Potassium and magnesium and cognitive decline in middle-aged adults
Abstract
BACKGROUND:
Increase in dementias globally is a burden to patients, caregivers, the healthcare system, and the communities in which they live. Understanding nutritional patterns and how they may impact the prevention of these conditions will be critical moving forward. The known impact of minerals such as potassium and magnesium on conditions such as hypertension, oxidative stress, and inflammation – all of which directly impact cognitive health – warrant further study as to their potential direct effects on cognitive function.
OBJECTIVE:
To determine if low potassium and magnesium blood levels and dietary intakes are associated with cognitive decline in middle-aged adults over a 6-year span.
METHODS:
Linear regression models were used to describe the associations between potassium and magnesium intakes and cognitive function scores of participants in the Atherosclerosis Risk in Communities Study (ARIC) dataset over 6 years of follow up. Associations with blood values were also assessed. Variables controlled for included total HEI score, a measure of dietary quality. 9,044 participants were included. All linear regression models were run with a 95% confidence interval.
RESULTS:
Levels of blood potassium and magnesium, in univariate as well as in multivariate analysis were found to have no significant association with cognitive decline. Likewise, intake levels of both minerals were shown to have no significant association with cognitive function.
CONCLUSIONS:
In 9,044 participants, ages 44 to 66, potassium and magnesium intake and blood serum levels were found to have no significant association with cognitive decline in fully controlled models over 6 years in the ARIC cohort.
1Introduction
Cognitive decline is defined by the APA Dictionary of Psychology as “the reduction in one of more cognitive abilities, such as memory, awareness, judgement, and mental acuity, across the adult lifespan” [1]. Although some cognitive decline is a part of healthy aging, cognitive decline that is non-normative, the focus of this study, can be indicative of dementias and Alzheimer’s disease [1]. It is for this reason that cognitive decline has been noted as an important issue worldwide since 2008 when the WHO included dementia as part of its Mental Health Gap Action Programme [2]. With nearly 10 million cases added annually, by 2030 an estimated 82 million people worldwide will be living with dementia with an expected increase to 152 million by 2050 [3]. An estimated $818 billion dollars is spent globally on dementia with approximately 85% of the cost burden falling to families and socially related costs as opposed to medical care [3]. Even mild cognitive decline, the earliest objective evidence of cognitive deficits, [4] is of concern given the marked increase in the prevalence and cost of these milder cases. By 2010 it was clear that genetic factors play a role in dementias, [5] and more robust study was encouraged toward prevention of cognitive decline, a precursor to dementia [2, 5]. A decade later extensive research has pointed to twelve risk factors of which five are known to be impacted by diet (obesity, hypertension, physical inactivity, alcohol intake, and diabetes) [5, 6].
The known impact of diet on obesity, hypertension, and diabetes has resulted in investigations into diets and cognitive decline. Most notably the Mediterranean Diet has demonstrated positive impacts on prevention of cognitive decline [7]. The Dietary Approaches to Stop Hypertension (DASH) Diet, similar to the Mediterranean Diet, encourages the intake of foods rich in potassium and magnesium. Studies have shown a positive effect on cognitive function through the DASH Diet [8, 9] though fewer studies have been conducted on DASH compared to the Mediterranean Diet [10]. Both the Mediterranean and DASH Diets have been demonstrated to reduce inflammation and oxidative stress [11, 12]. Inflammation and oxidative stress are both contributors to hypertension, diabetes, and obesity, and are also known factors in mild and severe cognitive decline [13].
Micronutrients can play a role in reducing and mitigating inflammation and oxidative stress. Potassium and magnesium are both important micronutrients found not only in animal food sources, but also in a wide variety of plant-sources such as legumes, leafy greens, nuts, potatoes, fruits, and vegetables all of which are included in the Mediterranean diet. Studies have pointed to risk of dementia, especially vascular dementia, being lower in those with higher intakes of magnesium and potassium [14]. Recommended dietary allowances (RDA) of magnesium for middle-aged women between the ages of 44 and 66 is 320 mg/day and 420 mg/day for men. The adequate intake (AI) for potassium for middle-aged women is 2,600 mg/day and 3,400 mg/day for men [15].
Magnesium has gained attention in cognitive decline studies due to the biochemical and physiological impacts in which magnesium deficiencies result. Magnesium deficiency has been linked to free radical production, oxidative tissue damage, inflammation, and vasospasm in cerebral arteries [9]. Furthermore, low magnesium has been shown to increase the risk of stroke, atrial fibrillation, diabetes and other cardiovascular diseases [14, 16]. Normal blood magnesium levels for adults are 1.5–2.0 mEq/L [17].
Potassium’s impact on cognition is less clear than magnesium’s, with various studies resulting in contradictory findings. One explanation for this may be that potassium has a protective effect only in early stages of dementia [14, 18]. A study on Mexican-Americans found a connection between serum potassium levels and mild cognitive impairment [18]. Normal blood potassium levels for adults are 3.5–5.0 mEg/L [19]. Though evidence supports potassium and magnesium having an effect on cognition, it is unclear to what degree, under what circumstance, and by what mechanism [14]. Further research of these minerals and cognition needs to account for design weaknesses of previous studies and isolate the unique contributions of magnesium and potassium.
The objective of this study is to investigate the impact of both dietary and blood levels of potassium and magnesium on non-normative cognitive decline within the Atherosclerosis Risk In Communities (ARIC) cohort.
2Materials and methods
2.1Participants
This publication was prepared using the Atherosclerosis Risk In Communities (ARIC) Research Materials obtained through the National Heart Lung Blood Institute (NHLBI) Biologic Specimen and the Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the ARIC research groups or the NHLBI. The Institutional Review Board of Appalachian State University approved the acquisition and use of the ARIC dataset [20].
The ARIC Study is a prospective epidemiologic study sponsored by the NHLBI. ARIC was designed to reveal causes of atherosclerosis through the enlistment of 15,792 participants from four U.S. communities (Washington County, MD, Forsyth County, NC, the city of Jackson, MS, and select suburbs of Minneapolis, MN). ARIC methodology details are described elsewhere [21].
Robust examinations were conducted on each participant including the capture of information of particular interest to this publication including cognitive function, dietary intake, certain covariates and blood plasma measurements of potassium and magnesium. Baseline for participants was established between 1987 and 1989 at Visit 1 with follow-up examinations occurring between 1990 to 1992 for Visit 2, 1993 to 1995 for Visit 3, and 1996 to 1998 for Visit 4. Food frequency questionnaire (FFQ) data from Visits 1 and 3 were used for assessing mineral intakes; data from Visits 1 and 2 were used for blood plasma mineral levels; cognitive function changes were assessed from Visits 2 and 4; relevant covariate data from Visit 1 were utilized for the current study.
2.2Dietary intake
Trained interviewers administered a modification of the Willet FFQ [22] to assess dietary intake. Ninety nutrients and food constituents were analyzed through the FFQ including those of interest in this study, potassium intake and magnesium intake. Participants were asked how often on average they had consumed each food during the preceding year with nine responses possible ranging from “almost never” to “greater than 6 times a day”. Daily nutrient intake was calculated by multiplying the nutrient content of the specified portion of each food item by the frequency of its estimated daily consumption and summing over all items. For this study, the HEI-2015 scoring [23, 24] was adapted to the ARIC FFQ [25]. A minor change was required in calculating the fatty acid HEI component score using vegetable oil (grams per day) from the nutrient data rather than the sum of mono and poly unsaturated fatty acids. The HEI-2015 was assessed with higher scores being healthier [23–25]. Determination of the relationship between magnesium and potassium intake and cognitive function score was controlled by using the HEI-2015 score as a measure of dietary quality [25]. Both magnesium and potassium in the diet come from generous fruit and vegetable intake often reflecting a generally healthy diet. In order to separate the unique effects of these dietary minerals from that of a generally healthy diet, HEI-2015 was used as a covariate.
2.3Cognitive assessment
Following the method used by Root et al. [26] the change in cognitive function over 6 years between visits 2 and 4 were calculated. Cognitive function was measured using three cognitive tests: delayed word recall test, the revised Wechsler Adult Intelligence Scale (WAIS-R) digit symbol subtest, and the word fluency test of Multilingual Aphasia Examination [27]. Trained interviewers administered cognitive tests in a quiet room and in standardized order. Interviewers were recorded on tape during the administration of the tests with each subject then a sample of testing sessions was reviewed for consistency of mean scores captured by various interviewers [28].
The delayed word recall test is scored on a 10-point scale giving one point for each of the 10 common nouns recalled by the participant following a 5-minute interval. The 5-minute interval is marked by the engagement of the participant in a psychometric test after which the participant is asked to recall the 10 common nouns by incorporating them into sentences to obtain their score. This test measures verbal learning and recent memory and showed high 6-month test-retest reliability [29].
The revised WAIS-R digit symbol subtest [30] is a timed psychomotor performance test where participants are given a symbol key, pencil, and paper then asked to translate numbers 1–9 to their symbol. Scores are based on the correct number of numbers translated to symbols in a 90 second time frame, with 93 as the highest score possible. For most adults, the test is not affected by learning, memory, or intellectual ability and has a high reliability for short-term test-retest in middle-aged individuals.
The word fluency test prompts participants through three trials to use given letters (F, A, and S) to form as many words as the participant is able in 60 seconds. The total number of words generated over the three attempts is the score value given to the participant. Though sensitive to linguistic impairments and cognitive decline in the elderly, the immediate test-retest reliability of the word fluency test has been demonstrated [31].
In this study the three test scores were combined to create a unified score of cognitive decline over 6 years. The difference of the test scores at visit 2 from visit 4 was taken for each test and divided by the standard deviation of the difference. A final score of change in cognitive function was created by summing the three standardized scores for each subject [26]. Subjects, on average, slightly declined in cognitive function over time (mean = –0.56).
2.4Other measurements
Standardized interviews were used to obtain self-reported data from participants at visit 1, including education level, ethnicity, and current smoking status. Education level was grouped by years of education completed in three categories: less than a high school degree, a high school degree, or a 4-year college degree. Ethnicity was reported as either Black or non-Black. Current smoking status was determined to be “yes” if participants answered “yes” to both questions: “Have you ever smoked cigarettes?”; “Do you now smoke cigarettes?” Ethanol measurements were used to control for alcohol intake in the current study. Ethanol intake was determined through the FFQ’s solicitation of how many grams of ethanol were consumed for each participant on a weekly basis [26].
Biospecimens measuring plasma levels of potassium and magnesium were collected by ARIC certified technicians at Visits 1 and 2 [32]. An average of the plasma measures for potassium and magnesium from Visits 1 and 2 were determined for each participant.
Participants who responded “yes” to the question, “Have you ever been told by a physician that you had a stroke, slight stroke, transient ischemic attack or TIA?” at either visit 1 or visit 4 were combined and controlled for within the linear regression [33].
2.5Statistical analysis
Participants were excluded based upon lack of data for nutrient intake, blood chemistry, covariates, or cognitive function test scores. Data from visits 1 through 4 were used for analysis of the population.
Linear regression models were created for potassium and magnesium intake versus the cognitive function change scores while controlling for age, gender, race, total caloric intake, education level, alcohol intake, smoking status, stroke diagnosis, and total HEI-2015 score. For blood levels of potassium and magnesium, the linear regression models created were the same as intake except that the HEI-2015 score was not included.
3Results
After exclusions were accounted for, a total of 9,044 participants were included in the present study for the dietary analysis and 9,074 participants were included in the blood analysis (Fig. 1). Table 1 illustrate the cohort’s demographic make-up including education level, current smoking statues (19.8%), and reported stroke at visit 1 (1.4%). The predominantly male (55.1%) cohort of middle-aged adults (44–66 years old) were consuming a typical American diet (HEI = 60±9) [34]. Most participants identified as White leaving only 17.2% of the present study’s cohort with identification as Black. Mean potassium and magnesium blood chemistry results fell within the normal range for the subjects [35]. The mean cognitive function change score shows a decline over six years as would be expected with the aging process of the participants.
Fig. 1
Cohort flow chart.
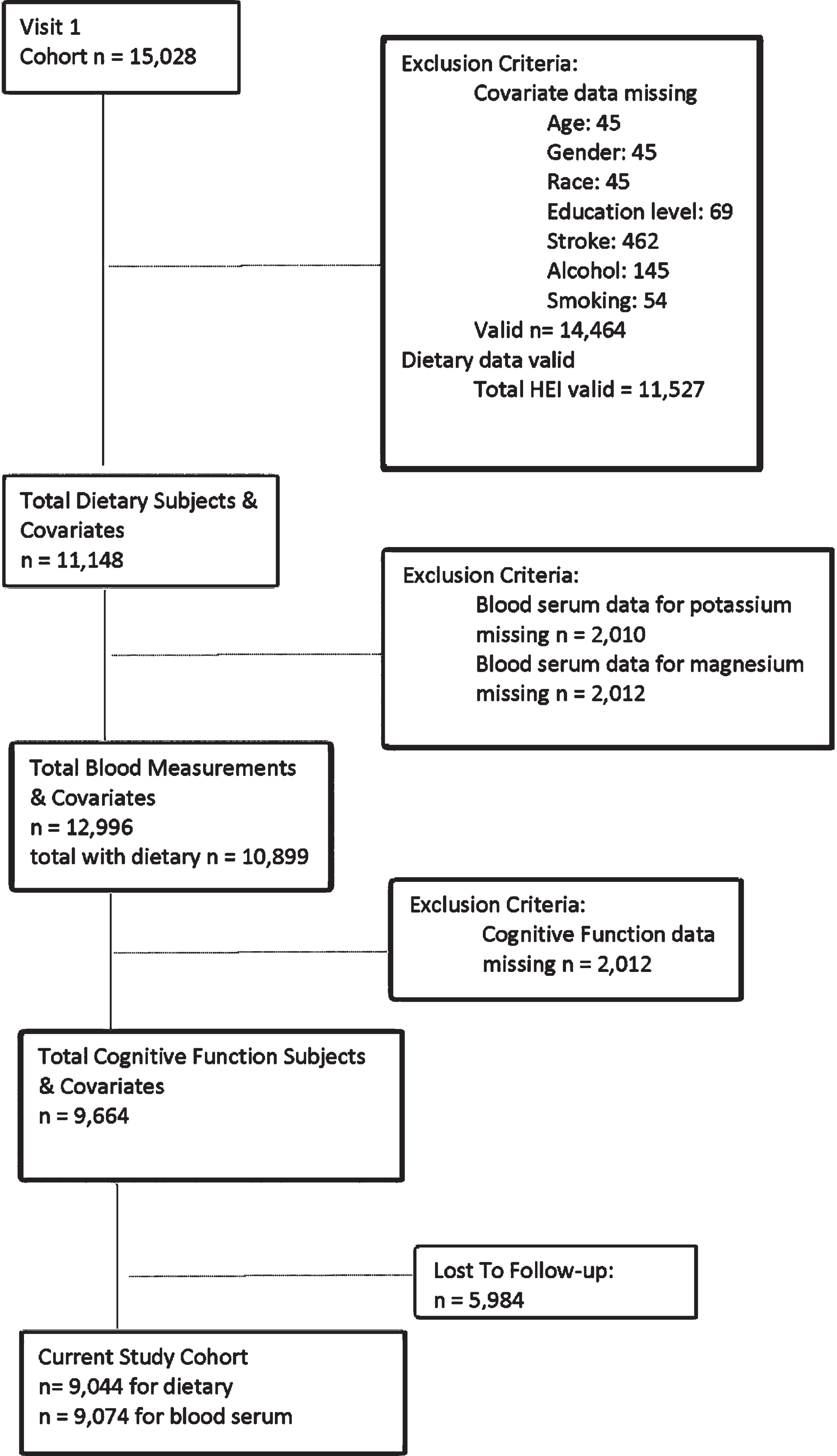
Table 1
Descriptive characteristics of the participants from ARIC in the present analysis (n = 9,044)
| Characteristic | Frequency | Percent | ||
| Male | 4980 | 55.1 | ||
| Black | 1557 | 17.2 | ||
| College graduate | 3615 | 40.0 | ||
| Current smoker | 1792 | 19.8 | ||
| Stroke diagnosis | 128 | 1.4 | ||
| Characteristic | Mean | Standard Deviation | Minimum | Maximum |
| Age at visit 1 (years) | 54.1 | 5.7 | 44 | 66 |
| Alcohol Intake (grams/week) | 42.9 | 90.7 | 0.0 | 1481 |
| Total HEI score (%) | 60.0 | 9.18 | 24.2 | 88.3 |
| Total energy (kcals) | 1617 | 517 | 537 | 4086 |
| Blood levels of potassium (mmol/L) | 4.31 | 0.35 | 2.65 | 5.80 |
| Potassium intake (mg/day) | 2680 | 830 | 520 | 7870 |
| Blood levels of magnesium (mEq/L) | 1.63 | 0.13 | 0.85 | 2.15 |
| Magnesium intake (mg/day) | 256 | 82 | 59 | 792 |
| Standardized cognitive function score | –0.56 | 1.85 | –12.21 | 12.70 |
Table 2a and 2b describe the linear regressions performed on participants’ blood serum potassium levels (Table 2a) and potassium intake levels (Table 2b). Linear regression models controlling for age, race, gender, stroke at visit 1, alcohol intake and current smoking status clearly demonstrate the ARIC participants’ blood potassium levels were not significantly tied to cognitive function score outcomes (p-value = 0.259). Likewise, potassium intake in the participants was shown to be non-significantly associated with cognitive decline in multivariate models that included total HEI-2015 scores (p-value = 0.659).
Table 2a
Association between blood levels of potassium and standardized cognitive function scores
| Model | B1 | 95% Confidence Interval | p-value |
| Univariate | –0.087 | –0.189, 0.015 | 0.093 |
| Demographica | –0.071 | –0.175, 0.034 | 0.185 |
| Multivariateb | –0.062 | –0.169, 0.045 | 0.259 |
Table 2b
Association between potassium intake and standardized cognitive function scores
| Model | B2 | 95% Confidence Interval | p-value |
| Univariate | –0.028 | –0.073, 0.016 | 0.217 |
| Demographica | –0.018 | –0.062, 0.027 | 0.438 |
| Multivariatec | –0.025 | –0.100, 0.049 | 0.507 |
| Multivariate w/ HEId | 0.021 | –0.071, 0.113 | 0.659 |
1: Beta represents change in Cognitive Function score per increase in 1 mmol/l of potassium in the blood. 2: Beta represents change in Cognitive Function score per 1000 mg increase in potassium intake per day. a: Demographic Linear Regression controls for age, gender, race. b: Multivariate Linear Regression controls for demographics, education level, stroke diagnosis, alcohol intake, and smoking status. c: Multivariate Linear Regression controls for demographics, education level, stroke diagnosis, alcohol intake, smoking status, and total calories. d: Multivariate Linear Regression w/HEI-2015 controls for intake multivariate variables and HEI-2015 Score.
Linear regression model results for magnesium blood serum levels and magnesium intake through dietary measures can be seen in Table 3a and 3b. Similarly, to the assessment outcomes for potassium, magnesium findings were nonsignificant. Magnesium blood levels were shown to be more impactful than potassium on cognitive function, but ultimately proved to be nonsignificant (p-value = 0.085) over a six-year assessment in multivariate models. Additionally, all multivariate linear regressions which also included HEI-2015 scores produced findings which were not significant (0.916).
Table 3a
Association between blood levels of magnesium and standardized cognitive function scores
| Model | B3 | 95% Confidence Interval | p-value |
| Univariate | 0.189 | –0.088, 0.466 | 0.181 |
| Demographica | 0.218 | –0.063, 0.500 | 0.128 |
| Multivariateb | 0.252 | –0.035, 0.539 | 0.085 |
Table 3b
Association between magnesium intake and standardized cognitive function scores
| Model | B4 | 95% Confidence Interval | p-value |
| Univariate | –0.040 | –0.086, 0.005 | 0.080 |
| Demographica | –0.028 | –0.074, 0.017 | 0.223 |
| Multivariatec | –0.037 | –0.111, –0.037 | 0.327 |
| Multivariate w/ HEId | 0.005 | –0.087, 0.097 | 0.916 |
3: Beta represents change in Cognitive Function score per increase in 1 mmol/l of potassium in the blood. 4: Beta represents change in Cognitive Function score per 100 mg increase in magnesium intake per day. a: Demographic Linear Regression controls for age, gender, race. b: Multivariate Linear Regression controls for demographics, education level, stroke diagnosis, alcohol intake, and smoking status. c: Multivariate Linear Regression controls for demographics, education level, stroke diagnosis, alcohol intake, smoking status, and total calories. d: Multivariate Linear Regression w/HEI-2015 controls for intake multivariate variables and HEI-2015 Score.
4Discussion
Cognitive decline within the ARIC population of middle-aged adults was not found to be directly associated with potassium and magnesium when assessed through blood serum and dietary intake. Interestingly, when linear regression was performed with magnesium blood serum levels and cognitive function scores the p-value moved closer to significance as we considered demographics and then again when demographics and multivariate were considered (univariate: p-value 0.181; demographic: p-value 0.128; multivariate: p-value 0.085). In the remaining analyses, the opposite trend occurred.
Though race and education were controlled for in this study, the overall design of the current study did not specifically allow for comparison of genders, race, socio-economic level, or education levels on HEI scores and associations between potassium and magnesium towards cognitive decline. Further studies to compare the aforementioned are needed to more fully inform existing research on dietary minerals and cognitive decline. Age can be closely associated with health implications as well. Most cognitive decline studies have been conducted on older populations, but to understand how nutrition specifically relates to prevention of cognitive decline, dementia, or the severity of such conditions, studies might need to be conducted in younger populations, ideally over longer periods of time. Further study attempting to isolate the biological implications of low potassium and magnesium through diet and blood serum on cognitive health and risk for cognitive decline as a result is needed in younger populations. Furthermore, socio-cultural conditions which affect health outcomes and access to proper nutrition should be considered in younger population studies moving forward [36].
Previous studies have shown mixed results when examining the effects of magnesium and potassium on cognitive decline. In 2018 a study looking at community-dwelling older adults (74±3 years) demonstrated that though a higher incident of cognitive decline was associated with a higher potassium/sodium dietary ratio, potassium intake was not independently associated with cognitive decline [37]. However, when two Mexican-American studies (FRONTIER and HABLE) were considered in a 2018 case-control study comparing 510 participants, high blood potassium was shown to be a predictor of mild-cognitive decline (OR = 3.1; p-value = 0.018) [18]. Participants were ≥50 years of age and individuals with mild cognitive impairment and normal controls were comparable to each other in health status and socio-economic makeup. Studies focusing on magnesium have been more consistent in their findings. A 2020 study on low serum magnesium in the ARIC cohort found that the lowest quintile of serum magnesium within the study had a higher incident rate (24%) of dementia compared to those in the top quintile, but low midlife serum magnesium did not appear to impact rates of cognitive decline [16].
Hypertension has been shown to predict cognitive decline and when present in midlife it has some of the strongest evidence for cause of cognitive decline [6, 38]. The presence of hypertension impairs cerebral blood flow because it compromises arterial structures involved in this process leading to inflammation and oxidative stress [39]. Both magnesium and potassium have been recognized, independently, as minerals that have an impact on hypertension. Individuals with a low-level of either mineral tend to have elevated blood pressures and those with higher levels have better blood pressure readings [40, 41]. In addition, both minerals have been shown to promote protection against hypertension and stroke [14]. Potassium-sparing diuretics have been shown to not only intervene in hypertension, but also have a positive impact on cognitive function [42]. Dietary sodium is known to impact cerebrovascular function and blood flow; thus, the known functions of potassium point strongly to it as a potential counter to the effects of high sodium [37] and hypertension. It was beyond the scope of this study to control for hypertension medications, however future research would benefit from well-designed studies which consider the interrelationship hypertension medications have on potassium and magnesium and vice versa.
One of the strengths of this study was the examination of blood serum levels of the minerals, potassium and magnesium, as well as the dietary intake effects on cognitive decline. Previous studies investigating these two minerals either looked at dietary intake and cognitive decline or blood serum levels as opposed to looking at both. Furthermore, most studies looked at magnesium and potassium separate from each other. HEI scores can be thought of as a grade for an individual or cohort, which signifies how well they are meeting the Dietary Guidelines set forth by the USDA. As of 2021 the average HEI-2015 score for an American 60+ years of age was 61 out of a possible high score of 100 and of the typical 18- to 64-year-old they score a 58 out of 100 [34]. The current cohort falls within this range (HEI = 60±9) making this a strength of the current study. More importantly, the use of HEI-2015 allowed for the current study to control for a generally healthy diet while comparing mineral intakes with cognitive outcomes making the current studies use of HEI scores a unique strength. Previous studies which showed an association between low potassium and magnesium in cognitive decline were often not designed to demonstrate potassium and magnesium as independent risk factors as opposed to simply markers for a generally healthy diet.
Several limitations can be noted from the current study. First, the wider the intake range the more likely an association would be clear. The current study was limited by a narrow range of potassium and magnesium intakes. The blood serum range for potassium was 2.65–5.80 (mmol/L) with a standard deviation of 0.35. For magnesium there was a blood serum range of 0.85–2.15 (mEq/L) with a standard deviation of 0.13. Another limitation of the current study is the short follow-up period (6 years) limiting the examination of associations over a longer period. Further, the cohort age span was 44–66 years of age limiting the exploration into the association between potassium and magnesium and cognitive decline over earlier periods of the lifespan. The design of the ARIC study, whose data we borrowed, limited the social determinants of health variables we could use. We did not explore, for example, socioeconomic factors like insurance instability. Lastly, the original design of the ARIC study only included two races: blacks and whites. This limits the ability of the current study in its representation of American racial demographics. Furthermore, the black population for the ARIC study was taken from Mississippi and represented a lower economic population than their white counterparts represented in the study who were taken from three other communities in the US (Washington County, MD, Forsyth County, NC, and select suburbs of Minneapolis, MN) with increased socio-economic advantages.
The results presented on the current study contributes to the existing data examining the impact of dietary minerals on cognitive decline in middle-age populations. Though the current study results were not significant, future studies may examine the interaction of these minerals with hypertension in the onset of MCI and cognitive decline. Further studies of the socio-cultural factors are needed as well as studies of the role of hypertension and hypertension medications.
Acknowledgments
The authors acknowledge the work of Drew Hilton, MS, RD for his work creating a stroke variable and calculations of HEI-2015 scores to create an HEI-2015 variable used in the current study.
Funding
The authors report no funding.
Conflict of interest
The authors have no conflict of interest to report.
References
[1] | VandenBos GR , American Psychological A. APA dictionary of psychology. Washington, DC: American Psychological Association; (2015) . |
[2] | Organization WH. Dementia: A public health priority. International WHOaAsD, editor. Geneva: World Health Organization; (2012) , p. 102. |
[3] | Organization WH. Risk reduction of cognitive decline and dementia WHO guidlines. (2019) . |
[4] | Reisberg M . Global Deterioration Scale [internet]. In: Inc. GR, editor. Geriatric Examination Tool Kit, (2005) . |
[5] | Daviglus ML , Bell CC , Berrettini W , Bowen PE , Connolly ES , Cox NJ , Dunbar-Jacob JM , Granieri EC , Hunt G , McGarry K , Patel D , Potosky AL , Sanders-Bush E , Silberberg D , Trevisan M , National Institutes of Health State-of-the-Science Conference Statement: Preventing Alzheimer’s Disease and cognitive decline NIH Consensus State Sci Statements; (2010) , Contract No.: 4. |
[6] | Livingston G , Huntley J , Sommerlad A , Ames D , Ballard C , Banerjee S , Brayne C , Burns A , Cohen-Mansfield J , Cooper C , Costafreda SG , Dias A , Fox N , Gitlin LN , Howard R , Kales HC , Kivimaki M , Larson EB , Ogunniyi A , Orgeta V , Ritchie K , Rockwood K , Sampson EL , Samus Q , Schneider LS , Selbaek G , Teri L , Mukadam N . Dementia prevention, intervention, and care: report of the Lancet Commission. Lancet. (2020) ;396: (10248):413–46. |
[7] | Capurso C , Bellanti F , Lo Buglio A , Vendemiale G . The Mediterranean Diet slows down the progression of aging and helps to prevent the onset of frailty: A narrative review. Nutrients. (2019) ;12: (1):1–34. |
[8] | Berendsen AAM , Kang JH , van de Rest O , Feskens EJM , de Groot LCPGM , Grodstein F . The Dietary Approaches to Stop Hypertension Diet, cognitive function, and cognitive decline in American older women. J Am Med Dir Assoc. (2017) ;18: (5):427–32. |
[9] | Dominguez LJ , Barbagallo M . Nutritional prevention of cognitive decline and dementia. Acta Biomed. (2018) ;89: (2):276–90. |
[10] | van de Rest O , Berendsen AA , Haveman-Nies A , de Groot LC . Dietary patterns, cognitive decline, and dementia: A systematic review. Adv Nutr. (2015) ;6: (2):154–68. |
[11] | Azzini E , Polito A , Fumagalli A , Intorre F , Venneria E , Durazzo A , Zaccaria M , Ciarapica D , Foddai MS , Mauro B , Raguzzini A , Palomba L , Maiani G . Mediterranean Diet effect: An Italian picture. Nutrition Journal. (2011) ;10: :1–8. |
[12] | Siervo M , Lara J , Chowdhury S , Ashor A , Oggioni C , Mathers JC . Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br J Nutr. (2015) ;113: (1):1–15. |
[13] | McGrattan AM , McGuinness B , McKinley MC , Kee F , Passmore P , Woodside JV , McEvoy CT . Diet and inflammation in cognitive ageing and Alzheimer’s Disease. Curr Nutr Re. (2019) ;8: (2):53–65. |
[14] | Cherbuin N , Kumar R , Sachdev PS , Anstey KJ . Dietary mineral intake and risk of mild cognitive impairment: The PATH Through Life Project. Front Aging Neurosci. (2014) ;6: (FEB):4. |
[15] | Services USDoAaUSDoHaH. Dietary guidelines for Americans, 2020-2025. In Services USDoAaUSDoHaH, editor. 9th ed (2020) |
[16] | Alam AB , Lutsey PL , Gottesman RF , Tin A , Alonso A . Low serum magnesium is associated with incident dementia in the ARIC-NCS Cohort. Nutrients. (2020) ;12: (10):1–11. |
[17] | Wilson DD . Laboratory and Diagnostic Tests - Magnesium. New York: The McGraw-Hill Companies, Inc., The Professional Book Group; (2008) , pp. 2–383. |
[18] | Vintimilla RM , Large SE , Gamboa A , Rohlfing GD , O’Jile JR , Hall JR , O’Bryant SE , Johnson LA . The link between potassium and mild cognitive impairment in Mexican-Americans. Dement Geriatr Cogn Dis Extra. (2018) ;8: (1):151–7. |
[19] | Wilson DD . Laboratory and Diagnostic Tests - Potassium, Blood. New York: The McGraw-Hill Companies, Inc., The Professional Book Group; (2008) , pp. 2–453. |
[20] | The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. The ARIC investigators. Am J Epidemiol. (1989) ;129: (4):687–702. |
[21] | Atherosclerosis Risk in Communities study description Chapel Hill, NC: Atherosclerosis Risk in Communities; (1987) [Available from: https://sites.cscc.unc.edu/aric/description. |
[22] | Willett WC , Sampson L , Stampfer MJ , Rosner B , Bain C , Witschi J , Hennekens CH , Speizer FE . Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. (1985) ;122: (1):51–65. |
[23] | Health NIo. Overview and background of Healthy Eating Index (HEI) Bethesda, MD [updated May 13; cited 2022 6 July 2022]. Available from: https://epi.grants.cancer.gov/hei/. |
[24] | Krebs-Smith SM , Pannucci TE , Subar AF , Kirkpatrick SI , Lerman JL , Tooze JA , Wilson MM , Reedy J . Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. (2018) ;118: (9):1591–602. |
[25] | Root MM , Nielsen MT , Smith TP , Meaux KM . Healthy eating index-and physical activity and disabilities of old age. Nutr Healthy Aging. (2019) ;5: (1):61–9. |
[26] | Root M , Ravine E , Harper A . Flavonol intake and cognitive decline in middle-aged adults. J Med Food. (2015) ;18: (12):1327–32. |
[27] | Benton A , Hamsher K , Sivan A . Multilingual Aphasia Examination. Third Edit ed. Iowa City: AJA Associates; (1994) . |
[28] | ARIC cognitive function form Chapel Hill, NC2010 [Available from: https://sites.cscc.unc.edu/aric/sites/default/files/public/datasets/CNFA.pdf. |
[29] | Knopman DS , Ryberg S . A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. (1989) ;46: (2):141–5. |
[30] | Wechsler D . WAISR Manual, Cleveland, OH: The Psychological Coporation; (1981) . |
[31] | Frasen M . Multilingual aphasia examination. In: Keyser D, Sweetland R, editors. Test Critiques. Kansas City, MO: Test Corporation of America; (1986) )278–82. |
[32] | ARIC protocol manual 7: Blood collection and processing visit 2 Chapel Hill, NC1990 [2:Available from: https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/Blood_Collection_and_Processing.2_7.pdf. |
[33] | Author. ARIC TIA/Stroke form Chapel Hill, NC1987 Available from: https://sites.cscc.unc.edu/aric/sites/default/files/public/forms/TIAB.pdf. |
[34] | USDA Food and Nutrition Service. HEI Scores for Americans 2019 updated 28 April 2022. Available from: https://www.fns.usda.gov/hei-scores-americans. |
[35] | Shrimanker I , Bhattarai S . Electrolytes Treasure Island, FL2022 [updated 26 July 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541123/. |
[36] | Moody L , Chen H , Pan Y-X . Early-life nutritional programming of cognition - the fundamental role of epigenetic mechanisms in mediating the relation between early-life environment and leanring and memory process. Adv Nutr. (2017) ;8: (2):337–50. |
[37] | Nowak KL , Fried L , Jovanovich A , Ix J , Yaffe K , You Z , Chonchol M . Dietary sodium/potassium intake does not affect cognitive function or brain imaging indices. Am J Nephrol. (2018) ;47: (1):57–65. |
[38] | Prince M , Albanese E , Guerchet M , Prina M . World Alzheimer Report 2014: Dementia and risk reduction. An analysis of protective and modifiable factors London; (2014) . |
[39] | Pires PW , Dams Ramos CM , Matin N , Dorrance AM . The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. (2013) ;304: (12):H1598–614. |
[40] | Supplements NIoHOoD. National Institutes of Health Office of Dietary Supplements [Internet] Bethesda (MD): National Institutes of Health Office of Dietary Supplements (US); [updated 2 June 2022; cited 2022 14 July 2022]. Available from: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/. |
[41] | Supplements NIoHOoD. National Institutes of Health Office of Dietary Supplements [Internet] Bethesda (MD): National Institutes of Health Office of Dietary Supplements (US); [updated 2 June 2022. Available from: https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional/. |
[42] | Cisternas P , Lindsay CB , Salazar P , Silva-Alvarez C , Retamales RM , Serrano FG , Vio CP , Inestrosa NC . The increased potassium intake improves cognitive performance and attenuates histopathological markers in a model of Alzheimer’s disease. Biochim Biophys Acta. (2015) ;1852: (12):2630–44. |



