Abstract
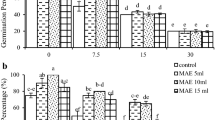
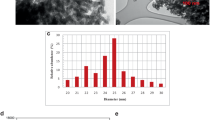
A study was conducted to evaluate the effect s of silver nanoparticles for ameliorating negative effects of salinity on germination and growth of Fenugreek seeds. In order to investigate salinity stress on Fenugreek germination indices, an experiment was carried out in Iran from October 2014 to November 2014 at Ferdowsi University of Mashhad, in the biotechnology Laboratory, to create salinity stress, sodium chloride (NaCl) at the levels of 0 (as control), 5, 10, 15 and 20 dS/m and Five levels of silver nanoparticles (0, 10, 20, 30 and 40 μg mL–1) on Fenugreek seed (Trigonella foenum-graecum); were tested Germination characteristics such as total germination (GT), Germination Speed Index (GSI), Shoot and Root of seedling long, Fresh Mass and Dry Mass were measured. Results showed the great effects of silver nanoparticles to improve salinity stress on Fenugreek seed germination. Results showed a significant reduction in germination percent and seedling growth due to the salinity stress while significantly increased with silicon nano-particles application. In without silver nanoparticle low level of salinity (0 dS/m) increased seed germination percentage, while the high levels (5, 10, 15 and 20 dS/m) inhibited the seed germination significantly. The results showed that the effect of AgNPs was significant on germination percentage in P ≤ 0.05. Overall, application of AgNPs was beneficial in improving salinity tolerance in the Fenugreek seedling and its application may stimulate the differences defense mechanisms of plants against salt toxicity.
Similar content being viewed by others
References
Ball, P., Natural strategies for the molecular engineer, Nanotechnology, 2002, vol. 13, pp. 15–28.
Beyer, E.M., A potent inhibitor of ethylene action in plants, Plant Physiol., 1976, vol. 58, no. 3, pp. 268–271.
Bliss, R.D., Plattaloia, K.A., and Thomson, W.W., Osmotic sensitivity in relation to salt sensitivity in germinating barley seeds, Plant Cell Environ., 1986, vol. 9, pp. 721–725.
Catalan, L., Balzarini, M., Taleisnik, E., Sereno, R., and Karlin, U., Effects of salinity on germination and seedling growth of Prosopis flexuosa (D.C.), Forest Ecol. Manage., 1994, vol. 63, pp. 347–357.
Chapman, V.J., Salt Marshes and Salt Deserts of the World, Stuttgart: J. Cramer Verlag, 1974.
Garthwaite, A.J., von Bothmer, R., and Colmer, T.D., Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl2 into the shoots, J. Exp. Bot., 2005, vol. 56, pp. 2365–2378.
Chen, X. and Schluesener, H.J., Nanosilver: Nano product in medical application, Toxicol. Lett., 2008, vol. 176, pp. 1–12.
FAO Agristat, 2007. http://www.fao.org. Cited June 10, 2010.
Freeman, C.E., Germination of response of a texas population of ocotillo (fouquieria splendens engelm) to constant temperature, water stress, ph and salinity, The American Midland Naturalist, 1973, vol. 89, pp. 252–256.
Fricke, W., Akhiyarova, G., Wei, W.X., et al., The short-term growth response to salt of the developing barley leaf, J. Exp. Bot., 2006, vol. 57, no. 5, pp. 1079–1095.
Hampson, C.R. and Simpson, G.M., Effects of temperature, salt, and osmotic potential on early growth of wheat (Triticum aestivum). 1. Germination, Can. J. Bot., 1990, vol. 68, pp. 524–528.
Hester, M.W., Mendelssohn, I.A., and McKee, K.L., Species and population variation to salinity stress in Panicum hemitomon, Spartina patens, and Spartina alterniflora: Morphological and physiological constraints, Environ. Exp. Bot., 2001, vol. 46, pp. 277–297.
Hong, F., Yang, F., Liu, C., Gao, Q., Wan, Z., Gu, F., Wu, C., Ma, Z., Zhou, J., and Yang, P., Influence of nano-TiO2 on the chloroplastaging of spinach under light, Biol. Trace Elem. Res., 2005, vol. 104, pp. 249–260.
Hu, L., Lu, H., Liu, Q.L., Chen, X.M., and Jiang, X.N., Overexpression of mtlD gene in transgenic Populus tomentosa improves salt tolerance through accumulation of mannitol, Tree Physiol., 2005, vol. 25, pp. 1273–1281.
Huang, J. and Redmann, R.E., Salt tolerance of Hordeum and Brassica species during germination and early seedling growth, Can. J. Plant Sci., 1995, vol. 75, pp. 815–819.
Kanai, M., Higuchi, K., Hagihara, T., et al., Common reed produces starch granules at the shoot base in response to salt stress, New Phytol., 2007, vol. 176, pp. 572–580.
Maggio, A., Miyazaki, S., Veronese, P., Fujita, T., Ibeas, J.I., Damsz, B., Narasimhan, M.L., Hasegawa, P.M., Joly, R.J., and Bressan, R.A., Does proline accumulation play an active role in stressinduced growth reduction, Plant J., 2002, vol. 31, pp. 699–712.
Maguire, J.D., Speed of germination—aid in selection and evaluation for seedling emergence and vigour, Crop Sci., 1962, vol. 2, pp. 176–177.
Mayer, A.M. and Mayber, P., The Germination of Seeds, Oxford: Pergamon Press, 1982.
Nel, A., Xia, T., Madler, L., and Li, N., Toxic potential of materials at the nanolevel, Science, 2006, vol. 311, pp. 622–627.
Neumann, P., Salinity resistance and plant growth revisited, Plant Cell Environ., 1997, vol. 20, pp. 1193–1198.
Graham, P.H. and Vance, C.P., Legumes: Importance and constraints to greater use, Plant Physiol., 2003, vol. 131, no. 3, pp. 872–877.
Roco, M.C., Broader societal issue on nanotechnology, J. Nanopart. Res., 2003, vol. 5, pp. 181–189.
Sharon, M., Choudhary, A.Kr., and Kumar, R., Nanotechnology in agricultural diseases and food safety, J. Phytol., 2010, vol. 2, no. 4, pp. 83–92.
Ungar I.A., Seed germination and seed-bank ecology of halophytes, in Seed Development and Germination, Kigel, J. and Galili, G., Eds., New York: Marcel Dekker, 1995, pp. 599–627.
Werner, J.E. and Finkelstein, R.R., Arabidopsis mutants with reduced response to NaCl and osmotic stress, Physiol. Plant., 1995, vol. 93, pp. 659–666.
Živković, S., Dević, M., Filipović, B., Giba, Z., and Grubišić, D., Effect of NaCl on seed germination in some Centaurium hill. species (Gentianaceae), Arch. Biol. Sci., 2007, vol. 59, no. 3, pp. 227–231.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Hojjat, S.S., Kamyab, M. The effect of silver nanoparticle on Fenugreek seed germination under salinity levels. Russ. Agricult. Sci. 43, 61–65 (2017). https://doi.org/10.3103/S1068367417010189
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068367417010189