Abstract
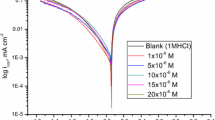
The inhibitive action of cysteine was investigated against the corrosion of aluminium alloy 6061-T6 (AA6061-T6) in a 0.5 M hydrochloric acidic medium in the temperatures range of 303–323 K using potentiodynamic polarization as well as electrochemical impedance spectroscopy methods. The electrochemical results of the former were validated by the latter. Cysteine showed mixed inhibitor behavior and exhibited good inhibition performance. The thermodynamic properties indicated mixed adsorption behavior of cysteine, which satisfied the Langmuir adsorption isotherm. The surface morphological investigation of both corroded and inhibited specimens was performed using a scanning electron microscopy and atomic force microscope, which revealed the adsorption of cysteine onto the alloy surface.









Similar content being viewed by others
REFERENCES
Davis, J.R., Corrosion of Aluminum and Aluminum Alloys, Materials Park, OH: ASM International, 1999, 1st ed.
Polmear, I.J., Light Alloys from Traditional Alloys to Nanocrystals, Oxford: Elsevier, 2006, 4th ed.
El-Shafei, A.A., Abd El-Maksoud, S.A., and Fouda, A.S., Corros. Sci., 2004, vol. 46, pp. 579–590.
Hamadi, L., Mansouri, S., Oulmi, K., and Kareche, A., Egypt. J. Petrol., 2018, vol. 27, pp. 1157–1165.
Oguzie, E.E., Li, Y., and Wang, F.H., Electrochim. Act-a, 2007, vol. 53, pp. 909–914.
Raja, A.S., Venkatesan, R., Sonisheeba, R., Thomas Paulraj, J., et al., Int. J. Adv. Res. Chem. Sci., 2014, vol. 1, pp. 101–109.
Wang, T., Wang, J., and Wu, Y., Corros. Sci., 2015, vol. 97, pp. 89–99.
Shkirskiy, V., Keil, P., Hintze-Bruening, H., Leroux, F., et al., Corros. Sci., 2015, vol. 100, pp. 101-112.
Mobin, M., Zehra, S., and Parveen, M., J. Mol. Liq., 2016, vol. 216, pp. 598–607.
Fontana, M.G., Corrosion Engineering, New York: McGraw-Hill, 1987, 3rd ed.
Khaled, K.F. and Al-Qahtani, M.M., Mater. Chem. Phys., 2009, vol. 113, pp. 150–158.
Riggs, Jr., O.L., Corrosion Inhibitors, Houston, TX: C.C. Nathan, 1973, 2nd ed.
Ateya, B.G., El-Khair, M.B.A., and Abdel-Hamed, I.A., Corros. Sci., 1976, vol. 16, pp. 163–169.
Abboud, Y., Abourriche, A., Saffaj, T., Berrada, M., et al., Appl. Surf. Sci., 2006, vol. 252, pp. 8178–8184.
Lee, E.J. and Pyun, S.I., Corros. Sci., 1995, vol. 37, pp. 157–168.
Noor, E.A., Mater. Chem. Phys., 2009, vol. 114, pp. 533–541.
Pinto, G.M., Nayak, J., and Nityananda Shetty, A., Mater. Chem. Phys., 2011, vol. 125, pp. 628–640.
Fakrudeen, S.P., Lokesh, H.B., Ananda Murthy, H.C., and Bheema Raju, V., IOSRJ. Appl. Chem., 2012, vol. 2, pp. 37–47.
Ashassi-Sorkhabi, H., Shabani, B. Aligholipour, B., and Seifzadeh, D., Appl. Surf. Sci., 2006, vol. 252, pp. 4039–4047.
Abd El Rehim, S.S., Hassan, H.H., and Amin, M.A., Mater. Chem. Phys., 2001, vol. 20, pp. 64–72.
Ech-chihbi, E., Belghiti, M.E., Salim, R., Oudda, H., et al., Surf. Interfaces, 2017, vol. 9, pp. 206–217.
Metikoš-Huković, M., Babič, R., and Grubač, Z., J. Appl. Electrochem., 1998, vol. 28, pp. 433–439.
Hsu, C.H. and Mansfeld, F., Corrosion, 2001, vol. 57, pp. 747–748.
Fouda, A.S., Mohamed, M.T., and Soltan, M.R., J. Electrochem. Sci. Technol., 2013, vol. 4, pp. 61–70.
Schorr, M. and Yahalom, J., Corros. Sci., 1972, vol. 12, pp. 867–868.
Ashassi-Sorkhabi, H., Shaabani, B., and Seifzadeh, D., Appl. Surf. Sci., 2005, vol. 239, pp. 154–164.
Abd El-Rehim, S.S., Ibrahim, M.A.M., and Khaled, K.F., J. Appl. Electrochem., 1999, vol. 29, pp. 593–599.
Bayol, E., Kayakırılmaz, K., and Erbil, M., Mater. Chem. Phys., 2007, vol. 104, pp. 74–82.
Kumari, P., Shetty, P., Rao, S.A., and Sunil, D., Trans. Indian Inst. Met., 2017, vol. 70, pp. 1139–1150.
Bereket, G. and Pinarbsi, A., Corros. Eng. Sci. Technol., 2004, vol. 39, pp. 308–312.
Abdel Ghanyl, N.A., El-Shenawy, A.E., and Hussien, W.A.M., Modern Appl. Sci., 2011, vol. 5, pp. 19–29.
Hackerman, N., Snavely, E.S., Jr., and Payne, J.S., Jr., J. Electrochem. Soc., 1966, vol. 113, pp. 671–686.
ACKNOWLEDGMENTS
The authors are grateful to Manipal Institute of Technology, Manipal Academy of Higher Education (A Deemed University), Manipal, India, for providing the laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Preethi Kumari P., Shetty, P., Nagalaxmi et al. Effect of Cysteine as Environmentally Friendly Inhibitor on AA6061-T6 Corrosion in 0.5 M HCl: Electrochemical and Surface Studies. Surf. Engin. Appl.Electrochem. 56, 624–634 (2020). https://doi.org/10.3103/S1068375520050087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375520050087