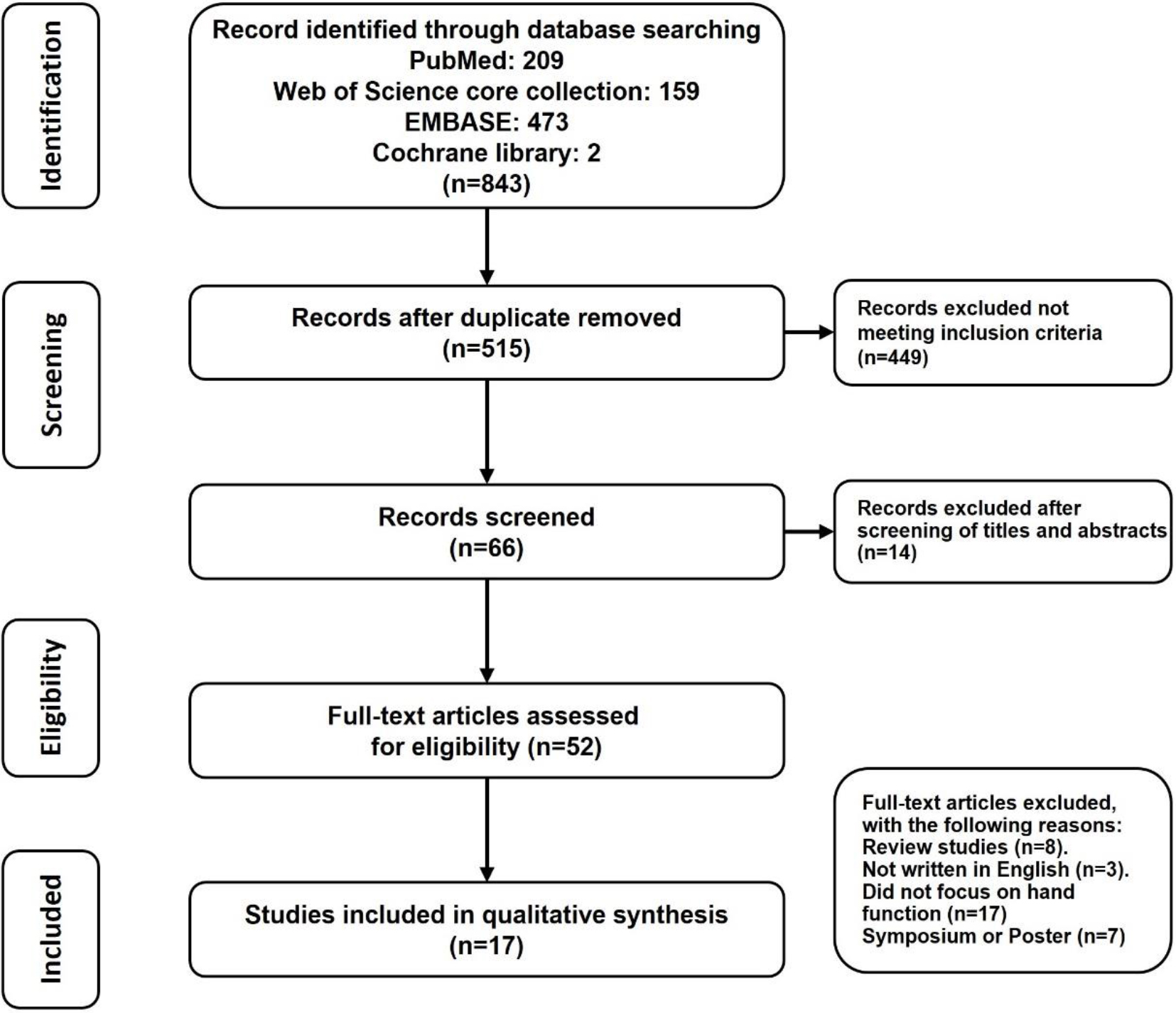
Seventeen studies that met the exclusion and selection conditions were used for the final analysis; four were longitudinal studies, and 13 were experimental studies. The title and abstract were reviewed to determine the relevance to the research topic. If the appropriateness of the paper was ambiguous, the full text was read to determine whether to include it.
2.2Inclusion and exclusion criteria
The exclusion criteria were 1) dissertation, 2) review paper, 3) paper without full-text, 4) conference poster, 5) survey paper, 6) research not targeting patients with dementia, and 7) qualitative research. The inclusion criteria were 1) studies of adult subjects with dementia, 2) studies of hand function in people with dementia, 3) full-text articles, and 4) studies published in English.
2.2.1Population
This study reviewed previous observational studies examining the relationship between hand motor function and dementia-related cognitive impairment. Hand function impairments caused by pathological disorders, such as musculoskeletal disorders or rheumatism, among the people living in the community and nursing homes were excluded.
2.2.2Study design
This systematic literature review analyzed longitudinal and experimental studies that compared the hand grip strength and hand agility of elderly people with AD symptoms and cognitive function problems with those without cognitive problems. Only studies that reported differences in the hand function through evaluation and measurements were included.
2.2.3Quality assessment
The titles and abstracts were reviewed for relevance to the study topic. Full-text publications of selected studies were reviewed for the inclusion criteria, and reasons for their exclusion were noted at this stage. In cases where the appropriateness of the paper was ambiguous, the full text was read and included. The second author was reconfirmed in the same way as the first author. If there was any disagreement during the review process, the final analysis target paper was determined through a discussion. For each of the final selected papers, the authors did not share their opinions when arranging the results. The opinions were combined and finalized after data analysis.
3.Data analysis
An evidence table was constructed to help organize and summarize the information of the studies included in the review. The information extracted was study setting, authors and year of publication, country, number of participants, anthropometric data, cognitive and hand grip strength and agility measurements, and primary outcomes. In order to maintain accuracy, data analysis was also conducted independently by two researchers and then exchanged and confirmed with each other. In case of differing opinions, the results were presented, and through consensus and discussion, the results were integrated and presented for narrative analysis.
4.Results
The search results and reasons for exclusion are presented in the PRISMA flow diagram in Fig. 1. Seventeen articles were assessed after applying the inclusion and exclusion criteria. The searched longitudinal studies revealed four studies, which were follow-up over more than four years, and 13 were quasi-experimental studies.
4.1Longitudinal studies
Four longitudinal studies reported in Table 1 comparisons of the hand grip strength and agility in older adults. Among them, in addition to the hand fine motor skill, some studies observed the activities of daily living and motor disability, but only hand agility was reviewed.
The hand grip strength was measured in one study using a Jamar dynamometer [16]. Various tools were used for hand dexterity. The tools used were researched using finger tapping using a nine-hole pegboard test, Purdue Pegboard Test (PPT), Grooved Pegboard Test (GPT), and Wacom, handwriting, finger tapping, keyboard typing, handwritten graphical task, and Vienna system test. Mini-Mental State Examination (MMSE), Dementia Rating Scale (DRS), Clinical Dementia Rating (CDR), Montreal Cognitive Assessment (MoCA), Global Deterioration Scale (GDS), and Mattis Dementia Rating Scale were used to evaluate dementia and cognitive function. The subjects were over 70 years old in the AD and over 60 years old in the other subjects.
Beeri et al. [16] reported that the hand strength is related to cognitive function and is the only independent motor factor for the incidence of dementia.
Hand dexterity was reported to be strongly related to the incidence of AD in four studies. Liou et al. [17] reported that a decrease in hand fine motor skills is highly likely to develop in moderate to severe dementia. de Paula et al. [18] showed that the mild cognitive impairment group exhibited a difference in the degree of decline in hand function compared to HE, but the degree was not high. On the other hand, the decline in hand function was reduced significantly in HE and AD patients.
Table 1
Longitudinal studies examining the association between hand cognitive perfomance
| Reference (first author and year of publication) | Dataset, country |
| Mean age | Duration (years) | Cognitive function tests | Hand strength | Hand dexterity | Main outcome: Association |
|---|---|---|---|---|---|---|---|---|
| de Paula [18] (2016) | Clinic, Brazil | HE: 20, aMCI: 34, MDaMCI: 32, AD: 38 | 74.2 | 4.0 | *DRS Brazil version | Not carried out | 9PT | 1. Significant group differences in hand dexterity were found between Healthy and MDaMC ( |
| Darweesh [29] (2017) | Population-based Rotterdam study, Netherland | 4,856 | 70.2 | 9.2 | *MMSE,*CAMDEX*NINCDS-ADRDA*NINDS-AIREN | Not carried out | PPT | 1. Using PPT for 4,856 normal people from 2000 to 2004, follow-up of patients with dementia and Parkinson’s disease until January 1, 2012.2. Diagnosed 227 dementia and 50 Parkinson’s disease over 9.2 years.3. The lower the PPT score, the higher the risk of developing dementia and Parkinson’s disease.4. Dexterity test can identify people at high risk for neurodegenerative diseases.5. 155 of 277 (56%) AD, 72 (26%) primary dementia diagnosis, 33 (12%) PD, 17 (6%) other cause PD.6. The lower the PPT score, the higher the risk of dementia and Parkinson’s disease, and the risk of degenerative diseases increased by 28% when the average PPT score decreased.7. The correlation between PPT score and degenerative disease was high in people under 70 years of age. Although there was no statistical significance, this phenomenon was evident in women.8. In the case of dementia, classification (continuous net reclassification index [NRI]) increased significantly in those aged 70 years or older, and discrimination (integrated discrimination improvement [IDI]) increased significantly in those aged 70 years or younger.9. Dexterity is strongly associated with the risk of both dementia and Parkinsonism. |
| Liou [17] (2020) | Taiwan Data Bank of Persons with Disability, Taiwan | mild AD: 11,935, moderate to severe AD: 20,883 | mild AD: 77.9 | 7.0 | *FUNDES-adult*WHODAS 2.0 | Not carried out | *Pen-holding*Buttoning*Knotting | 1. Moderate to severe AD has more difficulty in three performance skills than mild AD.2. Decreased fine motor skills of the hand have a significant risk of severe dementia. |
| Beeri [16] (2021) | Communities across metropolitan, USA | African American: 580, European American: 580, without dementia | 73.2 | 7.3 | *NINCDS-ADRDA*CFT | Jamar dynamometer | *PPT*Electronic tapper | 1. Each increase in global motor function score of 1 SD reduces the risk of AD by |
9PT, nine-hole pegboard test; AD, Alzheimer’s dementia; aMCI, single-domain amnestic MCI; CAMDEX, Cambridge examination for mental disorders of the elderly; CDR, clinical dementia rating; CFT, cognitive function test; DRS, dementia rating scale; FUNDES-Adult, functioning disability evaluation scale-adult version; GDS, global deterioration scale; GPT, grooved pegboard test; HE, healthy elderly; MCI, mild cognitive impairment; MDaMCI, multiple-domain amnestic MCI; MMSE, mini-mental state examination; MoCA, Montreal cognitive assessment; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; NINDS-AIREN, National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences; PPT, Purdue pegboard test; WHODAS, world health organization disability assessment schedule 2.0.
Table 2
Characteristics and results of included quasi-experimental research
| Reference (first author and year of publication) | Dataset, country |
| Mean age | Patient intervention/ control | Cognition/ diagnosis | Hand strength | Hand dexterity | Main outcome: Association |
|---|---|---|---|---|---|---|---|---|
| Saxton [19] (2000) | Community, clinic, USA | HE: 15, AD: 9, AlchD: 10, Alch non-D: 29 | HE: 70.8 | 4 groups (HE, AD, Alch AD, Alch non-AD) | *MMSE*NINCDS-ADRDA*DSM-IV*NARTR*CERAD | Not carried out | 1. Clock drawing.2. GPT. | 1. The pegboard and clock drawing were performed faster and more accurately by the dementia group than by the alcoholic dementia group. |
| Schroter [4] (2003) | Outpatient clinic, Germany | HC: 40, AD: 35, MCI: 39, DEP: 39, | HE: 65.6 | 4 groups (HE, AD, MCI, DEP), kinematic handwriting analysis | *MMSE*NINCDS-ADRDA*DSM-III-R | Not carried out | 1. Drawing concentric superimposed circles of 12 mm in diameter.2. Kinematic handwriting analysis. | 1. AD significantly increased velocity variation (V-SD) and relative velocity (V-Rel) than HC.2. MCI and AD exhibited a loss of fine motor performance.3. Compared to HCs, the movements of AD patients were significantly less automated, accurate, and regular. |
| Yan [5] (2008) | Hospital, USA | HE: 10, AD: 9, MCI: 9 | HE: 75.9 | 3 groups (HE, AD, MCI) | *MMSE*NINCDS-ADRDA | Not carried out | 1. Handwriting movements. | 1. Difficulty with AD or MCI on a fine motor task involving handwriting-like movements. Movement time increased significantly (coordination of fingers and wrist).2. MCI patients outperformed the AD patients in MT (speed) and movement jerk (smoothness).3. Using a fine motor task, such as handwriting, the present investigation supports using movement measures like velocity as an alternative diagnostic tool. |
| Suzumura [22] (2016) | Hospital, Community, Japan | HE: 13, AD/MCI: 14 | HE: 71.7 | 3 groups (HE, AD/MCI), magnetic sensing, finger tapping device | *MMSE*CDR | Not carried out | 1. Finger-to-thumb tapping. | 1. Regular distance and speed and high number of tappings in the healthy group, irregular distance and speed and tapping interval in the AD/MCI group.2. AD/MCI: Decreased index finger tapping speed in the non-dominant hand, a significant difference in bimanual tasks. It appears as a difference according to the sense of rhythm.3. Hand function declines according to the severity of dementia [1) values of total traveling distance, 2) dispersion of the duration the two fingers were in contact, and 3) rhythm perturbation, indicating] |
| Van Waes [30] (2017) | Netherlands | YA: 20, HE: 20, MCI: 8, MiAD: 4 | YA: 22.5 | 3 groups (YA, HE, MCI/MiAD), typing behavior | *NINCDS-ADRDA*MMSE*GDS | Not carried out | 1. A set of 16 bigrams characterizing.2. keystroke-logging program. | 1. Related to typing: It decreased in healthy elderly than adolescents, and MCI/AD decreased more.2. Typing copy test might provide valuable information in the diagnostic work-up of patients with neurodegenerative brain disorders. |
| Roalf [20] (2018) | USA | HE: 62, AD: 131, MCI: 46, PD: 63 | HE: 71.4 | 4 groups (HE, AD, MCI, PD), finger tapping | *MMSE | Not carried out | 1. Light beam finger and foot tapper test. | 1. AD, MCItotal finger taps decreased compared to the healthy group, PD was slightly higher than the healthy group.2. Finger tapping interval speed was longer in AD and MCI than in control and PD.3. Tapping IIV (intra-individual variability): Compared to the control, AD, MCI, and PD all appeared high, but PD had the most variables.4. Fine movement disorders have been proven in MCI and early AD; finger tapping is a way to differentiate between PD and AD. |
| Suzumura [23] (2018) | Hospital, Community, Japan | HE: 48, AD: 31, MCI: 15 | HEl: 73.6 | 3 groups (HE, AD, MCI), finger tapping | *MMSE | Not carried out | 1. Fingers in tapping marks on the just touch screen eight parameters. | 1. AD/control: Significant difference in tap response time fluctuation, rhythm, rhythm fluctuation, contact duration, contact duration fluctuation, and inter-hand divergence fluctuation.2. AD/MDI: finger function parameters of tap response time fluctuations and contact duration significant differences.3. Negative correlations (rhythmic tapping with alternating hands task): MMSE and contact duration, and MMSE and contact duration fluctuations.4. As AD progresses, the following decrease: finger dexterity parameters of response time, rhythm, contact duration, and interhand coordination, together with several parameters indicating declines in finger dexterity with increasing dementia severity.5. Decreased correlation between rhythm fluctuation and contact duration fluctuation and MMSE.6. Significant decrease in hand function when diagnosed with dementia. |
|
Table 2, continued | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference (first author and year of publication) | Dataset, country |
| Mean age | Patient intervention/ control | Cognition/ diagnosis | Hand strength | Hand dexterity | Main outcome: Association |
| Yu [24] (2019) | Taiwan | HE: 18, AD: 22, MCI: 14 | HE: 75.8 | 3 groups (HE, AD, MCI), computerized handwriting analysis | *CDR*NINCDS-ADRDA | Not carried out | 1. Two graphic and two handwriting tasks: (1) three 50-mm squares, (2) three diagonal crosses in a 50-mm square, (3) Chinese character “UTF8gbsn井,” pronounced “Jing,” in a row (5 copies) and a column (5 copies), (4) Chinese character “UTF8gbsn正,” pronounced “Zheng,” in a row (5 copies) and a column (5 copies). | 1. Movement fluency in handwriting tasks; Pause time per stroke (PTS), Ratio of In Air to On Paper time (RAPT), Ratio of In Air to On Paper trajectory length (RAPL).2. AD and aMCI groups showed significantly larger MSE than those of the HC group.3. The AD group showed larger RAPL and PTS than the HC group did.4. The character sizes in the AD and aMCI groups are larger than those in the HC group. The AD and aMCI groups showed significantly lower spatial accuracy than the HC group. |
| Van Deun [21] (2019) | Belgium | HE: 60, MiD: 31, MoD: 31 | HE: 82.1 | 3 groups (HE, MiD, MoD), hand dexterity | *MMSE*NINCDS-ADRDA*DSM-III-R | Not carried out | 1. PPT.2. Paratonia. | 1. In the Purdue Pegboard test PPT, dominant and non-dominant hands scored the highest in HC, the lowest in MiD, and the lowest in MoD.2. Paratonia has HC 0%, MiD 42%, and MoD 58% patients.3. Patients with paratonia showed low levels in all dominant, non-dominant, and bimanual scores in the Purdue Pegboard test PPT. |
| Suzumura [26] (2021) | Hospital, Japan | AD: 44, MCI: 20 | AD: 73.8 | 2 groups (AD, MCI), finger tapping task | *MMSE*NIA/AA | Not carried out | 1. Finger tapping device with magnetic sensors.2. Finger-tapping task. | 1. There is a significant difference between the dominant and non-dominant hand, and2. It is important to assess the dependency (simultaneous movements of both hands) and independence (alternate hands) when measuring both hands.3. In multiple regression analysis with MMSE score, the SD of distance rate of velocity peak in extending movements (No. 17) and the SD of the contact duration (No. 25) showed high coefficients.4. SD of contact duration has been reported to be related to MMSE score and finger function in dementia patients. 5. The degree of the standard deviation of contact time is high in the standard deviation regression coefficient.6. Tasks in the non-dominant hand showed the highest influence on the MMSE. |
| Kachouri [25] (2021) | Hospital, France | HE: 45, AD: 30 | HE: 73.5 | 2 groups (HE, AD) | *MMSE | Not carried out | 1. Draw one Archimedes spiral.2. Images including pressure, altitude, and velocity. | 1. HC: high-pressure values more than AD (65.7%).2. AD patients exhibit very low-velocity values (48%) more than HC (30%).3. A slower motion trend in AD since the early stage of the disease when drawing the spiral.4. The pertinence of pressure information in characterizing both HC and AD classes through hybrid images. |
| Hesseberg [31] (2021) | Community, Norway | MCI: 60, AD: 38 | MCI: 77.9 | 2 groups (AD, MCI), clock drawing test, trail making tests A and B | *MMSE*Winblad*CERAD | Jamar dynamometer | 1. Finger tapping test.2. GPT. | 1. No difference in hand function between MCI and AD groups.2. Executive function impairment causes decreased hand function.3. When the variables between the cognitive test and hand function were analyzed again, there was a difference of 64% in the variance in grip strength, 40% in the variance in the Grooved Pegboard (GPT), and 43% in the variance in finger tapping. |
| Anna [32] (2022) | Clinic, Poland | HE: 29, CD: 39 | HE: 75.1 | 2groups (HE, CD) | *MMSE | Not carried out | 1. Hand mobility: Motor Performance Series (MLS- Motorische Leisstung Serie) test battery from the Vienna Test System set- static and dynamic tasks using a contact pencil.2. S3-short form by Vasella. | 1. Comparing males and females for cognitive disorders/non-CD.2. M-Anova significance according to gender and MMSE status, but MLS-test score has no effect on sexually transmitted diseases and MMSE.3. Comparison of cognitive impairment and non-cognitive impairment groups without clearly distinguishing AD patients. |
AD, Alzheimer’s dementia; AlchD, Alcohol dementia; CD, cognitive disorders; CDR, clinical dementia rating; CERAD, Consortium to Establish a Registry of Alzheimer’s Disease; DEP, a major depression; DSM, Diagnostic and Statistical Manual of Mental Disorders; GDS, global deterioration scale; HE, healthy elderly; MCI, mild cognitive impairment; MiAD, mild AD; MMSE, mini-mental state examination; MoD, moderate dementia; NARTR, National Adult Reading Test-Revised; NIA/AA, National Institute on Aging-Alzheimer’s Association; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; PD, Parkinson’s disease; PPT, Purdue pegboard test; YA, young adult.
4.2Quasi-experimental research
Of the 13 studies, 11 investigated the differences in hand function according to the type and severity of dementia and the normal elderly or dementia subjects. These studies were analyzed and reported in Table 2. The other two studies compared the neuropsychological profiles and studied the transfer of learning. Of these two articles, only the part about hand function was extracted and used in this study. In addition, only the hand function results extracted from studies on alcohol-induced dementia symptoms [19], studies including depression groups [4], studies including Parkinson’s patients [20], and studies on dementia and hypotension [21] were analyzed in this study.
Among the targeted studies, three studies were divided into AD group and HE groups; two studies were classified into AD group and MCI groups; four studies were classified into three groups (normal cognitive group, AD group, and MCI group). The study subjects were over 60 years old in 12 studies, and subjects in their 20s, a group of young adults, were included in one study. Thirteen studies used MMSE to evaluate the cognitive function; NINCDS-ADRDA criteria were used in six studies, and CDR and GDS were additionally used to diagnose dementia. Five studies classified the cognitive function problems and dementia groups using only the MMSE.
Among 13 studies, one study measured handgrip strength using a Jamar dynamometer, and hand dexterity was measured using various types of tools in all 13 studies. The tools used to evaluate hand dexterity were two studies using finger tapping using the PPT, GPT, Wacom, handwriting, finger tapping, keyboard typing, handwritten graphical task, and Vienna system test.
The AD patients showed poor hand function in 13 studies. Schroter et al. [4] analyzed the pressure and handwriting motion while drawing a circle using a tablet. They reported that AD patients showed greater variation in speed and decreased automaticity and regularity than HE groups [4]. The HE group showed a constant interval and speed in the finger-to-them tapping motion. In contrast, irregular intervals and speeds were observed in the group with dementia and mild cognitive impairment, and the number of taps was also small [22]. Suzumura et al. [23] reported that the rhythm of hand movements and the time to contact the screen became irregular. The hand agility, reaction speed, rhythm, and coordination decreased as the dementia worsened as AD progressed. Yu et al. [24] reported irregular movement to and from the paper and space in the dementia group. They revealed amnestic MCI in the handwriting task, and the handwriting size was large. The AD group exhibited strong pressure on the tablet when drawing a spiral using a tablet [25]. Roalf et al. [20] also showed that the finger-tapping interval speed appeared longer in AD and MCI than in the control and PD. Suzumura et al. [26] stated that the time spent in contact with the tablet during finger tapping motion was longer and that the degree of activity in the non-dominant hand had a stronger influence on the cognitive function than in the dominant hand. The alcoholic dementia group took longer to use the pegboard than the non-alcoholic dementia group. On the other hand, the alcoholic dementia group required more time, but the difference was not significant. Nevertheless, the alcoholic dementia group and the AD group differed from the normal group [19].
5.Discussion
MCI, an early and transitional stage between normal brain aging and dementia, affects a range of cognitive or motor functions [27]. A previous study reported that the finger function of patients with dementia deteriorates in relation to fine finger movement control, finger tapping speed, and finger agility [28]. This study reviewed studies on hand function in dementia patients to determine the possibility of using it as a possible predictor of early diagnosis and disease progression of AD.
Longitudinal studies of hand motor dysfunction in AD patients are clinically meaningful because knowing the prognosis and cognitive abilities of AD patients with motor impairment at an early stage of the disease can assist in making treatment decisions [4]. In the four longitudinal studies reviewed in this paper, MMSE was used in three studies to evaluate dementia and cognitive function. In some cases, CT or MRI was used to diagnose dementia, but evaluation tools, such as the DRS, were used in many cases. In the hand function test, the grip strength and hand dexterity were measured together in a single study, and only hand dexterity was measured in three studies. Hand agility evaluation tools were used in various ways, but mainly to measure simple hand movements using a pegboard, and only one study measured the hand agility using electronic devices. In all four studies, there was a strong correlation between the hand dexterity and cognitive function, and the incidence of dementia was high when hand agility was low. They reported that hand agility was lower in dementia than mild cognitive impairment, and the decline in hand agility function in moderate dementia was highly likely to develop into severe dementia. Therefore, the decline in hand agility function appears to be a variable that can predict the occurrence or worsening of symptoms of dementia. On the other hand, there was no significant difference in hand agility between the HE group and the MCI group and between the MCI group and the AD group, making it difficult to understand the conversion of MCI to AD.
A functional hand can perform various activities, from writing and eating to communicative gestures and interactions with the environment around us [13]. The motor activity originates in the motor cortices, basal ganglia, and cerebellum. Voluntary and automatic movements originate in the motor cortex and basal ganglia. The cerebellum integrates vestibular, visual, proprioceptive and tactile sensory information, and using this integrated information. Appropriate adaptation to movement is transmitted through the corticospinal tract, vestibulospinal and reticulospinal tracts, and descending neural pathways originating from the brain stem. This results in an appropriate postural tone in the trunk and shoulder girdle, stabilizing the upper limb and allowing flexible wrist control and fine, dexterous movement of the fingers [13]. Nevertheless, motor control is a learned skill developed throughout life and occurs alongside cognitive functions, such as motivation, goals, and appropriate motor responses through similar experiences and tasks. The process that underpins this cognition accepts and integrates multiple senses, such as sight, hearing, and somatosensory, from a complex environment through afferent feedback. The sensory information then enters the cerebral cortex, which includes the motor cortex, premotor area, accessory cortex, and motor cortex [29].
In 13 experimental studies, the MMSE, which is used widely worldwide, was used to measure the cognitive function. The NINCDS-ADRDA criteria were used as a criterion for diagnosing dementia in 50% of the studies. In the experimental study, pressure during writing was measured, or complex motions were analyzed through hand motions, such as handwriting using a tablet or touching a specific point on the screen. In a study that analyzed pressure and handwriting motion while drawing circles on a tablet, AD patients showed greater variation in speed and less automaticity and regularity than HE groups. Compared to healthy controls, MCI patients had worse hand function in tasks involving fine and complex motor functions. In addition, the AD patients also showed motor dysfunction in tasks assessing gross motor control [4]. The AD group showed a longer movement time than HE group when performing handwriting tasks (forward or backward tilting movements) that require finger-wrist coordination [5]. In addition, in the finger-to-them tapping motion, the interval and speed were constant in the HE group, but the group with dementia and mild cognitive impairment showed irregular interval and speed, and the number of tappings was also small [22].
Suzumura et al. [23] reported that the sense of rhythm in the movement of the hand touching the mark on the screen and the time of contact with the screen becomes irregular. As AD progresses, the dementia worsens as the hand agility, reaction speed, sense of rhythm, and coordination decrease. Yu et al. [24] reported irregular movement to and from the paper and a space in the AD group and amnestic MCI groups in the handwriting task; the handwriting size was also large. When drawing a spiral using a tablet, the AD group said the pressure to press on the tablet was strong [25]. Roalf et al. [20] also showed that the finger tapping interval speed appeared longer in AD and MCI than in control and Parkinson’s patients. Suzumura et al. [26] said that the time spent in contact with the tablet during the finger-tapping motion was longer and that the degree of activity in the non-dominant hand had a stronger influence on the cognitive function than in the dominant hand. The alcoholic dementia group took longer to use the pegboard than the non-alcoholic dementia group, but the alcoholic dementia group needed more time, but the difference was not significant, and there was a difference between the HE group and the alcoholic dementia and AD groups [19].
Thirteen studies reported that the degree of hand agility was reduced in the MCI and AD groups compared to the HE group. In the four studies on the hand function through handwriting, the degree of automaticity, regularity, and rhythm in hand motion decreased in the AD group, suggesting that the difference in the regularity of the hand function motion is related to cognitive function. In these studies, the reaction time slowed as the cognitive function decreased; there was a difference in the equality of the time staying on the contact surface and the time the hand stayed in the space increased. Yan et al. [5] suggested that the speed in tasks, such as handwriting, could be substituted for diagnosing dementia. Therefore, the uniformity of motion speed in hand function appears to be a major factor in determining cognitive function.
6.Conclusion
Through studies related to dementia and hand function, hand dexterity is strongly related to dementia. In addition, in a simple hand motion test using a pegboard, there was no significant difference between the HE group and the MCI group, and between the MCI and AD groups. Third, in complex hand movements, AD showed a decrease in automatism, rhythm, and regularity, so complex movements, such as handwriting, can be used to predict the transition from MCI to AD and the progression from moderate dementia to severe dementia.
Conflict of interest
None to report.
References
[1] | 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. (2023) ; 19: (4): 1598-1695. |
[2] | Fabrigoule C, Rouch I, Taberly A, Letenneur L, Commenges D, Mazaux JM, Orgogozo JM, Dartigues JF. Cognitive process in preclinical phase of dementia. Brain. (1998) ; 121: : 135-141. |
[3] | Celsis P. Age-related cognitive decline, mild cognitive impairment or preclinical Alzheimer’s disease? Ann Med. (2000) ; 32: (1): 6-14. |
[4] | Schroter A, Mergl R, Burger K, Hampel H, Moller HJ, Hegerl U. Kinematic analysis of handwriting movements in patients with Alzheimer’s disease, mild cognitive impairment, depression and healthy subjects. Dement Geriatr Cogn Disord. (2003) ; 15: (3): 132-142. |
[5] | Yan JH, Rountree S, Massman P, Doody RS, Li H. Alzheimer’s disease and mild cognitive impairment deteriorate fine movement control. J Psychiatr Res. (2008) ; 42: (14): 1203-1212. |
[6] | MacDonald SW, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12-year cognitive change in older adults: Findings from the Victoria Longitudinal Study. Gerontology. (2004) ; 50: (2): 64-81. |
[7] | Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. (2007) ; 29: (1-2): 66-73. |
[8] | Svensson E. Hand function in children and in persons with neurological disorders: aspects of movement control and evaluation of measurements. Institutionen för samhällsmedicin och rehabilitering. (2009) . |
[9] | Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences. (2007) ; 30: (9): 464-472. |
[10] | Hooghiemstra AM, Ramakers I, Sistermans N, Pijnenburg YAL, Aalten P, Hamel REG, Melis RJF, Verhey FRJ, Olde Rikkert MGM, Scheltens P, Flier WM, on behalf of the 4C Study Group. Gait Speed and Grip Strength Reflect Cognitive Impairment and Are Modestly Related to Incident Cognitive Decline in Memory Clinic Patients With Subjective Cognitive Decline and Mild Cognitive Impairment: Findings From the 4C Study. J Gerontol A Biol Sci Med Sci. (2017) ; 72: (6): 846-854. |
[11] | Alfaro-Acha A, Al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. (2006) ; 61: (8): 859-865. |
[12] | Sayer AA, Kirkwood TB. Grip strength and mortality: A biomarker of ageing? Lancet. (2015) ; 386: (9990): 226-227. |
[13] | Metcalf CD, Irvine TA, Sims JL, Wang YL, Su AW, Norris DO. Complex hand dexterity: A review of biomechanical methods for measuring musical performance. Front Psychol. (2014) ; 5: : 414. |
[14] | Enoka RM. Neuromechanics of human movement. Human kinetics. (2008) . |
[15] | Song MJ. Meta-Analysis for Effect size of Single target studies with Maladaptive Behavior Intervention for Children with Developmental Delays. Meta-Analysis for Effect size of Single target studies with Maladaptive Behavior Intervention for Children with Developmental Delays. Journal of Special Education & Rehabilitation Science. (2020) ; 59: (1): 27-55. |
[16] | Beeri MS, Leurgans SE, Bennett DA, Barnes LL, Buchman AS. Diverse motor performances are related to incident cognitive impairment in community-dwelling older adults. Front Aging Neurosci. (2021) ; 13: : 717139. |
[17] | Liou WC, Chan L, Hong CT, Chi WC, Yen CF, Liao HF, Chen JH, Liou TH. Hand fine motor skill disability correlates with dementia severity. Arch Gerontol Geriatr. (2020) ; 90: : 104168. |
[18] | de Paula JJ, Albuquerque MR, Lage GM, Bicalho MA, Romano-Silva MA, Malloy-Diniz LF. Impairment of fine motor dexterity in mild cognitive impairment and Alzheimer’s disease dementia: Association with activities of daily living. Braz J Psychiatry. (2016) ; 38: (3): 235-238. |
[19] | Saxton J, Munro CA, Butters MA, Schramke C, McNeil MA. Alcohol, dementia, and Alzheimer’s disease: Comparison of neuropsychological profiles. J Geriatr Psychiatry Neurol. (2000) ; 13: (3): 141-149. |
[20] | Roalf DR, Rupert P, Mechanic-Hamilton D, Brennan L, Duda JE, Weintraub D, Trojanowski JQ, Wolk D, Moberg PJ. Quantitative assessment of finger tapping characteristics in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease. J Neurol. (2018) ; 265: (6): 1365-1375. |
[21] | Van Deun B, Van Den Noortgate N, Van Bladel A, Palmans T, Cambier D. The impact of paratonia on fine and gross motor function in older adults with mild and moderate dementia. Alzheimer Dis Assoc Disord. (2019) ; 33: (1): 54-61. |
[22] | Suzumura S, Osawa A, Nagahama T, Kondo I, Sano Y, Kandori A. Assessment of finger motor skills in individuals with mild cognitive impairment and patients with Alzheimer’s disease: Relationship between finger-to-thumb tapping and cognitive function. Japanese Journal of Comprehensive Rehabilitation Science. (2016) ; 7: : 19-28. |
[23] | Suzumura S, Osawa A, Maeda N, Sano Y, Kandori A, Mizuguchi T, Yin Y, Kondo I. Differences among patients with Alzheimer’s disease, older adults with mild cognitive impairment and healthy older adults in finger dexterity. Geriatr Gerontol Int. (2018) ; 18: (6): 907-914. |
[24] | Yu NY, Chang SH. Characterization of the fine motor problems in patients with cognitive dysfunction – A computerized handwriting analysis. Hum Mov Sci. (2019) ; 65: : 71-79. |
[25] | Kachouri M, Houmani N, Garcia-Salicetti S, Rigaud AS. A new scheme for the automatic assessment of Alzheimer’s disease on a fine motor task with Transfer Learning. Annu Int Conf IEEE Eng Med Biol Soc. (2021) ; 2021: : 3823-3829. |
[26] | Suzumura S, Kanada Y, Osawa A, Sugioka J, Maeda N, Nagahama T, Shiramoto K, Kuno K, Kizuka S, Sano Y, Mizuguchi T, Kandori A, Kondo I. Assessment of finger motor function that reflects the severity of cognitive function. Fujita Med J. (2021) ; 7: (4): 122-129. |
[27] | Yan JH, Dick MB. Practice effects on motor control in healthy seniors and patients with mild cognitive impairment and Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. (2006) ; 13: (3-4): 385-410. |
[28] | Slavin MJ, Phillips JG, Bradshaw JL, Hall KA, Presnell I. Consistency of handwriting movements in dementia of the Alzheimer’s type: A comparison with Huntington’s and Parkinson’s diseases. J Int Neuropsychol Soc. (1999) ; 5: (1): 20-25. |
[29] | Darweesh SK, Wolters FJ, Hofman A, Stricker BH, Koudstaal PJ, Ikram MA. Simple test of manual dexterity can help to identify persons at high risk for neurodegenerative diseases in the community. J Gerontol A Biol Sci Med Sci. (2017) ; 72: (1): 75-81. |
[30] | Van Waes L, Leijten M, Marien P, Engelborghs S. Typing competencies in Alzheimer’s disease: An exploration of copy tasks. Computers in Human Behavior. (2017) ; 73: : 311-319. |
[31] | Hesseberg K, Tangen GG, Pripp AH, Bergland A. Associations between cognition and hand function in older people diagnosed with mild cognitive impairment or dementia. Dementia and Geriatric Cognitive Disorders Extra. (2021) ; 10: (3): 195-204. |
[32] | Anna R, Jaroslaw F, Izabela W, Karolina L, Malgorzata K, Malgorzata S. Differences in the level of functional fitness and precise hand movements of people with and without cognitive disorders. Exp Aging Res. (2022) ; 48: (4): 351-361. |



