Combination of Ruthenium Dendrimers and Acoustically Propelled Gold Nanowires as a Platform for Active Intracellular Drug Delivery Towards Breast Cancer Therapy
A B S T R A C T
In this work, a new class of fluorescently labeled metallodendrimers based on ruthenium and possessing anticancer activity (FITC-CRD13) is combined with graphene oxide modified gold nanowires (GO-AuNWs). The resulting complexes were tested as active intracellular transporters being propelled by ultrasound field (US) and using breast cancer cells as a model. Energy dispersive X-ray spectroscopy analysis confirmed the successful modification of GO-AuNWs by dendrimers as shown by the uniform presence of ruthenium over the nanomotor structure corresponding to the ruthenium groups of FITC-CRD13. The binding of dendrimers to the surface of GO-AuNWs was accompanied by quenching their fluorescence signal. Upon the application of an ultrasound field (5 min, 2 V, 2.66 MHz), the complexes were propelled towards MCF7 breast cancer cells, detaching from the GO-nanomotor surface and thus recovering the dendrimer fluorescence signal. Fluorescence signal from US-treated samples was ~1.8 fold higher compared to passive controls. The results obtained in this work suggest that US-propelled AuNWs lead to faster cell internalization, hence accelerating the delivery of the carbosilane ruthenium dendrimers (CRD) payload inside MCF7 cells.
Keywords
Ruthenium-dendrimer, nanomotor, ultrasound, fluorescence, cancer
Introduction
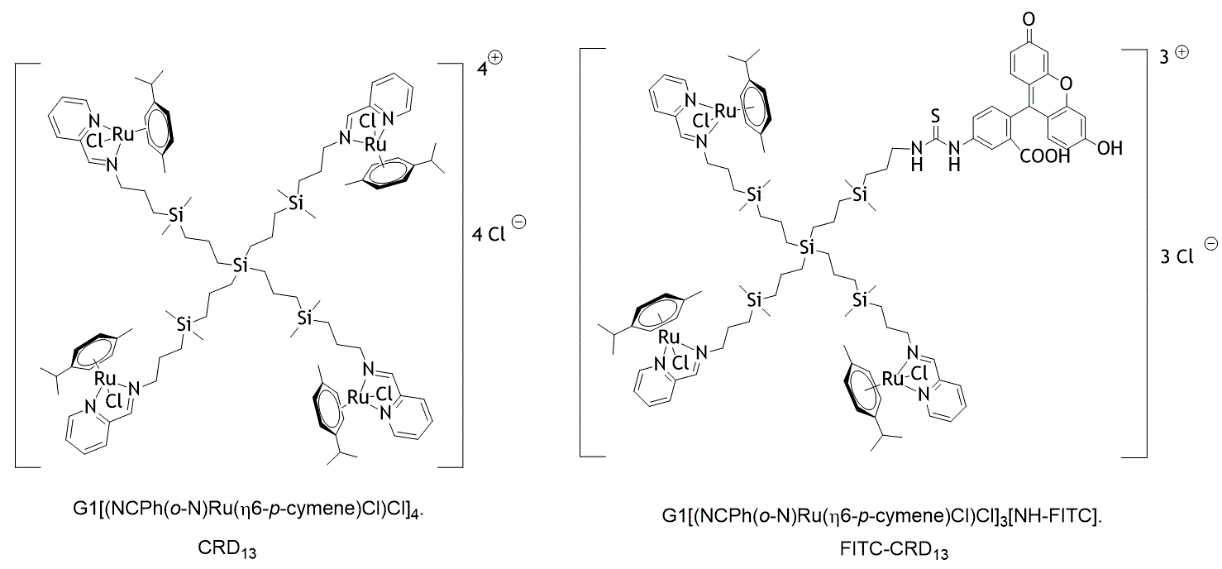
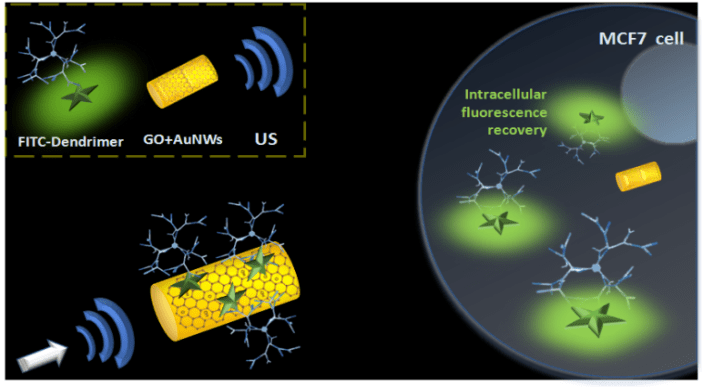
Dendrimers are highly branched nanoparticles with unique molecular properties, which make them promising carriers for delivery of various therapeutically active compounds into the disease affected cells. Recently, a new class of carbosilane metallodendrimers based on ruthenium (CRD) possessing anticancer activity has been synthesized [1-4]. At the same time, another group of engineered nanostructures named gold nanowires (AuNWs) have been intensively studied during the last years as active intracellular transporters (nanomotors) when placed under ultrasound (US) fields [5-7]. US-propelled nanomotors are attractive candidates to overcome biological barriers and physical limitations commonly faced by dendrimer carrier/cargo itself. The structure of CRD13 and fluorescently labeled ruthenium dendrimer (FITC-CRD13) is illustrated in (Figure 1). The proposed and inspected experimental concept is schematically illustrated in (Figure 2). Here, the FITC-CRD13 dendrimer is combined with graphene oxide (GO) modified-AuNWs [4]. GO is a two-dimensional crystal structure formed by a flat monolayer of carbon atoms arranged in a hexagonal lattice possessing many unique properties such as large surface area, good hydrophilicity and tunable optical properties. A large amount of oxygen-containing functional groups on the GO nanosheets provide easy modification of GO for various applications. For example, GO has been widely used in optically based biosensing applications due to its fluorescence quenching properties [6, 7]. Such fluorescence quenching properties have been used also in this work, being investigated in combination with the propulsion of US-propelled gold nanowires.
Briefly, when FITC-CRD13 binds to the GO sheets immobilized on the surface of AuNWs, the dendrimer fluorescence is quenched. Upon the application of an ultrasound field, FITC-CRD13-GO-AuNWs are acoustically propelled towards MCF7 breast cancer cells and actively internalized. Once inside the cell, the FITC-CRD13 quenched dendrimer detaches from the GO-nanomotor surface and the fluorescence signal is recovered (Figure 2). Detachment of denderimer from the surface of nanomotor might occur as a result of a complex intracellular network pathway, which is dependent on various determinants [8]. The most probably, due to the similarities with iron, ruthenium derivatives can specifically be transported towards tumor cells by transferrin receptor mediated endocytosis since these receptors are overexpressed on cancer cells. The receptor-mediated incorporation might result in the formation of disruptive endosomes (based on low endosomal pH~5.5) and/or is followed by interaction with DNA, two of the possible events that might take place and contribute to the further release of dendrimers from the nanomotor surface [9]. As a result, a recovery of the quenched fluorescence of dye-labeled dendrimer can be observed.
Figure 1: Structure of the carbosilane Ru(II)-metallodendrimers.
Material and Methods
I Dendrimers
Fluorescently labeled dendrimer (FITC-CRD13) was synthetized at the Organic and Inorganic Chemistry Department, University Alcala, Spain. Detailed synthesis of CRD13 and its heterofunctional ruthenium-terminated carbosilane dendrimer with FITC (FITC-CRD13) are described in [1, 4]. FITC-CRD13 with molecular weight 2234,66 g/mol was dissolved in H2O/DMSO (9/1) solution at 1 mM concentration and stored in the dark condition at 4°C before use.
Figure 2: Schematic illustration of ultrasound-propelled graphene oxide (GO) modified nanowires (AuNWs) loaded with fluorescently labeled ruthenium carbosilane dendrimers (FITC-CRD13) towards internalization in MCF7 breast cancer cells and active intracellular delivery of the loaded dendrimer.
Such combined system of anticancer dendrimers and active gold nanomotors gains a lot of potential for a) higher payloads loading and b) rapid intracellular delivery of therapeutic material; both representing important advantages for the potential treatment of many diseases.
II Gold nanowires (AuNWs) fabrication
Gold nanowires were prepared based on a template-directed electrodeposition protocol. A thin gold film was sputtered on one side of the porous polycarbonate membrane template containing 400 nm diameter pores to serve as a working electrode. This membrane was then assembled in a Teflon plating cell with aluminum foil serving as an electrical contact for the subsequent electrodeposition. Firstly, a sacrificial Ag layer was electrodeposited using a charge of 0.1 C and a potential of -0.90 V (vs an Ag/AgCl reference electrode, along with a Pt-wire as a counter electrode). Au was plated using commercial gold plating at -1 V using a charge of 1.5 C. The membrane was then mechanically polished using cotton tip applicators and alumina powder 3-4 μm. To remove any remaining Ag, a pure 8 M HNO3 solution was applied. The membrane was then dissolved in methylene chloride solution, followed by washing steps in isopropanol, ethanol and water (centrifuging 3 min at 8000 rpm between each washing step). The resulting AuNWs were separated from the solution by centrifugation (8000 rpm for 3 min) and washed repeatedly with ultrapure water. Between washing steps, the gold nanowires solution was briefly sonicated to ensure complete dispersion of nanomotors. All AuNWs were stored in ultrapure water at room temperature until use. All standard chemicals were of p.a. grade and used as supplied.
III Modification of gold nanowires with graphene oxide (GO)
The prepared AuNWs were covalently modified with GO through cysteamine linkage via carbodiimide chemistry using EDC/NHS coupling agents. AuNWs were firstly centrifuged (8000 rpm, 3 min), washed in ultrapure water and incubated overnight with 2 mM cysteamine solution, under gentle shaking. After the overnight incubation, cysteamine modified nanowires were washed 2 times with water (centrifuged, sonicated 3 s, vortexed) and incubated (1 h, under shaking) with a previously activated (during 30 min) GO solution: 0.005 mg/ml GO + 400 mM EDC + 100 mM NHS in MES buffer. GO-modified AuNWs were then washed twice in ultrapure water (being centrifuged, sonicated 3s, vortexed). Re-dispersed pellets of nanowires were characterized by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy analysis (EDX). SEM images of fabricated AuNWs were captured using a FEI Quanta 250 ESEM instrument (Hillsboro, Oregon, USA), using an acceleration voltage of 10 kV. EDX mapping analysis was carried out with an Oxford EDS detector attached to SEM instrument and controlled by Pathfinder software.
IV Modification of GO-nanowires with dendrimers
GO-modified AuNWs were then subjected for non-covalent functionalization with various concentrations of FITC-CRD13 dendrimer (10 μM, 100 μM) prepared in TRIS working buffer (Tris-HCl 20 mM, NaCl 100 mM, KCl 5 mM and MgCl2 5 mM, pH 7.4) by their simply mixing and overnight incubation at room temperature. Formed complexes were purified from unbound constituents by intensive centrifugation (no sonication step) using washing buffer (Tris-HCl 20 mM, NaCl 100 mM, EDTA 1 mM supplemented with 100 µg/mL of BSA, pH 7.4) until the fluorescence signal in supernatant reached the minimal background value. Final purified concentration of dendrimers was not determined. Prepared dendrimer-modified gold nanowires (FITC-CRD13+GO-AuNWs) were then tested for their ultrasound propelled delivery towards MCF7 breast cancer cells.
V Human breast cancer cells MCF-7
MCF-7 cells were purchased from the American Type Culture Collection (ATCC). They were cultured in Dulbecco´s Modified Eagle´s Medium (Hyclone) supplemented with 10% fetal bovine serum (FBS, Hyclone) and 1% penicillin/streptomycin (Thermo Fisher Scientific) at 37°C with 5% CO2. When the cells reached the confluence of 80%, they were subcultured by using the Trypsin-EDTA (Thermo Fisher Scientific, USA) and counted by cell counter (Fisher Scientific) for further applications. To determine the intracellular level of ruthenium dendrimers being combined with gold nanowires and propelled by ultrasound, mixture of 2 μl of the cell suspension (~1.0 106 cells/ml) and 2 μl of complexes (FITC-CRD13+GO-AuNWs) were prepared 2 times followed by the 5 min application of ultrasoud (2V, 2.66 MHz). Samples (8 μl) were then immersed into the 100 μl of growth media, placed into the 96 black well plate and fluorescence signal was checked at various time points (0.5 h, 4 h, overnight) using the fluorescence microplate reader. As a control, static samples without US treatment were involved; all performed in triplicates. After overnight incubation at 37 °C, the cells were visualized using the EVOS FL microscope (Thermo Fisher Scietific).
VI Ultrasound equipment
The acoustic cell setup consisted of a piezoelectric transducer (Ferroperm PZ26 disk 10 mm diameter, 0.5 mm thickness) responsible for the generation of ultrasound waves, attached by conductive epoxy glue to the bottom center of a steel plate (50 × 50 × 0.94 mm3); then the steel plate was covered with a 240 μm kapton tape protective layer and a sample reservoir at the center (5 mm). A glass slide was used to cover the reservoir for ultrasound reflection and to protect the sample. The continuous ultrasound sine wave was applied via a piezoelectric transducer, through an Agilent 15 MHz arbitrary waveform generator, in connection to a homemade power amplifier. The applied continuous sine waveform had a frequency of 2.66 MHz and voltage amplitude 2V.
Results and Discussion
The main idea of this work was to prepare and characterize a novel nanomotor-based platform suitable for higher payload and accelerated drug delivery towards cancer cells. We were focused to newly synthesized carbosilane dendrimers with ruthenium arene fragments [1]. Carbosilane dendrimers are water-soluble, monodisperse and stable molecules that are able to carry various therapeutically active compounds and genes as previously described [2]. Integrating ruthenium into their structure makes them promising drug delivery system with improved antitumor potential [3]. Therapeutically active compounds as well as drug carriers needs to face and deal with various physical limitations and biological barriers that appear on their delivery route before reaching the desired targets. Accelerated delivery of transported material represents a helpful strategy to overcome these limitations. Therefore, we combined ruthenium dendrimers with acoustically propelled gold nanowires, which are intensively studied in the field of drug delivery as active transporters called nanomotors [5-7]. Nanomotors in general, are micro/nanoscale structures able to convert chemical or external energy into mechanical motion. We have focused this strategy to acoustically propelled nanomotors. Ultrasound, with major advantages of long lifetime, noninvasive, contact free, and great biocompatibility, have been one of the most probable potential toward in vivo drug delivery and clinical diagnosis [10].
I Fabrication and characterization of GO-modified gold nanowires with immobilized FITC-CRD13 ruthenium dendrimers
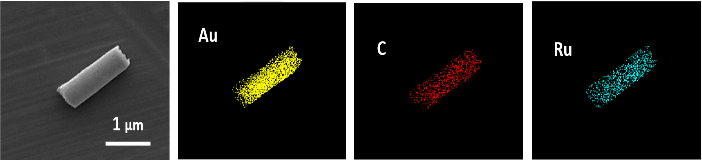
As a first step, we prepared gold nanowires (AuNWs) following the template electrodeposition method described above. To examine the structural morphology and material analysis of the nanowires, scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) analysis were performed. SEM image of a gold nanowire shows a wire with approximately 1.7 μm length and diameter of 400 nm. To note, diameter reflects the pore size of the polycarbonate membrane template used during fabrication process. As a second step, gold surface of AuNWs was functionalized with graphene oxide (GO). This was done via the covalent linkage with pre-self-assembled cysteamine molecules via carbodiimide chemistry. Using EDC/NHS coupling agents, cysteamine amine groups were coupled with the carboxylic acid groups of GO. Immobilized GO provides a suitable scaffold for the further adsorption/electrostatic immobilization of cargo molecules onto the surface of AuNWs. As a third immobilization step, positively charged ruthenium dendrimers were attached to the GO-AuNWs by mean of non-covalent interactions. EDX analysis demonstrated the successful composition and distribution of the functionalized nanowires as shown by the presence and distribution of elemental gold (yellow) and carbon (red). Modification of nanowires with dendrimers is confirmed from the presence of ruthenium corresponding to the ruthenium groups of FITC-CRD13 (blue) as well as from the quenched fluorescence signal upon the interaction with GO; carbon-based materials such as GO are known as common fluorescence quenchers.
Figure 3: Scanning electron microscopy and Energy dispersive X-ray (EDX) material analysis of graphene oxide modified gold nanowires with immobilized FITC-CRD13 ruthenium dendrimers. EDX images show the presence and distribution of gold (Au; yellow), carbon (C; red), and ruthenium (Ru; blue).
II Fluorescence based study of ultrasound propelled delivery of gold nanowires with immobilized Ruthenium dendrimers
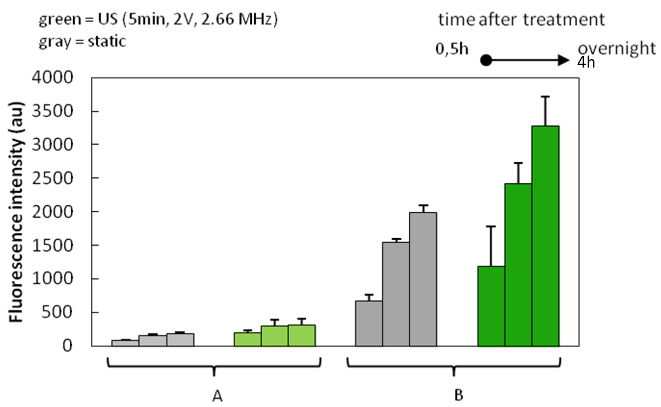
After the successful fabrication, purification and characterization of GO-modified gold nanowires with the confirmed presence of ruthenium dendrimers, prepared conjugates were further examined for their ultrasound propelled delivery toward breast cancer cells. Conjugates were introduced into the solution of MCF cells followed by the application of an ultrasound field (5 min, 2 V, 2.66 MHz) or left as US-untreated (considered as static conditions). Figure 4 shows results from the fluorescence intensity measurements that were carried out at various time points (0,5 h, 4 h, overnight) recorded after the US-(non)treatment.
Figure 4: Fluorescence intensity of MCF7 breast cancer cells incubated with GO-AuNWs nanomotors modified with fluorescently labeled ruthenium dendrimers FITC-CRD13. Initial concentrations of dendrimers used during the conjugation process were A) 10 μM and B) 100 μM upon the application of an ultrasound field (green; 5 min, 2 V, 2.66 MHz) or at static conditions (gray) reported at various recording time points (0,5h, 4h, overnight). It can be seen that US-treated samples had higher fluorescence intensity in comparison with the static controls. The fluorescence signal from US-treated samples was ~1.8 fold higher compared to that obtained from passive controls. This suggests that application of ultrasound accelerated delivery and decomplexation of dendrimers from the gold nanowires, leads to the higher fluorescence recovery detection. Fluorescence intensity increased also with increasing reading time, indicating continuous process of decomplexation and cellular internalization. Like iron, ruthenium can form complexes with transferrin and albumin. While binding to albumin may contribute to the targeted delivery of ruthenium compounds to cancer tissues due to the combination of leaky blood capillaries and lack of lymphatic drainage in tumors, binding to transferrin may constitute an “active” targeting route. In fact, the iron transport protein transferrin (Tf) is crucial in tumor development since highly proliferative tumors have higher demand for iron than normal tissues, resulting in the overexpression of the transferrin receptor. The receptor-mediated incorporation of Tf results in the formation of endosomes having low pH (ca. 5.5) with respect to the physiological one, which is supposed to trigger the release of the ruthenium compounds inside the cells [8]. In the cancer cells, like other metals with anticancer properties, ruthenium can interact and damage DNA [8, 9]. Taking all together, an accumulation pathway for ruthenium compounds inside the cells is a highly complex network which is dependent on various determinants [8].
By increasing the initial concentration of dendrimer (the concentration used for complexation; the final purified concentration was not determined) from 10 μM to 100 μM, fluorescence signal increased; samples with higher initial concentration were then used for fluorescence microscopy analysis.
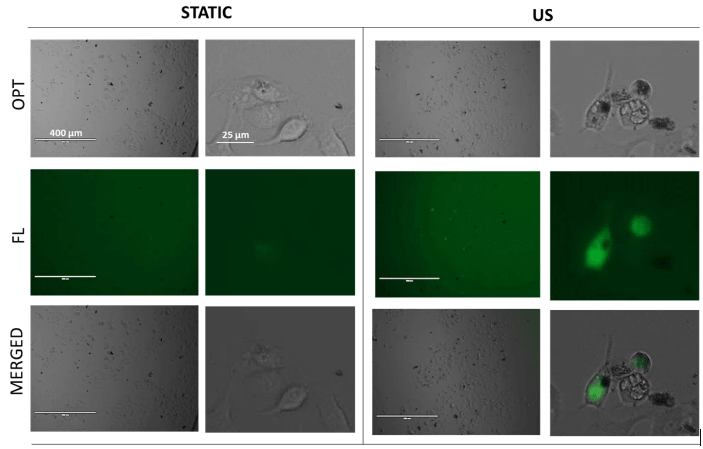
Figure 5: Overnight reported fluorescence intensity of MCF7 breast cancer cells incubated with GO-AuNWs nanomotors modified with fluorescently labeled ruthenium dendrimers FITC-CRD13; with initial conjugation concentration of dendrimers 100 μM, at static conditions and upon the application of ultrasound (US; 5 min, 2V, 2.66 MHz).
Figure 5 shows MCF7 cells overnight incubated with prepared complexes being US-non-treated (static) and those undergoing 5 min application of US treatment. For static samples, low fluorescence recovery was observed. On the other hand, cells treated with ultrasound propelled nanomotors displayed higher fluorescence recovery signal; fluorescence was observed inside the cells, suggesting successful and accelerated cellular internalization of dendrimers.
Conclusion
To summarize, a new class of fluorescently labeled ruthenium dendrimers FITC-CRD13 was combined with graphene oxide modified gold nanowires (GO-AuNWs). Such combination was performed with the aim to create anticancer drug delivery platform possessing higher payload, acceleration and fluorescence based turn off/on signal biodetection. Energy dispersive X-ray spectroscopy analysis confirmed successful modification of nanowires by dendrimers. In fluorescence-based study of the acoustically propelled FITC-CRD13+GO-AuNWs, approximately ~1.8 fold higher fluorescence recovery of US-treated samples compared to passive controls was observed, suggesting that ultrasound propulsion leads to faster cell internalization, hence accelerating the delivery of CRD13 dendrimers inside MCF7 breast cancer cells.
Acknowledgement
This work was supported by the European Commission within the project FORMILK under grant agreement number 690898/H2020-MSCA-RISE-2015, and by Slovak Research and Development Agency, APVV (Projects No. SK-PL-18-0080 and APVV-14-0267) and by Polish Ministry of Science and Higher Education). This work was also supported by CTQ2017-86224-P (MINECO), Consortium IMMUNOTHERCAN-CM B2017/BMD-3733 (CAM), NANODENDMED II-CM ref B2017/BMD-3703 and project SBPLY/17/180501/000358 JCCM and CCGP2017-EXp/023(UAH). CIBER-BBN is an initiative funded by the VI National R&D&i Plan 2008–2011, Iniciativa Ingenio 2010, Consolider Program, CIBER Actions and financed by the Instituto de Salud Carlos III with assistance from the European Regional Development Fund. This work has been supported partially by a EUROPARTNER “Strengthening and spreading international partnership activities of the Faculty of Biology and Environmental Protection for interdisciplinary research and innovation of the University of Lodz Programme: NAWA International Academic Partnership Programme”. This article/publication is based upon work from COST Action CA 17140 "Cancer Nanomedicine from the Bench to the Bedside" supported by COST (European Cooperation in Science and Technology). Gulcin Bolat acknowledges the postdoctoral fellowship from the Scientific and Technological Research Council of Turkey (TUBITAK 2219).
Article Info
Article Type
Short CommunicationPublication history
Received: Tue 20, Aug 2019Accepted: Fri 13, Sep 2019
Published: Wed 20, Nov 2019
Copyright
© 2023 Tibor Hianik. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2019.04.08
Author Info
Berta Esteban-Fernández de Ávila Hua Gong Joseph Wang Natalia Sanz del Olmo Paula Ortega Francisco Javier de la Mata Gulcin Bolat Sylwia Michlewska Tibor Hianik Zuzana Garaiová
Corresponding Author
Tibor HianikFaculty of Mathematics, Physics and Informatics, Comenius University, Bratislava, Slovakia
Figures & Tables
References
- Maroto-Diaz M, Elie BT, Gómez-Sal P, Pérez-Serrano J, Gómez R et al. (2016) Synthesis and anticancer activity of carbosilane metallodendrimers based on arene ruthenium (II) complexes. Dalton Trans 45: 7049-7066. [Crossref]
- Michlewska S, Ionov M, Maroto-Diaz M, Szwed A, Ihnatsyeu-Kachan A et al. (2018) Ruthenium dendrimers as carriers for anticancer siRNA. J Inorg Biochem 181: 18-27.
- Michlewska S, Ionov M, Maroto-Diaz M, Szwed A, Ihnatsyeu-Kachan A et al. (2019) Ruthenium dendrimers against acute promyelocytic leukemia: in vitro studies on HL-60 cell. Future Med Chem 11: 1741-1756. [Crossref]
- Michlewska S, Kubczak M, Maroto-Díaz M , Sanz Del Olmo N, Ortega P et al. (2019) Synthesis and characterization of FITC labelled ruthenium dendrimer as a perspective anticancer drug. Biomolecules 9. [Crossref]
- Abdelmohsen Loai KEA, Peng F, Tu Y, Wilson DA (2014) Micro- and nano-motors for biomedical applications. J Mat Chem B 2: 2395-2408.
- Esteban-Fernández de Ávila B, Martín A, Soto F, Lopez-Ramirez MA, Campuzano S et al. (2015) Single cell real-time miRNAs sensing based on nanomotors. ACS Nano 9: 6756-6764. [Crossref]
- Esteban-Fernández de Ávila B, Angel C, Soto F, Lopez-Ramirez MA, Báez DF et al. (2016) Acoustically propelled nanomotors for intracellular siRNA delivery. ACS Nano 10: 4997-5005. [Crossref]
- Spreckelmeyer S, Orvig Ch, Casini A (2014) Cellular transport mechanisms of cytotoxic metallodrugs: An overview beyond cisplatin. Molecules 19: 15584-15610. [Crossref]
- Chen H, Parkinson JA, Parsons S, Coxall RA, Gould RO et al. (2002) Organometallic ruthenium(II) diamine anticancer Ccomplexes: Arene-nucleobase stacking and stereospecific hydrogen-bonding in guanine adducts. J Am Chem Soc 124: 3064-3082. [Crossref]
- Xu T, Xu LP, Zhang X. (2017) Ultrasound propulsion of micro-/nanomotors. Appl Mat Today 9: 493-503.
