Abstract
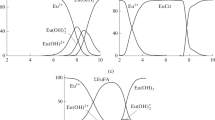
The efficiency of the sorption of Eu(III) by various forms of Mg,Al- and Zn,Al-layered double hydroxides (LDH) from model and natural waters at various pH was estimated for concentrating this metal ions and its subsequent spectrophotometric determination. It was found that the almost complete extraction of Eu(III) is reached on the carbonate form of Mg,Al-LDH by a deposition mechanism because of the formation of poorly soluble compounds of this element (carbonate and hydroxide of Eu(III) at pH ≥ 6.2, which correlates with the calculated Eu(III) speciation in aqueous solution in the presence of carbonate ions. The distribution coefficients of Eu(III) are 1.4 × 106 cm3/g (the pH of the initial solution is ≥7.0; the pH of the solution after the sorption is ≥ 6.87), which is indicative of a high efficiency of this sorbent with respect to this metal ion. For Mg,Al- and Zn,Al-LDH intercalated with organic complex-forming ligands—citrate and ethylenediaminetetraacetate ions, the dominant mechanism Eu(III) extraction from aqueous media is the complex formation. The distribution coefficients of Eu(III) on these sorbents are (0.26—22.71) × 103 cm3/g. For concentrating Eu(III) and its spectrophotometric determination in natural waters, it was proposed to use the carbonate form of Mg,Al-LDH with its subsequent decomposition by a nitric acid solution after the sorption. It was shown that the major components of natural waters and the constituents of this sorption materials do not affect the correctness and accuracy of the determination of Eu(III) by this method with arsenazo III (at pH 2.6 ± 0.1). The linearity range of the calibration graph is 1–50 μg Eu(III) in 50 cm3 of the final solution cm3 (λ = 650 nm). The proposed method of concentrating and determining Eu(III) was tested on samples of model, tap, and surface waters (the sorbent weight is 0.100 g, and the water sample volume is 500 cm3).


Similar content being viewed by others
REFERENCES
Meshkova, S.B., Topilova, Z.M., and Gerasimenko, G.I., Polymethylmethacrylate as a sorbent for effective recovery of lanthanides from solutions and highly sensitive luminescence determination of europium and terbium in waters, Zh. Anal. Khim., 1993, vol. 48, no. 1, pp. 65–72.
Dyul’dya, S.V., Bratchenko, M.I., and Skorobogatov, M.A., Europium radionuclides as radiation sources for gamma-radiation technologies: Modeling of absorbed dose distributions in homogeneous media, Vopr. At. Nauki Tekh., Ser.: Fiz. Radiats. Povrezhdenii Radiats. Materialoved., 2004, no. 3 (85), pp. 128–140.
Zakharov, I.S., Kontrosh, L.V., Khramov, A.V., and Shumilov, O.I., Environmental hazard of rare earth metals, Izv. S.-Peterb. Gos. Elektrotekh. Univ., LETI, 2018, no. 8, pp. 91–97.
Solovykh, G.N., Golinskaya, L.V., and Kanunikova, E.A., Rare earth metals as a factor of mutagenicity, Gig. Sanit., 2012, no. 3, pp. 23–25.
Guidelines for Drinking-Water Quality, Geneva: World Health Org., 2017, 4th ed.
Stoyanov, A.O., Stoyanova, I.V., Chivireva, N.A., and Antonovich, V.P., Determination of different valence forms of cerium and europium (a review), Metody Ob’ekty Khim. Anal., 2013, vol. 8, no. 3, pp. 104–118.
Gaiduk, O.V., Gudzenko, L.V., Ivkova, T.I., Pantaler, R.P., and Blank, A.B., Control of the content of activating cerium, neodymium and europium additives in scintillation materials using spectrophotometric method, Visn. Khark. Nats. Univ., 2008, vol. 820, no. 16 (39), pp. 15–21.
Vasilechko, V., Grishchuk, G., Nizhnik, O., and Kalichak, Ya., Acid-modified Transcarpathian clinoptilolite as a sorbent for the extraction of trace amounts of europium (III), Visn. Lviv. Univ., Ser.: Khim., 2015, no. 56 (1), pp. 192–202.
Lukashova, M.S., Kharchenko, S.G., Belikov, K.N., Bryleva, E.Yu., Grebenyuk, N.N., Shcherbakov, I.B., and Kalchenko, V.I., Sorption preconcentration of Eu(III) ions from Csl solutions by silica gel Impregnated with 5,11,17,23-tetrakis (diisoprpoxyphosphorylmethyl) 25,26,27,28-tetrahydroxythiacalix[4]arene, Metody Ob’ekty Khim. Anal., 2015, vol. 10, no. 3, pp. 143–149.
Mahmoud, M.R. and Someda, H.H., Mg–Al layered double hydroxide intercalated with sodium lauryl sulfate as a sorbent for 152+154Eu from aqueous solutions, J. Radioanal. Nucl. Chem., 2012, vol. 292, pp. 1391–1400.
Pshinko, G.N., Kosorukov, A.A., Puzyrnaya, L.N., and Goncharuk, V.V., Layered double hydroxides intercalated with EDTA as effective sorbents for U(VI) recovery from wastewater, Radiochemistry, 2011, vol. 53, no. 3, pp. 303–307.
Pshinko, G.N., Puzyrnaya, L.N., Shunkov, V.S., Kosorukov, A.A., and Demchenko, V.Ya., Removal of radiocesium from aqueous media with zinc–aluminum layered double hydroxide intercalated with copper (II) hexacyanoferrate, Radiochemistry, 2018, vol. 60, no. 4, pp. 395–399.
Puzyrnaya, L.N., Pshinko, G.N., Zub, V.Ya., and Zuy, O.V., Removal of Cu(II), Co(II), and Cd(II) from water solutions by layered double hydroxides with different [Mg(II)]/[Fe(III)] molar ratio, Bull. Mater. Sci., 2020, vol. 43, no. 3, pp. 1–6.
Puzyrnaya, L.N., Pshinko, G.N., Yatsik, B.P., Zub, V.Ya., and Kosorukov, A.A., Extraction of U(VI) from aqueous media with layered Zn,Al and Mg,Al double hydroxides intercalated with citrate Ions and with their magnetic nanocomposites, Radiochemistry, 2020, vol. 62, no. 1, pp. 50–61.
Upor, E., Mohai, M., and Novak, G., Photometric Methods in Inorganic Trace Analysis, Amsterdam: Elsevier, 1985.
Ho, Y.S. and McKay, G., The kinetics of sorption of divalent metal ions onto sphagnum moss peat, Water Res., 2000, vol. 34, no. 3, pp. 735–742.
Spahiu, K. and Bruno, J., A Selected Thermodynamic Database for REE to be Used in HLNW Performance Assessment Exercises: SKB Technical Report 95-35, Stockholm: Nucl. Fuel Waste Manage. Comp., 1995.
Runde, W., Chemical interactions of actinides in the environment, Los Alamos Sci., 2000, no. 26, pp. 392–411.
Khimiya i tekhnologiya redkikh i rasseyannykh elementov (Chemistry and Technology of Rare and Scattered Elements), Bol’shakov, K.A., Ed., Moscow: Vysshaya Shkola, 1979.
Stumpf, T., Curtius, H., Walther, C., Dardenne, K., Ufer, K., and Fanghänel, T., Incorporation of Eu(III) into hydrotalcite: A TRLFS and EXAFS study, Environ. Sci. Technol., 2007, vol. 41, pp. 3186–3191.
Brown, G. and Gastache, M.C., Mixed magnesium-aluminum hydroxides, Clay Miner., 1967, vol. 7, no. 2, pp. 193–201.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Glyanchenko
About this article
Cite this article
Pshinko, G.N., Puzyrnaya, L.N., Shunkov, V.S. et al. Carbonate Form of Mg,Al-Layered Double Hydroxides for Concentrating Eu(III) and Its Subsequent Analytical Determination in Natural Aqueous Media. J. Water Chem. Technol. 42, 365–372 (2020). https://doi.org/10.3103/S1063455X20050094
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X20050094