Novel Simulation Model of Non-Muscle Invasive Bladder Cancer: A Platform for a Virtual Randomized Trial of Conservative Therapy vs. Cystectomy in BCG Refractory Patients
Abstract
Introduction: There have been no randomized controlled trials (RCTs) evaluating the clinical or economic benefit of mitomycin C intravesical therapy vs. radical cystectomy in patients with high-risk non-muscle invasive bladder cancer (NMIBC). We used the Archimedes computational model to simulate RCT comparing radical cystectomy versus intravesical mitomycin C (MMC) therapy to evaluate the clinical and economic outcomes for BCG-refractory NMIBC as well demonstrate the utility of computer based models to simulate a clinical trial.
Methods: The Archimedes model was developed to generate a virtual population using the Surveillance Epidemiology and End Results database, other clinical trials, and expert opinions. Patients selected were diagnosed with NMIBC (<cT2 disease) who recurred or progressed despite BCG therapy and were randomized to 1) immediate radical cystectomy vs. 2) MMC induction intravesical therapy. Clinical (progression, overall survival, and disease specific survival) and economic outcomes were reported.
Results: A total of 1300 virtual patients were evaluation. Progression to MIBC in the MMC treatment arm was 30% over the lifetime. Disease specific death at 5 years was 1.6% and 8.7% for the immediate cystectomy and MMC treatment arms respectively; while, overall death was 17.8% and 23.8% at 5 years. Over a 5-year period the average cost of immediate cystectomy was $64,675 vs $68,517 in the MMC arm.
Conclusion: Immediate radical cystectomy after BCG failure for NMIBC has improved survival and is more cost-effective when compared to those undergoing MMC. Simulation of clinical trials using computational models similar to the Archimedes model can overcome shortcomings of real-world clinical trials and may prove useful in the face of current medical cost-conscious era.
INTRODUCTION
High risk (high grade, cTa, cT1, and cTis), non-muscle invasive bladder cancer (NMIBC) is principally managed with transurethral resections (TUR) and adjuvant intravesical bacillus Calmette-Guerin (BCG) therapy. However, disease recurs or progresses in approximately 40%–50% of patients [1]. Available management options for patients with recurrent or progressive high grade NMIBC include radical cystectomy (RC), additional intravesical therapy typically with chemotherapy, and clinical trial entry [2]. Some advocate RC in lieu of additional intravesical therapy as BCG failure portends poor oncologic outcomes when treated with bladder preservation and long-term survival in patients undergoing cystectomy with cTa/T1/Tis disease ranges from 80%–88% [3, 4]. Furthermore, cystectomy may be more cost-effective than intravesical therapy when used as first line treatment in certain high risk settings of NMIBC [5].
Mitomycin C (MMC) is predominantly utilized as treatment for patients with intermediate and high risk NMIBC as well as for patients who recur or progress despite intravesical BCG therapy [6]. There have been no randomized controlled trials (RCTs) evaluating the clinical or economic benefit of mitomycin C intravesical therapy over radical cystectomy in high risk NMIBC populations largely due to the expected challenges in patient accrual, real ethical concerns, expected costs of such a trial, and the willingness of investigators to participate [7].
In response to the difficulty in conducting RCTs, virtual clinical trials, which utilize computer algorithms to simulate clinical outcomes, have been under development [8]. The Archimedes Model, a large-scale integrated simulation model of human physiology, diseases, interventions and healthcare systems, has the ability to incorporate co-morbid conditions as well as human behavior (i.e. compliance with treatments, maintaining physician appointments etc) into medical models [8]. The Archimedes model has previously been utilized to study cost effectiveness of breast cancer and colorectal cancer screening [9, 10]. Using the Archimedes model we simulated an RCT comparing radical cystectomy versus intravesical MMC therapy to evaluate the clinical and economic outcomes for BCG-refractory patients with NMIBC and to demonstrate the utility of computer based models to simulate a clinical trial.
METHODS
We evaluated the cost-effectiveness of treatments for BCG-refractory bladder cancer patients using the Archimedes Model, a large-scale integrated simulation model of human physiology, diseases, interventions and healthcare systems [8]. The core of the Archimedes Model is a set of mathematical equations that represent physiological outcomes at the clinical level (i.e., at the level of detail of basic medical tests, clinical trials, and patient charts) and care utilization in the health care system. Currently, the Archimedes Model integrates several medical conditions including diabetes, congestive heart failure, coronary artery disease, stroke, hypertension, obesity, asthma, COPD and cancers of the breast, lung, colon and bladder. The Archimedes Model furthermore has the ability to factor in co-morbid conditions into the model as well as incorporating aspects of human behavior (i.e. compliance with treatments, maintaining physician appointments etc).
The NMIBC model was developed by at team at Archimedes with a panel of bladder cancer experts and Endo Pharmaceuticals Inc. The model integrates information from many data sources including: Surveillance Epidemiology and End Results (SEER) database, patient level data from the Urocidintrademark clinical trial, meta-analyses from clinical trials, large retrospective datasets and expert opinions into an integrated framework Fig. 1. The supplementary appendix further details the components and assumptions made in the NMIBC model. The NMIBC model consists of the following components:
1) Patient generation: Virtual patient population, which is constructed from SEER case listings
2) Natural history: Natural history of NMIBC which captures recurrence, progression and survival. Figure 2 demonstrates pathways for tumor progression used in the model.
3) Intervention: Health care systems which captures interactions between patients and the health care system, clinical practices, and care interventions (drugs, diagnostic tests, etc)
4) Costs: tracks costs of procedures, tests, and treatments related to bladder cancer, thereby enabling economic analyses
Virtual trial setup
Population generation
Virtual NMIBC patients were constructed using SEER case listings. After initial diagnosis of NMIBC, patients were treated with standard of care as defined by the 2011 National Comprehensive Cancer Network (NCCN) guidelines and were given appropriate intravesical therapy based on NCCN guidelines. Patients selected for inclusion into the study were diagnosed with high risk NMIBC (<cT2 disease) who recurred (at least one bladder tumor of the same or lower histological grade and T-stage as the primary (initial) tumor) or progressed (diagnosis of a new tumor within the bladder that is of higher histological grade or T-stage than the primary tumor) despite BCG therapy. BCG recurrence was defined as tumor recurrence of high-grade cTa, cT1, or Tis NMIBC within 1 year of BCG therapy. All patients were assumed to have received intravesical therapy in accordance with the recommendations of the 2011 NCCN guidelines.
Trial arms
Simulated patients who met inclusion criteria were randomized to radical cystectomy or MMC induction intravesical therapy. Patients who were randomized to the MMC arm underwent surveillance and follow-up as recommended by the NCCN guidelines and may have undergone cystectomy if they developed either a new tumor of any stage or muscle invasive bladder cancer. We assumed 100% compliance to the treatment arms defined and followed patients until death.
Costs
The cost of tests, procedures, and treatments were based on Medicare reimbursement rates. A payor perspective was used with costs discounted by 3% . Results of the clinical trial simulation are imported into the Archimedes Outcomes Analyzer, a data-viewing tool that allows users to adjust certain parameters, such as costs and patient compliance. For the present study parameters included: population size, discount rate, and the cost of TURBT, cytology, cystoscopy, cystectomy, and intravesical therapies. The changes in health outcomes, cost and cost-effectiveness were performed. A separate analysis was performed that took into account the time value of money with a discount rate of 3% .
RESULTS
A total of 1300 virtual patients were eligible for the trial with a mean age of 69 and 73% of patients being male. Table 1 displays a summary of demographic and tumor characteristics. At initial diagnosis 51% of patients had cT1 disease, 46% of patients had more than one tumor, and 19% had concomitant CIS. Prior to the start of the virtual trial, 58% of patient had cT1 as their worse cTstage on TURBT with an average of 1.8 tumor recurrences, and an average tumor recurrence time of 0.4 years.
Clinical outcomes
Clinical outcomes for the immediate cystectomy and the conservative MMC treatment arms are summarized in Table 2. The progression to MIBC in the conservative treatment arm was 22% at year 1, 24% at year 3 and 27% at year 5, with an overall rate of 30% over the lifetime. The cumulative incidence of cystectomy in the conservative arm was 52.7% in year 1 and 62.8% at year 5. Disease specific death at 5 years was 1.6% and 8.7% for the immediate cystectomy and conservative treatment arms respectively; while, overall death was 17.8% and 23.8% at 5 years (Figs. 3 and 4). The lifetime risk of bladder cancer death of 14.2% vs. 1.9% in the MMC and RC arms respectively.
Economic outcomes
Over a 5-year period the average cost of cystectomy was $64,675, dominated by the cost of initial cystectomy; while, patients in the conservative arm incurred average total costs of $68,517 at 5 years. The cost of intravesical therapy at 5 years in the conservative group was $2365 at 5 years. Surveillance of NMIBC, which included TURBT at time of randomization, was $3086 vs. $7590 at 5 years for immediate cystectomy and conservative treatment arms respectively.
Clinical-economic outcomes
Table 3 summarizes the differences in clinical-economic outcomes. Over a 5-year time period immediate cystectomy saves 0.19 life years and $3585 in total bladder cancer cost compared to the conservative arm (assuming a 3% discount rate). Over a lifetime cystectomy saves 0.94 life years and $9937.
DISCUSSION
We report on the first virtual clinical trial in the bladder cancer literature to date. Using the Archimedes model, 1300 BCG refractory patients were randomized to RC or MMC while factoring in co-morbid conditions, natural history of disease, treatment characteristics, and aspects of human behavior. We found that 1 in 3 patients progressed to MIBC in the MMC arm with a lifetime risk of bladder cancer death of 14.2% vs. 1.9% in the RC arm. Overall economic outcomes were more favorable in the RC group with lifetime saving of 0.94 life years and $9937.
Due to the heterogeneity of and low number of clinical trials in BCG refractory patients, comparison of our study population with other series is difficult; however, the simulated study population has similar clinical characteristics to prior studies. The study population of 1300 virtual patients is significantly higher than most published series on BCG refractory patients which range from 20–100 patients [11–15]. Our virtual series contained 58% of patients with clinical stage T1 urothelial carcinoma prior to randomization which falls within the range of previous BCG failure trials where 47%–78% had cT1 tumors [11, 15]. Thirty-seven percent of patients had cTis prior to the start of the virtual trial which falls within the range of 31%–77% from prior studies [11, 15].
There was no progression to muscle invasion in the immediate cystectomy group as it was assumed that there was no upstaging to muscle invasion; while, there was a 27% and 30% progression to muscle invasion at 5 years and over lifetime, respectively, for patients in the MMC group. This is lower than in the recent Phase II clinical trial comparing salvage intravesical gemcitabine (33% progression) vs. additional induction BCG (37.5% progression) in patients with recurrent NMIBC despite BCG at 2 years follow-up [11]. The rate of cystectomy was 62% at 5 years which is higher than previously published clinical trials which range from 33% to 56% at a follow-up of 1.5 to 3 years [11–15]. The differences are largely attributable to the fact that in our series we assumed perfect compliance and follow-up as well as strict adherence to NCCN guidelines, which are more aggressive than real-world settings.
The cumulative incidence of death for the RC group was 17.8% (82% survival) at 5 years which is similar to previously reported studies which report 5 year survival of 80%–90% for patients who undergo RC with non-muscle invasive disease [4]. The cumulative incidence of death was higher (24% ) in the MMC group at 5 years and is certainly attributed to the 27% of patients who progress to MIBC. Overall, virtual patients in the present study have a more favorable survival compared with patients from other series. Lerner et al. performed a subgroup analysis of the SWOG 8507 trial for patients who did not completely respond to BCG therapy reporting 5 year overall survival rates of 33% and 50% for patients who were >61 years of age with prior intravesical chemotherapy vs. >61 years of age and no prior intravesical chemotherapy respectively [3].
Bladder cancer has been shown to be the most expensive cancer to treat from diagnosis to death because of the requirement of lifelong surveillance and treatment [16]. Kulkarni et al. conducted a cost-analysis of immediate RC vs. intravesical BCG therapy and demonstrated more favorable clinical outcomes and lower costs with immediate RC in healthy patients age of <60 years; while, the most cost effective therapy in patients aged >70 was initial intravesical therapy. The present series revealed a more favorable cost for immediate RC over MMC with $9937 savings over a lifetime. Most of the costs associated with immediate RC were realized upfront at the time of cystectomy with an average cost of $61,997 at year one. While the average age of the population was 70 years old, there was still a cost benefit with more aggressive therapy with RC.
While the Archmides model incorporated data from several established sources (SEER, Urocidin trial, BCG failure clinical trials), the results rely on computer algorithms, which are dependent on the manner in which the mathematical models were programmed and on various clinical assumptions made (i.e. performance of TURBT to remove all disease, efficacy of MMC etc.). The reproducibility of the results generated from such a computer-based model created by a different company/programming group would be difficult. The present study assumed perfect compliance and follow-up per NCCN guidelines, which are more aggressive than real-world settings and likely resulted in more favorable simulated clinical outcomes. In the immediate cystectomy group there it was assumed that there was no upstaging to muscle invasion, which may favorably bias the survival in this arm. Although cystectomy appears favored based on the clinical/economic data, we did not study impacts on QOL based health related outcomes (largely related to sexual dysfunction and urinary diversions).
With improved computing ability and processing technology, virtual trials such as the current study may be a supplement and possibly an alternative to performing traditional clinical trials, which can be cost-prohibitive, have poor accrual, and require a long duration for results. Perhaps virtual clinical trials could be performed as an initial feasibility assessment prior to dedicating time, effort, and financial resources for a dedicated RCT. This simulation lends support to utilizing the Archimedes model prior to undertaking any clinical treatment trial to assess the feasibility and likelihood of a clinically and statistically meaningful result. While results of this virtual trial seem promising, they would still need to be confirmed in a real world setting. Nonetheless, the simulation environment can overcome some of the shortcomings of real-world clinical trials such as the inability to have a placebo arm, incorrect enrollment of patients (e.g., due to sites not properly following inclusion/exclusion criteria), missing data, protocol deviations, failure to achieve expected accrual. Virtual trials can also help to fill in the “gaps” in the clinical trials landscape specifically for certain therapies not tested in the literature, elderly population or other populations not previously investigated, certain combinations of therapies not previously tested, and trials precluded due to ethical restrictions, different protocols/care guidelines, and cost.
FUNDING
None.
Appendices
The supplementary Appendix is available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-150020.
REFERENCES
1 | Babjuk M, Oosterlinck W, Sylvester R(2011) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the updateEuropean Urology59: 9971008 |
2 | Yates DR, Brausi MA, Catto JWF(2012) Treatment options available for bacillus Calmette-Guérin failure in non-muscle-invasive bladder cancerEuropean Urology62: 10881096 |
3 | Lerner SP, Tangen CM, Sucharew H(2009) Failure to achieve a complete response to induction BCG therapy is associated with increased risk of disease worsening and death in patients with high risk non-muscle invasive bladder cancerURO27: 155159 |
4 | Stein JP, Lieskovsky G, Cote R(2001) Radical cystectomy in the treatment of invasive bladder cancer: Long-termresults in 1,054 patientsJournal of Clinical Oncology19: 666675 |
5 | Kulkarni GS, Alibhai SMH, Finelli A(2009) Cost-effectiveness analysis of immediate radical cystectomy versus intravesical Bacillus Calmette-Guerin therapy for high-risk, high-grade (T1G3) bladder cancerCancer115: 54505459 |
6 | Sylvester RJ, Oosterlinck W, van der Meijden APM(2004) A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: A meta-analysis of published results of randomized clinical trialsThe Journal of Urology171: 21862190 |
7 | Malmström PU, Wijkström H, Lundholm C(1999) 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study GroupJURO161: 11241127 |
8 | Schlessinger L, Eddy DM(2002) Archimedes: A new model for simulatinghealth care systems— the mathematical formulationJournalof Biomedical Informatics35: 13750 |
9 | Dinh TA, Rosner BI, Atwood JC(2011) Health benefits andcost-effectiveness of primary genetic screening for Lynch syndromein the general populationCancer Prevention4: 1922 |
10 | Noah-Vanhoucke J, Green LE, Dinh TA(2011) Cost-effectiveness of chemoprevention of breast cancer using tamoxifen in a postmenopausal US populationCancer117: 33223331 |
11 | Di Lorenzo G, Perdonà S, Damiano R(2010) Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancerCancer116: 18931900 |
12 | Gunelli R, Bercovich E, Nanni O(2007) Activity of endovesical gemcitabine in BCG-refractory bladder cancer patients: A translational studyBr J Cancer97: 14991504 |
13 | Steinberg G, Bahnson R, Brosman S(2000) Efficacy and safety ofvalrubicin for the treatment of Bacillus Calmette-Guerinrefractory carcinoma in situ of the bladder. The Valrubicin Study GroupJURO163: 761767 |
14 | Gacci M, Bartoletti R, Cai T(2006) Intravesical Gemcitabine in BCG-Refractory T1G3 Transitional Cell Carcinomaof the Bladder: A Pilot StudyUrologia Internationalis76: 106111 |
15 | Dalbagni G, Russo P, Bochner B(2006) Phase II trial of intravesical gemcitabine in bacille Calmette-Guérin–refractory transitional cell carcinoma of the bladderJ Clin Oncol24: 1827292734 |
16 | Botteman MF, Pashos CL, Redaelli A(2003) The health economics of bladder cancer: A comprehensive review of the published literaturePharmacoeconomics21: 13151330 |
Figures and Tables
Fig.1
Clinical Progression Framework.
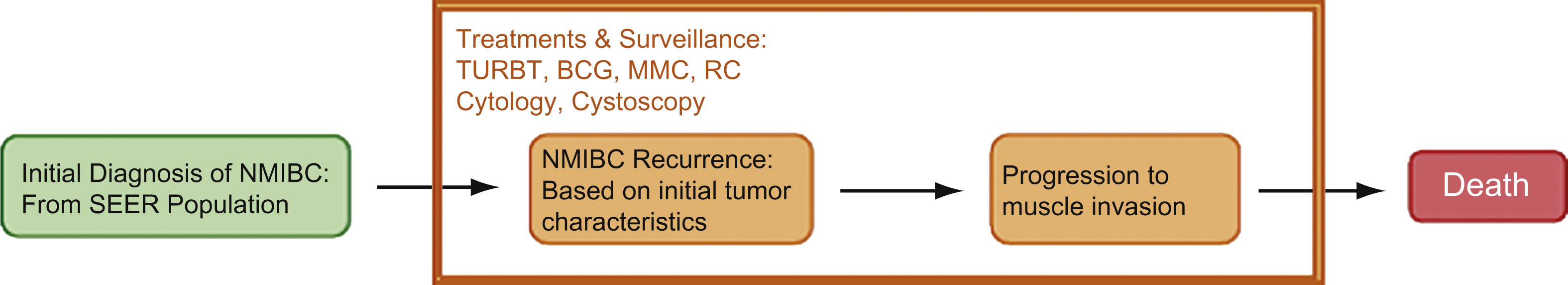
Fig.2
Pathways for Tumor Progression in Non-muscle Invasive Bladder Cancer. LG – Low Grade, HG – High Grade, CIS – Carcinoma in Situ.
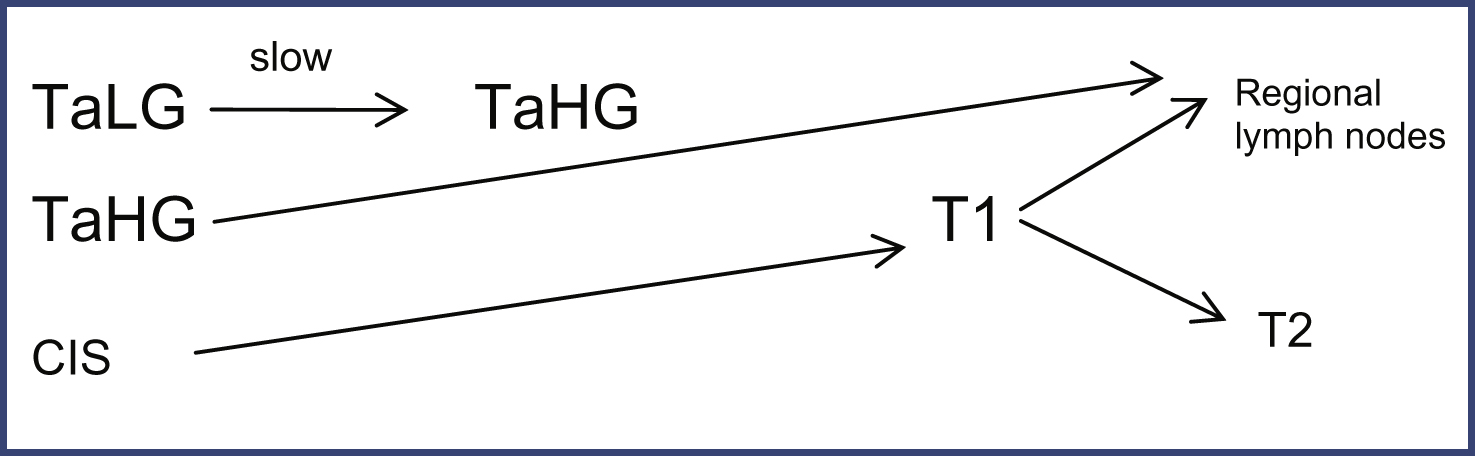
Fig.3
Cumulative risk of bladder cancer death.
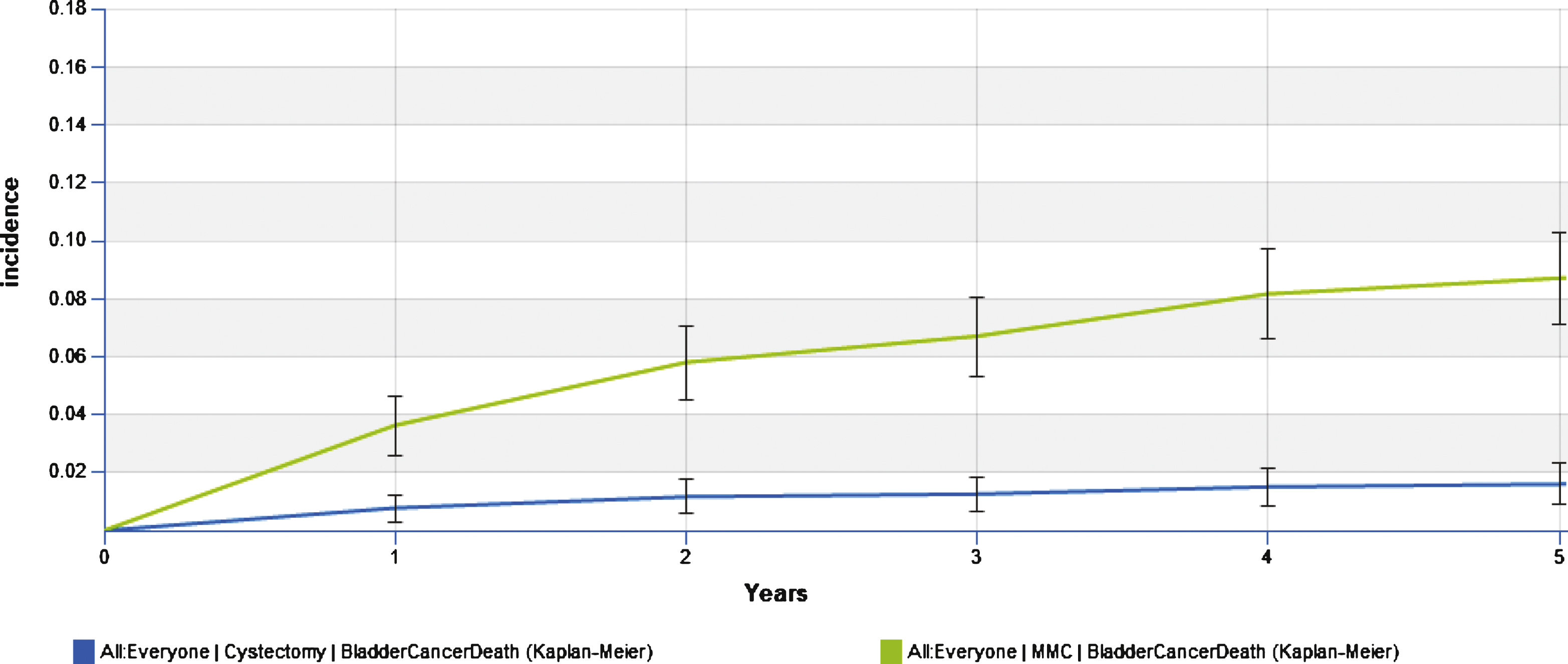
Fig.4
Cumulative risk of overall death.
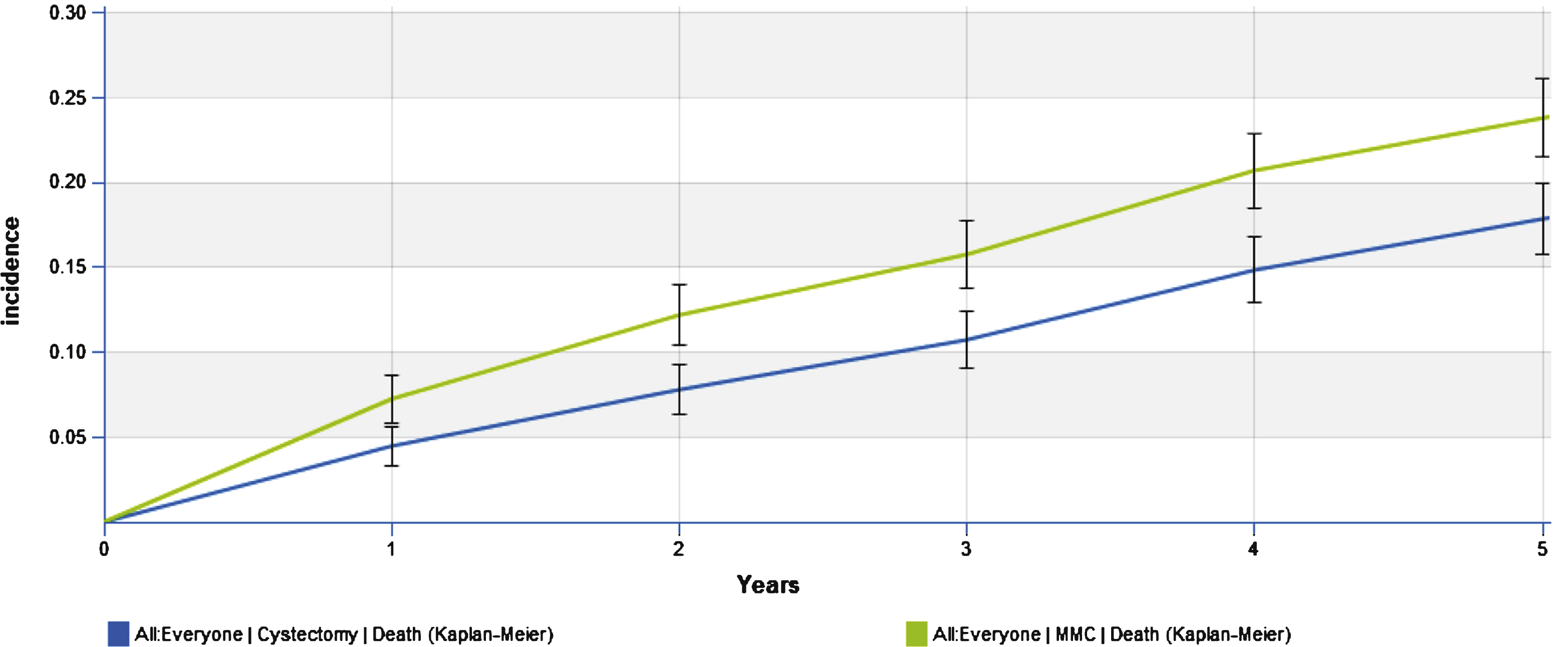
Table 1
Patient characteristics
| Risk factors | Virtual trial | ||
| Demographics | Population size | 1300 | |
| Average age at primary diagnosis of NMIBC (years) | 69 | ||
| Male fraction | 73% | ||
| Tumor characteristics at initial | Distribution of | Ta | 39% |
| T-stage at | Tis | 10% | |
| diagnosis of NMIBC | primary diagnosis | T1 | 51% |
| Fraction of patients with concomitant CIS at primary diagnosis | 19% | ||
| Fraction of patients with 2 or more tumors | 46% | ||
| Disease history | Worst T-stage prior to trial | TaHG | 22% |
| start (including tumors found | Tis | 20% | |
| at the last TURBT) | T1 | 58% | |
| Average number of | 1.8 | ||
| tumor recurrences | |||
| prior trial start | |||
| Average time form | 0.4 | ||
| previous tumor recurrence | |||
| to trial start (years) | |||
| Characteristics of tumors | T-stage of tumors | TaHG | 49% |
| found by the last TURBT | CIS | 37% | |
| prior to trial start | T1 | 14% | |
| 2 or more tumors | 14% | ||
| Tumor size | >3cm | 46% |
Table 2
Clinical outcomes for cystectomy and conservative treatment arms
| Outcomes (per persons) | Immediate cystectomy arm | Conservative treatment arm | |
| Cumulative incidence of progression to MIBC | Year 1 | 0% | 22% |
| Year 5 | 0% | 27% | |
| Life time | 0% | 30% | |
| Cumulative incidence of bladder cancer death | Year 1 | 0.8% | 3.6% |
| Year 5 | 1.6% | 8.7% | |
| Life time | 1.9% | 14.2% | |
| Cumulative incidence of death | Year 1 | 4.5% | 7.26% |
| Year 5 | 17.8% | 23.8% | |
| Life time | 100% | 100% | |
| Cumulative incidence of cystctomy | Year 1 | 100% | 52.7% |
| Year 5 | 100% | 62.8% | |
| Year 30 | 100% | 68.8% | |
| Cumulative bladder cancer costs (not discounted) | Year 1 | $64,083 | $55,265 |
| Year 5 | $64,675 | $68,517 | |
| Life time | $64,798 | $77,174 | |
| Total costs of surveillance of NMIBC (TURBT, cytology and cystoscopy) * | Year 1 | $3,086 | $5,886 |
| Year 5 | $3,086 | $7,590 | |
| Life time | $3,086 | $9,407 | |
| Total costs of intravesical therapies (MMC and BCG) | Year 1 | $0 | $1,791 |
| Year 5 | $0 | $2,049 | |
| Life time | $0 | $2,365 | |
| Total costs of cystectomy, MIBC treatment and bladder cancer death | Year 1 | $60,997 | $47,589 |
| Year 5 | $61,589 | $58,879 | |
| Life time | $61,712 | $65,402 |
Table 3
Clinical and economic outcomes associated with bladder cancer between immediate cystectomy and conservative treatment
| Difference in economic outcomes between immediate cystectomy arm and conservative arm and conservative treatment arm | |
| Delay (in years) of cystectomy for patients undergoing cystectomy in the conservative treatment arm | Mean: 1.1 |
| Median: 0.3 | |
| Average life years gained per person | 5 Year time horizon: 0.22 LY |
| Lifetime: 1.4 LY | |
| Average discounted life years gained per person (3% annually) | 5 year time horizon: 0.19 LY |
| Lifetime: 0.94 LY | |
| Average bladder cancer cost saved per person | 5 year time horizon: $3,842 |
| Lifetime: $12,375 | |
| Average discounted bladder cancer cost saved per person (3% annually) | 5 year time horizon: $3,585 |
| Lifetime: $9,937 | |



