Single Nucleotide Polymorphisms in COL1A2 Gene and Dental Fluorosis Among 4 and 8-Year-Old Nigerian Children
A B S T R A C T
Aim: To determine the association between single nucleotide polymorphisms (SNPs) within the COL1A2 gene and dental fluorosis among 4- and 8-year-old Nigerian children.
Methods: A cross-sectional study was undertaken among 125 four and eight-year-old Nigerian children living in naturally fluoridated areas of Ibadan, Nigeria. Drinking and cooking water samples were collected for F analysis. Buccal mucosa swabs were collected from all children and genomic DNA extracted. Presence or absence of the SNP within the COL1A2 gene was identified by PCR and DNA sequencing for 70 of the participants.
Results: The median (minimum, maximum) F concentration of drinking and cooking water were 0.05 (<0.1, 3.0) mg/L and 0.01 (<0.1, 4.0) mg/L respectively. The majority of the study participants (52.9%) were heterozygous for the SNP. There was a statistically significant association between F concentration in drinking water and the occurrence of dental fluorosis (p=0.04). F concentration in drinking water was the only statistically significant predictor of dental fluorosis (p=0.03, OR=3.64(CI=1.11-11.94)) after adjusting for F concentration in cooking water and SNPs. The risk of dental fluorosis tended to increase with the presence of SNPs AA and AC (RR > 1) but this association was not statistically significant.
Conclusion: The majority of the study participants had the heterozygote SNP AC genotype of COL1A2 gene. F concentration in drinking water was the only statistically significant predictor of dental fluorosis. The risk of dental fluorosis tended to increase with the presence of SNPs AA and AC (RR > 1) but was not statistically significant.
Keywords
Single nucleotide polymorphisms, COL1A2 gene, dental fluorosis, fluoride
Introduction
Numerous studies show that fluoride (F) plays a key role in the prevention and control of dental caries, predominantly through its topical rather than its systemic effect [1, 2]. Topical oral exposure to low concentrations of F can help prevent demineralisation and promote remineralisation of early carious lesions [2]. However, excessive accumulation of F in the body can cause serious health problems in hard tissues (skeleton and teeth) as well as in soft tissues such as the liver, kidney, brain and pancreas [3-5]. In the teeth, excessive ingestion of F can increase the risk of development of dental fluorosis, a disturbance of enamel formation which is clinically significant since it is responsible for aesthetic problems, dentinal sensitivity, wear, dentofacial anomalies as well as predisposition to dental caries [6, 7]. In a review of F and dental caries prevention in children, Lewis (2014) reported that artificial community water fluoridation schemes have led to markedly decreased rates of dental caries globally [8].
However, although mild fluorosis can be found in artificially fluoridated areas, severe fluorosis is usually only reported in naturally fluoridated areas [9]. Conversely, some studies have reported a high prevalence of dental fluorosis even in communities with low F drinking water concentrations (<0.5 mg/L) and this is thought to be due to the influence of fluoride from other sources or other internal influences acting directly on fluorosis development [10, 11]. These influences include factors affecting the bioavailability of ingested F and an individual’s genetic background [12, 13].
A potential way of reducing some of the burden of dental fluorosis is to identify susceptible populations within the community. There have been several indications of a potential influence of genetics on the susceptibility to dental fluorosis, however, less has been done to explore the effect of a gene polymorphism on susceptibility. Polymorphisms in calcium or bone metabolism related genes such as oestrogen, calcitonin, and osteocalcin might be associated with dental fluorosis in some Chinese populations [14]. A polymorphism in the gene that codes for the Collagen 1 (A2) (COL1A2); accession number NM_00089.3) protein has previously been identified as being associated with an increased risk of developing dental fluorosis in populations exposed to high F. The polymorphism identified is at position 1919 in the mRNA sequence of COL1A2, and causes a missense mutation from a C to an A, resulting in an amino acid residue change from alanine to aspartic acid at position 483 of the protein formed [15]. This previous study which was carried out in a Chinese population with high F exposure provided both support for the plausibility of a role for genetic factors in aetiology of dental fluorosis as well as preliminary evidence for a specific role for COL1A2 [15].
The authors reported an association between the COL1A2 polymorphism (rs414408) and dental fluorosis only in high F areas which is suggestive of gene-environment interaction. However, since the China study was carried out in areas of 2 ppm water F, further studies are needed to confirm this finding and to investigate the impact of this polymorphism in different study populations. Further mutations in the COL1A2 gene have also been linked to a wide spectrum of diseases of bone, cartilage and blood vessels [16]. Other have also reported significant associations between bone phenotypes such as bone mineral density and content and specific genes, including COL1A2 [17-19]. This COL1A2 gene may have the potential to act as a biomarker which could be used to identify high-risk populations that are genetically susceptible to dental fluorosis, which would help to guide clinical and public health decisions concerning the optimal use of F at community and individual level. The current study aimed to determine the association between SNPs within the COL1A2 gene and dental fluorosis among 4 and 8-year-old Nigerians with and without dental fluorosis, residing in areas with different water F concentrations.
Materials and Methods
The study proposal was approved by the Ethics Committee, Newcastle University, UK and the University of Ibadan Ethical Review Board and written informed consent was obtained from parents or legal guardians of the study participants. A cross-sectional study was conducted in Ibadan, Nigeria where dental fluorosis has been previously reported [20]. The fluoride concentration of water in Ibadan ranges between 0.07 ppm and 2.13 ppm [21]. Cluster sampling of 302 four-year-olds and 322 eight-year-olds of both genders was undertaken, in randomly selected nursery and primary schools respectively, as part of a larger project, from which a 20% subsample (n=65 four-year-olds and 60 eight-year-olds) who met inclusion criteria and then randomly selected from the main sample to participate in this present study [22]. Inclusion criteria for the included being healthy and residence in study area since birth. Samples of drinking and cooking waters consumed by study participants were obtained for F analysis by direct method using a F –ion selective electrode [21, 23].
A clinical dental examination was undertaken by an examiner who had been trained and calibrated in the diagnosis of dental fluorosis using the Thylstrup and Fejerskov index with the support of appropriate reference and calibration materials, using a wooden spatula, dry gauze and a disposable mouth mirror (DenLite Illuminated Dental Mirror, Miltex Inc. USA) [24]. The participant rinsed their oral cavity with clean water and a buccal mucosa swab was taken by the dental examiner by rubbing a non-invasive swab matrix (Isohelix DNA Buccal Swab – SK-1S) on the mucosa of the participant’s cheek 5 to 10 times. The swab head was placed into a labelled 5ml tube containing RNAlater solution (Ambion), a storage media that stabilizes and protects cellular RNA.
Genomic DNA was extracted from the buccal mucosa using PureLink kit (Life Technologies, UK). All DNA samples were subjected to PCR using oligonucleotide primers generating a 500 bp product containing the SNP of interest ((forward primer 5’ GGGATCCTCGGCCCCGCTGGAAAAGAA 3’ and reverse primer 5’ CCGAATTCACCTTTATCACCGTTTTTGCCA 3’; Integrated DNA services (IDT, UK)). The PCR was undertaken in reaction mixtures each containing 1 μg of genomic DNA and 12.5 µl of Taq Master Mix (containing 2 X buffer, Taq, polymerase, Mg2+ and dNTPs; NE Bioline). To the mixture, 0.5 µM of forward and 0.5 µM of reverse primers were added and water, up to a volume of 25 µl was added. The PCR was performed on T-Gradient thermocyclers (Gene Amp PCR System, Whatman biometra, Goettingen, Germany) at 95oC for 5 minutes, 95oC for 30 seconds, 58oC for 30 seconds, and 72oC for 60 seconds over 30 cycles. PCR negative reactions were carried out at the same time, where reactions contained no genomic DNA.
After PCR amplification, 8μL of the respective PCR products were electrophoresed on a 1.0% w/v agarose gel in 1 Tris-Acetate-Ethylenediaminetetraacetic acid (TAE) and 10μl of gel red nucleic acid stain (Phenix RGB – 4103). Gels were visualised on a UV trans-illuminator and images were taken. Of the 125 genomic samples, only 70 PCR products were deemed adequate to be sequenced. Sequencing was carried out by MWG-Eurofins, Milton Keynes, UK using the sequencing primer 5’ GTCCAGCCAATCCAATGTTGCC 3’. Sequencing chromatograms were inspected visually to identify the SNP.
Data for the 70 participants whose DNA were sequenced were entered into Statistical Package of Social Sciences (SPSS) software version 22 for data analysis. Frequencies and proportions were generated. A Chi-square test was used to investigate the association between categorical variables at (p<0.05). Binary regression analysis was undertaken to estimate the relationship between the dichotomous dependent variables (presence/absence of dental fluorosis) and the explanatory independent variables (type of SNP and F concentration in drinking and cooking water) at p<0.05.
Results
The mean (SD) F concentrations of drinking and cooking water were 0.25 mg/l (0.48) and 0.25 mg/l (0.64) respectively. The median (minimum, maximum) F concentration of drinking and cooking waters were 0.05 (<0.1, 3.0) mg/l and 0.01 (<0.1, 4.0) mg/l respectively.
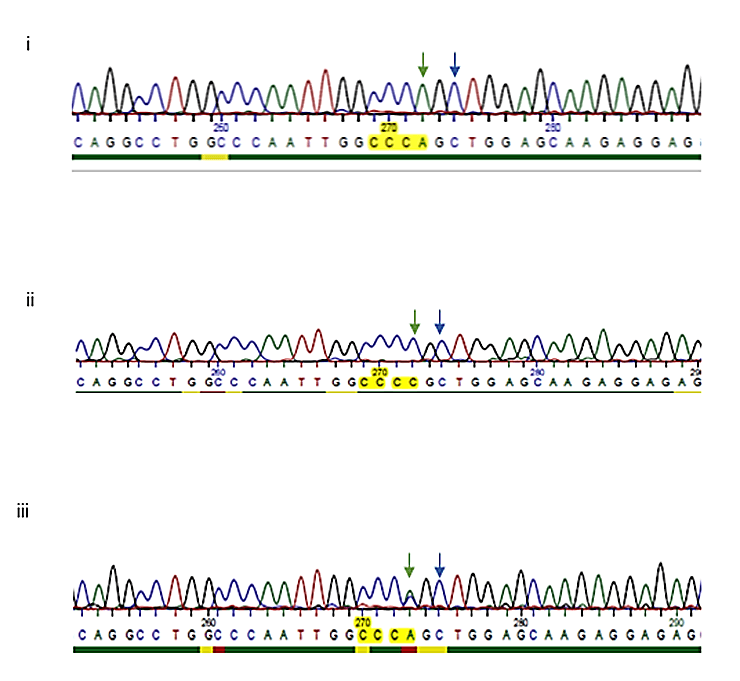
Figure 1: DNA sequences showing the substitution region, SNP rs412777 (i) AA or ii) CC or iii) AC (identified by the double peak)), identified by the green arrow. The previously identified SNP (rs414408) is identified on the chromatograms by a blue arrow.
Sequencing of the COL1A2 PCR product and comparison with the SNP database identified an allele change of an A to a C, at position 1917bp in the COL1A2 gene (resulting in a proline to proline at position 482 on the protein) [25]. This SNP has previously been identified as part of a study investigating osteogenesis imperfecta (OI) types I or III but to our knowledge has not specifically been looked at in relation to fluorosis risk, (rs412777) [26]. The previously identified C to an A SNP at position 1919 (rs414408) was not observed in any of our samples analysed in this population. The base associated at this position was recorded as either ‘AA’, ‘CC’ or ‘AC’. Heterozygous samples were visualised as 2 over laying peaks. Figure 1 shows three representative chromatograms, from participants who carried the ‘‘AA’ base (i), CC’ base (ii) and ‘AC’ base (iii).
Table 1 shows the distribution of the SNPs. The majority of the study participants 37/70 (52.9%) had the heterozygote SNP AC. There was a statistically significant association between F concentration in drinking water and the occurrence of dental fluorosis in this study population (Table 2, p=0.04). Participants who drank water that contained between 0.21 – 3.0 mg/l F had a significantly increased risk of dental fluorosis compared with those who drank water containing < 0.20 mg/l F (RR = 3.61, CI: 1.19, 10.95). There was no statistically significant association between F concentration in cooking water and the occurrence of dental fluorosis (p=0.15).
Table 1: Distribution of Single Nucleotide Polymorphism (SNP) among study participants (n=70).
|
Single Nucleotide Polymorphism |
Number |
(%) |
|
AC |
37 |
(52.9) |
|
CC |
24 |
(34.2) |
|
AA |
9 |
(12.9) |
|
Total |
70 |
(100.0) |
Table 2: Relationship between fluoride concentration in drinking and cooking water and dental fluorosis (n=70).
|
Fluoride concentration (mg/l) |
Dental fluorosis |
Total |
P value |
RR (95% CI) |
|
|
Present No. (%) |
Absent No. (%) |
No. (%) |
|||
|
Drinking water 0.21 – 3.0 < 0.20 |
10 (45.5) 9 (18.7) |
12 (54.5) 39 (81.3) |
22 (100.0) 48 (100.0) |
0.04
|
3.61 (1.19, 10.95) Ref |
|
Total |
19 (27.1) |
51 (72.9) |
70 (100.0) |
|
|
|
Cooking water 0.21 – 4.0 < 0.20 |
8 (40.0) 11 (22.0) |
12 (60.0) 39 (78.0) |
20 (100.0) 50 (100.0) |
0.15
|
2.36 (0.77, 7.22) Ref |
|
Total |
19 (27.1) |
51 (72.9) |
70 (100.0) |
|
|
RR: Relative Risk; Ref: Reference category.
When looked at independently to drinking or cooking water consumption, there was no statistically significant association between single nucleotide polymorphism type and the occurrence of dental fluorosis (p>0.05), however the risk of dental fluorosis appeared to increase with the presence of ‘AA’ when compared with ‘CC’ plus ‘AC’ as well as in ‘AC’ when compared with ‘AA’ plus ‘CC’ (RR > 1) (Table 3).
As expected, F concentration in drinking water was a statistically significant predictor of dental fluorosis (p=0.03, OR=3.64 (CI=1.11-11.94)) (Table 4). No other explanatory variables were statistically significant predictors of dental fluorosis. A binary regression model made the correct prediction for 75.7% of the children having dental fluorosis or not. The Nagelkerle R2 value from the model was 0.20 i.e. 20% of the variability in the dependent variable was accounted for by the independent variables.
Table 3: Association between single nucleotide polymorphisms (SNPs) and dental fluorosis among study participants (n=70).
|
Single Nucleotide Polymorphisms |
Dental fluorosis Present Absent |
p |
**RR (95% CI) |
|||
|
No. |
% |
No. |
% |
|||
|
CC AA AC |
4 3 12 |
21.1 16.8 63.3 |
20 6 25 |
39.2 11.8 49.0 |
0.257 0.696 0.421 |
0.51 (0.19,1) 1.27 (0.46, 3.51) 1.48 (0.66, 2.31) |
|
Total |
19 |
100.0 |
51 |
100.0 |
|
|
RR: Relative Risk; **Calculated by comparing SNP AA vs CC plus AC, SNP CC vs AA plus AC and SNP AC vs CC plus AA in a 2x2 Table.
Table 4: Binary regression analysis model for dental fluorosis among 4 and 8-year-olds (n=70).
|
Predictors |
Dental fluorosis (R2=0.20a; % Predicted = 75.7%) |
||||
|
B |
Sig (p) |
ORc Exp (B) |
95% CI |
||
|
Lower |
Upper |
||||
|
F Concentration Drinking Water (mg/l) |
1.29 |
0.03b |
3.64 |
1.11 |
11.94 |
|
F Concentration Cooking Water (mg/l) |
0.43 |
0.49 |
1.53 |
0.46 |
5.08 |
|
*Single nucleotide polymorphism SNP AA SNP AC |
1.450.65 |
0.05 0.48 |
0.24 0.52 |
0.55 0.08 |
1.01 3.24 |
*SNP CC was the reference category; aNagelkerke R2; bStatistically significant at p < 0.05; cOdds ratio.
Discussion
Dental fluorosis is a major public health problem in some areas in Nigeria due to excessive ingestion of F from drinking water in which excessive amounts of F occur naturally in some areas [27]. The mechanism by which F, an environmental element, causes dental fluorosis has been reported to be influenced by genetic factors [28, 29]. Despite the potential influence of genetics in the susceptibility of dental fluorosis, research is limited in this area and only very few human studies have looked at polymorphisms within specific genes. Single nucleotide polymorphisms are the commonest form of sequence variation and account for more than 90% of all variations present in the human genome [13]. Several case-control studies have been undertaken to determine whether polymorphism is associated with dental fluorosis susceptibility or resistance and, if associated, then whether the homozygous or heterozygous genotypes have played a role as a protective or risk factor for dental fluorosis [13].
Some studies on SNPs as genetic markers for susceptibility of fluorosis showed that genetic variants in some candidate genes like Collagen type 1 alpha 2 (COL1A2), Calcitonin receptor gene (CTR), Estrogen receptor (ESR), Catechol-o-methyltransferase (COMT), Glutathione S-transferase pi 1(GSTP1), Matrix metallopeptidase 2 (MMP-2), PRL (Prolactin), Vitamin D receptor (VDR), Myeloperoxidase (MPO) and PTH Bst BI could increase or decrease the risk of fluorosis among the exposed individuals in endemic areas [14, 15, 30-37]. This present study proposed that a single nucleotide polymorphism in the COL1A2 gene may influence the occurrence of dental fluorosis among children exposed to different concentrations of drinking and cooking waters in Nigeria.
In the current study, the F concentration in drinking and cooking water varied widely from <0.01 to 3.0 mg/l and <0.01 to 4.0 mg/l respectively. This wide variability in fluoride concentration in the waters might be due to water samples collected during different seasons since water collected from shallow wells during the rainy season are usually lower in F than those collected during the dry season [38]. In addition, some of this variability would have been due to water samples collected from shallow wells and aquifers, in agreement with a previous study, that also reported a high variability in F concentration in water obtained from shallow wells [39]. Dental fluorosis and skeletal fluorosis can occur from prolonged intake of drinking waters containing F at a concentration above 1.5 mg/l and 3.0 mg/l respectively [40]. However, dental fluorosis can also occur in tropical communities exposed to apparently appropriate F concentrations in drinking water [10, 11]. In this present study, participants who drank water containing between 0.21 – 3.0 mg/l F had increased risk of dental fluorosis compared with those who drank water containing < 0.20 mg/l indicating a statistically significant association between the F concentration in water and occurrence of dental fluorosis.
The sensitive methodology involving sequencing used in our study allowed us to identify an actual SNP change within the COL1A2 region. In a previous study of COL1A2 as a predictor for fluorosis risk, the SNP rs414408 was identified as the risk allele (C>A missense mutation), however the method for detection relied upon the ability of a restriction enzyme PvuII to digest the PCR product. The different SNP identified in our current study, would also disrupt this enzymic digestion reaction (Figure 1) and we propose that our results are in agreement with this previous study, that individuals with the PvuII restriction site at this region in the COL1A2 gene have a higher relative risk of developing dental fluorosis (i.e. AA allele, RR 1.27; AC allele RR 1.48). Further studies of the Chinese cohort, with sequencing to identify the actual SNP need to be carried out to confirm this theory [15].
Several studies have reported a relationship between F concentration in drinking water and the occurrence of dental fluorosis. Similarly, in this present study, fluoride in drinking water was the main predictor of dental fluorosis [41, 42]. The risk of having dental fluorosis increased significantly (P=0.04) with increasing F concentration in drinking water. Although not statistically significant, the risk of having dental fluorosis appeared to be higher in the presence of the AC and AA allele rather than CC.
Huang and colleagues studied the interactions between COL1A2 gene and dental fluorosis in high and low community water F areas, while this current study looked at the interactions between the gene and F concentration in drinking and cooking water consumed by study participants [15]. Studying the interactions between SNPs and F concentration in water alone cannot provide adequate information about gene-environment interaction in the occurrence of dental fluorosis since there are other influential environmental factors in aetiology. Therefore, further studies on the relationship between SNPs and other environmental factors such as diet and actual F exposure in the occurrence of dental fluorosis are needed. In addition, there is also a need to undertake a larger study in this Nigeria population. COL1A2 SNPs may be useful markers for the differential risk of dental fluorosis, which, being a complex condition, is likely influenced by several genes. The relationship between SNP and the severity of dental fluorosis was not explored in this current study.
The limitation of this study is that the actual F exposure at the time or age when individuals are more susceptible to the development of dental fluorosis is unknown. In addition, the small sample size in this study may prevent the findings from being extrapolated, therefore further studies among larger study populations are strongly recommended.
Conclusion
F concentrations in the Nigerian drinking and cooking waters analysed varied from <0.1 to 4.0 mg/L. The majority of the study participants had the heterozygote genotype of COL1A2 gene. F concentration in drinking water was the only statistically significant predictor of dental fluorosis. The risk of dental fluorosis tended to increase with the presence of SNPs AA and AC (RR > 1) but was not statistically significant in this small study population.
Acknowledgements
The study was funded by the Commonwealth Scholarship Commission and Newcastle University, UK.
Article Info
Article Type
Research ArticlePublication history
Received: Mon 22, Jun 2020Accepted: Sat 01, Aug 2020
Published: Mon 10, Aug 2020
Copyright
© 2023 Ruth Valentine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JDOA.2020.02.04
Author Info
Olushola Ibiyemi Anne Maguire Fatemeh Vida Zohoori Simon Kometa Ruth Valentine
Corresponding Author
Ruth ValentineCentre for Oral Health Research, School of Dental Sciences, Newcastle University, UK
Figures & Tables
Table 1: Distribution of Single Nucleotide Polymorphism (SNP) among study participants (n=70).
|
Single Nucleotide Polymorphism |
Number |
(%) |
|
AC |
37 |
(52.9) |
|
CC |
24 |
(34.2) |
|
AA |
9 |
(12.9) |
|
Total |
70 |
(100.0) |
Table 2: Relationship between fluoride concentration in drinking and cooking water and dental fluorosis (n=70).
|
Fluoride concentration (mg/l) |
Dental fluorosis |
Total |
P value |
RR (95% CI) |
|
|
Present No. (%) |
Absent No. (%) |
No. (%) |
|||
|
Drinking water 0.21 – 3.0 < 0.20 |
10 (45.5) 9 (18.7) |
12 (54.5) 39 (81.3) |
22 (100.0) 48 (100.0) |
0.04
|
3.61 (1.19, 10.95) Ref |
|
Total |
19 (27.1) |
51 (72.9) |
70 (100.0) |
|
|
|
Cooking water 0.21 – 4.0 < 0.20 |
8 (40.0) 11 (22.0) |
12 (60.0) 39 (78.0) |
20 (100.0) 50 (100.0) |
0.15
|
2.36 (0.77, 7.22) Ref |
|
Total |
19 (27.1) |
51 (72.9) |
70 (100.0) |
|
|
RR: Relative Risk; Ref: Reference category.
Table 3: Association between single nucleotide polymorphisms (SNPs) and dental fluorosis among study participants (n=70).
|
Single Nucleotide Polymorphisms |
Dental fluorosis Present Absent |
p |
**RR (95% CI) |
|||
|
No. |
% |
No. |
% |
|||
|
CC AA AC |
4 3 12 |
21.1 16.8 63.3 |
20 6 25 |
39.2 11.8 49.0 |
0.257 0.696 0.421 |
0.51 (0.19,1) 1.27 (0.46, 3.51) 1.48 (0.66, 2.31) |
|
Total |
19 |
100.0 |
51 |
100.0 |
|
|
RR: Relative Risk; **Calculated by comparing SNP AA vs CC plus AC, SNP CC vs AA plus AC and SNP AC vs CC plus AA in a 2x2 Table.
Table 4: Binary regression analysis model for dental fluorosis among 4 and 8-year-olds (n=70).
|
Predictors |
Dental fluorosis (R2=0.20a; % Predicted = 75.7%) |
||||
|
B |
Sig (p) |
ORc Exp (B) |
95% CI |
||
|
Lower |
Upper |
||||
|
F Concentration Drinking Water (mg/l) |
1.29 |
0.03b |
3.64 |
1.11 |
11.94 |
|
F Concentration Cooking Water (mg/l) |
0.43 |
0.49 |
1.53 |
0.46 |
5.08 |
|
*Single nucleotide polymorphism SNP AA SNP AC |
1.450.65 |
0.05 0.48 |
0.24 0.52 |
0.55 0.08 |
1.01 3.24 |
*SNP CC was the reference category; aNagelkerke R2; bStatistically significant at p < 0.05; cOdds ratio.
References
- J D Featherstone (1999) Prevention and reversal of dental caries: role of low level fluoride. Community Dent Oral Epidemiol 27: 31-40. [Crossref]
- F V Zohoori, G Whaley, P J Moynihan, A Maguire (2014) Fluoride intake of infants living in non-fluoridated and fluoridated areas. Br Dent J 216: E3. [Crossref]
- J Elliott, J H Scarpello, N G Morgan (2001) Effects of tyrosine kinase inhibitors on cell death induced by sodium fluoride and pertussis toxin in the pancreatic beta-cell line, RINm5F. Br J Pharmacol 132: 119-126. [Crossref]
- Xianzhi Xiong, Junling Liu, Weihong He, Tao Xia, Ping He et al. (2007) Dose-effect relationship between drinking water fluoride levels and damage to liver and kidney functions in children. Environ Res 103: 112-116. [Crossref]
- Ming Zhang, Aiguo Wang, Weihong He, Ping He, Bayi Xu et al. (2007) Effects of fluoride on the expression of NCAM, oxidative stress and apoptosis in primary cultured hippocampal neurons. Toxicology 236: 208-216. [Crossref]
- L Aine, M C Backström, R Mäki, A L Kuusela, A M Koivisto et al. (2000) Enamel defects in primary and permanent teeth of children born prematurely. J Oral Pathol Med 29: 403-409. [Crossref]
- Maria Contaldo, Dario Di Stasio, Rossella Santoro, Luigi Laino, Letizia Perillo et al. (2015) Non-invasive in vivo visualization of enamel defects by reflectance confocal microscopy (RCM). Odontology 103: 177-184. [Crossref]
- Charlotte W Lewis (2014) Fluoride and dental caries prevention in children. Pediatr Rev 35: 3-15. [Crossref]
- J P Yadav, Suman Lata, Sudhir K Kataria, Sunil Kumar (2009) Fluoride distribution in groundwater and survey of dental fluorosis among school children in the villages of the Jhajjar District of Haryana, India. Environ Geochem Health 31: 431-438. [Crossref]
- I D Brouwer, O B Dirks, A De Bruin, J G Hautvast (1988) Unsuitability of World Health Organization guidelines for fluoride concentrations in drinking water in Senegal. Lancet 67: 822-825. [Crossref]
- E S Akpata, Z Fakiha, N Khan (1997) Dental fluorosis in 12 - 15 years-old rural children exposed to fluorides from well drinking waters in the Hail region of Saudi Arabia. Community Dent Oral Epidemiol 25: 324-327. [Crossref]
- Cerklewski FL (1997) Fluoride bioavailability – Nutritional and clinical aspects. Nut Res 17: 907-929.
- Sreemanta Pramanik, Depanwita Saha (2017) The genetic influence in fluorosis. Environ Toxicol Pharmacol 56: 157-162. [Crossref]
- Yue Ba, Huizhen Zhang, Gang Wang, Shibao Wen, Yuejin Yang et al. (2011) Association of dental fluorosis with polymorphism of estrogen receptor gene in Chinese children. Biol Trace Elem Res 143: 87-96. [Crossref]
- Hui Huang, Yue Ba, Liuxin Cui, Xuemin Cheng, Jingyuan Zhu et al. (2008) COL1A2 gene polymorphisms (Pvu II and Rsa I), serum calciotropic hormone levels, and dental fluorosis. Community Dent Oral Epidemiol 36: 517-522. [Crossref]
- R Dalgleish (1997) The human type I collagen mutation database. Nucleic Acids Res 25: 181-187. [Crossref]
- Miia Suuriniemi, Anitta Mahonen, Vuokko Kovanen, Markku Alén, Sulin Cheng (2003) Relation of PvuII site polymorphism in the COL1A2 gene to the risk of fractures in prepubertal Finnish girls. Physiol Genomics 14: 217-224. [Crossref]
- Marcia C Willing, James C Torner, Trudy L Burns, Kathleen F Janz, Teresa Marshall et al. (2003) Gene polymorphisms, bone mineral density and bone mineral content in young children: the Iowa Bone Development Study. Osteoporos Int 14: 650-658. [Crossref]
- F Y Deng, M Y Liu, M X Li, S F Lei, Y J Qin et al. (2003) Tests of linkage and association of the COL1A2 gene with bone phenotypes' variation in Chinese nuclear families. Bone 33: 614-619. [Crossref]
- Ibiyemi, J O Taiwo (2011) Psychosocial aspect of anterior tooth discoloration among adolescents in Igboo-ora, Southwestern Nigeria. An Ibd Pg Med 9: 94-99. [Crossref]
- Olushola Ibiyemi, Fatemeh V Zohoori, Ruth A Valentine, Simon Kometa, Anne Maguire (2018) Prevalence and extent of enamel defects in the permanent teeth of 8-year-old Nigerian Children. Community Dent Oral Epidemiol 46: 54-62. [Crossref]
- Ibiyemi O (2016) Factors associated with the occurrence of developmental defects of enamel and dental fluorosis among 4 and 8-year-olds Nigerian children. PhD Thesis Newcastle University.
- E A Martínez Mier, J A Cury, J R Heilman, B P Katz, S M Levy et al. (2011) Development of gold standard ion-selective electrode-based methods for fluoride analysis. Caries Res 45: 3-12. [Crossref]
- A Thylstrup, O Fejerskov (1978) Clinical appearance of dental fluorosis in permanent teeth in relation to histologic changes. Community Dent Oral Epidemiol 6: 315-238. [Crossref]
- SNP database (2019).
- Aleksandra Augusciak Duma, Joanna Witecka, Aleksander L Sieron, Magdalena Janeczko, Jacek J Pietrzyk et al. (2018) Mutations in the COL1A1 and COL1A2 genes associated with osteogeneisis imperfacta (OI) types I or II. Acta Biochim Pol 65: 79-86. [Crossref]
- Enosakhare S Akpata, I S Danfillo, E C Otoh, J O Mafeni (2009) Geographical mapping of fluoride levels in drinking water sources in Nigeria. Afr Health Sci 9: 227-233. [Crossref]
- Yan Zhang, Qiaomei Yan, Wu Li, Pamela K DenBesten (2006) Fluoride down-regulates the expression of matrix metalloproteinase-20 in human fetal tooth ameloblast-lineage cells in vitro. Eur J Oral Sci 114: 105-110. [Crossref]
- Q Yan, Y Zhang, W Li, P K Denbesten (2007) Micromolar fluoride alters ameloblast lineage cells in vitro. J Dent Res 86: 336-340. [Crossref]
- Miao Jiang, Lihong Mu, Yingxiong Wang, Wei Yan, Yongzhuo Jiao (2015) The relationship between AluI polymorphisms in the calcitonin receptor gene and fluorosis endemic to Chongqing, China. Med Princ Pract 24: 80-83. [Crossref]
- Shun Zhang, Xiaofei Zhang, Hongliang Liu, Weidong Qu, Zhizhong Guan et al. (2015) Modifying effect of COMT gene polymorphism and a predictive role for proteomics analysis in children’s intelligence in endemic fluorosis area in Tianjin, China. Toxicol Sci 144: 238-245. [Crossref]
- Junhua Wu, Wei Wang, Yang Liu, Jing Sun, Yan Ye et al. (2015) Modifying role of GSTP1 polymorphism on the association between tea fluoride exposure and the brick-tea type fluorosis. PLoS One 10: e0128280. [Crossref]
- Junrui Pei, Bingyun Li, Yang Liu, Xiaona Liu, Mang Li et al. (2017) Matrix metallopeptidase-2 gene rs2287074 polymorphism is associated with Brick Tea skeletal fluorosis in Tibetans and Kazaks. China. Sci Rep 7: 40086. [Crossref]
- Bing Yun Li, Yan Mei Yang, Yang Liu, Jing Sun, Yan Ye et al. (2017) Prolactin rs1341239 T allele may have protective role against the brick tea type skeletal fluorosis. PLoS One 12: e0171011. [Crossref]
- Dan Yang, Yang Liu, Yanru Chu, Qing Yang, Wei Jiang et al. (2016) Association between vitamin D receptor gene FokI polymorphism and skeletal fluorosis of the brick-tea type fluorosis: a cross sectional, case control study. BMJ Open 6: e011980. [Crossref]
- Ting Zhang, Ke Ren Shan, Xi Tu, Yan He, Jin Jing Pei et al. (2013) Myeloperoxidase activity and its corresponding mRNA expression as well as gene polymorphism in the population living in the coal-burning endemic fluorosis area in Guizhou of China. Biol Trace Elem Res 152: 379-386. [Crossref]
- Shibao Wen, Anqi Li, Liuxin Cui, Qi Huang, Hongyang Chen et al. (2012) The Relationship of PTH Bst BI polymorphism, calciotropic hormone levels, and dental fluorosis of children in China. Biol Trace Elem Res 147: 84-90. [Crossref]
- World Health Organization (WHO) (1984) Guidelines for drinking water quality. WHO Geneva.
- F V Zohouri, A J Rugg Gunn (2000) Sources of dietary fluoride intake in 4-year-old children residing in low, medium and high fluoride areas in Iran. Int J Food Sci Nutr 51: 317-326. [Crossref]
- World Health Organization (WHO) (2000) Air quality guidelines. World Health Organization (WHO), Regional Publications, European Series, Regional Office for Europe, Copenhagen, Denmark Chapter 6.5.
- D F Cortes, R P Ellwood, D M O'Mullane, J R Bastos (1996) Drinking water fluoride levels, dental fluorosis and caries experience in Brazil. J Public Health Dent 56: 226-228. [Crossref]
- Zoran Mandinic, Marijana Curcic, Biljana Antonijevic, Momir Carevic, Jelena Mandic et al. (2010) Fluoride in drinking water and dental fluorosis. Sci Total Environ 408: 3507-3512. [Crossref]
