Evaluation of the efficacy and safety of robot-assisted and video assisted thoracic surgery for early non-small cell lung cancer: A meta-analysis
Abstract
BACKGROUND:
Radical resection of lung cancer and chemotherapy are the main methods for the treatment of early lung cancer, but surgical treatment is still the key and preferred method.
OBJECTIVE:
To evaluate the efficacy and safety of robotic-assisted thoracic surgery (RATS) and video assisted thoracic surgery (VATS) for non-small cell lung cancer (NSCLC).
METHODS:
The clinical cohort studies on the comparison of the effects of RATS and VATS in the treatment of NSCLC published in Web of Science, PubMed, The National Library of Medicine (NLM), China National Knowledge Infrastructure (CNKI) and Wanfang database from January 1, 2015 to December 31, 2022 were searched. Two researchers independently screened the literature, extracted the data, such as operation time, intraoperative conversion rate, intraoperative blood loss, number of lymph nodes dissected, and evaluated the quality of the included literature based on the Newcastle-Ottawa Scale (NOS). RevMan 5.3 software was used for Meat analysis.
RESULTS:
A total of 18 articles and 21,802 subjects were included. The results of the meta-analysis showed that the intraoperative blood loss of RATS was significantly less than that of VAS, and the difference was statistically significant [MD
CONCLUSION:
Compared with VATS, the number of lymph nodes dissected in RATS was significantly more, and the removal of lesions and lymph nodes was more thorough and accurate. More flexible and precise operation avoids the injury of important blood vessels during operation, effectively reduces the amount of blood loss during operation, shortens the indwelling time of thoracic drainage tube, and is conducive to postoperative rehabilitation of patients.
1.Introduction
According to the global cancer statistics report released by the International Agency for Research on Cancer (IARC) in 2020, the incidence and mortality of lung cancer are still high, which seriously endangers human life and health and brings great challenges to the global disease burden [1]. Radical resection of lung cancer and chemotherapy are the main methods for the treatment of early lung cancer, but surgical treatment is still the key and preferred method [2]. Non-small cell lung cancer (NSCLC) accounts for about 80% of all lung cancers, and about 75% of patients are already in the middle and advanced stage when detected, with a very low 5-year survival rate [3]. Taking NSCLC as an example, the most effective method of treatment is surgical resection of the lesion tissue. Traditional thoracotomy has a greater trauma to patients, and larger surgical incisions increase postoperative pain and affect the prognosis of patients [4]. In recent years, with the emergence and development of thoracoscopic technology, video-assisted thoracic surgery (VATS) has been widely used. Relevant studies have reported that VATS has more obvious advantages than traditional thoracotomy in the treatment of lung cancer [5, 6]. VATS has gradually developed into a routine procedure for the treatment of early lung cancer.
However, in thoracoscopic surgery, the surgeon’s surgical experience is very important and depends on the tacit cooperation between the surgeon and the handrail. However, due to the long running-in time, the learning curve is long. In addition, the lack of flexibility of thoracoscopic surgical instruments and unclear intraoperative vision also limit the further development of thoracoscopic technology [7]. In recent years, with the emergence of robotic surgery system, it has brought another significant change in the history of minimally invasive surgery [8]. In 2002, Melfi et al. [9] firstly reported robot-assisted thoracic surgery (RATS). At present, RATS has been proved to have a more stable and clear 3D vision, unique anti-tremor system and the ability of remote surgery, making it safer when completing fine operations [10]. However, some researchers still propose that RATS is not mature enough, mechanical operation is not stable enough and expensive [11].
With the continuous development of minimally invasive surgery, robotic and thoracoscopic surgery have been widely used in clinical practice and become the main treatment for lung cancer surgery. However, the current research evidence has inconsistent results for the clinical efficacy and safety evaluation of the two procedures, for both VATS and RATS, there are advantages and disadvantages and controversial. Therefore, this study retrieved the literature on robot-assisted and thoracoscopic-assisted treatment of early NSCLC published in the past five years, extracted relevant clinical indicators, and combined analysis to comprehensively and objectively evaluate the effectiveness and safety of robot-assisted and thoracoscopic-assisted surgery for early NSCLC. We intend to use meta-analysis to analyze and compare the real clinical effects of VATS and RATS in the treatment of NSCLC, in order to make a more objective evaluation of the efficacy and safety of the two procedures.
2.Materials and methods
2.1Search strategy
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance manual, three English databases, PubMed, Web of Science and The National Library of Medicine (NLM), and two Chinese databases, China National Knowledge Infrastructure (CNKI) and Wanfang, were systematically searched. The search time limit was from January 1, 2015 to December 31, 2022. The search was performed using a combination of subject words and free words. The search terms included Da-Vinci, robot, video-assisted, thoracoscopic, lung cancer, pulmonary neoplasm, and pulmonary cancer. In order to avoid literature omission, the target literature was obtained by reading the relevant systematic review.
2.2Inclusion and exclusion criteria
Inclusion criteria: (1) The subjects were NSCLC patients; (2) Intervention measures: the experimental group was treated with Da Vinci robot-assisted thoracic surgery (RATS), and the control group was treated with video assisted thoracic surgery (VATS). (3) Outcome indicators reflecting the effectiveness and safety of surgery were reported, including operation time, intraoperative conversion rate, intraoperative blood loss, number of lymph nodes dissected; postoperative mortality, postoperative recurrence rate, postoperative complication rate, postoperative chest drainage time, postoperative hospital stay.
Exclusion criteria: (1) The study subjects were not simple NSCLC patients, but lymph nodes, mediastinal tumors, esophageal cancer, small cell lung cancer, etc. (2) Conference articles, case reports, systematic reviews and other research literature.
2.3Study selection and data extraction
Two reviewers independently reviewed the abstracts and full text of each article according to inclusion of exclusion criteria. For disagreements between the two reviewers, a third reviewer was recruited for discussion until consensus was achieved. After literature screening, two reviewers independently respectively extracted the following information: (1) The basic characteristics of the included studies: including the first author, the scope of the study year, the year of publication, the country, and the type of research; (2) Baseline data of the included subjects: sample size and average age of the experimental group and the control group; (3) Outcome indicators: including operation time, intraoperative conversion rate, intraoperative blood loss, number of lymph nodes dissected; postoperative mortality, postoperative recurrence rate, postoperative complication rate, postoperative chest drainage time, postoperative hospital stay.
2.4Assessments of methodological quality
The Newcastle-Ottawa Scale (NOS) [12] was used to evaluate the quality of the included literature. The scale was evaluated from eight aspects: the representativeness of the study population, the comparability between groups, the adequacy of the evaluation of the results, the adequacy of the follow-up time and the integrity of the follow-up. The full score was 9 points, and the total score was 7 points and above for high-quality literature, 5 points and below for low-quality articles.
2.5Statistical analysis
Meta-analysis was performed using Revman 5.3 software. The odds ratio (OR) was used as the effect index for qualitative variables, and the mean deviation (MD) was used as the effect index for quantitative variables. Each effect size was expressed as point estimate and 95% confidence interval (CI). If the original literature only provided the median (interquartile range), the formula was used to convert it into mean and standard deviation and included in the analysis [13]. Heterogeneity test was used to determine the size of heterogeneity by the test of
3.Results
3.1Study characteristics
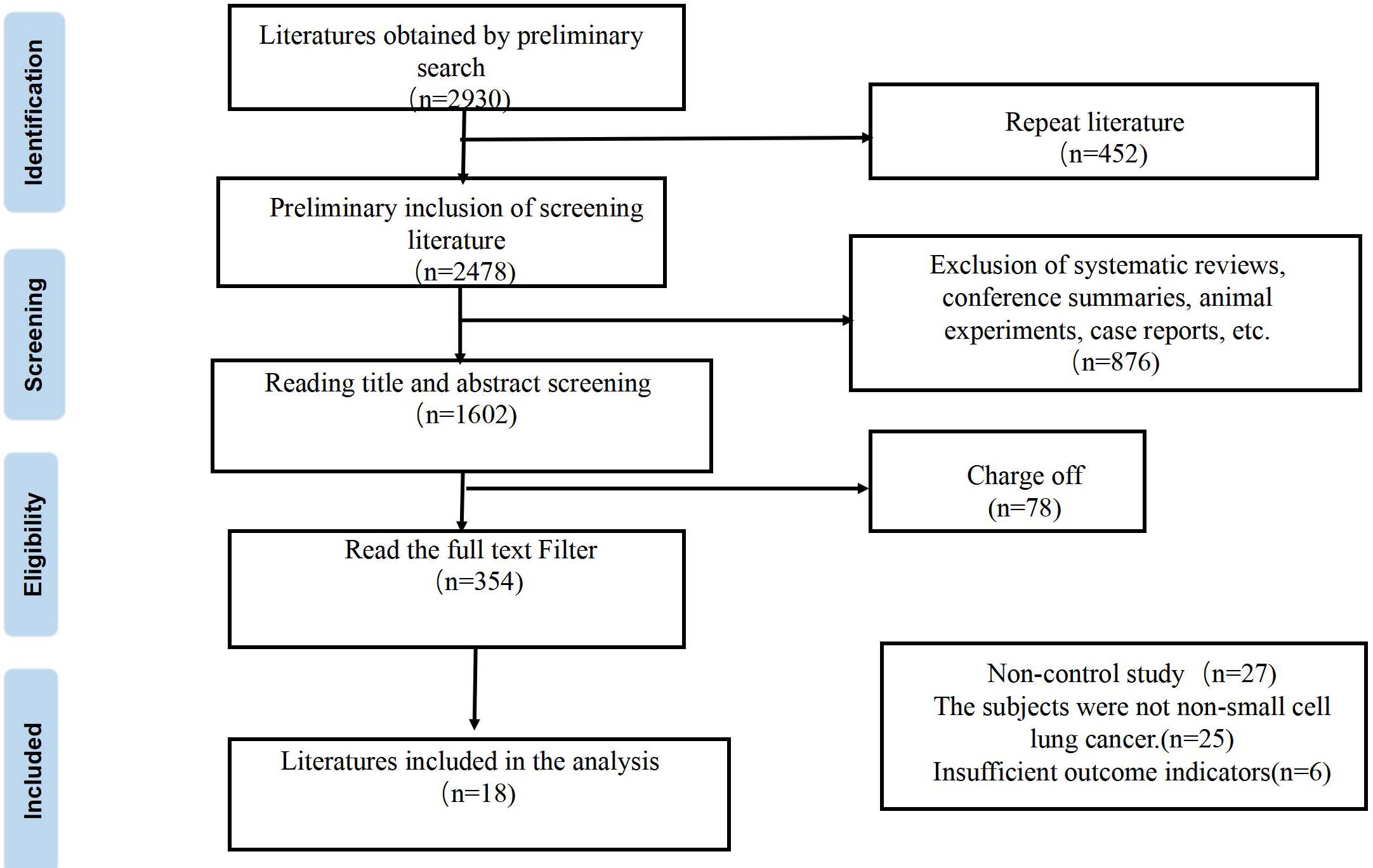
A total of 2930 relevant literatures were retrieved in this study. After systematic screening, 18 eligible literatures were finally included [2, 7, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29], with a total of 21,802 subjects, 4,862 in the experimental group and 1,6940 in the control group. The flow chart of literature retrieval and screening is shown in Fig. 1.
Figure 1.
Literature screening flow chart.
3.2Literature quality evaluation
The basic characteristics and quality evaluation of the included literature are shown in Table 1. Finally, 18 articles were included, including 4 prospective cohort studies [13, 23, 24, 28] and 14 retrospective cohort studies [2, 7, 14, 15, 16, 17, 18, 19, 20, 21, 22, 25, 26, 27]. Among them, 1 was from Japan [15], 1 from France [28], 5 from the United States [2, 14, 16, 21, 27], and the rest were from China [7, 13, 17, 18, 19, 20, 22, 23, 24, 25, 26]. Among the included literatures, 17 literatures with NOS score
Table 1
Basic characteristics of included studies and literature quality evaluation
| Included literature | Study year range | Publish time | Publish country | Research type | Sample size | Mean age (year) | Outcome index | Literature quality evaluation grade | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental group | Control group | Experimental group | Control group | |||||||
| Li Qingyuan et al. [13] | 2019–2020 | 2021 | China | Prospective cohort study | 60 | 60 | 54.7 | 55.5 | 1, 5, 6, 8 | 8 |
| Veluswamy et al. [14] | 2008–2013 | 2020 | America | Retrospective cohort study | 338 | 1230 | 73 | 72. | 7, 8 | 9 |
| Haruki et al. [15] | 2011–2018 | 2020 | Japan | Retrospective cohort study | 49 | 49 | 70 | 68 | 1, 2, 3, 5, 8 | 9 |
| Kneuertz et al. [16] | 2012–2017 | 2020 | America | Retrospective cohort study | 245 | 118 | 65.3 | 64.5 | 3, 4, 6, 8 | 9 |
| Zhou et al. [17] | 2011–2018 | 2020 | China | Retrospective cohort study | 50 | 50 | 54.7 | 57.7 | 1, 2, 3, 5, 6, 8 | 7 |
| Zhang et al. [18] | 2015–2019 | 2020 | China | Retrospective cohort study | 257 | 257 | 53.5 | 52.2 | 1, 2, 4, 5, 8 | 7 |
| Qiu et al. [19] | 2012–2017 | 2020 | China | Retrospective cohort study | 40 | 38 | 61.4 | 61.7 | 1, 2, 3, 4, 5, 6, 7, 8 | 8 |
| Ma Jilong et al. [20] | 2016–2017 | 2019 | China | Retrospective cohort study | 37 | 43 | 61.1 | 58.4 | 1, 2, 3, 4, 5, 6 | 7 |
| Li et al. [21] | 2014–2017 | 2019 | China | Retrospective cohort study | 36 | 85 | 57.2 | 59.7 | 1, 3, 4, 5, 6, 8 | 9 |
| Merritt et al. [22] | 2014–2018 | 2019 | America | Retrospective cohort study | 114 | 114 | 64.8 | 62.5 | 1, 3, 4, 6, 8 | 9 |
| Liu Xingchi et al. [23] | 2012–2017 | 2018 | China | Retrospective cohort study | 134 | 213 | 62.1 | 61.3 | 2, 3, 6 | 8 |
| Yang Lun et al. [24] | 2016–2016 | 2018 | China | Prospective cohort study | 22 | 51 | 60.7 | 51 | 1, 2, 3, 6 | 7 |
| Yang et al. [25] | 2002–2012 | 2017 | China | Prospective cohort study | 172 | 141 | 68 | 67.5 | 4, 6, 7, 8 | 7 |
| Bao et al. [26] | 2014–2015 | 2016 | China | Retrospective cohort study | 69 | 69 | 58.6 | 59.9 | 1, 2, 4, 5, 6, 7, 8 | 7 |
| Yang et al. [27] | 2010–2012 | 2016 | China | Retrospective cohort study | 1938 | 1938 | 68 | 69 | 4, 6, 7 | 8 |
| Mungo et al. [28] | 2007–2014 | 2016 | America | Retrospective cohort study | 53 | 80 | 66 | 67.5 | 4, 7, 8 | 9 |
| Louie et al. [29] | 2009–2013 | 2016 | America | Retrospective cohort study | 1220 | 12376 | 69 | 68 | 1, 6, 7, 8 | 8 |
| Mahieu et al. [30] | 2009–2013 | 2015 | France | Prospective cohort study | 28 | 28 | 62 | 59 | 4, 7, 8 | 6 |
Note: 1
3.3Meta-analysis results of main outcome indicators
3.3.1Operation time
A total of 11 articles compared the operation time of RATS and VATS. The results of heterogeneity evaluation showed that there was a high degree of heterogeneity among the studies (
Figure 2.
Meta-analysis of RATS and VATS operation time.
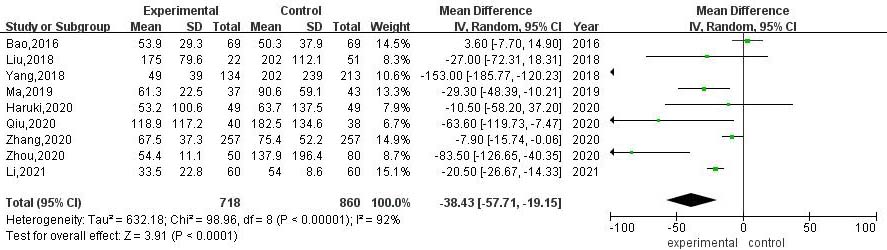
3.3.2Intraoperative blood loss
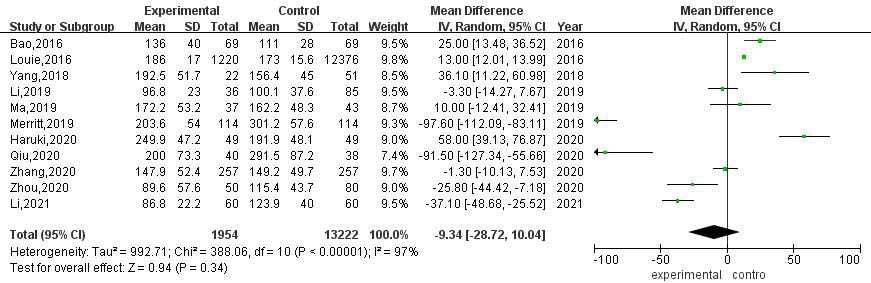
A total of 9 articles compared the intraoperative blood loss between RATS and VATS. The results of heterogeneity evaluation showed that there was a high degree of heterogeneity among the studies (
Figure 3.
Meta-analysis of intraoperative blood loss between RATS and VATS.
3.3.3Number of lymph nodes dissected during operation
A total of 9 articles compared the number of lymph nodes dissected during RATS and VATS. The results of heterogeneity evaluation showed that there was a high degree of heterogeneity among the studies (
Figure 4.
Meta-analysis of the number of lymph nodes dissected during RATS and VATS.
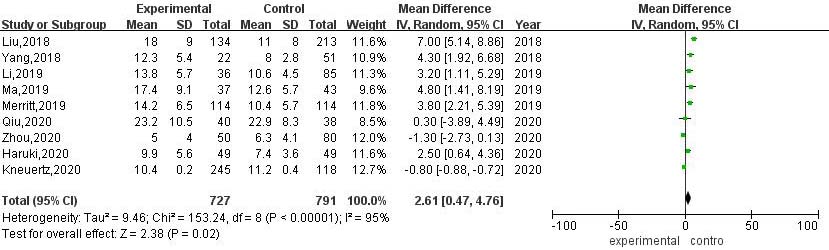
Figure 5.
Meta-analysis of the rate of intraoperative conversion to thoracotomy between RATS and VATS.
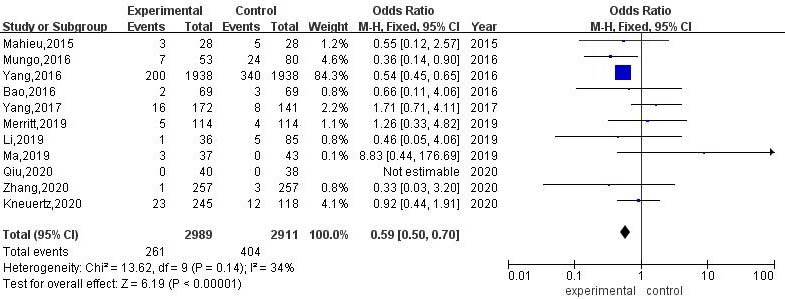
3.3.4Intraoperative transit thoracotomy rate
A total of 11 articles compared the rate of conversion to thoracotomy between RATS and VATS. The results of heterogeneity evaluation showed that there was low heterogeneity among the studies (
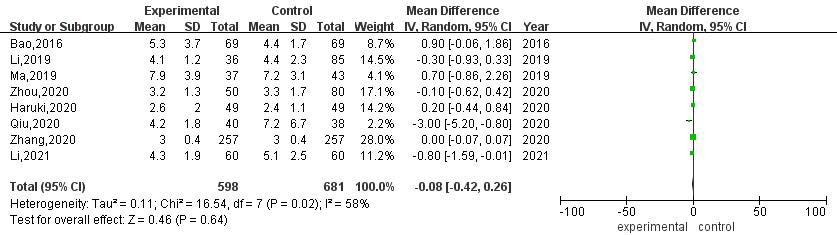
3.3.5Postoperative chest drainage time
A total of 8 articles compared the postoperative thoracic drainage time between RATS and VATS. The heterogeneity evaluation results showed that there was moderate heterogeneity among studies (
Figure 6.
Meta-analysis of thoracic drainage time after RATS and VATS.
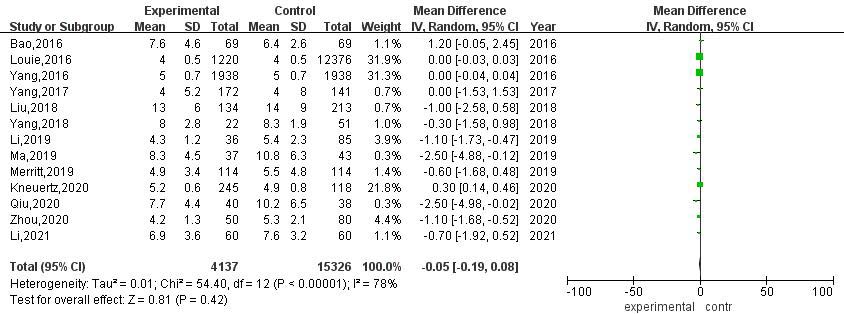
3.3.6Hospital stay after operation
A total of 13 articles compared the postoperative hospital stay between RATS and VATS. The results of heterogeneity evaluation showed that there was a high degree of heterogeneity among the studies (
Figure 7.
Meta-analysis of postoperative hospital stay after RATS and VATS.
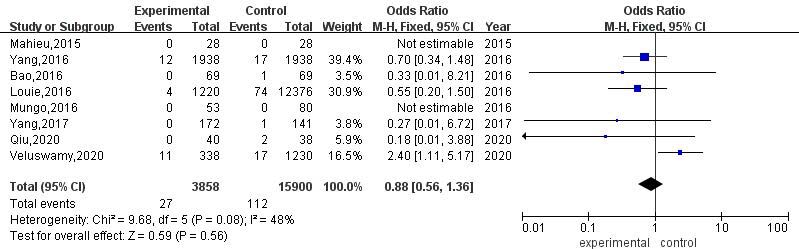
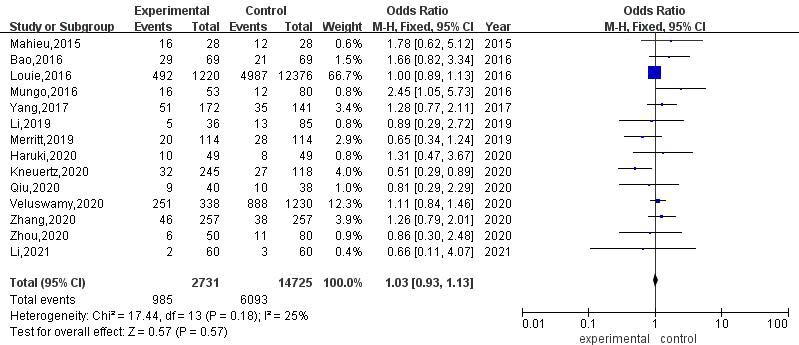
3.3.7Postoperative mortality
A total of 8 articles compared the postoperative mortality of RATS and VATS. The results of heterogeneity evaluation showed that there was low heterogeneity among the studies (
Figure 8.
Meta-analysis of postoperative mortality after RATS and VATS.
3.3.8Postoperative morbidity
A total of 14 articles compared the incidence of postoperative complications between RATS and VATS. The results of heterogeneity evaluation showed that there was low heterogeneity among the studies (
Figure 9.
Meta-analysis of the incidence of postoperative complications after RATS and VATS.
3.4Sensitivity analysis
Among the 9 studies that included intraoperative blood loss, after excluding 1 study [23], sensitivity analysis showed that the heterogeneity among the included studies was reduced by 14%, and the intraoperative blood loss of RATS was still significantly lower than that of VATS [MD
4.Discussion
Systematic lymph node dissection is the most important part of radical resection of lung cancer. The number of dissection groups and the number of dissections not only remove lymph nodes that may have occult metastasis, but also provide more specimens, reduce the rate of missed diagnosis of pathological examination, and improve the accuracy of pathology, so as to obtain more accurate Tumor Node Metastasis staging, provide a reliable basis for postoperative guidance and treatment, and enable patients to obtain better survival benefits [30, 31]. The Da Vinci robotic surgical system manipulator has 7 degrees of freedom, higher flexibility and stability, and can achieve 360∘ surgical operation, which has unique advantages in cleaning lymph nodes. The results of this study showed that compared with VATS, the number of lymph nodes dissected during RATS was significantly higher, and the difference was statistically significant, indicating that RATS was more thorough and accurate in the removal of lesions and lymph node dissection.
The common reasons for conversion to thoracotomy in minimally invasive radical resection of lung cancer include: (1) the operation space is narrow, and the surgeon cannot continue the operation; (2) intraoperative injury of blood vessels, resulting in bleeding; (3) Severe lymph node calcification and extensive pleural adhesions [32]. When the above situation occurs during thoracoscopic surgery, it is mostly converted to thoracotomy, thereby continuing to complete the remaining surgical procedures. The results of this meta-analysis showed that the rate of intraoperative conversion to thoracotomy in RATS was lower than that in VATS, and the difference was statistically significant. The possible reason is that the Da Vinci robotic surgical system can provide the surgeon with a high-definition 3D surgical field that is magnified by 10 to 15 times. The unique inward turning wrist system and the multi-dimensional freely moving robotic arm can make the surgical instruments achieve hand-eye coordination similar to traditional thoracotomy [33, 34]. At the same time, it can filter the adverse effects of the operator’s hand tremor on the operation, making it safer when performing fine operations in small spaces such as pleural roof and diaphragm angle [35]. At the same time, the results of this study showed that the intraoperative blood loss of RATS was significantly less than that of VAS, and the difference was statistically significant, suggesting that RATS surgery is more minimally invasive and less traumatic to the body. This is due to the unique advantages of the RATS surgical system, which can largely avoid the tremor of manual operation, and is more flexible when performing fine operations in deep tissues and narrow spaces, thus greatly reducing damage to surrounding tissues. In addition, the three-dimensional visual field provided by it can clearly show the complex anatomical structure around the mediastinum and hilum of the lung, thus greatly reducing the damage of the operating arm to the blood vessels and surrounding tissues during the operation, effectively reducing the amount of intraoperative blood loss, and also reducing the risk of intraoperative thoracotomy to a certain extent.
This study found that there was no significant difference in the operation time, postoperative thoracic drainage time, postoperative hospital stay, postoperative mortality and postoperative complication rate between RATS and VATS groups. By analyzing the differences of each index, it can be found that in terms of postoperative thoracic drainage time, the time of RATS is shorter than that of VATS, but the difference is not statistically significant, which is consistent with the previous research results [36]. Patients undergoing pneumonectomy need routine indwelling thoracic drainage tube after operation. The purpose is to drain pleural effusion and gas, maintain intrathoracic negative pressure, and promote lung recruitment. However, there is no uniform extubation time. The traditional view is that the thoracic drainage volume is less than 100 mL/d before extubation [37]. According to this standard, it will not only prolong the indwelling time of the thoracic drainage tube, limit the patient ’s early ambulation, but also increase the risk of wound infection, which is not conducive to the postoperative rehabilitation of patients. With the introduction of the concept of enhanced recovery after surgery (ERAS), this traditional extubation time is no longer suitable for clinical work [38]. Most of the studies [39, 40, 41] found that compared with the traditional extubation group (thoracic drainage volum
There are some limitations in this study: (1) The included literature does not involve information on patient race, tumor diameter, and tumor stage; (2) The included literature includes different surgical methods, including lobectomy, segmentectomy and wedge resection of the lung, and the differences in this area will also have a certain impact on the surgical results; (3) The sample size of some studies is small, which may have a certain impact on the final results; (4) The study did not discuss the difference in the cost of RATS and VATS, and the cost of surgery directly affected the choice of surgical methods to a large extent.
5.Conclusion
The results of this study showed that compared with VATS, the number of lymph nodes dissected during RATS was significantly higher, and the clearance of lesions and lymph node dissection were more thorough and accurate. More flexible and precise operation avoids the damage of important blood vessels during operation, effectively reduces the amount of blood loss during operation, shortens the indwelling time of thoracic drainage tube, and is conducive to postoperative rehabilitation of patients. However, due to the limitations of this study, multi-center randomized controlled trials are still needed to verify the conclusions.
Availability of data and materials
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Competing interests
None of the authors have any personal, financial, commercial, or academic conflicts of interest to report.
Funding
This study did not receive funding in any form.
Author contributions
WP conceived the study; FYH, QHF and HP participated in its design, data analysis and statistics; and WHF, LC and LXC helped draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Not applicable.
References
[1] | Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for36 cancers in 185 countries. CA Cancer J Clin. (2021) ; 71: (3): 209-249. |
[2] | Mungo B, Hooker CM, Ho JS, et al. Robotic versus thoracoscopic resection for lung cancer: Early results of a new robotic program. J Laparoendosc Adv Surg Tech A. (2016) ; 26: (4): 243-248. |
[3] | Hong M, Sun WH, Lu M, Zhong TL, Chen TY, Zhao YD, Hong N, Zhu Y, Ding YY. Analysis of the cluster efficacy and prescription characteristics of traditional Chinese medicine intervention for non-small cell lung cancer based on a clustering algorithm. Technol Health Care. (2023) Mar 23. |
[4] | Flores RM, Alam N. Video-assisted thoracic surgery lobectomy (VATS), open thoracotomy, and the robot for lung cancer. Ann Thorac Surg. (2008) ; 85: (2): S710-S715. |
[5] | Zhao Y, Cong B, Zhao X, et al. Meta-analysis of lymph node dissection and survival between total thoracoscopic lobectomy and thoracotomy lobectomy. Chinese Journal of Thoracic and Cardiovascular Surgery. (2014) ; 30: (8): 467-472. |
[6] | Grogan EL, Jones DR. VATS lobectomy is better than open thoracotomy: What is the evidence for short-term outcomes? Thorac Surg Clin. (2008) ; 18: (3): 249-258. |
[7] | Ma J, Jin D, Han S. A case-control study of Da Vinci robotic and thoracoscopic radical resection of lung cancer in patients with early lung cancer. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery. (2019) ; 26: (01): 48-52. |
[8] | Walker WS, Carnochan FM, Pugh GC, et al. Thoracoscopicpulmonary lobectomy. Early operative experience and preliminary clinical results. J Thorac Cardiovasc Surg. (1993) ; 106: (6): 1111-1117. |
[9] | Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg. (2002) ; 21: (5): 864-868. |
[10] | Nakamura H, Taniguchi Y. Robot-assisted thoracoscopic surgery for lung cancer. Kyobu Geka. (2016) ; 69: (8): 650-654. |
[11] | Nakamura H, Taniguchi Y. Robot-assisted thoracoscopic surgery: Current status and prospects. Gen Thorac Cardiovasc Surg. (2013) ; 61: (3): 127-132. |
[12] | Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Symposium on Systematic Reviews: Beyond the Basics. (2014) . |
[13] | Luo D, Wan X, Liu J. How to convert sample size, median, extreme, or quartile to mean and standard deviation. Chinese Journal of Evidence-Based Medicine. (2017) ; 17: (11): 1350-1356. |
[14] | Li Q, Tao S, Kang B, et al. Effects of robotic and thoracoscopic lobectomy on trauma and lymphocyte subsets in patients with non-small cell lung cancer. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery. (2021) ; 28: (3): 299-304. |
[15] | Veluswamy RR, Whittaker Brown SA, Mhango G, et al. Comparative effectiveness of robotic-assisted surgery for resectable lung cancer in older patients. Chest. (2020) ; 157: (5): 1313-1321. |
[16] | Haruki T, Kubouchi Y, Takagi Y, et al. Comparison of medium-term survival outcomes between robot-assisted thoracoscopic surgery and video-assisted thoracoscopic surgery in treating primary lung cancer. Gen Thorac Cardiovasc Surg. (2020) ; 68: (9): 984-992. |
[17] | Kneuertz PJ, D’Souza DM, Richardson M, et al. Long-termoncologic outcomes after robotic lobectomy for early-stage non-small-cell lung cancer versus video-assisted thoracoscopic andopen thoracotomy approach. Clin Lung Cancer. (2020) ; 21: (3): 214-224. |
[18] | Zhou Q, Huang J, Pan F, et al. Operative outcomes and long-termsurvival of robotic-assisted segmentectomy for stage? A lung cancer compared with video-assisted thoracoscopic segmentectomy. Transl Lung Cancer Res. (2020) ; 9: (2): 306-315. |
[19] | hang, Chen C, Hu J, et al. Early outcomes of robotic versus thoracoscopic segmentectomy for early-stage lung cancer: A multi-institutional propensity score-matched analysis. J Thorac Cardiovasc Surg. (2020) ; 160: (5): 1363-1372. |
[20] | Qiu T, Zhao Y, Xuan Y, et al. Robotic sleeve lobectomy forcentrally located non-small cell lung cancer: A propensity score-weighted comparison with thoracoscopic and open surgery. JThorac Cardiovasc Surg. (2020) ; 160: (3): 838-846. |
[21] | Li C, Hu Y, Huang J, et al. Comparison of robotic-assisted lobectomy with video-assisted thoracic surgery for stage B-IIIAnon-small cell lung cancer. Transl Lung Cancer Res. (2019) ; 8: (6): 820-828. |
[22] | Merritt RE, Kneuertz PJ, D’Souza DM. Successful transition torobotic-assisted lobectomy with previous proficiency inthoracoscopic lobectomy. Innovations (Phila). (2019) ; 14: (3): 263-271. |
[23] | Liu X, Xu S, Liu B, et al. Efficacy analysis of da Vinci robotic surgery for stage I non-small cell lung cancer. Chinese Journal of Lung Cancer. (2018) ; 21: (11): 849-856. |
[24] | Yang L, Yu B, Zhang Z, et al. Comparison of Da Vinci robot and thoracoscopic radical surgery in the treatment of non-small cell lung cancer. Acta Academiae Medicinae Weifang. (2018) ; 40: (1): 73-75. |
[25] | Yang HX, Woo KM, Sima CS, et al. Long-term survival based onthe surgical approach to lobectomy for clinical stageInon small cell lung cancer: Comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg. (2017) ; 265: (2): 431-437. |
[26] | Bao F, Zhang C, Yang Y, et al. Comparison of robotic and video-assisted thoracic surgery for lung cancer: A propensity-matched analysis. J Thorac Dis. (2016) ; 8: (7): 1798-1803. |
[27] | Yang CF, Sun Z, Speicher PJ, et al. Use and outcomes of minimally invasive lobectomy for stageI non-small cell lung cancer in thenational cancer data base. Ann Thorac Surg. (2016) ; 101: (3): 1037-1042. |
[28] | Louie BE, Wilson JL, Kim S, et al. Comparison of video-assisted thoracoscopic surgery and robotic approaches for clinical stageIand Stage II non-small cell lung cancer using the society of thoracic surgeons database. Ann Thorac Surg. (2016) ; 102: (3): 917-924. |
[29] | Mahieu J, Rinieri P, Bubenheim M, et al. Robot-assisted thoracoscopic surgery versus video-assisted thoracoscopic surgeryfor lung lobectomy: Can a robotic approach improve short-term outcomes and operative safety? Thorac Cardiovasc Surg. (2016) ; 64: (4): 354-362. |
[30] | Lardinois D, Suter H, Hak ki H, et al. Morbidity, survival, and site of rec u r rence a f ter med ia st i na l ly mph-node d i ssec t ion ver s u s systematic sa mpl ing a f ter complete resection for non-sma l l cel l lung cancer. Ann Thorac Surg. (2005) ; 80: (1): 268-275. |
[31] | Bof fa DJ, Kosi nsk i AS, Pau lS, et al. Ly mph node eva luat ion by open or video-assisted approaches in 11,500 anatomic lung cancer resections. A nn Thorac Surg. (2012) ; 94: (2): 347-353. |
[32] | Liang H, Liang W, Zhao L, et al. Robotic versus video-assistedlobectomy/segmentectomy for lung cancer: A meta-analysis. AnnSurg. (2018) ; 268: (2): 254-259. |
[33] | Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg. (2014) ; 97: (6): 1901-1907. |
[34] | Kim DH, In TS, Jung KS. Effects of robot-assisted trunk control training on trunk control ability and balance in patients with stroke: A randomized controlled trial. Technol Health Care. (2022) ; 30: (2): 413-422. |
[35] | Ye G, Liu Y, Zhang C, et al. Comparison of the effectiveness of da Vinci robot and thoracoscopic segmentectomy in the treatment of early non-small cell lung cancer. Chinese Journal of Robotic Surgery. (2021) ; 2: (1): 1-9. |
[36] | Lee HS, Jang HJ. Thoracoscopic mediastinal lymph node dissection for lung cancer. Semin Thorac Cardiovasc Surg. (2012) ; 24: (2): 131-141. |
[37] | Tomlinson MA, Treasure T. Insertion of a chest drain: How to doit. Br J Hosp Med. (1997) ; 58: (6): 248-252. |
[38] | Yao F, Wang J, Yao J, et al. Early chest tube removal afterthoracoscopic esophagectomy with high output. J LaparoendoscAdv Surg Tech A. (2016) ; 26: (1): 17-22. |
[39] | Zhang TX, Zhang Y, Liu ZD, et al. The volume threshold of 300versus 100 ml/day for chest tube removal after pulmonary lobectomy: A meta-analysis. Interact Cardiovasc Thorac Surg. (2018) ; 27: (5): 695-70252. |
[40] | Xie HY, Xu K, Tang JX, et al. A prospective randomized, controlledtrial deems a drainage of 300 mL/day safe before removal of the lastchest drain after video-assisted thoracoscopic surgery lobectomy. Interact Cardiovasc Thorac Surg. (2015) ; 21: (2): 200-20553. |
[41] | Zhou J, Li K, Li Y, et al. A randomized controlled study on the timing of removal of closed thoracic drainage tube after resection of lung cancer and esophageal cancer. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery. (2019) ; 26: (9): 853-857.54. |
[42] | Liao HC, Yang SM, Hung MH, et al. Thoracoscopic surgerywithout drainage tube placement for peripheral lung nodules. AnnThorac Surg. (2020) ; 109: (3): 887-893. |
[43] | Lesser T, Doenst T, Lehmann T, et al. Lung bioposy withoutpleural drainage. Dtsch Arztebl Int. (2019) ; 116: (19): 329-334. |



