J.ophthalmol.(Ukraine).2018;4:17-25.
|
https://doi.org/10.31288/oftalmolzh201841725 Received: 01 June 2018; Published on-line: 31 August 2018 Comparing bioelectrical activity of the central retina among myopic patients operated for rhegmatogenous retinal detachment complicated by choroidal detachment Alibet Yassine, Ophthalmologist, Postgraduate Student, V.S. Ponomarchuk, Dr Sc (Med), Prof., N.I. Khramenko, Cand Sc (Med), G.V. Levytska, Cand Sc (Med) Filatov Institute of Eye Disease and Tissue Therapy; Odessa (Ukraine) E-mail: alibet.yassine@gmail.com TO CITE THIS ARTICLE: Alibet Yassine, Ponomarchuk VS, Khramenko NI, Levytska GV. Comparing bioelectrical activity of the central retina among myopic patients operated for rhegmatogenous retinal detachment complicated by choroidal detachment. J.ophthalmol.(Ukraine).2018;4: 17-25. https://doi.org/10.31288/oftalmolzh201841725
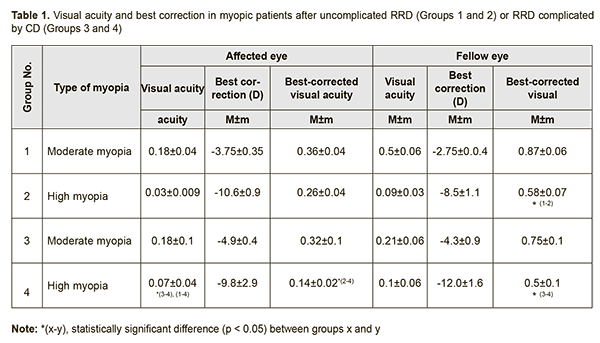
Background: Rhegmatogenous retinal detachment (RRD) is a serious incapacitating disease requiring surgical treatment. Trophic disturbances in the central and peripheral retina are characteristic for myopia, which is known to be a major risk factor for RRD. Purpose: 1) To investigate the bioelectrical activity of the central retina after successful surgery for combined RRD and CD in patients differing in the degree of myopia, and 2) to compare the characteristics with those of normal eyes and myopic patients successfully operated for uncomplicated RRD. Materials and Methods: Fifty two patients (52 eyes) were included into the study 3 months after undergoing a single successful vitrectomy with gas tamponade, either for uncomplicated RRD (32 eyes), or combined RRD and CD (20 eyes). They were divided into four groups: Groups 1 and 2 (moderate and high myopes after surgery for uncomplicated RRD; 21 and 11 patients, respectively), and Groups 3 and 4 (moderate and high myopes after surgery for combined RRD and CD; 9 and 11 patients, respectively). Fourteen age-matched individuals (28 eyes) without any ocular or systemic disease were enrolled as controls (Group 5). Photopic cone response and photopic 30 Hz flicker cone response were recorded to examine the bioelectrical activity of the central retina. Results: A-wave and b-wave implicit times of the photopic cone response (a) reflect conductance related to the photoreceptor and inner layers, respectively, of the central retina, and (b) were 25% longer and 28% longer, respectively, for myopic eyes after surgery for either uncomplicated RRD or combined RRD and CD, than for normal eyes. A-wave amplitude of the photopic cone response (a) reflects the summed bioelectrical activity of retinal photoreceptor cones, and (b) in moderately or highly myopic eyes after surgery for uncomplicated RRD was 1.9-fold and 3.5-fold lower, respectively, and in moderately or highly myopic eyes after surgery for combined RRD and CD, 3.9-fold and 6.6-fold lower, respectively, than in normal eyes. B-wave amplitude of the photopic cone response (a) reflects the summed bioelectrical activity of the retinal inner layers, and (b) in moderately or highly myopic eyes after surgery for uncomplicated RRD was 1.6-fold and 2.8-fold lower, respectively, whereas in those after surgery for combined RRD and CD, 3.8-fold lower, than in normal eyes. Amplitude of photopic 30 Hz flicker cone response (a) reflects the bioelectrical activity of cones, and (b) in moderately myopic eyes after surgery for uncomplicated RRD was 1.6-fold lower, whereas in myopic eyes after surgery for combined RRD and CD, 2.8-fold lower, than in normal eyes. We found direct correlations between BCVA and b-wave and a-wave amplitudes of the photopic cone response (r = 0.61 and r = 0.54, respectively), and amplitude of the 30 Hz flicker response (r = 0.53), and an inverse correlation (r=-0.53) between BCVA and a-wave implicit time of the photopic cone response for myopic eyes after surgery for either uncomplicated RRD or combined RRD and CD. Conclusion: Bioelectrical activity of the central retina was found to be more decreased in highly myopic eyes after surgery for combined RRD and CD than in other myopic eyes of the study. Keywords: rhegmatogenous retinal detachment, choroidal detachment, electroretinography Introduction The rate of rhegmatogenous retinal detachment (RRD) complications in-volving choroidal detachment (CD), hypotony and/or uveitis varies from 0.5-4% [1, 2]. Alterations in ciliary body function and morphology are characteristic for this form of retinal detachment and, certainly, play a major role in the development of intraocular inflammation [3, 4]. RRD is one of the most serious incapacitating disorders, requires surgical treatment, and its annual incidence has been reported to be 12.05 per 100,000 population (95% confidence interval, 11.35-12.70) [5]. In addition, it is a serious retinal condition, and even in anatomically successful surgeries (with complete re-attachment and rempdelling) for RRD, functional results may vary from patient to patient. Although there are a variety of surgical techniques for RRD, good functional results are achieved in only 30-80% of cases [6]. After RRD surgery, patients often complain of inadequate vision, metamorphopsia [7], and/or abnormal color perception. It has been reported that the retina, like the central nervous system, has a significant capacity for remodeling its cellular architecture [8]. This remodeling process includes the retraction of axons by rod photoreceptors, outgrowth of horizontal cell neurites, and Müller cell proliferation and structural reorganization [9]. In RRD patients, upon successful reattachment, the photoreceptor outer segments almost fully regenerate and the retinal-pigmented epithelium (RPE) can achieve good contact with the retina [10]. If degeneration of the photoreceptor layer occurs, downstream cellular changes are initiated throughout the retina. These morphological changes are often associated with impairment of retinal function as can be seen by the loss of electroretinographic (ERG) responses, which often are proportional to the area of the detachment [11]. It is reasonable to hypothesize that impaired choroidal attachment may exacerbate the degenerative process associated with worsening of visual function. It has been experimentally found that outer retinal ischemia due to impaired blood circulation may result in increased apoptosis [12]. We have demonstrated previously [14] that, in myopic eyes after surgery for combined RRD and choroidal detachment (CD), there was a sharp decline in a combined response of the peripheral and midperipheral retinal layers. Purpose: 1) To investigate the bioelectrical activity of the central retina after successful surgery for combined RRD and CD in eyes differing in the degree of myopia, and 2) to compare the characteristics with those of normal eyes and myopic eyes successfully operated for uncomplicated RRD. Materials and Methods Fifty two patients (52 eyes) were included into the study 3 months after undergoing a single successful procedure, either for uncomplicated RRD (32 patients, 32 eyes), or combined RRD and CD (20 patients, 20 eyes). Patients were divided into four groups: Group 1 (moderate myopes (−3.25D to −6D) after surgery for uncomplicated RRD; 21 patients), Group 2 (high myopes (> −6D) after surgery for uncomplicated RRD; 11 patients), Group 3 (moderate myopes after surgery for combined RRD and CD; 9 patients), and Group 4 (high myopes after surgery for combined RRD and CD; 11 patients). Patients with uncomplicated RRD were comparable with those with combined RRD and CD with regard to period of detachment (19.2±8.3 days vs 17.5±13.2 days), type of surgery (vitrectomy, retinal laser photocoagulation, perfluorpropane tamponade) and age (53.8±17 years). Operated eyes demonstrated a reftactive error that was comparable to the fellow eyes, and peripheral retinal degeneration was observed in all eyes. Fourteen age-matched individuals (28 eyes) without any ocular or systemic disease were enrolled as controls (Group 5). Exclusion criteria were history of previous vitreoretinal surgery, ocular trauma, glaucoma, age-related macular degeneration, epi- or submacular mem-brane, macular tear, diabetic or vascular retinal disorders. Operated and fellow eyes were assessed for bioelectric retinal activity. A full field (Ganzfeld) ERG examination was performed using electrophysiological test unit Retiscan according to ISCEV Standard ERG protocols [13], which included photopic cone response and photopic 30Hz flicker cone response. The responses were evoked by flashes of different intensities and durations as per ISCEV Standard ERG protocols [13] after the patient was duly prepared for the procedure and underwent adaptation. Data base development and analysis was performed using Statistica 8.0 (StatSoft, Tulsa, OK, USA) software. Distributions were tested for normality using histogram plots and the Kolmogorov-Smirnov test. The parametric Student's t test was used to determine significant differences between two normally distributed samples. Wilcoxon signed-rank test and Mann-Whitney U test and median (Me) values were used for the comparison of two samples when the underlying distributions were not normal. Non-parametric Spearman correlation coefficient (rs) was used to determine the linear relationship between the variables. Results There was no difference between the two groups of moderate myopes (Group 1 and Group 3) and between the two groups of high myopes (Group 2 and Group 4) with regard to uncorrected visual acuity (UCVA) in the affected eye (0.18±0.09 and 0.04±0.01, respectively; Table 1). In addition, there was no difference between the two groups of moderate myopes with regard to best-corrected visual acuity (BCVA; 0.34±0.09). There was, however, a significant difference between the two groups of high myopes (Group 2 and Group 4), with regard to BCVA in the affected eye (0.26±0.04 vs 0.14±0.02, p < 0.05).
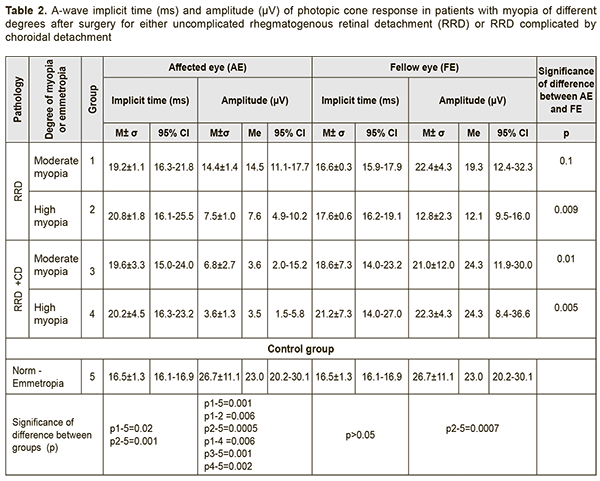
Therefore, in patients who had undergone surgery for either RRD alone or combined RRD and CD, the BCVA in the affected eye was lower than normal, and depended on the refractive error, and on whether the patient had a history of RRD alone or combined RRD and CD. The BCVA in the affected eye in high myopes who had undergone surgery for combined RRD and CD was 46% lower (p = 0.04) than in those who had undergone surgery for RRD only, and 61% lower (p = 0.001) than in moderate myopes of Groups 1 and 3. The BCVA in the fellow eye in moderate myopes of Group 1 was 0.87±0.06, which was 33% higher than in high myopes of Group 2 (0.58±0.07, p < 0.05). There was no significant difference in the BCVA in the fellow eye between patients who had undergone surgery for combined RRD and CD (Groups 3 and 4) and those who had undergone surgery for RRD only (Groups 1 and 2; Table 1). Next, we used photopic ERG (phERG) to examine the bioelectrical activity of the central retina. A-wave in the phERG reflects the state of photoreceptors of cones, pre-sumably from the central retina. A-wave implicit time reflects the speed o bioelectric processes and is measured from the time of the flash to the photoreceptor response peak. The a-wave implicit time in myopes who had undergone surgery for uncomplicated RRD and combined RRD and CD was 19.2±1.1 ms and 20.8±1.8 ms, respectively, which was 25% higher than in controls (mean value, 16.5±1.3 ms; 95% CI, 16.1 ms to 16.9 ms, p < 0.05). We found an inverse correlation (r=-0.5; p < 0.05) between BCVA and a-wave implicit time for the photopic ERG in the affected eye (Table 2).
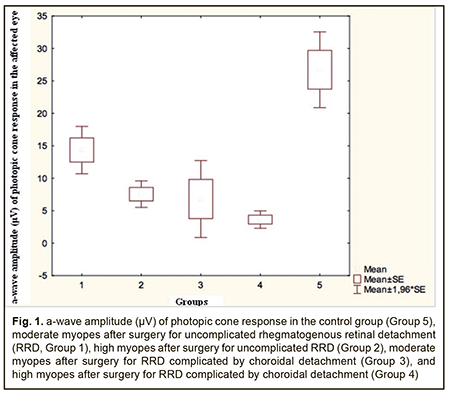
A-wave amplitude of the photopic ERG reflects the summed bioelectrical activity of retinal photoreceptor cones. The a-wave amplitude of the photopic ERG in the control group was 26.7±11.1 μV (95% CI, 20.2 μV to 30.1 μV). In moderate myopes who had undergone surgery for RRD only (Group 1), the a-wave amplitude was 14.4±1.4 μV, which was 1.9-fold lower than in normal eyes (p=0.001; Table 2). In high myopes who had undergone surgery for RRD only, the a-wave amplitude was 7.5±1.0 μV, which was 3.5-fold lower (p=0.0005) and 2.0-fold lower (p=0.006) than in normal eyes and Group 1, respectively. There was no difference in a-wave amplitude between affected and fellow eyes in Group 1, but the a-wave amplitude in the affected eye was 1.7-fold lower than in the fellow eye (p = 0.009) in Group 2. In moderate myopes who had undergone surgery for combined RRD and CD, the a-wave amplitude in the affected eye was 6.8±2.7 μV, which was 3.9-fold lower (p=0.001) and 3.1-fold lower (p=0.01) than in normal eyes and the fellow eye, respectively (Table 2). In high myopes who had undergone surgery for combined RRD and CD, the a-wave amplitude in the affected eye was 6.8±2.7 μV (95% CI, 1.5 ms to 5.8 ms), which was 6.6-fold lower (p=0.002) and 6.2-fold lower (p=0.005) than in normal eyes and the fellow eye, respectively. Therefore, a-wave implicit time of photopic ERG in all myopic eyes after surgery for either uncomplicated RRD or combined RRD and CD was 25% (p < 0.05) longer than in normal eyes, which reflects markedly slowed conduction velocity in retinal cones. A-wave amplitude of photopic ERG in myopic eyes after surgery for uncomplicated RRD and combined RRD and CD was 1.9-3.5-fold lower and 3.9-6.6-fold lower, respectively, than in normal eyes, which reflects a markedly reduced summed bioelectrical activity of retinal cones (Fig. 1).
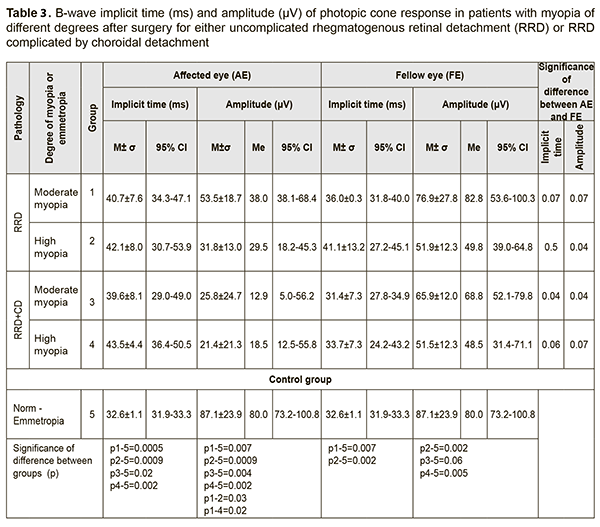
B-wave in the photopic ERG reflects the state of the inner retinal layer (bipolar cells and Muller cells associated with cones) located presumably in the central retina. The mean b-wave implicit time in the photopic ERG in the control group was 32.6±1.1 ms (95% CI, 31.9 ms to 33.3 ms). The b-wave implicit time in moderate myopes and high myopes who had undergone surgery for uncomplicated RRD was 40.7±7.6 ms and 42.1±8.0 ms, respectively, which was 25% higher (p=0.0005) and 31% higher (p = 0.0009), respectively, than in controls (Table 3). In Group 3 and Group 4, the b-wave implicit time was 39.6±8.1 ms and 43.5±4.4 ms, respectively, which was 24% higher (p=0.02) and 34% higher (p = 0.002), respectively, than in controls (Table 3). In total groups 1 to 4, the 95% CI for b-wave implicit time was 37.9 ms to 44.7 ms, which was 28% longer than in controls.
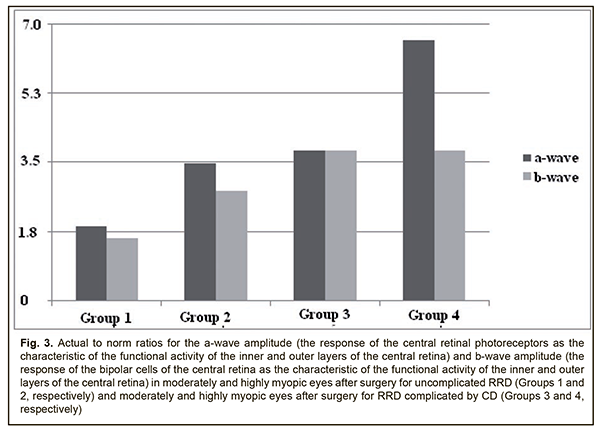
We found an inverse correlation (r=-0.6; p < 0.05) between BCVA and b-wave implicit time for the photopic ERG in the affected eye. B-wave amplitude of the photopic ERG reflects the summed bioelectrical activity of inner retinal cells. The mean b-wave amplitude in the control group was 87.1±23.9 μV. In Group 1 and Group 2, the b-wave amplitude was 53.5±18.7 μV and 31.8±13.0 μV, respectively, which was 1.6-fold lower (p=0.007) and 2.8-fold lower (p = 0.0009), respectively, than in controls (Fig. 2). It should be noted that a decline in the function of inner retinal cells becomes greater with an increase in myopia: in high myopes (Group 2), the b-wave amplitude was 1.7-fold lower (p=0.03) than in moderate myopes (Group 1) who had undergone surgery for uncomplicated RRD (Fig. 2). In high and moderate myopes who had undergone surgery for combined RRD and CD, the mean b-wave amplitude in the affected eye was 23.6±21.9 μV, which was 3.8-fold lower (p=0.002) and 2.5-fold lower (p=0.04) than in normal eyes and the fellow eye, respectively. High variability (91.7%) confirms functional instability of the photopic system in myopes who had undergone surgery for combined RRD and CD (Table 3, Fig. 3).
Therefore, in myopes who had undergone surgery for uncomplicated RRD, and in those who had undergone surgery for combined RRD and CD, the photopic function of the inner retina was 1.6-2.8-fold lower and 3.8-fold lower, respectively, than in controls. We found a direct correlation (r=0.54; p < 0.05) between BCVA and b-wave implicit time for the photopic ERG in the affected eye. 30Hz flicker ERG reflects the state of retinal cones (Table 4). The ampli-tude of a flicker ERG in the control group was 54.3±18.5 μV (95% CI, 43.6 μV to 65.0 μV). In moderate myopes who had undergone surgery for uncomplicated RRD, the amplitude of a flicker ERG in the affected eye was 33.1±16.5 μV, which was 1.6-fold lower (p = 0.005) than in controls. There was no statistically significant difference in the amplitude of a flicker ERG in the affected eye among Groups 2 to 4, and the median amplitude in these groups was 21.5±12.0 μV, which was 2.5-fold lower (p = 0.005) than in controls. In addition, in groups 1, 2, 3 and 4, the amplitude in the fellow eye was 2.0-fold lower (p = 0.04), 1.7-fold lower (p = 0.02), 2.1-fold lower (p = 0.04), and 2.0-fold lower (p = 0.06), respectively, than in the operated eye.
Conclusion First, a-wave and b-wave implicit times of the photopic cone response (a) reflect conductance related to the photoreceptor and inner layers, respectively, of the central retina, and (b) were 25% longer and 28% longer, respectively, for myopic eyes after surgery for either uncomplicated RRD or combined RRD and CD, than for normal eyes. Second, a-wave amplitude of the photopic cone response (a) reflects the summed bioelectrical activity of retinal photoreceptor cones, and (b) in moderately or highly myopic eyes after surgery for uncomplicated RRD was 1.9-fold and 3.5-fold lower, respectively, and in moderately or highly myopic eyes after surgery for combined RRD and CD, 3.9-fold and 6.6-fold lower, respectively, than in normal eyes. Third, b-wave amplitude of the photopic cone response (a) reflects the summed bioelectrical activity of the retinal inner layers, and (b) in moderately or highly myopic eyes after surgery for uncomplicated RRD was 1.6-fold and 2.8-fold lower, respectively, whereas in those after surgery for combined RRD and CD, 3.8-fold lower, than in normal eyes. Fourth, amplitude of photopic 30 Hz flicker cone response (a) reflects the bioelectrical activity of cones, and (b) in moderately myopic eyes after surgery for uncomplicated RRD was 1.6-fold lower, whereas in myopic eyes after surgery for combined RRD and CD, 2.8-fold lower, than in normal eyes.
Finally, we found direct correlations between BCVA and b-wave and a-wave amplitudes of the photopic cone response (r = 0.61 and r = 0.54, respectively), and 30 Hz flicker response amplitude (r = 0.53), and an inverse correlation (r=-0.53) between BCVA and a-wave implicit time of the photopic cone response for myopic eyes after surgery for either uncomplicated RRD or combined RRD and CD. References
|