Impact of Early Non-Invasive Ventilation in Amyotrophic Lateral Sclerosis: A multicenter Randomized Controlled Trial
Abstract
Background and objective:
Forced vital capacity (FVC) less than 50% of predicted is one of the main parameters used for Non-Invasive Ventilation (NIV) initiation in Amyotrophic Lateral Sclerosis (ALS). Recent studies suggest that higher values of FVC could be considered as a threshold. The aim of this study is to evaluate whether early use of NIV improves the prognosis of ALS patients compared with standard initiation.
Methods:
This is a randomized, parallel, multicenter, open-label, controlled clinical trial, with recruitment at the ALS outpatient multidisciplinary units of six Spanish hospitals. Patients were included when their FVC reached the 75% threshold and were randomized by computer, stratifying by center in an allocation ratio of 1:1 to Early NIV (FVC below 75%) or Standard NIV (FVC below 50%) initiation. The primary outcome was time to death or tracheostomy.
Trial registration number ClinicalTrials.gov: NCT01641965.
Results:
Between May 2012 and June 2014, 42 patients were randomized to two groups, 20 to Early NIV and 22 to Standard NIV initiation. We found differences in survival in favor of the intervention group: an incidence of mortality (2.68 [1.87–5.50] vs. 3.33 [1.34–4.80] person-months) and a median survival (25.2 vs. 19.4 months), although without reaching statistical significance (p = 0.267).
Conclusions:
This trial did not reach the primary endpoint of survival; nevertheless, it is the first Randomized Controlled Trial (RCT) to demonstrate the benefits of early NIV in slowing the decline of respiratory muscle strength and reducing adverse events. Although not all the results reached statistical significance, all the analyzed data favor early NIV. In addition, this study demonstrates good tolerance and compliance with early NIV without quality of sleep impairment. These data reinforce the early respiratory evaluation of ALS patients and NIV initiation with an FVC of around 75%.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a devastating, progressive neurodegenerative disease of unknown cause characterized by a loss of motor neurons in the spinal cord, brainstem and motor cortex. Progressive muscular weakness and atrophy results in disability and ultimately death, usually within three to five years after diagnosis. Currently, only one drug, riluzole, has been approved worldwide for the treatment of ALS, but its benefits are modest [1]. However, other treatments have been approved in some countries. Edaravone has shown a reduction in the decline of ALSFRS [2]; and AMX0035 has shown promising results for both functional and survival outcomes in a long-term analysis [3], results pending confirmation in a phase 3 study in Europe.
Respiratory failure as a result of progressive respiratory muscle weakness is the main cause of death in more than 80% of patients [4]. Over the last decade, Non Invasive Ventilation (NIV) has proven to be an efficient treatment for respiratory failure, improving the survival and quality of life of these patients [5, 6].
When to initiate NIV remains unclear [7]. Although it is widely accepted that NIV should be started prior to the development of hypercapnia (meaning a severe weakness of respiratory musculature), the most appropriate method of screening with a high pre-test probability to detect early respiratory insufficiency is still uncertain. Forced vital capacity (FVC) is one of the main parameters used in clinical practice, although varies between countries, being the indication of NIV when FVC is between 80% and 50% of predicted [8].
Some studies suggest that higher FVC values could be a better threshold to initiate NIV in ALS, describing increased benefits of early NIV initiation with FVC higher than 65% [9] or higher than 75% [10]. Some studies even suggest starting NIV with normal lung function (FVC higher than 80% of predicted) [11].
All of these studies were retrospective or non-randomized and, to the best of our knowledge, there are no data available from any Randomized Control Trial (RCT) to confirm these promising results.
Therefore, we decided to carry out an RCT to test the hypothesis that the early use of NIV, in the initial phase of respiratory muscle weakness (defined as a slight decrease in the FVC, lower than 75% of predicted), improves the prognosis of ALS patients, compared with the standard initiation of NIV in our country (FVC lower than 50% of predicted).
MATERIAL AND METHODS
Design and Participants
This is a multicentre, randomized, open-label, controlled clinical trial with a parallel treatment design, conducted from May 2012 to June 2014. Patients were recruited at the ALS outpatient multidisciplinary units of six Spanish tertiary hospitals. Patients with an ALS diagnosis and slightly decreased respiratory function (FVC below or equal to 75%) were randomly assigned to Early NIV or Standard NIV groups. The design of the study is represented in Fig. 1.
Fig. 1
CONSORT 2010 Flow Diagram.
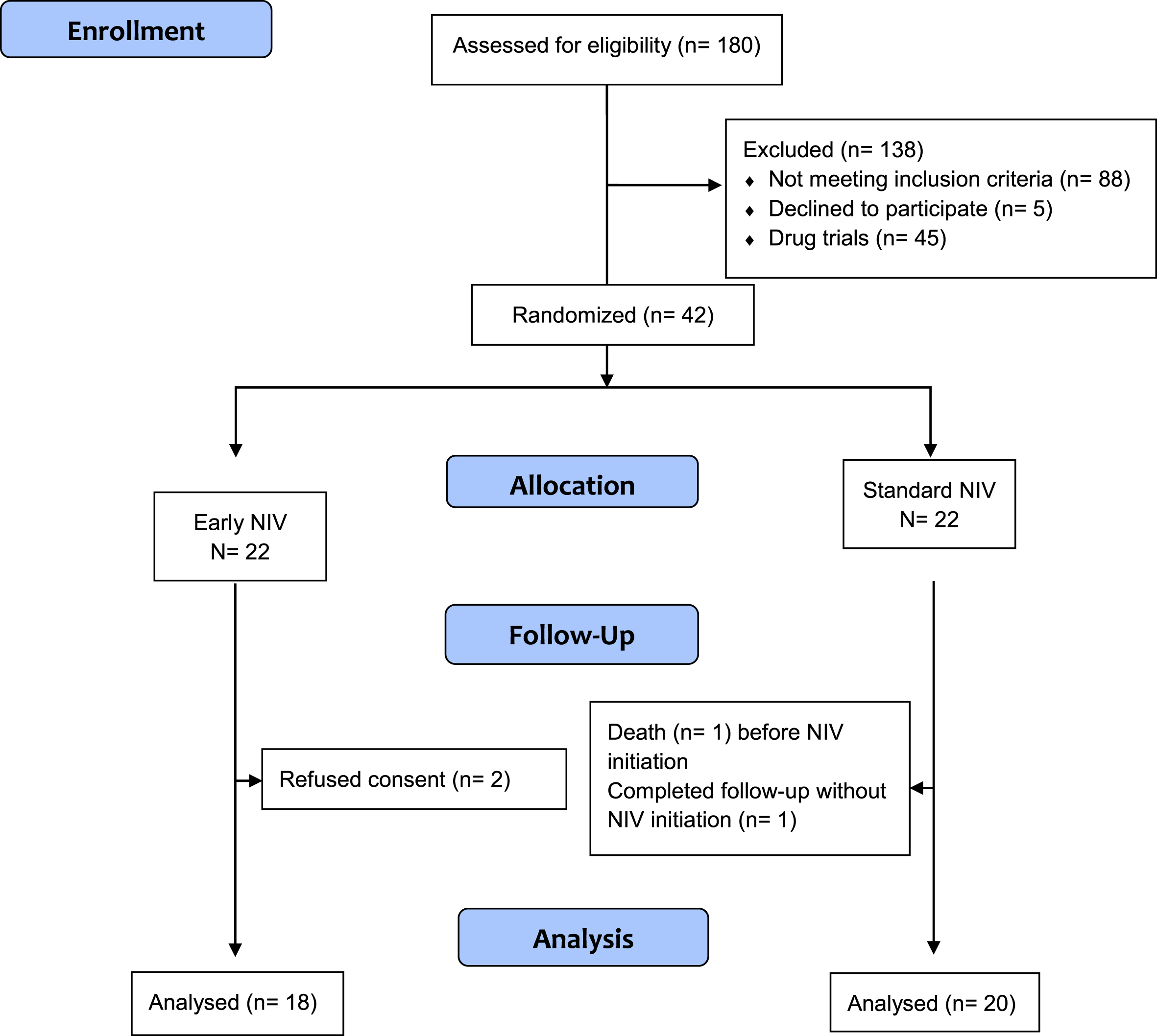
All the patients evaluated at the outpatient clinics in the multidisciplinary ALS units were eligible to participate in the study. The inclusion criteria were: (1) Definite or probable ALS diagnosis according to El Escorial Criteria [12]; (2) FVC below or equal to 75% (with recorded FVC greater than 75% within the six previous months). Exclusion criteria were: (1) indication of NIV according to standard criteria (PaCO2 above 45 mmHg, FVC below 50%, orthopnoea); (2) cognitive impairment that prevents the patient understanding and performing the study procedures including giving informed consent, and technically acceptable pulmonary function tests (FVC, Maximum Inspiratory Pressure (MIP), Sniff Nasal Inspiratory Pressure (SNIP), Cough Peak Flow (CPF)); (3) major comorbidity (not ALS-related) that can shorten life expectancy; (4) refusal of NIV treatment; (5) previous respiratory or cardiac diseases with known impaired spirometry; (6) ALS with a slow disease progression (more than three years), and (7) participation in another clinical trial.
The study protocol and subsequent amendments were approved by the Clinical Research Ethics Committee of all the participant centres. Written informed consent was obtained from all patients. The research was conducted according to the principles of the World Medical Association Declaration of Helsinki.
Randomisation and masking
Subjects were randomized to Early NIV or Standard NIV. They were assigned a randomization code and a study intervention code through a randomization plan. A computerized randomization was performed and the randomization plan was carried out by blocks, stratifying by centre at an allocation ratio of 1:1.
Patients in the Early group started NIV immediately, while patients in the Standard NIV group started when at least one criterion was fulfilled: FVC less than 50%, orthopnea or daytime hypercapnia.
The use of a “sham” NIV was rejected since previous studies in ALS have documented its doubtful efficacy (only 35% of the patients in the sham group believed that they were receiving active treatment) [13]. Therefore, an open-label approach was adopted.
Procedures/study protocol
Initial assessment
Patients were recruited from the ALS outpatient multidisciplinary units of each participating centre. When patients FVC reached the 75% threshold of the predicted value a screening visit was performed. Demographic data, medical history, symptoms, lung function, nocturnal oximetry and the measurement of daytime gas exchange were collected. After eligibility verification and informed signed consent was obtained, randomization was performed.
Intervention
Both groups initiated NIV treatment with a bi-level positive airway pressure device (VIVO 40, BREAS Medical AD, Sweden) operating in pressure support assisted/control mode. The minimum EPAP was 4 cmH2O, with a minimum pressure support 10 cmH2O (IPAP min 14 cmH2O), the maximum IPAP was adjusted according to the tolerance and needs of the patient. In the Early NIV patients, without or with minimum impairments, IPAP was adjusted according to tolerance; while in the Standard NIV group, IPAP was increased until normal nocturnal oximetry and daytime arterial PaCO2 under 45 mmHg during ventilation were achieved. A minimum back-up respiratory rate of 10 to 12 breaths/min was set. The remaining parameters were adjusted according to patient tolerability. Nasal or facial mask were used according to the presence of oral leaks and to obtain the greatest possible patient comfort.
Initial NIV indication was nocturnal in both groups of patients but increasing use of NIV was recommended according to symptoms or the presence of hypercapnia related to the progression of respiratory muscle weakness. If hypercapnia persisted, a reassessment of ventilator parameters was carried out to correct arterial blood gases and nocturnal oximetry during NIV.
Treatment tolerance and adherence through a device internal clock were assessed with a close follow-up of the side effects, which were treated immediately.
Follow-up
All included patients were visited every three months, repeating all the outcome measurements. In patients with NIV, compliance and side effects were assessed and corrected. All the patients had the ALS Unit telephone number and could contact us whenever they needed.
Outcomes
The primary study outcome was the difference in survival between groups, measured by the time from randomization until death or tracheostomy.
The secondary outcomes were progression of respiratory muscle weakness assessed by the rate of decline in FVC, MIP and SNIP, cough effectiveness measured by the CPF; gas exchange evolution measured by arterial blood gases while breathing room air and nocturnal desaturation evaluated by domiciliary nocturnal pulse oximetry; quality of sleep assessed by the Pittsburgh Sleep Quality Index (PSQI), adherence to NIV assessed by the machine counter clock, and tolerance to NIV assessed through the registration of the side effects of the NIV (skin wounds related to mask pressure, oropharyngeal dryness, aerophagia, rhinitis).
Other endpoints were general disease progression assessed by the ALS functional rating scale (ALSFRS-R), and quality of life assessed by the SF-36 questionnaire.
All Adverse Events (AE) and Serious Adverse Events (SAE) were recorded, specifically the number and severity of respiratory decompensations that required unplanned medical assistance (classified according to attention requirements; mild: outpatient clinics, moderate: emergency room or severe: hospital admission) (see Appendix).
Sample size
Since there are no previous similar studies, we based our estimation on two criteria: a) based on inclusion with FVC around 75% and the natural history of the progressive decline in FVC, which is estimated at 2–2.4% per month [24], estimating than the standard group would start treatment one year after the intervention group; b) the difference in survival estimated in retrospective studies evaluating the efficacy of early NIV [6, 7], we assumed an increase in survival function of 20% at one year in the Early NIV group compared to the Standard NIV (See Study design in Appendix). Thus, assuming a recruitment period of 12 months with a constant recruitment rate, a minimum follow-up period of 18 months and a dropout rate of 20%, a total of 72 patients, 36 in each group, were needed to detect a 20% survival difference at one year with an alpha risk of 0.05 and a beta risk of 0.20.
Statistics
Differences between groups in terms of demographic data, baseline clinical features and outcome measurements at each visit were compared by means of parametric t-tests or non-parametric U Mann-Whitney test for continuous variables, while Chi-Squared tests, or Fisher exact-tests were used for categorical variables.
The survival curves for each intervention group were analyzed by Kaplan-Meier’s estimate of the survival function and compared by a bilateral Log-rank test. To explore the time evolution of the outcomes within each study group, a generalized linear mixed model was used. Additionally, the t-test was used to compare differences between groups the first three controls.
Finally, the correlation between FVC and the other functional respiratory variables and nocturnal desaturation were measured at each visit, by Pearson or Spearman’s correlation coefficients.
For all variables, two-sided bilateral tests were be used with a level of significance of 5%. The statistical analysis was performed with SAS statistical software (SAS Institute, version 9.2).
RESULTS
Patients
We assessed 180 patients, 42 of whom met the eligibility criteria. 20 were randomised to the Early NIV group and 22 to the Standard NIV group.
Two patients assigned to the Early NIV group were not included in the analysis as they withdrew their informed consent before NIV initiation, and two patients in the Standard NIV group were not included because one died and the other completed the follow-up without reaching NIV initiation criteria (Fig. 1).
The two study arms had similar characteristics at baseline (ITT population) (Table 1) without significant differences. The mean age of patients was 60 years. At enrolment, they had a quite well-preserved ALS functional rate, good nutritional status, normal daytime gas exchange and mean FVC 68% (SD 5). Time from disease onset to diagnostic was similar in both groups, and the population had mainly limb onset.
Table 1
Baseline demographics and disease characteristics
| Early NIV (n = 20) | Standard NIV (n = 22) | |
| Age, years (SD) | 59.8 (13.7) | 62.1 (9.6) |
| Sex, % female | 45% | 59% |
| BMI, Kg/m2 (SD) | 27.1 (4.1) | 26.3 (4.6) |
| Time from onset to dg, months (range) | 7.40 (5.88–15.68) | 7.40 (4.04–12.43) |
| Time from dg to inclusion, months (range) | 8.47 (1.95–13.88) | 9.58 (2.16–15.82) |
| Site onset, limb% | 85% | 86.36% |
| ALSFRS-R (SD) | 33.3 (7) | 34.3 (7) |
| FVC, % predicted (SD) | 68 (5) | 68.8 (4.8) |
| MIP, cmH2O (SD) | 54.2 (25.4) | 51.7 (17.7) |
| Mean CT90, % (SD) | 8.16 (16.07) | 4.0 (6.76) |
| Mean nSaO2, % (SD) | 92.8 (1.6) | 93.4 (1.6) |
| PaCO2, mmHg (SD) | 37.5 (2.4) | 38.8 (2.8) |
BMI: Body Mass Index, dg: diagnostic, ALSFRS-R: Revised ALS Functional Rating Scale, FVC: Forced Vital Capacity, MIP: Maximal Inspiratory Pressure, CT90% : nocturnal saturation time below 90%, nSaO2: nocturnal oxygen saturation, PaCO2: daytime hypercapnia.
Disease progression
Global ALS progression assessed by the ALSFRS scale was similar in both groups without significant differences. All patients were taking Riluzole throughout the study. The number of patiets with feeding tubes were similar throughout the study in both groups without statistical significance (χ2 = 0.382).
NIV settings and time to NIV
There were no significant differences between groups in the number of patients who initiated NIV in hospital admission or in an ambulatory setting, in the type of mask or in the respiratory backup rate. There were significant differences in inspiratory and expiratory pressures, with these being higher in the Standard NIV group (Table 2).
Table 2
NIV initiation parameters
| Early NIV (n = 18) | Standard NIV (n = 20) | p | |
| Hospital initiation, % | 50% | 75% | 0.104 |
| Facial mask, % | 66.66% | 85% | 0.173 |
| IPAP, cmH2O (SD) | 13.8 (1.07) | 15.1 (1.8) | 0.02 |
| EPAP, cmH2O (SD) | 4 (0) | 4.5 (1.09) | 0.037 |
| BUR, n (SD) | 13.2 (1.9) | 13.6 (2.0) | 0.57 |
BUR: Backup Respiratory Rate, SD: Standard Deviation.
When we examined time to NIV initiation, we found that the Standard group initiated NIV at 194.5 (SD 99.3) days, six months later than the Early NIV group.
Primary outcome: time to event
Median follow-up was 16 months in the Standard group and 18.5 months in the Early group. The incidence of mortality in the Standard NIV group was 3.33 [1.87–5.50] persons/month with a median survival of 19.4 months while in the Early NIV group it was 2.68 [1.34–4.80] persons/month with a median survival of 25.2 months. Figure 2 shows the ITT event-free survival of both groups, which was not statistically significant (Gehan-Breslow test p = 0.267).
Fig. 2
Kaplan-Meier survival curves by groups.
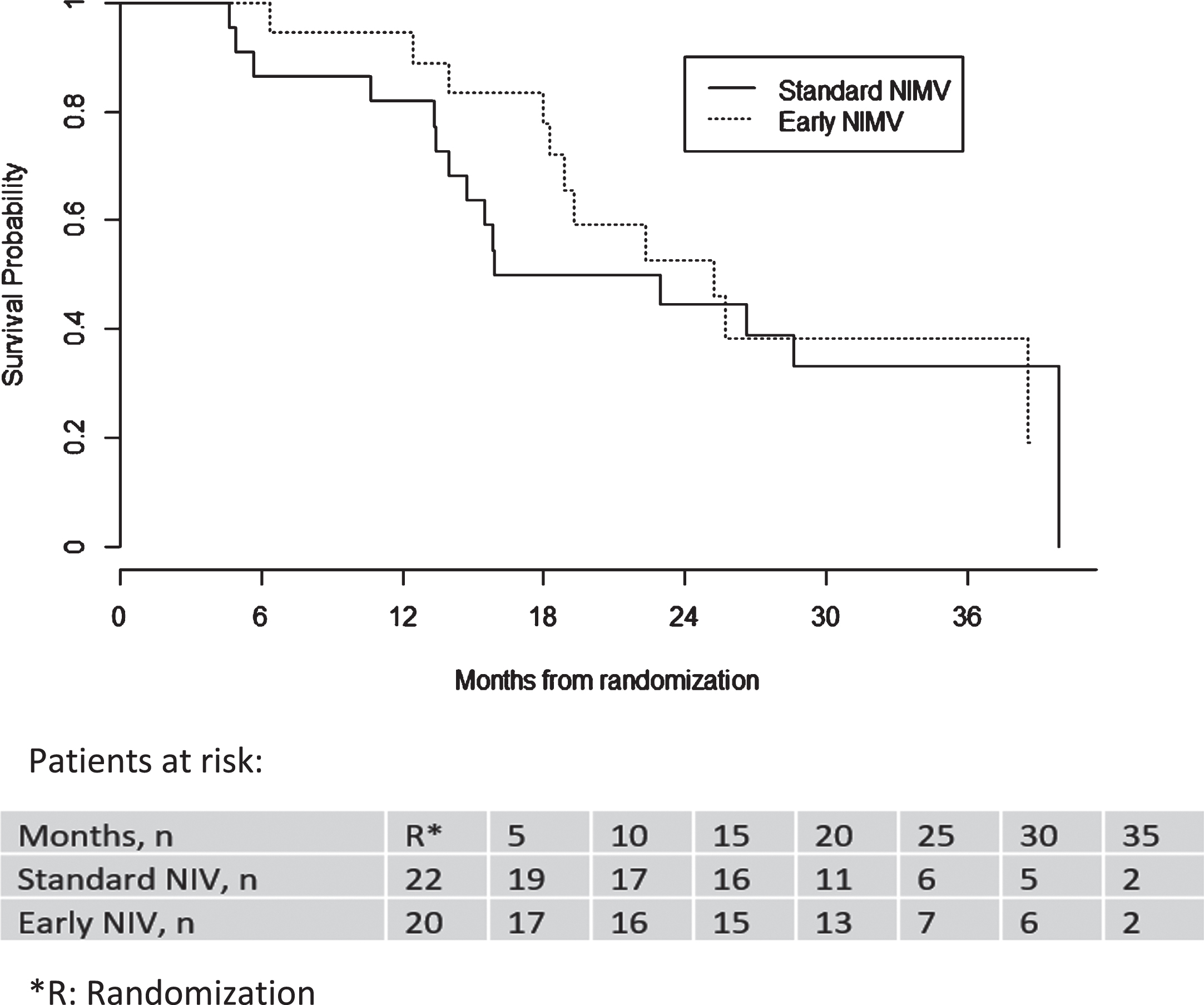
A Cox regression model was applied to compare the risk of mortality with Standard in relation to Early treatment. A statistically significant association was found between the value of FVC% throughout the study and death, which suggests a protective effect of FVC% ; likewise, a statistically significant association was found between bulbar involvement and death, although given the small number of patients with bulbar involvement, caution is required when interpreting its magnitude. See the Appendix for more details.
SECONDARY OUTCOMES
Respiratory function
Figure 3 shows the differences in the evolution curves of FVC, MIP and SNIP at the baseline and the successive controls every three months.
Fig. 3
Evolution of respiratory function parameters. (3A) FVC, (3B) SNIP, (3 C) PIM, (3D) CPF.
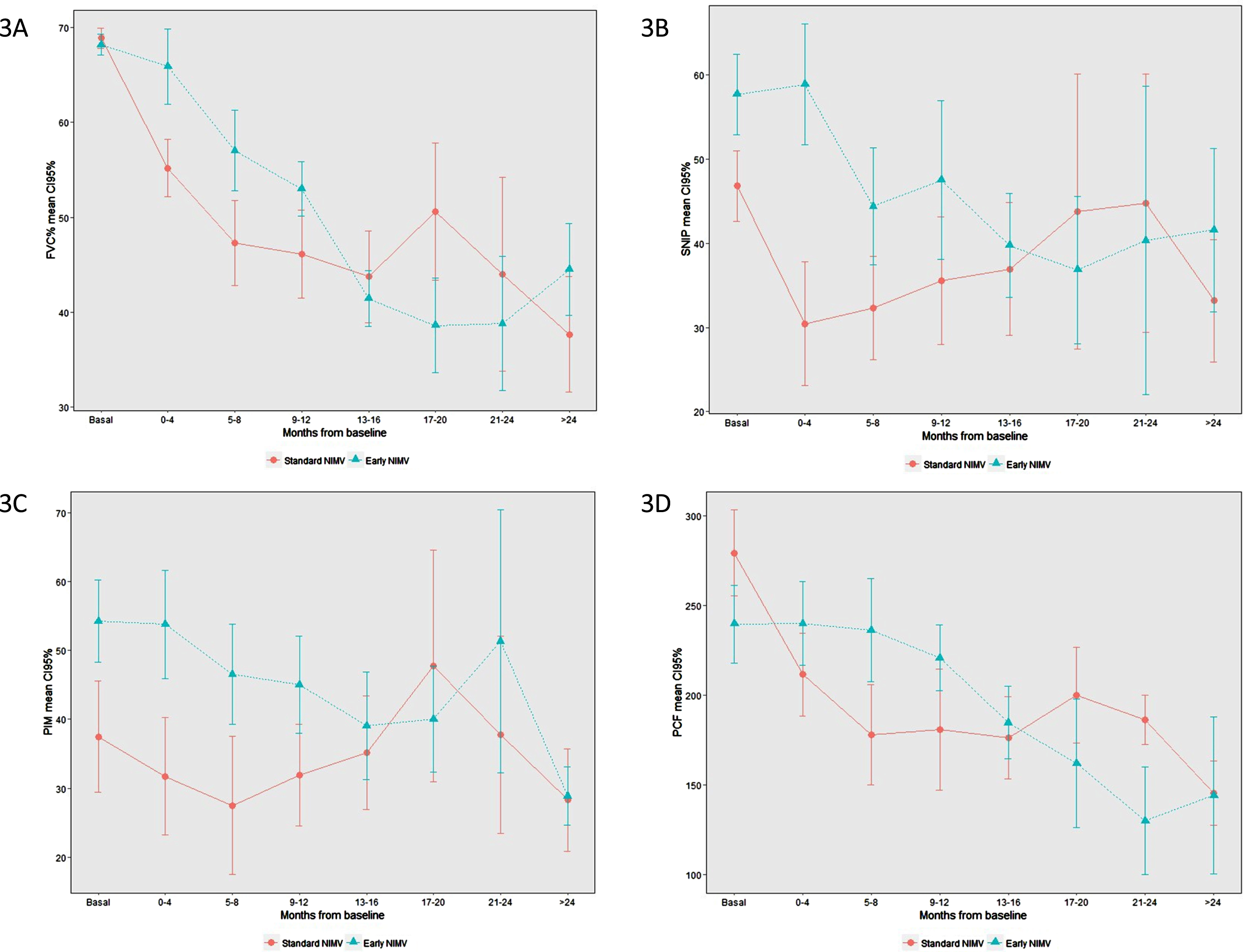
Differences in FVC of around 20 points can be observed in favour of the Early group between controls one to three. FVC and SNIP differences are statistically significant at control one, but not at controls two and three. MIP and CPF also show a difference, though it is not statistically significant (Table 3).
Table 3
Comparison of respiratory function parameters in the first three controls
| Standard | Early | p* | |
| Baseline | |||
| FVC% (SD) | 68.87 (4.9) | 68.20 (5) | 0.6595 |
| MIP, cmH2O | 37.48 | 54.24 | 0.104 |
| SNIP, cmH2O | 46.8 | 57.67 | 0.0961 |
| Control 1 (3 months) | |||
| FVC% (SD) | 54.83 (13.2) | 65.09 (15.3) | 0.0429 |
| MIP, cmH2O | 29.96 | 50.25 | 0.062 |
| SNIP, cmH2O | 27.3 | 56.4 | 0.0051 |
| Control 2 (6 months) | |||
| FVC% (SD) | 47.34 (18.9) | 56.36 (15.1) | 0.1396 |
| MIP, cmH2O | 26.71 | 46.68 | 0.1225 |
| SNIP, cmH2O | 34.53 | 44.20 | 0.3071 |
| Control 3 (9 months) | |||
| FVC% (SD) | 45.33 (18.5) | 52.75 (11.5) | 0.2202 |
| MIP, cmH2O | 33.11 | 44.24 | 0.3103 |
| SNIP, cmH2O | 38.09 | 46.15 | 0.502 |
*t-test.
From 12 months onwards, the results are highly variable, largely due to the small number of patients in relation to both deaths and bulbar impairment, which prevented the correct performance of the test.
There was a good correlation between FVC and MIP, and FVC and SNIP, but not between FVC and nocturnal desaturation (See Appendix).
Gas exchange
There were no significant differences between groups in pCO2 and pO2, nor in nocturnal desaturation (Appendix), although there was an increase of pCO2 in the Standard group.
Adverse events
The number of adverse events was significantly greater in the Standard group (n = 70) versus Early groups (n = 49). None of these events were related to treatment. The intensity of events was also higher in the Standard than in the Early group (Wilcoxon p = 0.0325).
Regarding respiratory events, there were more in the Standard group than in the Early group without reaching statistical significance. The number of patients that suffered a respiratory event was greater in the Standard group, this difference being almost significant as was the number of severe events (Table 4).
Table 4
Total and Respiratory adverse events
| Early NIV | Standard NIV | P | |
| R. events, n | 12 | 24 | 0.34* |
| R. patients, n (%) | 7 (35) | 15 (68) | 0.065** |
| Severity, n (%): | |||
| Mild/Moderate | 10 (83) | 15 (62) | |
| Severe | 2 (17) | 9 (38) | 0.07** |
*Chi2; **Fisher; R. events: number of total respiratory events; R. patients: number of patients with respiratory events.
The main respiratory events were respiratory infections (n = 16 Standard, n = 3 Early); followed by pneumonia (n = 6 Standard, n = 5 Early) and aspiration (n = 2 Standard, n = 2 Early). One patient in the Early group suffered respiratory failure after the placement of GRP.
Adherence, quality of sleep and side effects
There were no differences in adherence between groups, with good adherence from the first control after NIV initiation, when the average use of NIV was 5.28 hours (SD 2.65) in the Standard group and 5.19 hours (SD 3.58) in the Early group.
There were no significant differences in quality of sleep assessed by the Pittsburgh Quality Sleep Index (PSQI), through it was slightly higher in the Early group (p = 0.36). There were also no differences in physical SF-36 nor in mental SF-36 (See Appendix).
Finally, there were no differences in the number of NIV related side effects between groups (Table 7 - Appendix). These were mainly mild and in no case did they lead to treatment withdrawal. The most frequent side effect in both groups was oropharyngeal dryness, which was effectively controlled with a humidifier.
DISCUSSION
This is the first randomized controlled trial to show the benefits of early NIV on respiratory outcomes, slowing the progressive decline in respiratory muscle strength and reducing the number and severity of respiratory events, while treatment adherence and tolerance were good without worsening sleep or overall quality of life.
Moreover, our RCT shows a trend towards an increase of survival in patients with Early NIV initiation, however, it was not able to reach statistical significance, probably as a result of not having reached the estimated simple size. The main limitation in recruiting patients was competition with drug trials (dexpramipexole, masitinib, and docosahexaenoic acid (DHA)), which were running at the same time as our trial, and two new trials which were pending when we decided to close the inclusion period.
Until now, NIV has proven to be the best treatment for respiratory insufficiency in ALS. The initiation of NIV in early stages has been suggested in recent years since FVC is a major prognostic factor for survival [6, 14].
Two initial studies reported increased survival when NIV was started with FVC clearly over 50%. In a retrospective study, Lechtzin and colleagues [9] described a survival rate from time of diagnosis nearly one year longer in the early group (mean FVC 74%) than in the control group (mean FVC 48%). Likewise, Carratú and colleagues [10] reported a significantly better one-year survival rate in patients treated with early NIV (mean FVC 65%) compared to patients that did not accept or did not tolerate NIV, as well as reporting a slowing in the progressive decline of FVC in treated patients.
Two recent retrospective studies, with a large number of patients, also showed the benefits of early NIV treatment. Vitacca and colleagues [15] compared NIV initiation below 80% FVC with NIV over 80% FVC in 194 patients, finding a mortality rate of 35% at 36 months from diagnosis in the Early group versus 52% in Standard group. In the same way, Khamankar et colleagues [11] described a significant increased survival from diagnosis in NIV users than in non-users in 474 patients, and consistently increased survival according to FVC initiation threshold (<50% : 20.3 m.,≥50% : 23.6 m. or≥80% : 25.36 m).
Despite the rather impressive results of these studies, important differences should be highlighted that lend value to the results of our study. First, its retrospective or non-controlled design. Second, the analysed survival based on time from disease diagnosis, which can overestimate the effects of NIV. Finally, the homogeneity of the included study population, since none of the afore-mentioned studies excluded patients with slow disease progression, such as flail leg or flail arm, which have markedly prolonged survival rates [16].
Our randomized controlled trial excluded patients with a slow progression, without differences between groups in time from onset symptoms to diagnosis. Patients were at the same disease stage at inclusion and also had similar disease progression throughout the study, as reflected by the ALSFRS score both at inclusion and throughout the study. Finally, survival times are analysed from NIV initiation to ensure they mainly reflect the effects of NIV.
One of the possible mechanisms to explain the benefits of early NIV is a reduction in the progressive decrease in FVC through the resting of the respiratory muscles that present incipient denervation, avoiding fatigue and the development of hypoventilation [17]. In fact, our study is the first to demonstrate a slower FVC decline in an RCT. Patients on early NIV maintained their FVC value, while in those who do not start NIV, it declined very quickly during the first controls. Patients in the control group met the criteria to start NIV at an average time of six months, which was earlier than expected (See the Appendix for Protocol design), suggesting that once there is an incipient respiratory muscle impairment, it progresses rapidly unless NIV treatment is started.
Our results support the recommendations of some recent guidelines to start NIV when FVC is below 80% [18, 19]. Swiss guidelines [20] recommend the initiation of NIV based only on symptoms, which may be inaccurate as respiratory symptoms often go unnoticed. On the other hand, some studies recommend initiating NIV with FVC higher than 80%, which is probably unnecessary as they have a normal lung function, and better survival could be confounded by long-term phenotypes and better FVC.
Despite these clinical recommendations and the increasing evidence in favor of early NIV, real-world practice shows that NIV is frequently initiated with FVCs below 50% and mostly on hypercapnic patients [21, 22]. Therefore, in addition to defining the FVC threshold, the most important issue is probably to assess and follow-up respiratory function from disease diagnosis in order to adequately identify the onset of respiratory muscle deterioration [5].
Different measures have been proposed for respiratory assessment, and some studies suggest that other techniques as more sensitive for the early detection of respiratory impairment, such as MIP [23], SNIP [24] and nocturnal desaturation [25]. Morgan and colleagues [26] observed a better sensitivity with SNIP (97%) than FVC (58%). In our study we found a good correlation between FVC and SNIP and FVC and MIP, but not between FVC and nocturnal desaturation. We have not specifically studied the sensitivity of each of the techniques, but we think FVC is the best option since it is the easiest, most reproducible, low-cost technique and accessible to all health levels, including home, while MIP and SNIP are more complex techniques with more variability and are only accessible at specialized centers.
Another possible effect of early NIV on survival is the prevention of nocturnal hypoventilation with hypercapnia and respiratory acidosis, which may reduce the impairment of muscle contractility [27]. We found differences in PaCO2 level evolution in favor of the early group, although these differences were not significant.
Finally, one important effect of NIV may be the prevention of severe acute respiratory decompensations reducing the risk of death or intubation [28]. The principal causes of death in ALS patients are respiratory infections [29] and the progression of respiratory muscle impairment (diaphragm insufficiency) [30]. Our study found that a higher number of patients on Standard NIV had respiratory complications and, more importantly, these were more severe than in the early NIV group.
One of the main fears regarding early NIV is the lack of compliance or treatment rejection in probably asymptomatic patients. Our study clearly shows no differences in adherence, with good adherence from the first control and an average use of NIV of more than five hours. In the same way, tolerance was good without differences in side effects and, moreover, good quality of sleep and quality of life were reported, which is in line with recent publications [31].
Moreover, a recent randomized trial conducted to evaluate the use and tolerability of Early NIV [32] reported good results. Nevertheless, in comparison, the average use of NIV was very low (three hours or less in both groups), with low pressures (pressure-support of 4) and a follow-up of a small number of patients with NIV (n = 35). As in our study, a lower decline in FVC was found in the Early NIV group.
Mechanical ventilation can be a traumatic and intimidating step [33], and may be associated by the patient with end of life or with tracheostomy. In our opinion, it is important how therapy is presented to patients and how convinced the staff is, as well as compliance reinforcement.
In conclusion, FVC is probably the best option to assess respiratory function in ALS patients and the optimal time to start NIV is when FVC is less than 80% and around 75%. At this stage there is a slight affection of respiratory muscles, and this is when NIV can do its job: maintaining muscle strength, slowing the decline in FVC, and preventing nocturnal hypoventilation and respiratory complications, and thus prolonging survival.
Strengths and limitations
The main strength of this study lies in its design as a randomized controlled trial, conducted in tertiary hospitals and its multicentre character. In addition, the ALS phenotype population was very homogeneous. The study was led by pulmonologists with extensive experience in NIV management in ALS.
The most important limitation is the low number of participants (42 out of 72) as we failed to reach the estimated sample size, which probably influenced the lack of statistical significance of the results. As mentioned previously, the main reason for this was competition with drug trials, which is the usual scenario in multidisciplinary ALS units. In this situation, patients were either entered into a drug trial before they met our trial criteria or, given both options, they generally chose the drug trial because of the chance, however remote, of a cure. In this sense, it’s important to highlight the difficulty in conducting studies like this, both now and in the future. Few more studies like this will be conducted, not only because of competition with drug trials, their complexity, and high costs; but also with many centers opting for an earlier start of NIV.
CONCLUSION
This is the first and only randomised control trial to date to show that NIV initiation in early stages of respiratory failure in ALS may improve survival, suggesting that NIV should be initiated when FVC falls below 80% and not 50%, as was considered standard care previously.
Our findings highlight the importance of assessing respiratory function from diagnosis and encourage the early initiation on NIV in order to extend survival, slow the decline of muscle strength, improve the quality of sleep and reduce severe complications. Although our study is not totally conclusive, further RCT will be very difficult to repeat.
Trial registration. Trial registration number ClinicalTrials.gov: NCT01641965.
ACKNOWLEDGMENTS
Cristian Tebe contributed to statistical analysis. Miriam Sellés and Irma Sala from Fundació Miquel Valls monitored the trial. We would also like to thank Fundació Miquel Valls for their general support.
FUNDING
This study received Health Research Fund (FIS) from Carlos III Health Institute (ISCIII), and a grant from Catalan Society of Pneumology (SOCAP).
DATA AVAILABILITY
Qualified researchers can request access to individual patient level data and data dictionary. The study protocol will be available in the Appendix. This data will be available upon publication and will be shared on mail after IP approval.
STATEMENTS AND DECLARATIONS
MS declares no conflicts of interest. NG declares no conflicts of interest. AC declares no conflicts of interest. EP declares no conflicts of interest. JMRGM has received fees as a speaker, advisory board member, research grants from AstraZeneca, Boeringer-Ingelheim, Chiesi, GSK, Grifols, Novartis, Sanofi. No conflicts of interest to declare in relation to the article. SMB declares no conflicts of interest. ML has received personal fees for lectures from Philips and Resmed. No conflicts of interest in relation to the article. MC has received speaker fees from AstraZeneca, Bial, Chiesi, CSL Behring, GlaxoSmithKline, Menarini, and Grifols, and consulting fees from GlaxoSmithKline and Bial; none of them in relation to this article. AA declares no conflicts of interest. MP has received consulting fees from Biogen, Roche and Novartis. EF declares no conflicts of interest.
REFERENCES
[1] | Miller RG , Mitchell JD , Lyon M , Moore DH . Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). In: Miller RG, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; (2007) . |
[2] | Abe K , Aoki M , Tsuji S , Itoyama Y , Sobue G , Togo M , et al. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. (2017) ;16: (7):505–12. |
[3] | Paganoni S , Hendrix S , Dickson SP , Knowlton N , Macklin EA , Berry JD , et al. Long-term survival of participants in the CENTAUR trial of sodium phenylbutyrate-taurursodiol in amyotrophic lateral sclerosis. Muscle Nerve. (2021) ;63: (1):31–9. |
[4] | Spataro R , Lo Re M , Piccoli T , Piccoli F , La Bella V . Causes and place of death in Italian patients with amyotrophic lateral sclerosis. Acta Neurol Scand. (2010) ;122: (3). |
[5] | Farrero E , Prats E , Povedano M , Martinez-Matos JA , Manresa F , Escarrabill J . Survival in Amyotrophic Lateral Sclerosis With Home Mechanical Ventilation. Chest. (2005) ;127: (6):2132–8. |
[6] | Bourke SC , Tomlinson M , Williams TL , Bullock RE , Shaw PJ , Gibson GJ . Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: A randomised controlled trial. Lancet Neurology. (2006) ;5: (2). |
[7] | Morelot-Panzini C , Bruneteau G , Gonzalez-Bermejo J . NIV in amyotrophic lateral sclerosis: The ‘when’ and ‘how’ of the matter. Respirology. (2019) ;24: (6):521–30. |
[8] | Miller RG , Jackson CE , Kasarskis EJ , England JD , Forshew D , Johnston W , et al. Practice Parameter update: The care of the patient withamyotrophic lateral sclerosis: Multidisciplinary care, symptommanagement, and cognitive/behavioral impairment (an evidence-basedreview): Report of the Quality Standards Subcommittee of theAmerican Academy of Neurology. Neurology. (2009) ;73: (15). |
[9] | Lechtzin N , Scott Y , Busse AM , Clawson LL , Kimball R , Wiener CM . Early use of non-invasive ventilation prolongs survival in subjects with ALS. Amyotrophic Lateral Sclerosis. (2007) ;8: (3):185–8. |
[10] | Carratù P , Spicuzza L , Cassano A , Maniscalco M , Gadaleta F , Lacedonia D , et al. Early treatment with noninvasive positivepressure ventilation prolongs survival in Amyotrophic LateralSclerosis patients with nocturnal respiratory insufficiency. Orphanet J Rare Dis. (2009) ;4: (1):10. |
[11] | Khamankar N , Coan G , Weaver B , Mitchell CS . Associative Increases in Amyotrophic Lateral Sclerosis Survival Duration With Non-invasive Ventilation Initiation and Usage Protocols. Front Neurol. (2018) ;9: . |
[12] | Brooks BR . El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. (1994) ;124: (Suppl):96–107. |
[13] | Gruis KL , Brown DL , Weatherwax KJ , Feldman EL , Chervin RD . Evaluation of sham non-invasive ventilation for randomized, controlled trials in ALS. Amyotrophic Lateral Sclerosis. (2006) ;7: (2):96–9. |
[14] | Czaplinski A , Yen AA , Appel SH . Forced vital capacity (FVC) as an indicator of survival and disease progression in an ALS clinic population. J Neurol Neurosurg Psychiatry. (2006) ;77: (3). |
[15] | Vitacca M , Montini A , Lunetta C , Banfi P , Bertella E , De Mattia E , et al. Impact of an early respiratory care programme with non-invasive ventilation adaptation in patients with amyotrophic lateral sclerosis. Eur J Neurol. (2018) ;25: (3):556–e33. |
[16] | Wijesekera LC , Mathers S , Talman P , Galtrey C , Parkinson MH , Ganesalingam J , et al. Natural history and clinical features of the flail arm and flail leg ALS variants. Neurology. (2009) ;72: (12):1087–94. |
[17] | Georges M , Morélot-Panzini C , Similowski T , Gonzalez-Bermejo J . Noninvasive ventilation reduces energy expenditure in amyotrophiclateral sclerosis. BMC Pulm Med. (2014) ;14: (1):17. |
[18] | Andersen PM , Abrahams S , Borasio GD , de Carvalho M , Chio A , Van Damme P , et al. EFNS guidelines on the Clinical Management of Amyotrophic Lateral Sclerosis (MALS) –revised report of an EFNS task force. Eur J Neurol. (2012) ;19: (3):360–75. |
[19] | Georges M , Perez T , Rabec C , Jacquin L , Finet-Monnier A , Ramos C , et al. Proposals from a French expert panel for respiratory care in ALS patients. Respir Med Res. (2022) ;81: :100901. |
[20] | Janssens JP , Michel F , Schwarz EI , Prella M , Bloch K , Adler D , et al. Long-Term Mechanical Ventilation: Recommendations of the Swiss Society of Pulmonology. Respiration. (2020) ;99: (10):867–902. |
[21] | Georges M , Golmard JL , Llontop C , Shoukri A , Salachas F , Similowski T , et al. Initiation of non-invasive ventilation in amyotrophic lateral sclerosis and clinical practice guidelines: Single-centre, retrospective, descriptive study in a national reference centre. Amyotroph Lateral Scler Frontotemporal Degener. (2017) ;18: (1–2):46–52. |
[22] | Vitacca M , Vianello A . Respiratory Outcomes of Patients With Amyotrophic Lateral Sclerosis: An Italian Nationwide Survey. Respir Care. (2013) ;58: (9):143341. |
[23] | Terzano C , Romani S . Early use of non invasive ventilation in patients with amyotrophic lateral sclerosis: What benefits? Eur Rev Med Pharmacol Sci. (2015) ;19: (22):4304–13. |
[24] | Tilanus TBM , Groothuis JT , TenBroek-Pastoor JMC , Feuth TB , Heijdra YF , Slenders JPL , et al. The predictive value of respiratoryfunction tests for non-invasive ventilation in amyotrophic lateralsclerosis. Respir Res. (2017) ;18: (1):144. |
[25] | Pinto A , de Carvalho M , Evangelista T , Lopes A , Sales-Luís L . Nocturnal pulse oximetry: A new approach to establish theappropriate time for non-invasive ventilation in ALS patients. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. (2003) ;4: (1):31–5. |
[26] | Morgan RK , McNally S , Alexander M , Conroy R , Hardiman O , Costello RW . Use of Sniff Nasal-Inspiratory Force to Predict Survival in Amyotrophic Lateral Sclerosis. Am J Respir Crit Care Med. (2005) ;171: (3):269–74. |
[27] | Metha S , Hill NS . Noninvasive Ventilation. Am J Respir Crit Care Med. (2001) ;163: (2):540–77. |
[28] | Ward S , Chatwin M , Heather S , Simonds AK . Randomised controlled trial of non-invasive ventilation (NIV) for nocturnal hypoventilation in neuromuscular and chest wall disease patients with daytime normocapnia. Thorax. (2005) ;60: (12). |
[29] | Corcia P , Pradat P , Salachas F , Bruneteau G , le Forestier N , Seilhean D , et al. Causes of death in a post-mortem series of ALS patients. Amyotrophic Lateral Sclerosis. (2008) ;9: (1):59–62. |
[30] | Yang R , Huang R , Chen D , Song W , Zeng Y , Zhao B , et al. Causes and places of death of patients with amyotrophic lateral sclerosis in south-west China. Amyotrophic Lateral Sclerosis. (2011) ;12: (3):206–9. |
[31] | Jackson CE , Heiman-Patterson TD , Sherman M , Daohai YU , Kasarskis EJ , Heiman-Patterson T , et al. Factors associated with Noninvasive ventilation compliance in patients with ALS/MND. Amyotroph Lateral Scler Frontotemporal Degener. (2021) ;22: (S1):40–7. |
[32] | Jacobs TL , Brown DL , Baek J , Migda EM , Funckes T , Gruis KL . Trial of early noninvasive ventilation for ALS. Neurology. (2016) ;87: (18):1878–83. |
[33] | Mitsumoto H , Rabkin JG . Palliative Care for Patients With Amyotrophic Lateral Sclerosis. JAMA. (2007) ;298: (2):207. |



