J.ophthalmol.(Ukraine).2017;5:39-44.
|
https://doi.org/10.31288/oftalmolzh201753944 Serum adiponectin levels in obese type 2 diabetic patients with diabetic retinopathy N.V. Malachkova1, Cand Sc (Med), M.L. Kyryliuk2, Dr Sc (Med), Prof., I.V. Komarovska1, Post-grad Student 1Pirogov National Medical University Vinnytsia (Ukraine) 2Department of Neuroendocrinology, Ukrainian Research Center for Endocrine Surgery, Transplantation of Endocrine Organs and Tissues of Ministry of Public Health of Ukraine Kyiv (Ukraine) E-mail: natali.malachkova@mail.ru Background. Diabetic retinopathy (DR) is one of the main complications of diabetes mellitus, the main cause of irreversible blindness in patients of working age in industrialized countries, has a high incidence rates and refers to neovascular eye diseases. At the present time, additional factors that affect the sensitivity to chronic hyperglycemia, the formation of microvascular complications, in particular, DR, include obesity and obesity-associated hormones of adipose tissue (adipokines: leptin, adiponectin, resistin, etc.). Purpose. To investigate the serum adiponectin levels in patients at different stages of DR in type 2 diabetes mellitus (T2DM) and obesity. Material and Methods. Study involved 99 patients, divided into 2 groups. The 1-st group (control group) consisted of 23 persons with obesity without T2DM (both male and female subjects; mean age, 57.03 ± 4.91 years), the 2-nd group consisted of 76 patients with T2DM, obesity and DR (both male and female subjects; mean age, 59.98 ± 4.17 years; mean duration of diabetes, 10.01 ± 2.81 years; mean glycated hemoglobin (HbA1C) level, 10.94 ± 2.08%), subdivided into 3 subgroups: with minimal and mild non-proliferative DR, with moderate to severe non-proliferative DR, with proliferative DR. The concentration of serum adiponectin was determined by ELISA kit. Statistical analysis included one- and two-factor analysis of variance. Results. Patients with mild non-proliferative DR had somewhat lower (worst) adiponectin levels among patients aged 60 and below with DM subcompensation. The lowest serum adiponectin levels were common for moderate to severe non-proliferative DR among T2DM patients aged above 60 with duration of diabetes of 10 years or less and with T2DM compensation. Among T2DM patients with proliferative DR, the worst serum adiponectin levels were common for T2DM patients aged 60 and below with duration of diabetes of less than 10 years and with T2DM compensation. Considering statistic values of serum adiponectin levels for this stage, it should be noted that, for conditionally combined proliferative DR with severe and moderate DR, statistically significant changes (р=0.007) consisted in the decreased serum adiponectin levels in T2DM compensation. Conclusions. Minimal and mild non-proliferative DR is characterized by a significant lower serum adiponectin level compared with the subsequent stages in subcompensation of T2DM. Key-words: diabetic retinopathy, type 2 diabetes mellitus, obesity, adiponectin. Background Our previous studies on the state of carbohydrate metabolism and insulin resistance in patients with diabetic retinopathy (DR) in type 2 diabetes mellitus (T2DM) and obesity have shown that all patients with DR had chronic hyperglycemia with decompensated T2DM and the greatest proportion of patients with adverse indices of insulin resistance and with reduced sensitivity to insulin had proliferative DR [23]. According to the literature, in DR patients, hyperglycemia can induce the damage of retinal vascular endothelial cells, ischemia and adhesion of leukocytes to vascular endothelium [29, 30], synthesis of proangiogenic factors and cytokine excess, which results in small vessel functioning disorders and abnormal neovascular formations [10]. One of these cytokines is an adipose tissue-secreted adipokine, in particular adiponectin, modulating metabolic response. The absence of adiponectin in mice has been found to develop the insulin resistance, obesity, hyperglycemia, arterial hypertension and endothelial dysfunction [21, 25]. There are clinical studies on screening the relationship between plasma adiponectin concentration and DR severity [11, 24]. However, it is not always easy to interpret the findings of the adiponectin level test in T2DP patients with DR, which is associated with various test designs and methodological approaches to evaluating the hormone’s concentration in blood. The purpose of the present paper was to study serum adiponectin levels in obese patients with type 2 diabetes mellitus and different stages of diabetic retinopathy. Material and Methods Ninety-nine patients (112 eyes), divided into two groups, were involved in the study. Group 1 (controls) consisted of 23 overweight or obese persons without T2DM (both male and female subjects; mean age, 57.03±4.91); Group 2 comprised 76 obese T2DM patients with DR (both male and female subjects; mean age, 59.98±4.17; mean DM duration, 10.01±2.81 years; mean glycated hemoglobin (HbA1C) level, 10.94±2.08%). Inclusion criteria were age >18; T2DM; DR; and obesity (or overweight). Exclusion criteria were an endocrine and somatic disease leading to obesity; acute infectious disease; type 1 diabetes mellitus; cancer; comorbidity decompensation; mental disorders; administration of neuroleptics and antidepressants; proteinuria; clinically significant maculopathy; optic nerve damage; glaucoma; and cataract. The study followed the tenets of the Declaration of Helsinki of the World Medical Association (Seoul, 2008) and corresponding orders of Ministry of Health of Ukraine (No 281 dated November, 01, 2000; No 355 dated September, 25, 2002; No1118 dated December, 21, 2012). Obesity in the groups was detected according to body mass index (BMI). Glycemic control treatment included diet plus metformin (type 1); diet plus metformin and oral glycemic control agents (OGCA) (type 2); diet plus metmorfin and insulin therapy (type 3). HbA1C levels were determined by high-performance liquid chromatography. The target HbA1C level was determined following the recommendations of International Diabetes Federation (IDF) [13]. Serum adiponectin levels were measured spectrophotometrically with an ELISA reader using Human Adiponectin ELISA Kit. Serum adiponectin level was considered normal if it was ? 0.1 µg/ml and ? 10.0 µg/ml. Ophthalmologic examination included visual acuity, tonometry, Humphrey perimetry, biomicroscopy, ophthalmoscopy, Goldmann contact lens fundus examination, OCT, fundus camera examination and photography. If required, fluoresceing angiography was performed. Retinopathy was graded according to the Diabetic Retinopathy Disease Severity Scale (Wilkinson et al. 2003). According to this classification, DR patients were subdivided into three subgroups: mild non-proliferative DR (Subgroup 2A), moderate to severe non-proliferative DR (Subgroup 2B), and severe proliferative DR (Subgroup 2C). One-way and two-way ANOVA was used for statistical comparison. Data were analyzed using Kruskal-Wallis test or Fisher’s exact test (for parametric and non-parametric data respectively). Statistical data are presented as mean ± SEM and 95% confidence interval (CI). P < 0.05 was considered as significant. Statistical analysis was performed using SPSS 9.0 (SPSS Inc., Chicago, IL, USA). Results One-way ANOVA was used to analyze serum adiponectin levels in T2DM obese patients depending on the stage of diabetic retinopathy. Mean serum adiponectin levels in the groups are given in Table 1. It should be noted that mean serum adiponectin levels in all groups were below reference ranges for non-obese patients, males and females. ANOVA showed no statistical difference in mean serum adiponectin levels among patients in Control group and in Subgroups 2A and 2B with a certain decrease in its level in Subgroup 2A.
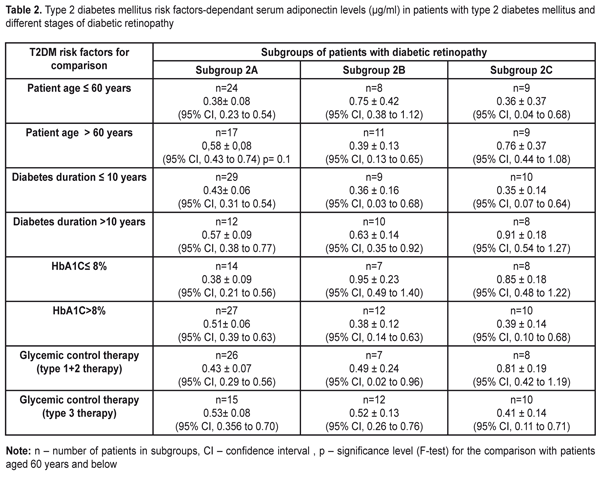
A post-hoc two-way ANOVA to test for the effects of patient’s age, duration of diabetes, HbA1C level and type of glycemic control therapy revealed a number of details (Table 2).
The minimal values for mean serum adiponectin levels among patients aged 60 and below were observed in patients with proliferative DR whereas among patients aged above 60, they were observed in patients of Subgroup 2B. Additionally, the minimal (worst) values for mean serum adiponectin levels (and their CIs) were observed in patients below 60 with proliferative DR and the maximal (best) values for mean serum adiponectin levels were also observed in proliferative DR in patients above 60. Mean serum adiponectin levels trended to be increased in the patients aged 60 and below compared with the older group. Analysis of serum adiponectin levels in patients with different stages of DR in dependence on the duration of diabetes showed the lowest values for mean serum adiponectin levels among patients with duration of diabetes of less than 10 years (and CIs) in Subgroups 2B and 2C and among patients with duration of diabetes of more than 10 years in Subgroup 2A. The highest mean serum adiponectin levels were noted in proliferative DR patients with duration of diabetes of more than 10 years. The lowest mean serum adiponectin levels (hypoadiponectinemia) among diabetic patients with HbA1C ? 8% were observed in patients of Subgroup 2A, whereas among diabetic patients with HbA1C > 8% they were observed in patients of Subgroup 2B. And the lowest (worst) serum adiponectin level was noted in patients with HbA1C > 8% of Subgroup 2B, whereas the highest (best) one was observed in patients with HbA1C ? 8% of Subgroup 2B. Statistically significant difference (р=0.007) was revealed when comparing Subgroup 2A with Subgroup 2B+2C for HbA1C ? 8%, 0.38±0.09 µg/ml vs 0.89±0.14 µg/ml, respectively, (р=0.007) and when comparing Subgroups 2B+2C for HbA1C ? 8% and HbA1C > 8%, 0.89±0.14 µg/ml vs 0.39±0.11 µg/ml, respectively, (р=0.007). Decreased serum adiponectin levels among diabetic patients with HbA1C > 8% could advance the mild non-proliferative stage of diabetic retinopathy to subsequent stages. A type of glycemic control therapy for T2DM did not have statistically significant effect on mean serum adiponectin levels in all DR stages studied. The lowerst values of mean serum adiponectin levels among patients with type 1 and type 2 glycemic control therapy (oral glycemic control agents) were observed in Subgroup 2A and among patients with type 3 glycemic control therapy (metmorfin and insulin therapy) they were observed in proliferative DR patients of Subgroup 2C. In general, the best (highest) mean serum adiponectin levels were observed in proliferative DR patients taking tablet agents and the worst ones were in patients getting insulin therapy. Discussion Diabetic retinopathy is one of the main complications of diabetes mellitus and the leading course of blindness in working age persons in developed industrial countries [32]. DR has high incidence rates and refers to neovascular eye diseases including retrolental fibroplasias (retinopathy of prematurity) and age-related macular degeneration, in which new vascular formation occurs under hypoxia or metabolic abnormalities which affect energy transfer. A lot of factors (insufficient control of blood glucose level, hypertony, dyslipidemia) have been found to have an effect on progression of diabetic retinopathy; kidney diseases are also important in this regard and can contribute to DR development [14, 18, 34]. Although the duration is the most important risk factor (as for DR) [14], not all patients with insufficient glycemic control developed retinopathy (especially its proliferative stage) over time. And, contrary, strict glycemic control is not always able to prevent from DR development. However, researchers share the same opinion that duration of diabetes mellitus and degree (severity) of hyperglycemia are the main risk factors for DR development [2]. Results of some studies using a family cluster principle assume that there are additional components that have an effect on sensitivity to chronic hyperglycemia, in particular, a hereditary factor [7] and, based on to-date data, hormones of adipose tissue (leptin, adiponectin, resistin, etc.). Adiponectin (APN, also called Acrp30, apM1) is a protein which is secreted by adypocytes, with the most abundant gen transcript 1 (apM1) [22], plays an important role in formation of antiatherogenic anti-inflammatory agents and insulin [6, 8, 31, 33], controls insulin sensitivity of tissues, and can also be involved in non-specific inflammation process [16, 17, 36], being a significant modulator of metabolic disorders and vascular diseases. Our analysis of the hormonal predictors (like adiponectin, an adipose tissue-specific hormone) of diabetic retinopathy development revealed that patients with mild non-proliferative DR had somewhat lower (worst) adiponectin levels among patients aged 60 and below with DM subcompensation. The lowest serum adiponectin levels were common for moderate to severe non-proliferative DR among T2DM patients aged above 60 with duration of diabetes of 10 years or less and with T2DM compensation. Among T2DM patients with proliferative DR, the worst serum adiponectin levels were common for T2DM patients aged 60 and below with duration of diabetes of less than 10 years and with T2DM compensation. Considering statistic values of serum adiponectin levels for this stage, it should be noted that, for conditionally combined proliferative DR and severe and moderate non-proliferative DR, statistically significant changes (р=0.007) consisted in the decreased serum adiponectin levels in T2DM compensation. Analyzed the finding of the present study, we can conclude that the decreased adiponectin levels are common for all stages of DR and have somewhat distribution characteristics due to risk factors and a stage of the disease. The absence of the protective effect of adiponectin makes vascular walls unprotected, which may contribute to DR development at early stage (mild proliferative DR) in patients aged below 60 and enhance the pathogenic effect of chronic hyperglycemia at later DR stages when hyperglycemia control is worsened. Since adiponectin has an anti-inflammatory effect and can increase glucose tolerance levels, we believe that hypoadiponectinemia can contribute to pathogenesis of T2DM and development of vascular complications [5, 9]. An interesting fact is that diabetes sensitivity locus in human is located on 3q27 chromosome where an adiponectin gene is placed [35]. Impaired adiponectin secretion causes increased insulin resistance [19]; adiponectin gene polymorphisms are associated with retinopathy in diabetic patients [37]. Based on the to-date data in the literature, plasma adiponectin concentrations get reduced in obesity, insulin resistance, T2MD, ischemic heart disease, and arterial hypertension [1, 4, 12, 15, 20], which completely correlates with our findings. Adiponectin receptor 2, AdipoR2, (but not AdipoR1) reduces vascular changes caused by ischemic heart disease [28]. Some studies have shown that adiponectin has anti-inflammatory features and, thus, can inhibit atherogenesis [8, 26, 27]. Adiponectin dose-dependently inhibits tumor necrosis factor, stimulates monocyte adhesion to the arterial endothelium in human [27]. Besides, adiponectin inhibits binding and macrophage uptake of oxidized low density lipoproteins (LDL) [26]. Decreased adiponectin levels can stimulate the oxidase activity of nicotinamide adenine dinucleotide phosphate (NADPH) in human arterial walls, which leads to DR development [3]. The data presented above give reason to believe that adiponectin can have a protective action against development of diabetic vascular complications. In general, adiponectin can be considered as a useful marker of insulin resistance, a modulator of main DR drivers, and a pleiotropic effect agent. In DR, adiponectin can also serve as a marker of retinal damage since it is a mediator of angiogenesis and its increased level in certain cases can also be assessed as a marker of adiponectin resistance [16] (like leptin resistance). Conclusions 1. Hypoadiponectinemia is common for all DR stages in obese patients with type 2 diabetes mellitus. 2. One-way ELISA showed confidence interval of 0.35-0.73 µg/ml for serum adiponectin levels in obese T2DP patients with DR. 3. Mild non-proliferative DR is characterized by significantly lower serum adiponectin levels compared to subsequent stages in T2DM subcompensation. References: 1.Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y. Decreased plasma adiponectin concentration in patients with essential hypertension. American Journal of Hypertension. 2003; 16: 72–5. 2.Aiello LM. Perspectives on diabetic retinopathy. American Journal of Ophthalmology. 2003; 136: 122–35 3.AntonopoulosAS, MargaritisM, CoutinhoP, et al. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes.2015; 64: 2207–19 4.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and Biophysical Research Communications. 1999; 257: 79–83. 5.Be?towski J. Adiponectin and resistin – new hormones of white adipose tissue. Medical Science Monitor. 2003; 9: RA55–61. 6.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–9. 7.Diabetes Control and Complications Trial Research Group, Clustering of long-term complications in families with diabetes in the Diabetes Control and Complications Trial. Diabetes. 1997; 46: 1829–39. 8.D?ez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003; 148: 293–300. 9.Fernandez-Real JM, Botas-Cervero P, Lopez-Bermano A, Casamitjana R, Funahashi T, Delgado E, Kihara S, RicartW. Adiponectin is independently associated with glycosylated haemoglobin. European Journal of Endocrinology. 2012; 150: 201–5. 10.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005; 438: 960–6. 11.Hadjadj S, Aubert R, Fumeron F, et al. Increased plasma adiponectin concentrations are associated with microangiopathy in type 1 diabetic subjects. Diabetologia. 2005; 48: 1088–92. 12.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, NishidaM, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000; 20: 1595–99. 13.International Diabetes Federation.Managing older people with Type 2 Diabetes. Global Guideline.2013. 14.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study: II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Archives of Ophthalmology. 1984; 102: 520–6. 15.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003; 23: 85–9. 16.Kuo Jane Z, Guo Xiuqing, Klein Ronald, Klein Barbara E, Genter Pauline, Roll Kathryn, Yang Hai, Goodarzi Mark O, Rotter Jerome I, Chen Yii-Der Ida, Ipp Eli. Adiponectin, Insulin Sensitivity and Diabetic Retinopathy in Latinos With Type 2 Diabetes. JCEM. 2015; 100(9): 3348-55 17.Kuo JZ, Guo X, Klein R, et al. Systemic soluble tumor necrosis factor receptors 1 and 2 are associated with severity of diabetic retinopathy in Hispanics. Ophthalmology. 2012; 119: 1041–46 18.Leslie RD, Pozzilli P. An introduction to new advances in diabetes. DiabetesMetabolism Research and Reviews. 2002; 18Suppl 1: S1–S6. 19.LianK, DuC, LiuY, ZhuD, YanW, ZhangH, HongZ, LiuP, ZhangL, PeiH, ZhangJ, GaoC, XinC, ChengH, XiongL, TaoL.Impaired adiponectin signaling contributes to disturbed catabolism of branched-chai n amino acids in diabetic mice. Diabetes. 2015; 64: 49–59. 20.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002; 360: 57–8. 21.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nature Medicine. 2002; 8: 731–7. 22.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most Abundant Gene Transcript 1). Biochemical and Biophysical Research Communications. 1996; 221: 286–9. 23.Malachkova N. V., Komarovskaya I. V., Kyryliuk M. L. Uroven' glikemii i insulinorezistentnosti u bol'nyh s diabeticheskoj retinopatiej, saharnym diabetom 2-go tipa i ozhireniem[Blood glucose level and insulin resistance in patients with type 2 diabetic mellitus, diabetic retinopathy and obesity]. Mezhdunarodnyi Endokrinologicheskii Zhurnal (IEJ). 2017; 3(13): 27-32. DOI:10.22141/2224-0721.13.3.2017.104108 (InRussian) 24.Matsuda M, Kawasaki F, Yamada K, et al. Impact of adiposity and plasma adipocytokines on diabetic angiopathies in Japanese Type 2 diabetic subjects. Diabet Med.2004;21:881–8. 25.Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Nagasawa A, Kobayashi H, Hiraoka H, Komai N, Kaibe M, Rakugi H, Ogihara T, Matsuzawa Y. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003; 42: 231–4. 26.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001; 103: 1057–63. 27.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999; 100: 2473–76. 28.Parker-DuffenL, NakamuraK, SilverM, Zuria gaMA, MacLauchlanS, AprahamianTR, WalshK. Divergent roles for adiponectin receptor 1 (AdipoR1) and AdipoR2 in mediating revascularization and metabolic dysfunction in vivo. J. Biol. Chem. 2014; 289: 16200–13. 29.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002; 288: 2579–2588. 30.Spranger J, Pfeiffer AF. New concepts in pathogenesis and treatment of diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2001; 109 Suppl 2: S438–S450. 31.Stefan N, Stumvoll M. Adiponectin–its role in metabolism and beyond. Horm Metab Res. 2002; 34: 469–74. 32.Tielsch JM, Sommer A, Witt K, Katz J, Royall RM. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Archives of Ophthalmology. 1990; 108: 286–290. 33.Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med. 2002; 80: 696–702. 34.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. British Medical Journal. 1998; 317: 703–13. 35.Vionnet N, Hani El-H, Dupont S, Gallina S, Francke S, Dotte S, De Matos F, Durand E, Lepretre F, Lecoeur C, Gallina P, Zekiri L, Dina C, Froguel P. Genome wide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q2-qter and independent replication of a type diabetes locus on chromosome 1q2-q24. American Journal of Human Genetics. 2000; 67: 1470–80. 36.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013; 2: 133–141. 37.Zietz B, Buechler C, Kobuch K, Neumeier M, Sch?lmerich J, Sch?fler A. Serum levels of adiponectin are associated with diabetic retinopathy and with adiponectin gene mutations in Caucasian patients with diabetes mellitus type 2. Exp Clin Endocrinol Diabetes. 2008; 116: 532–6.
|