Abstract
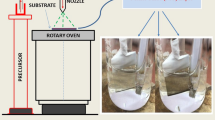
In this study, ZnS thin films were fabricated using an electrodeposition method from an aqueous electrolytic bath on a molybdenum (Mo) substrate and a spray pyrolysis method on a glass substrate. The potential range in which alloy electrodeposition of Zn and S could be carried out in a solution containing ZnSO4, Na2S2O3 and Na3C6H5O7 was determined by cyclic voltammetry. It was revealed that the thin film fabricated by the electrodeposition method was of ZnS and ZnO compounds and its microstructure was porous. The thin film prepared by the spray pyrolysis was smooth, with nano-grains. The band gap of the thin film fabricated by the spray pyrolysis was 3.72 eV, which was higher than that for the thin film fabricated by electrodeposition: 3.64 eV. Finally, the thin film fabricated by the spray pyrolysis was pure ZnS with a hexagonal structure, whereas that by electrodeposition was of ZnS (with a cubic structure) and ZnO compounds.
Similar content being viewed by others
References
Mandati, S., Sarada, B., Dey, S., and Joshi, S., Electron. Mater. Lett., 2015, vol. 11, no. 4, pp. 618–624.
Li, F.-Y., Dang, X.-Y., Zhang, L., Liu, F.-F., et al., Optoelectron. Lett., 2014, vol. 10, no. 4, pp. 266–268.
Xiang, J., Huang, X., Lin, G., Tang, J., Ju, C., and Miao, X., J. Electron. Mater., 2014, vol. 43, no. 7, pp. 2658–2666.
Chou, S.-H., Hsiao, Y.-J., Fang, T.-H., and Chou, P.-H., J. Mater. Eng. Perform., 2015, vol. 24, no. 6, pp. 2282–2286.
Sharbati, S., Keshmiri, S., McGoffin, J.T., and Geisthardt, R., Appl. Phys. A: Mater. Sci. Process., 2015, vol. 118, no. 4, pp. 1259–1265.
Witte, W., Hariskos, D., and Powalla, M., Thin Solid Films, 2011, vol. 519, no. 21, pp. 7549–7552.
Guo, P., Jiang, J., Shen, S., and Guo, L., Int. J. Hydrogen Energ., 2013, vol. 38, no. 29, pp. 13097–13103.
Weinhardt, L., Heske, C., Umbach, E., Niesen, T.P., et al., Appl. Phys. Lett., 2004, vol. 84, no. 16, pp. 3175–3177.
Mishra, A.K., Mishra, S.K., Pandey, S.P., and Mishra, K.L., Macromol. Symp., 2015, vol. 347, no. 1, pp. 49–51.
Chen, X., Liu, W., Zhang, G., Wu, N., Shi, L., and Pan, S., Adv. Mater. Sci. Eng., 2015, vol. 2015, pp. 1–8.
Magerramov, A.M., Ramazanov, M.A., and Mustafaeva, A.K., Surf. Eng. Appl. Electrochem., 2010, vol. 46, no. 3, pp. 281–284.
Yang, Y., Zheng, Y., Cao, W., Titov, A., et al., Nat. Photon., 2015, vol. 9, no. 4, pp. 259–266.
Park, S., Kim, S., Ko, H., and Lee, C., J. Electroceram., 2014, vol. 33, nos. 1–2, pp. 75–81.
Sivakumar, P., Gaurav Kumar, G.K., Sivakumar, P., and Renganathan, S., J. Nanostruct. Chem., 2014, vol. 4, no. 3, pp. 1–9.
La Porta, F.A., Ferrer, M.M., de Santana, Y.V.B., Raubach, C.W., et al., J. Alloy Compd., 2013, vol. 556, pp. 153–159.
Zhang, Y.C., Wang, G.Y., Hu, X.Y., and Chen, W.W., Mater. Res. Bull., 2006, vol. 41, no. 10, pp. 1817–1824.
Kumar, S. and Verma, N., J. Electron. Mater., 2015, vol. 44, no. 8, pp. 2829–2834.
Kumar, V., Sharma, M.K., Sandhub, G.S., Sharma, S., et al., IWPSD 2007, Int. Workshop, Mumbai: Inst. Electr. Electron. Eng., 2007.
Murali, K., IOSR J. Appl. Phys., 2014, vol. 6, no. 3, pp. 9–14.
Hennayaka, H. and Lee, H.S., Thin Solid Films, 2013, vol. 548, pp. 86–90.
Mkawi, E., Ibrahim, K., Ali, M., Farrukh, M., et al., J. Electron. Mater., 2015, vol. 44, no. 10, pp. 3380–3387.
Hwang, D., Ahn, J., Hui, K., and Son, Y., Nanoscale Res. Lett., 2012, vol. 7, no. 1, pp. 1–7.
McCloy, J.S. and Potter, B.G., Opt. Mater. Express., 2013, vol. 3, no. 9, pp. 1273–1278.
Xin, Z.-J., Peaty, R.J., Rutt, H.N., and Eason, R.W., Semicond. Sci. Technol., 1999, vol. 14, no. 8, pp. 695–698.
Lopez, M., Espinos, J., Martin, F., Leinen, D., et al., J. Cryst. Growth, 2005, vol. 285, no. 1, pp. 66–75.
Tokuda, T. and Yoshino, K., Phys. Status Solidi C, 2013, vol. 10, nos. 7–8, pp. 1102–1106.
Wei, A., Liu, J., Zhuang, M., and Zhao, Y., Mater. Sci. Semicond. Process., 2013, vol. 16, no. 6, pp. 1478–1484.
Heidari, G., Tavakoli, H., and Mousavi Khoie, S.M., J. Mater. Eng. Perform., 2010, vol. 19, no. 8, pp. 1183–1188.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Izi, M., Heidari, G., Mousavi Khoie, S.M. et al. Comparison of ZnS thin films fabricated by electrodeposition and spray pyrolysis methods. Surf. Engin. Appl.Electrochem. 53, 245–249 (2017). https://doi.org/10.3103/S1068375517030085
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375517030085