Abstract
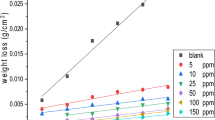
The corrosion inhibition effect of three different types of surfactants, namely, dodecyl trimethyl ammonium chloride (DTAC), octyl phenol poly (ethylene glycol ether) (TX-100), and dioctyl sodium sulfosuccinate (AOT) have been utilized as corrosion inhibitors for AISI 1037 carbon steel in 0.5 mol L–1 hydrochloric acid. The inhibition efficiency of the surfactants was studied via chemical and electrochemical techniques. The results proved that the order of protection efficiency is: DTAC > TX-100 > AOT. Also, the data revealed that the inhibition efficiency increases by increasing the concentrations of surfactants and decreases by increasing the temperature of the medium. Polarization curves indicated that the investigated surfactants are mixed-kind inhibitors. The adsorption of these surfactants on the AISI 1037 surface follows the Langmuir adsorption isotherm. The calculated free energy from the Langmuir adsorption isotherm indicated that the investigated surfactants are adsorbed on AISI 1037 via mixed adsorption, i.e. physisorption and chemisorption, in 0.5 mol L–1 HCl. The results of different tests are in excellent agreement.








Similar content being viewed by others
REFERENCES
Fouda, A.S., Zaki, E.G., and Khalifa, M.A., Some new nonionic surfactants based on propane tricarboxylic acid as corrosion inhibitors for low carbon steel in hydrochloric acid solutions, J. Bio- Tribo-Corros., 2019, vol. 5, no. 2, p. 1.
Atta, A.M., El-Mahdy, G.A., Allohedan, H.A., and Elsaeed, S.M., Application of nonionic surfactants based on rosin as corrosion inhibitor for tubing steel during acidization of petroleum oil and gas wells, Int. J. Electrochem. Sci., 2015, vol. 10, p. 8.
Hegazy, M.A., El-Etre, A.Y., El-Shafai, M., and Berry, K.M., Novel cationic surfactants for corrosion inhibition of carbon steel pipelines in oil and gas wells applications, J. Mol. Liq., 2016, vol. 214, p. 347.
Abdallah, M., Al-Jahdaly, B.A., and Al-Malyo, O.A., Evaluation of some nonionic surfactants derived from hydroquinol compounds as corrosion inhibitors for carbon steel in hydrochloric acid, Int. J. Electrochem. Sci., 2019, vol. 10, no. 3, p. 2740.
Sliem, M.H., Afifi, M., Radwan, A.B., Fayyad, E.M., et al., AEO7 surfactant as an eco-friendly corrosion inhibitor for carbon steel in HCl solution, Sci. Rep., 2019, vol. 9, p. 2319.
Zaafarany, I.A., Corrosion inhibition of mild steel in hydrochloric acid solution using cationic surfactant olyel-amido derivatives, Int. J. Electrochem. Sci., 2018, vol. 8, no. 7, p. 9531.
Deyab, M.A. and El-Rehim, S.A., On surfactant-polymer association and its effect on the corrosion behavior of carbon steel in cyclohexane propionic acid, Corros. Sci., 2012, vol. 65, p. 309.
Hany, M.A., Soliman, K.H., and Tantawy, A., Novel synthesized Schiff Base-based cationic gemini surfactants: Electrochemical investigation, theoretical modeling, and applicability as biodegradable inhibitors for mild steel against acidic corrosion, J. Mol. Liq., 2017, vol. 232, p. 478.
Sayed, R.A., Saleh, M.M., Al-Omair, A.M., Hany, M., et al., Efficient route synthesis of new polythiazoles and their inhibition characteristics of mild-steel corrosion in acidic chloride medium, J. Mol. Struct., 2019, vol. 118, p. 452.
Zhang, R. and Somasundaram, P., Advances in adsorption of surfactants and their mixtures at solid/solution interfaces, Adv. Colloid Interface Sci., 2006, vol. 123, p. 213.
Rafique, M.Z.A., Saxena, N., Khan, S., and Quraishi, M.A., Influence of surfactants on the corrosion inhibition behavior of 2-aminophenyl-5-mercapto-1-oxa-3, 4-diazole (AMOD) on mild steel, Mater. Chem. Phys., 2008, vol. 107, nos. 2–3, p. 528.
Fuchs-Godec, R., Effects of surfactants and their mixtures on inhibition of the corrosion process of ferritic stainless steel, Electrochim. Acta, 2009, vol. 54, p. 2171.
Tang, Y., Yao, L., Kong, C., Yang, W., et al., Molecular dynamics simulations of dodecyl amine adsorption on iron surfaces in aqueous solution, Corros. Sci., 2011, vol. 53, p. 2046.
Kornberg, B., Surfactant mixtures, Curr. Opin. Colloid Interface Sci., 1997, vol. 2, no. 5, p. 456.
Free, M.L., A new corrosion inhibition model for surfactants that more closely accounts for actual adsorption than traditional models that assume physical coverage is proportional to inhibition, Corros. Sci., 2004, vol. 46, p. 3101.
Free, M.L., Understanding the effect of surfactant aggregation on corrosion inhibition of mild steel in acidic medium, Corros. Sci., 2002, vol. 44, p. 2865.
Zhu, Y., Free, M.L., and Yi, G., Electrochemical measurement, modeling, and prediction of corrosion inhibition efficiency of ternary mixtures of homologous surfactants in salt solution, Corros. Sci., 2015, vol. 98, p. 417.
Peme, T., Olasunkanmi, L., Bahadur, I., Adekunle, A., et al., Adsorption and corrosion inhibition studies of some selected dyes as corrosion inhibitors for mild steel in acidic medium: gravimetric, electrochemical, quantum chemical studies and synergistic effect with iodide ions, Molecules, 2015, vol. 20, no. 9, p. 16004.
Fouda, A.S., Ibrahim, H., Rashwan, S., El-Hossiany, A., et al., Expired drug (pantoprazole sodium) as a corrosion inhibitor for high carbon steel in hydrochloric acid solution, Int. J. Electrochem. Sci., 2018, vol. 13, p. 6327.
Fouda, A.S., El-Dossoki, F.I., El-Hossiany, A., and Sello, E.A., Adsorption and anticorrosion behavior of expired meloxicam on mild steel in hydrochloric acid solution, Surf. Eng. Appl. Electrochem., 2020, vol. 56, no. 4, p. 491.
Inglezakis, V.J. and Zorpas, A.A., Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems, Desalin. Water Treat., 2012, vol. 39, nos. 1–3, p. 149.
Fouda, A.S., Rashwan, S.M., Shaban, S.M., Ibrahim, H.E., et al., Evaluation of a novel cationic surfactant based on 2-(2(dimethylamino) ethoxy)ethanol as a corrosion inhibitor for carbon steel 1018 in 1.0 M HCl solution, Egypt. J. Petroleum, 2018, vol. 27, no. 3, p. 295.
Fouda, A.S., Abd El-Maksoud, S.A., El-Hossiany, A., and Ibrahim, A., Evolution of the corrosion-inhibiting efficiency of novel hydrazine derivatives against corrosion of stainless steel 201 in acidic medium, Int. J. Electrochem. Sci., 2019, vol. 14, p. 6045.
Aslam, R., Mobin, M., Zehra, S., Obot, I.B., et al., N, N′-dialkylcystine gemini and monomeric N-alkyl cysteine surfactants as corrosion inhibitors on mild steel corrosion in 1M HCl solution: A comparative study, ACS Omega, 2017, vol. 2, no. 9, p. 5691.
Fouda, A.S., El-Maksoud, S.A., Belal, A.A.M., El-Hossiany, A., et al., Effectiveness of some organic compounds as corrosion inhibitors for stainless steel 201 in 1 M HCl: Experimental and theoretical studies, Int. J. Electrochem. Sci., 2018, vol. 13, no. 10, p. 9826.
Abd-Elaal, A.A., Elbasiony, N.M., Shaban, S.M., and Zaki, E.G., Studying the corrosion inhibition of some prepared nonionic surfactants based on 3-(4-hydroxyphenyl) propanoic acid and estimating the influence of silver nanoparticles on the surface parameters, J. Mol. Liq., 2018, vol. 249, p. 304.
Fouda, A.S., El-Gharkawy, E., Ramadan, H., and El-Hossiany, A., Corrosion resistance of mild steel in hydrochloric acid solutions by clinopodiumacinos as a green inhibitor, Biointerface Res. Appl. Chem., 2021, vol. 11, no. 2, p. 9786.
Zhu, Y. and Freem, M.L., Effects of surfactant aggregation and adsorption on steel corrosion inhibition in salt solution, Polym. Sci., 2015, vol. 1, p. 1.
Bahrami, M.J., Hosseini, S.M.A., and Pilvar, P., Experimental and theoretical investigation of organic compounds as inhibitors for mild steel corrosion in sulfuric acid medium, Corros. Sci., 2010, vol. 52, p. 2793.
Fouda, A.S., Mahmoud, W.M., and Elawayeb, K.M.A., Unused clopidogrel drug as eco-friendly corrosion inhibitor for carbon steel in aqueous media, Prot. Met. Phys. Chem. Surf., 2017, vol. 53, no. 1, p. 139.
Fouda, A.S., Eissa, M., and El-Hossiany, A., Ciprofloxacin as eco-friendly corrosion inhibitor for carbon steel in hydrochloric acid solution, Int. J. Electrochem. Sci., 2018, vol. 13, p. 11096.
Fouda, A.S., Abd El-Ghaffar, M.A., Sherif, M.H., Taher El-Habab, A., et al., Novel anionic 4-tert-octyl phenol ethoxylate phosphate surfactant as corrosion inhibitor for C-steel in acidic media, Prot. Met. Phys. Chem. Surf., 2020, vol. 56, no. 1, p. 189.
Yousef, W.M., Reda, Y., and Eldesoky, A.M., Electrochemical studies on the corrosion behaviour of reinforcing steel in the presence of cyanoacetamide derivatives as corrosion inhibitors, Int. J. Electrochem. Sci, 2018, vol. 13, p. 12172.
Chen, Y., Chen, X.H., Liu, Y.W., Yang, Z.N., et al., Evaluation of physical and chemical adsorption using electrochemical noise technique for methylene blue on mild steel, J. Chem. Thermodyn., 2018, vol. 126, p. 147.
Fouda, A.S., Abdel Azeem, M., Mohamed, S.A., El-Hossiany, A., et al., Corrosion inhibition and adsorption behavior of nerium oleander extract on carbon steel in hydrochloric acid solution, Int. J. Electrochem. Sci., 2019, vol. 14, p. 3932.
Fouda, A.S., Attia, A.M., and Rashed, A.M., Corrosion inhibition of mild steel in aqueous solutions using nonionic surfactants, Prot. Met. Phys. Chem. Surf., 2017, vol. 53, no. 4, p. 743.
Fouda, A.S., Al-Hazmi, N.E., El-Zehry, H.H., and El-Hossainy, A., Electrochemical and surface characterization of Chondria macrocarpa extract (CME) as save corrosion inhibitor for aluminum in 1 M HCl medium, J. Appl. Chem., 2020, vol. 9, no. 3, p. 362.
Srivastava, V., Haque, J., Verma, C., Singh, P., et al., Amino acid-based imidazolium zwitterions as novel and green corrosion inhibitors for mild steel: Experimental, DFT and MD studies, J. Mol. Liq., 2017, vol. 244, p. 340.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
About this article
Cite this article
Abdelmonem, H., Al-Bonayan, A.M. & Fouda, A.EA. Some Surfactants as Corrosion Inhibitors for Carbon Steel in Acidic Solutions. Surf. Engin. Appl.Electrochem. 58, 412–423 (2022). https://doi.org/10.3103/S1068375522040020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375522040020