Abstract
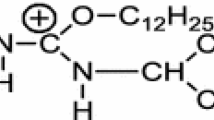
The adsorption of ionic surfactants on the water/air or water/hydrocarbon interface is considered. One form of the well-known Gibbs equation takes into account the surface excess of the amphiphilic ion in the compact layer, or monolayer, Γ m2 (2D adsorption), and the differential of the electrical potential of this layer. This expression is modified using some simplifying assumptions. The dependence of the surface tension, σ, on the activity of the amphiphilic ion, a 2, degree of gegen-ions binding in the compact layer, β, and Γ m2 is transformed into the following relationship: \(- \frac{{d\sigma }} {{RTd\ln a_2 }} = \Gamma _2^m \left\{ {2 - (1 - \beta )\frac{{d\ln \Gamma _2^m }} {{d\ln a_2 }}\left[ {\frac{1} {{\left[ {1 - \left( {\Gamma _2^m /\Gamma _2^{m\infty } } \right)} \right]^{1 + t} }} - \frac{{2b}} {{RT}}\Gamma _2^m } \right]} \right\}.\) Here Γ m2 denotes the Γ m2 value at complete filling of the adlayer, t = −1, 0, or +1 for the two-phase model of partition, for immobile or mobile monolayer respectively, b is the cohesion constant; both the long-tailed ion and the gegen-ion are single-charged. The usefulness of the proposed equation is discussed.
Similar content being viewed by others
References
Davies, J.T. and Rideal, E.K., Interfacial Phenomena, N.Y.-London: Academic Press, 1961, p. 480.
Shinoda, K., Nakagawa, T., Tamamushi, B.-i., and Isemura, T., Colloidal Surfactants, New York and London: Academic Press, 1963, p. 319.
Adamson, A.W., Physical Chemistry of Surfaces (Russian translation), Moscow: Mir, 1979.
Jaycock, M.J. and Parfitt, G.D., Chemistry of Interfaces (Russian translation), Moscow: Mir, 1984.
Chattoraj, D.K. and Birdi, K.S., Adsorption and the Gibbs Surface Excess, N.Y.-London: Plenum Press, 1984, p. 471.
Rusanov, A.I., Micellization in Surfactant Solutions, Reading: Harwood Academic Publishes, 1997, p. 326.
Lyklema, J., Fundamentals of Interface and Colloid Science, vol. III: Liquid-Fluid Interfaces, San Diego-London: Academic Press, 2000, p. 805.
Kralchevsky, P.A., Danov, K.D., and Denkov, N.D., Chemical physics of colloid systems and interfaces, in Handbook of Surface and Colloid Chemistry, 2009, ch. 7, pp. 197–377.
Poberezhnyi, V.V., Sotskova, T.Z., and Kulskiy, L.A., Electrostatic interactions in the ionic surfactant adsorption theory, Chemistry and Technology of Water (in Russian), 1996, vol. 18, pp. 570–591.
Lu, J.R., Thomas, R.K., and Penfold, J., Surfactant layers at the air/water interface: structure and composition, Adv. Coll. Int. Sci., 2000, vol. 84, pp. 143–304.
Prosser, A.J. and Franses, E.I., Adsorption and surface tension of ionic surfactants at the air-water interface: review and evaluation of equilibrium models, Coll. Surf., Ser. A, 2001, vol. 178, pp. 1–40.
Fainerman, V.B. and Vollhardt, D., Equilibrium and dynamics of 2D aggregating mixed monolayers consisting of soluble and insoluble amphiphiles, in Studies in Interface Science, Oxford: Elsevier, 2002, vol. 16, ch. 3, pp. 105–160.
Fainerman, V.B. and Lucassen-Reynders, E.H., Adsorption of single and mixed ionic surfactants at fluid interfaces, Adv. Coll. Int. Sci., 2002, vol. 96, pp. 295–323.
Gibbs, J.W., Thermodynamic Works (Russian translation), ed. V.K. Semenchenko, Moscow-Leningrad: Gos. Izdat. Tekhn.-Theoret. Lit., 1950.
Beattie, J.K. and Djerdjev, A.M., The Pristine oil/water interface: surfactant-free hydroxide-charged emulsions, Angew. Chem. Int. Ed., 2004, vol. 43, pp. 3568–3571.
Usui, S. and Healy, T.W., Zeta potential of insoluble monolayer of long-chain alcohol at the air-aqueous solution interface, J. Coll. Int. Sci., 2001, vol. 240, pp. 127–132, Ch. 1, pp. 1–71.
Mchedlov-Petrossyan, N.O., Vodolazkaya, N.A., and Kamneva, N.N., Acid-base equilibrium in aqueous micellar solutions of surfactants, in Micelles: Structural Biochemistry, Formation and Functions and Usage, N.Y.: Nova Publishers, 2013, p. 352, Ch. 1, pp. 1–71.
Brooks, J.H. and Pethica, B.A., Comparison of spread monolayers and adsorbed monolayers at an n-heptane/aqueous sodium chloride interface, Trans. Faraday Soc., 1965, vol. 61, pp. 571–576.
Rusanov, A.I. and Fainerman, V.B., Surface tension of surfactant solutions and characterization of micelles (in Russian), Doklady AN USSR, 1989, vol. 308, pp. 651–654.
Huang, X., Wang, Y., Dong, C., et al., Structure of adsorbed layers of nitrophenoxy-tailed quaternary ammonium surfactants at the air/water interface studied by neutron reflection, J. Coll. Int. Sci., 2008, vol. 325, pp. 114–121.
Eastoe, J., Nave, S., Downer, A., et al., Adsorption of ionic surfactants at the air-solution interface, Langmuir, 2000, vol. 16, pp. 4511–4518.
Bell, G.R., Li, Z.X., Bain, C.D., et al., Monolayers of hexadecyltrimethylammonium p-tosylate at the air-water interface, I. Sum-frequency spectroscopy, J. Phys. Chem., Ser. B, 1998, vol. 102, pp. 9461–9672.
Simon-Kutscher, J., Gericke, A., and Hühnerfuss, H., Effect of bivalent Ba, Cu, Ni, and Zn cations on the structure of octadecanoic acid monolayers at the air-water interface as determined by external infrared reflection/3-absorption spectroscopy, Langmuir, 1996, vol. 12, pp. 1027–1034.
Bae, S., Haage, K., Wantke, K., and Motschmann, H., On the factor in Gibbs equation for ionic surfactants, J. Phys. Chem., Ser. B, 1999, vol. 103, pp. 1045–1050.
Hall, D.G., Pethica, B.A., and Shinoda, K., The interpretation of surface tension data for solutions of ionic surfactants in the presence of electrolyte, Bull. Chem. Soc. Jpn., 1975, vol. 48, pp. 324–326.
Hall, D.G., The effect of electrolyte on ionic surfactant adsorption, Coll. Surf., Ser. A, 1994, vol. 90, pp. 285–288.
Warszyński, P., Barzyk, W., Lunkenheimer, K., and Fruhner, H., Surface tension and surface potential of Na n-dodecyl sulfate at the air-solution interface: model and experiment, J. Phys. Chem., Ser. B, 1998, vol. 102, pp. 10948–10957.
Davies, J.T., The distribution of ions under a charged monolayer, and surface equation of state for charged films, Proc. Roy Soc. London, Ser. A, 1951, vol. 208, pp. 224–247.
Sotskova, T.Z., Poberezhnij, V.Ya., Bazhenov, Yu.F., and Kul’sky, L.A., Investigation of potentials of the air-solution of ionogen surfactant interface (in Russian), Kolloidny Zhurn., 1983, vol. 45, pp. 108–113.
Haydon, D.A., A study of the relation between electrokinetic potential and surface charge density, Proc. Roy Soc. London, Ser. A, 1960, vol. 258, pp. 319–328.
Payens, Th.A.J., Ionized monolayers, Philips Res. Rep., 1955, vol. 10, pp. 425–481.
Cockbain, E.G., The adsorption of sodium dodecyl sulphate at the oil-water interface and application of the Gibbs equation, Trans. Faraday Soc., 1954, vol. 50, pp. 874–881.
Haydon, D.A. and Taylor, F.H., On adsorption at the oil/water interface and the calculation of electrical potentials in the aqueous surface phase, I: Neutral molecules and a simplified treatment of ions, Philos. Trans. Royal Soc., Ser. A, 1960, vol. 252, pp. 225–248.
Bell, G.M., Levine, S., and Pethica, B.A., The surface pressure of ionized monolayers, Trans. Faraday Soc., 1962, vol. 58, pp. 904–917.
Payens, T.A.J., The surface pressure of ionized monolayers: a comment, J. Coll. Int. Sci., 1970, vol. 33, pp. 480–481.
Bell, G.M., Levine, S., Pethica, B.A., and Stephens, D., The surface pressure of ionized monolayers: a reply to payens, J. Coll. Int. Sci., 1970, vol. 33, pp. 482–483.
Hachisu, S., Equation of state of ionized monolayers, J. Coll. Int. Sci., 1970, vol. 33, pp. 445–454.
Middleton, S.R., Pallas, N.R., Mingins, J., and Pethica, B.A., Thermodynamics of ionized monolayers: surface manometry on very low density spread mono-layers of sodium octadecyl sulfate at the air/water interface and analysis of ionic double layer contributions to the isotherms, J. Phys. Chem., Ser. C, 2001, vol. 115, pp. 8056–8063.
Huibers, P.D.T., Quantum-chemical calculations of the charge distribution in ionic surfactants, Langmuir, 1999, vol. 15, pp. 7546–7550.
Yan, P. and Xiao, J.-X., Polymer-surfactant interaction: differences between alkyl sulfate and alkyl sulfonate, Coll. Surf., Ser. A, 2004, vol. 244, pp. 39–44.
James, R.O., Application of site binding or surface complexation models to the estimation double layer free energy and of the surface pressure of ionizable and ionized surfaces, Coll. Surf., 1981, vol. 2, pp. 201–220.
Borwankar, R.P. and Wasan, D.T., Equilibrium and dynamics of adsorption of surfactants fluid-fluid interfaces, Chem. Eng. Sci., 1988, vol. 43, pp. 1323–1337.
Kalinin, V.V. and Radke, C.J., An ion-binding model for ionic surfactant adsorption at aqueous-fluid interfaces, Coll. Surf., Ser A, 1996, vol. 114, pp. 337–350.
Datwani, S.S. and Stebe, K.J., Monolayer penetration by a charged amphiphile: equilibrium and dynamics, Coll. Surf., Ser. A, 2001, voll. 192, pp. 109–129.
Kolev, V.L., Danov, K.D., Kralchevsky, P.A., et al., Comparison of the vand der Waals and Frumkin adsorption isotherms for sodium dodecyl sulfate at various sal concentrations, Langmuir, 2002, vol. 18, pp. 9106–9109.
Danov, K.D. and Kralchevsky, P.A., The Standard free energy of surfactant adsorption at air/water and oil/water interfaces: theoretical vs. empirical approaches, Coll. J., 2012, vol. 74, pp. 172–185.
Prosser, A.J. and Franses, E.I., New thermodynamic/electrostatic models of adsorption and tension equilibria of aqueous ionic surfactant mixtures: application to sodium dodecyl sulfate/sodium dodecyl sulfonate systems, J. Coll. Int. Sci., 2003, vol. 263, pp. 606–615.
Davies, J.T., Catalysis and reaction kinetics at liquid interfaces, Adv. Catal., 1954, vol. 6, pp. 1–65.
Markin, V.S., Volkova-Gugeshashvili, M.I., and Volkov, A.G., Adsorption at liquid interfaces: the generalized Langmuir isotherm and interfacial structure, J. Phys. Chem., Ser. B, 2006, vol. 110, pp. 11415–11420.
Langmuir, I., The distribution and orientation of molecules, Third Colloid Symp. Monogr., 1925, pp. 48–75.
Frumkin, A., Die Kapillarkurve der höheren Fettsäuren und die Zustandsgleichung der Oberflächenschicht, Z. phys. Chem., 1925, vol. 116, pp. 466–484.
Lu, J.R., Simister, E.A., Thomas, R.K., and Penfold, J., Structure of the surface of a surfactant solution above the critical micelle concentration, J. Phys. Chem., 1993, vol. 97, pp. 13907–13913.
Gilányi, T., Varga, I., and Mészáros, R., Specific counterion effect on the adsorption of alkali decyl sulfate surfactants at air/solution interface, Phys. Chem. Chem. Phys., 2004, vol. 6, pp. 4338–4346.
Warszyn-ski, P., Lunkenheimer, K., and Czichocki, G., Effect of counterion on the adsorption of ionic surfactants at fluid-fluid interfaces, Langmuir, 2002, vol. 18, pp. 2506–2514.
Aleiner, G.S. and Us’yarov, O.G., Electrical double layer at ionic surfactant solution-air interface, Coll. J., 2010, vol. 72, pp. 731–736.
Su, T.J., Lu, J.R., Thomas, R.K., and Penfold, J., Neutron reflection from counterions at the surface of a soluble surfactant solution, J. Phys. Chem., Ser. B, 1997, vol. 101, pp. 937–943.
Su, T.J., Thomas, R.K., and Penfold, J., Neutron reflection from counterions at the surface formed by a charged insoluble monolayer, Langmuir, 1997, vol. 13, pp. 2133–2142.
Mchedlov-Petrossyan, N.O., Protolytic equilibrium in lyophilic nano-sized dispersions: differentiating influence of the pseudophase and salt effects, Pure Appl. Chem., 2008, vol. 80, pp. 1459–1510.
Salley, D.J., Weith, A.J., Argyle, A.A., and Dixon, J.K., Measurements of the adsorption of surface-active agents at a solution/air interface by a radiotracer method, Proc. Roy Soc. London, Ser. A, 1950, vol. 203, pp. 42–55.
Flengas, S.N. and Rideal, E., A study of adsorption at the surface of dilute soap solutions by means of radioactive tracers, Trans. Faraday Soc., 1959, vol. 55, pp. 339–349.
Tajima, K., Muramatsu, M., and Sasaki, T., Radiotracer studies on adsorption of surface active substance at aqueous surface, I: Accurate measurements of adsorption of tritiated sodium dodecylsulfate, Bull. Chem. Soc. Jpn., 1970, vol. 43, pp. 1991–1998.
Tajima, K., Radiotracer studies on adsorption of surface active substance at aqueous surface, III: The effect of salts on the adsorption of sodium dodecylsulfate, Bull. Chem. Soc. Jpn., 1971, vol. 44, pp. 1767–1771.
Cross, A.W. and Jayson, G.G., The effect of small quantities of calcium on the adsorption of sodium dodecyl sulfate and calcium at the gas-liquid interface, J. Coll. Int. Sci., 1994, vol. 162, pp. 45–51.
Poberezhnij, V.Ya., Sotskova, T.Z., and Kul’sky, L.A., Adsorption of an ionogen surfactant and electrical surface properties of the liquid-gas interface (in Russian), Kolloidny Zhurn., 1982, vol. 44, pp. 41–46.
Poberezhnij, V.Ya. and Kul’sky, L.A., Electrosurface properties of the ionogen surfactant solution-air interface (in Russian), Kolloidny Zhurn., 1984, vol. 46, pp. 735–742.
Pethica, B.A., Are electrostatic potentials between regions of different chemical composition measurable? The Gibbs-Guggenheim principle reconsidered, extended and its consequences revisited, Phys. Chem. Chem. Phys., 2007, vol. 9, pp. 6243–6262.
Cheng, T., Chen, Q., Li, F., and Sun, H., Classic force field for predicting surface tension and interfacial properties of sodium dodecyl sulfate, J. Phys. Chem., Ser. B, 2010, vol. 114, pp. 13736–13744.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Mchedlov-Petrossyan, N.O. Adsorption of ionic surfactants on water/air interface: One more transformation of the Gibbs equation. Surf. Engin. Appl.Electrochem. 50, 173–182 (2014). https://doi.org/10.3103/S1068375514020100
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375514020100