Papillary Renal Cell Carcinoma: Current Evidence and Future Directions
Abstract
Papillary renal cell carcinoma (pRCC) comprises 15-20% of all patients with renal cell carcinoma (RCC). Although in the localized setting where pRCC appears to have better outcomes than clear cell RCC (ccRCC), patients with metastatic pRCC have significantly worse outcomes than patients with metastatic ccRCC. Because of the overall rarity of pRCC, there have been less research and clinical trials devoted to this subtype. Therefore, treatment of pRCC has generally been extrapolated from approved therapies for ccRCC. Recent data shows promise with newer tyrosine kinase inhibitors, and there is emerging evidence on their combination with immune checkpoint inhibitors. However, more dedicated clinical trials to pRCC are urgently needed, as response rates and outcomes still lag behind ccRCC. This review summarizes the pathophysiology, genetic features, the evolution of treatment approaches since the systemic cytokine era, and current challenges of managing pRCC.
INTRODUCTION
Traditionally, renal cell carcinoma (RCC) has been divided into two principal groups based on histology: clear cell versus non-clear cell (the latter is now increasingly known as variant histology). While clear cell RCC (ccRCC) is characterized by a clear cytoplasm on histology, the non-clear cell RCC (nccRCC) term includes a wide variety of histological and clinical presentations. Papillary RCC (pRCC) is the most common subtype of nccRCC, accounting for approximately 15–20% of all cases of RCC and derives its name from the distinctive papillary architecture seen on histology.
Conventional cytogenetics provided early evidence that the phenotypic distinctions between RCC variants have an underlying genetic basis. For instance, ccRCC frequently demonstrates loss of chromosome 3p, leading to inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene. In contrast, pRCC does not have 3p loss and is often genetically characterized by gains of chromosomes 7 and 17 [1].
Comprehensive molecular profiling, such as that performed by The Cancer Genome Atlas (TCGA), has further confirmed the genetic underpinnings of the phenotypic differences between histological renal cell carcinoma subtypes [2]. On the one hand, ccRCC harbors frequent mutations in VHL, PBRM1, BAP1 and SETD2 among other genes. Additionally, ccRCC shows increased expression of angiogenesis and hypoxia related genes, driven by VHL/hypoxia inducible factor (HIF) pathway activation, as well as heightened immune response signatures [3]. On the other hand, pRCC is characterized by mutations in MET, SETD2, NF2, KDM6A and other genes and displays a unique upregulation of amino acid metabolic pathways and retention of cilia-associated gene expression [4].
Single-cell RNA sequencing studies, with their higher resolution and precision, have validated that the cell of origin for most pRCC tumors is the proximal tubule epithelium [5]. A recent single-cell transcriptomic analysis further demonstrated that while the majority of pRCC cases arise from proximal tubule cells, a small subset may originate from principal cells of the collecting duct [6].
Given its lower prevalence relative to ccRCC, demographic data on pRCC is more limited but some studies suggest key distinctions from its more prevalent counterpart. Multiple analyses indicate patients with pRCC tend to be older at diagnosis than patients with ccRCC, while the age at initial presentation is similar between all the other kidney cancer subtypes [7]. pRCC also shows a tighter association with end-stage renal disease (ESRD) compared to ccRCC [7]. Additionally, African American patients appear disproportionately affected by pRCC versus ccRCC, even when accounting for increased ESRD rates in this population [7–11]. This predilection towards African Americans implicates potential genetic factors. Regarding outcomes, an analysis of the International Metastatic RCC Database Consortium from 2002–2019 found that metastatic pRCC had significantly worse overall survival (OS) compared to metastatic ccRCC [12]. However, for localized disease, some studies suggest pRCC may have better survival outcomes than localized ccRCC [13]. More research is still needed to refine demographic and survival profiles for pRCC versus ccRCC. Current evidence indicates key epidemiological and prognostic distinctions are likely tied to the unique molecular pathogenesis of pRCC.
In this review, we summarize key developments in the clinical management of pRCC. We discuss pRCC pathology, the evolution of treatment approaches from the systemic cytokine era to the current targeted therapies and immune checkpoint inhibitors, current standards for management of advanced and localized disease, as well as current challenges in management. Finally, we explore emerging therapies and biomarkers that may enable more precise, subtype-specific treatment approaches for pRCC in the future.
SUBTYPES OF PRCC
Papillary renal cell carcinoma (pRCC) was first clearly delineated as a distinct entity from other renal tumors in a 1976 report describing 34 cases [14]. However, it was not until 1997 that pRCC was further subclassified into type 1 and type 2 based on histologic differences [15]. Historically, type 1 pRCC was characterized by papillae covered with a single layer of small cuboidal cells with scant cytoplasm, while type 2 pRCC displayed papillae covered by large eosinophilic cells arranged in pseudostratified layers [15]. This historical classification of type 1 versus type 2 pRCC has fallen out of favor with increasing knowledge of their genetic and molecular features. Recent molecular studies, like TCGA study, suggest that type 2 pRCC may not constitute a single well-defined entity, but rather individual subgroups with a different molecular background [4, 16]. Therefore, the 5th World Health Organization (WHO) classification of adult renal tumors no longer divides pRCC into type 1 and type 2. The older term of type 1 is now called variant pRCC, and what used to be classified as type 2 is now organized into well-defined categories such as FH-deficient RCC, SMARCB1-deficient RCC or MiTF family RCC,etc. [17].
TREATMENT OF METASTATIC PAPILLARY RENAL CELL CARCINOMA
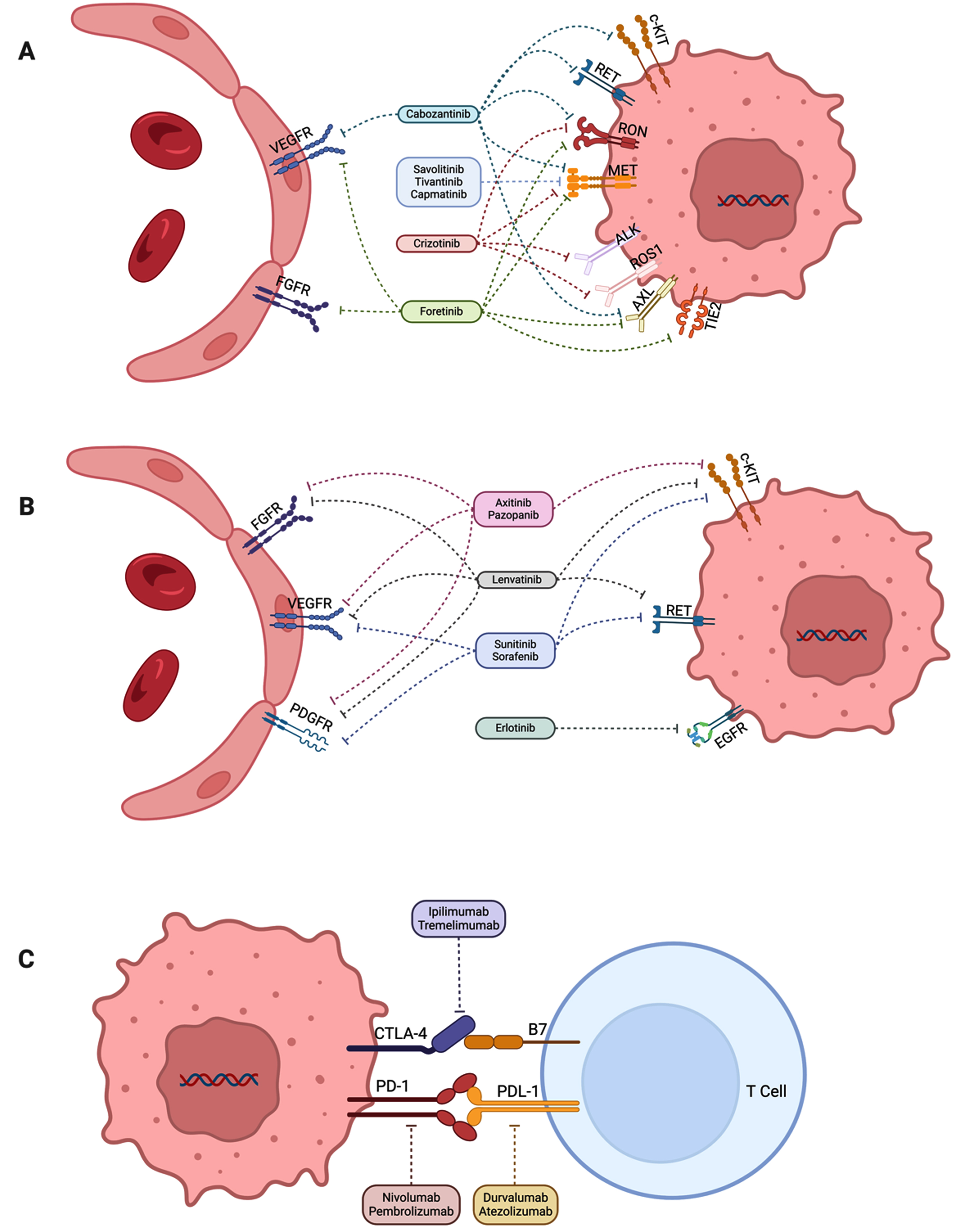
We will review the evidence in the different eras of conventional RCC therapy for pRCC beginning with the cytokine era through the current immune checkpoint inhibitor and tyrosine kinase inhibitor era. A summary of the reported clinical trials involving management of metastatic pRCC is provided in Table 1. Figure 1 outlines the key targets for pRCC and their therapies.
Table 1
Notable clinical trials with published results for papillary renal cell carcinoma
| Trial | Enrollment criteria | Number of evaluable patients with papillary RCC | Intervention | Results |
| NCT00065468 (ARCC) [26] | No prior systemic therapy | 55 | Temsirolimus vs. IFN-alfa | median PFS: 5.9 vs. 2.1 months (HR 0.52; 95% CI, 0.29–0.91) median OS: 10.9 vs. 5.7 months (HR 0.50; 95% CI, 0.27–0.94) |
| NCT00830895 [27] | Prior anti-VEGF therapies allowed | 29 | Everolimus | ORR: 6.9% median PFS: 3.4 months median OS: 10.9 months |
| NCT00688753 (RAPTOR) [28] | No prior systemic therapy | 88 | Everolimus | ORR: 1% median PFS: 4.1 months (95% CI, 3.6–5.5) median OS: 21.4 months (95% CI, 15.4–28.4) |
| NCT01399918 [30] | No prior systemic therapy | 37 | Everolimus with bevacizumab | ORR: 35% median PFS: 13.7 months (95% CI, 10.8–16.4) median OS: 33.9 months (95% CI, 23.3–71.9) |
| NCT02915783 [31] | No prior systemic therapy | 20 | Temsirolimus with lenvatinib | ORR: 15% median PFS: 9.2 months (95% CI, 3.5-NE) median OS: 11.7 months (95% CI, 8.1-NE) |
| NCT00111020 [33] | Prior systemic therapy allowed | 107 | Sorafenib | ORR: 3% |
| Molina et al. [34] | Prior systemic therapy allowed | 8 | Sunitinib | ORR: 0% median PFS: 5.6 months (95% CI, 1.4–7.1) |
| Tannir et al. [35] | Prior systemic therapy allowed | 25 | Sunitinib | ORR: 0% median PFS: 1.6 months (95% CI, 1.4–5.4) |
| Lee et al. [36] | No prior systemic therapy | 22 | Sunitinib | ORR: 36% |
| NCT00541008 (SUPAP) [37] | No prior systemic therapy | 61 | Sunitinib | ORR: 13% for type 1, 11% for type 2median PFS: 6.6 months (95% CI, 2.8–14.8) for type 1, 5.5 months (95% CI, 3.8–7.1) for type 2 median OS: 17.8 months (95% CI, 5.7–26.1) for type 1, 12.4 months (95% CI, 8.2–14.3) for type 2 |
| NCT01185366 (ESPN) [38] | No prior systemic therapy | 27 | Sunitinib vs. everolimus | median PFS: 5.7 vs. 4.1 months median OS: 16.6 vs. 14.9 months |
| NCT01108445 (ASPEN) [39] | No prior systemic therapy | 70 | Sunitinib vs. everolimus | ORR: 24% vs. 5% median PFS: 8.1 vs. 5.5 months (HR 1.6; 80% CI, 1.1–2.3) |
| NCT01538238 [42] | Prior systemic therapy allowed | 18 | Pazopanib | ORR: 39% median PFS: 17.3 months (95% CI, 14.8–19.8) |
| NCT01798446 [45] | Prior temsirolimus exposure | 26 | Axitinib | ORR: 40% median PFS: 3.5 months (95% CI, 0–10.9) median OS: 8.3 months (95% CI, 4.1–12.5) |
| NCT02489695 (AXIPAP) [46] | No prior systemic therapy | 42 | Axitinib | ORR: 28% median PFS: 6.6 months (95% CI, 5.5–9.2) median OS: 18.9 months (95% CI, 12.8-NR) |
| SWOG S0317 [48] | No prior chemotherapy or immunotherapy | 45 | Erlotinib | ORR: 11% median OS: 27 months (95% CI, 13–36) |
| NCT01130519 [49] | Prior systemic therapy allowed | 83 | Erlotinib with bevacizumab | ORR: 51% |
| NCT00726323 [51] | Prior exposure to sunitinib allowed | 74 | Foretinib | ORR: 13.5% median PFS: 9.3 months (95% CI, 6.9–12.9) |
| NCT01524926 (CREATE) [53] | Prior systemic therapy allowed | 27 | Crizotinib | ORR: 50% for 4 MET + patients, 6.3% for 16 MET- patients |
| NCT02127710 [55] | No prior MET inhibitors allowed | 109 | Savolitinib | ORR: 18% for MET + patients, 0% for MET- patients median PFS: 6.2 months for MET + patients, 1.4 months for MET- patients |
| NCT03091192 (SAVOIR) [56] | No prior MET inhibitors allowed | 60 | Savolitinib vs. sunitinib | ORR: 27% vs. 7% median PFS: 7.0 vs. 5.6 months (HR 0.71; 95% CI, 0.37–1.36; p = 0.31) median OS: NR vs. 13.2 months (HR 0.51; 95% CI, 0.21–1.17; p = 0.11) |
| NCT01688973 (SWOG S1107) [58] | No prior MET inhibitors allowed | 50 | Tivantinib with erlotinib vs. tivantinib | ORR: 0% vs. 0% median PFS: 3.9 vs. 2.0 months median OS: 11.3 vs. 10.3 months |
| NCT02019693 [60] | Prior systemic therapy allowed including MET inhibitor | 20 | Capmatinib | ORR: 15% |
| NCT02761057 (PAPMET) [68] | Up to one prior systemic therapy excluding VEGF and MET inhibitor | 147 (90 for cabozantinib vs. sunitinib) | Cabozantinib vs. sunitinib (savolitinib and crizotinib arms closed) | ORR: 23% vs. 4% (p = 0.010) median PFS: 9.0 vs. 5.6 months (HR 0.60; 95% CI, 0.37–0.97; p = 0.019) median OS: 20.0 vs. 16.4 months (HR 0.84; 95% CI, 0.47–1.51) |
| NCT02853344 (KEYNOTE-427 cohort B) [74] | No prior systemic therapy | 118 | Pembrolizumab | ORR: 28.8% median PFS: 5.5 months (95% CI, 3.9–6.9) median OS: 31.5 months (95% CI, 25.5-NR) |
| NCT02596035 (CheckMate 374) [75] | Prior systemic therapy allowed | 24 | Nivolumab | ORR: 8.3% |
| NCT03117309 (HCRN GU16-260-Cohort B) [76] | No prior systemic therapy | 19 | Nivolumab (part A), then with ipilimumab if refractory to monotherapy (part B) | ORR: 5% in part A |
| NCT03170960 (COSMIC-021) [82] | No prior ICIs or MET inhibitors | 15 | Cabozantinib with atezolizumab | ORR: 47% median PFS: 8.1 months (95% CI, 2.7–18.4) median OS: 31.8 months (95% CI, 6.1-NR) |
| NCT03635892 (CA209-9KU) [84] | No prior ICIs | 32 | Cabozantinib with nivolumab | ORR: 47% |
| NCT02819596 (CALYPSO) [85] | No prior ICIs or MET inhibitors | 41 | Savolitinib with durvalumab | ORR: 29% median PFS: 4.9 months (95% CI, 2.5–10) median OS: 14.1 months (95% CI, 7.3–30.7) |
| NCT04704219 (KEYNOTE-B61) [87] | No prior systemic therapy | 93 | Lenvatinib with pembrolizumab | ORR: 54% median PFS: 17.5 months (95% CI, 15-NR) |
| NCT04413123 [88] | No prior ICIs or cabozantinib | 20 | Ipilimumab, nivolumab, and cabozantinib | ORR: 20% |
RCC=renal cell carcinoma, ORR = objective response rate, PFS = progression-free survival, OS = overall survival, IFN = interferon, VEGF = vascular endothelial growth factor, TKI = tyrosine kinase inhibitor, ICI = immune checkpoint inhibitor.
Fig. 1
Key molecular targets in papillary renal cell carcinoma. Figure was developed courtesy of BioRender®. (A) Tyrosine kinase inhibitors targeting MET among other receptors. (B) Tyrosine kinase inhibitors not targeting MET receptor. (C) Immune checkpoint inhibitors.
Cytokine era
One of the first large retrospective cohort studies to evaluate outcomes and survival associated with systemic therapy (including interleukin-2, interferon-alfa-2a, chemotherapy, etc.) for patients with nccRCC that included a significant number of pRCC was a single-institution study in the United States (US) from 1985–2001 [18]. The study evaluated 64 patients with nccRCC, including 18 (28%) with pRCC. For patients with pRCC, 2 received interleukin-2, 1 received interferon-alfa-2a, and 3 received a combination of both cytokines. Overall, systemic therapies including cytokines for patients with pRCC were minimally effective. The median OS was only 5.5 months (95% CI, 4–12) for patients with pRCC, which was much lower compared to 11 months (95% CI, 8–15) and 29 months (95% CI, 19–59) for collecting duct and chromophobe subtypes, respectively. This study was one of the first to emphasize that the prognosis was poor for metastatic pRCC.
In another single-institution retrospective study conducted in Germany, 164 patients with metastatic RCC (mRCC) were treated with at least one cycle of immunochemotherapy with IL-2, IFN-alfa, and 5-fluorouracil from 2001 to 2005 [19]. The majority received radical nephrectomy as well. The cohort included 131 with ccRCC and 22 with pRCC. No patients with pRCC had any clinical response with immunochemotherapy, compared to 28.8% of patients with ccRCC.
The PERCY QUATTRO trial enrolled 492 patients from 2000 to 2004 with RCC to compare medroxyprogesterone acetate, IFN-alfa, IL-2, or a combination of both cytokines [20]. There was no significant difference in OS between patients treated with IFN-alfa patients and non-IFN-alfa as well as those treated with IL-2 versus non-IL-2 regimens. Notably, there was no objective response seen for any of the patients in this study with nccRCC, including 21 patients with pRCC.
These studies provided insights that metastatic pRCC had a worse prognosis than the other subtypes of RCC and that pRCC was more resistant to cytokines compared to ccRCC.
Targeted therapy - mTOR pathway inhibition
mTOR is a serine/threonine kinase that regulates cell growth and metabolism, and it is activated downstream of the PI3K/AKT pathway [21]. The mTOR signaling pathway is known to contribute to cancer cell proliferation and survival, and its pathway is often activated in RCC [22]. HIF protein expression, important for tumorigenesis of RCC, is dependent on mTOR activation [22]. In preclinical and murine models, inhibition of mTOR was found to block mRNA translation of HIF1A and to induce growth arrest of kidney cancer cells [23]. Interestingly, HIF1A nuclear expression seems to be significantly higher in pRCC in comparison with ccRCC [24], although this will need further validation.
The phase 3 ARCC trial (NCT00065468) enrolled 626 previously untreated patients with mRCC randomized to either IFN-alfa, temsirolimus (specific inhibitor of the FKBP-12 intracellular protein to suppress mTOR signaling), or the combination of IFN-alfa and temsirolimus from 2003–2005 [25]. Patients on temsirolimus monotherapy had a significantly longer median OS (10.9 vs. 7.3 months; HR 0.73; 95% CI 0.58–0.92; p = 0.008) than patients on IFN-alfa monotherapy, while the combination of temsirolimus and IFN-alfa did not significantly improve median OS over IFN-alfa monotherapy (8.4 vs. 7.3 months; HR 0.96; 95% CI, 0.76–1.20; p = 0.70). Subgroup analyses of this trial showed that 339 patients (82%) of patients had ccRCC and 73 patients (18%) had nccRCC, including 55 patients (11%) with pRCC [26]. For the 55 patients with pRCC, 25 received temsirolimus monotherapy and 30 received IFN-alfa monotherapy. The median progression-free survival (PFS) and median OS statistically favored the temsirolimus arm (5.9 vs. 2.1 months; HR 0.52; 95% CI, 0.29–0.91 and 10.9 vs. 5.7 months; HR 0.50; 95% CI, 0.27–0.94, respectively). This analysis showed temsirolimus to be favorable over IFN-alfa monotherapy regardless of the RCC histology.
A phase 2 trial (NCT00830895) conducted in Korea to evaluate everolimus for patients with nccRCC enrolled 49 patients (29 had pRCC) from 2009 to 2011, including 23 treated with prior anti-vascular endothelial growth factor (VEGF) therapies [27]. For the patients with pRCC, 6.9% (2/29) had a partial response (PR), 48% (14/29) had stable disease (SD), and 41% (12/29) had progressive disease (PD) as best response, for an objective response rate (ORR) of 6.9% (2/29). Patients with pRCC had a shorter median PFS (3.4 months) and median OS (10.9 months) compared to patients with chromophobe or collecting duct subtypes. For the overall cohort, the ORR was 10.2%, and the median PFS and the median OS were 5.2 months and 14.0 months, respectively. The trial showed efficacy of everolimus for patients with nccRCC with or without prior anti-VEGF treatment.
RAPTOR was a phase 2 trial (NCT00688753) for European patients with previously untreated pRCC to receive everolimus [28]. It was the first large prospective study dedicated to pRCC, enrolling patients from 2009 through 2012. In the intention-to-treat (ITT) population involving 88 patients, the median PFS was 4.1 months (95% CI, 3.6–5.5) and median OS was 21.4 months (95% CI, 15.4–28.4). The ORR was just 1% (95% CI, 0–5), while the disease control rate (DCR) was 66%. Comparing type 1 (n = 14) versus type 2 (n = 43) histology, the outcomes were better for type 1. The median PFS was 7.9 months (95% CI, 2.1–11.0) for type 1 and 5.1 months (95% CI, 3.3–5.5) for type 2, and the median OS was 28.0 months (95% CI, 7.6-NR) for type 1 and 24.2 months (95% CI, 15.8–32.8) for type 2. The adverse event profile was overall consistent with previous studies of everolimus for ccRCC. The trial demonstrated that everolimus was an effective frontline therapy for patients with metastatic pRCC.
The combination therapy of everolimus with bevacizumab, a humanized VEGF monoclonal antibody, was evaluated in patients with treatment-naïve nccRCC in a single-center phase 2 study (NCT01399918) [29]. In the 37 patients with papillary features (including 14 pRCC, 24 unclassified RCC with papillary features, and 1 translocation-associated RCC with papillary features), the ORR was 35%, the median PFS was 13.7 months (95% CI, 10.8–16.4), and the median OS was 33.9 months (95% CI, 23.3–71.9) [30]. This trial proposed that combination therapy of everolimus with bevacizumab could be a frontline option for pRCC.
Another phase 2 trial (NCT02915783) evaluated the combination of temsirolimus with lenvatinib as frontline therapy for patients with nccRCC from 2017 to 2019 [31]. Thirty-one patients were enrolled, including 20 patients with pRCC. Three of the 20 patients achieved a PR as best response (ORR 15%); the median PFS was 9.2 months (95% CI, 3.5-NE), and the median OS was 11.7 months (95% CI, 8.1-NE). For all 31 patients, the ORR was 26%, median PFS was 9.2 months (95% CI, 5.5-NE), and median OS was 15.6 months (95% CI, 9.2-NE).
These above studies demonstrated mTOR monotherapy as well as in combination with other agents was more effective than what was seen historically with cytokines for pRCC, but outcomes were still dismal.
Targeted therapy – tyrosine kinase pathway inhibition
Sunitinib and sorafenib
Multitarget tyrosine kinase pathway inhibitors (TKIs) like sunitinib and sorafenib have been studied for efficacy in pRCC in retrospective series and prospective trials.
One study evaluated 41 patients with pRCC receiving either sunitinib or sorafenib as their initial TKI therapy in five cancer institutions across the US and France from 2002 to 2006 [32]. Thirteen out of 41 patients received sunitinib and 28 patients received sorafenib; 2/12 patients (17%) had evaluable response with sunitinib, while 0/28 patients (0%) had treatment response with sorafenib. The overall median PFS was 7.6 months, including 11.9 months for sunitinib and 5.1 months for sorafenib (p < 0.001). The study concluded that sunitinib may be more effective than sorafenib in patients with pRCC, although the effectiveness of sunitinib was minimal.
The safety and efficacy of sorafenib in both frontline and refractory settings for patients with RCC generally excluded from clinical trials was evaluated in a trial (NCT00111020) with 2504 patients in the US and Canada from 2005 to 2006 [33]. There were 202 patients with nccRCC in the trial, including 158 with pRCC. For the 107 evaluable patients with pRCC for best response, there were 0% (0/107) complete response (CR), 3% (3/107) PR, 81% (87/107) SD, and 16% (17/107) PD, for an ORR of 3% (3/107) and DCR of 84% (90/107). This study showed some activity of sorafenib in patients with pRCC.
Another phase 2 trial of sunitinib in nccRCC enrolled 23 patients with nccRCC, including 8 patients with pRCC, from 2007 to 2009 before it was discontinued early due to poor accrual [34]. None of these 8 patients had a response with sunitinib, and the median PFS was 5.6 months (95% CI, 1.4–7.1). In another phase 2 trial, 57 patients with nccRCC were enrolled, including 27 patients with pRCC (2 type 1, 11 type 2, and 14 not otherwise specified [NOS]), from 2007 to 2010 [35]. Of the 25 evaluable patients, there were no responses, 48% (12/25) had SD, and 52% (13/25) had PD; the median PFS in patients with pRCC was 1.6 months (95% CI, 1.4–5.4), and the median PFS for the entire cohort of 55 evaluable patients was 2.7 months (95% CI, 1.4–5.4). A multicenter phase 2 trial in Korea enrolled 31 patients with nccRCC, including 22 patients with pRCC, from 2008 to 2011 to receive sunitinib [36]. The 22 patients with papillary subtype included 17 type 2 and 5 NOS. In terms of efficacy, there were 36% PR (8/22), 50% (11/22) SD, 4.5% (1/22) PD, and 2 not evaluable. These small phase 2 trials showed some efficacy of sunitinib in a previously treated pRCC patient population.
SUPAP (NCT00541008) was the first phase 2 single-arm clinical trial that evaluated sunitinib as frontline therapy for 61 patients with pRCC, which included 15 with type 1 and 46 with type 2 pRCC [37]. Patients were enrolled from 2007 to 2011, with a median follow-up time of 51.4 months. For type 1 pRCC, there were 2/15 (13%) patients with PR and 10/15 (67%) with SD, the median PFS was 6.6 months (95% CI, 2.8–14.8), and the median OS was 17.8 months (95% CI, 5.7–26.1). For type 2 pRCC, there were 5/46 (11%) patients with PR and 25/46 (54%) with SD; the median PFS was 5.5 months (95% CI, 3.8–7.1), and the median OS was 12.4 months (95% CI, 8.2–14.3). The outcome for type 2 histology was worse compared to type 1 histology, although it was notable that 24% of patients with type 2 had liver metastases compared to none with type 1. Sunitinib was established as a potential frontline option for pRCC.
Two multicenter randomized phase 2 clinical trials compared sunitinib against everolimus as frontline therapy, ESPN (NCT01185366) and ASPEN (NCT01108445). ESPN enrolled 73 patients with nccRCC or ccRCC with sarcomatoid features in the US from 2010 to 2013, randomized to receive either sunitinib or everolimus as frontline therapy with crossover at PD [38]. Of the 27 patients with pRCC from 68 total evaluable patients, 14 received sunitinib and 13 received everolimus. The median PFS was 5.7 months (95% CI, 1.4–19.8) with sunitinib versus 4.1 months (95% CI, 1.5–7.4) with everolimus and the median OS was 16.6 months (95% CI, 5.9-NA) versus 14.9 months (95% CI, 7.1–22.7),respectively.
The ASPEN trial enrolled 109 patients with nccRCC in the US, Canada, and the United Kingdom from 2010 to 2013 to receive either frontline sunitinib or everolimus [39]. There were 70 patients with pRCC, including 33 in the sunitinib arm (4 with type 1 histology) and 37 in the everolimus arm (2 with type 1 histology). The ORR was 24% (8/33) for the sunitinib arm compared to 5% (2/37) for the everolimus arm. The median PFS for pRCC was 8.1 months with sunitinib versus 5.5 months with everolimus (HR 1.6; 80% CI, 1.1–2.3) and 8.3 months versus 5.6 months (HR 1.41; 80% CI, 1.03–1.92) in the overall cohort of nccRCC. Both these trials established non-inferiority of sunitinib compared to everolimus as frontline systemic therapy for patients with nccRCC including pRCC.
Pazopanib
Pazopanib is another multitarget tyrosine kinase pathway inhibitor including PDGFR, VEGFR, fibroblast growth factor receptor (FGFR), and c-KIT [40]. The Italian retrospective study PANORAMA analyzed 37 patients with nccRCC with frontline pazopanib from 2010 to 2015 [41]. There were 19 patients with pRCC including 8 type 1, 7 type 2, and 4 NOS. The ORR was 21% (no CR) and the DCR was 95% for pRCC. The study suggested pazopanib could be an effective and feasible option for patients with nccRCC.
A single-arm phase 2 trial (NCT01538238) enrolled 29 patients with nccRCC to receive pazopanib in Korea from 2012–2014, including 19 patients with pRCC [42]. For the 18 evaluable patients with pRCC, there were 39% (7/18) PR, 50% (9/18) SD, and 11% (2/18) PD, for an ORR of 39% (7/18). The median PFS was 17.3 months (95% CI, 14.8–19.8), and the median OS was not reached at the time of reporting. The trial showed effectiveness and tolerability of pazopanib for patients withnccRCC.
Axitinib
Axitinib is a multi-kinase inhibitor targeting VEGFR1-3, PDGFR, and KIT, with more specificity for VEGFR compared to the previous generation TKIs like sunitinib and pazopanib [43]. The AXIS phase 3 trial (NCT00678392) confirmed axitinib to have superiority over sorafenib as a second-line therapy (progression on either sunitinib, bevacizumab plus interferon-alfa, temsirolimus, or cytokines) based on median PFS (6.7 vs. 4.7 months; HR 0.665; 95% CI, 0.544–0.812; p < 0.0001) for ccRCC only [44].
A phase 2 trial (NCT01798446) enrolled 40 patients with nccRCC with prior temsirolimus exposure in Korea from 2013 to 2016 [45]. There were 26 patients with pRCC, including 24 type 2, 1 type 1, and 1 NOS. For these 26 patients, there were 40% (10/25) PR, 24% (6/25) SD, 36% (9/25) PD for an ORR of 40% (10/25); the median PFS was 3.5 months (95% CI, 0–10.9), and the median OS was 8.3 months (95% CI, 4.1–12.5). Axitinib emerged as a promising second-line therapy for nccRCC.
AXIPAP (NCT02489695) was a phase 2 trial that evaluated axitinib in the frontline setting for patients with pRCC in France [46]. The trial enrolled 44 patients with pRCC between 2015 and 2018, with 13 type 1, 30 type 2, and 1 NOS. Among 42 evaluable patients for best objective response, there were 29% (12/42) PR, 62% (26/42) SD, and 9.5% (4/42) PD, for an ORR of 29% (12/42). Notably, there was just 8% (1/13) PR for type 1 patients compared to 36% (10/28) PR for type 2 patients (10/28). The median PFS for the cohort was 6.6 months (95% CI, 5.5–9.2), and the median OS was 18.9 months (95% CI, 12.8-NR). The trial suggested axitinib was effective as frontline therapy for patients with pRCC, especially for type 2 histology.
Erlotinib
Erlotinib is an endothelial growth factor receptor (EGFR) inhibitor. Prior preclinical data indicated that renal tumor cell lines including papillary subtype with VHL wild type expression demonstrated tumor cell growth inhibition with an EGFR inhibitor compared to VHL-mutated ccRCC tumor cell lines [47]. The Southwest Oncology Group (SWOG) S0317 phase 2 trial registered 52 patients with pRCC with no prior treatment between 2005 and 2006; of the 45 evaluable patients, there was no CR, 11% (5/45) PR, and 53% (24/45) SD for an ORR of 11% (5/45) and DCR of 64% (29/45) [48]. The median OS was 27 months (95% CI, 13–36). The study did not meet the primary endpoint goal (ORR 20%). It demonstrated the activity of erlotinib for some patients with pRCC, but VHL status could not be used as a predictor. Another phase 2 trial (NCT01130519) evaluated the combination of bevacizumab and erlotinib for 83 patients with pRCC (including 42 with hereditary leiomyomatosis with a fumarate hydratase deficiency associated with type 2 pRCC) with up to two prior VEGF TKIs [49]. The ORR was 51% (42/83), and it was higher for the HLRCC cohort (64%, 27/42) than the sporadic cohort (37%, 15/41), showing the combination was acceptable especially for HLRCC.
Foretinib
Foretinib is a multi-kinase inhibitor including MET, VEGFR2, PDGFR, TIE-2, RON, and AXL [50]. A phase 2 trial (NCT00726323) enrolled 74 patients with pRCC from 10 cancer centers in the US [51]. Prior exposure to sunitinib was allowed. There were two cohorts: cohort A (37 patients) received foretinib 240 mg once daily for the first five days of a 14-day cycle, and cohort B (37 patients) received foretinib 80 mg daily. Overall, the ORR was 13.5% (10/51), all being PR. The median PFS was 9.3 months (95% CI, 6.9–12.9) with slight improvement for cohort A (median PFS 11.6 months; 95% CI, 6.9–12.9) compared to cohort B (median PFS 9.1 months; 95% CI, 5.8–10.9), and the median OS was not reached in either cohort. For patients with germline MET mutation, foretinib was more effective (5/10 with germline mutation compared to 5/57 without germline mutation). Although the predefined primary endpoint of this study (ORR of 25%) was not achieved, this was one of the first studies suggesting MET inhibitors could be effective for patients with MET-mutated pRCC.
Crizotinib
Crizotinib is a multi-kinase inhibitor including MET, ALK, ROS1, and RON [52]. The phase 2 trial CREATE (NCT01524926) evaluated patients with advanced tumors with MET and/or ALK alterations, and it included a pRCC type 1 cohort [53]. In this cohort, 27 patients with type 1 pRCC were enrolled between 2012 and 2016 from eight European countries. Among these 27 patients, there were 4 patients with MET+mutations, 19 MET-, and 4 questionable. Of the 4 MET+patients, there were 50% (2/4) PR and 25% (1/4) SD (ORR 50%). The two responders had a duration of response of 21.8 and 37.3 months, with the latter being a case of MET amplification. Of 16 evaluable MET- patients, there were 6.3% (1/16) PR and 69% (11/16) SD (ORR 6.3%). The trial showed patients with MET-altered tumors could achieve a good response to MET inhibitors.
Savolitinib
In contrast to the previously discussed MET inhibitors that also inhibit other tyrosine kinase pathways, savolitinib is a highly selective MET inhibitor in an ATP-competitive manner [54]. A phase 2 trial (NCT02127710) evaluated savolitinib in 109 patients with pRCC irrespective of prior treatment (excluding prior/current MET inhibition) from 2014 through 2016 [55]. There were 16 type 1, 68 type 2, and 25 unclassifiable. In terms of MET status, there were 44 MET+, 46 MET-, and 19 MET unknown. For the overall cohort of 109 patients, the ORR was 7.3% (8/109). Importantly, the ORR was 8/44 (18%, all of whom had PR) for MET+ patients, and 0/46 (0%) for MET- patients. The SD rate was 22/44 (50%) for MET+ patients, and 11/46 (24%) in MET- patients. The median PFS for patients with MET+ tumors was 6.2 months compared to 1.4 months for patients with MET- tumors (HR 0.33; 95% CI, 0.20–0.52; p < 0.001). Overall, savolitinib was well tolerated and showed promise of savolitinib as an option for patients with MET-driven pRCC, but the study was closed early due to poor accrual.
The results of this phase 2 trial led to the phase 3 trial SAVOIR (NCT03091192), which was an open-label, randomized trial comparing savolitinib versus sunitinib in patients with MET-driven pRCC (prior VEGF TKI therapies allowed), with a primary endpoint of PFS [56]. The study aimed to enroll 180 patients, but recruitment was terminated early due to accrual challenges, and only 60 patients were enrolled between 2017 and 2018, with 33 patients randomized to savolitinib and 27 patients to sunitinib. The median PFS was 7.0 months with savolitinib and 5.6 months with sunitinib (HR 0.71; 95% CI, 0.37–1.36; p = 0.31), and the median OS was not reached with savolitinib versus 13.2 months (HR 0.51; 95% CI, 0.21–1.17; p = 0.11) with sunitinib. The ORR was 27% (9/33) for savolitinib, compared to only 7% (2/27) for sunitinib, with no CR for either arm. Despite the challenges faced by SAVOIR, it highlighted the importance of a MET inhibitor that could target MET-driven pRCC and ensured savolitinib to be further evaluated in a future trial.
Tivantinib
Tivantinib is a selective MET inhibitor in an ATP-independent manner [57]. A phase 2 trial evaluated tivantinib with or without erlotinib for patients with pRCC, attempting to determine if dual MET and EGFR inhibition would be synergistic [58]. A total of 50 patients were enrolled in the US between 2012 and 2014, with 25 patients in the tivantinib monotherapy arm and 25 patients in the combination arm. Prior systemic therapy was allowed. In the tivantinib monotherapy arm, there were 2 type 1, 11 type 2, and 12 unassigned. In the tivantinib with erlotinib combination arm, there were 1 type 1, 10 type 2, and 14 unassigned. There were no responses in either arm. The median PFS was 3.9 months with tivantinib and erlotinib (95% CI, 1.8–7.3) vs. 2.0 months with tivantinib alone (95% CI, 1.8–3.0), and the median OS was 11.3 months (95% CI, 6.7–21.9) vs. 10.3 months (95% CI, 7.3–15.7), both trending in favor of the combination arm but not statistically significant. Notably, there were 16/35 patients (46%) with adequate tumor tissue DNA, and 1/16 (6%) had a MET mutation, which may have influenced theresults.
Capmatinib
Capmatinib is a highly selective MET inhibitor [59] that was evaluated in a phase 2 trial (NCT02019693) in 20 patients with type 1 pRCC enrolled between 2014 and 2019 [60]. There were 15% (3/20) PR and 35% (7/20) SD (ORR of 15% and DCR of 50%). Molecular alterations of MET for this study were not reported.
Cabozantinib
Cabozantinib is a multi-kinase inhibitor including VEGFR2, MET, RET, KIT, AXL, RON, and FLT3 [61]. Cabozantinib is approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in second/subsequent line therapy for ccRCC based on the phase 3 trial METEOR (NCT01865747), which showed a statistically significant improvement in both median PFS and median OS over everolimus [62, 63]. Cabozantinib is also regulatory approved as frontline therapy for previously untreated ccRCC, based on the phase 2 trial CABOSUN (NCT01835158), which showed a significantly prolonged median PFS over sunitinib [64, 65].
Cabozantinib was studied in a retrospective cohort of patients with nccRCC [66]. There were 30 patients with nccRCC, of which 17 patients had pRCC, including 3 type 1, 11 type 2, and 3 NOS. Twenty-six of the 30 patients had received prior VEGF TKIs. Fifteen of the 17 patients with pRCC were evaluable for measurable disease, and there were 13% (2/15) PR and 87% (13/15) SD. A multi-institutional retrospective study involving 21 centers in the US and one in Belgium included 112 patients with nccRCC treated with cabozantinib between 2015 and 2018 [67]. Cabozantinib was used in various treatment lines. There were 66 patients (59%) with pRCC. The ORR was 27% (18/66) including one patient with CR after 6 months of therapy, and there were 30 patients with SD. The median PFS was 6.9 months (95% CI, 4.6–10.1), and the 12-month OS was 46% (95% CI, 31–60). The retrospective study suggested cabozantinib could provide a clinically meaningful benefit for patients with pRCC even with prior VEGF TKI progression and warranted evaluation with a clinical trial.
PAPMET (NCT02761057) was a multi-arm phase 2 trial exclusively for patients with pRCC who had received up to one prior therapy (excluding VEGF and MET inhibitors) and were randomized to one of four arms: sunitinib, cabozantinib, crizotinib, or savolitinib [68]. One hundred and forty-seven eligible patients from 65 centers in the US and Canada were enrolled from 2016 through 2019. The savolitinib (n = 29) and crizotinib (n = 28) arms were halted due to prespecified futility analysis, while the sunitinib (n = 46) and cabozantinib (n = 44) arms completed accrual. The study showed superiority for cabozantinib over sunitinib in terms of the median PFS (9.0 vs. 5.6 months; HR 0.60; 95% CI, 0.37–0.97; p = 0.019), and ORR (23% vs. 4%, p = 0.010). Five percent (2/44) in the cabozantinib group achieved a CR, compared to none for the other three arms. The median OS for cabozantinib was 20.0 months compared to 16.4 months for sunitinib (HR 0.84; 95% CI, 0.47–1.51). PAPMET was the first prospective trial to show superiority of another VEGF TKI oversunitinib.
Immune checkpoint inhibitor era
CheckMate 025 (NCT01668784) was the landmark phase 3 trial that showed superior OS with nivolumab compared to everolimus in patients with ccRCC in the second-line setting after prior anti-angiogenic therapy [69]. Frontline ipilimumab with nivolumab showed significant OS benefit compared to sunitinib for intermediate- and poor-risk ccRCC in CheckMate 214 (NCT02231749) [70]. Both trials excluded all patients with nccRCC histologies.
One of the earliest multi-institutional retrospective studies was for 41 patients with nccRCC who received at least one dose of nivolumab monotherapy (with 92% of patients having received at least one prior line of therapy) between 2015 and 2017 [71]. This included 16 patients with pRCC, and there were 14% (2/14) PR, 21% (3/14) SD, 64% (9/14) PD, and 2 not evaluable. For the entire cohort of nccRCC, the ORR was 20%, with no CR. Another multi-institutional retrospective study of 43 patients with nccRCC including 14 patients with pRCC who received immune checkpoint inhibitor (ICI) monotherapy or in combination with either another ICI or VEGF TKI prior to July 2016 [72]. The ORR for the pRCC cohort was 29% (4/14), including 7% (1/14) CR, 21% (3/14) PR, 29% (4/14) SD, and 43% (6/14) PD.
Another multi-institutional retrospective study in pRCC evaluated ICI efficacy (95% of cases using nivolumab monotherapy, as well as atezolizumab and avelumab monotherapy) in various treatment lines in 57 patients across 15 centers in Europe from February 2016 through January 2019 [73]. There were 2 patients with type 1 not evaluable due to death from PD before evaluation. For 14 patients with type 1, there were 0% (0/14) CR, 14% (2/14) PR, 43% (6/14) SD, and 43% (6/14) PD. For 34 patients with type 2, there were 6% (2/34) CR, 3% (1/34) PR, 24% (8/34) SD, and 68% (23/34) PD. For 7 unclassified, there were 0% (0/7) CR, 14% (1/7) PR, 57% (4/7) SD, and 29% (2/7) PD. The overall ORR for 55 evaluable patients was 11% (6/55), and the median OS was 14.6 months (95% CI, 9.0-NR).
These retrospective studies, although limited by heterogeneity in terms of the number of prior treatment lines, showed that the efficacy of ICI monotherapy for pretreated pRCC was modest. Predictive biomarkers are needed to select the patients who will be durable responders to ICI monotherapy.
Dedicated prospective trials exclusively focusing on pRCC have been lacking due to the rare nature of this subset and challenges with accrual. The first clinical trial to evaluate frontline ICI therapy in patients with nccRCC was the KEYNOTE-427 (NCT02853344) cohort B [74]. There were 165 patients with nccRCC enrolled who received frontline pembrolizumab monotherapy, including 118 patients with pRCC. For these patients with pRCC, there were 5.9% (7/118) CR, 22.9% (27/118) PR, 33.1% (39/118) SD, and 32.2% (38/118) PD, and 5.9% (7/118) not assessed/not evaluable, for an ORR of 28.8% and a DCR of 47.5%. The median PFS was 5.5 months (95% CI, 3.9–6.9), and the median OS was 31.5 months (95% CI, 25.5-NR). The results from cohort B showed promising efficacy in pembrolizumab for pRCC.
CheckMate 374 (NCT02596035) was a phase 3/4 trial to evaluate the safety of nivolumab monotherapy in different patient cohorts including nccRCC, with prior systemic therapies allowed [75]. Between 2015 to 2016, 44 patients with nccRCC were enrolled, including 24 patients with pRCC. Two patients with pRCC had PR (ORR of 8.3%).
HCRN GU16-260-Cohort B (NCT03117309) is a phase 2 trial evaluating nivolumab for treatment-naive nccRCC (Part A), and those who progressed were allowed to enter Part B of salvage ipilimumab and nivolumab followed by nivolumab maintenance [76]. There were 35 patients enrolled between 2017 and 2019 in the initial analysis, including 19 patients with pRCC. For patients with pRCC, the ORR for patients in part A was only 5% (1/19), although this patient had a CR. Notably, this patient also had sarcomatoid histology.
These clinical trials involving ICI monotherapy suggested it was plausible to provide patients with pRCC this option, but the response could be quite variable and difficult to predict.
Combination of immune checkpoint inhibitors and tyrosine kinase inhibitors
The current era of the combination of ICI and TKI was ushered in with the success of pembrolizumab and axitinib as frontline therapy over sunitinib for ccRCC in terms of median PFS and median OS based on the phase 3 trial KEYNOTE-426 (NCT02853331) [77]. These were followed in quick succession by other positive phase 3 trials of combination ICI and TKI over sunitinib monotherapy for ccRCC, including avelumab and axitinib based on JAVELIN Renal 101 (NCT02684006) [78], nivolumab and cabozantinib based on CheckMate 9ER (NCT03141177) [79], and pembrolizumab and lenvatinib based on CLEAR (KEYNOTE-581, NCT02811861) [80]. The success of ICI and TKI for ccRCC inspired the evaluation in patients with nccRCC as well.
COSMIC-021 (NCT03170960) is a multicenter phase 1b trial to assess the combination of cabozantinib and atezolizumab in multiple metastatic solid tumors, including ccRCC and nccRCC. Results from the cohort of mRCC have been reported, with a total of 32 patients with nccRCC (15 patients with pRCC) enrolled between 2017 and 2019 [81]. One prior VEGF TKI therapy was allowed, and no prior MET-targeting TKIs or ICIs were allowed. In an updated analysis at the 2023 ASCO Genitourinary Cancers Symposium, for pRCC, the median PFS was 8.1 months (95% CI, 2.7–18.4) and the median OS was 31.8 months (95% CI, 6.1-NR) [82].
The CA209-9KU trial (NCT03635892) evaluated the efficacy of cabozantinib and nivolumab in patients with nccRCC with up to one prior systemic therapy excluding ICIs [83]. In an analysis of 47 patients with nccRCC enrolled between 2018 and 2020, there were 32 patients with pRCC. The ORR for pRCC was 47% (15/32). For the cohort of 40 patients with papillary, unclassified, or translocation-associated RCC, the ORR was 47.5%, the median PFS was 12.5 months (95% CI, 6.3–16.4), and the median OS was 28 months (95% CI, 16.3-NR). In an updated analysis presented at the 2023 ASCO Annual Meeting, the longer follow-up continued to show efficacy for this combination, with 3% (1/32) CR, 44% (14/32) PR, 53% (17/32) SD, and no PD in the pRCC cohort [84].
CALYPSO (NCT02819596) is a multi-arm phase 2 trial evaluating savolitinib, durvalumab, the combination of savolitinib and durvalumab, and the combination of durvalumab and tremelimumab for patients with either treatment-naïve or previously treated ccRCC or pRCC. Results for the savolitinib and durvalumab combination arm of 41 patients with pRCC were reported, including 17 with MET-driven tumors [85]. The ORR was 29% (12/41), which did not meet the primary endpoint (minimum 50%), halting further treatment. For the 41 patients with pRCC, the median PFS was 4.9 months (95% CI, 2.5–10), and the median OS was 14.1 months (95% CI, 7.3–30.7). Notably, for the MET-driven pRCC, the median PFS was 12.0 months (95% CI, 2.9–19.4), and the median OS was 27.4 months (95% CI, 9.3-NR). Despite the primary endpoint not being met, the promising results seen in the MET-driven patients has inspired the open-label, randomized, three-arm phase 3 trial SAMETA (NCT05043090) evaluating the combination of savolitinib with durvalumab versus sunitinib versus durvalumab as frontline therapy [86]. This trial is expected to enroll 220 patients with MET-driven pRCC, with PFS as the primary outcome.
KEYNOTE-B61 (NCT04704219) is a single-arm international phase 2 trial to evaluate the combination of lenvatinib with pembrolizumab as frontline therapy for nccRCC, and 158 patients with nccRCC were enrolled from 2021 to 2022 [87]. For the 93 patients with pRCC, the ORR was 54% (50/93) with 8.9% (8/93) CR, 45% (42/93) PR, 31% (29/93) SD, 9.7% (9/93) PD, and 5 not assessable. The median PFS for patients with pRCC was 17.5 months (95% CI, 15-NR). Overall, the study showed this combination is an effective option.
A single-arm phase 2 trial (NCT04413123) is enrolling patients with nccRCC (up to one prior line of VEGF TKI therapy eligible and excluding ICI or cabozantinib) to receive the triplet combination of ipilimumab, nivolumab, and cabozantinib [88]. The primary endpoint is ORR. Preliminary results for the pRCC cohort (n = 20) presented showed 25% (5/20) PR, 55% (11/20) SD, and 20% (4/20) PD, for an ORR of 25% (5/20).
These trials, although small in design, suggest that the combination of an oral targeted therapy with immunotherapy is the most promising strategy to treat patients with metastatic pRCC, with improvement over either agent alone. This concept has spurred the design of larger active and recruiting trials dedicated to pRCC evaluating a TKI with ICI.
Additional active and recruiting clinical trials of interest
There are several other clinical trials involving patients with pRCC that are underway, including STELLAR-304 (NCT05678673), SUNNIFORECAST (NCT03075423), PAPMET2 (NCT05411081), LENKYN (NCT04267120), PAXIPEM (NCT05096390), and ICONIC (NCT03866382). Additional registered clinical trials are enrolling patients with nccRCC including pRCC involving novel agents as the goal to improve outcomes for these patients continues. These include the use of palbociclib (CDK4/CDK6 inhibitor), sasanlimab (PD-1 inhibitor), orellanine (mycotoxin), and CBM588 (Clostridium butyricum probiotic). These trials are summarized in Table 2.
Table 2
Notable active and recruiting trials with papillary renal cell carcinoma awaiting results
| Trial | Phase | Enrollment criteria | Intervention | Primary outcome measures | Estimated primary completion date |
| NCT05043090 (SAMETA) | 3 | No prior systemic therapy | Savolitinib with durvalumab vs. durvalumab vs. sunitinib | PFS | May 2025 |
| NCT05678673 (STELLAR-304) | 3 | No prior systemic therapy | Zanzalintinib with nivolumab vs. sunitinib | PFS, ORR | July 2025 |
| NCT03075423 (SUNNIFORECAST) | 2 | No prior systemic therapy | Ipilimumab with nivolumab vs. standard of care | OS at 12 months | November 2023 |
| NCT05411081 (PAPMET2) | 2 | No prior cabozantinib, and no prior ICIs within 6 months | Cabozantinib with atezolizumab vs. cabozantinib | PFS | July 2027 |
| NCT04267120 (LENKYN) | 2 | No prior systemic therapy | Lenvatinib with pembrolizumab | ORR | July 2024 |
| NCT05096390 (PAXIPEM) | 2 | No prior systemic therapy | Axitinib with pembrolizumab vs. axitinib | ORR at 6 months | October 2024 |
| NCT03866382 (ICONIC) | 2 | Prior systemic therapy allowed | Ipilimumab, nivolumab, and cabozantinib | ORR | February 2025 |
| NCT04071223 (RadiCal) | 2 | Prior systemic therapy allowed | Radium-223 with cabozantinib vs. cabozantinib | Symptomatic skeletal-event free survival | October 2024 |
| NCT05665361 | 1/2 | Prior systemic therapy allowed | Palbociclib with sasanlimab | R2PD, DLT, ORR | June 2025 |
| NCT05287945 | 1/2 | Prior systemic therapy allowed | Orellanine | DLT, MTD, RP2D | February 2025 |
| NCT05122546 | 1 | No prior systemic therapy | CBM588 with cabozantinib and nivolumab vs. cabozantinib and nivolumab | Change in Bifidobacterium composition of stool | October 2024 |
PFS = progression-free survival, ORR = objective response rate, OS = overall survival, R2PD = recommended phase 2 dose, DLT = dose-limiting toxicities, MTD = maximum tolerated dose.
PREDICTIVE AND PROGNOSTIC BIOMARKERS
Unlike the International Metastatic RCC Database Consortium (IMDC) model for metastatic ccRCC, there are currently no prognostic models specifically for metastatic pRCC due to its lower prevalence. In addition, like ccRCC, no molecular biomarkers have been clinically validated to predict prognosis or response to therapies in pRCC.
The role of MET molecular status as a predictive biomarker for MET-targeted therapy in pRCC is being investigated. The phase 2 trial of savolitinib showed patients with MET-driven pRCC had a much better ORR and median PFS than non-MET-driven pRCC (p = 0.002 and p < 0.001, respectively) [55]. The phase 3 trial SAVOIR with the selective MET inhibitor savolitinib versus sunitinib showed improved overall response rates but no PFS or OS benefit with savolitinib, irrespective of MET status [56]. However, this study was not designed to evaluate MET as a predictive biomarker and was forced to terminate early. A recent retrospective analysis found that the presence of MET genetic alterations did not predict differential outcomes with current standard therapies compared to MET-wild type tumors [89]. Ongoing studies such as SAMETA are evaluating savolitinib specifically in MET-driven pRCC to validate MET’s utility as a predictive biomarker for response to MET-targeted agents.
CONCLUSIONS
It is evident that patients with metastatic pRCC have worse outcomes than patients with metastatic ccRCC. In the days of the cytokine era, patients with pRCC overall did not respond to these treatments. Targeted therapy against the mTOR pathway had slightly improved success, but ORR generally remained in the single digits. Targeting various receptor tyrosine kinase receptor pathways such as VEGF and MET finally yielded better outcomes. ICIs combined with VEGF and MET TKIs so far are the most promising with ORR up to 30–50%, which is a major improvement over use of either VEGF TKI or ICI as a single agent. However, effective therapies for pRCC still lag far behind those for ccRCC. Fortunately, more clinical trials dedicated to enrolling patients with pRCC are underway. Still, there is much to learn about pRCC in order to design more effective agents. As genetic and molecular features become better characterized, prognostic and predictive markers will hopefully someday help guide management and determine future therapy options.
ACKNOWLEDGMENTS
The authors have no acknowledgements.
FUNDING
The authors report no funding.
AUTHOR CONTRIBUTIONS
AJ, CSH, SG: performance, interpretation of data, writing, and conception.
CONFLICT OF INTEREST
SG is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review. SG has consultancy for Bristol Myers Squibb, Merck, Bayer, Pfizer, EMD Sorono, Seattle Genetics, Gilead Sciences, Foundation Medicine, Guardant, and Astellas; Speaker bureau for Seattle Genetics, Bristol Myers Squibb and Gilead Sciences. Research grant/funding (institution) from Bristol Myers Squibb, Roche, Merck, Seattle Genetics, QED, Novartis, Moderna, EMD Sorono, Acrivion and Pfizer; and is a shareholder of Moderna, Nektar Therapeutics and BioNTech.
REFERENCES
[1] | Bentz M , Bergerheim US , Li C , Joos S , Werner CA , Baudis M , et al. Chromosome imbalances in papillary renal cell carcinoma and first cytogenetic data of familial cases analyzed by comparative genomic hybridization. Cytogenetics and Cell Genetics. (1996) ;75: (1):17–21. |
[2] | Ricketts CJ , De Cubas AA , Fan H , Smith CC , Lang M , Reznik E , et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Reports. (2018) ;23: (1):313–26.e5. |
[3] | Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. (2013) ;499: (7456):43–9. |
[4] | Linehan WM , Spellman PT , Ricketts CJ , Creighton CJ , Fei SS , Davis C , et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. (2016) ;374: (2):135–45. |
[5] | Zhang Y , Narayanan SP , Mannan R , Raskind G , Wang X , Vats P , et al. Single-cell analyses of renal cell cancers reveal insights into tumor microenvironment, cell of origin, and therapy response. Proc Natl Acad Sci U S A. (2021) ;118: (24). |
[6] | Wang Q , Zhang Y , Zhang B , Fu Y , Zhao X , Zhang J , et al. Single-cell chromatin accessibility landscape in kidney identifies additional cell-of-origin in heterogenous papillary renal cell carcinoma. Nature Communications. (2022) ;13: (1):31. |
[7] | Lipworth L , Morgans AK , Edwards TL , Barocas DA , Chang SS , Herrell SD , et al. Renal cell cancer histological subtype distribution differs by race and sex. BJU Int. (2016) ;117: (2):260–5. |
[8] | Sankin A , Cohen J , Wang H , Macchia RJ , Karanikolas N . Rate of renal cell carcinoma subtypes in different races. International Braz J Urol: Official Journal of the Brazilian Society of Urology. (2011) ;37: (1):29–32; discussion 3-4. |
[9] | Purdue MP , Moore LE , Merino MJ , Boffetta P , Colt JS , Schwartz KL , et al. An investigation of risk factors for renal cell carcinoma by histologic subtype in two case-control studies. Int J Cancer. (2013) ;132: (11):2640–7. |
[10] | Qi P , Tsivian M , Abern MR , Passoni NM , McGinley KF , Polascik TJ . Clinicopathological characteristics and outcomes of surgically excised renal masses in African Americans. Urol Oncol. (2014) ;32: (5):555–60. |
[11] | Batai K , Harb-De la Rosa A , Zeng J , Chipollini JJ , Gachupin FC , Lee BR . Racial/ethnic disparities in renal cell carcinoma: Increased risk of early-onset and variation in histologic subtypes. Cancer Med. (2019) ;8: (15):6780–8. |
[12] | Dudani S , de Velasco G , Wells JC , Gan CL , Donskov F , Porta C , et al. Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association With Survival. JAMA Network Open. (2021) ;4: (1):e2021869. |
[13] | Wagener N , Edelmann D , Benner A , Zigeuner R , Borgmann H , Wolff I , et al. Outcome of papillary versus clear cell renal cell carcinoma varies significantly in non-metastatic disease. PLoS One. (2017) ;12: (9):e0184173. |
[14] | Mancilla-Jimenez R , Stanley RJ , Blath RA . Papillary renal cell carcinoma: a clinical, radiologic, and pathologic study of 34 cases. Cancer. (1976) ;38: (6):2469–80. |
[15] | Delahunt B , Eble JN . Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. (1997) ;10: (6):537–44. |
[16] | Haake SM , Weyandt JD , Rathmell WK . Insights into the Genetic Basis of the Renal Cell Carcinomas from The Cancer Genome Atlas. Molecular cancer research: MCR. (2016) ;14: (7):589–98. |
[17] | Hes O , Michalová K , Pivovarčíková K . New insightsin the new WHO classification of adult renal tumors. CeskoslovenskaPatologie. (2022) ;67: (4):187–91. |
[18] | Motzer RJ , Bacik J , Mariani T , Russo P , Mazumdar M , Reuter V . Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. (2002) ;20: (9):2376–81. |
[19] | Herrmann E , Brinkmann OA , Bode ME , Bierer S , Köpke T , Bögemann M , et al. Histologic subtype of metastatic renal cellcarcinoma predicts response to combined immunochemotherapy withinterleukin 2, interferon alpha and 5-fluorouracil. Eur Urol. (2007) ;51: (6):1625–31; discussion 31-2. |
[20] | Negrier S , Perol D , Ravaud A , Chevreau C , Bay JO , Delva R , et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer. (2007) ;110: (11):2468–77. |
[21] | Saxton RA , Sabatini DM . mTOR Signaling in Growth, Metabolism, and Disease. Cell. (2017) ;168: (6):960–76. |
[22] | Battelli C , Cho DC . mTOR inhibitors in renal cell carcinoma. Therapy. (2011) ;8: (4):359–67. |
[23] | Thomas GV , Tran C , Mellinghoff IK , Welsbie DS , Chan E , Fueger B , et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. (2006) ;12: (1):122–7. |
[24] | Strikic A , Kokeza J , Ogorevc M , Kelam N , Vukoja M , Dolonga P , et al. Differential expression of HIF1A and its downstream target VEGFA in the main subtypes of renal cell carcinoma and their impact on patient survival. Front Oncol. (2023) ;13: :1287239. |
[25] | Hudes G , Carducci M , Tomczak P , Dutcher J , Figlin R , Kapoor A , et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. (2007) ;356: (22):2271–81. |
[26] | Dutcher JP , de Souza P , McDermott D , Figlin RA , Berkenblit A , Thiele A , et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. (2009) ;26: (2):202–9. |
[27] | Koh Y , Lim HY , Ahn JH , Lee JL , Rha SY , Kim YJ , et al. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma. Ann Oncol. (2013) ;24: (4):1026–31. |
[28] | Escudier B , Molinie V , Bracarda S , Maroto P , Szczylik C , Nathan P , et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer. (2016) ;69: :226–35. |
[29] | Voss MH , Molina AM , Chen YB , Woo KM , Chaim JL , Coskey DT , et al. Phase II Trial and Correlative Genomic Analysis of Everolimus Plus Bevacizumab in Advanced Non-Clear Cell Renal Cell Carcinoma. J Clin Oncol. (2016) ;34: (32):3846–53. |
[30] | Feldman DR , Ged Y , Lee CH , Knezevic A , Molina AM , Chen YB , et al. Everolimus plus bevacizumab is an effective first-line treatment for patients with advanced papillary variant renal cell carcinoma: Final results from a phase II trial. Cancer. (2020) ;126: (24):5247–55. |
[31] | Hutson TE , Michaelson MD , Kuzel TM , Agarwal N , Molina AM , Hsieh JJ , et al. A Single-arm, Multicenter, Phase 2 Study of Lenvatinib Plus Everolimus in Patients with Advanced Non-Clear Cell Renal Cell Carcinoma. Eur Urol. (2021) ;80: (2):162–70. |
[32] | Choueiri TK , Plantade A , Elson P , Negrier S , Ravaud A , Oudard S , et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. (2008) ;26: (1):127–31. |
[33] | Stadler WM , Figlin RA , McDermott DF , Dutcher JP , Knox JJ , Miller WH Jr , et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. (2010) ;116: (5):1272–80. |
[34] | Molina AM , Feldman DR , Ginsberg MS , Kroog G , Tickoo SK , Jia X , et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. (2012) ;30: (1):335–40. |
[35] | Tannir NM , Plimack E , Ng C , Tamboli P , Bekele NB , Xiao L , et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. (2012) ;62: (6):1013–9. |
[36] | Lee JL , Ahn JH , Lim HY , Park SH , Lee SH , Kim TM , et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol. (2012) ;23: (8):2108–14. |
[37] | Ravaud A , Oudard S , De Fromont M , Chevreau C , Gravis G , Zanetta S , et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: a phase II study (SUPAP) by the French Genitourinary Group (GETUG)†. Ann Oncol. (2015) ;26: (6):1123–8. |
[38] | Tannir NM , Jonasch E , Albiges L , Altinmakas E , Ng CS , Matin SF , et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur Urol. (2016) ;69: (5):866–74. |
[39] | Armstrong AJ , Halabi S , Eisen T , Broderick S , Stadler WM , Jones RJ , et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. (2016) ;17: (3):378–88. |
[40] | Miyamoto S , Kakutani S , Sato Y , Hanashi A , Kinoshita Y , Ishikawa A . Drug review: Pazopanib. Japanese Journal of Clinical Oncology. (2018) ;48: (6):503–13. |
[41] | Buti S , Bersanelli M , Maines F , Facchini G , Gelsomino F , Zustovich F , et al. First-Line PAzopanib in NOn-clear-cell Renal cArcinoMA: The Italian Retrospective Multicenter PANORAMA Study. Clin Genitourin Cancer. (2017) ;15: (4):e609–e14. |
[42] | Jung KS , Lee SJ , Park SH , Lee JL , Lee SH , Lim JY , et al. Pazopanib for the Treatment of Non-clear Cell Renal Cell Carcinoma: A Single-Arm, Open-Label, Multicenter, Phase II Study. Cancer Research and Treatment. (2018) ;50: (2):488–94. |
[43] | Bukowski RM . Third generation tyrosine kinase inhibitors and their development in advanced renal cell carcinoma. Front Oncol. (2012) ;2: :13. |
[44] | Rini BI , Escudier B , Tomczak P , Kaprin A , Szczylik C , Hutson TE , et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. (2011) ;378: (9807):1931–9. |
[45] | Park I , Lee SH , Lee JL . A Multicenter Phase II Trial of Axitinib in Patients With Recurrent or Metastatic Non-clear-cell Renal Cell Carcinoma Who Had Failed Prior Treatment With Temsirolimus. Clin Genitourin Cancer. (2018) ;16: (5):e997–e1002. |
[46] | Negrier S , Rioux-Leclercq N , Ferlay C , Gross-Goupil M , Gravis G , Geoffrois L , et al. Axitinib in first-line for patients with metastatic papillary renal cell carcinoma: Results of the multicentre, open-label, single-arm, phase II AXIPAP trial. Eur J Cancer. (2020) ;129: :107–16. |
[47] | Perera AD , Kleymenova EV , Walker CL . Requirement for the von Hippel-Lindau tumor suppressor gene for functional epidermal growth factor receptor blockade by monoclonal antibody C225 in renal cell carcinoma. Clin Cancer Res. (2000) ;6: (4):1518–23. |
[48] | Gordon MS , Hussey M , Nagle RB , Lara PN Jr , Mack PC, Dutcher J, et al. Phase II study of erlotinib in patients with locally advanced or metastatic papillary histology renal cell cancer: SWOG S0317. J Clin Oncol. (2009) ;27: (34):5788–93. |
[49] | Srinivasan R , Gurram S , Al Harthy M , Singer EA , Sidana A , Shuch BM , et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. Journal of Clinical Oncology. (2020) ;38: (15_suppl):5004. |
[50] | Kataoka Y , Mukohara T , Tomioka H , Funakoshi Y , Kiyota N , Fujiwara Y , et al. Foretinib (GSK1363089), a multi-kinase inhibitor of MET and VEGFRs, inhibits growth of gastric cancer cell lines by blocking inter-receptor tyrosine kinase networks. Invest New Drugs. (2012) ;30: (4):1352–60. |
[51] | Choueiri TK , Vaishampayan U , Rosenberg JE , Logan TF , Harzstark AL , Bukowski RM , et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol. (2013) ;31: (2):181–6. |
[52] | Sahu A , Prabhash K , Noronha V , Joshi A , Desai S . Crizotinib: A comprehensive review. South Asian Journal of Cancer. (2013) ;2: (2):91–7. |
[53] | Schöffski P , Wozniak A , Escudier B , Rutkowski P , Anthoney A , Bauer S , et al. Crizotinib achieves long-lasting disease control inadvanced papillary renal-cell carcinoma type 1 patients with METmutations or amplification. EORTC 90101 CREATE trial. Eur J Cancer. (2017) ;87: :147–63. |
[54] | Jia H , Dai G , Weng J , Zhang Z , Wang Q , Zhou F , et al. Discovery of (S)-1-(1-(Imidazo[1,2-a]pyridin-6-yl)ethyl)-6-(1-methyl-1H-pyrazol-4-yl)-1H-[1,2,3]triazolo[4,5-b]pyrazine (volitinib) as a highly potent and selective mesenchymal-epithelial transition factor (c-Met) inhibitor in clinical development for treatment of cancer. J Med Chem. (2014) ;57: (18):7577–89. |
[55] | Choueiri TK , Plimack E , Arkenau HT , Jonasch E , Heng DYC , Powles T , et al. Biomarker-Based Phase II Trial of Savolitinib in Patients With Advanced Papillary Renal Cell Cancer. J Clin Oncol. (2017) ;35: (26):2993–3001. |
[56] | Choueiri TK , Heng DYC , Lee JL , Cancel M , Verheijen RB , Mellemgaard A , et al. Efficacy of Savolitinib vs Sunitinib in Patients With MET-Driven Papillary Renal Cell Carcinoma: The SAVOIR Phase 3 Randomized Clinical Trial. JAMA Oncol. (2020) ;6: (8):1247–55. |
[57] | Munshi N , Jeay S , Li Y , Chen CR , France DS , Ashwell MA , et al. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther. (2010) ;9: (6):1544–53. |
[58] | Twardowski PW , Tangen CM , Wu X , Plets MR , Plimack ER , Agarwal N , et al. Parallel (Randomized) Phase II Evaluation of Tivantinib (ARQ197) and Tivantinib in Combination with Erlotinib in Papillary Renal Cell Carcinoma: SWOG S1107. Kidney Cancer. (2017) ;1: (2):123–32. |
[59] | Liu X , Wang Q , Yang G , Marando C , Koblish HK , Hall LM , et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res. (2011) ;17: (22):7127–38. |
[60] | Leger PD , Girardi DdM , Al Harthy M , Friend JC , Mac L , Purcell E , et al. A phase II study of the selective MET kinase inhibitor INC280 in advanced papillary renal cell cancer. Journal of Clinical Oncology. (2020) ;38: (15_suppl):5075. |
[61] | Yakes FM , Chen J , Tan J , Yamaguchi K , Shi Y , Yu P , et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. (2011) ;10: (12):2298–308. |
[62] | Choueiri TK , Escudier B , Powles T , Mainwaring PN , Rini BI , Donskov F , et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. (2015) ;373: (19):1814–23. |
[63] | Choueiri TK , Escudier B , Powles T , Tannir NM , Mainwaring PN , Rini BI , et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. (2016) ;17: (7):917–27. |
[64] | Choueiri TK , Halabi S , Sanford BL , Hahn O , Michaelson MD , Walsh MK , et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol. (2017) ;35: (6):591–7. |
[65] | Choueiri TK , Hessel C , Halabi S , Sanford B , Michaelson MD , Hahn O , et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur J Cancer. (2018) ;94: :115–25. |
[66] | Campbell MT , Bilen MA , Shah AY , Lemke E , Jonasch E , Venkatesan AM , et al. Cabozantinib for the treatment of patients with metastatic non-clear cell renal cell carcinoma: A retrospective analysis. Eur J Cancer. (2018) ;104: :188–94. |
[67] | Martínez Chanzá N , Xie W , Asim Bilen M , Dzimitrowicz H , Burkart J , Geynisman DM , et al. Cabozantinib in advancednon-clear-cell renal cell carcinoma: a multicentre, retrospective, cohort study. Lancet Oncol. (2019) ;20: (4):581–90. |
[68] | Pal SK , Tangen C , Thompson IM Jr , Balzer-Haas N, George DJ, Heng DYC, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. (2021) ;397: (10275):695–703. |
[69] | Motzer RJ , Escudier B , McDermott DF , George S , Hammers HJ , Srinivas S , et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. (2015) ;373: (19):1803–13. |
[70] | Motzer RJ , Tannir NM , McDermott DF , Arén Frontera O , Melichar B , Choueiri TK , et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. (2018) ;378: (14):1277–90. |
[71] | Koshkin VS , Barata PC , Zhang T , George DJ , Atkins MB , Kelly WJ , et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer. (2018) ;6: (1):9. |
[72] | McKay RR , Bossé D , Xie W , Wankowicz SAM , Flaifel A , Brandao R , et al. The Clinical Activity of PD-1/PD-L1 Inhibitors in Metastatic Non-Clear Cell Renal Cell Carcinoma. Cancer Immunol Res. (2018) ;6: (7):758–65. |
[73] | de Vries-Brilland M , Gross-Goupil M , Seegers V , Boughalem E , Beuselinck B , Thibault C , et al. Are immune checkpoint inhibitors a valid option for papillary renal cell carcinoma? A multicentre retrospective study. Eur J Cancer. (2020) ;136: :76–83. |
[74] | McDermott DF , Lee JL , Ziobro M , Suarez C , Langiewicz P , Matveev VB , et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma. J Clin Oncol. (2021) ;39: (9):1029–39. |
[75] | Vogelzang NJ , Olsen MR , McFarlane JJ , Arrowsmith E , Bauer TM , Jain RK , et al. Safety and Efficacy of Nivolumab in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma: Results From the Phase IIIb/IV CheckMate 374 Study. Clin Genitourin Cancer. (2020) ;18: (6):461–8.e3. |
[76] | Atkins MB , Jegede OA , Haas NB , McDermott DF , Bilen MA , Stein M , etal. Phase II study of nivolumab and salvage nivolumab/ipilimumab intreatment-naïve patients with advanced non-clear cell renalcell carcinoma (HCRN GU16-260-Cohort B). J Immunother Cancer. (2023) ;11: (3). |
[77] | Rini BI , Plimack ER , Stus V , Gafanov R , Hawkins R , Nosov D , et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) ;380: (12):1116–27. |
[78] | Motzer RJ , Penkov K , Haanen J , Rini B , Albiges L , Campbell MT , et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) ;380: (12):1103–15. |
[79] | Choueiri TK , Powles T , Burotto M , Escudier B , Bourlon MT , Zurawski B , et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2021) ;384: (9):829–41. |
[80] | Motzer R , Alekseev B , Rha SY , Porta C , Eto M , Powles T , et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. (2021) ;384: (14):1289–300. |
[81] | Pal SK , McGregor B , Suárez C , Tsao CK , Kelly W , Vaishampayan U , et al. Cabozantinib in Combination With Atezolizumab for AdvancedRenal Cell Carcinoma: Results From the COSMIC-021 Study. J Clin Oncol. (2021) ;39: (33):3725–36. |
[82] | McGregor BA , Agarwal N , Suárez C , Tsao K , Kelly WK , Pagliaro LC , et al. Cabozantinib in combination with atezolizumab in non-clearcell renal cell carcinoma: Extended follow-up results of cohort 10of the COSMIC-021 study. Journal of Clinical Oncology. (2023) ;41: (6_suppl):684. |
[83] | Lee CH , Voss MH , Carlo MI , Chen YB , Zucker M , Knezevic A , et al. Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non-Clear-Cell Renal Cell Carcinoma and Genomic Correlates. J Clin Oncol. (2022) ;40: (21):2333–41. |
[84] | Lee C-H , Fitzgerald KN , Voss MH , Carlo MI , Knezevic A , Peralta L , et al. Nivolumab plus cabozantinib in patients with non-clear cell renal cell carcinoma: Updated results from a phase 2 trial. Journal of Clinical Oncology. (2023) ;41: (16_suppl):4537. |
[85] | Suárez C , Larkin JMG , Patel P , Valderrama BP , Rodriguez-Vida A , Glen H , et al. Phase II Study Investigating the Safety and Efficacyof Savolitinib and Durvalumab in Metastatic Papillary Renal Cancer(CALYPSO). J Clin Oncol. (2023) ;41: (14):2493–502. |
[86] | Choueiri TK , Xu W , Poole L , Telaranta-Keerie A , Hartmaier R , Powles T . SAMETA: An open-label, three-arm, multicenter, phase III study of savolitinib + durvalumab versus sunitinib and durvalumab monotherapy in patients with MET-driven, unresectable, locally advanced/metastatic papillary renal cell carcinoma (PRCC). Journal of Clinical Oncology. (2022) ;40: (16_suppl):TPS4601-TPS. |
[87] | Albiges L , Gurney H , Atduev V , Suarez C , Climent MA , Pook D , et al. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial. Lancet Oncol. (2023) ;24: (8):881–91. |
[88] | McGregor BA , Huang J , Xie W , Xu W , Bilen MA , Braun DA , et al. Phase II study of cabozantinib (Cabo) with nivolumab (Nivo) and ipilimumab (Ipi) in advanced renal cell carcinoma with variant histologies (RCCvh). Journal of Clinical Oncology. (2023) ;41: (16_suppl):4520. |
[89] | Albiges L , Heng DYC , Lee JL , Walker S , Mellemgaard A , Ottesen L , et al. Impact of MET status on treatment outcomes in papillary renal cell carcinoma: A pooled analysis of historical data. Eur J Cancer. (2022) ;170: :158–68. |



